Abstract
Context
Maintaining excellent cure rates in pediatric Hodgkin lymphoma while minimizing toxicity.
Objective
To evaluate the efficacy of 4 cycles of vinblastine, Adriamycin, methotrexate, and prednisone (VAMP) in patients with favorable risk Hodgkin lymphoma who achieve a complete response after 2 cycles and do not receive radiotherapy.
Design, Setting, and Patients
Multi-institutional, unblinded, non-randomized single group phase II clinical trial to assess the need for radiotherapy based on early response to chemotherapy. Eighty-eight eligible patients with Hodgkin lymphoma stage I and II (< 3 nodal sites, no B symptoms, mediastinal bulk, or extranodal extension) enrolled between March 3, 2000 through December 9, 2008. Data frozen March 12, 2012.
Interventions
Patients who achieved a complete response (n=47) after 2 cycles received no radiotherapy, and those with less than complete response (n=41) were given 25.5 Gy involved field radiotherapy.
Main Outcome Measures
2-year event-free survival was the primary outcome measure. A 2-year event-free survival of greater than 90% was desired, and 80% was considered to be unacceptably low.
Results
Two-year event-free survival was 90.8% (95% CI, 84.7% – 96.9%); for patients who did not require radiotherapy it was 89.4% (95% CI, 80.8% – 98%), compared with 92.5% (95% CI, 84.5% – 100%) for those who did (P=0.61). Most common acute side effects were neuropathic pain (2% of patients), nausea/vomiting (3% of patients), neutropenia (32% of cycles), and febrile neutropenia (2% of patients). Nine patients (10%) were hospitalized 11 times (3% of cycles) for febrile neutropenia or non-neutropenic infection. Long term side effects after radiotherapy were asymptomatic compensated hypothyroidism in 9 patients (10%), osteonecrosis and moderate osteopenia in 2 patients each, subclinical pulmonary dysfunction in 12 patients (26%) and asymptomatic left ventricular dysfunction in 4 patients (5%). No second malignant neoplasms were observed.
Conclusions
Among patients with favorable risk Hodgkin lymphoma and a complete early response to chemotherapy, the use of limited therapy resulted in a high rate of 2-year EFS.
INTRODUCTION
Currently more than 90% of children with favorable-risk Hodgkin lymphoma will achieve long-term survival. However, studies demonstrate excess mortality among patients followed beyond 10 years from diagnosis as a result of late toxicities of therapy1;2 including the development of second malignant neoplasms and non-neoplastic treatment complications.3 Risk-adapted combined-modality therapy (combined chemo- and radiotherapy according to predetermined risk stratification) has therefore been tailored to minimize therapy while maintaining excellent outcome.4–8 Response-adapted therapies (tailored according to early initial response) aim to identify patients for whom it would be safe to reduce radiation therapy dose, volume, or both.4;6;9;10
We previously reported the results of treatment with 4 cycles of vinblastine, Adriamycin, methotrexate, and prednisone (VAMP) chemotherapy and low-dose, involved field radiotherapy,6;11 and we identified a group of patients with a favorable profile including early complete response to VAMP chemotherapy who might be curable without irradiation. We now report the results of a multi-institutional, non-randomized study testing the efficacy of VAMP with or without radiotherapy based on early response to chemotherapy for children with favorable risk Hodgkin lymphoma. Evaluation of quality of life during and after treatment was a secondary objective and will be reported in a forthcoming manuscript.
PATIENTS AND METHODS
Study Population and Eligibility Criteria
This trial was open to accrual from March 2, 2000 through February 10, 2009. There were no competing trials open at participating institutions during the study period and no eligible patient chose not to participate. The study was designed to assess whether a 2-year event-free survival >90% could be maintained in children with Hodgkin lymphoma and favorable risk features treated with 4 cycles of VAMP with or without radiotherapy according to their early response after 2 cycles. Eligibility criteria included age <21 years, previously untreated Hodgkin lymphoma, Ann Arbor stage12 IA or IIA, non-bulky mediastinal mass (ratio of the size of the mass compared to the widest intrathoracic diameter measured on an upright postero-anterior chest radiograph <1/3), no extranodal extension of disease, and fewer than 3 involved nodal regions.13 Race and ethnicity are routinely captured on every patient and defined according to open-ended self-reported categories. The protocol was approved by the institutional review board at each participating institution and monitored by the St. Jude Children’s Research Hospital Data and Safety Monitoring Board. Informed consent was obtained from parents, legal guardians, or patients, as appropriate.
Staging, Treatment, Early Response Evaluation, and Follow-up
Initial evaluation included history and physical examination, complete blood count, C-reactive protein or erythrocyte sedimentation rate, renal and hepatic function, lactate dehydrogenase, alkaline phosphatase, albumin, chest radiograph, contrast-enhanced computed tomography (CT) of neck, chest, abdomen, and pelvis, and functional imaging (gallium, later replaced by [18F]fluoro-2-deoxyglucose positron emission tomography [PET]). Bone marrow biopsies were not performed.
Chemotherapy consisted of four 28-day cycles of VAMP: vinblastine 6 mg/m2, Adryamicin 25 mg/m2, and methotrexate 20 mg/m2 administered intravenously on days 1 and 15, and oral prednisone 40 mg/m2 on days 1 through 14. During therapy patients were evaluated weekly.
All patients underwent early response evaluation by CT and functional imaging of originally involved sites of disease just before the third cycle of VAMP. Complete response was defined as a negative gallium- or PET-scan and either ≥75% reduction of the sum of the products of the perpendicular diameters of the lesions of all measurable or evaluable disease, or return of nodes to their normal size. A partial response required at least a 50% reduction in the size of the measurable lesions of the original tumor volume, or gallium or PET avidity persistence in nodal masses despite ≥75% reduction in volume. Less than 50% reduction in the size of the measurable lesions was defined as stable disease, and an increase of more than 25% of the original tumor volume or appearance of new areas of disease represented progressive disease.14
There was no blinding in this study. Radiotherapy assignment was based on early response to chemotherapy. Patients with a complete response at early response evaluation completed therapy following the fourth chemotherapy cycle. Patients who achieved less than a complete response after two cycles received 25.5 Gy involved field radiotherapy (IFRT) in 17 fractions of 1.5 Gy beginning 2 to 4 weeks after completion of all chemotherapy and included treatment of initially involved nodes and surrounding nodal region.
Follow-up evaluations included history and physical examination, chest radiograph, and laboratory examinations every 3 months during the first year, every 4 months during the second and third year, every 6 months up to 5 years off therapy and yearly thereafter. PET and CT scans were performed at 1 and 2 years off therapy and as clinically indicated. Ongoing assessments for late treatment complications include annual evaluations of growth and pubertal development, thyroid, pulmonary, and cardiac function and pregnancy outcomes. Relapses were confirmed by biopsy. Following relapse, patients are observed for survival. Retrieval therapy (cytoreductive chemotherapy, additional radiotherapy with or without consolidative autologous stem-cell transplant) was not specified and was administered according to investigator preference.
Statistical Methods
Phase II study to determine if the 2-year event-free survival is >90% or if there is evidence that the true 2-year event-free survival is <80%. Accrual of 87 patients was required to test the hypothesis with a type I error rate of 5% and 80% power. We also report 5-year outcome estimates as post-hoc analyses.
The one-sample binomial test was used to test whether the proportion of patients event-free at 2 years was significantly different than the unacceptably low rate of 80% and also the desired rate of 90%. Demographic and disease characteristics were compared according to early response to therapy using a two-tailed Fisher’s exact test (categorical variables) or Wilcoxon rank sum test (continuous variables).15 Event-free survival was defined as the time from study enrollment to date of treatment failure (relapse or progressive disease) or date of last follow-up for non-relapsing patients. Event-free and overall survival distributions were estimated using the method of Kaplan-Meier,16 and reported with 95% confidence intervals (CI). Demographic and disease characteristics were examined as predictors of event-free survival using Cox proportional hazards regression.17 Each factor was examined univariately as there were too few events to include multiple predictors in a model. The adequacy of each model was assessed using graphical and numerical methods derived from cumulative sums of martingale residuals over follow-up times and covariate values. Early response to therapy (complete response vs. <complete response), was treated as a time-dependent covariate. These analyses were post-hoc and have limited power due to small number of events and should be considered exploratory. SAS Version 9.2 was used for statistical analysis. All reported p-values are two-sided and considered to be statistically significant if less than 0.05.
RESULTS
Patients were enrolled at St. Jude Children’s Research Hospital (n=44, 48%), Stanford University Medical Center (n=14, 15%), Dana-Farber Cancer Institute (n=19, 21%), Massachusetts General Hospital (n=13, 14%), and Maine Medical Center (n=1, 1%). Of 91 patients enrolled, 3 were ineligible and not considered in this report. Two of these patients did not meet criteria for favorable risk disease, while pathology review of the third patient failed to confirm the diagnosis of Hodgkin lymphoma. Thus, 88 patients are the focus of this report. Sixty-seven percent of the cohort was male (n=59), 88% Caucasian, 8% African-American. The median age at diagnosis was 13.9 years (range, 4.4 – 20.6 years). Histologic subtype revealed classical Hodgkin lymphoma in 56 (64%) and nodular lymphocyte predominant Hodgkin lymphoma in 32 (36%). Thirty-nine (44%) patients had stage IA, 35 (40%) patients had a mediastinal mass, and 13 (15%) had peripheral bulk disease (≥ 6 cm).
Early Response to Therapy
Forty-seven patients (53%) achieved a complete response after 2 cycles of VAMP and finished therapy without IFRT. Among the 41 (47%) who did not achieve a complete response (39 partial responses, 2 stable diseases), 39 received radiotherapy according to protocol, one withdrew consent for participation and received radiotherapy elsewhere, and one had early disease progression prior to radiotherapy and received retrieval therapy. Demographic and disease characteristics according to early response to therapy are listed in Table 1. There was no difference in distribution according to gender, race, or age. Presence of peripheral bulk disease did not differ between groups. Patients with nodular sclerosing Hodgkin lymphoma were less likely to achieve a complete response (63%, P<.001), as were patients with stage IIA disease (71%, P=.01) and those with a mediastinal mass (59%, P=.001).
Table 1.
Demographic and Disease Characteristics According to Early Response to Therapy
| Complete Response | No Complete Response | ||||
|---|---|---|---|---|---|
| Characteristic | No. of Patients | % | No. of Patients | % | P |
| No. of Patients | 47 | 41 | |||
| Gender | |||||
| Male | 35 | 74 | 24 | 59 | .17 |
| Female | 12 | 26 | 17 | 41 | |
| Race | |||||
| Caucasian | 40 | 85 | 37 | 90 | |
| African American | 4 | 9 | 3 | 7 | .53a |
| Asian | 2 | 4 | 0 | 0 | |
| Other | 1 | 2 | 1 | 2 | |
| Age | |||||
| Median (years) | 13.2 | 14.0 | .97 | ||
| Range (years) | 4.7–20.6 | 4.4–19.1 | |||
| Histology | |||||
| Nodular sclerosing | 11 | 23 | 26 | 63 | <.001b |
| Mixed cellularity | 5 | 11 | 5 | 12 | |
| Lymphocyte rich | 2 | 4 | 1 | 2 | |
| Classical, not otherwise specified | 3 | 6 | 3 | 7 | |
| Nodular Lymphocyte predominant | 26 | 55 | 6 | 15 | <.001c |
| Stage | |||||
| IA | 27 | 57 | 12 | 29 | .010 |
| IIA | 20 | 43 | 29 | 71 | |
| Mediastinal mass | |||||
| Absent | 36 | 77 | 17 | 41 | .001 |
| Present | 11 | 23 | 24 | 59 | |
| Bulkd | |||||
| Absent | 43 | 91 | 32 | 78 | .13 |
| Present | 4 | 9 | 9 | 22 | |
Comparison of Caucasian vs. other races
Comparison of nodular sclerosing vs. other histologies
Comparison of lymphocyte predominant vs. other histologies
Peripheral lymph node disease ≥ 6 cm
Follow–up and Outcome
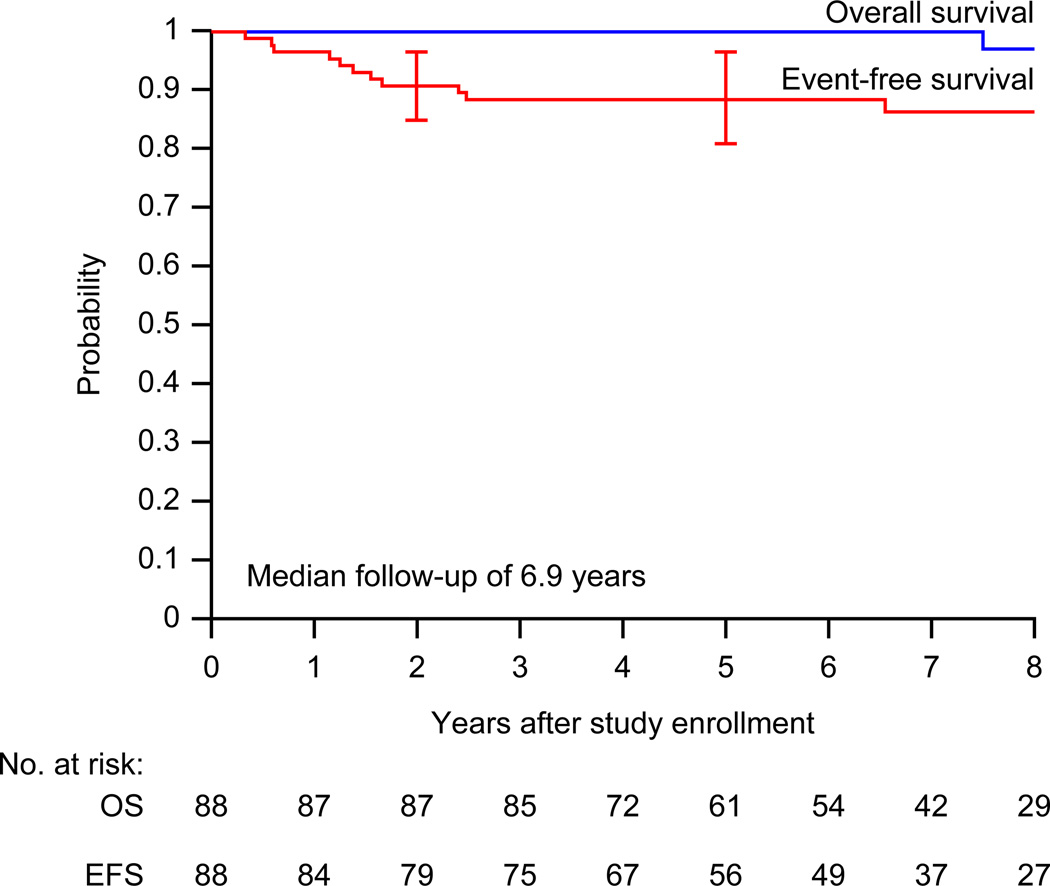
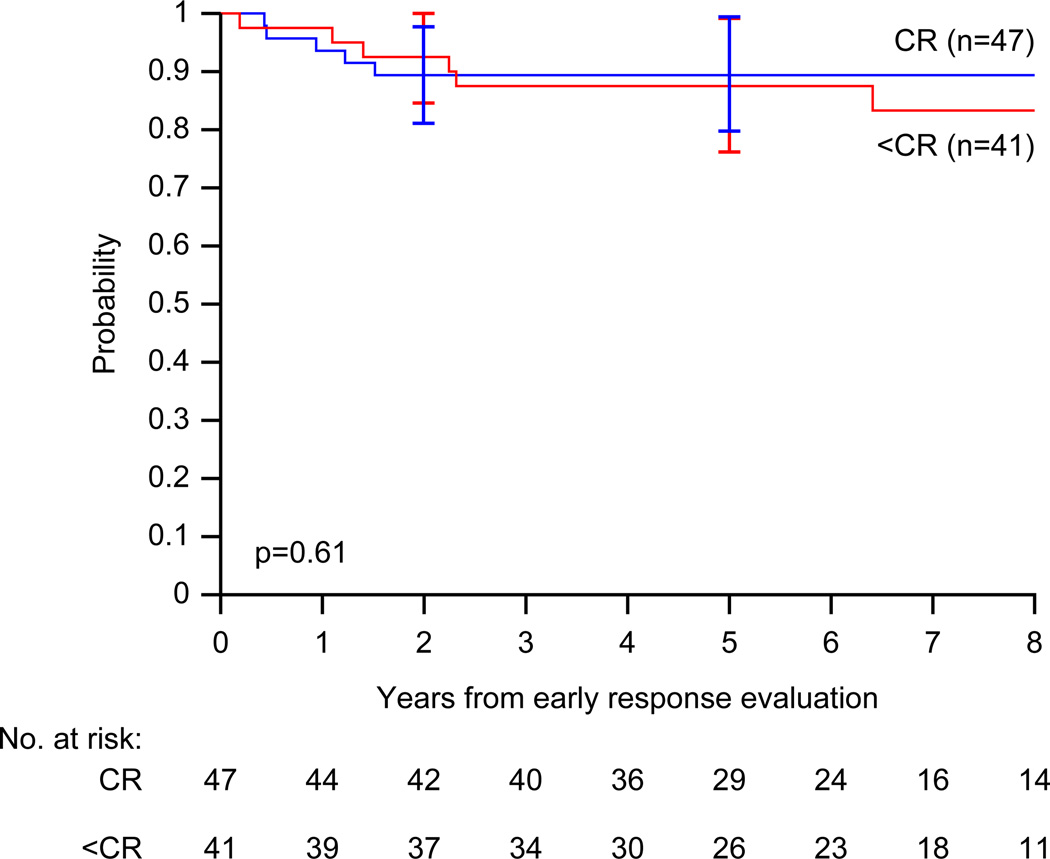
All but one patient are alive at the time of the analysis. The median follow-up for survivors is 6.9 years (range, 2.5 – 11.4 years) excluding the patient who withdrew consent prior to radiotherapy. At the time of analysis (data current to March 12, 2012), 80% of patients had been seen or contacted within the past year, and 91% within the past 18 months. Figure 1 shows overall- and event-free survival distributions for the study cohort. The estimated 2-year event-free survival is 90.8% (95% CI, 84.7% – 96.9%). The proportion of patients event-free at 2 years (90.8%) is significantly different than 80% (p=0.011) but not significantly different than 90% (p=0.98). Figure 2 shows event-free survival distributions according to early response to therapy. The 2-year event-free survival for patients who achieved early complete response and did not receive IFRT is 89.4% (95% CI, 80.8% – 98.0%), compared to 92.5% (95% CI, 84.5% – 100%) for those who did not achieve complete response.
Figure 1.
Overall survival (OS) and event-free survival (EFS) distributions for the whole cohort of children with favorable-risk Hodgkin lymphoma treated with VAMP (vinblastine, doxorubicin, methotrexate, and prednisone) with or without low-dose field radiotherapy (n=88). One patient with nodular lymphocyte predominant Hodgkin lymphoma died more than five years after original diagnosis and combined relapse with transformation to diffuse large B-cell lymphoma. Curves have been truncated at 8 years.
Figure 2.
Event-free survival (EFS) distributions for children with favorable-risk Hodgkin lymphoma according to their early response to therapy: complete response (complete response, n=47) versus less than complete response (< complete response, n=41) after treatment with VAMP (vinblastine, doxorubicin, methotrexate, and prednisone) with or without low-dose, involved-field radiotherapy. Curves have been truncated at 8 years. The p-value was derived using Cox proportional hazards regression.
Excluding the patient who withdrew consent early, 56 of 87 patients (64%) had at least 5 years of follow-up. The 5-year event-free survival is 88.5% (95% CI, 80.7% – 96.3%), and the 5-year overall survival 100%; 1 patient died approximately 7.5 years after study enrollment. The clinical characteristics of the 11 patients who experienced treatment failure (median, 17 months; range, 4 – 79 months) are summarized in Table 2. Non-irradiated patients had an estimated 5-year event-free survival of 89.4% (95% CI, 79.0% – 99.8%), similar to irradiated patients (5-year event-free survival, 87.5% (95% CI, 75.7% – 99.3%). There was no evidence that early complete response was a significant predictor of event-free survival (p=0.61). All non-irradiated patients who recurred did so at a previously involved site and were successfully retrieved with chemotherapy and IFRT without stem-cell transplantation (see Table 2). Sites of failure in 5 of the 6 patients with less than complete response at early response evaluation also included previously irradiated sites. Four of these patients received high-dose chemotherapy and autologous stem-cell transplantation; one patient with a localized late relapse (30 months after original diagnosis) was retrieved with chemotherapy and IFRT. The remaining patient developed recurrent nodular lymphocyte predominant Hodgkin lymphoma concurrent with transformation to diffuse large B-cell lymphoma and succumbed to refractory disease.
Table 2.
Patients with Disease Progression or Recurrence
| Patient No. |
Age at diagnosis (years) |
Stage | Histology | Sites of initial Disease | Early Response |
Time to failure (months) |
Sites of Failure | Retrieval Therapy | Auto- SCT |
Outcome from failure (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.8 | IIA | nLP | R iliac, R inguinal/femoral | CR | 20 | R inguinal/femoral | Stanford V + IFRT | No | NED, 70 |
| 2 | 4.6 | IA | NS | L neck | CR | 17 | L neck | Stanford V + IFRT | No | NED, 30 |
| 3 | 11.2 | IIA | nLP | R+L neck | CR | 7 | Mediastinum, R+L neck | Stanford V + IFRT | No | NED, 33 |
| 4 | 8.9 | IIA | nLP | R+L neck | CR | 7 | R neck | BEACOPP + IFRT | No | NED, 78 |
| 5 | 11.3 | IIA | nLP | R axilla, L neck | CR | 14 | L neck | 6 × COPP + IFRT | No | NED, 33 |
| 6 | 17.6 | IIA | NS | Mediastinum, L neck | PR | 30 | L neck | Stanford V + IFRT | No | NED, 93 |
| 7 | 15.5 | IIA | HL NOS | R axilla, R neck | PR | 4 | R axilla, R neck | 3 × MIED + IFRT | Yes | NED, 68 |
| 8 | 7.0 | IIA | MC | R+L neck | SD | 19 | Mesenteric/porta hepatis, Para-aortic, Spleen, Retroperitoneal | 3 × GV, 1×IV + IFRT | Yes | NED, 29 |
| 9 | 14.5 | IA | NS | L axilla | PR | 15 | R Lung, Mediastinum | IV and GV + IFRT | Yes | NED, 27 |
| 10 | 13.9 | IIA | NS | L neck, Mediastinum | PR | 29 | Bone marrow, Bony Pelvis, Mesenteric/ porta hepatis, Paraaortic, Spleen, Vertebra | BEACOPP + IFRT | Yes | NED, 75 |
| 11a | 9.6 | IIA | nLP | R+L neck | PR | 79 | Liver, Mesenteric/ porta hepatis | As per ANHL01P1 | No | DOD, 11 |
Abbreviations: Auto-SCT, autologous stem-cell transplant; nLP, nodular lymphocyte predominant; NS, nodular sclerosing; HL NOS, Hodgkin lymphoma not otherwise specified; MC, mixed cellularity; CR, complete response, PR, partial response, SD, stable disease; Stanford V, prednisone, vinblastine, doxorubicin, nitrogen mustard, vincristine, bleomycin, and etoposide; IFRT, involved field radiotherapy; COPP, cyclophosphamide, vincristine, prednisone, and procarbazine; MIED, methotrexate, ifosfamide, etoposide, and dexamethasone; GV, gemcitabine and vinorelbine; IV, ifosfamide and vinorelbine; NED, no evidence of disease, DOD, dead of disease.
Patient’s disease transformed to diffuse large B-cell lymphoma upon relapse
Prognostic factors for treatment failure are presented in Table 3. Neither patient characteristics (gender, race, and age) nor tumor characteristics (histology, stage, presence of a mediastinal mass or peripheral lymph node bulk) predicted failure, although the power to detect such prognostic factors is limited by the relatively small number of patients and events.
Table 3.
Prognostic Factors for Treatment Failure in Children with Favorable Risk Hodgkin Lymphoma
| No. of Patients | |||||||
|---|---|---|---|---|---|---|---|
| Factor | All (n=88) |
Failed (n=11) |
Univariate Analysis | ||||
| n | % | n | % | HR | 95% CI | P | |
| Gender | |||||||
| Male | 59 | 67 | 8 | 73 | 1.41 | 0.37–5.31 | 0.61 |
| Female | 29 | 33 | 3 | 27 | 1.0 | - | - |
| Race | |||||||
| Caucasian | 77 | 88 | 9 | 82 | 0.58 | 0.13–2.69 | 0.49 |
| Afro-American | 7 | 8 | 1 | 9 | |||
| Asian | 2 | 2 | 0 | 0 | 1.0 | - | - |
| Other | 2 | 2 | 1 | 9 | |||
| Age at Study Enrollment (years) | |||||||
| Median | 13.9 | 11.3 | 0.92a | 0.80–1.07 | 0.28 | ||
| Range | 4.4–20.6 | 4.7–17.7 | |||||
| Histology | |||||||
| Nodular sclerosing | 37 | 42 | 4 | 36 | 0.77b | 0.23 – 2.62b | 0.67b |
| Nodular lymphocyte predominant | 32 | 36 | 5 | 45 | 1.50c | 0.46 – 4.91c | 0.50c |
| Mixed cellularity | 10 | 11 | 1 | 9 | - | - | - |
| Other histologies | 9 | 10 | 1 | 9 | - | - | - |
| Stage | |||||||
| IA | 39 | 44 | 2 | 18 | 1.0 | - | - |
| IIA | 49 | 56 | 9 | 82 | 3.77 | 0.82–17.47 | 0.090 |
| Mediastinal mass | |||||||
| Absent | 53 | 60 | 8 | 73 | 1.0 | - | - |
| Present | 35 | 40 | 3 | 27 | 0.53 | 0.14–1.99 | 0.35 |
| Bulkd | |||||||
| Absent | 75 | 85 | 8 | 73 | 1.0 | - | - |
| Present | 13 | 15 | 3 | 27 | 2.33 | 0.62–8.80 | 0.21 |
| Early Response to Therapy | |||||||
| CR | 47 | 53 | 5 | 45 | 0.73 | 0.22–2.40 | 0.61 |
| < CR | 41 | 47 | 6 | 55 | 1.0 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; CR, complete response.
One-year increment
Comparison of nodular sclerosing vs. all other histologies combined
Comparison of nodular lymphocyte predominant vs. all other histologies combined
Peripheral lymph node disease ≥ 6 cm
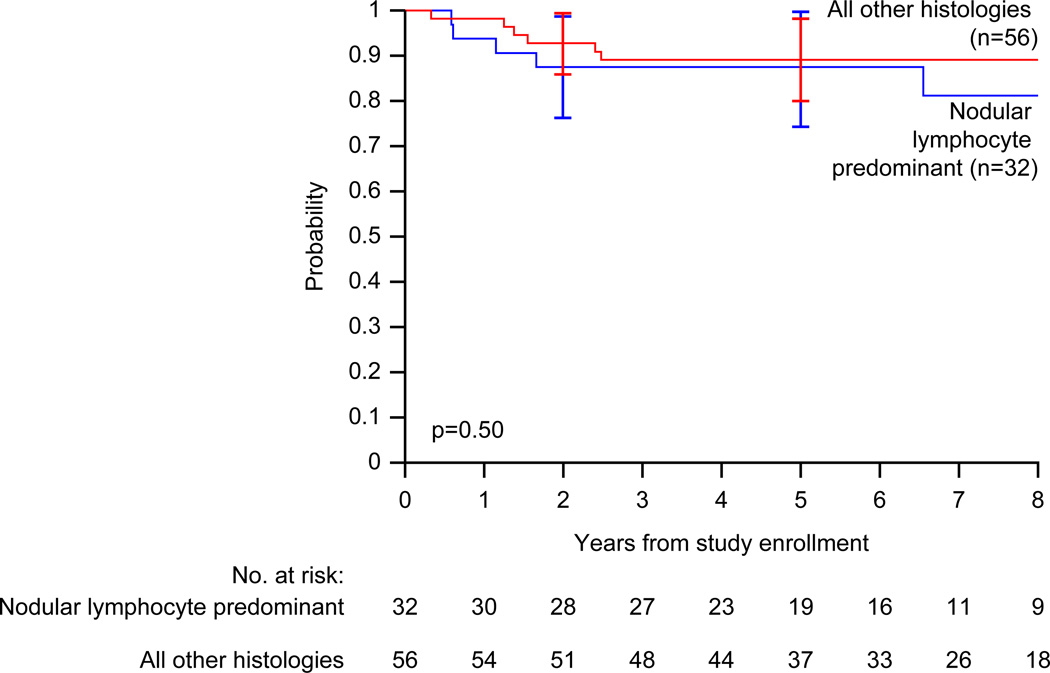
The estimated 5-year event-free survival for patients with nodular lymphocyte predominant Hodgkin lymphoma is 87.5% (95% CI, 74.0% – 100%), compared with 89.1% (95% CI, 79.7% – 98.5%; p=0.50) for classical Hodgkin lymphoma (Figure 3).
Figure 3.
Event-free survival (EFS) distributions for nodular lymphocyte predominant Hodgkin lymphoma (n=32) versus classical Hodgkin lymphoma (all other histologies, n=56) in children treated with VAMP (vinblastine, doxorubicin, methotrexate, and prednisone) with or without low-dose field radiotherapy. Curves have been truncated at 8 years. The p-value was derived using Cox proportional hazards regression.
Acute and Long-Term Toxicity
Therapy was well tolerated without major complications. Delay or dose modifications due to toxicity were rare. The most common side effects were neuropathic pain (2% of patients) and nausea/vomiting (3% of patients), readily managed with supportive care. Neutropenia (absolute neutrophil count < 1000/dL) was observed in 60% of patients (32% of cycles), and febrile neutropenia in 2% of patients (0.9% of cycles). Nine patients (10%) were hospitalized eleven times (3% of cycles) for febrile neutropenia (3 hospitalizations) or non-neutropenic infection. Granulocyte colony stimulating factors were not used.
No second malignant neoplasm occurred except for a histology transformation in a patient with nodular lymphocyte predominant Hodgkin lymphoma. Nine of 88 patients (10%) developed asymptomatic compensated hypothyroidism 16–30 months from study enrollment. All of them had received IFRT to the neck. Two patients developed osteonecrosis, and 2 others developed moderate osteopenia. Twelve patients (26%) developed subclinical pulmonary dysfunction following thoracic radiation, most commonly asymptomatic mild to moderate pulmonary diffusion deficits (n=8; DLCO 57 – 74% of predicted). Four patients (5%; 2 irradiated and 2 non-irradiated) experienced asymptomatic left ventricular dysfunction (shortening fractions <30%; range, 24% – 29%) at a median of 35 months after study enrollment (range, 8 – 86 months). Two of them (1 irradiated and 1 non-irradiated) recovered shortening fraction to ≥ 30%, and the other two to 29% and 28% respectively. All remained asymptomatic. Among patients >18 years old (23 females and 38 males), 6 babies were conceived by 3 patients (2 females and 1 male) resulting in 4 live births.
COMMENT
To our knowledge, this is the first trial in which a select group of children with favorable risk Hodgkin lymphoma experienced a high rate of 2- and 5-year event-free survival without exposure to radiotherapy, alkylating agent, epipodophyllotoxin, or bleomycin chemotherapy and a relatively low cumulative dose of anthracyclines. The desire to avoid late treatment complications—particularly those resulting from high doses of irradiation—has motivated most treatment modifications for pediatric Hodgkin lymphoma. Early trials established the effectiveness of combined-modality therapy featuring multi-agent chemotherapy and lower cumulative doses of radiation to involved sites of disease.18–20 To avoid radiation complications altogether, chemotherapy-only trials were developed prescribing multiple courses of nitrogen mustard, vincristine, procarbazine, and prednisone (MOPP) or derivatives.21–23 These regimens proved to be effective in achieving long term remissions.24–26 However, most trials featured high cumulative doses of alkylating agents, anthracyclines, or bleomycin leading to increased morbidity from myelosuppression, cardiopulmonary and gonadal toxicity, and secondary leukemia. As a result, contemporary combined-modality trials focus on balancing efficacy and toxicity of therapy. Investigators have also sought to identify patients with favorable features who would be candidates for therapy reductions. These efforts led to the evaluation of a response-based radiation approach, which was first undertaken in Stanford pediatric protocols prescribing lower doses of radiation to patients with good response to MOPP.27 This experience has shaped our consortium trials since.6;28;29
The Children’s Cancer Group study CCG594230 and the GPOH-HD954 study (confirmed in the GPOH-HD20025 study) pursued response-based trials in the mid 1990’s aimed to omit radiation. The GPOH trials had comparable outcomes for non-irradiated favorable risk patients who achieved complete response after 2 cycles of vincristine, procarbazine, prednisone, and doxorubicin (OPPA; for girls) or vincristine, etoposide, prednisone, and doxorubicin (OEPA; for boys), compared to those who achieved less than complete response and received radiotherapy. A marked event-free survival advantage with combined-modality therapy was only appreciated in intermediate and high risk groups, while overall survival remained comparable.4 In the CCG study, patients who achieved a complete response after all chemotherapy were randomized to 21 Gy IFRT or no additional treatment (eTable1). There was a 3-year event-free survival advantage for the irradiated group (100% vs. 89%); however, survival in the two groups was identical (3-year overall survival 100%), raising the concern of the number of children needed irradiated to prevent one relapse. This is particularly pertinent in our study where patients developing relapse after chemotherapy-only were successfully retrieved with standard multi-agent chemotherapy and IFRT without high-dose chemotherapy or stem-cell transplant. As a result, more than 50% of patients on our study could be spared radiotherapy, and the majority could be cured without exposure to leukemogenic agents.
We previously reported the results using VAMP chemotherapy and low-dose IFRT.6;11 The 5-year event-free and overall survival for the entire cohort were 93% and 99%, respectively. Results of the current study are similar with a 5-year event-free and overall survival of 89% and 100%, respectively. In the present study, the 5-year event-free survival for patients with classical Hodgkin lymphoma was 89%, with no difference in outcome between those treated with and without radiotherapy (p=0.61).
Historically, patients with nodular lymphocyte predominant Hodgkin lymphoma have a favorable outcome and have been treated on regimens suitable for classical Hodgkin lymphoma; however, there are several reports in the adult and pediatric literature suggesting that such patients can be cured with less therapy. In adults, radiotherapy-only approaches are favored;31;32 however, the required doses of 30 to 36 Gy would result in significant musculoskeletal toxicity in children. Most children with nodular lymphocyte predominant Hodgkin lymphoma do well regardless of therapy chosen; toxicity remains the main concern for more involved treatment approaches.33–35 Remarkably, a substantial proportion of children with limited-stage, completely-resected nodular lymphocyte predominant Hodgkin lymphoma achieve long-term remission with surgical resection alone without additional therapy.37 In view of these results, the outcome for patients with nodular lymphocyte predominant Hodgkin lymphoma on our study who were treated without irradiation was disappointing. Twenty-six of 32 patients were early responders and thus treated without irradiation. Four of them (15%) relapsed, compared with no treatment failures in our prior experience with a combined-modality approach wherein all such patients were irradiated.6 In the current study, none of the ten patients with nodular lymphocyte predominant Hodgkin lymphoma who had undergone complete resection relapsed; thus, it is possible that many of them could have been spared chemotherapy altogether. In contrast, patients with stage II (and unresected) nodular lymphocyte predominant Hodgkin lymphoma who did not receive radiotherapy are at increased risk of relapse. Whether or not the omission of alkylating agents from the VAMP regimen can account for the less favorable result is speculative. However, it appears that even low dose irradiation may be beneficial for children with nodular lymphocyte predominant Hodgkin lymphoma who are treated with chemotherapy regimens that omit alkylating agents.
A limitation of this study is the relatively small sample size limiting the power to assess differences between study sites and limiting subgroup analyses. Thus, it would be important to confirm the results in a larger cohort. Our results suggest that a risk-adapted response-based approach may be very effective and well tolerated for a selected group of patients with favorable risk Hodgkin lymphoma. Such patients can achieve high 2-year event-free survival without alkylating agent, bleomycin, or epipodophyllotoxin chemotherapy, and more than half without radiotherapy. Future studies should consider further tailoring of radiotherapy reserving irradiation for patients who remain PET positive at early response evaluation.
Supplementary Material
Acknowledgements
Funding/Support: Study supported by the National Institutes of Health Cancer Support Core Grant (CA-21765) and the American Lebanese Syrian Associated Charities (ALSAC).
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
The authors would like to thank editorial support by David Galloway, BS, ELS, staff scientific editor at St Jude Children’s Research Hospital, Memphis, TN, whose services are available to all investigators free of charge.
Footnotes
Previous Presentation: Presented in part at the annual meeting of the American Society of Clinical Oncology (ASCO), June, 2011.
Access to data: Dr Metzger had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Online-Only Material: eTables1 is available at http://www.jama.com.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Independent Statistical Analysis: Dr. Wu performed the statistical analysis of the data with the assistance of Ms Billups.
Data and Safety Monitoring Board: Frank Baylis, MD, Chair of the Data and Safety Monitoring Board, is Professor of Pediatrics, Perelman School of Medicine at the University of Pennsylvania and Director of Clinical Research, Center for Childhood Cancer Research in Philadelphia, PA. James Boyett, PhD, St Jude Data and Monitoring Board Executive Secretary, St Jude Children’s Research Hospital, Memphis, TN.
Reference List
- 1.Hudson MM, Jones D, Boyett J, Sharp GB, Pui CH. Late mortality of long-term survivors of childhood cancer. J Clin Oncol. 1997;15:2205–2213. doi: 10.1200/JCO.1997.15.6.2205. [DOI] [PubMed] [Google Scholar]
- 2.Hudson MM, Poquette CA, Lee J, et al. Increased mortality after successful treatment for Hodgkin's disease. J Clin Oncol. 1998;16:3592–3600. doi: 10.1200/JCO.1998.16.11.3592. [DOI] [PubMed] [Google Scholar]
- 3.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorffel W, Luders H, Ruhl U, et al. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin's disease in children and adolescents: analysis and outlook. Klin Padiatr. 2003;215:139–145. doi: 10.1055/s-2003-39372. [DOI] [PubMed] [Google Scholar]
- 5.Mauz-Korholz C, Hasenclever D, Dorffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010;28:3680–3686. doi: 10.1200/JCO.2009.26.9381. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson SS, Link MP, Weinstein HJ, et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin's disease. J Clin Oncol. 2007;25:332–337. doi: 10.1200/JCO.2006.08.4772. [DOI] [PubMed] [Google Scholar]
- 7.Landman-Parker J, Pacquement H, Leblanc T, et al. Localized childhood Hodgkin's disease: response-adapted chemotherapy with etoposide, bleomycin, vinblastine, and prednisone before low-dose radiation therapy-results of the French Society of Pediatric Oncology Study MDH90. J Clin Oncol. 2000;18:1500–1507. doi: 10.1200/JCO.2000.18.7.1500. [DOI] [PubMed] [Google Scholar]
- 8.Tebbi CK, Mendenhall N, London WB, Williams JL, de Alarcon PA, Chauvenet AR. Treatment of stage I, IIA, IIIA(1) pediatric Hodgkin disease with doxorubicin, bleomycin, vincristine and etoposide (DBVE) and radiation: A Pediatric Oncology Group (POG) study. Pediatr Blood Cancer. 2005;46(2):198–202. doi: 10.1002/pbc.20546. [DOI] [PubMed] [Google Scholar]
- 9.Kung FH, Schwartz CL, Ferree CR, et al. POG 8625: a randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with Stages I, IIA, IIIA1 Hodgkin Disease: a report from the Children's Oncology Group. J Pediatr Hematol Oncol. 2006;28:362–368. doi: 10.1097/00043426-200606000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz CL, Constine LS. Early Response-based Therapy for Children with Hodgkin Lymphoma: A Surrogate for Using Biology to Effect Cure and Minimize Toxicity. Educational Book 2010. 2010:391–396. ASCO. [Google Scholar]
- 11.Donaldson SS, Hudson MM, Lamborn KR, et al. VAMP and low-dose, involved-field radiation for children and adolescents with favorable, early-stage Hodgkin's disease: results of a prospective clinical trial. J Clin Oncol. 2002;20:3081–3087. doi: 10.1200/JCO.2002.12.101. [DOI] [PubMed] [Google Scholar]
- 12.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971;31:1860–1861. [PubMed] [Google Scholar]
- 13.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brepoels L, Stroobants S, De WW, et al. Hodgkin lymphoma: Response assessment by revised International Workshop Criteria. Leuk Lymphoma. 2007;48:1539–1547. doi: 10.1080/10428190701422414. [DOI] [PubMed] [Google Scholar]
- 15.Gehan EA. A generalized wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52:203–223. [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Cox DR. Regression models and life-tables. J.Roy.Stat.Soc. B. 1972;34:187–220. [Google Scholar]
- 18.Hudson M, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent (COP/ABVD) chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin's disease. J Clin Oncol. 1993;11:100–108. doi: 10.1200/JCO.1993.11.1.100. [DOI] [PubMed] [Google Scholar]
- 19.Hunger SP, Link MP, Donaldson SS. ABVD/MOPP and low-dose involved-field radiotherapy in pediatric Hodgkin's disease: the Stanford experience. J Clin Oncol. 1994;12:2160–2166. doi: 10.1200/JCO.1994.12.10.2160. [DOI] [PubMed] [Google Scholar]
- 20.Weiner MA, Leventhal B, Brecher ML, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin's disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol. 1997;15:2769–2779. doi: 10.1200/JCO.1997.15.8.2769. [DOI] [PubMed] [Google Scholar]
- 21.Behrendt H, Brinkhuis M, Van Leeuwen EF. Treatment of childhood Hodgkin's disease with ABVD without radiotherapy. Med Pediatr Oncol. 1996;26:244–248. doi: 10.1002/(SICI)1096-911X(199604)26:4<244::AID-MPO4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 22.Hakvoort-Cammel FG, Buitendijk S, Heuvel-Eibrink M, Hahlen K. Treatment of pediatric Hodgkin disease avoiding radiotherapy: excellent outcome with the Rotterdam-HD-84-protocol. Pediatr Blood Cancer. 2004;43:8–16. doi: 10.1002/pbc.20031. [DOI] [PubMed] [Google Scholar]
- 23.Van Den BH, Zsiros J, Behrendt H. Treatment of childhood Hodgkin's disease without radiotherapy. Ann Oncol. 1997;8(Suppl 1):15–17. [PubMed] [Google Scholar]
- 24.Baez F, Ocampo E, Conter V, et al. Treatment of childhood Hodgkin's disease with COPP or COPP-ABV (hybrid) without radiotherapy in Nicaragua. Ann Oncol. 1997;8:247–250. doi: 10.1023/a:1008200210674. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs P, King HS, Karabus C, Hartley P, Werner D. Hodgkin's disease in children. A ten-year experience in South Africa. Cancer. 1984;53:210–213. doi: 10.1002/1097-0142(19840115)53:2<210::aid-cncr2820530204>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Olweny CL, Katongole-Mbidde E, Kiire C, Lwanga SK, Magrath I, Ziegler JL. Childhood Hodgkin's disease in Uganda: a ten year experience. Cancer. 1978;42:787–792. doi: 10.1002/1097-0142(197808)42:2<787::aid-cncr2820420251>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Donaldson SS, Link MP. Combined modality treatment with low-dose radiation and MOPP chemotherapy for children with Hodgkin's disease. J Clin Oncol. 1987;5:742–749. doi: 10.1200/JCO.1987.5.5.742. [DOI] [PubMed] [Google Scholar]
- 28.Friedmann AM, Hudson MM, Weinstein HJ, et al. Treatment of unfavorable childhood Hodgkin's disease with VEPA and low-dose, involved-field radiation. J Clin Oncol. 2002;20:3088–3094. doi: 10.1200/JCO.2002.03.051. [DOI] [PubMed] [Google Scholar]
- 29.Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin's disease. J Clin Oncol. 2004;22:4541–4550. doi: 10.1200/JCO.2004.02.139. [DOI] [PubMed] [Google Scholar]
- 30.Nachman JB, Sposto R, Herzog P, et al. Randomized Comparison of Low-Dose Involved-Field Radiotherapy and No Radiotherapy for Children With Hodgkin's Disease Who Achieve a Complete Response to Chemotherapy. J Clin Oncol. 2002;20:3765–3771. doi: 10.1200/JCO.2002.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Nogova L, Reineke T, Eich HT, et al. Extended field radiotherapy, combined modality treatment or involved field radiotherapy for patients with stage IA lymphocyte-predominant Hodgkin's lymphoma: a retrospective analysis from the German Hodgkin Study Group (GHSG) Ann Oncol. 2005;16:1683–1687. doi: 10.1093/annonc/mdi323. [DOI] [PubMed] [Google Scholar]
- 32.Wirth A, Yuen K, Barton M, et al. Long-term outcome after radiotherapy alone for lymphocyte-predominant Hodgkin lymphoma: a retrospective multicenter study of the Australasian Radiation Oncology Lymphoma Group. Cancer. 2005;104:1221–1229. doi: 10.1002/cncr.21303. [DOI] [PubMed] [Google Scholar]
- 33.Karayalcin G, Behm FG, Gieser PW, et al. Lymphocyte predominant Hodgkin disease: clinico-pathologic features and results of treatment--the Pediatric Oncology Group experience. Med Pediatr Oncol. 1997;29:519–525. doi: 10.1002/(sici)1096-911x(199712)29:6<519::aid-mpo1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 34.Murphy SB, Morgan ER, Katzenstein HM, Kletzel M. Results of little or no treatment for lymphocyte-predominant Hodgkin disease in children and adolescents. J Pediatr Hematol Oncol. 2003;25:684–687. doi: 10.1097/00043426-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Sandoval C, Venkateswaran L, Billups C, Slim M, Jayabose S, Hudson MM. Lymphocyte-predominant Hodgkin disease in children. J Pediatr Hematol Oncol. 2002;24:269–273. doi: 10.1097/00043426-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Straus DJ, Portlock CS, Qin J, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood. 2004;104:3483–3489. doi: 10.1182/blood-2004-04-1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.