Abstract
Varenicline promotes smoking cessation and reduces urges to smoke. However, the mechanisms associated with these effects and their time course are not well characterized. One mechanism may be extinction, but the duration of the current dosing protocol may not be sufficient. We examined the effect of extended pre-treatment with varenicline on smoking behavior among 17 non-treatment seeking adult smokers. Using a within-subjects, double-blind, placebo-controlled crossover design, participants received standard dosing of varenicline for 21 days, followed by a 14-day washout period and 21 days of placebo; order counterbalanced. Cigarettes per day (CPD), smoking topography, smoking urges (QSU), and side effects were assessed every three days. Biomarkers (e.g. nicotine metabolites) were collected on days 1, 7, and 21. There was a significant drug by time interaction indicating a reduction in CPD during varenicline phase (between days 10–21), but no reduction during placebo. Varenicline also led to reductions in nicotine metabolites and urges to smoke. Among this sample of non-treatment seeking smokers, varenicline significantly reduced smoking behavior. Results have important treatment implications because changes in CPD and craving did not occur until after the typical one-week run-up period. This suggests that a longer duration of pre-treatment may be beneficial for some smokers.
Keywords: Cigarette smoking, varenicline, nicotine, positive reinforcement, smoking cessation
Introduction
Varenicline, a partial agonist at the α4β2 nicotinic acetylcholine receptor (nAChR) and a full agonist at the α7 nAChRs, is an effi-cacious treatment for smoking cessation (Gonzales et al., 2006; Mihalak et al., 2006). Preclinical data demonstrate that varenicline's agonist effects produce a moderate amount of dopamine release (Coe et al., 2005; Rollema et al., 2007), resulting in reductions in withdrawal and craving (Brandon et al., 2011; Patterson et al., 2009; Perkins et al., 2010). Varenicline binds to α4β2 nAChRs with higher affinity than nicotine, and so the rewarding effects of nicotine are also reduced (Le Foll et al., 2011). Several human laboratory studies have demonstrated these effects (Brandon et al., 2011; Franklin et al., 2011; Sofuoglu and Mooney, 2009).
Varenicline's effects on smoking behavior and subjective response to smoking have been partially explained through an extinction framework (Rollema et al., 2007). Specifically, when the reinforcing properties of smoking are reduced (i.e. by blocking nAChRs), the frequency of the behavior (i.e. smoking behavior) should also decrease. Two recent studies have shown that extend-ing the pre-treatment period (i.e. the duration of time medication is taken prior to making a quit attempt) to four weeks results in a greater reduction in smoking behavior prior to a quit attempt and an increase in cessation rates compared to a one-week run up, suggesting that the standard dosing regimen may not be sufficient to extinguish smoking behavior (Hajek et al., 2011; Hawk et al., 2012). In animals, a longer duration of treatment with varenicline resulted in a greater reduction in nicotine self-administration (Le Foll et al., 2011). Thus, there is support showing that varenicline increases abstinence rates by promoting extinction of smoking behavior.
Despite these converging lines of evidence in support of the extinction hypothesis, no study to date has systematically tested these behavioral mechanisms with respect to the time course of responses to varenicline. Laboratory studies typically administer varenicline for four days to three weeks and generally assess two or three timepoints (Ashare and McKee, 2012; Brandon et al., 2011; Sofuoglu et al., 2009). To better understand the time course of the effects of varenicline, it is necessary to repeatedly assess changes in smoking behavior during a longer course of treatment. Furthermore, varenicline's effects on smoking behavior and urges to smoke prior to a quit attempt may be important for identifying the optimal quit date in order to maximize treatment success (Hughes et al., 2011). The current study employed a placebo-controlled cross-over design to assess the time course of changes in smoking behavior. We hypothesized that three weeks of treatment with varenicline, compared to placebo, would reduce self-reported cigarettes per day, total puff volume measured via smoking topography, urges to smoke for positive reinforcement, and biological (e.g. nicotine metabolites) measures. Understanding these processes may be important for improving varenicline's effects on abstinence rates.
Methods and materials
Participants
Prospective participants were those who responded to local adver-tisements (e.g. newspaper and internet) to participate in a research study. Smokers were eligible if they were not currently seeking treatment for smoking cessation but reported an intention to quit smoking in the next six months; were between 21 and 65 years old; smoked ≥10 cigarettes/day for the past five years. Exclusion criteria included: currently receiving treatment for nicotine dependence; inability to provide a baseline CO reading>10 ppm; smoking menthol cigarettes; history or current treatment of sub-stance abuse; self-reported alcohol use>25 drinks/week; history or current diagnosis of psychosis, major current depression, bipolar disorder, ADHD, schizophrenia, or other DSM-IV Axis I disorders (assessed via Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) on Day 1); recent (past 30 days) use of psychotropic medication; serious medical conditions (e.g. dia-betes, kidney function impairment, uncontrolled hypertension); and pregnancy or planned pregnancy.
Procedures
Overview of study design
This was a within-subject, double-blind, placebo-controlled crossover design. The study consisted of two 21-day phases during which participants were randomized to receive either varenicline or placebo (order counterbalanced), with a minimum 14-day washout period between phases. On Days 1, 4, 7, 10, 14, 18, and 21 of each phase, participants completed study visits to assess smoking behavior, craving, biomarkers of nicotine exposure, medication adherence, and side effects. During each phase and the washout period, participants were instructed to follow their urges and smoke as they wished. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and all procedures were approved by the Institutional Review Board of the University of Pennsylvania.
Eligibility screening
Initial eligibility was determined during a telephone interview by a trained research technician. Participants meeting preliminary eligibility criteria scheduled a screening session during which informed consent was given, eligibility require-ments were reviewed, and a urine drug screen (and pregnancy test for females) and a blood pressure reading was taken. Eligible participants were then randomized to receive either varenicline or placebo during the first phase.
Study visits
All visits were scheduled between 8 am and 10 am. Participants arrived at approximately the same time of day for each visit (+/− 1 hr) and provided a carbon monoxide breath sample, a urine sample (on Days 1, 7, and 21 for nicotine and cotinine; on Day 1 only, drug screen, pregnancy screen for females), reported the number of cigarettes smoked since 00:01 that day, provided used cigarette filters collected since previous visit, and completed self-report questionnaires for smoking urges and withdrawal. On Day 1 of each phase, participants received study medication for that period and were instructed to follow their urges and smoke as they wished. Varenicline and matching placebo were provided by Pfizer. Dosing followed the standard regimen for varenicline: 0.5 mg orally once daily for Days 1–3; 0.5 mg orally twice daily for Days 4–7; 1.0 mg orally twice daily for Days 8–21. Study medication was administered in the presence of study staff at each visit and medication adherence, which was 97% during both periods, was assessed by pill counts. Side effects were moni-tored at each visit, consistent with previous varenicline research in our center (Patterson et al., 2009). During each visit, participants smoked a cigarette on a topography machine. On Days 1, 7, and 21 participants completed a longer four-hour laboratory session during which additional measures were collected (data not reported here). During their final visit, participants were asked whether they had received varenicline during the first or second phase. Although 65% correctly identified when they were on active medication, this did not influence any outcome measure.
Washout period
At the end of the first 21-day phase, there was a minimum 14-day washout period to ensure complete elimina-tion of the drug between phases. Participants were instructed to resume their pre-enrollment smoking practices and to maintain a daily cigarette consumption log as part of their recruitment/appointment calendar.
Dependent measures
Smoking behavior
The primary dependent measure of smoking consumption was the self-reported number of cigarettes smoked per day recorded on a Timeline Followback (TLFB) (Sobell and Sobell, 1992) calendar for each day between visits. To confirm the number of cigarettes smoked, participants were asked to collect used cigarette filters and bring them to each visit in a re-sealable date-labeled bag provided for each study day. The correlations between self-reported number of cigarettes and cigarette filters returned at each were high (all rs>0.80, ps<0.001). The number of cigarettes per day between each visit was averaged to create a more stable estimate of cigarette consumption. The resulting six timepoints were used in subsequent analyses (e.g. Visit 1 cigarettes per day (CPD)=mean of Day 1, Day 2, and Day 3; Visit 2 CPD=mean Day 4, Day 5, and Day 6, etc.).
During each visit, participants smoked one of their preferred brand cigarettes under ad libitum conditions on a smoking topography device (Borgwaldt KC (recently Plowshare Technologies), Richmond, VA, USA) in a smoking-approved ventilated room. Puff volume, puff velocity, interpuff interval, and peak velocity were assessed. Total puff volume, defined as the sum of all puffs, was used as the primary dependent measure (Strasser et al., 2007, 2011). Next, total puff volume was multiplied by the number of cigarettes per day reported on the TLFB to form a composite measure of `daily smoking behavior.' This measure was computed for each day and was used to further characterize daily smoking behavior by allowing adjustments in smoking while estimating for the total smoking behavior for each day; similar compensation models for daily smoking behavior have been previously proposed (Benowitz et al., 2005; Strasser et al., 2011).
Craving
Craving for cigarettes was assessed with the 32-item Questionnaire of Smoking Urges (QSU) (Tiffany and Drobes, 1991) during each study visit. Each item is rated on a Likert-type scale from 1 (strongly disagree) to 7 (strongly agree). The QSU consists of two factors: Factor 1 reflects the desire to smoke for pleasure and Factor 2 reflects urges to smoke to relieve withdrawal-related negative affect. In addition to creating mean scores for each factor, we also created a QSU total score of all 32 items. Internal consistency for each scale across all timepoints was high (Cronbach's α > 0.95, 0.85, and 0.95 for Factor 1, Factor 2, and QSU total, respectively).
Biomarkers of nicotine exposure
Nicotine and cotinine measure- ments were conducted using a modification of a method described previously (Rangiah et al., 2011). Briefly, urine (250 μL) was mixed with 0.5 mL of phosphate buffer (100 mM, pH 6.8) followed by the addition of 10 μL of internal standard solution (10 μL containing 100 μg/mL of each deuterated internal standard). The internal standard was also added to standard curve and quality control samples. The samples were incubated with β-glucuronidase enzyme (3000 units/mL) for 12 hours at 37°C for the total (free + conjugates) analytes. Solid-phase extraction (SPE) columns (Oasis MCX) were pre-conditioned with 1 mL each of methanol, water and ammonium formate buffer (20 mM, pH 2.5). The pH of the samples was reduced to ~3 by adding 10 μL of 50% formic acid before loading on the SPE columns. The SPE columns were then washed with 1 mL ammonium formate buffer (20 mM, pH 2.5) and analytes were eluted with 500 μL methanol/aqueous ammonium hydroxide (95/5). After elution, the pH of the eluate was reduced to ~4 by adding 30 μL of 50% formic acid and 10 μL of the resulting solution was analyzed by liquid chromatography-multiple reaction monitoring/mass spectrometry as described previously (Rangiah, et al., 2011).
Side effects
The Symptoms Evaluation Checklist (SEC) consists of 17 items representing potential drug side effects (e.g. headache, nausea, fatigue). Symptoms are rated on a scale ranging from 0 (none) to 3 (severe). Several summary scales can be generated from this instrument; we used the total symptom count which is the sum of the number of non-zero responses across all 17 items. Nausea is the most commonly reported side effect of vare- nicline (Cahill et al., 2011; Sofuoglu et al., 2011), and because of this we examined this item separately.
Data analysis
Repeated-measures analysis of covariance (ANCOVA) models were used to assess treatment effects on smoking behavior (i.e. cigarettes per day, total puff volume, and daily smoking behavior), craving, and biomarkers of nicotine exposure. In each model, drug (varenicline vs placebo) and time were within-subject factors. For cigarettes per day and daily smoking behavior, the timepoints (Visit 1, Visit 2, Visit 3, Visit 4, Visit 5, Visit 6) reflect the fact that the number of cigarettes per day was averaged between each visit. For total puff volume, craving, and biomarkers, the timepoints (Day 1, Day 4, Day 7, Day 10, Day 14, Day 18, Day 21) reflect the fact that these data were collected on discrete days. Treatment order was included as a between-subjects factor in all models to account for order effects. Age and Fagerstrom Test for Nicotine Dependence (FTND) scores were included as covariates in primary models. To correct for vio- lations of the sphericity assumption in testing effects involving time, Huynh-Feldt epsilon adjustments were used (Huynh and Feldt, 1970). We had a priori hypotheses about the drug by time interaction, and so follow-up simple effects analyses were used to examine the effects of time on varenicline and placebo in separate models. Exploratory post hoc analyses examined the specific time course of the effects of varenicline. Evidence that varenicline may have carryover effects (Patterson et al., 2009; Turner et al., 2011) prompted us to conduct post hoc tests to assess whether treatment order influenced cigarette consumption. In addition, we explored the association between changes in urges to smoke and cigarette consumption with Pearson correlations.
Results
Participant characteristics
During initial phone screens, 82 eligible participants scheduled a medical screening visit, of which 53 attended (64%), and 41 were determined eligible and expressed interest in participating in the study. For the laboratory sessions, 29 participants completed the first laboratory session, and of those, 23 participants returned for the follow up session (80%), which is consistent with previous attrition rates and similar to our projection for this study. Seventeen of the 23 participants (3 female) completed all visits and assessments and are the basis of our analysis below. The majority of participants described themselves as Caucasian (94%), 53% had never been married, more than half (59%) had a college degree, and 53% were employed at least part-time. Participants ranged from 22 to 64 years old (mean=43 years; SD=15) and had an average body mass index (BMI) of 26.1 (SD=5.8). Participants reported that on average they smoked 18 cigarettes per day (SD=5.6), began smoking at age 17 years (SD=7), and were moderately nicotine dependent (FTND mean=5.1, SD=2). At the eligibility screening visit, the average carbon monoxide (CO) breath level was 26 ppm (SD=10.6).
As a result of the high rate of attrition, we conducted preliminary analyses comparing participants who completed the study to those who did not. Compared to those who completed the study, participants who were lost to attrition tended to be younger (mean=35 years, SD=12) and have lower baseline CO levels (mean=20.4, SD=11), F(1,40)=3.6 and 3.1, p=0.06 and 0.09, respectively. The two groups did not differ on any other characteristic.
Effect of treatment on behavior
Cigarette consumption
Figure 1 depicts mean (SE) for cigarettes per day for all study visits by drug phase. For cigarettes per day, there was an overall significant drug × time interaction, Huynh-Feldt F(5,65)=3.6, p=0.006, ηp2=0.22. Separate contrasts revealed that the drug × time linear interaction was significant, F(1,13)=17.4, p=0.001, ηp2=0.57. Post hoc comparisons indicated that in the varenicline phase, there was no change in cigarettes per day between Visits 1 (mean=16.1, SE=0.86) and 3 (mean=15.1, SE=1.3; Days 1 through 9), F(1,13) = 1.1, p=0.35, ηp2=0.16. Cigarettes per day began to decrease at Visit 4 (14.1, SE=1.3) and continued to decrease through Visit 6 (mean=12.8, SE=1.3), F(1,13)=4.8, p=0.02, ηp2=0.57. Overall, there was a 23% reduction in cigarettes per day during the varenicline phase. In contrast, there was a non-significant reduction in cigarettes per day during the placebo phase (mean difference=1.7, SE=1.1), F<1. Similar results were observed when examining cigarettes per day by discrete timepoints (i.e., the final day between each visit). The drug × time interaction was significant, F(1,13)=8.8, p=0.01, ηp2=0.4. The means (SE) for the varenicline period were: 15.2(0.65); 15.5(1.2); 15.2(0.95); 14.8(1.6); 14.5(1.4); 13.3(1.3); and 12.4(1.3). For the placebo period, the means (SE) were: 15.8(1.2); 16.3(1.0); 15.0(1.1); 15.8(1.3); 16.7(1.2); 14.2(1.6); and 13.4(1.7). Despite the fact that participants smoked fewer cigarettes during the second phase compared to the first phase, treatment order × drug interaction F(1,13)=11.5, p=0.005, ηp2=0.47, treatment order had no effect on the critical drug x time interaction, p=0.22.
Figure 1.
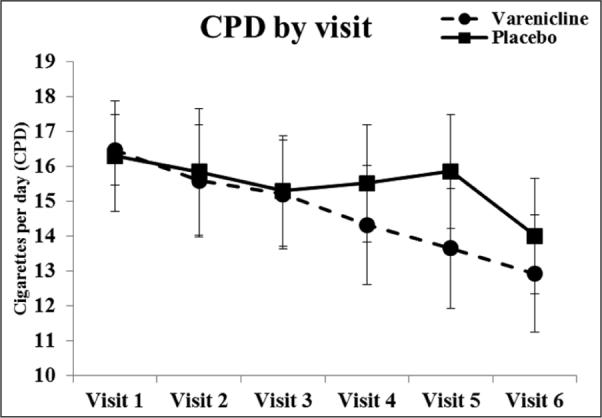
Cigarettes per day (CPD) across time during the varenicline and placebo phase.
Smoking topography
Although there were main effects of age and treatment order on total puff volume, F(1,11)=5.8, p=0.034 and F(1,11)=8.9, p=0.012, respectively, the main effects of drug and time and their interaction were not significant, all ps>0.22. Despite randomizing treatment order across participants, those who received placebo first demonstrated lower total puff volume (mean=523.4, SE=41), regardless of drug or time compared to those who received varenicline first (mean=684.6, SE=33).
Daily smoking behavior
For the composite measure of `daily smoking behavior' (product of cigarettes per day and total puff volume), the Huynh-Feldt adjusted drug × time interaction was significant, F(6,66)=2.4, p=0.036, ηp2=0.18. Similar to cigarettes per day, the drug × time linear contrast was significant, F(1,13)=7.5, p=0.02, ηp2=0.40. Simple effects post hoc tests suggested that during varenicline, there was a significant reduction in total smoking behavior from Visit 1 (Day 1) (mean=9375.4, SE=538) to Visit 6 (Day 21) (mean=6564.4, SE=999), F(1,13)=8.2, p=0.014, ηp2=0.38. In contrast, there was no significant change in total smoking behavior during placebo from Visit 1 (mean=9550.8, SE=1097) to Visit 6 (mean=7892.3, SE=1068), F < 1.
Effect of treatment on craving
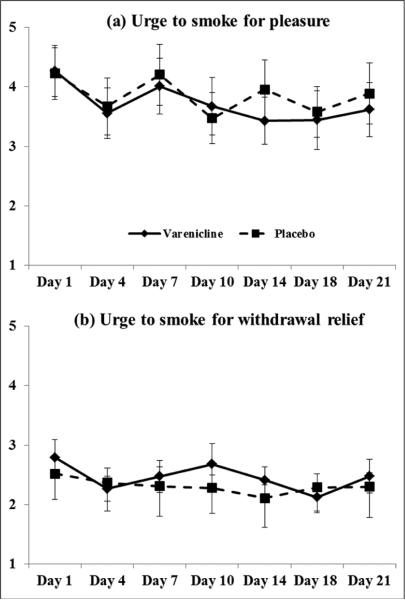
Figure 2 depicts QSU Factor 1 and 2 scores by time for each drug phase. Based on our hypothesis that varenicline may reduce smoking behavior through a decrease in the anticipation of pleasure from smoking, we predicted that decreases in urges to smoke would be primarily due to reductions in QSU Factor 1 (`anticipation of pleasure from smoking'). Although the drug × time interaction was not significant, F(1,11)=1.2, p=0.30, QSU Factor 1 tended to decrease across time, F(1,11)=2.9, p=0.11, ηp2=0.21. Unprotected follow-up tests indicated that decreases in `urges to smoke for pleasure' (QSU Factor 1) only occurred in the varenicline phase, F(1,13)=6.0, p=0.03, ηp2=0.32. Between Day 1 and 10, there were no significant changes in QSU Factor 1 scores, ps>0.22. Beginning at Day 14, there were significant decreases in urges to smoke for pleasure in the varenicline phase, F(1,13)=11.8, p<0.01.
Figure 2.
(a) Urges to smoke for pleasure (QSU Factor 1) and (b) urges to smoke for withdrawal relief (QSU Factor 2) across time during the varenicline and placebo phase.
QSU: Questionnaire of Smoking Urges.
There were no effects of drug or changes across time for QSU Factor 2 (`urges to smoke for withdrawal relief'), Fs <1. For the total QSU score, the repeated measures ANCOVA model revealed no significant changes in overall urges to smoke as a function of time or drug, ps>0.17. However, the pattern of means suggested a greater reduction in craving scores during varenicline (mean difference=0.55, SE=0.24) compared to placebo (mean difference=0.32, SE=0.27).
Effect of treatment on biomarkers of nicotine exposure
Table 1 contains means (SD) for all drug × time cells for nicotine metabolites. For total metabolites, there was an overall effect of time, F(1,12)=5.1, p=0.045, ηp2=0.32, such that the total urinary metabolites decreased from Day 1 to Day 21, irrespective of drug condition, drug and drug × time interaction, Fs <1. For urinary nicotine, the drug × time quadratic interaction was marginal, F(1,12)=3.6, p=0.08, ηp2=0.24. Consistent with self-reported cigarettes per day, there was a significant decrease in urinary nicotine in the varenicline phase, F(1,12)=12.3, p=0.004, ηp2=0.51, but not in the placebo phase, F<1. Although cotinine tended to decrease across time this effect was not statistically significant, F(1,12)=3.1, p=0.10, ηp2=0.21. The overall effect of drug and the drug × time interaction were not significant, Fs <1. There were no significant changes in CO breath levels either as a function of drug or time, Fs <1.
Table 1.
Nicotine metabolites for each drug × time cell (N=17).
| Drug phase |
||||||
|---|---|---|---|---|---|---|
| Placebo |
Varenicline |
|||||
| Day 1 | Day 7 | Day 21 | Day 1 | Day 7 | Day 21 | |
| Total metabolitesa | 110 (14) | 74 (11) | 79 (14) | 111 (15) | 106 (13) | 81 (9) |
| Nicotineb | 20.0 (6) | 12.4 (3) | 18.0 (6) | 24.0 (5) | 17.1 (4) | 13.8 (3) |
| Cotinine | 19.0 (2) | 13.8 (2) | 12.0 (2) | 16.9 (2) | 16.8 (2) | 14.0 (2) |
| CO (ppm) | 30 (4) | 28.6 (3) | 25 (2) | 23.2 (2) | 24.1 (2) | 22 (2) |
CO: carbon monoxide; ppm: parts per million. Values are mean (SE), adjusted for age and nicotine dependence; all units are μM except where noted.
Time, p<0.05.
Drug × time interaction, p<0.10.
Side effects
There were no significant main effects or interactions for drug and time on the side effects summary score, Fs<1.2, ps>0.30. Nausea ratings were marginally higher in the varenicline phase compared to placebo, F(1,12)=4.3, p=0.06, ηp2=0.27 and this effect appeared to vary across time, drug × time quadratic interaction, F(1,12) = 5.7, p=0.034, ηp2=0.32. However, the increase in nausea appeared to be driven by a small subset of participants. Indeed, the majority of participants reported no nausea during varenicline (n=14). Only three smokers reported mild or moderate nausea and no one reported severe nausea. There were no significant changes in nausea ratings in the placebo phase, F(1,12)=1.2, p=0.31. Importantly, there were no differences in medication adherence between individuals who reported nausea (mean compliance=99%) and individuals who did not (mean compliance=98%), nor were there differences between the placebo phase and the varenicline phase (96% vs 97%), F<1.
Association between changes in urges to smoke and cigarette consumption
Post hoc correlation analyses were conducted to explore the association between the time course of varenicline's effects on urges to smoke and cigarette consumption. We observed a reduction in cigarettes per day beginning at Visit 3 (i.e. Day 10), and so we were interested in whether a reduction in urge to smoke prior to Day 10 was related to changes in cigarette consumption. Thus, we examined the relationship between decreases in urges to smoke from Day 1 to Day 10 and subsequent reductions in cigarettes per day from Day 10 to Day 20. Both QSU factors were included and the varenicline and placebo phases were examined separately. Results revealed that decreases in urges to smoke for pleasure (QSU Factor 1) were significantly associated with greater reductions in cigarette consumption (r=0.60, p=00.01) during the varenicline phase, but not the placebo phase (r=−0.06, p=0.82). Changes in urges to smoke to reduce negative affect and decreases in cigarettes per day were not related in either phase (rs<0.33, ps>0.21).
Discussion
We examined the time course of smoking behavior during 21 days of varenicline and placebo in order to elucidate the mechanisms by which varenicline extinguishes smoking behavior. The key finding is that varenicline reduced cigarettes smoked per day, compared to placebo. This finding was supported by decreases in nicotine metabolites and reduced urges to smoke for pleasure. There were no observed changes to urges to smoke for withdrawal relief. Although smoking topography was not sensitive to treatment effects, we did observe a reduction in the composite measure of daily smoking behavior during varenicline providing additional support for the hypothesis that varenicline alters smoking behavior. Few participants reported nausea, suggesting that changes in smoking behavior are likely not to be attributable to side effects of varenicline. The current data also provide insight into differences in the time course of changes across measures. These findings highlight the importance of examining underlying behavioral mechanisms by which treatments work to enhance their efficacy.
One of the strengths of the current design was the inclusion of multiple assessments during each 21-day treatment period. Typically, smokers are told to set a quit date seven days after starting medication (Fiore et al., 2008). During the varenicline phase, we did not observe a significant change in cigarette consumption until Day 10. Following this initial reduction, the number of cigarettes per day continued to gradually decrease during the remainder of the varenicline phase. According to current treatment recommendations, the target quit day would have occurred three days prior to the observed decrease in CPD in the current study. Reducing cigarette consumption prior to a quit attempt may be important for reducing the severity of withdrawal symptoms and urges to smoke (Hughes et al., 2011). Furthermore, pre-treatment with nicotine replacement therapy enhances abstinence rates and may reduce urges to smoke, suggesting that the effect of pre-treatment may not be specific to varenicline (Rose, 2011; Rose et al., 2006; Shiffman and Ferguson, 2008). Although no study to our knowledge has tested this hypothesis, the current data indicate that the time course of the reduction in CPD may have important implications for one's ability to remain abstinent.
Hughes et al. (2011) also propose that a pre-quit reduction in cigarette consumption may decrease dependence on cigarettes, which may enhance the likelihood that a smoker will make a quit attempt. Consistent with this hypothesis and the current data, there is mounting evidence that a longer duration of pre-treatment with varenicline may promote abstinence among smokers (Hajek et al., 2011). In that study, 35% of the varenicline group reduced cigarette consumption by 50% prior to a quit attempt, compared to only 10% who received placebo during the 3-week pre-treatment phase. Furthermore, 'reducers' who received extended treatment with varenicline had higher 12-week abstinence rates compared to non-reducers and those receiving standard treatment (Hajek et al., 2011). As a comparison to Hajek et al. (2011), we conducted similar analyses and observed a comparable number of reducers in both the varenicline (30%) and placebo phase (11%). To further characterize these participants, we found that reducers smoked fewer cigarettes per day (17 vs 21 CPD), tended to have lower nicotine dependence scores (3.8 vs 5.6), and tended to wait longer to smoke the first cigarette of the day. Of note, all but one of the 11 non-reducers reported smoking their first cigarette within the first 30 minutes of waking. Evidence suggests that the first cigarette of the day predicts overall smoke exposure (Strasser et al., 2009), cotinine levels (Muscat et al., 2009), and ability to quit smoking (Baker et al., 2007). Although these analyses were purely exploratory to help characterize these groups, they may have important treatment implications and provide directions for future research.
Hughes et al. (2011) also highlight another important aspect of the current study – the need to understand how those with low motivation to quit may respond to treatment. Certainly, it is important to demonstrate treatment efficacy in clinical trials, but only about 8% of the US population reports planning to quit in the next month (Boyle et al., 2000; Wewers et al., 2003). The current study adds to the relatively small literature on the effects of varenicline on non-treatment seeking smokers and provides important information regarding the generalizability of existing treatments to a broader population of smokers. In the present sample, treatment with varenicline resulted in a 23% reduction in cigarette consumption and daily smoking behavior (the product of cigarettes per day and total puff volume). This is consistent with previous work in non-treatment seeking smokers, suggesting that varenicline reduced smoking behavior by 29% compared to 17% for placebo (Hughes et al., 2011); we observed a comparable 15% reduction during the placebo phase. Furthermore, smokers who were treated with varenicline made a quit attempt sooner than those who received placebo, 17 days vs 24 days (Rennard et al., 2012) and made significantly more quit attempts in a 6-month period compared to placebo (Hughes et al., 2011). In contrast to previous studies (e.g. Hughes et al., 2011; Rennard et al., 2012), we instructed participants to follow their urges and smoke as they wished and we did not provide brief counseling or encourage smokers to consider a quit attempt. Despite this difference, we observed comparable changes in smoking behavior following treatment with varenicline. It is worth noting that we were able to identify changes in smoking behavior and urges to smoke at Day 10 and 14, respectively. Because these changes immediately precede the Day 17 quit attempt in the Rennard et al. (2012) study, collectively these results may indicate an important pattern of events at a critical time period. Thus, a longer duration of pre-treatment with varenicline may reduce smoking behavior and prompt a quit attempt among ambivalent smokers in addition to promoting abstinence among motivated smokers (Hughes et al., 2011).
In general, evidence suggests that varenicline reduces reinforcement from smoking and urges to smoke (Brandon et al., 2011; Gonzales et al., 2006) and the current data support this hypothesis. We observed decreases in urges to smoke for pleasure during the varenicline phase, but not the placebo phase, despite the fact that the overall interaction was not significant. Similar to smoking behavior, changes in urges to smoke did not emerge until well after the standard one-week run-up period (Day 14). Hughes et al. (2011) suggest that this decrease in pleasure from smoking may lead to extinction of smoking and increase confidence in the ability to quit. Therefore, we conducted an exploratory post hoc analysis which revealed that during the varenicline period, early decreases in urges to smoke for pleasure were associated with greater subsequent reductions in cigarette consumption. This relationship was not evident during the placebo phase nor was there a relationship between urges to smoke to reduce negative affect and changes in cigarettes per day in either phase.
Also consistent with our hypothesis, there were few changes in urges to smoke for withdrawal relief during either the varenicline or placebo phase. The few studies that have found that varenicline reduces urges to smoke to relieve negative affect tested smokers following at least 12 hours of abstinence (Ashare and McKee, 2012; Brandon et al., 2011). Since smokers in the current study were not trying to quit or remain abstinent, it is not surprising that we did not observe reductions in urges to smoke for withdrawal relief. Nevertheless, we were interested in examining whether specific items from QSU Factor 1 were sensitive to the effects of varenicline. An exploratory set of analyses revealed that only three items significantly decreased during the varenicline phase: `I have no desire for a cigarette right now', `I would not enjoy a cigarette right now', and `A cigarette would not be very satisfying right now.' We speculate that this cluster of items reflects a more sensitive measure of the anticipated positive reinforcement from smoking and may be one way in which varenicline promotes extinction. However, these tentative conclusions require replication in future studies.
Limitations
Several limitations of the current study warrant mention. First, attrition rates were high with only 17 of the 29 who attended the first visit completing all sessions. We considered whether the non-completers differed in potentially important ways from those who completed the study. Other than marginal differences in age and baseline CO levels, there were no other significant differences between the two groups on any other demographic or smoking characteristic. It is also important to note that the final sample consisted primarily of Caucasian males, which may limit the generalizability of the present findings. Second, although drug order was randomized across participants and accounted for in all analyses, previous work suggests that varenicline may have carryover effects (Patterson et al., 2009; Turner et al., 2011). However, in the current study, participants' cigarette consumption at Visit 1 of each phase did not vary according to treatment order. Rather, all participants reported smoking fewer cigarettes at the start of their second phase. Nevertheless, future studies can address possible treatment carryover effects by using a longer washout period or a between-subjects manipulation. Third, biomarkers (metabolites, CO) were less sensitive to the effects of varenicline than cigarette consumption. Nicotine metabolites were only assessed at three timepoints (Days 1, 7, and 21), which may have limited our ability to detect changes that corresponded with the reduction in cigarette consumption observed at Day 10. Thus, it may be useful to assess biomarkers more frequently in future work.
Conclusions
The current results have important implications for treatment. Namely, the fact that changes in CPD and urges to smoke did not occur until Days 10 and 14, respectively, suggests that some smokers may benefit from a longer pre-cessation duration of treatment in order to extinguish a complex behavior such as cigarette smoking. Furthermore, reductions in urges to smoke were related to subsequent decreases in cigarette consumption. Importantly, these changes occurred among non-treatment seeking smokers, which indicates that extended use of varenicline may reduce smoking behavior and perhaps prompt a quit attempt, even among less motivated smokers. The current data add to a growing body of literature, which advocates for developing more personalized treatment strat-egies (Patterson et al., 2008), including allowing smokers to choose their own quit date (Rennard et al., 2012). The present findings provide important information regarding the time course of varenicline's effects on smoking behavior, which may provide insight into the mechanisms by which varenicline promotes cessation.
Acknowledgements
We thank Caryn Lerman for her comments on a previous version of this manuscript.
Funding This research was supported by Global Research Award for NicotineDependence (GRAND) from Pfizer (GA30523L) and grants from the National Cancer Institute and the National Institutes on Drug Abuse at the National Institutes of Health: R01 CA120594, R01 CA130961, P30 ES013508, and P50 CA143187.
References
- Ashare RL, McKee SA. Effects of Varenicline and Bupropion on cognitive processes among nicotine-deprived smokers. Exp Clin Psychopharmaco. 2012;20:63–70. doi: 10.1037/a0025594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Bernert JT, et al. Carcinogen exposure during short-term switching from regular to `light' cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14:1376–1383. doi: 10.1158/1055-9965.EPI-04-0667. [DOI] [PubMed] [Google Scholar]
- Boyle P, Gandini S, Robertson C, et al. Characteristics of smokers' attitudes towards stopping: survey of 10,295 smokers in representative samples from 17 European countries. Eur J Public Health. 2000;10:5–14. [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology. 2011;218:391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2011;2:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha-4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. clinical practice guideline. US Department of Health and Human Service. Public Health Service; Rockville, MD: 2008. [Google Scholar]
- Franklin T, Wang Z, Suh JJ, et al. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha-4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hajek P, McRobbie HJ, Myers KE, et al. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171:770–777. doi: 10.1001/archinternmed.2011.138. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Jr, Ashare RL, Lohnes SF, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther. 2012;91:172–180. doi: 10.1038/clpt.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Rennard SI, Fingar JR, et al. Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial. Nicotine Tob Res. 2011;13:955–964. doi: 10.1093/ntr/ntr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Conditions under which mean square ratios in repeated measurements designs have exact F-distributions. J Am Stat Assoc. 1970;65:1582–1589. [Google Scholar]
- Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, et al. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol. 2011 Sep 23;:1–10. doi: 10.1017/S1461145711001398. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Stellman SD, Caraballo RS, et al. Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiol Biomarkers Prev. 2009;18:3415–3420. doi: 10.1158/1055-9965.EPI-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Schnoll R, Wileyto E, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Mercincavage M, Fonte CA, et al. Varenicline's effects on acute smoking behavior and reward and their association with subsequent abstinence. Psychopharmacology. 2010;210:45–51. doi: 10.1007/s00213-010-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangiah K, Hwang WT, Mesaros C, et al. Nicotine exposure and metabolizer phenotypes from analysis of urinary nicotine and its 15 metabolites by LC-MS. Bioanalysis. 2011;3:745–761. doi: 10.4155/bio.11.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S, Hughes J, Cinciripini PM, et al. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res. 2011;14:343–350. doi: 10.1093/ntr/ntr220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine preloading: the importance of a pre-cessation reduction in smoking behavior. Psychopharmacology. 2011;217:453–454. doi: 10.1007/s00213-011-2350-0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, et al. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:S22–S33. [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG. Nicotine patch therapy prior to quitting smoking: a meta-analysis. Addiction. 2008;103:557–563. doi: 10.1111/j.1360-0443.2008.02138.x. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and bio-chemical methods. Humana Press; Totowa, New Jersey: 1992. [Google Scholar]
- Sofuoglu M, Duffey D, Mooney ME. Varenicline increases smoking abstinence at 6 months to a year compared with placebo or bupropion; nausea is the most commonly reported adverse effect. Evid Based Med. 2011;16:113–114. doi: 10.1136/ebm1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Herman A, Mooney M, et al. Varenicline attenuates some of the subjective and physiological effects of intravenous nicotine in humans. Psychopharmacology. 2009;207:153–162. doi: 10.1007/s00213-009-1643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M. Subjective responses to intravenous nicotine: Greater sensitivity in women than in men. Exp Clin Psychopharmaco. 2009;17:63–69. doi: 10.1037/a0015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Benowitz NL, Pinto AG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20:234–238. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Lerman C, Sanborn PM, et al. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86:294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Tang KZ, Sanborn PM, et al. Behavioral filter vent blocking on the first cigarette of the day predicts which smokers of light cigarettes will increase smoke exposure from blocked vents. Exp Clin Psychopharmacol. 2009;17:405–412. doi: 10.1037/a0017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers ME, Stillman FA, Hartman AM, et al. Distribution of daily smokers by stage of change: Current Population Survey results. Prev Med. 2003;36:710–720. doi: 10.1016/s0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]