Abstract
Background
Influx of extracellular Ca2+ into human lung mast cells (HLMCs) is essential for the FcεRI-dependent release of preformed granule-derived mediators and newly synthesized autacoids and cytokines. However, the identity of the ion channels underlying this Ca2+ influx is unknown. The recently discovered members of the CRACM/Orai ion channel family that carries the Ca2+ release–activated Ca2+ current are candidates.
Objectives
To investigate the expression and function of CRACM channels in HLMCs.
Methods
CRACM mRNA, protein, and functional expression were examined in purified HLMCs and isolated human bronchus.
Results
CRACM1, -2, and -3 mRNA transcripts and CRACM1 and -2 proteins were detectable in HLMCs. A CRACM-like current was detected following FcεRI-dependent HLMC activation and also in HLMCs dialyzed with 30 μM inositol triphosphate. The Ca2+-selective current obtained under both conditions was blocked by 10 μM La3+ and Gd3+, known blockers of CRACM channels, and 2 distinct and specific CRACM-channel blockers—GSK-7975A and Synta-66. Both blockers reduced FcεRI-dependent Ca2+ influx, and 3 μM GSK-7975A and Synta-66 reduced the release of histamine, leukotriene C4, and cytokines (IL-5/-8/-13 and TNFα) by up to 50%. Synta-66 also inhibited allergen-dependent bronchial smooth muscle contraction in ex vivo tissue.
Conclusions
The presence of CRACM channels, a CRACM-like current, and functional inhibition of HLMC Ca2+ influx, mediator release, and allergen-induced bronchial smooth muscle contraction by CRACM-channel blockers supports a role for CRACM channels in FcεRI-dependent HLMC secretion. CRACM channels are therefore a potential therapeutic target in the treatment of asthma and related allergic diseases.
Key words: CRACM, Orai, Ca2+, asthma, mast cell, histamine, leukotriene C4, cytokine, GSK-7975A, Synta-66
Abbreviations used: [Ca2+]i, Intracellular free Ca2+; CRAC, Ca2+ release–activated Ca2+; DMSO, Dimethyl sulfoxide; HLMCs, Human lung mast cells; LTC4, Leukotriene C4; MC, Mast cell
Mast cells play a major role in the pathophysiology of asthma and related allergic diseases such as rhinitis, urticaria, and anaphylaxis.1 Allergens and many nonimmunological stimuli activate complex signaling cascades in mast cells that lead to the secretion of a plethora of autacoid mediators, cytokines, and proteases.1 Excess release of these mediators contributes to complex immunopathologies and symptoms. Current putative clinical inhibitors of human mast cell (MC) mediator release, such as cromoglycate and β2-adrenoceptor agonists, are ineffective in some tissues such as the skin,2 and in lung they show weak activity and/or rapid tachyphylaxis/desensitization to the effects both in vitro and in vivo.2-5 A novel potent inhibitor of mast cell secretion that maintains its activity on chronic administration would therefore be of great benefit for the treatment of asthma and allergy.
Ion channels are emerging as attractive targets for the functional modulation of inflammatory and structural nonexcitable cells.6,7 Channels carrying Ca2+, K+, and Cl− regulate diverse cell processes including secretion,8 proliferation,9 adhesion,10 and migration.11 Influx of extracellular Ca2+ is an essential requirement for the IgE-dependent release of both preformed (granule-derived) mediators and newly generated autacoids and cytokines from mast cells.12 Receptor-mediated signaling in many nonexcitable cells including mast cells initiates an initial rise in intracellular Ca2+ because of its release from endoplasmic reticulum stores. The resulting store depletion induces Ca2+ entry through the plasma membrane, a process termed store-operated Ca2+ entry.13 The Ca2+ current passing through the plasma membrane is known as the Ca2+ release–activated Ca2+ (CRAC) current, and it is believed to play a central role in many physiological processes such as gene transcription, proliferation, and cytokine release.13,14 The Ca2+ release–activated Ca2+ current has been well characterized electrophysiologically in several cells including rodent mast cells,15 and the molecular components of the CRAC channel have been recently identified. STIM1 senses the endoplasmic reticulum Ca2+ concentration and transmits this information to the CRAC-channel pore.16 CRACM1 (also known as Orai1) was subsequently identified as the Ca2+-selective pore-forming protein in the plasma membrane.17-20 Mammalian cells express 2 further homologs—CRACM2 and CRACM321—which also carry CRAC currents but exhibit distinct functional properties.21 CRACM1 may form heterodimeric channels with CRACM2 and CRACM3.21
Studies in a CRACM1 knockout mouse suggested that CRACM1 function is essential for mast cell degranulation, leukotriene C4 (LTC4) release, and TNFα production following IgE-dependent activation, while CRACM2 regulates T-cell responses.22 However, whether CRACM channels operate in human MCs is not known. Because of the profound differences between rodent mast cells and human MCs in terms of pharmacology, mediator content, immunological responsiveness, and ion channel expression,23 it cannot be assumed that findings regarding Ca2+ channels in rodent mast cells can be extrapolated to humans. In this study, we have therefore examined the expression and function of CRACM channels in human lung mast cells (HLMCs).
Methods
Full experimental details are provided in the Methods section in this article’s Online Repository at www.jacionline.org.
Human MC purification and cell culture
All human subjects gave written informed consent, and the study was approved by the Leicestershire Research Ethics Committee. HLMCs were purified from macroscopically normal human lung (n = 11 donors) obtained within 1 hour of resection for lung cancer as described previously.24 Final HLMC purity was more than 99%, and viability was more than 97%. HLMCs were cultured as described previously.25
The human MC line HMC-1 (a gift from Dr J. Butterfield, Mayo Clinic, Rochester, Minn) was cultured in Iscove’s modified Dulbeccos’s medium as described previously.24 HEK293 cells were cultured in Dulbecco modified Eagle medium (Invitrogen, Paisley, United Kingdom) containing 10% FCS.
RT-PCR and quantitative RT-PCR
RT-PCR and quantitative RT-PCR were used to examine CRACM mRNA expression in HLMCs. Full details including primer sequences are provided in this article’s Online Repository at www.jacionline.org.
Analysis of CRACM protein expression
Analysis of CRACM protein expression was undertaken by using Western blot. Full experimental details and information on the antibodies used are provided in this article’s Online Repository at www.jacionline.org.
Patch-clamp electrophysiology
The whole-cell variant of the patch-clamp technique was used as described previously.26 Currents in some experiments were also evoked by using a ramp protocol consisting of a continuous voltage ramp from −120 to +120 mV. Further details are provided in this article’s Online Repository at www.jacionline.org.
The CRACM-channel blockers GSK-7975A and Synta-6627 (gifts from GlaxoSmithKline, Stevenage, United Kingdom), Gd3+, and La3+ were added directly to the recording chamber as required. GSK-7975A is compound 36 from patent WO 2010/1222089.
Ca2+ imaging
Changes in intracellular-free Ca2+ ([Ca2+]i) were monitored fluorometrically by use of the Ca2+-sensitive probe Fura-2, as described previously.8 Baseline measurements of HLMC [Ca2+]i were recorded as the mean of the 6 values preceding the addition of an anti-FcεRIα antibody (Fisher Scientific, Loughborough, United Kingdom). The postactivation value of [Ca2+]i was recorded as the mean of the 6 recordings taken immediately after the point of inflection following the rapid rise in [Ca2+]i.
HLMC activation for mediator release
Experiments were performed at 37°C. For the analysis of histamine and LTC4 release, 2 × 104 HLMCs in 80 μL were added to a 96-well V-bottom plate in triplicate, immediately followed by 10 μL of 10 times the final concentration of CRACM-channel blocker or dimethyl sulfoxide (DMSO) control. Plates were incubated for 10 minutes before the activation of cells by the addition of 10 μL of 10 times anti-FcεRIα antibody (final dilution 1:300). Plates were incubated for 30 minutes and centrifuged, and the supernatant was stored at −20°C for the measurement of mediator content. Control cell pellets were lyzed in ultrapure water for the determination of total histamine content. For the analysis of cytokine release, the final cell concentration was 0.666 × 106 cells/mL, and IgE-sensitized cells were activated with anti-IgE (Hybridoma Reagents Laboratory, Baltimore, Md; final concentration 2 μg/mL) for 16 hours before harvesting the culture supernatant.
Mediator assays
Histamine was measured by radioenzymatic assay and LTC4 by ELISA as described previously.24,26 The cytokines IL-5, IL-8, IL-13, and TNFα were measured blind by using the Meso Scale Discovery platform.
Allergen-induced bronchial smooth muscle contraction in isolated human bronchus
Lung tissue was obtained postmortem. Airways were dissected free of lung parenchyma and adjoining blood vessels. Secondary and tertiary bronchi, with cartilaginous walls and diameters of 3 to 10 mm, were cut spirally into strips 3 to 5 mm wide and then cut into pieces 10 to 15 mm long. The strips were passively sensitized overnight at room temperature (21°C) in atopic serum (20% v/v) in Krebs buffer. Before use, sensitized tissues were washed free of serum. Tissues were mounted under 1.5 g of resting tension in an immersion organ bath, maintained in oxygenated Krebs buffer solution at 37°C, and allowed to equilibrate for 30 to 45 minutes with 2 washes and retensioning if required.
Two preliminary “priming” contractions to 10 μM methacholine (Sigma, Poole, United Kingdom) were performed. The tissue was then incubated with Synta-66 (10 μM) or DMSO control (0.1% final concentration) for 1 hour. Grass allergen (Six grass mix, ALK-Abelló, Hungerford, United Kingdom) was then added cumulatively (0.1-30 U/mL final concentration), with contractions measured in milligrams tension. This was followed by a final measurement of contraction to 10 μM methacholine. Data were expressed as percentage of the initial 10 μM methacholine contraction.
Results
HMCs express CRACM1, -2, and -3 mRNAs
RT-PCR and quantitative RT-PCR experiments were performed to determine the expression of CRACM-channel mRNAs in HLMCs. Robust expression of CRACM1 (n = 8 donors), CRACM2 (n = 6 donors), and CRACM3 (n = 6 donors) was observed in all donors examined and also in HMC-1 cells (Fig 1, A and B). Normalizing the amount of each CRACM transcript to the amount of either β-actin mRNA (Fig 1, C) or 18S RNA (data not shown) revealed CRACM1 to be the most abundant of the 3 CRACM transcripts expressed in HLMCs. CRACM2 mRNA was the least abundantly expressed, with levels of CRACM3 being intermediate. Only the difference in levels of expression between CRACM1 and CRACM2 reached statistical significance (P = .0068; overall comparing all 3 transcripts: P = .0299). Similar relative amounts of CRACM transcripts were observed in HMC-1 cells (data not shown).
Fig 1.
HLMCs express CRACM1, CRACM2, and CRACM3 mRNAs. RT-PCR of CRACM1, -2, and -3 and rig/S15 mRNA transcripts using RNA purified from HLMCs (A) and from HMC-1 cells (B) in the presence (+) or absence (−) of reverse transcriptase. C, Quantitative RT-PCR of CRACM mRNA transcripts in HLMCs relative to β-actin transcripts (mean ± SEM; n = 6-8). CRACM1 versus CRACM2, P = .0068.
HLMCs express CRACM1 and -2 proteins
Western blotting of 3 HLMC lysates from 3 independent donors revealed the presence of bands close to the predicted molecular weight of CRACM1 (32.7 kDa) and CRACM2 (28.6 kDa) (Fig 2, A). A blocking peptide for CRACM2 was available and inhibited CRACM2 staining (Fig 2, B). Blotting for CRACM3 in HLMCs failed to convincingly demonstrate the presence of band(s) close to the predicted molecular weight of CRACM3 (31.5 kDa). A higher molecular weight band was however identified (Fig 2, C). The Western blotting of whole-cell lysates of HEK293 cells transiently transfected with a construct directing the expression of the myc epitope–tagged CRACM3 protein did reveal a band of the expected size, indicating that the band identified in HLMCs is likely to be nonspecific (Fig 2, D).
Fig 2.
HLMCs express CRACM1 and CRACM2 proteins. A, B, and C, Representative Western blots using the indicated antibodies and lysates of HMC-1 and HLMCs (Fig 2, A and B) or A-20 cells and HLMCs (Fig 2, C). Three independent mast cell lysates were analyzed. D, Western blots using the indicated antibodies and lysates of HEK293 cells transiently transfected with vectors directing the expression of CRACM1-Myc (lane 1), CRACM2-Myc (lane 2), or CRACM3-Myc (lane 3).
HLMCs express CRACM currents following store depletion with IP3
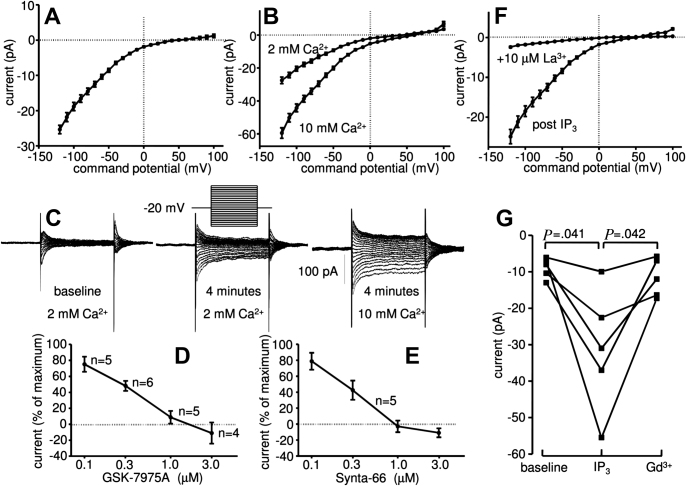
To investigate whether HLMCs expressed a Ca2+ current induced by store depletion, cells were dialyzed with 30 μM IP3. This resulted in the development of an inwardly rectifying current with the electrophysiological features of CRAC currents, the current carried by CRACM channels (Fig 3, A) in 90% of the cells tested. The subtracted IP3-dependent current (IP3 whole-cell current minus baseline whole-cell current) peaked at a mean of 25.4 ± 1.2 pA at −120 mV with a reversal potential of 49.9 ± 1.3 mV within 4 minutes of achieving the whole-cell configuration (n = 42 cells from 9 donors, P < .0001, compared with baseline for both current and reversal potential) (Fig 3, A). The IP3-dependent current was increased by 32.0 ± 3.2 pA by increasing extracellular Ca2+ to 10 mM (n = 6 cells; P = .004) (Fig 3, B), with reversal potential shifting to 59.6 ± 4.4 mV (P = .009). Inspection of the raw current obtained during voltage steps showed typical features of CRACM channels with current evoked immediately following each voltage step, and with mild decay over 100 ms (Fig 3, C). Similar results were seen by using voltage ramps (data not shown). Further pharmacological analysis of the current showed that it was blocked dose dependently by GSK-7975A (IC50 of 3.4 × 10−7 mol/L) (Fig 3, D) and Synta-66 (IC50 of 2.5 × 10−7 mol/L) (Fig 3, E). This is consistent with the blocking of CRACM channels by these compounds in previous studies27 (and unpublished data). In addition, the IP3-induced current was blocked by 10 μM La3+ (Fig 3, F) and 10 μM Gd3+ (Fig 3, G), consistent with the properties of CRACM channels.17,22,28 CRACM currents did not develop in control cells in the absence of IP3 (n = 9).
Fig 3.
HLMCs express CRACM-channel currents when dialyzed with IP3. Subtracted whole-cell patch-clamp current-voltage (I-V) curves from HLMCs 4 minutes after dialysis with IP3 in 2 mM external Ca2+ (mean ± SEM; n = 42 cells) (A) and in 2 mM and then 10 mM external Ca2+ (mean ± SEM; n = 6) (B). C, Representative raw current traces from a single cell in the presence of IP3 (voltage protocol shown inset). Inhibition of IP3-dependent whole-cell current by (D) GSK-7975A and (E) Synta-66 (n = 4-6) (a negative value implies a contribution from CRACM channels to the baseline whole-cell current) and (F) 10 μM La3+ (n = 12) and (G) 10 μM Gd3+.
HLMCs develop CRACM currents following activation with anti-IgE
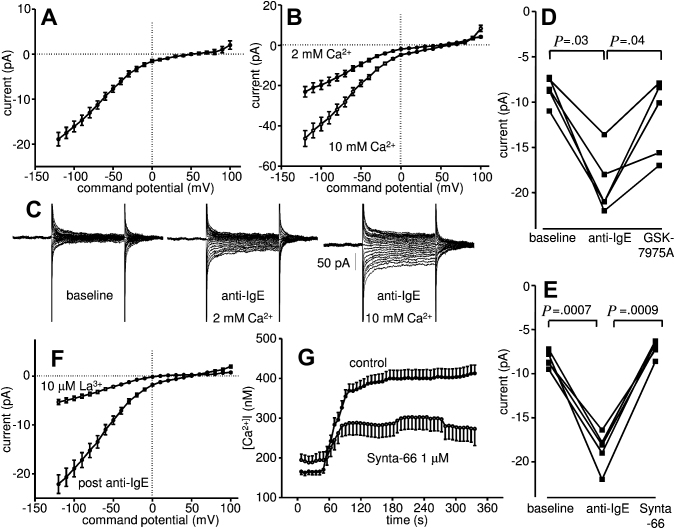
Cross-linking the high-affinity IgE receptor FcεRI with the addition of anti-IgE to the recording chamber induced the development of a current similar to that seen with IP3 in 90% of the HLMCs tested (Fig 4, A). The subtracted IgE-dependent current peaked at a mean of 18.9 ± 1.5 pA at −120 mV with a reversal potential of 49.1 ± 1.4 mV within 4 minutes of cell activation (n = 37 cells from 7 donors, P < .0001, compared with baseline for both current and reversal potential). The current was increased by 23.2 ± 3.9 pA by increasing extracellular Ca2+ to 10 mM (n = 7 cells; P = .003) (Fig 4, B), with a shift in reversal potential to 66.0 ± 3.9 mV (P = .005). Inspection of the raw current obtained during voltage steps again showed typical features of CRACM channels with current evoked immediately with each voltage step and with mild decay over 100 ms (Fig 4, C). Similar results were seen by using voltage ramps (data not shown). Further pharmacological analysis of the IgE-dependent current showed it was blocked by both 1 μM GSK-7975A (n = 5 cells; P = .04) (Fig 4, D) and 1 μM Synta-66 (n = 5 cells; P = .0009) (Fig 4, E). In addition, the IgE-dependent current was blocked by 10 μM La3+ (n = 11; P = .0003) (Fig 4, F). Taken together, these findings are consistent with the development of CRACM currents in HLMCs following IgE-dependent activation.
Fig 4.
HLMCs express CRACM-channel currents following FcεRI-dependent activation. Whole-cell patch-clamp current-voltage (I-V) curves of HLMCs within 4 minutes of FcεRI-dependent activation in (A) 2 mM external Ca2+ (mean ± SEM; n = 37 cells) and (B) 2 mM and then 10 mM external Ca2+ (mean ± SEM; n = 7). C, Representative raw current following FcεRI-dependent activation. Inhibition of IgE-dependent CRACM currents by (D) 1 μM GSK-7975A, (E) 1 μM Synta-66, and (F) 10 μM La3+ (n = 11 cells). G, Attenuation of the FcεRI-dependent increase in [Ca2+]i in HLMCs by 1 μM Synta-66.
CRACM-channel blockers attenuate IgE-dependent Ca2+ influx in HLMCs
Activation of HLMCs with anti-FcεRIα induced an acute increase in [Ca2+]i followed by a plateau phase as described previously8 (Fig 4, G). In total, 111 of 118 (94%) cells responded. In the absence of CRACM-channel blockers, [Ca2+]i increased from a mean baseline of 164.9 ± 9.0 to 375.6 ± 17.4 nM (n = 66 cells from 3 donors; P < .0001) following FcεRIα-dependent activation. In HLMCs from matched donors, [Ca2+]i increased from a mean baseline of 191.7 ± 16.4 to 287.0 ± 25.7 nM (n = 52 cells from 3 donors; P < .0001) with 1 μM Synta-66. There was a significant difference in the absolute change in [Ca2+]i in FcεRIα-activated control cells versus those activated in the presence of Synta-66 (P < .0001). In summary, 1 μM Synta-66 reduced the FcεRIα-dependent increase in [Ca2+]i by 54.8% ± 9.1%.
CRACM-channel blockers attenuate HLMC mediator release
Following activation with anti-FcεRIα in the presence of DMSO control, HLMCs released histamine (net 25.3% ± 4.8% of total histamine content, a marker of degranulation), 125.7 ± 32.5 ng/106 cells of LTC4 (a marker of arachidonic acid metabolism), and IL-5 (505.1 ± 142.7 pg/106 cells), IL-8 (8880 ± 3469 pg/106 cells), IL-13 (140.5 ± 78.7 pg/106 cells), and TNFα (618.3 ± 73.4 pg/106 cells). Both GSK-7975A and Synta-66 dose dependently attenuated the release of these mediators (P < .05 by repeated-measures ANOVA for all drugs and mediators with the exception of TNFα inhibition by Synta-66; P = .087) (Fig 5, A-C). Net IgE-dependent histamine release was reduced by 45.2% ± 2.5% (n = 5; P < .0001) and 38.8% ± 5.4% (n = 5; P < .0001) in the presence of 3 μM GSK-7975A and 3 μM Synta-66, respectively. A similar degree of inhibition was seen with the release of LTC4 (Fig 5, B) and the above cytokines (Fig 5, C).
Fig 5.

The CRACM-channel blockers GSK-7975A and Synta-66 inhibit HLMC histamine (A), LTC4(B), and cytokine release (C) dose-dependently following FcεRI-dependent activation. Mean ± SEM for percentage inhibition (n = 5 for histamine and LTC4, n = 3 for cytokines). P < .05 for all conditions except Synta-66 TNFα analyzed by repeated-measures ANOVA on raw data. ∗P < .05 compared with control using Bonferroni post hoc test.
CRACM blockade attenuates allergen-induced bronchial smooth muscle contraction in isolated human bronchus
Allergen-induced bronchoconstriction was assessed in isolated human bronchus. The application of allergen induced a dose-dependent increase in bronchial smooth muscle contraction (Fig 6) (geometric mean EC50 in DMSO control 0.825 [95% CI 0.50-1.35] grass allergen units/mL; maximal response 63.6% ± 4.1% of that induced by 10 μM methacholine; n = 4). In the presence of Synta-66 10 μM, there was a rightward shift in the allergen dose-response curve (EC50 4.14 [95% CI 1.72-9.96] grass allergen units/mL; P = .02). In 3 out of 4 experiments, there was a marked reduction in the maximal response (39.7% ± 7.0% of methacholine response for all data; P = .084). Synta-66 had no effect on methacholine-induced contraction (data not shown).
Fig 6.
The CRACM-channel blocker Synta-66 attenuates allergen-dependent human bronchial smooth muscle contraction (n = 4). P = .02 for rightward shift in allergen EC50.
Discussion
In spite of the absolute requirement for an influx of extracellular Ca2+ for the FcεRI-dependent release of preformed granule-derived mediators, newly generated leukotrienes and prostaglandins, and many cytokines in human MCs, the Ca2+ entry pathway has not been defined. Studies of knockout mice lacking CRACM1 function have shown that CRACM channels are essential for the influx of extracellular Ca2+ into rodent mast cells following their activation.22 In addition, indirect evidence has implicated CRACM channels as the means of Ca2+ influx in human MCs derived from nasal polyps.29 Here we show for the first time that HLMCs express CRACM1, -2, and -3 at the mRNA level, at least CRACM1 and -2 at the protein level, and following IgE-dependent activation, functional CRAC currents.
Our results are consistent with CRACM channels playing a role in the influx of extracellular Ca2+ into HLMCs following their activation. Two specific pharmacological blockers of CRACM channels—GSK-7975A and Synta-6627—reduced the increase in intracellular Ca2+ that occurs following ligation of FcεRIα and attenuated the release of histamine, LTC4, and several cytokines. The inhibition by these drugs occurred in the dose range of channel block demonstrated electrophysiologically. It takes approximately 5 to 10 times the IC50 of a channel blocker to inhibit 100% of the relevant channels. At 4 times the IC50, Synta-66 reduced FcεRI-dependent Ca2+ influx by 50% and at 10 times the IC50, both GSK-7975A and Synta-66 inhibited mediator release by up to 50%.
The biological relevance of these findings with respect to asthma is highlighted by the ability of Synta-66 to inhibit allergen-induced bronchial smooth muscle contraction in ex vivo passively sensitized bronchial tissue. The acute bronchoconstrictor smooth muscle response to allergen challenge is entirely dependent on the release of bronchospastic mediators from airway mast cells.30 In keeping with the attenuation of HLMC Ca2+ influx and mediator release observed with both Synta-66 and GSK-7975A, Synta-66 shifted the dose-response curve for allergen-dependent bronchial smooth muscle contraction 5-fold to the right and markedly reduced the maximal allergen-dependent response in 3 out of 4 donors. It should be noted that bronchial smooth muscle cells express CRACM1 and demonstrate store-operated Ca2+ currents,31 but it is unlikely that these currents in airway smooth muscle contribute to allergen-induced bronchoconstriction induced by mast cell mediators. This is because CRACM blockade had no effect on bronchial smooth muscle contraction induced directly by methacholine, which means that it is unlikely that it would inhibit the histamine and leukotriene-dependent contraction following allergen-dependent mast cell degranulation. Thus, the highly reproducible responses in both isolated HLMCs and tissue in the presence of CRACM-channel blockers suggests that the predominant site of activity of the CRACM inhibition in tissue is the mast cell.
Our results indicate that although important, CRACM channels may not be solely responsible for Ca2+ influx into activated HLMCs. The substantial residual histamine, LTC4, and cytokine secretion that we observe using high concentrations of blockers indicates that further Ca2+-permeable channels and/or receptors may play at least some role in Ca2+ influx into HLMCs. These results are in contrast to those from CRACM1 knockout mice where antigen-evoked Ca2+ influx into mast cells is reportedly reduced by 70% with the remaining Ca2+ influx being blocked by CRACM-channel inhibitors.22 Our results therefore highlight further the heterogeneity of mast cells from different species and underline the importance of studying human MCs rather than attempting to extrapolate results from rodent mast cells.
In addition to CRACM, mast cells express a number of other ion channels/receptors that may allow the entry of extracellular Ca2+. In rodents, the L-type voltage-gated Ca2+ channel Cav1.2 may be involved in Ca2+ influx independent of endoplasmic reticulum Ca2+ store emptying following mast cell activation.32 However, we have never observed a Cav-like current in HLMCs although these cells do express mRNA for Cav3.3 and the α2δ2 subunit.33 Our laboratory has also shown that HLMCs express the P2X receptors P2X1, P2X4, and P2X7, which although acting as nonselective cation channels can produce significant Ca2+ influx in response to nucleotides such as ATP.34 Finally, much attention has been focused on the potential role of canonical transient receptor potential channels in Ca2+ entry following cell activation that function as nonselective cation channels able to pass Ca2+. The potential role of all these channels will require further investigation.
Our work provides strong evidence for the expression of both CRACM1 and CRACM2, with CRACM1 transcripts present in significantly higher amounts. To assess the contribution of each channel to HLMC Ca2+ entry will require the use of knockdown strategies and the use of dominant negative mutants in future work. In mouse mast cells CRACM1 dominates, while in mouse T cells CRACM2 expression is the highest and CRACM1 is dispensable for cell function.22 However, in human T cells, CRACM1 is essential for cell function, and its complete absence results in one form of hereditary severe combined immune deficiency.17 Interestingly, while the expression of wild-type CRACM1 in T cells from patients with severe combined immune deficiency fully restores the CRAC current, expression of either CRACM2 and/or CRACM3 is reported to have little or no effect,35 demonstrating that these channels have distinct roles.
Given the relative abundance of CRACM3 mRNA transcripts in HLMCs, we were surprised not to be able to demonstrate CRACM3 protein expression by Western blotting. It is possible that CRACM3 is more sensitive to proteolysis than are its homologs. Proteolysis has been noted as a problem in the analysis of the protein expression of other mast cell ion channels.32 However, we have also been unable to demonstrate the expression of CRACM3 by flow cytometry (data not shown), suggesting that if it is expressed as a protein, it is expressed in relatively very low amounts.
In conclusion, we have demonstrated the presence of functional CRACM channels in HLMCs. Treatment of HLMCs with CRACM-channel blockers reduced the release of mediators and cytokines; CRACM channels are therefore a potential therapeutic target in the treatment of asthma and related allergic diseases.
Key messages.
-
•
The Ca2+ influx pathway required for FcεRI-dependent HLMC mediator release is not known.
-
•
HLMCs express CRACM ion channels that contribute to at least 50% of the Ca2+ influx required for FcεRI-dependent histamine, LTC4, and cytokine release, and allergen-induced bronchial smooth muscle contraction.
-
•
CRACM channels are a potential therapeutic target in the treatment of asthma and related allergic diseases.
Footnotes
This work was supported by a Wellcome Trust Project Grant (grant no. 087499) and was conducted in laboratories part funded by European Regional Development Fund no. 05567.
Disclosure of potential conflict of interest: P. Bradding has received travel grants from GlaxoSmithKline. The rest of the authors have declared that they have no conflict of interest.
Methods
Human mast cell purification and cell culture
All human subjects gave written informed consent, and the study was approved by the Leicestershire Research Ethics Committee. HLMCs were purified from macroscopically normal human lung (n = 11 donors) obtained within 1 hour of resection for lung cancer as described previously.E1 Final HLMC purity was more than 99%, and viability was more than 97%. HLMCs were cultured as described previously.E2 Cells were sensitized with human myeloma IgE 2.5 μg/mL (Merck Bioscience Ltd, Nottingham, United Kingdom) as required.
The human MC line HMC-1 (a gift from Dr J. Butterfield, Mayo Clinic, Rochester, Minn) was cultured in Iscove’s modified Dulbeccos’s medium (Invitrogen, Paisley, United Kingdom) as described previously.E1 HEK293 cells were cultured in Dulbecco modified Eagle’s medium (Invitrogen) containing 10% FCS.
RT-PCR and quantitative RT-PCR
Total RNA was isolated from cells by using an RNAqueous-4PCR kit (Applied Biosystems, Warrington, United Kingdom) according to the manufacturer’s instructions. HMC-1 cells (1 μg) or HLMCs (0.4-1 μg) of total RNA was used to generate cDNA by using random decamer primers and a Retroscript kit (Applied Biosystems). The following primer pairs were designed to amplify channel cDNA: CRACM1 5′GCCAGAGTTACTCCGAGGTG3 ′ and 5′TGACCGAGTTGAGATTGTGC3 ′, CRACM2 5′TCGACCCCTCTGCTCCTGCC3 ′ and 5′TGCGGGGACTCGCTGATGGA3 ′, and CRACM3 5′TCGGCCACGTACCGGGAGTT3′ and 5′GCCCAGGGCAGTGGAGAAGC3 ′. The predicted product sizes were 311, 390, and 405 bp respectively. Primers 5′TTCCGCAAGTTCACCTACC3 ′ and 5′CGGGCCGGCCATGCTTTACG3 ′ designed to amplify a 361-bp sequence from the small ribosomal subunit protein rig/S15 cDNA were used as a positive control. Pfu DNA Polymerase (Promega, Southampton, United Kingdom) was used for the PCR amplification.
For quantitative PCR, TaqMan probes for human CRACM1 (Hs00385627_m1), CRACM2 (Hs00259863_m1), and CRACM3 (Hs00752190_s1) were used together with TaqMan Gene Expression Master Mix (all Applied Biosystems). Reactions were run on a Stratagene Mx3000P (Agilent Technologies, Stockport, United Kingdom) real-time thermocycler. For some HLMC donors, limiting amounts of RNA precluded the analysis of CRACM1, -2, and -3 expression together in the same experiment. When this was the case, expression of 2 of the 3 CRACM transcripts was analyzed.
Analysis of CRACM protein expression
Cells were lyzed in Radio-Immunoprecipitation Assay (RIPA) buffer (50 mM Tris HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing protease inhibitors. Proteins were separated on 12% Bis-Tris Nu-Page gels (Invitrogen) and then blotted onto polyvinyidene fluoride membranes. Membranes were blocked with 5% nonfat milk in PBS and then probed with rabbit polyclonal antibodies recognizing CRACM1 (Alomone Labs Ltd, Jerusalem, Israel), CRACM2 (Alomone Labs Ltd), CRACM3 (AbD Serotec, Kidlington, United Kingdom), or a mouse monoclonal anti-c-Myc antibody (clone 9E10, Sigma, Poole, United Kingdom). In addition, the anti-CRACM2 antibody was used following preincubation with its immunogenic (blocking) peptide. Blots were subsequently probed with goat antirabbit immunoglobulins-horseradish peroxidise secondary antibody (Dako, Cambridge, United Kingdom) or goat antimouse IgG-horseradish peroxidise (Santa Cruz Biotechnology, Inc, Heidelberg, Germany) as appropriate. Immunoreactive bands were visualized by using Pierce ECL Western Blotting Substrate (Fisher Scientific, Loughborough, United Kingdom).
For the expression of c-Myc epitope–tagged CRACM channels, full-length CRACM1 (accession no. NM_032790), CRACM2 (accession no. NM_032831), and CRACM3 (accession no. NM_152288) were amplified from cDNA generated from total RNA purified from a single HLMC donor using Pfu DNA polymerase. cDNAs were then cloned in frame immediately following the c-Myc epitope tag in vector pCruz Myc (Santa Cruz Biotechnology Inc). HEK293 cells were transiently transfected with vectors directing the expression of c-Myc epitope–tagged CRACM channels using GeneJuice transfection reagent (Merck Bioscience Ltd) and harvested for lysis 24 hours after transfection.
Patch-clamp electrophysiology
The whole-cell variant of the patch-clamp technique was used.E3,E4 Patch pipettes were made from borosilicate fiber-containing glass (Clark Electromedical Instruments, Reading, United Kingdom), and their tips were heat polished, typically resulting in resistances of 4 to 6 MΩ. The standard pipette solution contained CsCl (140 mM), MgCl2 (2 mM), HEPES (10 mM), NaATP (2 mM), and GTP (0.1 mM), pH 7.3, with KOH. For IP3 experiments, 5 mM EGTA was also added to the pipette solution. For anti-IgE experiments, intracellular Ca2+ was buffered to 250 nM by using CaCl2 and ethyleneglycol-bis-(β-aminoethylether)-N,N,N′,N′-tetraacetic acid. The standard external solution contained NaCl (140 mM), KCl (5 mM), CaCl2 (2 mM), MgCl2 (1 mM), HEPES (10 mM), and glucose (5 mM), pH 7.3, with NaOH. For recording, mast cells were placed in 35-mm dishes containing standard external solution.
Whole-cell currents were recorded by using an Axoclamp 200A amplifier (Axon Instruments, Foster City, Calif), and currents usually evoked by applying voltage commands to a range of potentials in 10-mV steps from a holding potential of −20 mV. The currents were digitized (sampled at a frequency of 10 kHz), stored on computer, and subsequently analyzed by using pClamp software (Axon Instruments). Capacitance transients were minimized by using the capacitance neutralization circuits on the amplifier. Correction for series resistance was not routinely applied. Experiments were performed at 27°C, temperature being controlled by a Peltier device. Experiments were performed with a perfusion system (Automate Scientific, Inc, San Francisco, Calif) to allow solution changes, although drugs were added directly to the recording chamber. Currents in some experiments were also evoked by using a ramp protocol consisting of a continuous voltage ramp from −120 to +120 mV.
The CRACM-channel blockers GSK-7975A and Synta-66E5 (gifts from GlaxoSmithKline, Stevenage, United Kingdom), Gd3+ (gadolinium(III) chloride), and La3+ (lanthanum(III) chloride) were added directly to the recording chamber as required. GSK-7975A is compound 36 from patent WO 2010/1222089.
Ca2+ imaging
Changes in [Ca2+]i were monitored fluorometrically by use of the Ca2+-sensitive probe Fura-2. Cells were loaded with Fura-2 (Molecular Probes, Eugene, Ore) by incubation in normal physiological saline solution (130 mM NaCl, 5.6 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 11 mM glucose, 10 mM HEPES, pH 7.4, with NaOH) containing 2 μM fura-2-acetoxymethyl ester (Fura-2-AM) for 30 minutes at room temperature and then washed for 30 minutes. A coverslip with attached cells was mounted in a perfusion chamber at 37°C. The single-cell recording system has been described previously.E2 Data acquisition occurred at a rate of one dual-wavelength image every 6 seconds as the 340/380 nm ratio. This was converted to [Ca2+]i by using a commercially available calibration kit (Molecular Probes). Drugs were added to the dish as required. Baseline measurements of HLMC [Ca2+]i were recorded as the mean of the 6 values preceding the addition of an anti-FcεRIα antibody (Fisher Scientific). The postactivation value of [Ca2+]i was recorded as the mean of the 6 recordings taken immediately after the initial peak.
HLMC activation for mediator release
Experiments were performed at 37°C. For the analysis of histamine and leukotriene release, 2 × 104 HLMCs in a volume of 80 μL were added to a 96-well V-bottom plate in triplicate, immediately followed by 10 μL of 10 times the final concentration of CRACM-channel blocker or DMSO control. Plates were incubated for 10 minutes before the activation of cells by the addition of 10 μL of 10 times anti-FcεRIα antibody (final dilution of antibody 1:300). Plates were incubated for 30 minutes and centrifuged, and the supernatant was decanted and stored at −20°C for the measurement of mediator content. Control cell pellets were lyzed in ultrapure water for the determination of total histamine content.
For the analysis of cytokine release, the final cell concentration was 0.666 × 106 cells/mL, and IgE-sensitized cells were activated with anti-IgE (Hybridoma Reagents Laboratory, Baltimore, Md) (final concentration 2 μg/mL) for 16 hours before harvesting of the culture supernatant.
Mediator assays
Histamine was measured by radioenzymatic assay and LTC4 by ELISA as described previously.E1,E4 The cytokines IL-5, IL-8, IL-13, and TNFα were measured by using a human TH1/TH2 multiplex plate on a Sector Imager 6000 (Meso Scale Discovery, Gaithersburg, Md).
Allergen-induced bronchial smooth muscle contraction in isolated human bronchus
Lung tissue was obtained postmortem. Airways were dissected free of lung parenchyma and adjoining blood vessels. Secondary and tertiary bronchus, with cartilaginous walls and diameters of 3 to 10 mm, were cut spirally into strips approx 3 to 5 mm wide and then cut into pieces approximately 10 to 15 mm long. The strips were then passively sensitized overnight at room temperature (21°C) in atopic serum (20% v/v) in Krebs buffer. Before use, sensitized tissues were washed free of serum. Tissues were mounted under 1.5 g of resting tension in an immersion organ bath, maintained in oxygenated Krebs buffer solution at 37°C, and allowed to equilibrate for 30 to 45 minutes with 2 washes and retensioning if required.
Two preliminary “priming” contractions to 10 μM methacholine (Sigma, Poole, United Kingdom) were performed. The tissue was then incubated with Synta-66 (10 μM) or DMSO control (0.1% final concentration) for 1 hour. Grass allergen (Six grass mix, Alk-Abello, Hungerford, United Kingdom) was then added (0.1-30 U/mL final concentration), with contractions measured in milligrams tension. This was followed by the measurement of contraction to 10 μM methacholine. Data were expressed as % of the initial 10 μM methacholine contraction. Only limited quantities of viable human bronchus tissue were available, which allowed examination of the effects of only a single CRACM-channel blocker.
References
- 1.Bradding P., Walls A.F., Holgate S.T. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 2.Okayama Y., Benyon R.C., Rees P.H., Lowman M.A., Hillier K., Church M.K. Inhibition profiles of sodium cromoglycate and nedocromil sodium on mediator release from mast cells of human skin, lung, tonsil, adenoid and intestine. Clin Exp Allergy. 1992;22:401–409. doi: 10.1111/j.1365-2222.1992.tb03102.x. [DOI] [PubMed] [Google Scholar]
- 3.Chong L.K., Morice A.H., Yeo W.W., Schleimer R.P., Peachell P.T. Functional desensitization of beta agonist responses in human lung mast cells. Am J Respir Cell Mol Biol. 1995;13:540–546. doi: 10.1165/ajrcmb.13.5.7576689. [DOI] [PubMed] [Google Scholar]
- 4.Swystun V.A., Gordon J.R., Davis E.B., Zhang X., Cockcroft D.W. Mast cell tryptase release and asthmatic responses to allergen increase with regular use of salbutamol. J Allergy Clin Immunol. 2000;106:57–64. doi: 10.1067/mai.2000.107396. [DOI] [PubMed] [Google Scholar]
- 5.Giannini D., Carletti A., Dente F.L., Bacci E., Di Franco A., Vagaggini B. Tolerance to the protective effect of salmeterol on allergen challenge. Chest. 1996;110:1452–1457. doi: 10.1378/chest.110.6.1452. [DOI] [PubMed] [Google Scholar]
- 6.Bradding P. Mast cell ion channels. Chem Immunol Allergy. 2005;87:163–178. doi: 10.1159/000087643. [DOI] [PubMed] [Google Scholar]
- 7.Chandy K.G., Wulff H., Beeton C., Pennington M., Gutman G.A., Cahalan M.D. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffy S.M., Berger P., Cruse G., Yang W., Bolton S.J., Bradding P. The K+ channel IKCa1 potentiates Ca2+ influx and degranulation in human lung mast cells. J Allergy Clin Immunol. 2004;114:66–72. doi: 10.1016/j.jaci.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd M.C., Duffy S.M., Harris T., Cruse G., Schuliga M., Brightling C.E. KCa3.1 Ca2+ activated K+ channels regulate human airway smooth muscle proliferation. Am J Respir Cell Mol Biol. 2007;37:525–531. doi: 10.1165/rcmb.2006-0358OC. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra J.D., Kazen-Gillespie K., Hortsch M., Isom L.L. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J Biol Chem. 2000;275:11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- 11.Cruse G., Duffy S.M., Brightling C.E., Bradding P. Functional KCa3.1 K+ channels are required for human lung mast cell migration. Thorax. 2006;61:880–885. doi: 10.1136/thx.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church M.K., Pao G.J., Holgate S.T. Characterization of histamine secretion from mechanically dispersed human lung mast cells: effects of anti-IgE, calcium ionophore A23187, compound 48/80, and basic polypeptides. J Immunol. 1982;129:2116–2121. [PubMed] [Google Scholar]
- 13.Parekh A.B., Putney J.W., Jr. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 14.Lewis R.S. Calcium oscillations in T-cells: mechanisms and consequences for gene expression. Biochem Soc Trans. 2003;31:925–929. doi: 10.1042/bst0310925. [DOI] [PubMed] [Google Scholar]
- 15.Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S.L., Yu Y., Roos J., Kozak J.A., Deerinck T.J., Ellisman M.H. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S.H., Tanasa B. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 18.Vig M., Peinelt C., Beck A., Koomoa D.L., Rabah D., Koblan-Huberson M. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 20.Yeromin A.V., Zhang S.L., Jiang W., Yu Y., Safrina O., Cahalan M.D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lis A., Peinelt C., Beck A., Parvez S., Monteilh-Zoller M., Fleig A. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vig M., DeHaven W.I., Bird G.S., Billingsley J.M., Wang H., Rao P.E. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradding P. Human lung mast cell heterogeneity. Thorax. 2009;64:278–280. doi: 10.1136/thx.2008.106427. [DOI] [PubMed] [Google Scholar]
- 24.Sanmugalingam D., Wardlaw A.J., Bradding P. Adhesion of human lung mast cells to bronchial epithelium: evidence for a novel carbohydrate-mediated mechanism. J Leuk Biol. 2000;68:38–46. [PubMed] [Google Scholar]
- 25.Duffy S.M., Lawley W.J., Kaur D., Yang W., Bradding P. Inhibition of human mast cell proliferation and survival by tamoxifen in association with ion channel modulation. J Allergy Clin Immunol. 2003;112:970–977. doi: 10.1016/j.jaci.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Duffy S.M., Lawley W.J., Conley E.C., Bradding P. Resting and activation-dependent ion channels in human mast cells. J Immunol. 2001;167:4261–4270. doi: 10.4049/jimmunol.167.8.4261. [DOI] [PubMed] [Google Scholar]
- 27.Ng S.W., Di C.J., Singaravelu K., Parekh A.B. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem. 2008;283:31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 28.Hoth M., Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Capite J., Nelson C., Bates G., Parekh A.B. Targeting Ca2+ release-activated Ca2+ channel channels and leukotriene receptors provides a novel combination strategy for treating nasal polyposis. J Allergy Clin Immunol. 2009;124:1014–1021. doi: 10.1016/j.jaci.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Bradding P. Mast cells in asthma. In: Busse W.W., Holgate S.T., editors. Asthma & rhinitis. Blackwell Scientific Publications; Boston: 2000. pp. 319–338. [Google Scholar]
- 31.Peel S.E., Liu B., Hall I.P. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–749. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimaru T., Suzuki Y., Inoue T., Ra C. L-type Ca2+ channels in mast cells: activation by membrane depolarization and distinct roles in regulating mediator release from store-operated Ca2+ channels. Mol Immunol. 2009;46:1267–1277. doi: 10.1016/j.molimm.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Bradding P., Okayama Y., Kambe N., Saito H. Ion channel gene expression in human lung, skin, and cord blood-derived mast cells. J Leuk Biol. 2003;73:614–620. doi: 10.1189/jlb.1202602. [DOI] [PubMed] [Google Scholar]
- 34.Wareham K., Vial C., Wykes R.C., Bradding P., Seward E.P. Functional evidence for the expression of P2X1, P2X4 and P2X7 receptors in human lung mast cells. Br J Pharmacol. 2009;157:1215–1224. doi: 10.1111/j.1476-5381.2009.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwack Y., Srikanth S., Feske S., Cruz-Guilloty F., Oh-Hora M., Neems D.S. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
References
- Sanmugalingam D., Wardlaw A.J., Bradding P. Adhesion of human lung mast cells to bronchial epithelium: evidence for a novel carbohydrate-mediated mechanism. J Leuk Biol. 2000;68:38–46. [PubMed] [Google Scholar]
- Duffy S.M., Lawley W.J., Kaur D., Yang W., Bradding P. Inhibition of human mast cell proliferation and survival by tamoxifen in association with ion channel modulation. J Allergy Clin Immunol. 2003;112:970–977. doi: 10.1016/j.jaci.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Duffy S.M., Lawley W.J., Conley E.C., Bradding P. Resting and activation-dependent ion channels in human mast cells. J Immunol. 2001;167:4261–4270. doi: 10.4049/jimmunol.167.8.4261. [DOI] [PubMed] [Google Scholar]
- Ng S.W., Di C.J., Singaravelu K., Parekh A.B. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem. 2008;283:31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]