SUMMARY
Because of major advances in the treatment of HIV /AIDS, HIV-positive persons now live longer, healthier lives; however, hepatitis C virus (HCV) is increasingly recognized as a major cause of morbidity and mortality in this population. Among HCV-infected persons, HIV co-infection is associated with increased HCV RNA levels, increased hepatic inflammation and fibrosis, and more rapid progression to cirrhosis and end-stage liver disease. Compounding this problem are reduced HCV treatment response rates among HCV /HIV co-infected persons. Moreover, antiretroviral therapy used to suppress HIV replication is often associated with a paradoxical increase in HCV RNA levels, as well as hepatotoxicity. Despite the adverse clinical consequences of HCV/ HIV co-infection, the mechanisms by which these two viruses interact at the cellular level remain largely unexplored. This review focuses on the evidence demonstrating direct infection of hepatocytes by HIV, as well as the indirect mechanisms by which HIV may regulate HCV replication at the cellular level. A comprehensive understanding of virus–virus and virus–cell interactions is critical to the development of novel treatment strategies to combat HCV/ HIV co-infection.
Keywords: co-infection, hepatitis C virus, human immunodeficiency virus, liver
NATURAL HISTORY OF HCV /HIV CO-INFECTION
Over 170 million people worldwide are infected with hepatitis C virus (HCV), an enveloped, single-stranded RNA virus. Infection with HCV is a major cause of chronic liver disease, with 60–80% of patients developing chronic hepatitis and up to 20% of chronically infected persons developing cirrhosis within 20 years of infection [1]. HCV infection is also a major cause of hepatocellular carcinoma and the major reason for liver transplantation in the US. There is no effective vaccine for the prevention of HCV infection and current treatment regimens achieve sustained virological response in only 50% of treated individuals [2].
Because of shared routes of transmission, co-infection with HCV is increasingly recognized as a major cause of morbidity and mortality among HIV-positive persons [3]. In the US, 150 000 to 300 000 persons are infected with HIV and HCV, representing 15–30% of all HIV-infected persons and 5–10% of all HCV-infected persons [4,5].
The effects of HCV infection on HIV disease are not well understood but have been addressed in a small number of studies. Daar et al. reported that increased HCV RNA levels were associated with clinical progression to AIDS in haemophiliacs even after controlling for CD4 cell count and HIV RNA level [6]. In the Swiss HIV Cohort Study, HCV seropositivity was associated with progression to a new AIDS-defining illness or to death [7]. HCV seropositivity was also associated with a reduced CD4 cell recovery during antiretroviral therapy (ART). While these results have been generally supported by other clinical investigations [8–12], not all cohorts have found an association between HCV co-infection and HIV disease progression [13]. While the mechanisms by which HCV may impact HIV disease progression have not been elucidated, they may include intolerance to ART in the presence of HCV and / or HCV-induced immune activation that leads to HIV disease progression. Moreover, extrahepatic replication of HCV does occur [14]; therefore, HCV could significantly impair CD4 cell proliferation and function and consequently impact HIV disease progression.
In contrast, it is well established that HIV significantly alters the clinical course of HCV disease. Co-infection with HIV results in enhanced HCV replication as HCV RNA levels are significantly elevated in HCV /HIV co-infected persons compared with HCV mono-infected persons [15,16] and HIV seroconversion is associated with sustained increases in HCV viral load [17]. Paradoxically, ART initiation is frequently associated with sustained increases in HCV viral load, as well as hepatotoxicity [18–23]. The clinical course of HCV infection is also accelerated in HCV /HIV co-infected persons as they experience more advanced liver fibrosis and cirrhosis, as well as higher rates of progressive liver disease and death than do HCV mono-infected persons [3,24–32]. Importantly, HIV therapy appears to slow the progression of hepatic disease in co-infected persons (reviewed in [33]). Further compounding the clinical management of HCV disease, HIV co-infection is associated with decreased response rates to HCV treatment compared with HCV monoinfection [34–36]. Thus, HIV co-infection clearly has a negative impact on HCV pathogenesis.
THE IMMUNOLOGICAL RESPONSE TO HCV
It has been suggested that <10% of hepatocytes are infected with HCV [37] although data are limited and considerable variability may be possible. Factors that limit virus replication to a subset of hepatocytes may be the same factors involved in viral clearance. Type I interferon (IFNα and IFNβ) production is induced by viral infection in most cell types, including hepatocytes. Type 1 IFNs activate the expression of multiple IFN-stimulated genes (ISGs), including the RNA-dependent protein kinase (PKR), 2′,5′-oligoadenylate synthetase (OAS), RNase L, adenosine deaminase (ADAR) and the Mx protein GTPases. Several HCV proteins – core, E2, NS3 /4A and NS5A – have also been implicated in the inhibition of IFN-inducible genes and / or key components of IFN signalling pathways (reviewed in [38]). Furthermore, host immunological selection pressures may drive the outgrowth of viral variants capable of persisting despite antiviral treatment or the presence of an immunological response directed against HCV.
Strong and persistent CD8+ and CD4+ T-cell responses are critical for HCV clearance (reviewed in [39]). However, HCV-specific adaptive immune responses are infrequently detected during HIV co-infection, suggesting that virally mediated pathogenesis is at least partially the result of impaired cell-mediated immunity to HCV [40–42], although this phenomenon has not been confirmed in all studies [43,44]. Cytokine dysregulation, commonly observed among HIV-positive persons [45], may further contribute to ineffective anti-HCV immune responses and contribute to viral persistence. One possible explanation for increased HCV replication during HIV co-infection is that HCV infection may not be immunologically controlled in the face of HIV-mediated immunosuppression. However, at least one study found that HCV RNA levels were more strongly associated with HIV RNA levels than with CD4 cell counts [46]; thus, direct virus–virus interactions are also likely.
Immune restoration also appears to play an interesting, albeit poorly understood, role in HCV pathogenesis. The introduction of highly active antiretroviral therapy (HAART) has led to a remarkable reduction in the number of AIDS-related deaths. While HAART results in significantly decreased HIV RNA levels and subsequent increases in CD4 cell count, a paradoxical increase in HCV RNA levels is frequently observed among HCV /HIV co-infected persons initiating HAART (reviewed in [23]). Possible explanations for this phenomenon include enhanced lysis of HCV-infected cells by cytotoxic T lymphocytes and release of HCV particles into the plasma, reduced competition by HIV for HCV entry receptors, reduced HIV-mediated induction of endogenous IFNα that would otherwise antagonize HCV replication, increased cellular injury as a result of antiretroviral-induced hepatotoxicity and /or increased replication of HCV in extrahepatic cell types once HIV levels in those same cell types have been suppressed.
HIV DETECTION IN THE LIVER
Despite the adverse clinical consequences of HCV /HIV co-infection, the precise molecular mechanisms by which these two viruses interact with one another have not been fully explored. While the liver is known to clear many foreign pathogens efficiently, including RNA viruses [47–49], given their principle sites of replication, the opportunity for direct viral interactions between HIV and HCV would appear to be limited. Nonetheless, several lines of evidence suggest that several liver cell types can be productively infected with HIV (Table 1).
Table 1.
Evidence for HIV infection of the liver
Housset et al. investigated HIV infection of liver cells using liver samples from 17 infected patients [50]. Staining with a monoclonal antibody to HIV p24 was positive for seven samples, particularly in Kupffer cells. In five additional livers, HIV RNA was detected by in situ hybridization in inflammatory mononuclear cells and / or sinusoidal cells. A subsequent study amplified HIV DNA from nine of 14 liver samples from patients with AIDS [51]. HIV antigen and mRNA were also detected in Kupffer cells and hepatocytes, suggesting these hepatic cell types as potential sources of HIV replication in vivo.
It has been shown that the HepG2 hepatocyte-derived cell line expresses the CD4 receptor and produces infectious HIV particles in vitro – as measured by both p24 production and the ability of HepG2-produced HIV to infect T-cell lines [52]. Unfortunately, experiments assessing HIV infection of primary hepatocytes are extremely limited in the available literature. While hepatocytes are the primary site of HCV replication, additional liver cell types may support HIV, thus placing HIV in the proximity of actively replicating HCV. For instance, primary cultures of Kupffer cells can be productively infected with HIV as demonstrated by HIV antigen detection, the presence of viral particles and reverse transcriptase activity [53]. Similarly, primary cultures of human liver sinusoidal endothelial cells are also permissive for HIV replication as evidenced by staining for HIV antigens, reverse transcriptase activity and visualization of viral budding by electron microscopy [54]. Finally, hepatotropic variants of HIV have been identified in autopsied liver tissues [55], suggesting that HIV may adapt for efficient replication within the hepatic environment, although this issue has not be explored further.
Cao et al. measured the susceptibility of five hepatocyte-derived cell lines to in vitro infection with HIV [56]. Each cell line was productively infected with multiple strains of HIV as shown by detectable HIV p24 antigen in cell culture supernatants for up to 90 days in culture. When infected hepatocytes were serially passaged, p24 and infectious virus were no longer detectable after the second passage. However, proviral DNA was present until the fourth passage suggesting that HIV can integrate into hepatocyte DNA but that infection may become latent. Intracellular p24 antigen and viral mRNA were detected in each hepatocyte cell line 3 days after inoculation with HIV; approximately 20–50% of the hepatocytes expressed p24 protein. Interestingly, the authors were unable to detect CD4 protein or mRNA in the hepatocyte cell lines examined. An anti-CD4 monoclonal antibody and soluble CD4 did not block HIV infection of hepatocytes, further suggesting that HIV infection of hepatocytes could occur in a CD4-independent manner. In contrast, a subsequent attempt to infect the Huh7 cell line with HIV showed no evidence of HIV infection as measured by intracellular staining for p24 [57]. While the reasons for these discrepant results remain unclear, they may reflect the period of time after which HIV infection was assessed in target cells and / or methodological differences in the ability to detect HIV antigens in vitro.
More recently, replication of HIV in hepatic stellate cells (HSC) has been demonstrated by detection of p24 antigen and HIV mRNA. HIV infection, as well as viral proteins, also promoted HSC activation as measured by increased collagen I mRNA levels [58]. Collectively, these data suggest that HIV infection of hepatocytes, as well as other hepatic cell types, is possible. Thus, further exploration of how these interactions impact HCV /HIV pathogenesis is warranted.
HIV REGULATION OF HCV EXPRESSION
The ability of HIV to infect hepatocytes productively could have a profound effect on HCV replication. However, several possible indirect mechanisms by which these two viruses interact are also plausible (Fig. 1). For example, HIV gains entry into target cells by forming a complex consisting of its outer envelope glycoprotein (gp120), CD4 receptor and members of the chemokine co-receptor family [59]. Binding of HIV virions or soluble gp120 to CD4 receptors triggers a broad spectrum of signalling pathways that modulates the activation state of target cells. Similarly, gp120 engagement of chemokine receptors also results in activation of specific intracellular signalling pathways (reviewed in [60–62]). Relevant to understanding HCV /HIV co-infection at the cellular level, several groups have shown that hepatocytes express key HIV co-receptors including CD4, CCR5 and CXCR4 [57,63–65]. Moreover, several studies have demonstrated that expression of intrahepatic chemokine ligands and their receptors was associated with severe HCV-induced liver inflammation [66–68]. Recent data also suggest that CCR5Δ32 (a CCR5 deletion mutant that produces a nonfunctional receptor) is associated with reduced portal inflammation and milder fibrosis among HCV-positive, HIV-negative individuals [69]. These data support the concept that intrahepatic chemokine receptor expression and signalling could play a key role in regulating pathogenic events during chronic HCV infection.
Fig. 1.
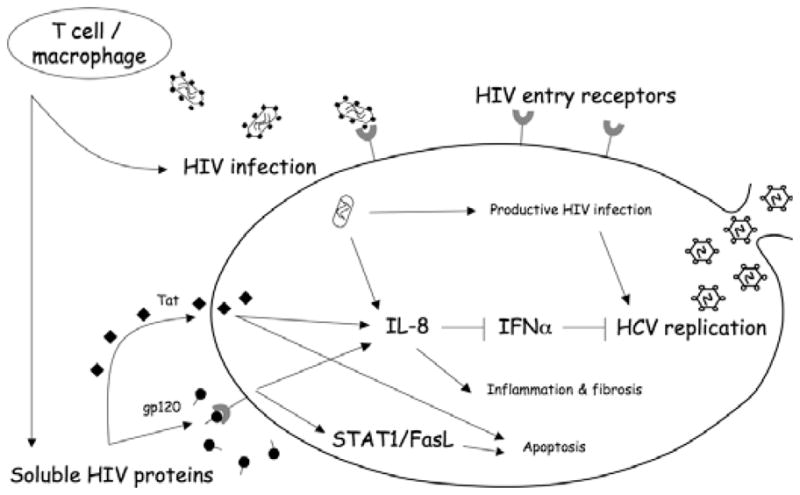
Potential mechanisms by which HIV may influence hepatocyte cell function and / or HCV replication. IL-8, interleukin 8; IFNα, interferon α; HCV, hepatitis C virus; STAT1, signal transducer and activator of transcription 1; FasL, fas ligand. Soluble HIV proteins include Tat (transactivator protein) and gp120 (envelope glycoprotein 120).
In addition to HIV infection of hepatocytes and / or other cell types, it has been hypothesized that hepatocytes exposed to HIV might be injured through an “innocent bystander” mechanism after cell-surface binding of viral proteins. Using an in vitro model that utilizes only viral proteins and uninfected hepatocytes, it was found that the HCV E2 and HIV gp120 envelope proteins cooperatively induce apoptosis in hepatocytes through the CXCR4 chemokine co-receptor [57,63]. Downstream signalling events involve upregulation of Fas ligand and degradation of the anti-apoptotic molecule AKT [63], as well as enhanced caspase expression [70] and activation of signal transducer and activator of transcription 1 (STAT1) [71]. In vivo studies also confirm higher hepatocyte Fas expression in HCV/ HIV co-infected patients compared with HCV mono-infected patients [72]. Moreover, these viral proteins activate hepatic expression of interleukin 8 (IL-8), a pro-inflammatory chemokine, through activation of p38 mitogen-activated protein kinase and the tyrosine kinase SHP2 [64]. Interestingly, IL-8 is an important mediator of hepatic inflammation and is known to antagonize the antiviral effects of IFNα [73–75]. Thus, the authors suggest that release of a potent inflammatory chemokine by hepatocytes could facilitate local inflammatory changes and, in turn, induce fibrosis and cirrhosis.
The effects of recombinant HIV proteins on HCV replication have been addressed using Huh7-2-3 cells that harbour a genotype 1b full-length HCV replicon [76]. HCV core antigen levels were elevated more than two-fold after incubation with HIV gp120; this effect was abrogated by neutralizing antibodies to CCR5 or CXCR4. HIV gp120 also suppressed type I IFN signalling, suggesting that HIV enhanced HCV replication in hepatocytes independent of its effects on T-cell immunity.
Another HIV protein – Tat – is secreted by HIV-infected cells and can be endocytosed by uninfected cells [77]. Tat has also been shown to enhance hepatocarcinogenesis in transgenic mice [78,79]. Collectively, these data convincingly demonstrate that HIV proteins can have a significant impact on the intrahepatic environment even in the absence of productive HIV infection.
EXTRAHEPATIC REPLICATION OF HCV
While hepatocytes are the major site of HCV replication, replication of HCV has also been demonstrated in several extrahepatic tissue and cell types (reviewed in [14]). Importantly, several of these cell types, including T lymphocytes and monocytes /macrophages, also support HIV replication, thereby providing additional reservoirs for interactions between these two viruses. Thus, HIV may influence the clinical progression of HCV disease through enhanced extrahepatic replication of HCV and / or antagonizing ISGs that would otherwise promote HCV clearance. Interestingly, several studies suggest that extrahepatic replication of HCV may occur frequently during HIV co-infection [80–83]. It has further been suggested that HIV infection may facilitate HCV replication in monocytes / macrophages by rendering them more susceptible to HCV infection and / or by increasing HCV replication [84]. While the precise molecular interactions between HIV and HCV have not been completely explored in extrahepatic cell types, these data suggest that non-hepatic cell types might also permit direct virus interactions and contribute to the pathogenesis of HCV/ HIV co-infection.
DEVELOPMENT OF IN VITRO MODELS OF HCV /HIV CO-INFECTION
Critical to the future identification and characterization of the molecular interactions between HIV and HCV is the development of novel in vitro HCV /HIV expression systems. However, no such systems have been fully developed to date. Cell lines transfected with the HCV polyprotein [85–88] offer one possibility, but are not capable of sustained RNA replication. Inoculation of chimpanzees with HCV RNA transcripts [87] and the severe combined immunodeficiency mouse model [89] support HCV infection but are cumbersome and prohibitively expensive. Thus, the development of self-replicating HCV RNA replicons revolutionized the study of HCV [90]. While this system yielded high levels of replication, it was restricted to a single, highly permissive cell line and required adaptive mutations to replicate efficiently, nor can the viral genome be easily manipulated [91,92]. More recently, tissue culture systems capable of producing infectious HCV particles have been described [93–96]. For the first time, the complete viral life cycle from receptor engagement to virion assembly and budding can be examined in vitro. While such systems are currently limited to HCV genotype 2 infection, this is a rapidly evolving area of investigation. For instance, compensatory mutations that result in increased viral replication are regularly identified [97] and additional replication systems will certainly be developed and refined in the near future.
CONCLUSIONS
Rapid HCV disease progression and lower HCV treatment response rates are of major clinical concern during HIV co-infection. The mechanism(s) associated with these observations remain(s) unknown. Nonetheless, there are considerable data to suggest that HIV can productively infect hepatocytes and / or other cell types present in the liver, as well as produce viral proteins capable of affecting hepatocyte function. Moreover, the extrahepatic replication of HCV would appear to provide additional cell types in which HIV and HCV may directly interact to influence each other’s replicative cycle. Future studies that expand our understanding of direct HIV–HCV interactions, as well as virus–cell interactions, will be critical to the development of novel treatment strategies to combat HCV /HIV co-infection.
Acknowledgments
This work was supported in part by NIAID R01 (AI 065256) and NIDDK K24 (DK 070528) Awards to KES and a NIDA R21 Award (DA 022148) to JTB.
Abbreviations
- HCV
hepatitis C virus
- ART
antiretroviral therapy
- IFN
interferon
- ISG
IFN-stimulated genes
- OAS
2′,5′-oligoadenylate synthetase
- HAART
highly active antiretroviral therapy
- HSC
hepatic stellate cells
References
- 1.Seeff L. Natural history of viral hepatitis, type C. Semin Gastrointest Dis. 1995;6:20–27. [PubMed] [Google Scholar]
- 2.Koike K. Antiviral treatment of hepatitis C: present status and future prospects. J Infect Chemother. 2006;12:227–232. doi: 10.1007/s10156-006-0460-0. [DOI] [PubMed] [Google Scholar]
- 3.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492– 497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 4.Sherman K, Rouster S, Chung R, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS clinical trials groups. Clin Infect Dis. 2002;34:831– 837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 5.Alter M, Druszon-Moran D, Nainan O, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 6.Daar E, Lynn H, Donfield S, et al. Hepatitis C viral load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis. 2001;193:589–595. doi: 10.1086/318539. [DOI] [PubMed] [Google Scholar]
- 7.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 8.Piroth L, Grappin M, Cuzin L, et al. Hepatitis C virus coinfection is a negative prognostic factor for clinical evolution in human immunodeficiency virus-positive patients. J Viral Hepat. 2000;7:302–308. doi: 10.1046/j.1365-2893.2000.00227.x. [DOI] [PubMed] [Google Scholar]
- 9.Piroth L, Duong M, Quantin C, et al. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS. 1998;12:381–388. doi: 10.1097/00002030-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Macias J, Pineda J, Lozano F, et al. Impaired recovery of CD4+ cell counts following highly active antiretroviral therapy in drug-naive patients coinfected with human immunodeficiency virus and hepatitis C virus. Eur J Clin Microbiol Infect Dis. 2003;22:675–680. doi: 10.1007/s10096-003-1015-2. [DOI] [PubMed] [Google Scholar]
- 11.Ruys T, Padmos J, Lange J, Wit F. Impaired recovery of CD4 cells in HIV-1 HCV co-infected patients using highly active antiretroviral therapy. XV International AIDS Conference; July 11–16, 2004; Bangkok, Thailand. [Google Scholar]
- 12.Anderson K, Guest J, Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta Cohort Study. Clin Infect Dis. 2004;39:1507–1513. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- 13.Rancinan C, Neau D, Saves M, et al. Is hepatitis C virus coinfection associated with survival in HIV-infected patients treated by combination antiretroviral therapy? AIDS. 2002;16:1357–1362. doi: 10.1097/00002030-200207050-00007. [DOI] [PubMed] [Google Scholar]
- 14.Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44:15–22. doi: 10.1002/hep.21283. [DOI] [PubMed] [Google Scholar]
- 15.Sherman KE, O’Brien J, Gutierrez AG, et al. Quantitative evaluation of hepatitis C virus RNA in patients with concurrent human immunodeficiency virus infections. J Clin Microbiol. 1993;31:2679–2682. doi: 10.1128/jcm.31.10.2679-2682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonacini M, Govindarajan S, Blatt LM, Schmid P, Conrad A, Lindsay KL. Patients co-infected with human immunodeficiency virus and hepatitis C virus demonstrate higher levels of hepatic HCV RNA. J Viral Hepat. 1999;6:203–208. doi: 10.1046/j.1365-2893.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- 17.Beld M, Penning M, Lukashov V, et al. Evidence that both HIV and HIV-induced immunodeificiency enhance HCV replication among HCV seroconverters. Virology. 1998;244:504–512. doi: 10.1006/viro.1998.9130. [DOI] [PubMed] [Google Scholar]
- 18.Ragni M, Bontempo F. Increase in hepatitis C viral load in hemophiliacs during treatment with highly active antiretroviral therapy. J Infect Dis. 1999;180:2027–2029. doi: 10.1086/315143. [DOI] [PubMed] [Google Scholar]
- 19.Pett S, Dore G, Fielden R, Cooper D. Cyclical hepatitis and early liver cirrhosis after hepatitis C seroconversion during pulsed antiretroviral therapy for primary HIV-1. AIDS. 2002;16:2364–2365. doi: 10.1097/00002030-200211220-00029. [DOI] [PubMed] [Google Scholar]
- 20.John M, Flexman J, French M. Hepatitis C virus-associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune response restoration disease? AIDS. 1998;12:2289–2293. doi: 10.1097/00002030-199817000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Chung R, Evans S, Yang Y, et al. Immune recovery is associated with persistent rise in hepatitis C RNA, infrequent liver test flares, and is not impaired by hepatitis C in coinfected subjects. AIDS. 2002;16:1915–1923. doi: 10.1097/00002030-200209270-00008. [DOI] [PubMed] [Google Scholar]
- 22.Rutschmann O, Negro F, Hirschel B, Hadengue A, Anwar D, Perrin L. Impact of treatment with human immunodeficiency virus (HIV) protease inhibitors on hepatitis C viremia in patients coinfected with HIV. J Infect Dis. 1998;177:783– 785. doi: 10.1086/517808. [DOI] [PubMed] [Google Scholar]
- 23.Cooper C, Cameron D. Review of the effect of highly active antiretroviral therapy on hepatitis C virus (HCV) RNA levels in human immunodeficiency virus and HCV coinfection. Clin Infect Dis. 2002;35:873–879. doi: 10.1086/342388. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Sierra C, Arizcorreta A, Diaz F, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36:491–498. doi: 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- 25.Mohsen A, Easterbrook P, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52:1035–1040. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poynard T, Mathurin P, Lai C, et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257–265. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 27.Soto B, Sanchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 28.Lesens O, Deschenes M, Steben M, Belanger G, Tsoukas G. Hepatitis C virus is related to progressive liver disease in human immunodeficiency virus-positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis. 1999;179:1254–1258. doi: 10.1086/314720. [DOI] [PubMed] [Google Scholar]
- 29.Ragni M, Belle S. Impact of human immunodeficiency virus infection on progress to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis. 2001;183:1112–1115. doi: 10.1086/319273. [DOI] [PubMed] [Google Scholar]
- 30.Tedaldi E, Baker R, Moorman A, et al. Influence of coinfection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2003;36:363–367. doi: 10.1086/345953. [DOI] [PubMed] [Google Scholar]
- 31.Yee T, Griffioen A, Sabin C, Dusheiko G, Lee C. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut. 2000;47:845–851. doi: 10.1136/gut.47.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puoti M, Bonancini M, Spinetti A, et al. Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J Infect Dis. 2001;183:134–137. doi: 10.1086/317644. [DOI] [PubMed] [Google Scholar]
- 33.Sulkowski MS, Benhamou Y. Therapeutic issues in HIV/ HCV-coinfected patients. J Viral Hepat. 2007;14:371–386. doi: 10.1111/j.1365-2893.2006.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiMartino V, Rufat P, Boyer N, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology. 2001;34:1193–1199. doi: 10.1053/jhep.2001.29201. [DOI] [PubMed] [Google Scholar]
- 35.Chung R, Andersen J, Volberding P, et al. Peginterferon alpha-2a plus ribavirin versus interferon alpha-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Olmeda M, Nunez M, Romero M, et al. Pegylated IFN-alpha2b plus ribavirin as therapy for chronic hepatitis C in HIV-infected patients. AIDS. 2003;17:1023–1028. doi: 10.1097/00002030-200305020-00011. [DOI] [PubMed] [Google Scholar]
- 37.Lanford R, Bigger C. Advances in model systems for hepatitis C virus research. Virology. 2002;293:1–9. doi: 10.1006/viro.2001.1316. [DOI] [PubMed] [Google Scholar]
- 38.Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 39.Sun J, Li K, Shata MT, Chan TS. The immunologic basis for hepatitis C infection. Curr Opin Gastroenterol. 2004;20:598– 602. doi: 10.1097/00001574-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Valdez H, Anthony D, Farukhi F, et al. Immune responses to hepatitis C and non-hepatitis C antigens in hepatitis C virus infected and HIV-1 coinfected persons. AIDS. 2000;14:2239–2246. doi: 10.1097/00002030-200010200-00004. [DOI] [PubMed] [Google Scholar]
- 41.Lauer G, Nguyen T, Day C, et al. Human immunodeficiency virus type 1-hepatitis C virus coinfection: intraindividual comparison of cellular immune responses against two persistent viruses. J Virol. 2002;76:2817–2826. doi: 10.1128/JVI.76.6.2817-2826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anthony D, Yonkers N, Post A, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–4916. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 43.Graham C, Curry M, He Q, et al. Comparison of HCV-specific intrahepatic CD4+ T cells in HIV /HCV versus HCV. Hepatology. 2004;40:125–132. doi: 10.1002/hep.20258. [DOI] [PubMed] [Google Scholar]
- 44.Alatrakchi N, Graham C, He Q, Sherman K, Koziel M. CD8+ cell responses to hepatitis C virus (HCV) in the liver of persons with HCV-HIV coinfection versus HCV monoinfection. J Infect Dis. 2005;191:702–709. doi: 10.1086/427778. [DOI] [PubMed] [Google Scholar]
- 45.Breen E. Pro- and anti-inflammatory cytokines in human immunodeficiency virus infection and acquired immunodeficiency syndrome. Pharmacol Ther. 2002;95:295– 304. doi: 10.1016/s0163-7258(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 46.Thomas D, Rich J, Schuman P, et al. Multicenter evaluation of hepatitis C RNA levels among female injection drug users. J Infect Dis. 2001;183:973–976. doi: 10.1086/319256. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Dailey P, Gettie A, Blanchard J, Ho D. The liver is a major organ for clearing simian immunodeficiency virus in rhesus monkeys. J Virol. 2002;76:5271–5273. doi: 10.1128/JVI.76.10.5271-5273.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz I, Neva FA. Relationship between blood clearance and viruria after intravenous injection of mice and rats with bacteriophage and polioviruses. J Immunol. 1965;94:833– 841. [PubMed] [Google Scholar]
- 49.Mims CA. Rift Valley Fever virus in mice. II. Adsorption and multiplication of virus. Br J Exp Pathol. 1956;37:110–119. [PMC free article] [PubMed] [Google Scholar]
- 50.Housset C, Lamas E, Brechot C. Detection of HIV1 RNA and p24 antigen in HIV-1-infected human liver. Res Virol. 1990;141:153–159. doi: 10.1016/0923-2516(90)90017-d. [DOI] [PubMed] [Google Scholar]
- 51.Cao Y, Dieterich D, Thomas P, Huang Y, Mirabile M, Ho D. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS. 1992;6:65–70. doi: 10.1097/00002030-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee R, Sperber K, Pizzella T, Mayer L. Inhibition of HIV-1 productive infection in hepatoblastoma HepG2 cells by recombinant tumor necrosis factor-α. AIDS. 1992;6:1127–1131. doi: 10.1097/00002030-199210000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Schmitt M, Steffan A, Gendrault J, et al. Multiplication of human immunodeficiency virus in primary cultures of human kupffer cells – possible role of liver macrophage infection in the physiopathology of AIDS. Res Virol. 1990;141:143–152. doi: 10.1016/0923-2516(90)90016-c. [DOI] [PubMed] [Google Scholar]
- 54.Steffan A, Lafon M, Gendrault J, et al. Primary cultures of endothelial cells from the human liver sinusoid are permissive for human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:1582–1586. doi: 10.1073/pnas.89.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van’t Wout A, Ran L, Kuiken C, Kootstra N, Pals S, Schuitemaker H. Analysis of the temporal relationshiop between human immunodeficiency virus type 1 quasispecies in sequentio blood samples and various organs obtained at autopsy. J Virol. 1998;72:488–496. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao Y, Friedman-Kein A, Huang Y, et al. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol. 1990;64:2553–2559. doi: 10.1128/jvi.64.6.2553-2559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vlahakis S, Villasis-Keever A, Gomez T, Bren G, Paya C. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis. 2003;188:1455– 1460. doi: 10.1086/379738. [DOI] [PubMed] [Google Scholar]
- 58.Tuyama A, Hong F, Schecter A, et al. HIV entry and replication in stellate cells promotes cellular activation and fibrogenesis: implications for hepatic fibrosis in HIV/ HCV co-infection. The 58th Annnual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. [Google Scholar]
- 59.Littman D. Chemokine receptors: keys to AIDS pathogenesis? Cell Death Differ. 1998;93:677–680. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- 60.Popik W, Pitha P. Exploitation of cellular signaling by HIV-1: unwelcome guests with master keys that signal their entry. Virology. 2000;276:1–6. doi: 10.1006/viro.2000.0581. [DOI] [PubMed] [Google Scholar]
- 61.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 62.Mellado M, Rodriguez-Frade J, Manes S, Martinez C. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19:397–421. doi: 10.1146/annurev.immunol.19.1.397. [DOI] [PubMed] [Google Scholar]
- 63.Munshi N, Balasubramanian A, Koziel M, Ganju R, Groopman J. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188:1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 64.Balasubramanian A, Ganju R, Groopman J. HCV and HIV envelope proteins collaboratively mediate IL-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278:35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- 65.Yoong K, Afford S, Jones R, et al. Expression and function of CXC and CC chemokines in human malignant liver tumors: a role for human monokine induced by g-interferon in lymphocyte recruitment to hepatocellular carcinoma. Hepatology. 1999;30:100–111. doi: 10.1002/hep.510300147. [DOI] [PubMed] [Google Scholar]
- 66.Apolinario A, Majano P, Alvarez-Perez E, et al. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861– 2870. doi: 10.1111/j.1572-0241.2002.07054.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang J, Holmes T, Cheung R, Greenberg H, He X. Expression of chemokine receptors on intrahepatic and peripheral lymphocytes in chronic hepatitis C infection: its relationship to liver inflammation. J Infect Dis. 2004;190:989–997. doi: 10.1086/423283. [DOI] [PubMed] [Google Scholar]
- 68.Kusano F, Tanaka Y, Marumo F, Sato C. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest. 2000;80:415–422. doi: 10.1038/labinvest.3780046. [DOI] [PubMed] [Google Scholar]
- 69.Hellier S, Frodsham A, Hennig B, et al. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003;38:1468–1476. doi: 10.1016/j.hep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 70.Balasubramanian A, Koziel M, Groopman J, Ganju R. Molecular mechanism of hepatic injury in coinfection with hepatitis C virus and HIV. Clin Infect Dis. 2005;41:S32–S37. doi: 10.1086/429493. [DOI] [PubMed] [Google Scholar]
- 71.Balasubramanian A, Ganju RK, Groopman JE. Signal transducer and activator of transcription factor 1 mediates apoptosis induced by hepatitis C virus and HIV envelope proteins in hepatocytes. J Infect Dis. 2006;194:670–681. doi: 10.1086/505708. [DOI] [PubMed] [Google Scholar]
- 72.Macias J, Japón MA, Sáez C, et al. Increased hepatocyte fas expression and apoptosis in HIV and hepatitis C virus co-infection. J Infect Dis. 2005;192:1566–1576. doi: 10.1086/491736. [DOI] [PubMed] [Google Scholar]
- 73.Polyak S, Khabar K, Rezeiq M, Gretch D. Elevated levels of interleukin-8 in serum are associated with hepatitis C infection and resistance to interferon therapy. J Virol. 2001;75:6209–6211. doi: 10.1128/JVI.75.13.6209-6211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Polyak S, Khabar K, Paschal D, et al. Hepatitis C virus nonstructural 5A protein induces interleukin-8, leading to partial inhibition of the interferon-induced antiviral response. J Virol. 2001;75:6095–6106. doi: 10.1128/JVI.75.13.6095-6106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khabar K, Al-Zoghaibi F, Al-Ahdal M, et al. The alpha chemokine, interleukin 8, inhibits the antiviral action of interferon α. J Exp Med. 1997;186:1077–1085. doi: 10.1084/jem.186.7.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin W, Kim S, Kim K, Chung R. HIV gp120 protein increases HCV replication in cell culture models. American Association for the Study of Liver Diseases; October 27–31, 2006; Boston, MA. [Google Scholar]
- 77.Frankel A, Pabo C. Cellular uptake of the tat protein from human immunodeficiency virus. Cell Death Differ. 1988;55:118–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 78.Vogel J, Hinrichs S, Napolitano L, Ngo L, Jay G. Liver cancer in transgenic mice carrying the human immunodeficiency virus tat gene. Cancer Res. 1991;51:6686–6690. [PubMed] [Google Scholar]
- 79.Altavilla G, Caputo A, Landredi M, Piola C, Barbanti-Brodano G, Corallini A. Enhancement of chemical carcinogenesis by the HIV-1 tat gene. Am J Pathol. 2000;157:1081–1089. doi: 10.1016/S0002-9440(10)64622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blackard J, Smeaton L, Hiasa Y, et al. Detection of hepatitis C virus (HCV) in serum and peripheral blood mononuclear cells of HCV-monoinfected and HIV / HCV-coinfected persons. J Infect Dis. 2005;192:258–265. doi: 10.1086/430949. [DOI] [PubMed] [Google Scholar]
- 81.Laskus T, Radkowski M, Piasek A, et al. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: evidence of active replication in monocytes /macrophages and lymphocytes. J Infect Dis. 2000;181:442–448. doi: 10.1086/315283. [DOI] [PubMed] [Google Scholar]
- 82.Laskus T, Radkowski M, Wang L, Vargas H, Rakela J. The presence of active hepatitis C virus replication in lymphoid tissue in patients coinfected with human immunodeficiency virus type 1. J Infect Dis. 1998;178:1189–1192. doi: 10.1086/515682. [DOI] [PubMed] [Google Scholar]
- 83.Laskus T, Radkowski M, Wang L, Vargas H, Rakela J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: specific detection of negative-strand viral RNA in various tissues. Hepatology. 1998;28:1398–1401. doi: 10.1002/hep.510280531. [DOI] [PubMed] [Google Scholar]
- 84.Laskus T, Radkowski M, Jablonska J, et al. Human immunodeficiency virus facilitates infection / replication of hepatitis C virus in native human macrophages. Blood. 2004;103:3854–3859. doi: 10.1182/blood-2003-08-2923. [DOI] [PubMed] [Google Scholar]
- 85.Korth M, Katze M. Evading the interferon response: hepatitis C virus and the interferon-induced protein kinase, PKR. Curr Top Microbiol Immunol. 2000;242:197–224. doi: 10.1007/978-3-642-59605-6_10. [DOI] [PubMed] [Google Scholar]
- 86.Gale M, Blakely C, Kwieciszewski B, et al. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yanagi M, Purcell R, Emerson S, Bukh J. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc Natl Acad Sci USA. 1997;94:8738–8743. doi: 10.1073/pnas.94.16.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taylor D, Shi S, Romano P, Barber G, Lai M. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 89.Mercer D, Schiller D, Elliott J, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 90.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 91.Blight K, Kolykhalov A, Rice C. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 92.Bukh J, Pietschmann T, Lohmann V, et al. Mutations that permit efficient replication of hepatitis C virus RNA in Huh-7 cells prevent productive replication in chimpanzees. Proc Natl Acad Sci USA. 2002;99:14416–14421. doi: 10.1073/pnas.212532699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wakita T, Pietschmann T, Kato T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong J, Gastaminza P, Cheng G, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heller T, Saito S, Auerbach J, et al. An in vitro model of hepatitis C virion production. Proc Natl Acad Sci USA. 2005;102:2579–2583. doi: 10.1073/pnas.0409666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindenbach B, Evans M, Syder A, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 97.Yi M, Ma Y, Yates J, Lemon S. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J Virol. 2007;81:629–638. doi: 10.1128/JVI.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


