Abstract
Fatigue is a frequent complaint in muscular dystrophies but it is yet not well defined or studied. We have examined the issue of muscle fatigue in a series of molecularly defined muscular dystrophies. A greater fatigability is seen in muscular dystrophy patients and can be an acute or chronic status. In Duchenne Muscular Dystrophy and beta-sarcoglycanopathy besides the alteration of dystrophin and/or sarcoglycan complex, a neuronal nitric oxide synthase depletion is frequently found and might correlate with post-exercise fatigability as well as with cardiac involvement. Therefore, it might be an important modulating factor of the severity of myopathy. In myotonic dystrophy, fatigue is a common complaint: muscle is involved and type 1 atrophy is a frequent feature; brain involvement and depressed mood might likely explain the extent of fatigue and daytime sleepiness commonly observed in these patients. Furthermore, in our observation in a series of 24 cases, muscle and brain can be independently involved in DM1 patients. These observations have profound impact on the type of physical therapy to be prescribed in such patients.
Keywords: Fatigue, Duchenne dystrophy, Limb girdle dystrophy, Myotonic dystrophy, Nitric oxide synthase
1. Introduction
Muscular dystrophies are hereditary disorders of skeletal muscle, but they may also involve the brain (i.e. myotonic dystrophy). Fatigue can be a frequent complaint even though its origin is variable [1]. It is well known that myopathic patients have difficulties to support an excessive or long-term physical activity; on the other hand the fatigability, met during the exercise of moderate or short-lived intensity and imposed by daily life, remains underestimated.
Fatigue can be an acute, i.e. the fatigue that follows an effort, or a chronic phenomenon. In the myopathic patient, fatigue can increase after muscle effort required for the realization of a task (i.e. climbing stairs) and/or the impossibility to realize this task. Thus, the increase of physical exhaustion for the energetic expense and for a given exercise can be considered as cause of the acute fatigue. The loss of muscle force or the loss of capacity to maintain a certain level of force at maximum level is another cause of chronic fatigue.
Fatigue can be related to many mechanisms on several sites of the motor axis, ranging from the motor cortex to the muscle. One can thus distinguish a “central fatigue” and a peripheral fatigue. The central fatigue implies that all the steps are localized upstream of the neuromuscular junction. The peripheral fatigue can be either due to coupling of excitement–contraction in muscle, availability of substrates or blood flow and exercise adaptation of vasodilatation by nitric oxide (NO) as well as to the possible modifications of the intracellular environment and disruption of contractile apparatus [2–6].
The acute fatigue can be consequent to a load of excessive work in a short time and in myopathic patients after an eccentric exercise. There is often rupture of the sarcolemma and loss of sarcoplasmic enzymes, i.e. creatine kinase (CK). Nevertheless, if these loads are repeated and if the recovery and muscle regeneration is insufficient on the quantitative or qualitative levels, the patient can suffer from a generalized fatigue, characterized by permanent weakness and more chronic symptoms. This fatigue can also degenerate in chronic exhaustion i.e. a “burnout phenomenon” that constitutes the ultimate state in which the fatigue sensation can persist several weeks despite apparent recovery.
Multiple factors contribute to reduced motor ability and increased sedentary behavior, including muscle wasting secondary to the muscular dystrophy process itself; fear of increased muscle damage leading to increasingly restricted mobility; higher energy cost is partly caused by secondary contractures, biomechanical problems including ankle retraction, clumsy gait, poor balance, foot and knee deformities, and the increased body fat mass induced by disuse inactivity, muscle atrophy. In myotonic dystrophy patients, particularly in DM1, where patients have an avoidant personality, reduced motivation is on turn accompanied by increased fatigue, depression, increased social barriers, and less social integration. Moreover, several muscle patients have a marked reduction in pulmonary capacity and lower peak of oxygen consumption, or suffer from night desaturation symptoms that cause day time “sleepiness” and fatigue. Both Duchenne and Becker muscular dystrophy patients might have decreased heart function, for a progressive cardiomyopathy, and decreased maximum ventilation. Therefore, part of the reduced physical ability is directly due to the progressive muscle disease, but the disease also leads to physical deconditioning that can lead to increased risks for the co-morbidity caused by cardio-pulmonary complications.
Exhaustion during exercise and fatigue are among common presenting symptoms in medical consultations of dystrophic patients, and represent the most significant factors that negatively influence the quality of life for patients with muscle diseases. Specific scales for evaluation of muscle fatigue are therefore useful in clinical trials and have to be added in clinical practice in patient management.
Our review covers the current state of the field, introduces new aspects of muscular dystrophy and myotonic dystrophy clinical characteristics and outlines recent developments in therapeutical efforts.
2. Variable muscular dystrophy dysfunction and pathogenesis
Quantitative assessment of muscle fatigue is not commonly carried out when evaluating myopathic patients, perhaps because of the complex nature and difficulty of defining and measuring fatigue. Few studies have systematically examined the problem using objective measurement techniques (MRI with STIR sequences [3], exercise induced fatigue, vascular effect on post-exercise activity by nitric oxide production, blood flow pre- and post-exercise). For instance, insufficient relief of local vasoconstriction in active muscle can lead to muscle edema and Duchenne muscular dystrophy (DMD) and limb girdle muscular dystrophy (LGMD) patients show muscle edema on MRI. Furthermore, the variability of pathogenetic mechanisms underlying the various forms of muscular dystrophy is well known and it may play a different role in determining muscle changes. In myotonic dystrophy fatty degeneration and myxedema are localized to anterior thigh muscles and posterior leg [3].
The sarcolemma of striated muscle fibers is characterized by the presence of the dystrophin–glycoprotein complex (DGC) that is composed of cytoskeletal proteins (dystrophin, syntrophins), the dystroglycan complex and the sarcoglycan (SG) complex. The DGC provides a mechanical linkage between laminin in the extracellular matrix and the intracellular F-actin cytoskeleton. The structural and functional integrity of this connection is crucial to stabilize the sarcolemma during contractions. The SG complex is composed of four glycoproteins (namely α-, β-, γ-, and δ-SG), whose mutant genes cause a group of autosomal recessive Limb-girdle muscular dystrophies (LGMD) called “sarcoglycanopathies” (LGMD2D, 2E, 2C and 2F, respectively). When one gene is mutated all the other SG components are secondarily reduced, the assembly of the SG complex is compromised, and the sarcolemma integrity and stability are lost. A similar pathogenetic mechanism leads to DMD, which is caused by mutations in the dystrophin gene, while in Becker dystrophy there is a residual abnormal dystrophin.
The DGC has also signaling roles due to its interaction with other proteins including neuronal nitric oxide synthase (nNOS), which is anchored at the sarcolemma [4–11].
Nitric oxide (NO) is a messenger molecule that in muscle regulates development, contractility and blood flow. NO is formed by nNOS activity, which rapidly transduces signaling events in a calcium-dependent manner. In adult muscle, nNOS is usually localized at the sarcolemma, the neuromuscular and myotendineous junctions, while in the soluble fraction, significant amounts of nNOS are associated with the muscle phosphofructokinase isoform, although the nature of this interaction is unclear. Regulation of nNOS activity depends on the interaction with various proteins: it may be inhibited by caveolin-3, a component of the DGC that might displace the activator calmodulin.
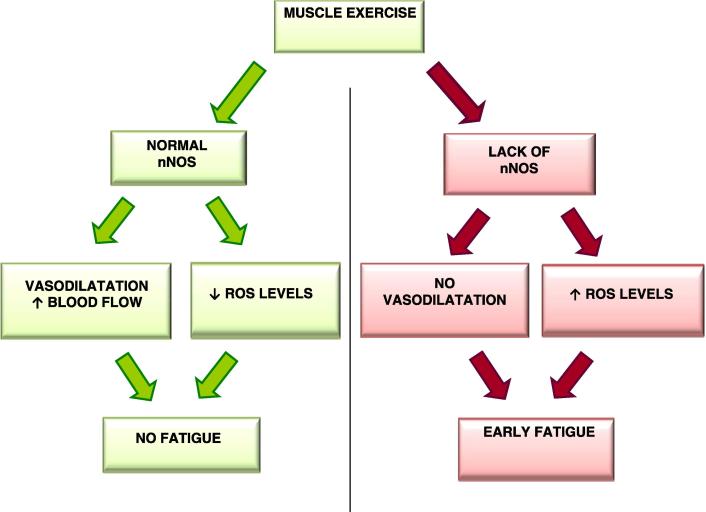
One physiological role of nNOS in normal muscle is the production of NO that mediates the inhibition of sympathetic vasoconstriction. The secondary loss of nNOS has been suggested to contribute to fiber degeneration in muscular dystrophies, both in Duchenne and Becker dystrophies, because the reduced production of nNOS would reduce the normal protective action of NO against local ischemia during contraction (vascular hypothesis) and increase the cellular susceptibility to superoxides (oxidative stress hypothesis) (Fig. 1).
Fig. 1.
Cascade of events consequent to muscle exercise when normal or defective nNOS is present.
Muscle from DMD patients, dystrophin-deficient mdx mice and α1-syntrophin knock-out mice showed absent nNOS at the sarcolemma, which however remained in the cytosol. Absent sarcolemmal nNOS was observed also in Becker muscular dystrophy (BMD) with dystrophin gene deletions removing the central rod domain (exons between 10 and 53), a region which is crucial for the interaction between nNOS and α1-syntrophin [12]. The possibility that the loss of sarcolemmal nNOS could be observed in disorders different from dystrophinopathies but having similar pathogenetic mechanism lead to an investigation in LGMD muscles. The sarcolemmal nNOS was found to be normal in an early study on α-sarcoglycanopathy, and reduced in a more extensive investigation on sarcoglycanopathies, suggesting that nNOS defect might contribute to muscle pathology. Among LGMD other than sarcoglycanopathies, only caveolinopathy (due to caveolin-3 gene mutations) has been investigated for nNOS expression in muscle and it was found to be either normal in patients with the rippling muscle disease phenotype, or severely reduced in patients with LGMD phenotype [6].
We analyzed the muscle biopsies from 32 patients affected with 7 different forms of molecularly defined LGMD and 5 patients with DMD, in order to investigate both the cytosolic nNOS expression and its sarcolemmal localization [6]. By evaluation of the role of nNOS in muscle pathology and finally in disease progression, we found evidence supporting a role of nNOS in modulating the disease phenotype, this could be of great importance for future therapeutic interventions in LGMD.
One interesting and still controversial issue is whether the loss of sarcolemmal nNOS, compounded with the defect of another muscle protein (i.e. dystrophin or sarcoglycans) might modulate the course of the disease. Earlier studies demonstrated that normal nNOS activity could reduce dystrophic symptoms: forced expression of nNOS reduced muscle pathology in mdx mice; nNOS transgene in the myocardium of mdx mice prevented the ventricular fibrosis and greatly reduced myocarditis; sarcolemmal nNOS was restored by the use of minidystrophin vector in mdx mice. Moreover, the level of nNOS expression seemed to be inversely correlated with the severity of the disease in caveolinopathy and in BMD muscle (Table 1). However, in animal models some experimental results suggested that a reduction of NOS activity would not significantly contribute to the dystrophic pathology: mdx mice inbreeded with NOS-null mutant mice showed no difference in muscle pathology compared with mdx mice; nNOS-null mice do not show muscular dystrophy; α1-syntrophin knock-out mice with loss of sarcolemmal nNOS did not show muscle degeneration [7].
Table 1.
Loss of sarcolemma-localized nNOS and types of muscular dystrophies.
| Disease | Type of fatigue | nNOS | Drugs |
|---|---|---|---|
| DMD | Peripheral | Absent | Steroids |
| BMD | Peripheral | Variable | – |
| LGMD2A | Peripheral | Normal | – |
| LGMD2B | Peripheral | Normal | – |
| LGMD2C–2F | Peripheral | Absent in complete primary SG defects | Steroids in LGMD2D |
| LGMD1C | Peripheral | Normal | – |
| Myotonic Dystrophy 1 | Central and peripheral | Required for DMPK expression during myogenesis | Mexiletine, anti-depressants |
3. Central and peripheral components of fatigue in myotonic dystrophy
Myotonic dystrophy type 1 (DM1) is caused by an unstable expansion of CTG repeats; the most common clinical features of DM1 include myotonia, muscle weakness and multisystemic involvement, i.e. heart and brain involvement. The majority of patients with DM1 complain of fatigue and day time sleepiness. In most DM1 patients fatigability is an abnormal sense of tiredness induced by mental or physical task. During motor tasks, fatigability is caused by the inability to sustain the required effort and may have in DM1 a central and/or peripheral component [13]. Central fatigue is a progressive reduction in voluntary muscle activation during exercise, related to cortical factors, while peripheral fatigue would result from an altered muscle function. Various electrophysiological techniques have been developed to appraise the respective involvement of these different factors to explain fatigue in such pathological condition. Despite the fact that exercise-induced fatigue is prominent in patients with DM1, limiting daily living activities and thereby affecting quality of life, this phenomenon remains poorly documented. Pathological studies of DM1 muscle have shown both preferential type 1 fiber atrophy and selective type 2 fiber loss, likely depending on the biopsied muscle and stage of disease severity, but the amount of fatigability is not proportional to muscle involvement or fiber atrophy.
Increased daytime sleepiness and fatigue are among the most frequent complaint in patients with DM1. Weak oro-pharyngeal and respiratory muscles leading to obstructive sleep apnea and alveolar hypoventilation have been regarded as causative factors of daytime sleepiness; however, there is increasing evidence that tiredness primarily results from central nervous system dysfunction rather than progressive respiratory weakness [14]. These considerations suggest that a study on parallel central nervous system and muscle involvement is needed to define the fatigability in DM1 patients. The only study linking NOS and myotonic dystrophy suggests that although for induction of dystrophia myotonica protein kinase (DMPK) expression during myogenesis, NOS is required, there is only a weak functional implication for such a generation and subsequent maintenance of DMPK expression [15].
4. Muscle involvement in muscular dystrophies and in myotonic dystrophy
To understand the molecular basis of exercise induced fatigue response, genetically defined muscle disorders are a field of investigation. Since secondary inflammation can be a feature of dystrophinopathy, one can speculate that fatigue is associated with muscle pain or to cytokine and CK release. During trials in DMD patients with Deflazacort or Prednisone [16], the exaggerated fatigue response in these patients was not attributed to inflammation or pain but to lack of muscle force and permanent weakness. Similar data were observed in some sarcoglycanopathy patients, treated with Deflazacort. Loss of sarcolemma-localized nNOS serves as a diagnostic indicator of substantial drop in activity in some forms of BMD or LGMD.
When a patient is diagnosed as having a muscular dystrophy, questions arise about the prognosis, possible interventions and genetics. Weakness and impaired cardio-respiratory functions are common in patients with muscular dystrophies. Training or aerobic exercise programs might maximize strength and ventilatory assistance as well as cardiorespiratory function and prevent additional disuse atrophy.
The benefit from strength training or aerobic exercise training in muscular dystrophies is not yet clear. The only two conditions in which strength training has been evaluated are myotonic dystrophy and facio-scapulo-humeral dystrophy [17].
In myotonic dystrophy and facio-scapulo-humeral dystrophy, moderate intensity strength training appears not to do harm but there is insufficient evidence to conclude that it offers benefit.
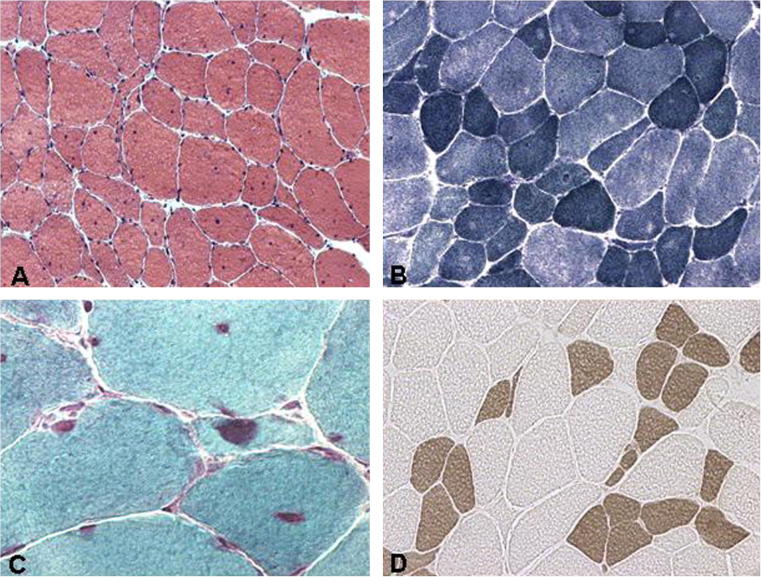
In order to further investigate the simultaneous cerebral and muscle impairment in DM1, we recruited 24 DM1 patients (13 males, 11 females, mean age 34 years, age range 15–67 years) in our neuromuscular center and studied the severity of muscle involvement using a muscle impairment rating scale (MIRS) [18]. MIRS scores varied between 1 and 5: 2 patients scored 1, 6 scored 2, 9 scored 3, 6 scored 4, 1 scored 5. Muscle biopsies were obtained for diagnostic purposes under local anesthesia after informed consent. We used muscle biopsy samples from routinely selected muscles: 11 from biceps brachii and 13 from vastus lateralis. Cryosections underwent a panel of routine stains including haematoxylin-eosin, modified Gomori trichrome, ATP-ase at pH 4.3 and 9.4, PAS and oxidative stains. A quantitative and qualitative morphometric analysis was done for muscle fiber atrophy and hypertrophy factors. In DM1 the characteristic change in muscle on conventional H&E stain is variation and highly increased number of multiple internal nuclei in muscle fibers (Fig. 2); on immunohistochemistry slow type 1 fibers are on average smaller than fast type 2 fibers.
Fig. 2.
Muscle pathology in DM1. Cross sections of muscle fibers showing with hematoxylin-eosin stain (A) several internal nuclei and size variability, with NADH-TR (B) central nuclei, type 1 fiber hypotrophy and some moth eaten fibers, with Gomori trichrome (C) sarcoplasmic masses, central nuclei and atrophic fibers, with acid ATP-ase (D), type 1 fiber atrophy and type 2 fiber hypertrophy.
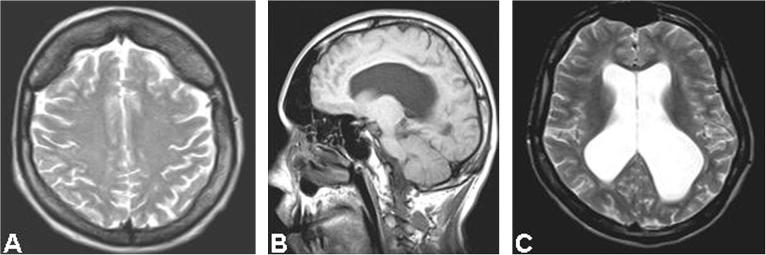
Furthermore, brain MRI scans were obtained in all 24 DM1 patients. The MRI studies were focused on white matter lesions. We adopted, as a new parameter, the total white matter lesion load, which is obtained from the average score calculated in both hemispheres and separated the values obtained in four different classes. We also investigated the presence of other abnormalities: cranial hyperostosis, overdevelopment of the synus, ventriculomegaly (Fig. 3). Only four patients had normal MRI imaging, while bilateral symmetrical focal or diffuse white matter abnormalities were found in 83% patients. Diffuse symmetrical lesions were present in the insular regions in 15 patients (62%) and in 12 of them a peculiar subcortical diffuse involvement of the polar region was detected. When we analyzed the distribution of CTG expansion classes, they correlated with lesion load by MRI. Also the distribution of muscle fiber atrophy factor classes was a function of CTG expansion; however, there was no significant correlation between classes of atrophy factor and classes of the total white matter lesion load. We concluded therefore, that greater CTG expansion size is a confirmed risk factor for more extensive cerebral and muscle impairment. However, our study indicates that muscle and brain are independently involved in DM1. As a consequence, we conclude that two distinct types of fatigue are possible in DM1: a central fatigue due to cortical atrophy and white matter lesions, and a peripheral fatigue due to atrophy of muscle fibers. These two fatigability components are differently expressed in the various patients.
Fig. 3.
Brain MRI images in DM1 showing cranial hyperostosis (A), enlargement of paranasal cavities (B) and ventriculomegaly (C).
There is currently no effective therapy for DM1; mexiletine, however, has been shown to be useful to control myotonia. Powerful and stable antisense oligonucleotides (AON) have been identified, most of them complementary to the CUG nucleotide expansion in DM1 that reduce products on the mutant DMPK allele and diminish ribonuclear foci sequestering muscle blind like protein (MBNL) and therefore inducing multiple aberrant splicing. These AON in animal models enter very efficiently skeletal muscle; however, the problem of the central component of fatigue in DM1 will not be solved by such an approach.
5. Cardiac and skeletal muscle: future therapeutic strategies in muscular dystrophies
Absence or severe reduction of nNOS expression at the sarcolemma was associated with severe early-onset muscular dystrophy in all cases and occurred with the presence of dilated cardiomyopathy in 2 of 3 sarcoglycanopathy patients, who were old enough to exhibit cardiomyopathy [6].
There is a correlation between NOS depletion, clinical severity and muscle fatigue. Limitation in the design studies in other muscular dystrophies prevents more general conclusions in these disorders. More research on fatigue is needed also in the field of muscular dystrophies and on the role of nNOS, that when absent seems an adverse modulating factor.
Furthermore, experimental evidence in animal models of sarcoglycanopathies showed that transgenic mice with α-SG and γ-SG deficiency had severe muscular dystrophy and cardiomyopathy, and that SG and DGC complexes were disrupted in skeletal, cardiac, and vascular smooth muscle, suggesting that a perturbation of vascular smooth muscle might be an additional mechanism that could contribute to the development of myopathy and cardiomyopathy. The NO was found to be increased in regions of cardiac tissue damage and altered membrane permeability in SG mutant mice. The absence of SG complex in the vascular smooth muscle might lead either to structural changes or to an impairment of metabolic and signaling pathways involved in the microvascular dysfunction; that is, high Ca++ levels and disturbance of the NOS pathway increase vasculature contractility, making cardiomyocytes more susceptible to intermittent ischemia. Indeed, the use of vasodilator drugs has been shown to prevent the development of acute myocardial necrosis in animal models of α- and γ-sarcoglycanopathy. The finding that absent sarcolemmal nNOS can be found not only in some Becker muscular dystrophy patients, but also in sarcoglycanopathies emphasizes the value of nNOS immunohistochemical analysis in LGMD and provides further insights for future therapeutic interventions. In LGMD due to alpha-sarcoglycan deficiency, Deflazacort reduced CK levels and maintained force [19]. Antioxidants that attenuate the superoxide attack and restore the bioactive NO level and exogenous NO donors or dietary addition of a NOS substrate such as l-arginine, may be useful approaches for the treatment of these disorders.
NOS is a key enzyme in the production of NO, a molecule that directly regulates vaso-relaxation and blood supply. Diverse forms of muscle disease have been clinically associated with unusual fatigue after exercise. The localization of nNOS at the plasma membrane of muscle has recently been shown to prevent muscle fatigue after exercise. These results emphasize the value of nNOS immunohistochemical analysis in muscular dystrophies and provide additional insights for future therapeutic interventions.
Lai et al. [2] showed that dystrophin anchors nNOS to the sarcolemma through a direct interaction with dystrophin spectrin-like repeats 16 and 17. Furthermore, in another recently reported study of mouse models of muscular dystrophy, phosphodiesterase 5A inhibitors were used to treat the downstream ischemia that is associated with nNOS mislocalization. Collectively, these findings promise a series of therapeutic interventions based on our understanding of exercise-induced muscle fatigue and its role in muscle dystrophy.
6. Conflict of interest
None.
Acknowledgments
Comitato Telethon Fondazione Onlus (Grant #GTB07001) and Fondazione Cariparo Project of excellence.
References
- 1.Feasson L., Camdessanche J.P., El Mandhi L., Calmels P., Millet G.Y. Fatigue et affection neuromusculaire. Ann Readapt Med Phys. 2006;49:289–300. doi: 10.1016/j.annrmp.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Lai Y., Thomas G.D., Yue Y. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stramare R., Beltrame V., Dal Borgo R. MRI in the assessment of muscular pathology: a comparison between limb-girdle muscular dystrophies, hyaline body myopathies and myotonic dystrophies. Radiol Med. 2010;115:585–599. doi: 10.1007/s11547-010-0531-2. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi Y.M., Rader E.P., Crawford R.W. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heydemann A., McNally E. NO more fatigue muscle. J Clin Invest. 2009;119:448–450. doi: 10.1172/JCI38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanin M., Tasca E., Nascimbeni A.C., Angelini C. Sarcolemmal neuronal nitric oxide synthase defect in limb girdle muscular dystrophy: an adverse modulating factor in the disease course? J Neuropathol Exp Neurol. 2009;68:383–390. doi: 10.1097/NEN.0b013e31819cd612. [DOI] [PubMed] [Google Scholar]
- 7.Percival J.M., Anderson K.N.E., Huang P., Adams M.E., Froehner S.C. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest. 2010;120:816–826. doi: 10.1172/JCI40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sander M., Chavoshan B., Harris S.A. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosbie R.H. NO vascular control in Duchenne muscular dystrophy. Nat Med. 2001;7:27–29. doi: 10.1038/83309. [DOI] [PubMed] [Google Scholar]
- 10.Chao D.S., Gorospe J.R., Brenman J.E. Selective loss of sarcolemmal nitric oxide synthase in Becker muscular dystrophy. J Exp Med. 1996;184:609–618. doi: 10.1084/jem.184.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells K.E., Torelli S., Lu Q. Relocalization of neuronal nitric oxide synthase (nNOS) as a marker for complete restoration of the dystrophin associated protein complex in skeletal muscle. Neuromuscul Disord. 2003;13:21–31. doi: 10.1016/s0960-8966(02)00191-8. [DOI] [PubMed] [Google Scholar]
- 12.Torelli S., Brown S.C., Jimenez-Mallebrera C. Absence of neuronal nitric oxide synthase (nNOS) as a pathological marker for the diagnosis of Becker muscular dystrophy with rod domain deletions. Neuropathol Appl Neurobiol. 2004;30:540–545. doi: 10.1111/j.1365-2990.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 13.Boërio D., Lefaucheur J.P., Bassez G., Hogrel J.Y. Central and peripheral components of exercise-related fatigability in myotonic dystrophy type 1. Acta Neurol Scand. 2012;125:38–46. doi: 10.1111/j.1600-0404.2011.01497.x. [DOI] [PubMed] [Google Scholar]
- 14.Minnerop M., Weber B., Schoene-Bake J.C. The brain in myotonic dystrophy 1 and 2: evidence for a predominant white matter disease. Brain. 2011;134:3527–3543. doi: 10.1093/brain/awr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrasco M., Canicio J., Palacin M., Zorzano A., Kaliman P. Identification of intracellular signaling pathways that induce myotonic dystrophy protein kinase expression during myogenesis. Endocrinology. 2002;143:3017–3025. doi: 10.1210/endo.143.8.8972. [DOI] [PubMed] [Google Scholar]
- 16.Angelini C., Peterle E. Old and new therapeutic developments in steroid treatment in Duchenne muscular dystrophy. Acta Myol. 2012;31:9–15. [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Kooi E.L., Vogels O.J., van Asseldonk R.J. Strength training and albuterol in facio scapulo humeral muscular dystrophy. Neurology. 2004;63:702–708. doi: 10.1212/01.wnl.0000134660.30793.1f. [DOI] [PubMed] [Google Scholar]
- 18.Ferrati C., Romeo V., Fanin M. Are brain and muscle independently involved in DM1? J Neurol. 2012;259(S1):S205. [Google Scholar]
- 19.Angelini C., Fanin M., Menegazzo E., Freda M.P., Duggan D.J., Hoffman E.P. Homozygous alpha-sarcoglycan mutation in two siblings: one asymptomatic and one steroid responsive mild limb-girdle muscular dystrophy patient. Muscle Nerve. 1998;21:769–875. doi: 10.1002/(sici)1097-4598(199806)21:6<769::aid-mus9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]