Abstract
Background
The aim of this study was to determine the characteristics of drug adherence in antidepressant-treated versus antidepressant-naïve patients using Drug Attitude Inventory (DAI)-10 scores for nonadherence, to examine the contribution of patient variables such as age, gender, education, prescription contents, side effects, and type of depression (melancholic, nonmelancholic, bipolar) to the reported DAI-10 score, and to examine the efficacy of pharmacist adherence instruction on adherence with antidepressant therapy.
Methods
The subjects were 71 antidepressant-treated inpatients (17 with melancholic depression, 35 with nonmelancholic depression, and 19 with bipolar depression) and 80 antidepressant-naïve inpatients. In the antidepressant-treated patients, self-management of drug intake and pharmacist adherence instruction was initiated after depressive symptoms were in remission, and pharmacist adherence instruction was conducted until the day of discharge.
Results
There were no significant differences in baseline characteristics between antidepressant-naïve and antidepressant-treated patients. In antidepressant-treated patients, the mean DAI-10 total score was significantly lower and awareness of side effects was significantly higher than in antidepressant-naïve patients who have never taken antidepressants, nor been referred to psychiatry services (according to pharmacist interviews and medical records). On the first day of self-management of drug intake, the DAI-10 total score in patients with melancholic and bipolar depression was significantly lower than that in patients with nonmelancholic depression. On the day of discharge, there was a significant improvement of DAI-10 total score in all antidepressant-treated patients, and the DAI-10 total score in patients with melancholic depression was significantly lower than that in patients with nonmelancholic depression. The limitation of the study was the small sample size and the fact that we followed only acute phase inpatients. However, the findings seem particularly robust in view of this.
Conclusion
Risk factors for nonadherence included side effects of antidepressant treatment and type of depression. The results presented here suggest that patients with melancholic depression may be more vulnerable to nonadherence, and that pharmacist adherence instruction may improve nonadherence in antidepressant-treated patients according to type of depression.
Keywords: adherence, antidepressants, pharmacist
Introduction
Depressive disorders increase the risk of failure at school, and rate of divorce, unemployment, suicide, and mortality.1 Recurrence of an episode of depression occurs in 85% of patients diagnosed with unipolar depression.2,3 Recurrence of depression, in turn, increases the likelihood that future episodes will be more severe, more frequent, and more difficult to treat.4 Antidepressant treatment reduces depressive symptoms, but premature discontinuation increases the risk of relapse. Therefore, it is widely believed that drug adherence is required to maintain the therapeutic benefits of antidepressant therapy. Many patients recognize the necessity of continued antidepressant treatment,5 but a significant percentage of individuals diagnosed with major mood disorders are nonetheless nonadherent.6 Previous reports have discussed the involvement of antidepressant nonadherence and factors such as gender, age, and side effects,1,7–11 but reports vary, with little agreement.
Over past decades, a considerable number of studies related to drug adherence have been conducted using the Drug Attitude Inventory (DAI)-10 (Table 1).12 The DAI-10 is a self-reported scale developed to measure subjective responses and attitudes of patients towards treatment, and is useful in clinical practice to assist in optimizing treatment. The DAI-10 has been used for evaluation of drug adherence in patients taking primarily antipsychotics, with few accounts of drug adherence in patients treated with antidepressants.13,14 In the psychiatric unit, this instrument is often utilized in pharmacist adherence instruction. The role of pharmacists in the medical field has grown in patient education for depressive patients,15 and pharmacist adherence instruction has proved to be beneficial for improving treatment outcomes in health care settings.16 However, the efficacy of pharmacist adherence instruction for improvement of antidepressant nonadherence is still limited.17 In summary, unanswered questions remain in the literature concerning risk factors for antidepressant nonadherence, and the impact of the DAI-10 and pharmacist adherence instruction on drug adherence improvement in antidepressant-treated patients.
Table 1.
Drug Attitude Inventory-10
|
The aim of this study was to determine the characteristics of drug adherence in antidepressant-treated versus antidepressant-naïve patients using the DAI-10 score of nonadherence, to examine the contribution of patient variables such as age, gender, education, contents of the prescriptions, side effects, and type of depression (melancholic, nonmelancholic, bipolar) to the reported DAI-10 score, and to examine the efficacy of pharmacist adherence instruction on antidepressant adherence.
Materials and methods
Patients and study description
This study was conducted at the Akita University Hospital. The study protocol was approved by the ethics committee of Akita University School of Medicine, and written informed patient consent was obtained prior to participation in the study. The study was a follow-up of subjects who were deemed suitable for self-management of drug intake, and was designed to assess the contribution of patient variables, such as drug side effects, contents of the prescription, and type of depression (specific diagnosis) on antidepressant adherence. We divided antidepressant-treated patients into three groups according to type of depression, ie, 17 with melancholic depression, 35 with nonmelancholic depression, and 19 with bipolar depression. Eighty antidepressant-naïve subjects (who have never taken antidepressants, nor been referred to psychiatry services (according to pharmacist interviews and medical records)) were matched by age, gender, and years of formal education, and were hospitalized in nonpsychiatric wards. No limitation was set regarding type of antidepressant therapy used in this study. A doctor determined whether patients were suitable for self-management of drug intake after depressive symptoms were in remission, and pharmacist adherence instruction was initiated. Pharmacist adherence instruction was conducted once per week and continued until the day of discharge. Drug intake was carefully monitored by a nurse. The pharmacist monitored the patients for potential side effects and efficacy of drug treatment and also gave instructions on the necessity for drug therapy. Pharmacist adherence instruction was done by the same pharmacist using a short information leaflet covering drug effects, side effects, and drug signature as supplementary material for discussion. Illness characteristics (type of depression, history of drug intake, and number of prior hospitalizations) were obtained from clinical interviews and medical records. All patients were given routine clinical care and hospitalized throughout the study. The DAI-10 was assessed on the first day of pharmacist adherence instruction and on the day of discharge. Antidepressant doses were converted to equivalent doses of imipramine.
We defined patients who have never taken an antidepressant as antidepressant-naïve. In antidepressant-naïve patients, selection for self-management of drug intake was determined by the doctor and contingent upon the absence of a history of antidepressant treatment. The pharmacist monitored the drug content of treatment, drug side effects, and drug adherence by DAI-10 scoring.
Measures
Side effects
The occurrence of side effects was determined via regularly scheduled evaluations and changes in vital signs. Patients were asked at each pharmacist adherence instruction session whether they noticed any changes in how they were feeling, or if they had had any uncomfortable experiences before starting self-management of drug intake. In addition, physical signs, including changes in vital signs, were considered potential side effects. As part of the informed consent process, patients were advised of the side effects most frequently occurring on antidepressant therapy.
Drug adherence
The DAI-10 scale was used to measure each patient’s subjective responses to antidepressant treatment. This self-reported scale has 10 items that the patient scores as either “true” or “false”. A response of “true” is considered a positive subjective response for six items (eg, “By staying on medications, I can prevent getting sick”). A response of “false” is considered a positive subjective response for the other four items (eg, “I feel weird, like a “zombie”, on medication”). A positive total score indicates a positive subjective response and attitude, and a negative total score indicates a negative subjective response and attitude.
Statistical analysis
The clinical characteristics of patients and the DAI-10 total score for each group were expressed as a median value (quartile 1 to quartile 3). The DAI-10 total score between antidepressant-naïve and antidepressant-treated patients was compared using the Mann–Whitney test. DAI-10 total scores for the three types of depression (melancholic, nonmelancholic, bipolar) were compared using the Kruskal–Wallis test followed by the Mann–Whitney test. The scores were analyzed for statistically significant differences using Bonferroni’s correction. The Wilcoxon signed-rank test was used to assess the alteration in DAI-10 total score. A P value < 0.05 was considered to be statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 19.0 for Windows (SPSS IBM Japan Inc, Tokyo, Japan).
Results
Patient profile
The characteristics of the patients are shown in Table 2. There were no significant differences in age, gender, and years of formal education between patients according to type of depression (melancholic, nonmelancholic, or bipolar).
Table 2.
Baseline characteristics of patients
| Variables | AD-naïve | Overall | NMD | MD | BD |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| n = 80 | n = 71 | n = 35 | n = 17 | n = 19 | |
| Age (years) | 59 (47–65) | 53 (43–53) | 53 (45–57) | 56 (52–63) | 47 (45–55) |
| Gender (n, %) | |||||
| Female | 42 | 40 | 19 | 10 | 11 |
| Male | 38 | 31 | 16 | 7 | 8 |
| Education (years) | 14 (12–26) | 14 (12–26) | 14 (12–26) | 14 (12–26) | 12 (12–26) |
| Total treated drugs (n) | |||||
| ADs | 0 | 122 | 71 | 25 | 26 |
| SSRI | 0 | 35 | 21 | 7 | 7 |
| SNRI | 0 | 27 | 13 | 7 | 7 |
| NaSSA | 0 | 32 | 22 | 5 | 5 |
| TCA | 0 | 9 | 4 | 3 | 2 |
| Others | 0 | 18 | 12 | 3 | 3 |
| Mood stabilizer | 0 | 27 | 10 | 6 | 11 |
| Antipsychotics | 0 | 22 | 8 | 3 | 11 |
| Benzodiazepines | 34 | 79 | 36 | 24 | 19 |
| Dose of ADs (mg/day) imipramine equivalents | 0 | 150 (125–175) | 150 (125–175) | 150 (125–175) | 125 (100–150)* |
| Median number of treated agents per one patient | |||||
| ADs | 0 | 2 (1–2) | 2 (1–2) | 1 (1–2)** | 1 (1–2)** |
| Mood stabilizer | 0 | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (0–1) |
| Antipsychotics | 0 | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (0–1)* |
| Benzodiazepines | 0 (0–1) | 1 (1–1) | 1 (1–1) | 1 (1–2) | 1 (1–1) |
| Period from hospitalization to direction by pharmacist (days) | 15 (11–21) | 17 (11–21) | 14 (12–19) | 16 (12–21) | |
| Number of conducted PAIs (n) | 4 (1–5) | 4 (1–5) | 4 (2–5) | 4 (2–5) | |
Notes: Values are presented as median [quartile 1 to quartile 3];
P < 0.017,
P < 0.003 versus patients with nonmelancholic depression (compared using the Kruskal–Wallis test followed by the Mann–Whitney test, and analyzed for statistical differences using Bonferroni’s correction).
Abbreviations: Overall, overall antidepressant-treated patients; MD, melancholic depression; NMD, nonmelancholic depression; BD, bipolar depression; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin noradrenaline reuptake inhibitor; NaSSA, noradrenergic and specific serotonergic antidepressant; TCA, tricyclic antidepressants; AD, antidepressants.
Profile of treated agents
As shown in Table 2, no antidepressants, mood stabilizers, or antipsychotics was prescribed for antidepressant-naïve patients, and 34 patients took benzodiazepine at baseline. Antidepressant treatment included selective serotonin reuptake inhibitors (n = 35), serotonin noradrenaline reuptake inhibitors (n = 27), noradrenergic and specific serotonergic antidepressants (n = 32), tricyclic antidepressants (n = 9), and other types of antidepressants (n = 18). Patient were treated with mood stabilizers (n = 27), antipsychotics (n = 22), or benzodiazepines (n = 79) at baseline.
The imipramine equivalent dose in patients with bipolar depression (125 mg/day) was significantly lower than that prescribed for other patients (150 mg/day, respectively, P < 0.017). There were no other significant differences found between patients in the groups according to whether they had melancholic, nonmelancholic, or bipolar depression. In patients with melancholic or bipolar depression, the median number of antidepressants per patient was 1 (1–2), which was significantly lower than that reported in patients with nonmelancholic depression [2 (1–2), P < 0.003]. In patients with bipolar depression, the median number of antipsychotics per patient [1 (0–1)] was significantly higher than for those with melancholic or nonmelancholic depression [0 (0–1), P < 0.017].
Pharmacist adherence instruction
In patients with melancholic, nonmelancholic, or bipolar depression, the time line from hospitalization to direction by a pharmacist was as follows: 14 (12–19) days in patients with melancholic depression, 7 (11–21) days in patients with nonmelancholic depression, and 16 (12–21) days in patients with bipolar depression. The day and frequency of pharmacist adherence instruction was as follows: 4 (1–5) days in patients with nonmelancholic depression, and 4 (2–5) days in patients with melancholic or bipolar depression (Table 2). There were no significant differences in these data among the antidepressant-treated patients.
Awareness of side effects
As shown in Table 3, side effects in antidepressant-naïve patients were sleepiness (13.75%), constipation (11.25%), dry mouth (8.75%), malaise (5%), dizziness (5%), cephalalgia (5%), dysuria and anorexia (3.75%), and hidrosis (2.5%). In all antidepressant-treated patients, the following side effects were significantly more common than in antidepressant-naïve patients: anorexia 9.86% (P < 0.05), sleepiness 61.97%, constipation 69.01%, dry mouth 76.06%, malaise 39.44%, dizziness 49.30%, cephalalgia 28.17%, dysuria 36.62%, and hidrosis 15.49% (P < 0.01). There were no significant differences in side effects among the antidepressant-treated patients.
Table 3.
Treatment-emergent side effects that occurred by discharge day
| Side effects | AD-naïve | Overall | NMD | MD | BD |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| n = 80 | n = 71 | n = 35 | n = 17 | n = 19 | |
| Sleepiness | 13.75 | 61.97** | 71.43 | 58.82 | 47.37 |
| Malaise | 5.00 | 39.44** | 42.86 | 47.06 | 26.32 |
| Dry mouth | 8.75 | 76.06** | 80.00 | 88.24 | 57.89 |
| Constipation | 11.25 | 69.01** | 54.29 | 52.94 | 110.53 |
| Dysuria | 3.75 | 36.62** | 31.43 | 52.94 | 31.58 |
| Dizziness | 5.00 | 49.30** | 34.29 | 29.41 | 94.74 |
| Cephalalgia | 5.00 | 28.17** | 22.86 | 35.29 | 31.58 |
| Hidrosis | 2.50 | 15.49** | 17.14 | 11.76 | 15.79 |
| Anorexia | 3.75 | 9.86* | 11.43 | 11.76 | 5.26 |
| Tremor | 0.00 | 8.45 | 8.57 | 5.88 | 10.53 |
| Akathisia | 0.00 | 5.63 | 5.71 | 5.88 | 5.26 |
Notes: Values are expressed as % of the total number of patients in each group;
P < 0.05,
P < 0.01 versus antidepressant-naïve patients (Mann–Whitney U test).
Abbreviations: AD, antidepressant; MD, melancholic depression; NMD, nonmelancholic depression; BD, bipolar depression.
DAI-10 total score in antidepressant-naïve versus antidepressant-treated patients
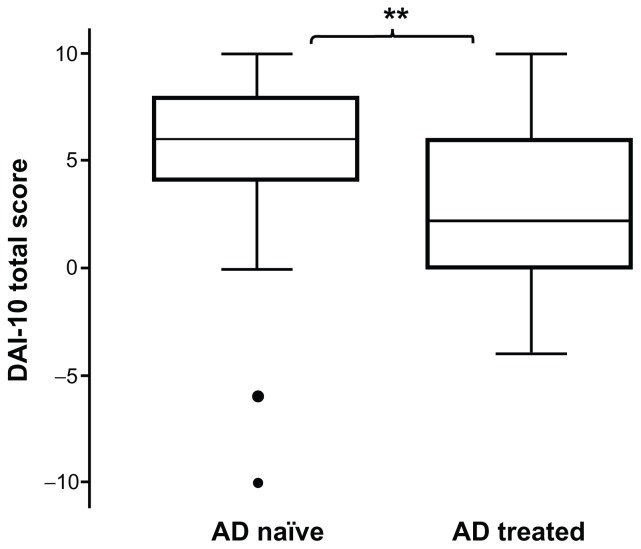
The total DAI-10 drug adherence score is reported for antidepressant-naïve and antidepressant-treated patients in Figure 1. The median total DAI-10 score was significantly lower in antidepressant-treated patients [6 (4–8)] than in antidepressant-naïve patients [2 (0–6), P < 0.01].
Figure 1.
Total score of DAI-10 in antidepressant-naïve and antidepressant-treated patients.
Notes: Values are presented as the median (quartile 1 to quartile 3). **P < 0.01 versus antidepressant-treated patients (Mann–Whitney U test).
Abbreviation: AD, antidepressant; DAI-10, Drug Attitude Inventory-10.
Comparison of DAI-10 total score among antidepressant-treated patients
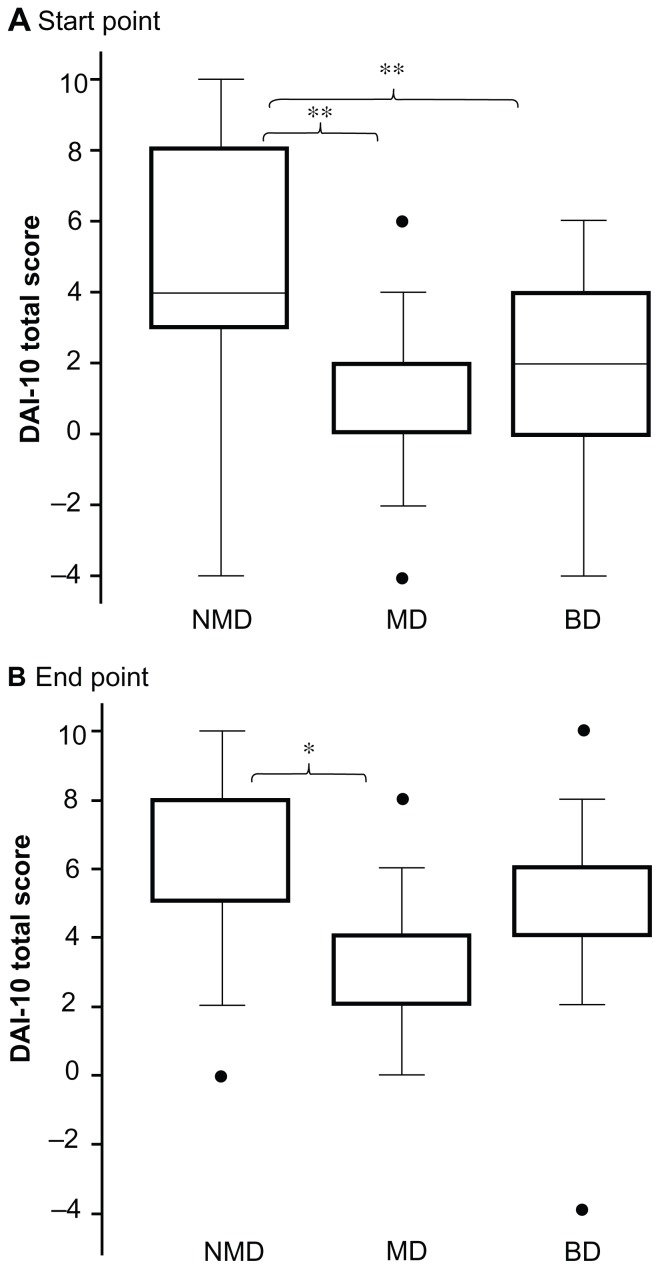
DAI-10 total scores for drug adherence among antidepressant-treated patients measured on the starting day of pharmacist adherence instruction and at discharge are shown in Figure 2. On the starting day of self-management of drug intake, the DAI-10 total scores for patients with melancholic depression [0 (0–2)] or bipolar depression [2 (0–4)] were significantly lower than those reported in patients with nonmelancholic depression [4 (3–8), P < 0.003, respectively, Figure 2A]. On the day of discharge, the DAI-10 total score in patients with melancholic depression [4 (2–4)] was still significantly lower than that in patients with nonmelancholic depression [8 (5–8), P < 0.017, Figure 2B].
Figure 2.
Total score for DAI-10 in antidepressant-treated patients. (A) Start point (start day of self-management of drug intake), (B) end point (discharge day).
Notes: Values are presented as the median (quartile 1 to quartile 3). *P < 0.017, **P < 0.003 versus patients with nonmelancholic depression (compared using the Kruskal–Wallis test followed by the Mann–Whitney test, and analyzed by statistical differences using Bonferroni’s correction).
Abbreviations: DAI-10, Drug Attitude Inventory-10; MD, melancholic depression; NMD, nonmelancholic depression; BD, bipolar depression.
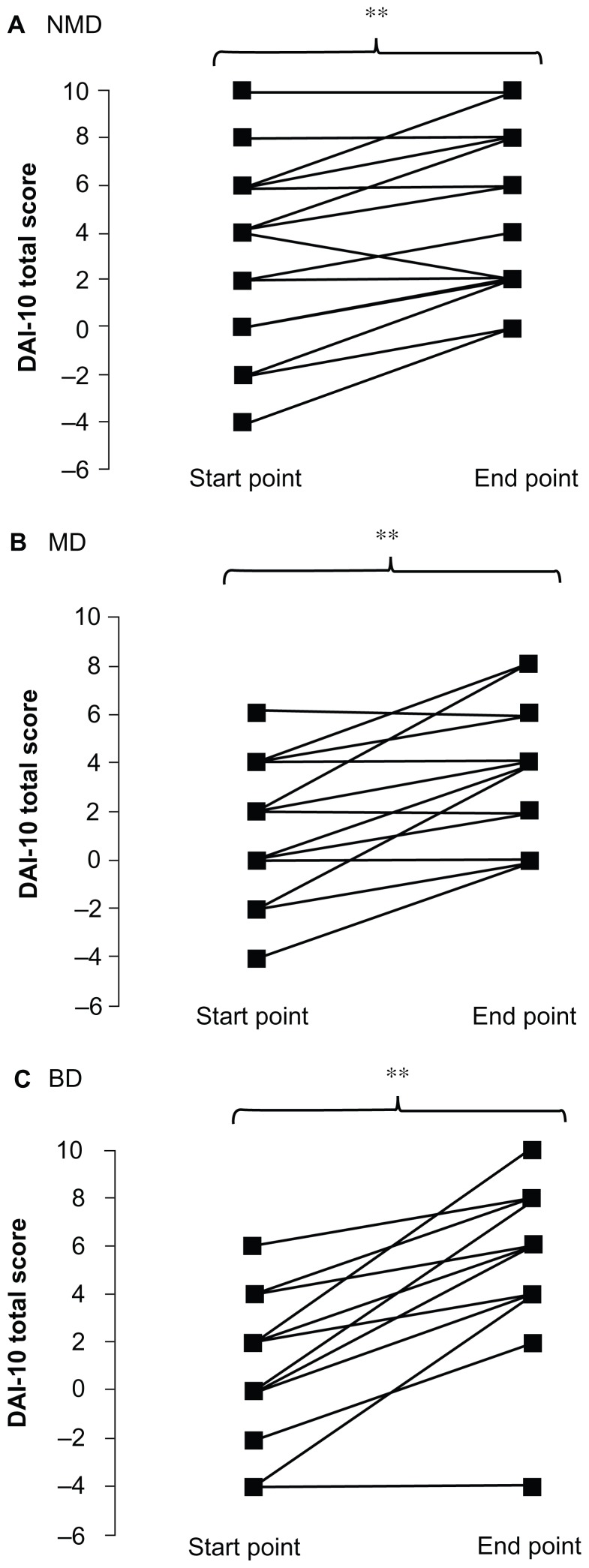
At discharge, the DAI-10 total score was significantly improved relative to the starting day of self-management of drug intake in all groups [nonmelancholic depression, 4 (3–8) versus 8 (5–8); melancholic depression, 0 (0–2) versus 4 (2–4); bipolar depression, 2 (0–4) versus 6 (4–6), respectively, P < 0.01, Figure 3].
Figure 3.
Alteration of DAI-10 total score start (start day of self-management of drug intake) and end point (discharge day). (A) Patients with nonmelancholic depression, (B) patients with melancholic depression, and (C) patients with bipolar depression.
Notes: *P < 0.05, **P < 0.01 versus DAI-10 total score start point (Wilcoxon signed-rank test).
Abbreviations: DAI-10, Drug Attitude Inventory-10; MD, melancholic depression; NMD, non melancholic depression; BD, bipolar depression.
Discussion
Our findings indicate that the DAI-10 score in antidepressant-treated patients was significantly lower than that reported in antidepressant-naïve patients. Furthermore, our data suggest that patients with melancholic depression may be more vulnerable to drug nonadherence relative to the other depression groups tested. In addition, pharmacist adherence instruction contributes to improvement of adherence in antidepressant-treated patients. This research supports previous reports that antidepressant-treated patients have significantly lower DAI-10 scores.8,9
Antidepressant-treated patients reported significantly more side effects, including dry mouth, dizziness, and constipation, than antidepressant-naïve patients; however, there was no significant difference in age, education, or gender between antidepressant-naïve and antidepressant-treated patients. It is reported that the side effects of antidepressant treatment can provoke negative attitudes towards drug compliance,9 and correlate with perceived harmfulness of antidepressants.7 The data presented here indicate that the incidence of side effects with antidepressants may be a risk factor for antidepressant nonadherence.
In the present study, the DAI-10 total score reported by patients with melancholic depression was significantly lower than that reported by patients with nonmelancholic depression; however, no significant differences were reported for age, gender, education, or awareness of side effects in antidepressant-treated patients. It is well known that a high concomitant number of prescribed drugs confounds drug adherence,8 and the number of agents used for melancholic depression is typically lower than for other types of depression. Our data suggest that patients with melancholic depression may be more vulnerable to antidepressant nonadherence. Melancholia occurs in the majority of depressed patients, plausibly constituting a specific vulnerability factor.18 The results presented here suggest that antidepressant-treated patients with melancholic depression may be more prone to the negative effects of antidepressant treatment on drug adherence. Vanelli and Oca-Perraillon1 report a relationship between nonadherence and conditioned fear that has implications for drug intake. It is possible that the occurrence of side effects associated with antidepressant compliance may provoke a fear of drug intake among patients with melancholic depression, leading to nonadherence. Additional research is required to determine if patients with melancholic depression are more vulnerable to antidepressant nonadherence, potential risk factors, and the role of conditioned fear in those effects.
It is reported that drug adherence does not correlate significantly with objective measures of medication use, such as pill counts and electronic monitoring.1,10,11 The efficacy of the DAI-10 is well established19 and has been proven to be useful in clinical practice. Our present results indicate that the DAI-10 has considerable validity for pharmacists in the evaluation of antidepressant adherence.
Our results indicate an improvement in DAI-10 total score in antidepressant-treated patients after pharmacist adherence instruction. In this study, depressive symptoms in all patients remitted on the start day of self-management of drug intake, and the dose and schedule of antidepressants and total number of tablets (data are not shown) were not altered after the start of pharmacist adherence instruction, suggesting that such instruction contributed to the improved DAI-10 score in antidepressant-treated patients.
Our results also indicate that antidepressant-treated patients with melancholic depression may be more vulnerable to nonadherence, or perhaps pharmacist adherence instruction is less effective in these patients. Our results suggest significant involvement of type of depression in the efficacy of pharmacist adherence instruction. The link between type of depression and antidepressant adherence is not well understood. Aikens et al7 pointed out the necessity to pay attention to individual variation in antidepressant adherence to improve interventions, but the involvement between types of depression and antidepressant adherence is not well understood. Our results suggest that pharmacist intervention tailored to the specific type of depression may improve nonadherence in antidepressant-treated patients as well as the effectiveness of pharmacist adherence instruction. A limitation of our study is the relatively small sample size and the fact that we only included antidepressant-treated inpatients. A larger sample and a longer trial will make clear risk factors of antidepressant nonadherence including side effects and type of depression.
Conclusion
We report significant nonadherence and side effects in hospitalized and routinely treated patients whose depressive symptoms remitted versus hospitalized antidepressant-naïve patients. In particular, patients with melancholic depression were significantly more nonadherent with antidepressant medication than patients with other types of depression. However, after the start of self-management of drug intake, pharmacist adherence instruction seemed to contribute to improved drug adherence until the time of discharge. We conclude that melancholic depression and antidepressant side effects are risk factors for antidepressant nonadherence in routine treatment of depression, and pharmacist adherence instruction can ameliorate antidepressant nonadherence.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vanelli M, Oca-Perraillon M. Role of patient experience in antidepressant adherence: a retrospective data analysis. Clin Ther. 2008;30:1737–1745. doi: 10.1016/j.clinthera.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 3.Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60:261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- 4.Kessing LV, Hansen MG, Andersen PK. Course of illness in depressive and bipolar disorders. Naturalistic study, 1994–1999. Br J Psychiatry. 2004;185:372–377. doi: 10.1192/bjp.185.5.372. [DOI] [PubMed] [Google Scholar]
- 5.Robinson DG, Woerner MG, Ma J, et al. Predictors of medication discontinuation by patients with first-episode schizophrenia and schizoaffective disorder. Schizophrenia Res. 2002;57:209–219. doi: 10.1016/s0920-9964(01)00312-7. [DOI] [PubMed] [Google Scholar]
- 6.Pope M, Scott J. Do clinicians understand why individuals stop taking lithium? J Affect Disord. 2003;74:287–291. doi: 10.1016/s0165-0327(02)00341-5. [DOI] [PubMed] [Google Scholar]
- 7.Aikens JE, Nease DE, Jr, Nau DP, Klinkman MS, Schwenk TL. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med. 2005;3:23–30. doi: 10.1370/afm.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty K, Avasthi A, Kumar S, Grover S. Attitudes and beliefs of patients of first episode depression towards antidepressants and their adherence to treatment. Soc Psychiatry Psychiatr Epidemiol. 2008;44:482–488. doi: 10.1007/s00127-008-0468-0. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty LR, Klein DN, Olino TM, Dyson M, Rose S. Increased waking salivary cortisol and depression risk in preschoolers: the role of maternal history of melancholic depression and early child temperament. J Child Psychol Psychiatry. 2009;50:1495–1503. doi: 10.1111/j.1469-7610.2009.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2002;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Velligan DI, Wang M, Diamond P, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58:1187–1192. doi: 10.1176/ps.2007.58.9.1187. [DOI] [PubMed] [Google Scholar]
- 12.Hogan TP, Awad AG, Eastwood RA. Self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13:177–183. doi: 10.1017/s0033291700050182. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Kim SY, Ahn YM, Kim YS. Subjective response to clozapine and risperidone treatment in outpatients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:301–305. doi: 10.1016/j.pnpbp.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Wolters HA, Knegtering H, van den Bosch RJ, Wiersma D. Effects and side effects of antipsychotic treatment in schizophrenia: pros and cons of available self-rating scales. Schizophr Res. 2009;112:114–118. doi: 10.1016/j.schres.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Rubio-Valera M, Serrano-Blanco A, Trave P, Penarrubia-Maria MT, Ruiz M, Pujol M. Community pharmacist intervention in depressed primary care patients (PRODEFAR study): randomized controlled trial protocol. BMC Public Health. 2009;9:284–292. doi: 10.1186/1471-2458-9-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanlon JT, Artz MB. Drug-related problems and pharmaceutical care. Med Care. 2001;39:109–112. doi: 10.1097/00005650-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Rubio-Valera M, Serrano-Blanco A, Magdalena-Belío J, et al. Effectiveness of pharmacist care in the improvement of adherence to antidepressants: a systematic review and meta-analysis. Ann Pharmacother. 2011;45:39–48. doi: 10.1345/aph.1P429. [DOI] [PubMed] [Google Scholar]
- 18.Stanghellini G, Bertelli M, Raballo A. Typus melancholicus: personality structure and the characteristics of major unipolar depressive episode. J Affect Disord. 2006;93:159–167. doi: 10.1016/j.jad.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Hogan TP, Awad AG. Subjective response to neuroleptics and outcome in schizophrenia: a re-examination comparing two measures. Psychol Med. 1992;22:347–352. doi: 10.1017/s0033291700030282. [DOI] [PubMed] [Google Scholar]