Abstract
Mitochondrial dysfunction after traumatic brain injury (TBI) is manifested by increased levels of oxidative damage, loss of respiratory functions and diminished ability to buffer cytosolic calcium. This study investigated the detrimental effects of lipid peroxyl radicals (LOO•) and lipid peroxidation (LP) in brain mitochondria after TBI by examining the protective effects of U-83836E, a potent and selective scavenger of LOO• radicals. Male CF1 mice were subjected to severe controlled cortical impact TBI (CCI-TBI) and treated with either vehicle or U-83836E initiated i.v. at 15 min post-injury. Calcium (Ca++) buffering capacity and respiratory function were measured in isolated cortical mitochondrial samples taken from the ipsilateral hemisphere at 3 and 12 h post-TBI, respectively. In vehicle-treated injured mice, the cortical mitochondrial Ca++ buffering capacity was reduced by 60% at 3 h post-injury (p < 0.001) and the respiratory control ratio was decreased by 27% at 12 h post-TBI, relative to sham, non-injured mice. U-83836E treatment significantly (p < 0.05) preserved Ca++ buffering capacity and attenuated the reduction in respiratory control ratio values. Consistent with the functional effects of U-83836E being as a result of an attenuation of mitochondrial oxidative damage, the compound significantly (p < 0.001) reduced LP-generated 4-hydroxynonenal levels in both cortical homogenates and mitochondria at both 3 and 12 h post-TBI. Unexpectedly, U-83836E also reduced peroxynitrite-generated 3-nitrotyrosine in parallel with the reduction in 4-hydroxynonenal. The results demonstrate that LOO• radicals contribute to secondary brain mitochondrial dysfunction after TBI by propagating LP and protein nitrative damage in cellular and mitochondrial membranes.
Keywords: 3-nitrotyrosine, 4-hydroxynonenal, lipid peroxidation, mitochondria, traumatic brain injury, U-83836E
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity in the developed world. It is estimated that every year in the U.S. there are 2 million new cases of TBI. Of those, more than 50 thousand people die and 70–90 thousand people suffer long term disability (Madikians and Giza 2006; Greve and Zink 2009). The lack of an effective neuroprotective or neurorestorative therapy for TBI makes it one of the major unmet medical needs. However, the hope for an effective treatment is derived from the fact that much of the post-traumatic damage to the injured brain is caused by a secondary injury cascade of pathochemical and pathophysiological events that exacerbates the primary mechanical TBI.
There is a plethora of data generated over the past three decades that consistently suggests that free radical generation and oxidative damage plays a major role in post-traumatic secondary injury to neurovascular structures after TBI (Hall et al. 2010). In particular, lipid peroxidation (LP)-mediated oxidative damage is initiated after TBI by the action of free radicals generated from reactive oxygen species and reactive nitrogen species (e.g. peroxynitrite) (Beckman and Koppenol 1996; Violi et al. 1999; Deng et al. 2007; Singh et al. 2007; Deng-Bryant et al. 2008), and is catalyzed by an iron-dependent mechanisms. However, lipid peroxyl radicals (LOO•) are the central propagators of LP reactions across cellular and mitochondrial membranes which is detrimental to their structure and function (Spiteller 2006). Moreover, 4-hydroxynonenal (4-HNE), one of the toxic aldehydic byproducts of LP (Esterbauer et al. 1991), has been shown to covalently bind to cellular and mitochondrial proteins further impairing their function. These primary and secondary effects of LP have been linked to a compromise in brain mitochondrial respiratory function (i.e. ATP generation) and calcium (Ca++) buffering (Xiong et al. 1997b; Singh et al. 2006; Deng et al. 2007), the latter constituting a major mechanism of cellular calcium homeostasis. Indeed, mitochondria are central to Ca++ homeostasis (Sullivan et al. 2005) by acting as an intracellular Ca++ buffer (Jouaville et al. 1998). However, the resulting mitochondrial Ca++ loading has been shown to induce mitochondrial generation of reactive oxygen species, reactive nitrogen species and their highly reactive free radicals (Brustovetsky et al. 2002; Lifshitz et al. 2003; Hansson et al. 2008). Thus, Ca++-loaded neural mitochondria become both the sniper and the target of these free radical bullets which initiate mitochondrial membrane LP and protein modifications resulting in irreversible loss of mitochondrial functions such as mitochondrial respiration, oxidative phosphorylation and ion transport rendering mitochondria dysfunctional (Gadelha et al. 1997; Kowaltowski and Vercesi 1999) and decreasing mitochondrial Ca++ buffering capacity (Singh et al. 2006). This deteriorated mitochondrial function and ultimately failure due largely to LP-related oxidative damage is strongly implicated in neuronal cell death (Sullivan et al. 1999).
The important role of LP in the pathobiology of TBI is supported by the fact that LP inhibitors such as the 21-aminosteroid tirilazad mesylate (i.e. U-74006F) (Hall 1988) and the 2-methylaminochroman U-78517F (Hall et al. 1991) have been shown to enhance neurological recovery in mice after experimental TBI. However, in those investigations, a direct link between their effects on neurological recovery and inhibition of post-traumatic oxidative damage was not demonstrated. Therefore, in the present work, we used the mouse CCI-TBI model and U-83836E (an enantiomer of the racemic U-78517F) (Hall et al. 1991), which is a potent, selective and dual mechanism LOO• scavenger (see Fig. 1), to pharmacologically test the hypothesis that LP is responsible for post-traumatic brain mitochondrial functional impairment. Specifically, we investigated whether the scavenging of LOO• radicals with U-83836E can preserve mitochondrial respiratory and Ca++ buffering capacity in the injured brain as a potential neuroprotective mechanism.
Fig. 1.
U-83836 is a 2-methylaminochroman that contains the phenolic chroman ring structure of vitamin E (α-tocopherol, left side of diagram) which can donate an electron to a lipid peroxyl radical (LOO •) converting it to a lipid hydroperoxide (LOOH) which in turn can be converted to a harmless lipid alcohol (LOH) by the action of the antioxidant enzyme glutathione peroxidase (GSHPx). The chroman radical is a weak, non-oxidizing free radical that can be re-reduced back to the phenolic hydroxyl by ascorbate or glutathione (GSH) so that it can reduce a second LOO•. On the right side of the diagram is shown the LOO• scavenging mechanism for the bis-pyrrolo pyrimidine portion of U-83836E. Initially, a LOO• binds to the 5 position of the pyrimidine ring as shown. This is hydrolyzed resulting in the formation of a lipid alcohol and a 5-hydroxy pyrimidine. This phenolic hydroxyl can react with an alkoxyl radical (LO•) radical converting it to a LOOH which in turn can be converted by either GSHPx or phospholipid GSHPx to a LOH. The right hand reaction sequence represents a catalytic scavenging mechanism in which the antioxidant bis-pyrrolo pyrimidine moiety is able to repeatedly react with LOO• and LO• radicals. The dual peroxyl scavenging mechanism together with the ability of the chroman phenol to be re-reduced and the bis-pyrollo-pyrimdine to react catalytically makes U-83836 a highly effective and potent lipid peroxidation inhibitor. U-83836E = HCl salt of U-83836.
Materials and methods
Materials
Magnesium chloride (MgCl2), sucrose, mannitol, EGTA, bovine serum albumin (BSA), HEPES potassium salt, Triton, Tris HCL, NaCl, EDTA, glycerol, protease inhibitors (Complete Mini™ Protease Inhibitor Cocktail tablet, Roche Diagnostics, Indianapolis, IN, USA). potassium phosphate monobasic anhydrous (KH2PO4), pyruvate, malate, ADP, oligomycin A, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone, and succinate were purchased from Sigma-Aldrich (St. Louis, MO, USA). BCA protein assay kit was purchased from Pierce (Rockford, IL, USA).
Animals
The present studies employed 210 young adult male CF-1 mice (Charles River, Portage, MI, USA) weighing 29–32 g. All animals were fed ad libitum and housed in the Division of Laboratory Animal Resources (DLAR) sector of the University of Kentucky Medical Center, which is fully accredited by AAALAC. The procedures followed protocols approved by the University of Kentucky’s Institutional Animal Care and Use Committee (IACUC).
Mouse model of focal (Controlled Cortical Impact) traumatic brain injury
Mice were initially anesthetized in a plexiglass chamber using 4.0% isoflurane, shaved, weighed and then placed in a stereotaxic frame (David Kopf, Tujunga, CA, USA). Throughout the injury procedure, mice were kept anesthetized by a constant flow of 3% isoflurane and oxygen both delivered via a nose cone. The head was positioned in the horizontal plane with the nose bar set at zero. A 2 cm sagittal incision is made in the scalp and the flap was retracted using hemostats to expose the skull. After that, a 4.0 mm craniotomy was made lateral to the sagittal suture and centered between bregma and lambda. The drilled skull cap at the craniotomy site was carefully removed to avoid inflicting damage to the dura.
A computer-controlled pneumatic impactor (Precision System Instruments TBI-030 Impactor, Fairfax Station, VA, USA) was used to generate the CCI-TBI. The impactor induced a non-penetrating, localized contusion of the cortex. The impactor tip was 3.0 mm in diameter with a slightly beveled edge. Injury severity is altered via independent adjustment of the impactor contact velocity and the depth of cortical deformation. In the present studies, the contact velocity of the impactor was set at 3.5 m/s, while the deformation depth was set at 1.0 mm as described previously (Scheff and Sullivan 1999; Singh et al. 2006; Deng-Bryant et al. 2008; Mbye et al. 2008). After injury, the craniotomy was closed by placement of a small disk of saline moistened Surgicil over the dura, followed by placement of a 6.0 mm diameter disk made of dental acrylic that was cemented in place with methyl methacrylate to close the craniotomy. The mice were then placed in a Hova-Bator Incubator (model 1583; Randall Burkey Co, Boerne, TX, USA), set at 37°C, for at least 20 min to prevent post-traumatic hypothermia. Consciousness (i.e. return of right reflex and mobility) was regained within 10 min after the injury. The survival time points for the injured mice were either 3 or 12 h depending on their group assignment.
U-83836E Preparation and dosing
U-83836E was purchased from the Biomol Company (Biomol International, LP, Plymouth Meeting, PA, USA) and freshly diluted in physiologic (0.9%) saline. For i.v. injections, dilutions were made to deliver 3 mg/kg in an injection volume of ≈ 0.12 mL where as for i.p. injections, dilutions were made to deliver 10 mg/kg in an injection volume of ≈ 0.30 mL. The employed dose was based upon the previously published effects of this compound in the mouse diffuse TBI model (Hall et al. 1991).
Ficoll-purified mitochondrial isolation
Brain mitochondria were isolated as described previously, with some modifications (Sullivan et al. 2003; Singh et al. 2007). Briefly, mice were deeply anaesthetized in a CO2 chamber, decapitated and the brains were rapidly harvested. The ipsilateral contused cortex was quickly dissected out on an ice-cold stage and pooled from four mice per N. The non-injured or contused cortices were homogenized in an ice-cold isolation buffer with 1 mmol/L EGTA (215 mmol/L mannitol, 75 mmol/L sucrose, 0.1% BSA, 20 mmol/L HEPES, 1 mmol/L EGTA; pH adjusted to 7.2 with KOH), using a Potter-Elvehjem homogenizer (Thermo Fisher Scientific Inc, Rockford, IL, USA). The homogenates were then subjected to differential centrifugation at 4°C. First, the homogenate was centrifuged twice at 1300 g for 3 min in an Eppendorf microcentrifuge (Eppendorf North America, Hauppauge, NY, USA) at 4°C to remove cellular debris and nuclei. The pellet was discarded and the supernatant further centrifuged at 13 000 g for 10 min. The crude mitochondrial pellet obtained after differential centrifugation was then subjected to nitrogen decompression to release synaptic mitochondria using a nitrogen cell disruption bomb (model 4639; Parr Instrument Co., Moline, IL, USA) cooled to 4°C, under a pressure of 1200 psi for 10 min (Brown et al. 2004; Singh et al. 2006). After nitrogen disruption the mitochondria were suspended in isolation buffer to a final volume of 0.75 mL and further laid on two preformed layers consisting of 2 mL of 7.5% ficoll and 2 mL of 10% ficoll in centrifuge tubes. The gradient was centrifuged at 100 000 g for 30 min at 4°C as previously described (Sullivan et al. 2003; Singh et al. 2007). The fraction accumulated (synaptosomal membranes and myelin) at the inter-phase of 7.5% and 10% ficoll was first removed and discarded then the rest of the supernatant was also carefully removed and discarded leaving the mitochondrial pellet at the bottom of the tube. The pellet was gently washed with isolation buffer without EGTA, and then resuspended in 0.5 mL of isolation buffer without EGTA, transferred to microcentrifuge tubes and topped off with isolation buffer without EGTA and centrifuged at 10 000 g for 10 min at 4°C. The final mitochondrial pellet was re-suspended in isolation buffer without EGTA to yield a concentration of ~ 10 mg/mL. The protein concentration was then determined using a BCA protein assay kit, measuring absorbance at 562 nm with a BioTek Synergy HT plate reader (Winooski, VT, USA).
Mitochondrial respiration measurement
Mitochondrial respiratory rates were measured using a Clark-type oxygen electrode in a sealed, thermostatically controlled, and continuously stirred chamber (Oxytherm System, Hansatech Instruments Ltd, Norfolk, UK), maintained at 37°C as described previously. Approximately 40–45 µg of isolated mitochondrial protein was placed in the chamber containing 250 µL of KCl-based respiration buffer (125 mmol/L KCl, 2 mmol/L MgCl2, 2.5 mmol/L KH2PO4, 0.1% BSA, 20 mmol/L HEPES at pH 7.2) and allowed to equilibrate for 1 min. Following that, complex-I substrates, 5 mmol/L pyruvate and 2.5 mmol/L malate, were added to monitor the state II respiratory rate. One minute later, two boluses of 150 µmol/L ADP were sequentially added to the mitochondria to initiate state III respiration for 2 min, followed by the addition of 2 µmol/L oligomycin to monitor state IV respiratory rate for an additional 2 min. To assess the uncoupled respiratory rate, 2 µmol/L carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone was added to the mitochondria in the chamber and oxygen consumption was monitored for another 2 min. This was followed by the addition of 10 mmol/L succinate to monitor complex II-driven respiration. The respiratory control ratio (RCR) was calculated by dividing state III oxygen consumption (defined as the rate of respiration in the presence of ADP, second bolus addition) by the state IV oxygen consumption (rate obtained in the presence of oligomycin).
Mitochondrial calcium buffering measurement
Mitochondrial Ca++ buffering measurements were conducted in a spectrofluorimeter (Shimadzu RF5301; Columbia, MD, USA) set at 37°C as described previously (Singh et al. 2006). The extra-mitochondrial levels of Ca++ were detected using the fluorescent Ca++-sensitive indicator calcium green 5 N (CaG5N) (Molecular Probes, Eugene, OR, USA) at a final concentration of 100 nmol/L with excitation and emission wavelengths 506 nm and 532 nm, respectively. The fluorescence quenching of the cationic indicator, 150 nmol/L tetramethyl rhodamine ethyl ester (TMRE), was used to crudely measure the mitochondrial transmembrane potential. The TMRE is accumulated and quenched inside energized mitochondria when the transmembrane potential goes up. The excitation wavelength was 550 nm for TMRE. The experiment was initiated by the sequential additions of mitochondria (100 µg) to a 2.0 mL cuvette containing KCl-based respiration buffer (125 mmol/L KCl, 2 mmol/L MgCl2, 2.5 mmol/L KH2PO4, 0.1% BSA, 20 mmol/L HEPES at pH 7.2), followed by 100 nmol/L CaG5N and 150 nmol/L TMRE. The reaction was initiated by energizing mitochondria with 5 mmol/L pyruvate and 2.5 mmol/L malate at 1 min, followed by the addition of 150 µmol/L ADP at 2 min and 1 µmol/L oligomycin at 3 min. Two minutes later, 32 mmol/L Ca++ was continuously infused, using a syringe pump, at rate of 0.5 µL/min. The infusion continued until the mitochondria were no longer capable of sequestering Ca++ as revealed by a dramatic increase in the extra-mitochondrial CaG5N fluorescent signal.
Preparation of mitochondrial samples for measurement of mitochondrial oxidative damage markers
A portion of the isolated ipsilateral cortical mitochondria from sham, vehicle-, U-83836E-treated mice was used for the quantitative measurements of 4-HNE as an index of LP and 3-nitrotyrosine (3-NT) as an index of protein nitration by western immunoblot as described below. Briefly, mitochondrial samples were centrifuged at 10 000 g for 10 min to get rid of isolation buffer. The pellets were re-suspended in ice-cold Triton lysis buffer (1% triton, 20 mmol/L tris HCL, 150 mmol/L NaCl, 5 mmol/L EGTA, 10 mmol/L EDTA, 10% glycerol) with protease inhibitors (Complete Mini™ Protease Inhibitor Cocktail tablet; Roche Diagnostics). Samples were then briefly sonicated, vortexed, and incubated in ice for 45 min. Following that, the samples were centrifuged at 19 064 g for 30 min at 4°C, and supernatants were collected for protein assay. Protein concentration was determined by Bio-Rad DC Protein Assay (Bio-Rad; Hercules, CA, USA).
Cortical homogenate preparation and protein assay
At 3 h post-TBI, the mice were deeply anesthetized with CO2, followed by decapitation. The ipsilateral contused cortical tissue was then rapidly dissected out on an ice-chilled stage as described above, and immediately transferred into pre-cooled Triton lysis buffer (1% triton, 20 mmol/L tris HCL, 150 mmol/L NaCl, 5 mmol/L EGTA, 10 mmol/L EDTA, 10% glycerol) with protease inhibitors (Complete Mini™ Protease Inhibitor Cocktail tablet, Roche Diagnostics). Samples were then briefly sonicated, vortexed and incubated in ice for 45 min. Following that the samples were centrifuged at 19 064 g for 30 min at 4°C, and supernatants were collected for protein assay. Protein concentration was determined by Bio-Rad DC Protein Assay.
Western-blotting analysis of oxidative damage in cortical homogenates and mitochondria
To measure 4-HNE as an index of LP and 3-NT as an index of protein nitration, aliquots of each sample (a total of 15 µg in a final volume of 15 µL) were run on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis Precast gel (12% Tris-Acetate Criterion™ XT Precast gel, Bio-Rad) and transferred onto a nitrocellulose membrane using a semi-dry electro-transferring unit set at 15 V for 30 min. Following gel transfer, membranes were incubated in a Tris-buffered saline blocking solution with 5% milk for 1 h at 21°C. For the detection of protein 3-NT, a mouse monoclonal anti-3-NT antibody (GenWay biotech, Inc, San Diego, CA, USA) was used at a dilution of 1 : 2000 with overnight incubation at 4°C. For detection of 4-HNE, a mouse monoclonal anti-HNE antibody (Japan Institute for the Control of Aging, JalCA; Fukuroi, Japan) was used at a dilution of 1 : 4000 with overnight incubation at 4°C. Positive bands were detected by a secondary IR-Dye 800CW conjugated goat anti-mouse antibody (Rockland Inc, Gilbertsville, PA, USA) at a dilution of 1 : 5000. All incubations and washing steps were performed according to the instructions given by the manufacturers. The intensity of the bands was visualized and quantified using a Li-Cor Odyssey Infrared Imager (Li-Cor; Lincoln, NE, USA). A standardized protein loading control was included in each blot to normalize the band densities so that quantitative comparisons could be made across multiple blots. This was made up of pooled brain tissue protein collected from previously run TBI mice that showed strong bands for 3-NT or 4-HNE.
Sample size and statistical analysis
For the immunoblotting (oxidative damage in whole cortical cell lysates and cortical mitochondria) and mitochondrial respiration experiments, the N for each group was 10 with four mice pooled in each N for mitochondrial respiration measurements. For the Ca++ buffering experiments, the N for each group was four with five mice pooled in each N. All data are presented as mean ± SD. Each analysis which included sham non-injured, vehicle-treated injured and U-83836E- treated injured groups was conducted with an initial one way anova. Where the anova was statistically significant, this was followed by Student-Neuman–Keuls (SNK) analysis of inter-group differences. A p < 0.05 was required for statistical significance.
Results
Effect of U-83836E on post-traumatic oxidative damage in cortical homogenates
We first examined the ability of U-83836E to antagonize oxidative damage in cortical homogenates at 3 h post-injury by administering 3mg/kg of U-83836E or vehicle i.v. at 15 min post-injury and measuring levels of 4-HNE and 3-NT by quantitative western blot. The anova showed a significant difference across the three experimental groups (4-HNE; F(2,28) = 18.052, p < 0.0001. 3-NT; F(2,28) = 22.584, p < 0.0001). As shown in Fig. 2, contused ipsilateral cortical tissue harvested from vehicle-treated mice had higher levels of 4-HNE (p < 0.001; Fig. 2a) and 3-NT (p < 0.001; Fig. 2b) compared with sham, non-injured mice. On the other hand, 4-HNE levels of ipsilateral contused cortical tissue harvested from U-83836E-treated mice were not different from those of shams (p > 0.05). Moreover, treatment of mice with 3 mg/kg U-83836E showed significant (p < 0.001) reduction in the levels of 4-HNE and 3-NT compared with vehicle-treated mice.
Fig. 2.
Attenuation of post-traumatic lipid peroxidation–derived 4-hydroxynonenal (4-HNE) (a) and protein nitration-related 3-nitrotyrosine (3-NT) (b) in cortical homogenates by U-83836E at 3 h following controlled cortical impact traumatic brain injury in male mice. Mice were administered 3 mg/kg of U-83836E i.v. 15 min post-injury and oxidative damage markers were measured 3 h following injury. The levels of 4-HNE and 3-NT were detected using western blotting, (c) and (d) respectively. Lanes were loaded with either sham (S), vehicle (V), U-83836E (U) or loading control (LC). Bars indicate group means ± SD. Statistical differences (one-way anova and Student-Neuman–Keuls post hoc test): *p < 0.001 versus sham, #p < 0.001 versus vehicle, n = 10/group.
Effect of U-83836E on post-traumatic cortical mitochondrial respiratory dysfunction
In a second set of experiments, we explored the ability of U-83836E to preserve mitochondrial bioenergetics (RCR and respiration rates) by administering 3 mg/kg of U-83836E or vehicle 15 min post-injury and measuring mitochondrial respiration using a Clarke-type oxygen electrode at 12 h post-injury. Fig. 3 presents the results of these experiments. The anova showed that there was a significant difference across the three experimental groups for both the RCRs (Fig. 3a; F(2,25) = 5.28, p < 0.0122) and state III respiratory rates (Fig. 3b; F(2,25) = 7.367, p < 0.003). The post hoc SNK analysis revealed that RCR values and state III respiration rates of mitochondria isolated from vehicle-treated injured mice were significantly (p < 0.05, p < 0.01, respectively) lower than those of mitochondria isolated from sham mice. Treatment of injured mice with 3 mg/kg U-83836E significantly (p < 0.05) improved mitochondrial state III respiration rates and the RCR values which were no different from those obtained in sham mice. While a small decrease was seen in the mean state IV values in the U-83836E-treated mice, this was not statistically significant. Thus, the improved RCRs are because of the maintenance of state III in the U-83836E-treated mice.
Fig. 3.
Effects of U-83836E on cortical mitochondrial bioenergetics at 12 h following severe controlled cortical impact traumatic brain injury in male mice: (a). respiratory control ratio (RCR); (b). State III and IV respiratory rates. Animals were administered 3 mg/kg U-83836E i.v. 15 min post-injury and mitochondria isolated using the Ficoll density-gradient isolation technique. Mitochondrial oxygen consumption was measured using a Clark-type oxygen electrode. RCR was calculated as the ratio of oxygen consumption in the presence of ADP (state III) and after the addition of oligomycin (state IV). Values = mean ± sd. Statistical differences (one-way anova and Student-Neuman–Keuls post hoc test): *p < 0.05 versus sham, #p < 0.05 versus vehicle, n = 10/group.
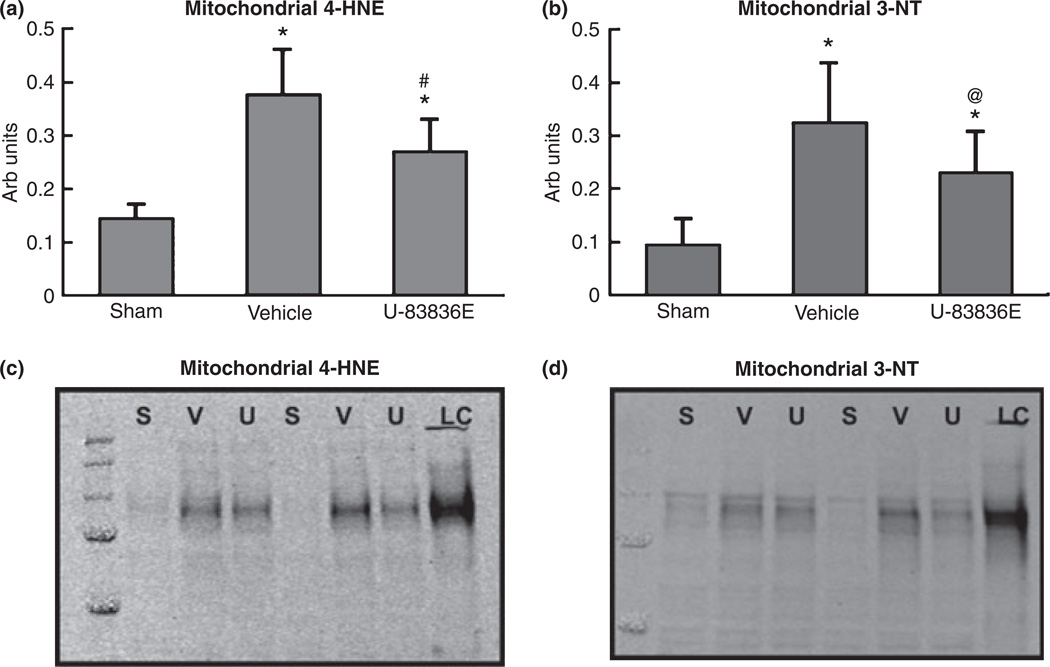
Effect of U-83836E on post-traumatic oxidative damage in cortical mitochondria
A portion of the isolated ipsilateral cortical mitochondria isolated from sham, vehicle treated and U-83836E-treated mice harvested at 12 h post-TBI for the respiratory function analyses (above) was used for quantitative analysis of 4-HNE and 3-NT, the results of which are provided in Fig. 4. The initial anova revealed that there were significant differences across groups for both 4-HNE (Fig. 4a; F(2,27) = 35.082, p < 0.0001) and 3-NT (Fig. 4b; F(2,27) = 19.948, p < 0.0001). Subsequent SNK analysis revealed that at 12 h post-TBI, mitochondria isolated from vehicle-treated mice had higher levels of LP-derived 4-HNE (p < 0.001) and protein nitration-derived 3-NT (p < 0.001) compared with sham, non-injured mice. On the other hand, treatment of mice with 3 mg/kg U-83836E showed a significant reduction in the levels of 4-HNE (p < 0.001) and 3-NT (p < 0.05) compared with vehicle-treated mice. Thus, there is a parallel incidence of both mitochondrial oxidative damage and loss of mitochondrial function (Fig. 3) after CCI-TBI which was antagonized by early post-traumatic U-83836E administration.
Fig. 4.
Attenuation of lipid peroxidation–derived 4-hydroxynonenal (4-HNE) (a) and protein nitration-related 3-nitrotyrosine (3-NT) (b) in mitochondria by U-83836E at 12 h following controlled cortical impact traumatic brain injury in male mice. Animals were administered 3 mg/kg of U-83836E i.v. 15 min post-injury and oxidative markers were measured 12 h following injury. The levels of 4-HNE and 3-NT were detected using western blotting, (c) and (d) respectively. Lanes were loaded with either sham (S), vehicle (V), U-83836E (U) or loading control (LC). Values = mean ± SD. Statistical differences (one-way anova and Student-Neuman–Keuls post hoc test): *p < 0.001 versus sham, #p < 0.001 versus vehicle, @ p < 0.05 versus vehicle n = 10.
Effect of U-83836E on post-traumatic cortical mitochondrial calcium buffering impairment after TBI
In a third set of experiments, the results of which are presented in Fig. 5, we investigated the ability of U-83836E (3 mg/kg i.v. at 15 min post-injury) to preserve mitochondrial Ca++ buffering capacity at 3 h post-injury. The anova demonstrated a significant difference across the sham, vehicle-treated and U-83836E-treated groups (F(2,9) = 9.298, p < 0.006). Subsequent SNK analysis revealed that the amount of Ca++ sequestered (before the onset of the membrane permeability transition) by mitochondria isolated from vehicle treated mice was significantly (p < 0.01) lower than that of mitochondria isolated from sham (craniotomy but no injury) mice. Treatment of injured mice with 3 mg/kg U-83836E i.v. followed by 10 mg/kg U-83836E i.p. significantly (p < 0.05) improved mitochondrial Ca++ buffering capacity compared with vehicle-treated mice.
Fig. 5.
Effects of U-83836E on Ca++ buffering capacity in mitochondria at 3 h following controlled cortical impact traumatic brain injury in male mice. Mice were administered 3 mg/kg U-83836E i.v. 15 min post-injury and mitochondria were isolated using the Ficoll density-gradient isolation technique. Part (a) shows a quantification of mitochondrial Ca++ buffering capacity across experimental groups. The Ca++ buffering capacity was reduced in vehicle-treated injured group compared to sham (non-injured) group and U-83836E treatment ameliorated this posttraumatic reduction in mitochondrial Ca++ buffering capacity. Mitochondrial Ca++ buffering was evaluated in a thermostatically-controlled, continuously stirred cuvette incubated in a Shimadzu RF-5301 spectrofluorimeter set at 37°C. Levels of extra-mitochondrial calcium were measured using Ca++-sensitive indicator calcium green 5 N (CaG5N), a fluorescent calcium-sensitive indicator. Malate and pyruvate (M/P) and ADP were loaded sequentially over 2 min followed by oligomycin (O) at 3 min. Two minutes after adding oligomycin, calcium (32 nmol/L) infusion started at a rate of 0.5µL/min. Part (b) is a typical trace that shows the differences in mitochondrial Ca++ uptake in sham (non-injured), vehicle-treated injured and U-83836E-treated injured mitochondria. Values = mean ± sd. Statistical differences (one-way anova and Student-Neuman–Keuls post hoc test): *p < 0.01 versus sham, #p < 0.05 versus vehicle, n = 4.
Discussion
The present work continues to validate free radical-induced LP damage as a key event in the pathobiology of post-traumatic brain secondary injury after TBI. The current work explored one element of LP which is the propagation of LOO•-mediated LP chain reactions. The results have demonstrated that scavenging LOO• radicals is able to protect both cortical tissue and mitochondria against oxidative damage after TBI. Specifically, U-83836E preserved mitochondrial function in terms of respiration rates, RCR values and Ca++ buffering capacity. The drug, U-83836E, which is a selective scavenger of LOO• was employed in this study so as to reveal the role of lipid peroxyl radicals in mitochondrial dysfunction after TBI.
Because U-83836E lacks any other neuroprotective mechanisms it provides a tool for selectively exploring the pathophysiological role of LP in post-TBI secondary injury (Hall et al. 1991). U-83836E is a synthetic dual mechanism LOO• radical scavenger that represents a chemical combination of the bis-pyrrolidino-pyrimidine functionality from the 21-aminosteroid LP inhibitor tirilazad with the LP-inhibiting chromanol ring structure of vitamin E (Hall et al. 1991). Both moieties are scavengers of LOO• radicals. The bis-pyrrolidino-pyrimidine can catalytically (repeatedly) scavenge LOO• while the electron-donating chromanol, after donating an electron to a LOO•, can be re-reduced by other physiological reducing agents such as glutathione or ascorbate. As a result of these dual LOO• scavenging mechanisms, U-83836E is one of the most potent inhibitors of free radical-induced, iron-catalyzed LP currently available for study. Moreover, the drug has been repeatedly shown to protect cultured neurons from a variety of oxidative insults and to do so in conjunction with an attenuation of LP-mediated damage (Grasbon-Frodl et al. 1996; Sureda et al. 1999; Karlsson et al. 2002; Kabadere et al. 2004; Thajeb et al. 2006). However this is the first report to show that U-83836E inhibits LP in an in vivo TBI model.
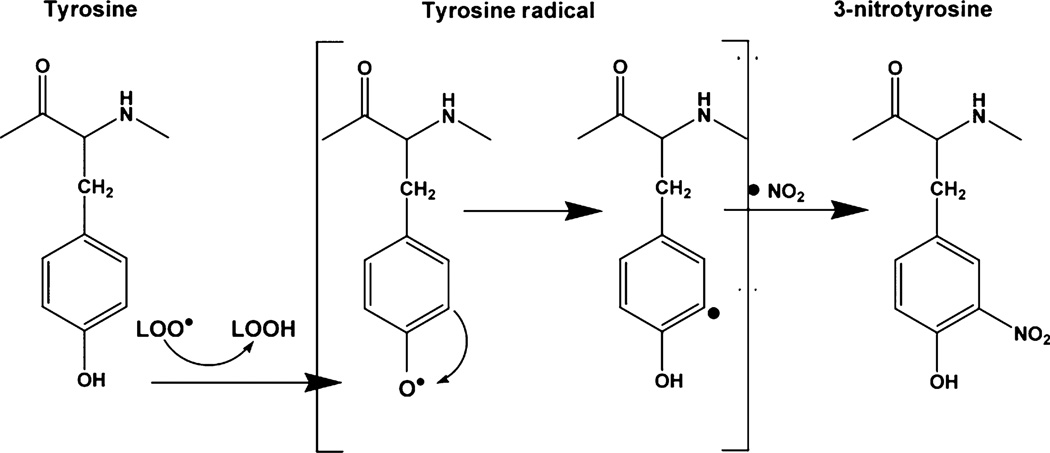
Unexpectedly, U-83836E also inhibited protein nitration (3-NT) in injured cortical tissue and mitochondria even though it does not interact directly with the reactive nitrogen species peroxynitrite. This ability to inhibit nitration-mediated oxidative damage has been reported for the pyrrolopyrimidine LP inhibitor U-101033E (Rohn and Quinn 1998). Peroxynitrite causes nitration of tyrosine phenol ring via its generation of the highly reactive nitrogen dioxide radical (·NO2) (Alvarez and Radi 2003). As illustrated in Fig. 6 the first step of nitration is the abduction of H atom from the phenol ring of tyrosine leading to the formation of a tyrosine radical. By means of electron rearrangement, the unpaired electron moves to Carbon 3. This is followed by nitration of carbon 3 by ·NO2 (Beckman and Koppenol 1996) leading to the formation of 3-NT which is routinely employed as a biomarker for nitrative oxidative damage (Denicola et al. 1996; Radi 1998; Alvarez and Radi 2003). Although the second step is mediated exclusively by ·NO2, the first step (oxidative removal of an electron from the phenolic ring) can be mediated by various reactive species like LOO•. Accordingly, as LOO• radicals can potentially initiate the process of tyrosine nitration, their scavenging by U-83836E most likely explains how U-83836E is able to reduce the levels of 3-NT in injured cortical tissue and mitochondrial proteins.
Fig. 6.
Mechanistic hypothesis of the contribution of lipid peroxyl radicals to peroxynitrite-mediated oxidative damage following traumatic brain injury (see Discussion).
We also have shown in this report that U-83836E is able to confer protection against mitochondrial dysfunction after TBI. Mitochondria are prone to both direct and indirect LP-mediated damage following TBI. Compelling evidence suggests that LP plays a central role in loss of Ca++ homeostasis after TBI. It has been shown that the accumulation of 4-HNE aggravates excitotoxicity by impeding glutamate uptake at synapses (Pedersen et al. 1999) which can potentially aggravate Ca++ dysregulation by prolonging Ca++ influxes into neuronal cells. Similarly, LP-mediated damage has been associated with Ca++ accumulation in endothelial cells following hydrogen peroxide exposure (Kimura et al. 1992). Lipid peroxidative damage has also been demonstrated to compromise the integrity of endothelial cells and increase vascular permeability, an effect that was successfully reversed by U-83836E and other LOO• radical scavengers (Shi et al. 1995). Moreover, LP compromises the ability of the cell to restore Ca++ homeostasis by damaging the ATPase within the cell membrane responsible for Ca++ extrusion (Hall and Springer 2004). The potentiation of Ca++ dysregulation by LP puts a huge burden on mitochondria which contribute to Ca++ homeostasis by uptake of cytosolic Ca++ (Budd and Nicholls 1996). Thus, the post-traumatic increase in cytosolic Ca++ leads to a comparable increase in mitochondrial Ca++ load that is detrimental to mitochondrial functions (Xiong et al. 1997a). Furthermore, Ca++ overloaded mitochondria display a high rate of free radical formation (Halliwell and Chirico 1993; Singh et al. 2006). The free radical-induced LP in mitochondrial membranes causes further deterioration of brain mitochondria, limiting their ability to buffer Ca++ (Xiong et al. 1997b; Singh et al. 2006; Deng-Bryant et al. 2008).
In conclusion, we hypothesize that U-83836E ameliorated TBI sequela in mitochondria via multiple indirect and direct avenues. First, U83836E may have mitigated the post-traumatic surge in cytosolic Ca++ mediated by glutamate by shielding glutamate uptake mechanisms against LP-mediated damage (Keller et al. 1997; Pedersen et al. 1999). Similarly, studies done in cell cultures have shown that LOO• radical scavengers inhibit oxidant-stimulated Ca++ influxes (Kimura et al. 1992; Munns and Leach 1995). Secondly, U-83836E has been shown to stabilize ATPase activity in the traumatized brain (Durmaz et al. 2003) which can potentially help the cell preserve Ca++ homeostasis. Thirdly, U-83836E has also been effective in abrogating Ca++ leak from endoplasmic reticulum following oxidant treatment (Racay et al. 1997). Such effects of U-83836E in maintaining cytosolic Ca++ homeostasis are central for preserving mitochondrial functions and mitigating free radical generation. In addition to preventing Ca++ dysregulation, U-83836E also protected mitochondrial proteins from aberrant modification by LP byproducts. High levels of LP in mitochondria have been always linked to mitochondrial dysfunction (Sullivan et al. 1999; Singh et al. 2006; Mbye et al. 2008). By protecting mitochondrial proteins, U-83836E enhanced mitochondrial respiration and Ca++ buffering capacity.
Abbreviations used
- 3-NT
3-nitrotyrosine
- 4-HNE
4-hydroxynonenal
- BSA
bovine serum albumin
- CaG5N
Ca++-sensitive indicator calcium green 5 N
- CCI
controlled cortical impact
- LOO•
lipid peroxyl radicals
- LP
lipid peroxidation
- RCR
respiratory control ratio
- SNK
Student-Neuman–Keuls
- TBI
traumatic brain injury
- TMRE
tetramethyl rhodamine ethyl ester
References
- Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Dorenbos KA, Modafferi EA, Geddes JW, Steward O. Nitrogen disruption of synaptoneurosomes: an alternative method to isolate brain mitochondria. J. Neurosci. Methods. 2004;137:299–303. doi: 10.1016/j.jneumeth.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J. Neurochem. 1996;66:403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- Deng Y, Thompson BM, Gao X, Hall ED. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng-Bryant Y, Singh IN, Carrico KM, Hall ED. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. 2008;28:1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Denicola A, Freeman BA, Trujillo M, Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch. Biochem. Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- Durmaz R, Kanbak G, Akyuz F, Isiksoy S, Yucel F, Inal M, Tel E. Lazaroid attenuates edema by stabilizing ATPase in the traumatized rat brain. Can. J. Neurol. Sci. 2003;30:143–149. doi: 10.1017/s0317167100053415. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Gadelha FR, Thomson L, Fagian MM, Costa AD, Radi R, Vercesi AE. Ca2+-independent permeabilization of the inner mitochondrial membrane by peroxynitrite is mediated by membrane protein thiol cross-linking and lipid peroxidation. Arch. Biochem. Biophys. 1997;345:243–250. doi: 10.1006/abbi.1997.0259. [DOI] [PubMed] [Google Scholar]
- Grasbon-Frodl EM, Andersson A, Brundin P. Lazaroid treatment prevents death of cultured rat embryonic mesencephalic neurons following glutathione depletion. J. Neurochem. 1996;67:1653–1660. doi: 10.1046/j.1471-4159.1996.67041653.x. [DOI] [PubMed] [Google Scholar]
- Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J. Med. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- Hall ED. Effects of the 21-aminosteroid U74006F on post-traumatic spinal cord ischemia in cats. J. Neurosurg. 1988;68:462–465. doi: 10.3171/jns.1988.68.3.0462. [DOI] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Braughler JM, Yonkers PA, et al. U-78517F: a potent inhibitor of lipid peroxidation with activity in experimental brain injury and ischemia. J. Pharmacol. Exp. Ther. 1991;258:688–694. [PubMed] [Google Scholar]
- Hall E, Vaishnav R, Mustafa A. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993;57:715S–724S. doi: 10.1093/ajcn/57.5.715S. discussion 724S–725S. [DOI] [PubMed] [Google Scholar]
- Hansson MJ, Mansson R, Morota S, et al. Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic. Biol. Med. 2008;45:284–294. doi: 10.1016/j.freeradbiomed.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Jouaville LS, Ichas F, Mazat JP. Modulation of cell calcium signals by mitochondria. Mol. Cell. Biochem. 1998;184:371–376. [PubMed] [Google Scholar]
- Kabadere S, Oztopcu P, Korkmaz S, Erol K, Uyar R. MgSO4 and lazaroid (U-83836E) partially protects glioma cells against glutamate toxicity in vitro. Acta Neurobiol. Exp. (Wars) 2004;64:461–466. doi: 10.55782/ane-2004-1528. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Emgard M, Brundin P. Comparison between survival of lazaroid-treated embryonic nigral neurons in cell suspensions, cultures and transplants. Brain Res. 2002;955:268–280. doi: 10.1016/s0006-8993(02)03601-6. [DOI] [PubMed] [Google Scholar]
- Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997;80:685–696. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Kimura M, Maeda K, Hayashi S. Cytosolic calcium increase in coronary endothelial cells after H2O2 exposure and the inhibitory effect of U78517F. Br. J. Pharmacol. 1992;107:488–493. doi: 10.1111/j.1476-5381.1992.tb12772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med. 1999;26:463–471. doi: 10.1016/s0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Friberg H, Neumar RW, et al. Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J. Cereb. Blood Flow Metab. 2003;23:219–231. doi: 10.1097/01.WCB.0000040581.43808.03. [DOI] [PubMed] [Google Scholar]
- Madikians A, Giza C. A clinician’s guide to the pathophysiology of traumatic brain injury. Indian J. Neurotra. 2006;3:9–17. [Google Scholar]
- Mbye LH, Singh IN, Sullivan PG, Springer JE, Hall ED. Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp. Neurol. 2008;209:243–253. doi: 10.1016/j.expneurol.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Munns PL, Leach KL. Two novel antioxidants, U74006F and U78517F, inhibit oxidant-stimulated calcium influx. Free Radic. Biol. Med. 1995;18:467–478. doi: 10.1016/0891-5849(94)00163-e. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Cashman NR, Mattson MP. The lipid peroxidation product 4-hydroxynonenal impairs glutamate and glucose transport and choline acetyltransferase activity in NSC-19 motor neuron cells. Exp. Neurol. 1999;155:1–10. doi: 10.1006/exnr.1998.6890. [DOI] [PubMed] [Google Scholar]
- Racay P, Kaplan P, Mezesova V, Lehotsky J. Lipid peroxidation both inhibits Ca(2+)-ATPase and increases Ca2+ permeability of endoplasmic reticulum membrane. Biochem. Mol. Biol. Int. 1997;41:647–655. doi: 10.1080/15216549700201691. [DOI] [PubMed] [Google Scholar]
- Radi R. Peroxynitrite reactions and diffusion in biology. Chem. Res. Toxicol. 1998;11:720–721. doi: 10.1021/tx980096z. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Quinn MT. Inhibition of peroxynitrite-mediated tyrosine nitration by a novel pyrrolopyrimidine antioxidant. Eur. J. Pharmacol. 1998;353:329–336. doi: 10.1016/s0014-2999(98)00399-9. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sullivan PG. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma. 1999;16:783–792. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- Shi F, Cavitt J, Audus KL. 21-aminosteroid and 2-(aminomethyl) chromans inhibition of arachidonic acid-induced lipid peroxidation and permeability enhancement in bovine brain microvessel endothelial cell monolayers. Free Radic. Biol. Med. 1995;19:349–357. doi: 10.1016/0891-5849(95)00049-4. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Hall ED. Peroxynitrite-mediated oxidative damage to brain mitochondria: protective effects of peroxynitrite scavengers. J. Neurosci. Res. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- Spiteller G. Peroxyl radicals: inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radic. Biol. Med. 2006;41:362–387. doi: 10.1016/j.freeradbiomed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Dube C, Dorenbos K, Steward O, Baram TZ. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann. Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Sureda FX, Gabriel C, Pubill D, Pallas M, Escubedo E, Camarasa J, Camins A. Effects of U-83836E on glutamate-induced neurotoxicity in dissociated rat cerebellar granule cells. Toxicol. Appl. Pharmacol. 1999;156:1–5. doi: 10.1006/taap.1998.8613. [DOI] [PubMed] [Google Scholar]
- Thajeb P, Kuo JS, Shyu WC, Lin SZ. Neuroprotection with methylaminochroman and lazaroid of embryonic ventral mesencephalic tegmental dopaminergic neurons in cold storage. J. Clin. Neurosci. 2006;13:467–470. doi: 10.1016/j.jocn.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Violi F, Marino R, Milite MT, Loffredo L. Nitric oxide and its role in lipid peroxidation. Diabetes Metab. Res. Rev. 1999;15:283–288. doi: 10.1002/(sici)1520-7560(199907/08)15:4<283::aid-dmrr42>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Gu Q, Peterson PL, Muizelaar JP, Lee CP. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma. 1997a;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Peterson PL, Muizelaar JP, Lee CP. Amelioration of mitochondrial function by a novel antioxidant U-101033E following traumatic brain injury in rats. J. Neurotrauma. 1997b;14:907–917. doi: 10.1089/neu.1997.14.907. [DOI] [PubMed] [Google Scholar]