Abstract
Purpose
In order to predict whether tumor markers assist in the histopathologic diagnosis of germ cell tumors (GCTs), we analyzed the correlation of beta human chorionic gonadotropin (bHCG) and alpha-fetoprotein (AFP) in serum and cerebrospinal fluid (CSF) samples at baseline and subsequent follow-up examinations.
Method
A retrospective study of patients diagnosed with intracranial GCTs between July 1985 and February 2011 at our institution was conducted to review clinical, surgical, radiological, laboratory and histopathologic data.
Results
Of 67 patients eligible for the study, 42 had germinomas and 25 non germinomatous GCTs. At baseline, serum and CSF AFP agreed in 97.9% of patients (Cohen’s Kappa 0.93). Baseline bHCG samples agreed in only 72.5% of patients (Cohen’s Kappa 0.46). In most cases, values were higher in serum for AFP and in CSF for bHCG. ROC curves estimated from logistic regression model indicated that CSF and serum samples had almost equal diagnostic utility, and the DeLong test showed that the difference in area under curves was not statistically significant. During follow-up (185 paired CSF and serum values from 43 patients), 90.3% of AFP values correlated between CSF and serum (Cohen’s Kappa 0.22, showing fair agreement). For bHCG, 96.2% of values agreed in serum and CSF (Cohen’s Kappa 0.61).
Conclusions
In some patients, intracranial GCTs can be diagnosed based solely upon positive serum AFP values. In addition, marker values from serum only may be sufficient to predict tumor relapse at interval follow-up examinations.
Keywords: germ cell tumors, tumor markers, beta human chorionic gonadotropin, alpha-fetoprotein, correlation of markers, diagnostic utility
Introduction
Germ cell tumors (GCTs), a heterogeneous group of gonadal or extragonadal tumors thought to arise from the aberrant migration and differentiation of primordial germ cells during embryogenesis, comprise approximately 3% of all pediatric cancers [3]. Extragonadal GCTs occur primarily along midline sites such as the mediastinum and sacrococcygeal region, and intracranially in the pineal and suprasellar regions [21]. Classified as germinomatous and non-germinomatous germ cell tumors (NGGCTs), intracranial GCTs are a rare but interesting group of tumors accounting for a small portion of all GCTs and only 3% of all pediatric brain tumors [3, 6, 7, 21]. Germinomas are highly chemo-radio sensitive, and therefore generally have a good prognosis, in contrast to NGGCTs which are generally viewed as less sensitive to radiation and chemotherapy with a consequently worse prognosis [3,6,7,21].
NGGCTs and a subpopulation of germinomas secrete specific proteins known as alpha-fetoprotein (AFP) and beta-human chorionic gonadotropin (bHCG), which can be used as tumor markers [17]. Tumor markers have been identified for a variety of cancers and integrated into the diagnosis and management of patients with these cancers [17]. AFP is a 70 kDa glycoprotein normally secreted by the fetus primarily in the yolk sac, gastrointestinal tract, and liver [4, 5]. bHCG is a 36.7 kDa glycoprotein normally secreted by placental tissues [17].
Specifically, AFP and bHCG have been useful clinical measures for patients with GCTs in regard to diagnosis, response to therapy, prognosis, and monitoring disease recurrence [9,17]. Moreover, AFP and bHCG [17] help to differentiate germinomas from NGGCTs. Elevated serum or CSF concentrations of AFP are strong predictors of NGGCTs [7], as are high concentrations ob bHCG; however, the latter can be mildly to moderately increased in germinomas [7]. AFP is a 70 kDa glycoprotein normally secreted by the fetus primarily in the yolk sac, gastrointestinal tract, and liver [4, 5]. Since AFP concentrations normally drop to adult levels by 1 year of life, elevated levels in adolescents and adults can be indicative of malignancy [9]. bHCG is a 36.7 kDa glycoprotein normally secreted by placental tissues [17]. Similarly, elevated serum concentrations of bHCG in males and non-pregnant females can be indicative of malignancy [11]. Clinically, AFP and bHCG have been useful markers in diagnosis, measuring response to therapy, prognosis, and monitoring disease recurrence of GCTs [9,17]. These tumor markers can help differentiate germinomas from NGGCTs. Elevated serum or CSF concentrations of AFP are strong predictors of NGGCTs [7]. bHCG can be mildly to moderately increased in germinomas, but very high concentrations suggest NGGCTs [7].
Most data on tumor markers in GCTs are from gonadal GCTs [9, 15, 16]. Although these tumors are thought to be similar to their intracranial counterpart in regard to histology, genetics, and response to therapy [6], some are significantly different [3]. It is important to validate the findings for extracranial GCT markers compared to those for intracranial GCT markers [8,10,12,13,19]. The location of intracranial GCTs allows the detection of AFP and bHCG in the cerebrospinal fluid (CSF) as well, thereby providing another relevant source to potentially diagnose and manage patients with intracranial GCTs. CSF samples, however are usually procured through lumbar punctures which carry with them the risk of complications, including infection, pain, spinal headache, CSF leak, and those associated with sedation [1], not to mention the increased costs associated with obtaining CSF samples. On the other hand, serum samples cost less and are less invasive to obtain. Although studies have begun to look at tumor markers specifically in intracranial GCTs [8,10,12,13,19], it remains to be determined whether it is necessary to assess the concentrations of tumor markers in both the CSF and serum for intracranial GCTs. In this study, we analyze how both these sources correlate at baseline, and during follow-up, and we described their diagnostic utility.
Methods
Study design and participants
This study was approved by the institutional review board of the St. Jude Children’s Research Hospital (St. Jude). A retrospective study of patients treated for intracranial GCTs at St. Jude from July 1985 to February 2011 was conducted. Medical records were reviewed for clinical, surgical, radiological, laboratory, and histopathologic data.
Tumor-related definitions
Surgical resection was considered gross total resection (GTR) when no visible tumor remained, near total resection (NTR) when > 90% of tumor was removed, subtotal resection (STR) when 50%–90% of tumor was removed, partial resection (PR) when 10%–49% of the tumor was removed, and biopsy if less than 10% of tumor was removed. Date of diagnosis was considered the first radiologic scan showing tumor. A metastasis was defined as the presence of a tumor distant (except for bifocal) from the primary tumor or by positive CSF tumor cell cytology. Bifocal tumors were defined as being simultaneously present in both the pineal and suprasellar regions [14]. Time to follow-up was considered time from diagnosis to last contact with patient.
Tumor marker assessment
The baseline tumor marker value was considered the value drawn closest to the second day of treatment, regardless of whether markers concentrations were measured at St. Jude or at another institution. Since the normal values used in previous studies vary, the normal values established by our institution were used: CSF-AFP ≤ 0.8 ng/mL, serum AFP ≤ 12 ng/mL, and bHCG ≤ 5 milli international units (mIU)/mL for both serum and CSF. Since many institutions use the international units for AFP instead of the mass units, it is worth mentioning that one IU equals 1.21 ng [20]. As the range of raw markers value is wide and sometimes included zeros, natural log of the raw values plus one (1) (i.e., ln(raw value + 1)) were used to transform the makers to better support the normality assumption. To more specifically compare CSF and serum samples at each time point during follow-up examinations, only data from patients whose CSF and serum samples had been collected within 5 days of each other, for both AFP and bHCG were included. Forty-three patients met these criteria with both AFP and bHCG values available for at least one follow-up exam. All values (n=185) were pooled to assess the correlation at follow-up.
Statistical analyses
Descriptive statistics and graphical tools were used to summarize general demographics and characteristics of the data. The McNemar test was employed to test the marginal homogeneity of outcomes of CSF and serum markers. The magnitude of agreement was further estimated by Cohen’s Kappa coefficient. The predictive ability of a given set of markers was described using receiver operating characteristics (ROC) curves estimated using logistic regression models [18]. ROC curves were compared by the DeLong test [18].
Results
Patient Characteristics
There were 67 patients (48 males and 19 females; median age at diagnosis, 11.8 years) eligible for the study. The characteristics of our cohort (Table 1) were consistent with those reported in previous studies for intracranial GCTs [3,6,7,21]. Histopathologic confirmation was not available for 15 patients; 2 of whom were diagnosed with germinoma based on the tumor’s bifocal occurrence; 3 of whom were diagnosed with germinoma in the setting of negative AFP values and only slightly elevated bHCG values; and 10 of whom were diagnosed with NGGCTs based on elevated concentrations of either bHCG (n=4) or both AFP and bHCG (n=6). The majority of patients (n=58, 86.6%) received cranio-spinal irradiation (CSI). Ninety-two percent (n=23) of patients with NGGCTs and 42.9% (n=18) of those with germinomas received chemotherapy. The median time of follow-up was 9.3 years (range, 0.4–24.4 years).
Table 1.
Patient (n=67) characteristics
| Patient Demographics | ||||
| Sex | Male | 48 | ||
| Female | 19 | |||
| Race | Caucasian | 54 | ||
| African American | 10 | |||
| Other | 3 | |||
| Age at diagnosis(years) [median (range)] | 11.8 (2.2–18.7) | |||
| Tumor description | ||||
| Diagnosis | Germinoma | 42 | ||
| NGGCT | 25 | |||
| Location | Pineal | 38 | ||
| Suprasellar | 22 | |||
| Bifocal | 7 | |||
| Metastatic at presentation | 21 | |||
| Initial Treatment | ||||
| Germinoma (42) | NGGCT (25) | Total (67) | ||
| Surgery | 37 | 15 | 52 | |
| Biopsy | 26 | 5a | 31 | |
| PR | 3 | 2 | 5 | |
| STR | 6 | 3 | 9 | |
| NTR/GTR | 2 | 5b | 7 | |
| No Surgery | 5c | 10d | 15 | |
| RT | 41 | 24 | 65e | |
| Focal only | 2f | 0 | 2 | |
| CSI+Focal Boost | 36 | 22 | 58 | |
| Whole Brain+Focal Boost | 0 | 1g | 1 | |
| WVI + Focal Boost | 3 | 1 | 4 | |
| No RT | 1 | 1 | 2 | |
| Additional Sterotactic radiosurgery | 0 | 4 | 4 | |
| Chemotherapy | 18 | 23h | 41 | |
| Follow-up | ||||
| Time of follow-up (years) [median (range)] | Median | 9.3 (0.4–24.4) | ||
| Status | NEDi | 57 | ||
| DOD | 5 | |||
| DNODj | 5 |
Abbreviations: NGGCT, non-germinomatous germ cell tumor; PR, partial resection;, STR, subtotal resection; NTR, near total resection; GTR, gross total resection; RT, radiation therapy; CSI, cranio-spinal irradiation; WVI, whole ventricular irradiation; NED, no evidence of disease; DOD, died of disease; DNOD died not of disease.
Five patients with NGGCT had a biopsy and then soon after had second surgery [partial (n=1), STR (n=1), NTR/GTR (n=3)] and for them the surgery after biopsy was considered the definite surgery; therefore, these patients were not included in the patient numbers for biopsy.
Five patients had GTR/NTR surgery after RT(n=1), chemo (n=3), or both (n=1).
Three patients were diagnosed by elevated bHCG and two by bifocal location.
Ten patients were diagnosed by elevated markers alone, with no indication for surgery.
One patient with germinoma and 1 with NNGCT did not receive RT, as they were 2 and 3 years old, respectively.
One patient treated outside St. Jude, and 1 patient treated in the low-risk branch of POG 9530 protocol.
Patient treated in 1985.
Two patients treated in 1985 did not receive chemotherapy.
Four patients were considered NED on the basis of negative markers but still had a residual mass likely consisting of benign fibrous tissue.
Five patients considered DNOD [endocrinopathies (n=2), second malignancy (n=1), VP shunt sepsis (n=1), and suicide (n=1)].
Correlation of Baseline Tumor Markers
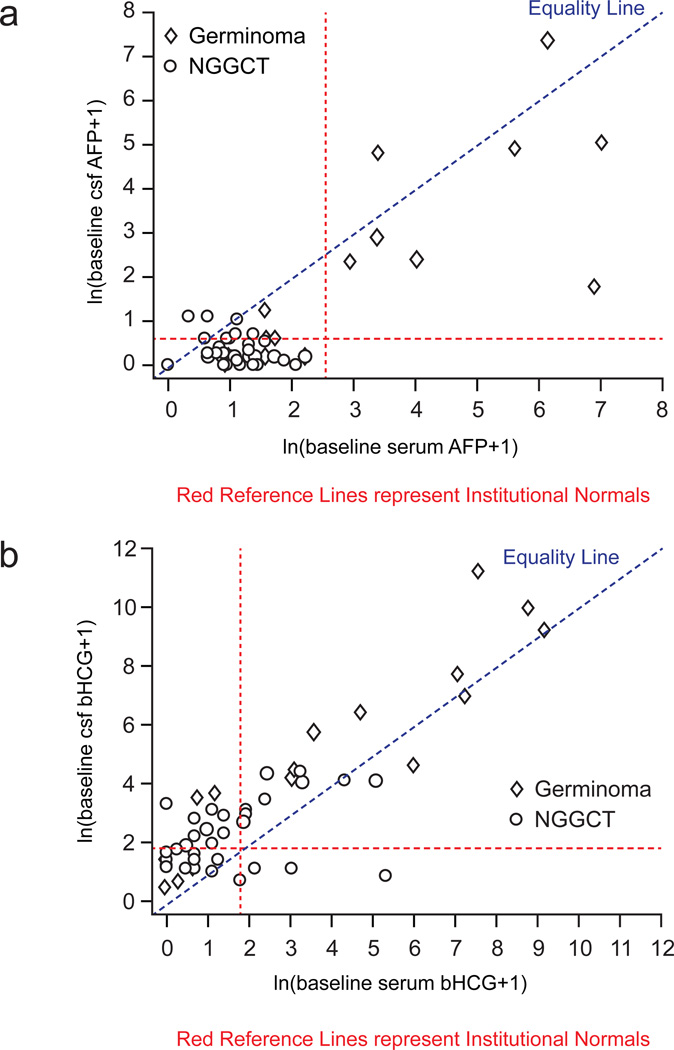
Table 2 compares baseline CSF AFP values with those in the serum. CSF and serum AFP values correlated in all but one patient (97.9%). The Cohen’s Kappa of agreement was high at 0.93. The one patient for whom CSF and serum values did not agree had a NGGCT; CSF was positive for AFP while the serum value was negative for AFP. Figure 1(a) presents the baseline AFP correlation graphically, allowing the data to be studied categorically as normal or abnormal, as well as continuously by measuring the magnitude. Almost all AFP values were on the serum side of the equality line, indicating that they are higher in the serum than the CSF.
Table 2.
Baseline Serum and CSF AFP (n=48)
| Serum | |||||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| CSF | Positive | 1 (2.1%) | 8 (16.7%) | Agree | 47 (97.9%) |
| Negative | 39 (81.3%) | 0 (0.0%) | Do not agree | 1 (2.1%) | |
Figure 1.
Table 3 compares baseline CSF and serum values for bHCG. Correlation for bHCG serum and CSF values was lower (n= 37; 72.5%) than that for AFP. A Cohen’s Kappa of agreement value of 0.46 suggested only moderate agreement of CSF and serum bHCG values. When values did not correlate, they were more often positive in CSF and negative in serum, accounting for 11 of 14 patients whose values did not correlate (78.6%). Three patients, however, did have a positive serum but a negative CSF value for bHCG. Interestingly, the baseline bHCG correlation plotted graphically (Figure 1b) showed a trend opposite to that seen for AFP – the majority (n=41) of bHCG values were higher in CSF than the serum.
Table 3.
Baseline Serum and CSF bHCG (n=51)
| CSF | |||||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Serum | Positive | 3 (5.9%) | 19 (37.3%) | Agree | 37 (72.5%) |
| Negative | 18 (35.3%) | 11 (21.6%) | Do not agree | 14 (27.5%) | |
Baseline tumor marker prediction of histopathologic diagnosis
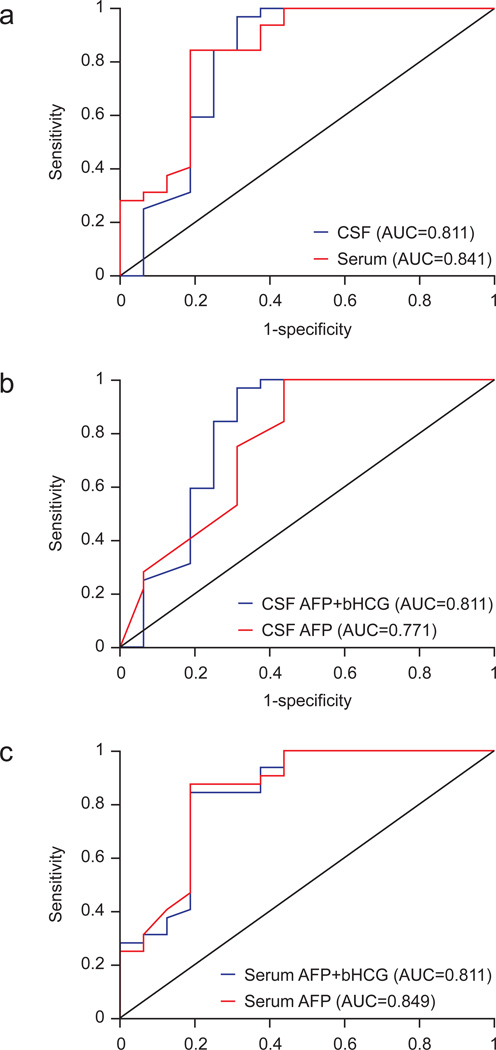
Since these tumor markers are used to diagnose intracranial GCTs, we employed logistic regression models to determine whether CSF or serum markers were better in assisting diagnosis. The CSF model includes both AFP and bHCG in the CSF to predict tumor pathology; the serum model uses serum values of AFP and bHCG. Values for both serum and CSF were available for 48 patients. A comparison of both models by ROC curves (Figure 2a) revealed a target decision probability of 0.5; the serum model correctly classified 39 of 48 (81.3%) patients, with a sensitivity of 93.8% and a specificity of 56.3%. Of these 39 patients, 31 (79.5%) had surgically confirmed pathology consistent with GCT. At a target decision probability of 0.5, the CSF model correctly classified 42 of 48 (87.5%) patients, with a sensitivity of 96.9% and a specificity of 62.5%. Of the 42 patients, 34 (81.0%) had GCT confirmed by surgical pathology. The DeLong test indicated that the difference between the areas under both ROC curves was not significant (P = 0.6411).
Figure 2.
In both CSF and serum samples, comparing regression models of AFP alone with the models of AFP and bHCG showed that addition of bHCG did not lead to a significant change in the ability to predict germinoma versus NGGCT (Figure 2b and 2c). The DeLong test showed that the difference was not statistically significant in the CSF (P = 0.4498) or serum (P = 0.4965).
Correlation of Tumor Markers During Follow-up
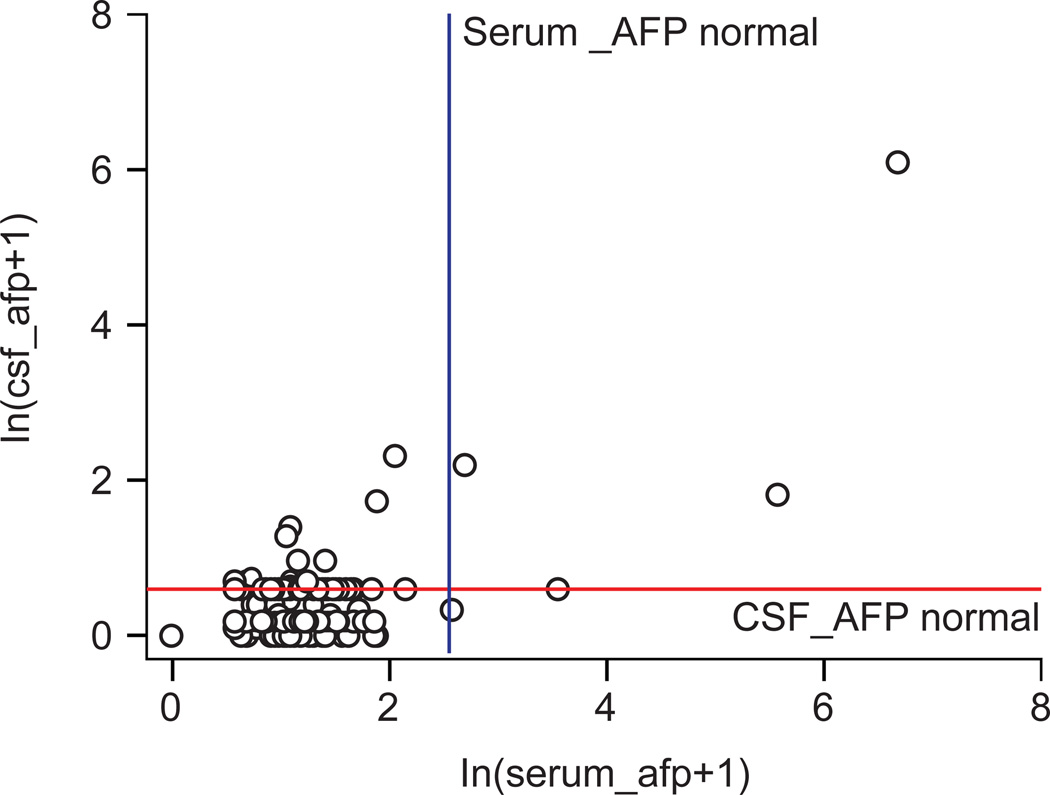
Pooled values (185 values in 43 patients) were analyzed to determine whether there was correlation in CSF and serum values. For AFP, 167 of 185 (90.3%) values correlated. The Cohen Kappa coefficient was 0.22, which indicated moderate agreement. Of 18 values that did not agree, 16 were positive in CSF and negative in serum (Table 4, Figure 3). The majority (n=164) of values agreed for negative values, but when AFP serum and CSF values were not in agreement, they were more often positive in CSF than in serum (16/18).
Table 4.
All Serum and CSF AFP values (n=185) from baseline and overtime from 43 patients
| CSF | AFP | ||||
|---|---|---|---|---|---|
| Serum | Negative | Positive | Total | ||
| Positive | 2 (1.1%) | 3 (1.6%) | 5 (2.7%) | Agree | 167 (90.3%) |
| Negative | 164 (88.6%) | 16 (8.6%) | 180 (97.3%) | Do not agree | 18 (9.7%) |
| Total | 166 (89.7%) | 19 (10.3%) | 185 | ||
Figure 3.
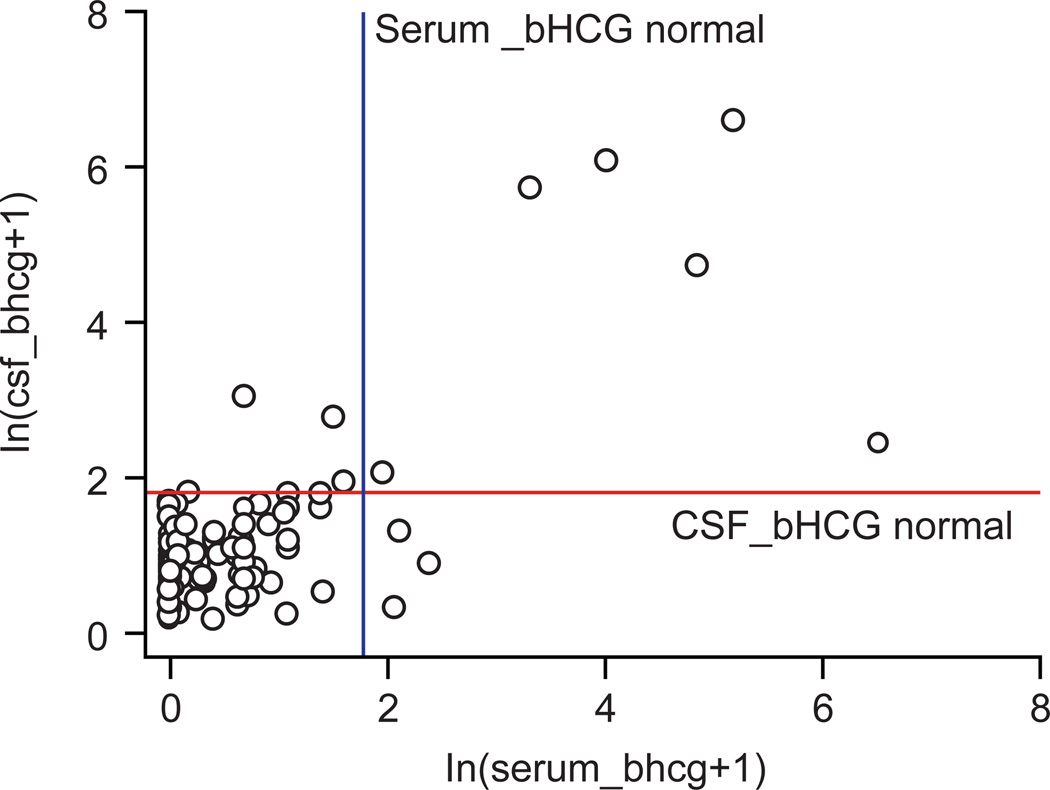
For bHCG, 178 of 185 (96.2%) values correlated in CSF and serum. The Cohen’s Kappa coefficient of 0.6117 indicated good agreement. As observed for AFP, the majority of values were negative for bHCG too (Table 5, Figure 4), but values that did not agree were almost evenly split (4/7 positive in CSF and negative in serum and 3/7 negative in CSF and positive in serum).
Table 5.
All Serum and CSF bHCG values (n=185) from baseline and overtime from 43 patients
| CSF | |||||
|---|---|---|---|---|---|
| Serum | Negative | Positive | Total | ||
| Positive | 3 (1.6%) | 6 (3.2%) | 9 (4.9%) | Agree | 178 (96.2%) |
| Negative | 172 (93.0%) | 4 (2.2%) | 176 (95.1%) | Do not agree | 7 (3.8%) |
| Total | 175 (94.6%) | 10 (5.4%) | 185 | ||
Figure 4.
Discussion
To our knowledge, our study is the first to address whether the tumor markers AFP and bHCG need to be measured from both the CSF and serum in all types of GCTs. Allen et al looked at the use of serum and CSF bHCG to diagnose pure germinoma in 60 patients from 2 prospective studies. They concluded [2] that histologic confirmation is still required for diagnosis of pure germinoma.
Our study indicates that AFP values at diagnosis correlate well between CSF and serum; the majority were negative as expected, reflecting the 63% of patients diagnosed with germinoma. Of the 40 patients with negative AFP values in the serum, only one patient had a positive AFP value in CSF and 8 patients had positive AFP values in both CSF and serum (Table 2 and Figure 1a). This small number of patients does not allow us to conclude that measurement of AFP in only the serum is sufficient; however, it does suggest that a diagnosis can be made on the basis of serum values alone in some patients. This observation will need to be confirmed in larger prospective studies. bHCG values correlated in only 72.5% of patients, suggesting that bHCG measurements are required in both CSF and serum.
Tumor markers are important in differentiating germinomas and NGGCTs. Our predictive model shows that serum and CSF samples are nearly equal in their ability to assist in diagnosis. It should be noted that we compared CSF versus serum samples – not AFP versus bHCG values – in predicting diagnosis. All 11 patients with positive AFP values (8 in both CSF and serum) had a diagnosis of NGGCT. The models also show that adding bHCG as a tumor marker to AFP does not improve the predictive ability of diagnosis. AFP alone is more clearly positive in NGGCTs, whereas bHCG can be elevated in both germinomas and NGGCTs.
Our study highlights that both markers are useful but not perfect in predicting histopathologic types of GCTs. Ideally, if markers could completely differentiate germinomas from NGGCTs, there would be little need for biopsies. Of the 15 patients who did not have a histopathologic diagnosis, the majority (n=13) did not undergo a biopsy because they were positive for characteristic markers from either CSF or serum.
Pooled values of AFP during follow-up showed fair agreement, and when values did not agree they were often positive in CSF and negative in serum. Pooled values of bHCG during follow-up were in good agreement, and those that did not agree were often positive in CSF and negative in serum. Overall, at baseline and subsequent follow-up exams in which serum and CSF tumor markers were not in agreement, values were often positive in CSF and negative in serum or showed mixed results. This suggests that measurements in CSF may be more sensitive than those in serum, but these positive values need to be analyzed from a clinical standpoint as well. Many values considered positive by CSF are close to threshold and represent isolated rises above normal that return back to normal in patients, suggesting CSF values may not only be more sensitive but also more prone to false positives. Further evaluation of the threshold values we use is needed. Meanwhile, we recommend repeating values that are near threshold in 4–6 weeks to confirm the trend.
Given the associated risk and cost of repeated lumbar puncture procedures during the course of treatment and follow-up evaluations, this study provides a starting point to explore if CSF values are even needed to make a diagnosis or if tumor marker concentrations in the serum alone have sufficient diagnostic power.
The trend of AFP elevation in serum and bHCG elevation in CSF in both baseline and follow-up analysis is interesting, but the reason for this remains unknown. This trend has been noted previously in intracranial GCTs [13, 19]. Rogers et al. offered a potential hypothesis involving the blood brain barrier to explain the increased levels of bHCG in the CSF [19].
Our results highlight the need to further investigate the correlation of serum and CSF markers at diagnosis and follow-up. Our study is limited by its retrospective design, long period of study, and small sample size. Nonetheless, it can be used for hypothesis generation for future large-scale prospective studies to evaluate potential elimination of CSF sampling, especially in the post-diagnosis period, and the need for surgical intervention for histologic confirmation. Finally, there is a need to continue the search for more sensitive and specific tumor markers with careful clinical evaluation of those measures.
Acknowledgments
This work was supported in part by Cancer Center Support Grant CA21765 from the National Cancer Institute, The Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC), and the American Lebanese Syrian Associated Charities (ALSAC). The authors would like to acknowledge Vani Shanker for the scientific editing of the manuscript and Julie Groff for support of the artwork.
References
- 1.Adler MD, Comi AE, Walker AR. Acute hemorrhagic complication of diagnostic lumbar puncture. Pediatric Emergency Care. 2001;17:184–188. doi: 10.1097/00006565-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Allen JC, Donahue B, Mathew N, et al. Can serum and/or lumbar CSF BHCG be used to diagnose A CNS germinoma. Neuro-Oncology. 2010;12:i29. [Google Scholar]
- 3.Bernstein L, Smith MA, Liu L, et al. Germ cell, trophoblastic and other gonadal neoplasm ICCC X. SEER Pediatric Monograph. 1994:125–137. [Google Scholar]
- 4.Blohm ME, Vesterling-Horner D, Calaminus G, Gobel U. Alpha-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatric Hematology and Oncology. 1998;15:135–142. doi: 10.3109/08880019809167228. [DOI] [PubMed] [Google Scholar]
- 5.Coakley J, Kellie SJ, Munas A, Cooke-Yarborough C. Interpretation of alpha-fetoprotein concentrations in cerebrospinal fluid in infants. Ann Clin Biochem. 2005;42:24–29. doi: 10.1258/0004563053026763. [DOI] [PubMed] [Google Scholar]
- 6.Echevarria ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: A Review. Oncologist. 2008;13:690–699. doi: 10.1634/theoncologist.2008-0037. [DOI] [PubMed] [Google Scholar]
- 7.Fujimaki T. Central nervous system germ cell tumors: classification, clinical features, and treatment with a historical review. Journal of Child Neurology. 2009;24:1439–1445. doi: 10.1177/0883073809342127. [DOI] [PubMed] [Google Scholar]
- 8.Fujimaki T, Mishima K, Asai A, Tabuchi K, Kobayashi M, Suzuki I, Kirino T. Levels of beta-human chorionic gonadotropin in cerebrospinal fluid of patients with malignant germ cell tumor can be used to detect early recurrence and monitor the response to treatment. Jpn J Clin Oncol. 2000;30:291–294. doi: 10.1093/jjco/hyd076. [DOI] [PubMed] [Google Scholar]
- 9.Gilligan TD, Seidenfeld J, Basch EM, et al. American society of clinical oncology practice guidelines on uses of serum tumor markers in adult males with germ cell tumors. Journal of Clinical Oncology. 2010;28:3388–3404. doi: 10.1200/JCO.2009.26.4481. [DOI] [PubMed] [Google Scholar]
- 10.Gregory JJ, Finlay JL. Alpha-fetoprotein and beta-human chorionic gonadotropin their clinical significance as tumour markers. Drugs. 1999;57:463–467. doi: 10.2165/00003495-199957040-00001. [DOI] [PubMed] [Google Scholar]
- 11.Honegger J, Mann K, Thierauf P, Zrinzo A, Fahlbusch R. Human chorionic gonadotropin immunoactivity in cystic intracranial tumours. Clinical Endocrinology. 1995;42:235–241. doi: 10.1111/j.1365-2265.1995.tb01870.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi T, Kumabe T, Kanamori M, et al. Logarithmic decrease of serum alpha-fetoprotein or human chorionic gonadotropin in response to chemotherapy can distinguish a subgroup with a better response among highly malignant intracranial non-germinomatous germ cell tumors. J Neurooncol. 2011;104:779–787. doi: 10.1007/s11060-011-0544-2. [DOI] [PubMed] [Google Scholar]
- 13.Kim A, Ji L, Balmaceda C, et al. The prognostic value of tumor markers in newly diagnosed patients with primary central nervous system germ cell tumors. Pediatr Blood Cancer. 2008;51:768–773. doi: 10.1002/pbc.21741. [DOI] [PubMed] [Google Scholar]
- 14.Lafay-Cousin L, Millar BA, Mabbott D, et al. Limited-field radiation for bifocal germinoma. Int J Radiat Oncol Biol Phys. 2006;65:486–492. doi: 10.1016/j.ijrobp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Mazumdar M, Bajorin DF, Higgins G, Higgins G, Motzer RJ, Bosl GJ. Predicting outcome to chemotherapy in patients with germ cell tumors: the value of rate of decline of human chorionic gonadotropin and alpha-fetoprotein during therapy. Journal of Clinical Oncology. 2001;19:2534–2541. doi: 10.1200/JCO.2001.19.9.2534. [DOI] [PubMed] [Google Scholar]
- 16.Murphy BA, Motzer RJ, Mazumdar M, et al. Serum tumor marker decline is an early predictor of treatment outcome in germ cell tumor patients treated with cisplatin and ifosfamide salvage chemotherapy. Cancer. 1994;73:2520–2526. doi: 10.1002/1097-0142(19940515)73:10<2520::aid-cncr2820731012>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Perkins GL, Slater ED, Sanders GK, Prichard JG. Serum tumor markers. American Family Physician. 2003;68:1075–8102. [PubMed] [Google Scholar]
- 18.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers PB, Sims EC, Plowman N. Blood to cerebrospinal fluid human chorionic gonadotropin-beta ratios in intracranial germ cell tumors. Neurosurg Focus. 1998;5(1):e4. doi: 10.3171/foc.1998.5.1.7. [DOI] [PubMed] [Google Scholar]
- 20.Sizaret P. Equivalence between international units and mass units of alpha-foetoprotein. Report of a collaborative study. Clinica Chemica Acta. 1979;96:59–65. [PubMed] [Google Scholar]
- 21.Villano JL, Virk IY, Ramirez V, Propp JM, Engelhard HH, McCarthy BJ. Descriptive epidemiology of central nervous system germ cell tumors: nonpineal analysis. Neuro-Oncology. 2010;12(3):257–264. doi: 10.1093/neuonc/nop029. [DOI] [PMC free article] [PubMed] [Google Scholar]