Abstract
Aim
To investigate LIN28B gene variants in children with idiopathic central precocious puberty (CPP).
Patients and Methods
We studied 178 Brazilian children with CPP (171 girls,16.8% familial cases). A large multiethnic group (1599 subjects; MEC cohort) was used as control. DNA analysis and biochemical in vitro studies were performed.
Results
A heterozygous LIN28B variant, p.H199R, was identified in a girl who developed CPP at 5.2 yrs. This variant was absent in 310 Brazilian control individuals, but it was found in the same allele frequency in women from the MEC cohort, independently of the age of menarche. Functional studies revealed that when ectopically expressed in cells the mutant protein was capable of binding pre-let-7 miRNA and inhibiting let-7 expression to the same extent as wild-type Lin28B protein. Other rare LIN28B variants (p.P173P, c.198+32_33delCT, g.9575731A>C and c.-11C>T) were identified in CPP patients and controls. Therefore, no functional mutation was identified.
Conclusion
In vitro studies revealed that the rare LIN28B p.H199R variant identified in a girl with CPP does not affect the Lin28B function in the regulation of let-7 expression. Although LIN28B SNPs were associated with normal pubertal timing, rare variations in this gene do not seem to be commonly involved in the molecular pathogenesis of CPP.
Keywords: LIN28B, central precocious puberty, let-7, microRNA, early and late menarche
Introduction
Central precocious puberty (CPP) results from the premature activation of the hypothalamic-pituitary-gonadal axis before the age of 8 years in girls and 9.5 years in boys (1). CPP has a striking predominance among girls, and most of these cases show no organic abnormality in the central nervous system, being considered as idiopathic (2, 3). It is known that genetic factors play a fundamental role in the timing of pubertal onset, as illustrated by the similar age at menarche among members of an ethnic group and in mother-daughter, monozygotic-twin, and sibling pairs (4). Interestingly, a 27.5% prevalence of familial cases has been reported in CPP, suggesting a genetic origin (5). Segregation analysis in these families suggested autosomal dominant transmission with incomplete, sex-dependent penetrance (5). Furthermore, activating mutations in kisspeptin and its receptor, a potent GnRH stimulator system, have recently been described as genetic causes of CPP in isolated cases (6, 7).
In 2009, four independent genome-wide association studies (GWAS) identified genetic variations clustered at 6q21 near or within the LIN28B gene, associated with timing of puberty (8-11). LIN28B is a human homolog of lin-28 of the nematode Caenorhabtidis elegans, which was originally identified as a heterochronic regulator of developmental timing (12). Deleterious mutations in lin-28 produce an abnormal rapid tempo of development through larval stages to adult cuticle formation (13). Conversely, enhancement of lin-28 expression by deletion of regulatory elements delays larval stage progression (12). In fact, transgenic mice overexpressing Lin28a exhibited increased body size, crow-rump length and delayed onset of puberty (14).
The lin-28 genes regulate the biogenesis of let-7 microRNA (miRNA) family members, controlling the time of developmental events (15). In vertebrates, let-7 miRNA controls Lin-28 translation, whereas Lin-28 is required for the correct timing of let-7 expression during normal development, constituting an autoregulatory loop (16). In the present study, we hypothesized that rare variants in LIN28B could cause more dramatic changes in the timing of puberty, resulting in new organic CPP
Patients and Methods
We studied 178 patients with CPP (171 girls and 7 boys), recruited from University Hospitals from Sao Paulo University Medical School, at Sao Paulo and Ribeirao Preto, Brazil. The protocol was approved by the Ethical Committees of Sao Paulo University. Written informed consent was obtained from all patients and/or their parents. The selected patients initiated puberty before 8 yr in girls and 9.5 yr in boys, with mean age at start of puberty of 5.4 ± 2 yrs (0.1-7.9). All patients had pubertal basal and/or GnRH-stimulated LH levels, advanced bone age and normal central nervous system magnetic resonance imaging. In thirty patients (16.8%) familial history of CPP was recalled.
Two distinct control populations were studied for LIN28B variant analyses. One population consisted of 310 Brazilian adults who had normal pubertal development at appropriated chronological age, according to a systematic questionnaire. In addition, a group of 1599 women from the Multiethnic Cohort (MEC) was also studied (17). They had normal and spontaneous puberty and were divided in two subgroups according to the age of menarche: early (less than 11 yr) or late (at 15 yr or older). Ancestry informative markers were previously genotyped in this panel and individuals whose self-reported racial/ethnic group did not match their estimated genetic ancestry were removed. Fourteen women who may have started oral contraceptives before their first period were excluded from analysis. In total, 1599 women had suitable genotype and phenotype data for analysis.
DNA analysis
Genomic DNA was extracted from peripheral blood leukocytes using standard procedures. The four exons and boundary regions of LIN28B (GenBank accession number - MIM 611044) were amplified by PCR and automatically sequenced in all patients with idiopathic CPP. In addition, the proximal promoter region (0.4 kb) was studied in 99 patients with CPP and in 110 Brazilian controls. Primers and PCR conditions are available upon request. All sequences were analyzed using CodonCode Aligner v. 3.5.2™. Genetic variations found in the patients were confirmed in both strands. In addition, the variations found in the patients were screened in control DNA samples. The splice predictor software program, NNSplice version 0.9 (www.fruitfly.org/seq_tools/splice.html) was used for analyzing aberrant RNAs.
The genotyping of a LIN28B variant (c.799A>G) in the MEC samples was performed using the Sequenom MassARRAY platform and the iPLEX genotyping protocol (Sequenom, Inc., San Diego, CA, USA).
Functional studies
Cloning
Human LIN28B was cloned into pFLAG-CMV2 vector (Sigma). LIN28B H199R was generated by site-directed mutagenesis using the QuickChange system (Stratagene). Pri-let-7g expression plasmid and the pSiCheck2-luciferase reporter containing the let-7 target sites were previously reported (15).
Cell culture and transfections
HEK293 and Hela cells were maintained in DMEM (Gibco, Invitrogen), supplemented with 10% FBS, Pen/Strep, L-Glutamine and Non-essential Amino Acids (Gibco, Invitrogen). All transfections were performed with Lipofectamine (Invitrogen) per manufacturer’s instructions.
Immunoprecipitation and Western blotting
Whole cell lysates were prepared using lysis buffer: 20 mM Tris/pH8.0, 137 mM NaCl, 1 mM EDTA, 1% Triton X100, 10% Glycerol, 1.5 mM MgCl2, 1 mM DTT, with protease inhibitors (Roche). Flag-immunoprecipitations were done using Flag-agarose beads (Sigma) for 90 min at 4C. Beads were washed with Buffer containing 300mM KCl. Elutions were performed with Flag peptide (Sigma). Affinity eluate was resolved on 4-12% Tris-Glycine-SDS gels (Invitrogen) and transferred to Immobilon-P PVDF Membrane (Millipore). Anti-Flag-HRP Antibody (Sigma, A8592) was used at 1:1000 dilution in 5% milk for an hour.
EMSA
EMSA was performed using a synthetic 5’-end radiolabeled 78-nt pre-let-7g RNA with final concentration of 0.5 μM (18). Affinity-purified wild-type and mutant Lin28B proteins from human cells were used. Complexes were resolved on native 3.5% or 5% polyacrylamide gels and visualized by autoradiography. Band intensities of scanned gels were quantified using ImageJ software and used to calculate percentage of probe bound. Percent active protein was determined using stoichiometric binding reactions.
RNA extraction and real time-PCR
RNA was harvested from transfected HEK293 cells using Trizol (Invitrogen) per manufacturer’s instructions. U18 small nuclear RNA (snRNA) was used as a normalizer. TaqMan microRNA assays (Applied Biosystems) were used to quantify mature miRNA expression as described previously (19).
Luciferase Assay
Hela cells do not express endogenous Lin28B, as previously demonstrated (20). These cells were grown on 6-well plates, co-transfected with 1 μg of the indicated expression plasmids together with 1 μg let-7-reporter gene. Cells were harvested 48 hours after transfection. All transfections were done in triplicate. Luciferase levels were analyzed using the Dual Luciferase Assay System (Promega) that contains an internal control. A siCHECK2 vector that expresses both Firefly luciferase and the Renilla luciferase from the same plasmid were used. The vector was engineered to contain a let-7 complementary site between the Renilla luciferase open reading frame and the polyA site. This reporter allowed monitoring of let-7 activity in cells by monitoring relative luciferase levels in the presence or absence of Lin28B expression.
Statistical analysis
Age at menarche was analyzed as a dichotomous trait using the software package PLINK v1.07 (21). Because the women from MEC cohort represented different racial/ethnic groups, the groups were analyzed separately using Fisher’s exact test for each group in which the variant allele was observed. The results were then combined in a sample-size weighted meta-analysis using the program METAL (22). Similar results were obtained using regression with racial/ethnic groups as a covariate, along with estimated genetic ancestry as previously described (22).
Results
DNA analysis of LIN28B gene in Central Precocious puberty
A heterozygous c.799A>G transition in exon 4 of the LIN28B gene, resulting in substitution of arginine for a histidine at position 199 (p.H199R), in the carboxy-terminal region of LIN28B was identified in one girl who developed precocious puberty at 5.2 yrs (Figure 1A). Both parents had normal pubertal development. Her father carried the same heterozygous variant and the mother was homozygous for the wild-type allele. The c.799A>G variant was not found in 310 Brazilian controls.
Figure 1.
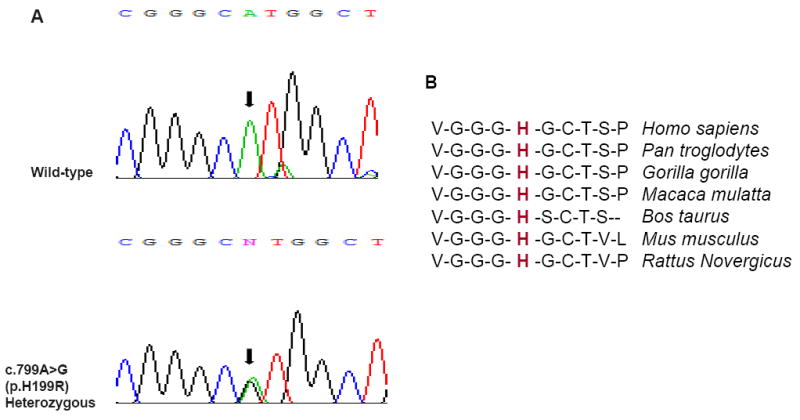
A, Nucleotide sequence of LIN28B gene showing the wild type and the variant identified in a girl with CPP. B, shows detailed LIN28B amino acid sequence alignment among species, showing that histidine in position 199 is highly conserved among mammals. The following were sequence sources: Homo sapiens (GenBank NP_002247.2), Pan troglodytes (GenBank XP_514123.1), Gorilla gorilla, Macaca mulatta (GenBank XP_001098284.1), Bos taurus (GenBank XP_872566.1), Mus musculus (GenBank NP_839991.1) and Rattus norvegicus (GenBank NP_859043.1).
In the 5’UTR, the heterozygous c.-11C>T substitution was identified in two patients with CPP and in one Brazilian control subject. In another patient, we identified an intronic heterozygous 2-nt deletion (c.198+32_33delCT), which was not identified in 110 Brazilian controls. The presence of the c.198+32_33delCT variant did not affect the splice site strength score, suggesting no aberrant splicing. In intron 2, we identified the previously reported polymorphism g.9575731A>C (rs17065417, previously known as rs59756390), in the heterozygous state in 18 patients with CPP. Finally, a heterozygous silent variant in exon 3, c.519C>T (P173P), was identified in one girl with CPP and absent in 110 controls. In silico analysis suggested that this silent variant was also unlike to result in abnormal splicing. The Table 1 summarizes the LIN28B variants identified in children with CPP and controls.
Table 1.
Genetic variants identified in LIN28B gene in 178 patients with CPP
| Variant | Gene Location | Status | Variant Frequency % | Functional Impact | |
|---|---|---|---|---|---|
| CPP | Controls | ||||
| c.-11C>T (rs76300431) | 5’ UTR | Heterozygous | 2.0# | 0.9* | Unknown (reported SNP) |
| c.198+32_33delCT | Intron 2 | Heterozygous | 1# | 0* | Unlike to affect splicing |
| g.9575731A>C (rs17065417) | Intron 2 | Heterozygous | 18# | - | Unknown (reported SNP) |
| c.519C>T (p.P173P) | Exon 3 | Heterozygous | 0.56 | 0 | Silent mutation (unlike to affect splicing) |
| c.799A>G(p.H199R) | Exon 4 | Heterozygous | 0.56 | 0/0.5** | It does not affect the function of Lin28B in the regulation of let-7 expression |
Analysis of the 5’UTR and intron 2 of Lin28B gene in 99 patients with CPP.
Brazilian control subgroup (110 individuals).
MEC cohort 1599.
Functional studies
Both Lin28A and Lin28B were previously shown to block let-7 miRNA processing (15, 23). To begin to examine the functional consequences of the p.H199R mutation, we generated plasmids for the overexpression of Flag-tagged wild-type Lin28B as well as the p.H199R variant Lin28B protein. We first examined the expression of these cDNAs and performed Flag-affinity purifications from HEK293 cells transfected with the corresponding expression plasmids and analyzed the immunoprecipitate by Western Blot. This analysis revealed that both the wild-type and variant Lin28B proteins were expressed at similar levels (Figure 2A). Interestingly, we reproducibly observed a slower migrating band that was detected by Western blot using either anti-Flag antibody (Figure 2A) or a specific antibody for Lin28B detection (data not shown). Strikingly, this slower migrating from of Lin28B was not detected for the H199R variant protein (Figure 2A). The identity of the slower migrating band for the wild-type Lin28B protein was confirmed by large-scale Flag-immunoprecipitation and mass spectrometry (data not shown). Together these data indicate that a portion of Lin28B may be posttranslationally modified and that the H199R variant abolishes this putative modification.
Figure 2.
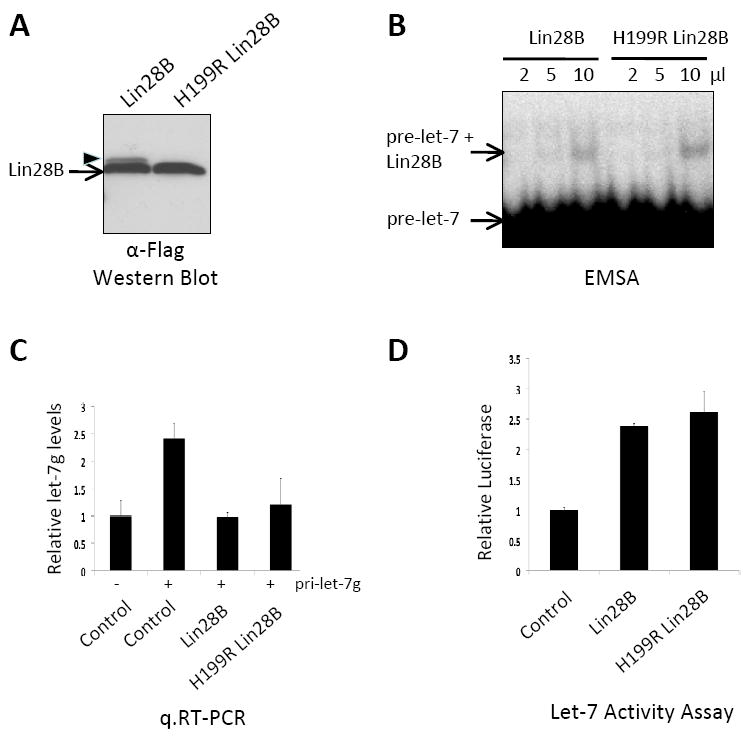
A, Analysis of Flag-immunopurified proteins by Western blot. B, EMSA performed with pre-let-7 and the indicated amount of Flag-immunopurified Lin28B and H199R Lin28B proteins. C, q.RT-PCR analysis of let-7g expression in transfected HEK293 cells. U18 quantitative RT-PCR was used as a normalizer. The transfection efficiency was confirmed by the observation that mature let-7 increased after pri-let-7 transfection. D, Luciferase activity assay using a let-7 responsive reporter gene. Hela cells were co-transfected with indicated expression plasmid together with the let-7 sensitive reporter plasmid. Relative Luciferase represents the Renilla:Firefly ratio normalized to 1 in the absence of Lin28B expression (control).
The relative RNA-binding affinity of the wild-type and H199R Lin28B proteins were measured by electromobility shift assay (EMSA) using radiolabeled synthetic pre-let-7 RNA. This analysis revealed that the both the wild-type and mutant Lin28B proteins display comparable pre-let-7 RNA-binding affinities (Figure 2B). Next, we assessed the ability of these proteins to inhibit let-7 expression. For these experiments HEK293 cells were transfected with a plasmid expressing pri-let-7g together with control, Lin28B, or H199R Lin28B expression vectors. We analyzed levels of mature let-7 miRNA expression by quantitative reverse transcriptase PCR (q.RT-PCR). Expression of wild-type or H199R Lin28B protein inhibited the accumulation of mature let-7g to a similar extent indicating that these proteins are equally capable of blocking let-7 biogenesis in these experiments (Figure 2C).
Finally, to further compare the capacity of wild-type and H199R Lin28B proteins to inhibit the let-7 miRNA pathway, we utilized a let-7 responsive reporter gene assay. For these experiments, we used a Luciferase construct engineered to contain functional let-7 target sites in the 3’UTR. Expression of luciferase from this construct is therefore normally repressed by let-7. Using this system, we found that co-expression of Lin28B together led to the expected de-repression of the let-7 reporter. Furthermore, expression of the mutant p.H199R Lin28B protein led to a comparable de-repression of the let-7 responsive luciferase reporter (Figure 2D). Together these experiments indicated that the p.H199R variant does not seem to have effect on the ability of the mutant protein to repress let-7 expression.
Screening of the p.H199R in MEC cohort
We tested whether the LIN28B variant c.799A>G (p.H199R) was associated with early age at menarche in a panel of 808 women with early at menarche and 791 women with late at menarche. Five women with early age at menarche (0.6%) and three women with late age at menarche (0.4%) were heterozygous (A/G); no homozygous for the rare allele (G/G) was observed (Table 2). There was no significant association with early or late age at menarche (overall p-value 0.771).
Table 2.
Analysis of the LIN28B variant c.799A>G (p.H199R) in 1599 women with early or late age at menarche.
| Non-Latina White | African-American | Japanese-American | Native Hawaiian | Latina | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Early | Late | Early | Late | Early | Late | Early | Late | Early | Late | ||
| A/A | 213 | 210 | 200 | 176 | 104 | 126 | 148 | 135 | 138 | 141 | 1591 |
| A/G | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 8 |
| G/G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Discussion
Recently, the first loci with common variation reproducibly associated with population variation in the timing normal of puberty were identified at 6q21 in or near LIN28B gene (24). In the current study, we investigated the role of genetic variants of LIN28B in the extreme phenotype involving early human pubertal onset. A group of 178 Brazilian children with CPP, mostly girls with mean pubertal onset at 5.4 yrs of age, was selected for sequencing analysis of the promoter and entire coding regions of LIN28B. Few rare heterozygous variants were identified, including two in the coding region (p.P173P and p.H199R), two intronic (c.198+32_33delCT and g.9575731A>C), and one in the 5’UTR (c. -11 C>T) (Table 1).
Among these variants, the p.H199R, identified in a girl with sporadic CPP, was considered a potential functional variant. The histidine at position 199, located in the carboxy-terminal region of the protein outside the RNA-binding domains, is highly conserved among mammalians (Figure1B) and it was absent in 310 Brazilian controls. In a previous study, sequencing of the LIN28B gene in 145 Finland children with constitutional delay of growth and puberty (CDGP) did not identify any allelic variant in LIN28B coding region, indicating that allelic variants in that region are very rare (25). More recently, the p.H199R variant was identified in one Danish patient with CPP and in one of the 132 controls (26).
Lin28 binds specifically to the terminal loop region of let-7 precursors, blocking the maturation of let-7 miRNAs in mouse embryonic stem cells and in mouse and human cancer cell lines (15, 18, 27). Interestingly, increased let-7 gene dosage causes precocious expression of adult fates during larval stages (27). We hypothesized that inactivating mutations in LIN28B could affect let-7 miRNA repression and consequently stimulate pubertal development in humans. To evaluate if the repression function of the p.H199R variant LIN28B was impaired, we performed in vitro biochemical assays. When ectopically expressed in cells, the mutant protein was capable of inhibiting let-7 miRNA expression to the same extent as wild-type Lin28B protein. Consistent with this finding, we also found that it was capable of binding pre-let-7 miRNA comparable to wild-type Lin28B (EMSA assays). Thus, it seems that this particular variant does not affect the function of Lin28B in the regulation of let-7 expression. Furthermore, this variant is located outside of the domains known to be required for the repression of let-7 miRNA maturation.
Other pleiotropic roles of Lin28 have been demonstrated in physiological and pathological processes. It remains possible that LIN28B has additional gene regulatory functions involved in the control of the timing of puberty in humans. In support of this notion, Lin28A has additional gene regulatory roles in the cell cytoplasm and can directly associate with several different mRNAs, including cell growth and metabolism (28). In addition, Lin28A and Lin28B are differentially localized in cells, with Lin28B being predominantly nuclear (20). It would be interesting to identify posttranslational modification(s) of the Lin28B protein that may be responsible for the altered gel migration patterns that we observed for the wild-type Lin28B protein but absent from the H199R protein. Therefore, our functional analysis cannot exclude that the p.H199R variant play a role in these other phenotypic traits. Even if the p.H199R variant leads to a yet unidentified functional consequence, its presence in the proband’s father argues against it causing CPP. Moreover, the genotypic frequency of the p.H199R (c.799A>G) variant was investigated in 1599 women with normal pubertal development, who were divided in early (<11 yrs) and late (>15 yrs) age at menarche. The G allele was identified in a low frequency (<1%) in both groups, with no statistical significance difference between the early and late menarche groups. Additionally, the p.H199R variant has been identified as a rare variant in less than 1% of the population in the 1000 genome pilot project, which provided a deep characterization of human genome sequence variation using different genome-wide sequencing with high-throughput platforms (29). Taken together these data suggest that the p.H199R variant does not play a causative role in the central precocious puberty phenotype.
Additionally, a heterozygous C>T substitution located 11 nucleotides before the translation start site was identified in two patients. To our knowledge the regulatory region of the LIN28B has not yet been characterized. This variation could affect the cis-regulatory control of gene expression, however, as it was also found in one of the control subjects and it is likely to be non-functional. In agreement with this observation, this variation was recently reported in another population and the reported allele frequency is comparable to the subjects analyzed in this study. In another CPP patient a 2-nt deletion in intron 2 was found. This variation was not observed in 110 healthy controls. The calculated splice strength score did not change, indicating that this mutation does not result in aberrant splicing. Therefore, it is unlike that this rare genetic variant affect LIN28B function.
In conclusion, we did not identify causative LIN28B mutations in patients with CPP. In vitro biochemical assays revealed that the missense p.H199R, found in one girl with CPP, does not affect the function of Lin28B in the regulation of let-7 expression. Thus, although GWAS had shown the association of common polymorphisms in LIN28B with variations in the timing of normal puberty, our data studying a large number of patients with CPP shows that mutations in LIN28B do not seem to be commonly involved in the molecular pathogenesis of CPP.
Acknowledgments
Funding: This work was supported by grants from Fundação de Amparo a Pesquisa do Estado de São Paulo – FAPESP, process # 05/04726 to A.C.L., 08/55953-4 to A.P.S.N. R.I.G was supported by grants from the US National Institute of General Medical Sciences (NIGMS) (1R01GM086386-01A1), The Harvard Stem Cell Institute, and the Emerald Foundation. R.I.G is a Pew Research Scholar. Work supported by NIH R01-HD048960 to M.R.P., J.N.H., and B.E.H.
Footnotes
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Carel JC, Leger J. Clinical practice. Precocious puberty. N Engl J Med. 2008 May 29;358(22):2366–77. doi: 10.1056/NEJMcp0800459. [DOI] [PubMed] [Google Scholar]
- 2.Delemarre-van de Waal HA. Secular trend of timing of puberty. Endocr Dev. 2005;8:1–14. doi: 10.1159/000084082. [DOI] [PubMed] [Google Scholar]
- 3.Partsch CJ, Sippell WG. Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens. Hum Reprod Update. 2001 May-Jun;7(3):292–302. doi: 10.1093/humupd/7.3.292. [DOI] [PubMed] [Google Scholar]
- 4.Palmert MR, Boepple PA. Variation in the timing of puberty: clinical spectrum and genetic investigation. J Clin Endocrinol Metab. 2001 Jun;86(6):2364–8. doi: 10.1210/jcem.86.6.7603. [DOI] [PubMed] [Google Scholar]
- 5.de Vries L, Kauschansky A, Shohat M, Phillip M. Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab. 2004 Apr;89(4):1794–800. doi: 10.1210/jc.2003-030361. [DOI] [PubMed] [Google Scholar]
- 6.Silveira LG, Noel SD, Silveira-Neto AP, Abreu AP, Brito VN, Santos MG, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010 May;95(5):2276–80. doi: 10.1210/jc.2009-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008 Feb 14;358(7):709–15. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE, et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009 Jun;41(6):724–8. doi: 10.1038/ng.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong KK, Elks CE, Li S, Zhao JH, Luan J, Andersen LB, et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet. 2009 Jun;41(6):729–33. doi: 10.1038/ng.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry JR, Stolk L, Franceschini N, Lunetta KL, Zhai G, McArdle PF, et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat Genet. 2009 Jun;41(6):648–50. doi: 10.1038/ng.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sulem P, Gudbjartsson DF, Rafnar T, Holm H, Olafsdottir EJ, Olafsdottir GH, et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat Genet. 2009 Jun;41(6):734–8. doi: 10.1038/ng.383. [DOI] [PubMed] [Google Scholar]
- 12.Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997 Mar 7;88(5):637–46. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 13.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984 Oct 26;226(4673):409–16. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet. 2010 Jul;42(7):626–30. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008 Apr 4;320(5872):97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol. 2003 Jun 15;258(2):432–42. doi: 10.1016/s0012-1606(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 17.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000 Feb 15;151(4):346–57. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piskounova E, Viswanathan SR, Janas M, LaPierre RJ, Daley GQ, Sliz P, et al. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem. 2008 Aug 1;283(31):21310–4. doi: 10.1074/jbc.C800108200. [DOI] [PubMed] [Google Scholar]
- 19.Hagan JP, Piskounova E, Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol. 2009 Oct;16(10):1021–5. doi: 10.1038/nsmb.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piskounova E, Polytarchou C, Thornton JE, LaPierre RJ, Pothoulakis C, Hagan JP, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011 Nov 23;147(5):1066–79. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007 Sep;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010 Sep 1;26(17):2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008 Aug;14(8):1539–49. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elks CE, Perry JR, Sulem P, Chasman DI, Franceschini N, He C, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet. 2010 Dec;42(12):1077–85. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tommiska J, Wehkalampi K, Vaaralahti K, Laitinen EM, Raivio T, Dunkel L. LIN28B in constitutional delay of growth and puberty. J Clin Endocrinol Metab. 2010 Jun;95(6):3063–6. doi: 10.1210/jc.2009-2344. [DOI] [PubMed] [Google Scholar]
- 26.Tommiska J, Sorensen K, Aksglaede L, Koivu R, Puhakka L, Juul A, et al. LIN28B, LIN28A, KISS1, and KISS1R in idiopathic central precocious puberty. BMC Res Notes. 2011;4:363. doi: 10.1186/1756-0500-4-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000 Feb 24;403(6772):901–6. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 28.Peng S, Chen LL, Lei XX, Yang L, Lin H, Carmichael GG, et al. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells. 2011 Mar;29(3):496–504. doi: 10.1002/stem.591. [DOI] [PubMed] [Google Scholar]
- 29.Durbin RM, Abecasis GR, Altshuler D, Auton A, Brooks LD, Gibbs RA, et al. A map of human genome variation from population-scale sequencing. Nature. 2010 Oct 28;467(7319):1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]


