Abstract
Background
High-intensity (high-pressure and high backup rate) noninvasive ventilation has recently been advocated for the management of stable hypercapnic chronic obstructive pulmonary disease (COPD). However, the relative contributions of high inspiratory pressure and high backup rate to ventilator adherence and physiological outcome have not been investigated.
Methods
Patients with stable hypercapnic COPD (daytime PaCO2 > 6 kPa) and nocturnal hypoventilation were enrolled. Patients were randomly allocated to high-pressure and high backup rate (high-intensity) and high-pressure and low backup rate (high-pressure) for a 6-week period. At the end of the first treatment period, patients were switched to the alternative treatment. The primary outcome measure was mean nightly ventilator usage.
Results
Twelve patients were recruited, with seven completing the 12-week trial protocol. The mean patient age was 71 ± 8 years, with a forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) of 50% ± 13% and FEV1 of 32% ± 12%. The baseline PaCO2 and PaO2 were 8.6 ± 1.7 kPa and 7.3 ± 1.4 kPa, respectively. There was no significant difference demonstrated in mean nightly ventilator usage between the high-intensity and high-pressure groups (difference of 4 minutes; 95% confidence interval −45 to 53; P = 0.9). Furthermore, there were no differences in any of the secondary endpoints, with the exception of the respiratory domain of the Severe Respiratory Insufficiency questionnaire, which was lower in the high-intensity arm than in the high-pressure arm (57 ± 11 versus 69 ± 16; P < 0.05).
Conclusion
There was no additional benefit, in terms of night-time ventilator adherence or any of the other measured parameters, demonstrated by addition of a high backup rate to high-pressure noninvasive ventilation. These data suggest that it is the high-pressure component of the high-intensity noninvasive ventilation approach that plays the important therapeutic role in the management of hypercapnic respiratory failure in COPD patients.
Keywords: chronic obstructive pulmonary disease, chronic respiratory failure, noninvasive ventilation
Introduction
Although uncontrolled detailed physiological studies have shown the short-term benefits of the addition of domiciliary noninvasive ventilation (NIV) to long-term oxygen therapy, the most recent trials comparing long-term oxygen therapy alone against long-term oxygen therapy and NIV in stable hypercapnic chronic obstructive pulmonary disease (COPD) have demonstrated limited additional benefit of NIV.1–4 The ventilator setup, in particular the low level of pressure support and backup rate frequency, have been suggested as major contributors to this lack of effect in larger clinical trials. A more recent crossover study showed that pressure-controlled ventilation with both high inflation pressures and high backup rate, termed high-intensity NIV, had short-term physiological benefit compared with low-intensity NIV.5 In addition to the lack of longer-term clinical outcome data in this recent study, these data do not differentiate between the effect of high inspiratory pressures and high backup rate. Furthermore, this high-intensity NIV strategy could be physiologically challenged as the most useful approach in patients with severe airways resistance because such patients require a prolonged expiratory phase to maximize lung emptying. The high-intensity approach, with a high backup frequency, results in a shortened expiratory time and incomplete lung emptying, which would be expected to have deleterious effects on pulmonary mechanics and respiratory muscle pressure-generating capacity as a consequence of lung hyperinflation.6 In addition, it had been shown by our group and others that neural respiratory drive is elevated in these patients, suggesting that there is little requirement for a high backup rate frequency.7,8 Concerns regarding patient acceptance of this high-intensity approach have been challenged by recent work from Dreher et al,5 who reported longer mean daily ventilator use with high-intensity than low-intensity NIV, suggesting good tolerability. We hypothesized that pressure support ventilation with high inspiratory pressures and low backup rate, termed high-pressure NIV, would provide equivalent levels of nightly ventilator usage compared with high-intensity NIV with high inflation pressures and high backup rate.
Materials and methods
Study design
We used a single-blind, randomized, two-treatment crossover design, similar to that of the previous high-intensity NIV study.5 Stable hypercapnic COPD patients with daytime partial pressure of arterial carbon dioxide (PaCO2) greater than 6 kPa were randomized, via sealed envelope allocation, to high-pressure support ventilation (low backup rate) or high-pressure controlled ventilation (high backup rate) nightly for 6 weeks. Baseline measurements were reassessed at 6 weeks, and subjects were then crossed over to the other arm, with final assessment at 12 weeks. Local research ethics committee approval was obtained (09/H0802/3) for this study which is registered at http://www.clinicaltrial.gov as NCT00994552.
Patients
The study was performed in a specialist tertiary home mechanical ventilation center. All patients provided their written informed consent. Patients with chronic hypercapnic respiratory failure (daytime PaCO2 > 6.0 kPa), nocturnal hypoventilation (transcutaneous carbon dioxide > 7.5 kPa or a rise in transcutaneous carbon dioxide of >1 kPa), a clinical diagnosis of COPD (forced expiratory volume in one second/forced vital capacity [FEV1/FVC] ratio < 70% and an FEV1 < 50% predicted), and symptoms compatible with nocturnal hypoventilation referred for assessment for nocturnal NIV were consecutively enrolled. Patients were all naïve to domiciliary NIV. All patients were established on optimal medical therapy prior to enrolment. Patients were excluded if they had underlying malignancy, severe cardiac dysfunction (left ventricular ejection fraction < 40%), or obstructive sleep apnea syndrome (based on limited attended respiratory polygraphy data9).
Measurements
Limited attended respiratory polygraphy with continuous oximetry, transcutaneous carbon dioxide (Tosca 500, Linde Medical Sensors, Basel, Switzerland), and chest and abdominal plethysmography were used. Objective sleep quality was assessed by 2 weeks of nocturnal actigraphy (Actiwatch AW4, CamNTech, Cambridge, UK) prior to each assessment. Health-related quality of life was measured using the Severe Respiratory Insufficiency questionnaire.10 Arterial blood gases were taken at least 4 hours after waking and whilst sitting comfortably on room air. Ventilator download data were used to calculate mean nightly ventilator usage, respiratory rate, and patient triggering rate.
Ventilator setup
NIPPY3 and NIPPY3+ ventilators (B&D ElectroMedical, Warwickshire, UK) were used for the duration of the study. Nasal and nasal-mouth masks were used according to patient comfort. In the pressure support ventilation mode, the backup rate frequency was set to the minimum setting of six breaths per minute. In the pressure-controlled ventilation mode, the backup rate frequency was set at two below the resting respiratory rate, with the patient breathing quietly at rest. The inspiratory and expiratory triggers were set at the standard factory settings and adjusted to patient comfort. The inspiratory time, applicable to the pressure-controlled ventilation mode, was set at 30% of the duty cycle, but this was modified according to patient tolerance. Oxygen therapy was entrained at a flow rate of 1–2 liters per minute in order to maintain oxygen saturations ≥ 88%. Other ventilator settings were achieved using an overnight titration protocol in keeping with local clinical practice (Figure E1, online data supplement) on the first night of ventilator usage. At treatment crossover, only the ventilator backup rate was adjusted, with other ventilator parameters remaining unchanged.
Data analysis and statistics
A sample size calculation was performed using the primary outcome, ie, NIV compliance, assuming a standard deviation of 90 minutes and a between-group difference of 90 minutes. To demonstrate a between-group difference of ≥90 minutes in a crossover design, with a power of 0.8 and a level of significance of 0.05, ten patients were required to be recruited and complete the trial. Allowing for a 20% dropout or noncompliance rate, a target of twelve patients was set. Secondary outcomes were arterial blood gas analysis, health-related quality of life, and actigraphy-assessed sleep parameters. Data were analyzed using paired t-tests, or when appropriate the nonparametric equivalent. Data are presented as the mean ± standard deviation if normally distributed or median (range) if not normally distributed. For all analyses, a P value <0.05 was considered to be statistically significant. Data analyses were conducted using the IBM Statistical Package for Social Sciences version 19 (IBM, Armonk, NY).
Results
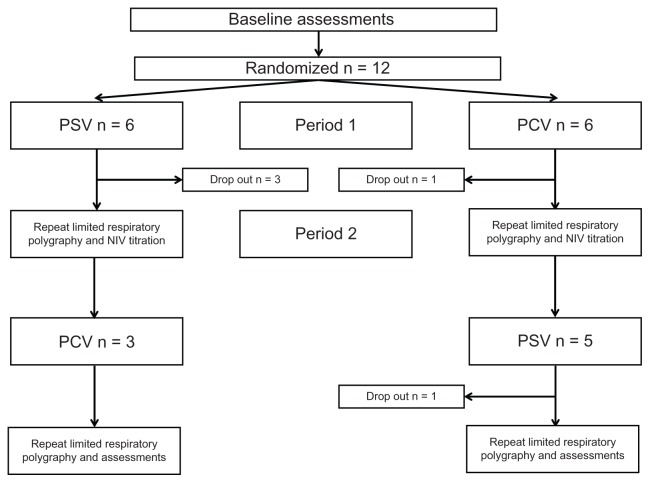
Twelve patients were recruited and seven patients completed the study, with equal numbers starting on pressure support ventilation and pressure-controlled ventilation. The mean age was 71 ± 8 years at enrolment for study completers. Baseline spirometry showed an FEV1 of 32% ± 12% with an FEV1/FVC 50% ± 13%. Baseline PaCO2 and PaO2 were 8.5 ± 1.8 kPa and 7.3 ± 1.5 kPa, respectively. Health-related quality of life, as measured by the Severe Respiratory Insufficiency questionnaire summary scale, was 52 ± 9 at baseline. Five patients withdrew from the study during the trial period (Figure 1). Baseline characteristics and reasons for study withdrawal are provide in the online data supplement (Table E1). There were no significant differences between those patients who completed and withdrew from the trial in terms of age, gender, anthropometrics, gas exchange, spirometry, or ventilator settings, with the exception of FVC (46% completers versus 69% withdrawers, P = 0.015).
Figure 1.
Consort recruitment and retention diagram.
Abbreviations: PCV, pressure-controlled (high-intensity) ventilation; PSV, pressure support (high pressure) ventilation; NIV, noninvasive ventilation.
Mean inspiratory positive airway pressure was 29 ± 2 cm H2O and mean expiratory positive airway pressure was 5 ± 3 cm H2O. There were no differences observed between the high-intensity and high-pressure groups in the primary outcome, ie, mean nocturnal ventilator usage (Table 1, Figure E2), or in the secondary outcomes, ie, gas exchange (Table 1, Figure E3), objective sleep quality (Table 2), and subjective sleep quality (Table 3). There were no between-group differences in health-related quality of life (Table 4, Figure E4), with the exception of the respiratory domain of the Severe Respiratory Insufficiency questionnaire (57 ± 11 versus 69 ± 16; P = 0.017). As expected by the trial design, the setup backup rate frequency (16 ± 2 versus 6 ± 0; P < 0.001) and breath frequency (16 ± 2 versus 14 ± 3; P = 0.002) were higher in the high-intensity group.
Table 1.
Respiratory and ventilator parameters following 6 weeks of high-pressure and high-intensity noninvasive ventilation
| High pressure NIV (PSV) | High intensity NIV (PCV) | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Mean nightly use (hours:minutes) | 6:37 ± 1:45 | 6:33 ± 2:14 | 0:04 (−0:45 to 0:53) | 0.851 |
| pH | 7.38 ± 0.01 | 7.38 ± 0.02 | 0.00 (−0.01 to 0.01) | 0.818 |
| PaCO2 (kPa) | 7.2 ± 0.8 | 7.0 ± 0.8 | 0.3 (−0.2 to 0.7) | 0.190 |
| PaO2 (kPa) | 7.9 ± 1.5 | 8.2 ± 1.3 | −0.3 (−0.7 to 0.03) | 0.070 |
| Serum bicarbonate (mmol/L) | 31 ± 4 | 30 ± 4 | 1 (−0.1 to 2.1) | 0.071 |
| Overnight mean tcCO2 (kPa) | 6.5 ± 1.2 | 6.5 ± 1.3 | −0.1 (−0.9 to 0.9) | 0.985 |
| Patient triggering rate (%) | 90 ± 9 | 25 ± 11 | 65 (51 to 79) | <0.001 |
| Bf (bpm) on ventilator | 14 ± 3 | 16 ± 2 | −2.6 (−1.4 to −3.7) | 0.002 |
Abbreviations: Bf, breathing frequency; CI, confidence interval; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; NIV, noninvasive ventilation; PSV, pressure support ventilation; PCV, pressure-controlled ventilation; tcCO2, transcutaneous carbon dioxide.
Table 2.
Sleep actigraphy following 6 weeks of high-pressure and high-intensity noninvasive ventilation
| High-pressure NIV (PSV) | High-intensity NIV (PCV) | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Actual sleep time (min) | 345 ± 91 | 329 ± 120 | 16 (−49 to 82) | 0.571 |
| Actual wake time (min) | 69 ± 35 | 83 ± 43 | −14 (−40 to 11) | 0.225 |
| Sleep efficiency (%) | 77 ± 12 | 73 ± 18 | 4 (−8 to 16) | 0.484 |
| Sleep latency (min) | 20 ± 16 | 21 ± 16 | −1 (−18 to 15) | 0.835 |
Abbreviations: CI, confidence interval; PSV, pressure support ventilation; PCV, pressure controlled ventilation; NIV, noninvasive ventilation.
Table 3.
Self-reported sleep comfort and sleep quality evaluated following 6 weeks of high-pressure and high-intensity noninvasive ventilation
| High-pressure NIV (PSV) | High-intensity NIV (PCV) | Mean difference | P value (95% CI) | |
|---|---|---|---|---|
| Sleep comfort VAS (mm) | 57 ± 29 | 58 ± 29 | −1 (−40 to 37) | 0.944 |
| Sleep quality (0–5 scale) | 3.3 ± 0.3 | 3.2 ± 0.6 | 0.1 (−0.3 to 0.7) | 0.638 |
Notes: Sleep comfort VAS: 0–100 mm (0, least comfortable; 100, most comfortable). Sleep quality: 0 to 5 (0, least quality; 5, best quality).
Abbreviations: CI, confidence interval; VAS, visual analog scale; PSV, pressure support ventilation; PCV, pressure-controlled ventilation; NIV, noninvasive ventilation.
Table 4.
Severe Respiratory Insufficiency questionnaire following 6 weeks of high-pressure and high-intensity noninvasive ventilation
| High pressure NIV (PSV) |
High intensity NIV (PCV) |
Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Respiratory | 69 ± 16 | 57 ± 11 | 12 (3 to 21) | 0.017 |
| Physical | 57 ± 27 | 60 ± 29 | −3 (−19 to 13) | 0.668 |
| Attendant symptoms and sleep | 56 ± 10 | 51 ± 11 | 5 (−1 to 11) | 0.070 |
| Social relationships | 70 ± 14 | 76 ± 12 | −6 (−19 to 7) | 0.297 |
| Anxiety | 74 ± 22 | 78 ± 23 | −4 (−18 to 10) | 0.557 |
| Psychological well-being | 72 ± 18 | 70 ± 24 | 2 (−11 to 16) | 0.679 |
| Social functioning | 72 ± 22 | 70 ± 24 | 2 (−2 to 7) | 0.283 |
| Summary scale | 67 ± 12 | 66 ± 16 | 1 (−4 to 7) | 0.571 |
Abbreviations: CI, confidence interval; PSV, pressure support ventilation; PCV, pressure controlled ventilation; NIV, noninvasive ventilation.
Discussion
This small, randomized, crossover trial could not demonstrate a difference in ventilator adherence between high-intensity and high-pressure NIV in the short-term treatment of stable chronic respiratory failure secondary to COPD. Furthermore, there was no clinical or physiological superiority demonstrated by high-intensity compared with high-pressure NIV. These data challenge the view that high-intensity NIV is required to achieve both physiological and clinical improvement in stable hypercapnic COPD, and suggest that high-pressure NIV may be equally effective.
Critique of the method
This is a small, randomized, crossover trial that was subject to a higher than expected dropout rate, as a result of which our recruitment did not match our sample size calculation. Although higher than anticipated at study inception, the dropout rate was comparable with that of similar studies in the literature.5,11 Despite this caveat, the mean difference in ventilator usage between the groups was only 4 minutes, with a standard deviation of 53 minutes, so it is highly unlikely that addition of three more patients would have changed the lack of difference in the primary outcome.
The study was designed to allow direct comparison with previous studies5 and, as such, a relatively short assessment duration was used without a between-treatment washout period. A carryover effect cannot be excluded and this may have contributed to the failure to detect a significant between-group difference in mean daily ventilator usage. However, this same study design when employed in the previous study showed a significant difference in daily ventilator usage, which was the predefined primary outcome of this study.5 Therefore, these data suggest that there is not a significant carryover effect of the treatment on outcome.
Efficacy of ventilation
Similar levels of compliance were achieved in the high-intensity and high-pressure NIV groups. The current data differ from those previously reported by Dreher et al5 in that mean nightly ventilator usage was lower in the current study. However, these values are similar to those normally seen at our own center in both clinical practice and randomized controlled trials, and may reflect differences in patient population and referral patterns, as well as in health care systems.6,12,13 The purported mechanism for the difference in compliance in the previous study, albeit lacking supportive data, was that the enhanced nocturnal control of gas exchange provided by high-intensity NIV resulted in enhanced subjective clinical improvement and thus promoted greater patient adherence with ventilator usage. The current study employed high-pressure NIV in both arms, and despite the differences in the backup rate, there was a similar degree of control of nocturnal carbon dioxide in both arms. These data support the theory that it is the driving pressure during NIV that is the predominant factor modifying the carbon dioxide level rather than breath frequency. The most recent study by Dreher et al supports this concept.11 These investigators showed that lower pressure support, but without a change in breath frequency, resulted in a rise in the nocturnal transcutaneous carbon dioxide level.
Effect of ventilatory mode on sleep quality
There were no differences observed between high-pressure and high-intensity NIV in either objective or subjective measures of sleep quality and quantity. Although the authors acknowledge that the current study lacks the sleep staging data provided by combined electroencephalography and polysomnography, they would like to highlight the benefits of sleep actigraphy monitoring over polysomnography data. Sleep actigraphy provides a measure of domiciliary sleep parameters, which could not be achieved with single-night inhospital polysomnography. As would be expected, given the ease and comfort of undertaking sleep actigraphy in the home setting, the mean total sleep time in the current data was longer than that recorded in single-night polysomnography studies, but with similar levels of sleep efficiency.11 Furthermore, such data support the concept that high-pressure and high-intensity NIV do not adversely affect sleep quality. Indeed, both high-pressure and high-intensity NIV are accepted by patients, as indicated by high levels of daily ventilator compliance.
Health-related quality of life improvements
There were similar improvements in health-related quality of life achieved using both high-intensity and high-pressure NIV, in line with other published data.5 However, in contrast with previous data, patients completing the high-pressure NIV treatment period had greater improvement in the respiratory complaints subdomain of the Severe Respiratory Insufficiency questionnaire than when completing the high-intensity treatment period. A possible explanation for this difference could be greater patient-ventilator asynchrony using the high-intensity NIV approach, given that there are previous data acknowledging greater patient-ventilator asynchrony during pressure-controlled ventilation.14
Implications for clinical trials to assess effectiveness of NIV in COPD
The role of NIV in addition to long-term oxygen therapy in enhancing the outcome for patients with severe COPD and chronic respiratory failure is still a focus of research. These patients have a very poor prognosis and currently we have little to offer them in terms of clinical management beyond standard inhaled therapies and long-term oxygen therapy. There are in progress a number of clinical trials in Europe that have been designed to assess the role of addition of domiciliary NIV to long-term oxygen therapy in patients with severe COPD. The data from the current study show that high-pressure NIV is similar to high-intensity NIV in terms of the short-term physiological outcome and clinical benefit. Both these approaches optimize the driving pressure to reduce hypercapnia and improve health-related quality of life without impairing sleep quality. Data from the current study have directly informed UK HoT-HMV (United Kingdom Home Oxygen Therapy vs Home Mechanical Ventilation in COPD), which is a multicenter, randomized, controlled trial of domiciliary NIV and home oxygen therapy against home oxygen therapy alone for the management of patients who remain persistently hypercapnic following a severe acute exacerbation of COPD (UKCRN 8059).
Conclusion
In conclusion, these data add further support for the use of a high-pressure strategy for domiciliary NIV in patients with severe stable COPD and chronic respiratory failure. Furthermore, we suggest that the use of a high backup rate may not be necessary to achieve clinical or physiological improvements in such patients. Data from ongoing, multicenter, randomized, controlled trials in Europe will further inform our management of these complex patients.
Ventilator setup protocol used at initiation of ventilation in either high-intensity or high-pressure arms.
Abbreviations: EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure; NIV; noninvasive ventilation.
Individual datum points for mean nightly ventilator usage during each 6-week trial period of high-intensity or high-pressure noninvasive ventilation.
Note: Data downloaded from ventilators using Bespoke® software (B&D ElectroMedical, Warwickshire, UK).
Individual datum points for arterial partial pressure of carbon dioxide (PaCO2) following a 6-week trial period in both the high-intensity and high-pressure arms.
Individual datum points for health-related quality of life as measured by the Severe Respiratory Insufficiency questionnaire summary scale (A) and Severe Respiratory Insufficiency respiratory complaints domain (B) following 6-week trial period in the high-intensity or high-pressure arms.
Abbreviations: SRI, Severe Respiratory Insufficiecy.
Table E1.
Individual baseline characteristics for all recruited patients
| Age (years) | Gender | FEV1 (l/%) | FVC (l/%) | FEV1/ FVC (%) | PaCO2 (kPa) | PaO2 (kPa) | Starting mode | Outcome | IPAP | EPAP | BUR (PCV) | BUR (PSV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 71 | M | 0.55/20 | 1.23/35 | 45 | 12.2 | 8.6 | PSV | Completed | 28 | 3 | 16 | 6 |
| 67 | M | 1.49/50 | 2.22/58 | 67 | 8.3 | 6.7 | PSV | Completed | 26 | 5 | 16 | 6 |
| 85 | F | 0.57/29 | 1.15/48 | 49 | 7.6 | 9.3 | PCV | Completed | 28 | 3 | 12 | 6 |
| 75 | M | 0.83/28 | 1.90/49 | 44 | 7.3 | 6.9 | PCV | Completed | 30 | 5 | 18 | 6 |
| 61 | F | 0.42/17 | 1.19/40 | 35 | 8.4 | 6.5 | PCV | Completed | 30 | 3 | 18 | 6 |
| 72 | M | 0.70/40 | 1.70/46 | 41 | 9.3 | 4.9 | PSV | Completed | 30 | 12 | 18 | 6 |
| 63 | M | 1.10/39 | 1.64/46 | 67 | 6.7 | 8.2 | PSV | Completed | 30 | 6 | 16 | 6 |
| 73 | F | 0.51/31 | 1.92/95 | 27 | 8.1 | 6.3 | PSV | Withdrew, claustrophobia | 20 | 5 | 6 | |
| 74 | M | 0.86/34 | 2.05/62 | 42 | 5.9 | 6.6 | PCV | Withdrew, acute exacerbation | 22 | 3 | 14 | 6 |
| 87 | M | 1.24/50 | 2.09/63 | 59 | 7.3 | 8.5 | PSV | Withdrew , intolerant of therapy | 28 | 3 | 6 | |
| 69 | M | 0.64/32 | 1.94/80 | 33 | 10.5 | 6.8 | PSV | Withdrew, intolerant of therapy | ||||
| 79 | F | 0.71/38 | 1.05/46 | 68 | 9.6 | 8.7 | PCV | Withdrew, prolonged central sleep apneas on PSV and withdrawn by clinical team | 28 | 6 | 12 | 6 |
Abbreviations: M, male; F, female; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; PSV, pressure support (high-pressure) ventilation; PCV, pressure-controlled (high-intensity) ventilation; IPAP, inspiratory positive airway pressure; EPAP, expiratory positive airway pressure; BUR, backup rate.
Acknowledgments
These data are published on behalf of the UK HoT-HMV Trial (UKCRN Trial 8059) group. The authors acknowledge the financial support of the Department of Health via the National Institute for Health Research Comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The study was supported in part by the National Institute for Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London, which partially fund MIP’s salary.
Footnotes
Disclosure
The Lane Fox Clinical Respiratory Physiology Group has received unrestricted research grants from: ResMed, Abingdon, Oxfordshire, UK; Philips-Respironics, Murrysville, PA; Fisher and Paykel Healthcare, Auckland, New Zealand; and B&D ElectroMedical, Stratford-upon-Avon, Warwickshire, UK. PM has received expenses for travel to conferences from Philips-Respironics. ACD has received fees for consultancy from Smiths Medical, Ashford, Kent, UK. NH has received fees for lecturing from Philips-Respironics and Fisher and Paykel. MIP has received fees for lecturing from Philips-Respironics. This study was supported by B&D ElectroMedical. Neither the study design, results, interpretation of the findings nor any other subject discussed in the submitted manuscript was dependent on support.
References
- 1.Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med. 1995;152:538–544. doi: 10.1164/ajrccm.152.2.7633704. [DOI] [PubMed] [Google Scholar]
- 2.Elliott MW, Mulvey DA, Moxham J, Green M, Branthwaite MA. Domiciliary nocturnal nasal intermittent positive pressure ventilation in COPD: mechanisms underlying changes in arterial blood gas tensions. Eur Respir J. 1991;4:1044–1052. [PubMed] [Google Scholar]
- 3.Clini E, Sturani C, Rossi A, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20:529–538. doi: 10.1183/09031936.02.02162001. [DOI] [PubMed] [Google Scholar]
- 4.McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64:561–566. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 5.Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax. 2010;65:303–308. doi: 10.1136/thx.2009.124263. [DOI] [PubMed] [Google Scholar]
- 6.Nickol AH, Hart N, Hopkinson NS, et al. Mechanisms of improvement of respiratory failure in patients with COPD treated with NIV. Int J Chron Obstruct Pulmon Dis. 2008;3:453–462. doi: 10.2147/copd.s2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy PB, Kumar A, Reilly C, et al. Neural respiratory drive as a physiological biomarker to monitor change during acute exacerbations of COPD. Thorax. 2011;66:602–608. doi: 10.1136/thx.2010.151332. [DOI] [PubMed] [Google Scholar]
- 8.Jolley CJ, Luo YM, Steier J, et al. Neural respiratory drive in healthy subjects and in COPD. Eur Respir J. 2009;33:289–297. doi: 10.1183/09031936.00093408. [DOI] [PubMed] [Google Scholar]
- 9.Scottish Intercollegiate Guidelines Network. Management of obstructive sleep apnoea/hypopnoea syndrome in adults. 2003. [Accessed October 21, 2012]. Available from: http://www.sign.ac.uk/pdf/sign73.pdf.
- 10.Ghosh D, Rzehak P, Elliott MW, Windisch W. Validation of the english severe respiratory insufficiency questionnaire. Eur Respir J. 2012;40:408–415. doi: 10.1183/09031936.00152411. [DOI] [PubMed] [Google Scholar]
- 11.Dreher M, Ekkernkamp E, Walterspacher S, et al. Noninvasive ventilation in COPD: impact of inspiratory pressure levels on sleep quality. Chest. 2011;140:939–945. doi: 10.1378/chest.11-0253. [DOI] [PubMed] [Google Scholar]
- 12.Murphy PB, Davidson C, Hind MD, et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax. 2012;67:727–734. doi: 10.1136/thoraxjnl-2011-201081. [DOI] [PubMed] [Google Scholar]
- 13.Mackie N, Weston N, Grey N, Davidson AC, Hart N. Two years mortality data of UK patients established on home mechanical ventilation (HMV): non-invasive ventilation cohort. Paper presented at the 13th International Conference on Home Mechanical Ventilation; Barcelona, Spain. March 15–17, 2012. [Google Scholar]
- 14.Adler D, Perrig S, Takahashi H, et al. Polysomnography in stable COPD under non-invasive ventilation to reduce patient-ventilator asynchrony and morning breathlessness. Sleep Breath. doi: 10.1007/s11325-011-0605-y. Published Online First: 4 November 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ventilator setup protocol used at initiation of ventilation in either high-intensity or high-pressure arms.
Abbreviations: EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure; NIV; noninvasive ventilation.
Individual datum points for mean nightly ventilator usage during each 6-week trial period of high-intensity or high-pressure noninvasive ventilation.
Note: Data downloaded from ventilators using Bespoke® software (B&D ElectroMedical, Warwickshire, UK).
Individual datum points for arterial partial pressure of carbon dioxide (PaCO2) following a 6-week trial period in both the high-intensity and high-pressure arms.
Individual datum points for health-related quality of life as measured by the Severe Respiratory Insufficiency questionnaire summary scale (A) and Severe Respiratory Insufficiency respiratory complaints domain (B) following 6-week trial period in the high-intensity or high-pressure arms.
Abbreviations: SRI, Severe Respiratory Insufficiecy.
Table E1.
Individual baseline characteristics for all recruited patients
| Age (years) | Gender | FEV1 (l/%) | FVC (l/%) | FEV1/ FVC (%) | PaCO2 (kPa) | PaO2 (kPa) | Starting mode | Outcome | IPAP | EPAP | BUR (PCV) | BUR (PSV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 71 | M | 0.55/20 | 1.23/35 | 45 | 12.2 | 8.6 | PSV | Completed | 28 | 3 | 16 | 6 |
| 67 | M | 1.49/50 | 2.22/58 | 67 | 8.3 | 6.7 | PSV | Completed | 26 | 5 | 16 | 6 |
| 85 | F | 0.57/29 | 1.15/48 | 49 | 7.6 | 9.3 | PCV | Completed | 28 | 3 | 12 | 6 |
| 75 | M | 0.83/28 | 1.90/49 | 44 | 7.3 | 6.9 | PCV | Completed | 30 | 5 | 18 | 6 |
| 61 | F | 0.42/17 | 1.19/40 | 35 | 8.4 | 6.5 | PCV | Completed | 30 | 3 | 18 | 6 |
| 72 | M | 0.70/40 | 1.70/46 | 41 | 9.3 | 4.9 | PSV | Completed | 30 | 12 | 18 | 6 |
| 63 | M | 1.10/39 | 1.64/46 | 67 | 6.7 | 8.2 | PSV | Completed | 30 | 6 | 16 | 6 |
| 73 | F | 0.51/31 | 1.92/95 | 27 | 8.1 | 6.3 | PSV | Withdrew, claustrophobia | 20 | 5 | 6 | |
| 74 | M | 0.86/34 | 2.05/62 | 42 | 5.9 | 6.6 | PCV | Withdrew, acute exacerbation | 22 | 3 | 14 | 6 |
| 87 | M | 1.24/50 | 2.09/63 | 59 | 7.3 | 8.5 | PSV | Withdrew , intolerant of therapy | 28 | 3 | 6 | |
| 69 | M | 0.64/32 | 1.94/80 | 33 | 10.5 | 6.8 | PSV | Withdrew, intolerant of therapy | ||||
| 79 | F | 0.71/38 | 1.05/46 | 68 | 9.6 | 8.7 | PCV | Withdrew, prolonged central sleep apneas on PSV and withdrawn by clinical team | 28 | 6 | 12 | 6 |
Abbreviations: M, male; F, female; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; PSV, pressure support (high-pressure) ventilation; PCV, pressure-controlled (high-intensity) ventilation; IPAP, inspiratory positive airway pressure; EPAP, expiratory positive airway pressure; BUR, backup rate.