Abstract
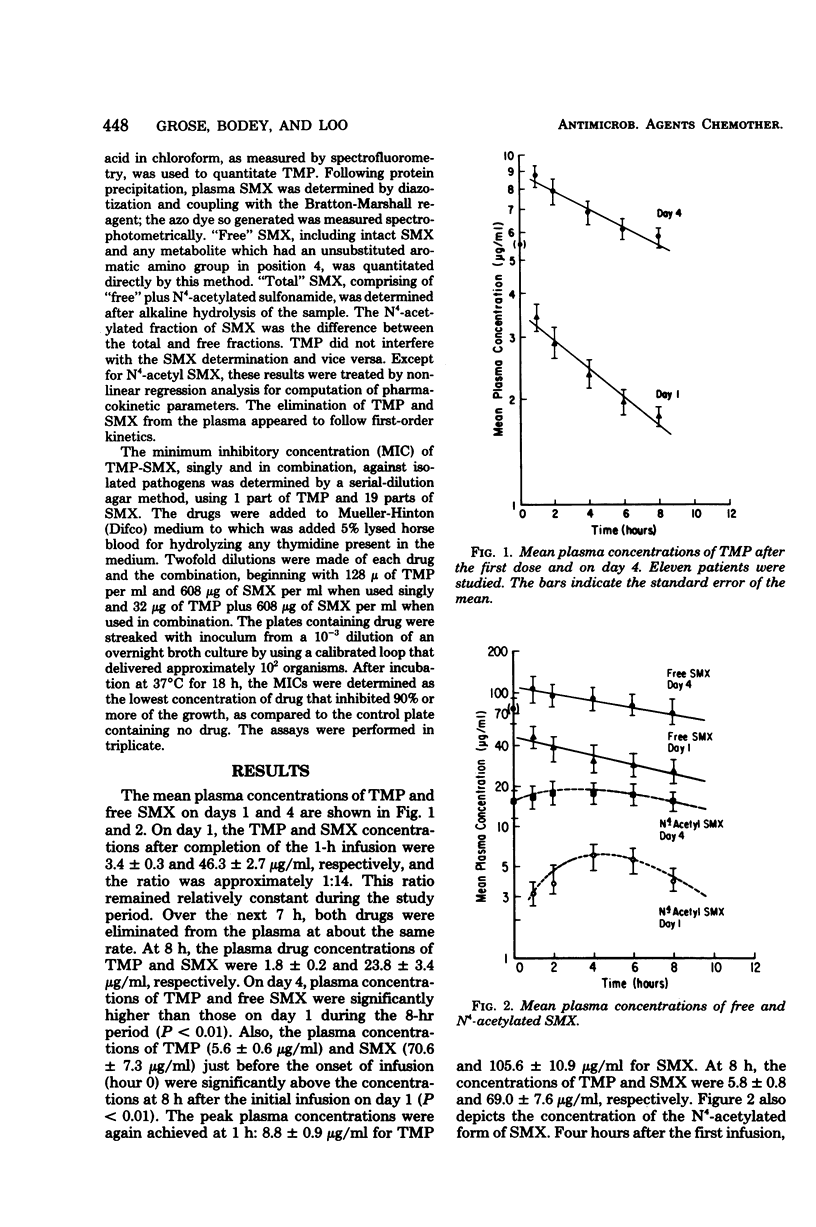
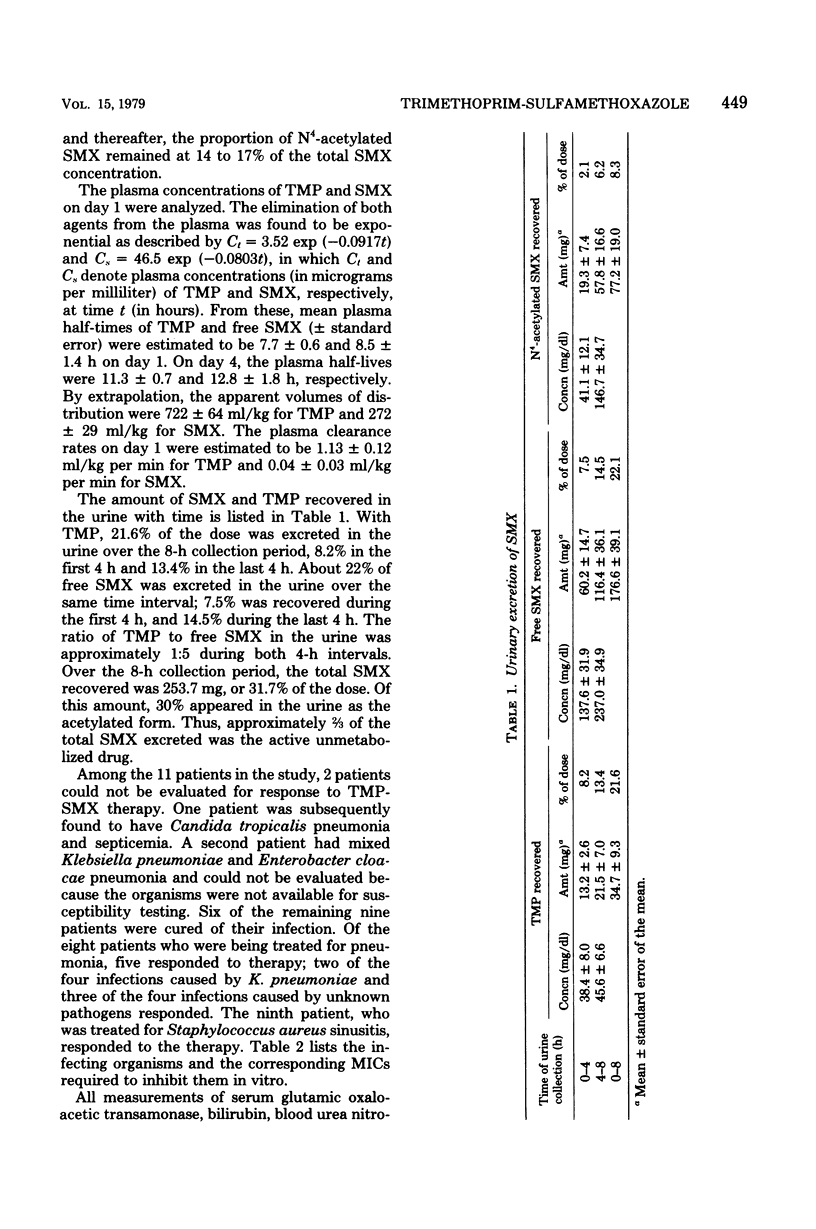
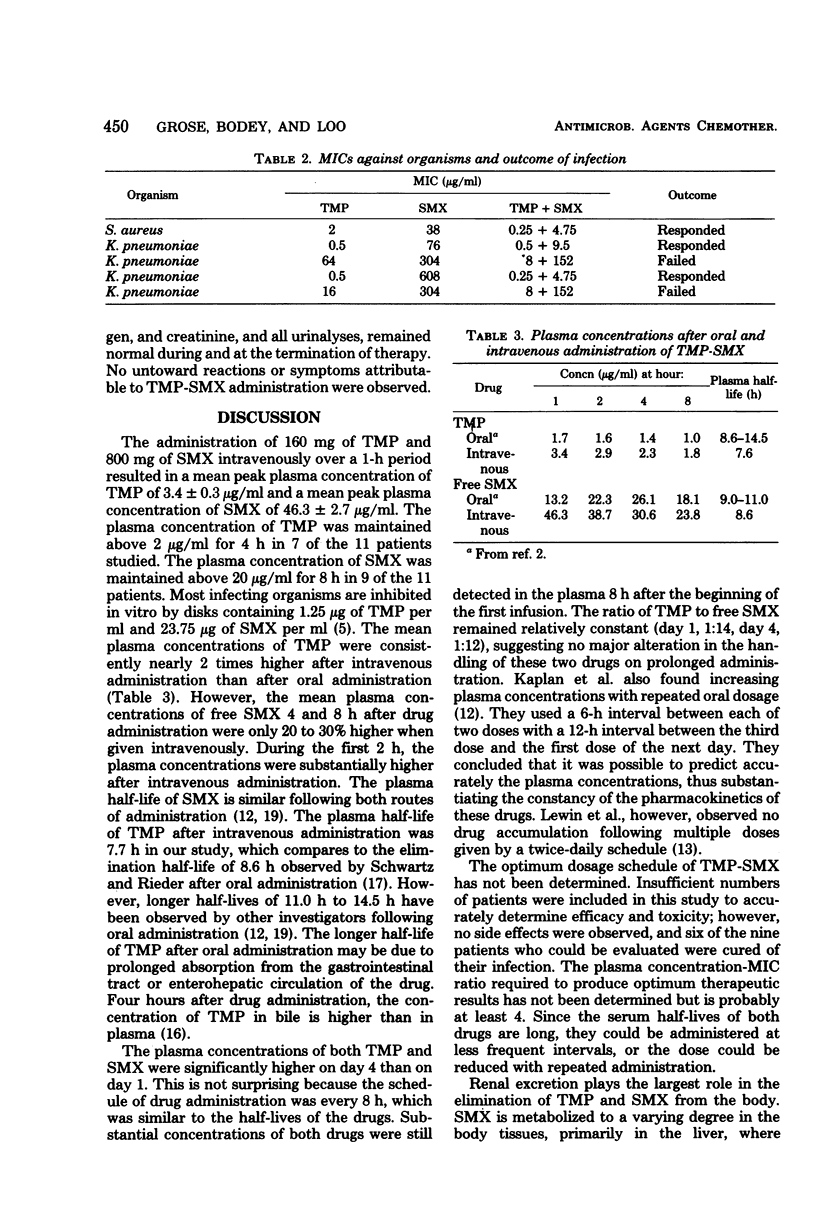
Pharmacokinetic studies of intravenously administered trimethoprim-sulfamethoxazole (TMP-SMX) were conducted in 11 patients with cancer while they received therapy with this drug combination for infection. Each patient received 160 mg of TMP and 800 mg of SMX every 8 h. The highest plasma concentrations of both agents were attained at the end of a 1-h infusion period, and the levels were maintained above 38 μg of free SMX and 2 μg of TMP per ml for 2 to 4 h on day 1. On day 4, these concentrations were exceeded at all time intervals of blood sampling. High concentrations of TMP and free SMX were recovered in the urine during the 8-h period. The plasma half-lives of TMP and free SMX, as determined during the first 8-h period, were 7.6 and 8.6 h, respectively. Compared with SMX, TMP had an approximately 2.5 times higher volume of distribution. This drug combination was well tolerated by the patients and unaccompanied by drug-related toxicity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. C., Finland M., Gold O., Wilcox C. Susceptibility of recently isolated pathogenic bacteria to trimethoprim and sulfamethoxazole separately and combined. J Infect Dis. 1973 Nov;128(Suppl):508–p. doi: 10.1093/infdis/128.supplement_3.s508. [DOI] [PubMed] [Google Scholar]
- Bach M. C., Gold O., Finland M. Absorption and urinary execretion of trimethoprim, sulfamethoxazole, and trimethoprim-sulfamethoxazole: results with single doses in normal young adults and preliminary observations during therapy with trimethoprim-sulfamethoxazole. J Infect Dis. 1973 Nov;128(Suppl):584–p. doi: 10.1093/infdis/128.supplement_3.s584. [DOI] [PubMed] [Google Scholar]
- Bushby S. R., Hitchings G. H. Trimethoprim, a sulphonamide potentiator. Br J Pharmacol Chemother. 1968 May;33(1):72–90. doi: 10.1111/j.1476-5381.1968.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby S. R. Trimethoprim-sulfamethoxazole: in vitro microbiological aspects. J Infect Dis. 1973 Nov;128(Suppl):442–p. doi: 10.1093/infdis/128.supplement_3.s442. [DOI] [PubMed] [Google Scholar]
- Grose W. E., Bodey G. P., Rodriguez V. Sulfamethoxazole-trimethoprim for infections in cancer patients. JAMA. 1977 Jan 24;237(4):352–354. [PubMed] [Google Scholar]
- Grunberg E., DeLorenzo W. F. Potentiation of sulfonamides and antibiotics by trimethoprim [2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine]. Antimicrob Agents Chemother (Bethesda) 1966;6:430–433. [PubMed] [Google Scholar]
- Hansen I., Lykkegaard Nielsen M., Bertelsen S. Trimethoprim in human saliva, bronchial secretion and lung tissue. Acta Pharmacol Toxicol (Copenh) 1973;32(5):337–344. [PubMed] [Google Scholar]
- Hitchings G. H. Species differences among dihydrofolate reductases as a basis for chemotherapy. Postgrad Med J. 1969 Nov;45(Suppl):7–10. [PubMed] [Google Scholar]
- Hughes D. T. Single-blind comparative trial of trimethoprim-sulphamethoxazole and ampicillin in the treatment of exacerbations of chronic bronchitis. Br Med J. 1969 Nov 22;4(5681):470–473. doi: 10.1136/bmj.4.5681.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. A., Weinfeld R. E., Abruzzo C. W., McFaden K., Jack M. L., Weissman L. Pharmacokinetic profile of trimethoprim-sulfamethoxazole in man. J Infect Dis. 1973 Nov;128(Suppl):547–p. doi: 10.1093/infdis/128.supplement_3.s547. [DOI] [PubMed] [Google Scholar]
- Lewin E. B., Klein J. O., Finland M. Trimethoprim-sulfamethoxazole: absorption, excretion, and toxicity in six children. J Infect Dis. 1973 Nov;128(Suppl):618–p. doi: 10.1093/infdis/128.supplement_3.s618. [DOI] [PubMed] [Google Scholar]
- Marks M. I. Pharmacokinetics and efficacy of trimethoprim-sulfamethoxazole in the treatment of gastroenteritis in children. Can Med Assoc J. 1975 Jun 14;112(13 Spec No):33–34. [PMC free article] [PubMed] [Google Scholar]
- Rieder J. Excretion of sulfamethoxazole and trimethoprim into human bile. J Infect Dis. 1973 Nov;128(Suppl):574–p. doi: 10.1093/infdis/128.supplement_3.s574. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Koechlin B. A., Weinfeld R. E. Spectrofluorimetric method for the determination of trimethoprim in body fluids. Chemotherapy. 1969;14(Suppl):22–29. doi: 10.1159/000220652. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Rieder J. Pharmacokinetics of sulfamethoxazole plus trimethoprim in man and their distribution in the rat. Chemotherapy. 1970;15(6):337–355. doi: 10.1159/000220701. [DOI] [PubMed] [Google Scholar]
- Welling P. G., Craig W. A., Amidon G. L., Kunin C. M. Pharmacokinetics of trimethoprim and sulfamethoxazole in normal subjects and in patients with renal failure. J Infect Dis. 1973 Nov;128(Suppl):556–p. doi: 10.1093/infdis/128.supplement_3.s556. [DOI] [PubMed] [Google Scholar]
- Wilfert C. M. Trimethoprim-sulfamethoxazole in children: pharmacokinetics and clinical studies. J Infect Dis. 1973 Nov;128(Suppl):613–p. doi: 10.1093/infdis/128.supplement_3.s613. [DOI] [PubMed] [Google Scholar]


