Abstract
Purpose
To investigate the efficacy and predictability of wavefront-guided laser in situ keratomileusis (LASIK) treatments using the iris registration (IR) technology for the correction of refractive errors in patients with large pupils.
Setting
Horus Vision Correction Center, Alexandria, Egypt.
Methods
Prospective noncomparative study including a total of 52 eyes of 30 consecutive laser refractive correction candidates with large mesopic pupil diameters and myopia or myopic astigmatism. Wavefront-guided LASIK was performed in all cases using the VISX STAR S4 IR excimer laser platform. Visual, refractive, aberrometric and mesopic contrast sensitivity (CS) outcomes were evaluated during a 6-month follow-up.
Results
Mean mesopic pupil diameter ranged from 8.0 mm to 9.4 mm. A significant improvement in uncorrected distance visual acuity (UCDVA) (P < 0.01) was found postoperatively, which was consistent with a significant refractive correction (P < 0.01). No significant change was detected in corrected distance visual acuity (CDVA) (P = 0.11). Efficacy index (the ratio of postoperative UCDVA to preoperative CDVA) and safety index (the ratio of postoperative CDVA to preoperative CDVA) were calculated. Mean efficacy and safety indices were 1.06 ± 0.33 and 1.05 ± 0.18, respectively, and 92.31% of eyes had a postoperative spherical equivalent within ±0.50 diopters (D). Manifest refractive spherical equivalent improved significantly (P < 0.05) from a preoperative level of −3.1 ± 1.6 D (range −6.6 to 0 D) to −0.1 ± 0.2 D (range −1.3 to 0.1 D) at 6 months postoperative. No significant changes were found in mesopic CS (P ≥ 0.08), except CS for three cycles/degree, which improved significantly (P = 0.02). Magnitudes of primary coma and trefoil did not change significantly (P ≥ 0.34), with a small but statistically significant increase in primary spherical aberration.
Conclusion
Wavefront-guided LASIK provides an effective correction of low to moderate myopia or myopic astigmatism in large pupil patients without deterioration of visual quality.
Keywords: large pupil, wavefront, LASIK
Introduction
Laser corneal refractive surgery has been shown to be a safe and reliable option for the correction of refractive errors.1 However, the induction of significant amounts of higher order aberrations (HOA) that may lead to a postoperative limitation of the visual quality (mainly primary coma and spherical aberration) has been described as a potential side effect of this surgical option.2–11 This aberrometric phenomenon has been associated with several factors, such as the use of inappropriate ablation algorithms, corneal biomechanical changes, flap creation in laser assisted in situ keratomileusis (LASIK) surgery, decentration of the ablation, or the effectivity loss of the peripheral laser rays coming into contact with the cornea in a nonorthogonal way with no energy compensation.12–17 Visual discomfort and night vision disturbances after keratorefractive surgery have been related to the magnitude of these induced HOAs.4,18,19 These aberration-related symptoms are pupil-dependent. It should be noted that the larger the pupil diameter, the higher the magnitude of HOA. This is the reason for considering the presence of a large mesopic pupil size as a potential risk factor for night vision complaints after refractive surgery.20
The introduction of wavefront-guided laser technology into the field of refractive surgery in 1999 represented a significant advancement in ophthalmology, allowing an optimized correction not only of spherocylindrical errors but also of HOA.21,22 Specifically, ocular or total wavefront-guided ablations have been shown to be effective in minimizing aberrations in eyes without previous unsuccessful or nonoptimized refractive surgeries.23–33 Patients with large mesopic pupil sizes seem to be ideal candidates for this type of procedure considering the commonly increased aberrometric profile in these type of eyes, and the potential of night vision disturbances after surgery.20 However, the larger the pupil, the higher the possibility of a significant pupil center displacement from mesopic conditions maintained during aberrometric measurements to photopic conditions during laser treatment. The recently developed iris registration (IR) technology compensates for such shift by means of axial registration considering the iris periphery as a reference for centration. IR also uses torsional registration based on identification of the individual iris details to compensate for torsional changes that might occur when patient’s position changes from sitting during aberrometric measurement to supine position during laser treatment. IR provides better axial and torsional registration, and this may be especially useful in eyes with large pupils.34
The aim of this prospective noncomparative study was to investigate the efficacy and predictability of wavefront-guided LASIK treatments using the IR technology for the correction of refractive errors in patients with large pupils.
Patients and methods
This prospective, noncomparative study included a total of 52 eyes from 30 consecutive laser refractive correction candidates at the Horus Vision Correction Center ([HVCC] Alexandria, Egypt). In all cases, wavefront-guided LASIK was indicated and performed due to the presence of a preoperative large pupil size by using the VISX STAR S4 IR excimer laser platform (Abbott Medical Optics Inc, Santa Ana, CA). This study was reviewed and approved by the Institutional Review Board at HVCC in Alexandria, Egypt. Written informed consent was obtained from all subjects before their participation in the study.
Inclusion and exclusion criteria
Inclusion criteria consisted of preoperative refractive errors ranging from +4.0 diopters (D) to −7.0 D of the spherical equivalent of myopia or hyperopia with astigmatism of up to 5.0 D at the spectacle plane, and pupil diameter of 8.0 mm or larger at 3 cd/m2 of illumination (mesopic),35 age of 18 years old or older, and stable refraction, which was defined as a change in the spherical equivalent within ±0.5 D over the last 12 months. Exclusion criteria were dry eye syndrome, irregular corneal topography patterns compatible with corneal ectatic disease, corneal scarring, history of herpetic eye disease, significant anterior and posterior segment pathologies, previous ocular surgeries, autoimmune disease, and any active ocular disease. Contact lens wearers were asked to discontinue the use of contacts at least 1 week for soft lenses and 3 weeks for hard or gas permeable lenses prior to the preoperative examination. If topographic features of contact lens warpage or unstable tear film were detected after that period, the patients were asked to discontinue wearing contact lenses for 1 or 2 additional weeks while using preservative-free artificial tear drops.
Preoperative evaluation and surgical planning
The preoperative evaluation included examining patients’ ocular and medical history with special attention to the above mentioned exclusion criteria, manifest and cycloplegic refraction measurements, uncorrected (UDVA) and best corrected distance visual acuity (CDVA) testing, pupil diameter under both photopic and mesopic conditions using the Colvard pupillometer35 (OASIS Medical, Inc, Glendora, CA), mesopic contrast sensitivity (CS) testing using the CVS-1000 chart (Vector-Vision Inc, Greenville, OH), corneal topography, anterior segment imaging using the Pentacam-HR system (Oculus Inc, Wetzlar, Germany), ultrasound pachymetry using the Nidek UP-1000 US pachymeter (Nidek Co, Ltd, Gamagori, Japan), slit-lamp examination, and applanation tonometry. Regarding the aberrometric analysis, the following parameters were analyzed and recorded: higher order root mean square (RMS), primary coma RMS (computed for the Zernike terms Z3±1), trefoil RMS, and the Zernike term corresponding to the primary spherical aberration (Z40) with its sign.
The WaveScan aberrometer (Hartmann-Shack wavefront sensor; Abbott Medical Optics Inc) was used for the preoperative measurement of wavefront aberrations as well as for the planning of the most optimum ablation profile in each case (wavefront-guided ablation profile). Target postoperative refraction was emmetropia in all eyes. The wavefront-guided customized ablation was designed and calculated using the commercially available software CustomVue™ from Abbott Medical Optics Inc. For this purpose, the aberrometric data as well as the manifest refraction, central corneal thickness, and intended flap pachymetry were introduced in the software. In all cases, the optical zone was set to 6.5 mm and the transition zone to 8.5 mm.
Surgical procedure
All surgical procedures were performed by the same surgeon at HVCC. The designed treatment with the CustomVue™ software was first loaded to the excimer laser computer and reviewed by the surgeon to confirm the data. The VISX STAR S4 IR excimer laser platform (0.65 mm spot size combined with the ActiveTrak 3D eye tracker; Abbott Medical Optics Inc) was used to perform all the LASIK treatments. After ablation pattern confirmation, a corneal flap was created by means of the mechanical microkeratome Moria M2 (Moria SA, Antony, France) and lifted prior to corneal laser ablation. The microkeratome rings were selected according to the preoperative k-reading for each individual eye (manufacturer’s recommendations) to create superior hinge flaps at a diameter of 9.0 mm. After this, the programmed treatment was applied on the stroma. All surgeries were performed under topical anesthesia. Regular topical postoperative treatment was administered to all patients in the form of topical antibiotics, topical steroids, and topical preservative-free artificial tears drops. No retreatments were performed in any case during the postoperative follow-up.
Postoperative follow-up
Patients were examined the day after surgery and were then scheduled to come back to the hospital after 1 week, as well as 1, 3, and 6 months postoperatively. Since then, regular examinations were recommended every year. On the first postoperative day, a detailed slit-lamp examination was performed to evaluate the flap position and the integrity of the cornea. UDVA and CDVA assessment, manifest refraction, and biomicroscopic examination were performed in the remaining visits. Likewise, mesopic CS and ocular wavefront aberrations were evaluated at the last follow-up visit. Efficacy and safety indexes were calculated using the visual acuities in decimal notation. The efficacy index was calculated as the ratio of the postoperative UDVA to the preoperative CDVA, whereas the safety index was calculated as the ratio of the postoperative CDVA to the preoperative CDVA. Furthermore, the magnitude of decentration was evaluated at the end of the follow up. The method of evaluating the ablation centration has been described.36 Briefly, on the preoperative map, the cursor was placed at the detected pupil center and the distance from the corneal vertex to the pupil center was recorded directly from the legend in both millimeters and the angle of semimeridians. On the difference map, between preoperative and 6 months postoperative, the cursor was placed in the center of the confluent blue zone, which represents the ablation center. The distance from the corneal vertex to the ablation center was recorded directly from the legend. The distance from the pupil center to the ablation center (amount of decentration) was calculated by vector analysis from the above data (ie, distance from the corneal vertex to the pupil center and from the corneal vertex to the ablation zone; Figure 1).
Figure 1.
Pentacam (Oculus Inc, Wetzlar, Germany) axial curvature difference map comparing pre- and postoperative curvature to assess treatment centration.
Refraction notation
The spherocylindrical refractions obtained before and after surgery were converted to vectorial notations using the power vector method described by Thibos and Horner.37 Using this procedure, any spherocylindrical refractive errors can be expressed by three dioptric powers: M, J0, and J45, with M being a spherical lens equal to the spherical equivalent of the given refractive error, and J0 and J45 are two Jackson crossed cylinders equivalent to the conventional cylinder. These numbers are the coordinates of a point in a three-dimensional dioptric space (M, J0, J45). The length of this vector is a measure of the overall blurring strength B of a spherocylindrical refractive error.
According to the power vector method, manifest refractions in conventional script notation (S [sphere], C [cylinder] × ϕ [axis]) were converted to power vector coordinates and overall blurring strength (B) by the following formulas:
| (1) |
| (2) |
| (3) |
| (4) |
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences software for Windows, version 15.0 (IBM Corporation, Armonk, NY). Normality of the data samples was evaluated by means of the Kolmogorov–Smirnov and Shapiro–Wilk tests. When parametric analysis was possible, the Student’s t-test for paired data was used for comparisons between the preoperative and postoperative data, whereas the Wilcoxon rank sum test was applied to assess the significance of such differences when parametric analysis was not possible. Differences were considered to be statistically significant when the associated P-value was <0.05. Correlation coefficients (Pearson or Spearman, depending if normality condition could be assumed) were used to assess the correlation between different variables. Furthermore, the standard graphs for reporting the outcomes in refractive surgery according to the Waring protocol38 were used for displaying and summarizing the main outcomes of this study.
Results
Mean patient age of the analyzed sample was 27.4 (standard deviation [SD] ± 3.1) years (range 19 to 32 years). Nine patients were males and 21 were females. Mean mesopic pupil diameter ranged from 8.0 mm to 9.4 mm, with a mean value of 8.6 (SD ± 0.41) mm. IR was enabled in 86% of cases during laser treatment. Table 1 summarizes the preoperative and postoperative visual, refractive, and aberrometric outcomes.
Table 1.
Summary of the visual, refractive, and aberrometric outcomes in the current series during the complete follow-up
| Outcome | Preoperative Mean (SD) Median (range) |
Postoperative Mean (SD) Median (range) |
P-value (Wilcoxon test) |
|---|---|---|---|
| LogMAR UDVA | 0.41 (0.18) | 0.01 (0.05) | <0.01 |
| 0.40 (0.05 to 0.70) | 0.00 (−0.08 to 0.10) | ||
| Manifest sphere (D) | −2.65 (1.67) | −0.07 (0.25) | <0.01 |
| −3.00 (−6.25 to 1.00) | 0.00 (−1.00 to 0.25) | ||
| Manifest cylinder (D) | −1.02 (0.71) | −0.19 (0.20) | <0.01 |
| −1.00 (−2.25 to 0.00) | −0.25 (−0.75 to 0.00) | ||
| J0 (D) | 0.32 (0.39) | 0.07 (0.10) | <0.01 |
| 0.35 (−0.81 to 1.11) | 0.01 (−0.11 to 0.34) | ||
| J45 (D) | 0.06 (0.36) | 0.003 (0.075) | 0.20 |
| 0.03 (−0.77 to 1.11) | 0.000 (−0.20 to 0.22) | ||
| B (D) | 3.25 (1.57) | 0.23 (0.27) | <0.01 |
| 3.68 (0.00 to 6.64) | 0.18 (0.00 to 1.43) | ||
| MRSE (D) | −3.15 (1.64) | −0.17 (0.28) | <0.01 |
| −3.63 (−6.63 to 0.00) | −0.13 (−1.38 to 0.13) | ||
| LogMAR CDVA | 0.02 (0.11) | −0.002 (0.060) | 0.11 |
| 0.00 (−0.08 to 0.40) | 0.000 (−0.08 to 0.15) | ||
| Primary coma RMS (μm) | 0.27 (0.14) | 0.25 (0.16) | 0.64 |
| 0.23 (0.10 to 0.75) | 0.24 (0.05 to 0.63) | ||
| Primary trefoil RMS (μm) | 0.24 (0.15) | 0.24 (0.18) | 0.35 |
| 0.18 (0.05 to 0.65) | 0.18 (0.08 to 0.77) | ||
| Primary spherical aberration (μm) | −0.02 (0.17) | 0.16 (0.23) | <0.01 |
| 0.01 (−0.45 to 0.38) | 0.20 (−0.45 to 0.62) |
Abbreviations: SD, standard deviation; UDVA, uncorrected distance visual acuity; J0 and J45, power vector components of manifest cylinder; MRSE, spherical equivalent; B, overall blurring strength of the manifest spherocylindrical error; CDVA, corrected distance visual acuity; RMS, root mean square; D, diopters.
Visual outcomes
As shown in Table 1, a significant improvement in LogMAR UDVA was found postoperatively (Wilcoxon test, P < 0.01); however, no significant changes were detected in LogMAR CDVA (Wilcoxon test, P = 0.11). Postoperative LogMAR UDVA was 0.1 (about 20/25) or better in all cases (100%), and 0.0 (about 20/20) or better in 44 eyes (84.62%) (Figure 2). Postoperative LogMAR CDVA was 0.1 (about 20/25) or better in all cases (100%), and 0.0 (about 20/20) or better in 48 eyes (92.31%).
Figure 2.
Comparative distribution of the preoperative CDVA and the UDVA at the end of the follow-up.
Abbreviations: CDVA, corrected distance visual acuity; UDVA, uncorrected distance visual acuity.
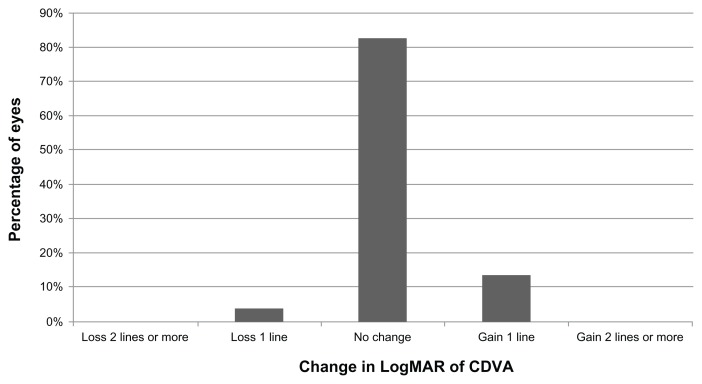
The overall efficacy and safety indices were 1.06 (SD ± 0.33) and 1.05 (SD ± 0.18), respectively. Postoperatively, losses of lines of CDVA were only observed in two eyes (3.85%) (Figure 3). In contrast, gains of lines of CDVA were detected in a total of seven eyes (13.46%) (Figure 3).
Figure 3.
Distribution of changes in postoperative CDVA during follow-up.
Abbreviation: CDVA, corrected distance visual acuity.
Refractive outcomes
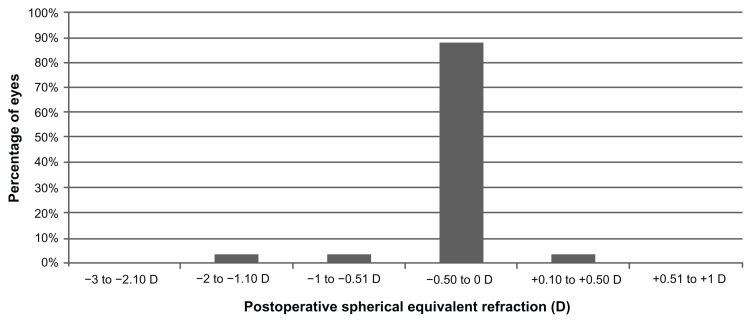
As shown in Table 1, statistically significant reductions in B and M values were found postoperatively (Wilcoxon test, P < 0.01). Almost all eyes (50 eyes, 96.15%) had a postoperative value of M within ±1.00 D of emmetropia (Figure 4), and 48 eyes (92.31%) had a postoperative M value within ±0.50 D of emmetropia (Figures 4 and 5). Figure 4 shows the achieved spherical equivalent correction plotted against the intended correction. A strong and statistically significant correlation was found among the achieved and the intended corrections (r = 0.97, P < 0.01). Regarding the astigmatic outcomes (Table 1), a statistically significant reduction was only detected postoperatively in the power vector component J0 (Wilcoxon test, P < 0.01).
Figure 4.
Distribution of the postoperative spherical equivalent during the whole follow-up.
Abbreviation: D, diopters.
Figure 5.
Scattergram showing the relationship between the achieved postoperative spherical equivalent correction and the intended.
Abbreviations: SE, spherical equivalent; D, diopters.
Contrast sensitivity outcomes
Figure 6 summarizes the monocular CS outcomes obtained in the analyzed sample under mesopic conditions preoperatively and postoperatively. No statistically significant changes were observed after surgery in the CS corresponding to the spatial frequencies of six (P = 0.68, Wilcoxon test), 12 (P = 0.08, Wilcoxon test), and 18 cycles/degree (P = 0.47, Wilcoxon test). Only a minimal but statistically significant improvement was observed for the lowest spatial frequency evaluated, three cycles/degree (P = 0.02, Wilcoxon test). Mesopic pupil diameter did not correlate with CS for any of the spatial frequencies analyzed (three cycles/degree, r = −0.15, P = 0.32; six cycles/degree, r = −0.11, P = 0.47; 12 cycles/degree, r = −0.19, P = 0.20; 18 cycles/degree, r = −0.21, P = 0.16).
Figure 6.
Mean monocular contrast sensitivity function measured under mesopic conditions preoperatively (gray line) and postoperatively (red line).
Ocular aberrometric outcomes
No statistically significant changes were observed in primary coma (P = 0.64, Wilcoxon test) and trefoil RMS (P = 0.34, Wilcoxon test) (Table 1). However, a small but statistically significant change in the magnitude of primary spherical aberration toward a more positive value was found postoperatively (P < 0.01, Wilcoxon test) (Table 1). Specifically, the mean change in this aberrometric parameter was 0.18 μm.
A weak but statistically significant correlation between postoperative primary coma RMS and CDVA was observed (r = 0.31, P = 0.03). However, no significant correlations of postoperative CDVA with postoperative trefoil RMS (r = 0.17, P = 0.24) and Z40 (r = −0.17, P = 0.26) were detected. Mesopic pupil size did not correlate with the magnitude of the postoperative HOA analyzed: primary coma (r = 0.16, P = 0.29), trefoil (r = 0.25, P = 0.10), and primary spherical aberration (r = 0.11, P = 0.47). Furthermore, no significant correlations were detected between postoperative CS and the magnitude of the HOA analyzed (−0.21 ≤ r ≤ 0.21, P ≥ 0.15). The change in M was also found to be significantly correlated with the change in magnitude of primary spherical aberration with surgery (r = −0.51, P < 0.01) (Figure 7). Figure 8 shows the aberrometric outcomes in one patient that presented an improvement or maintenance of HOA.
Figure 7.
Scattergram showing the relationship between the change with surgery in spherical equivalent (ΔM) and the associated change in the magnitude of the Zernike coefficient corresponding to the primary spherical aberration (ΔZ40).
Note: The adjusting line to the data obtained by means of the least-squares fit is shown (R2: 0.20): ΔZ ΔM 40 =0.030 − 0.052×.
Abbreviation: D, diopters.
Figure 8.
CustomVue™ software (Abbott Medical Optics Inc, Santa Ana, CA) showing the aberrometric outcomes in one patient that presented an improvement/ maintenance of HOA. (A) Shows wave-front maps, and (B) shows Zernike polynomials differences.
Note: Images used with permission of Abbott Medical Optics Inc, Santa Ana, CA.
Abbreviation: HOA, higher order aberrations.
Complications
No intraoperative complications occurred. Retreatment was not necessary during the follow-up period in any cases from the current series. Decentration analysis by means of corneal topography revealed the absence of decentrations.
Discussion
Excimer laser wavefront-guided ablations were developed and introduced in clinical practice with the aim of optimizing the outcomes obtained with keratorefractive procedures. Several studies have shown that this type of procedure is effective in correcting the spherocylindrical error and minimizing HOA in eyes without previous refractive surgeries.23–33 Therefore, this surgical procedure has the potential to reduce post-LASIK night vision problems, and may even result in improved post-LASIK vision. These types of treatments seem to be especially useful in patients with large pupil diameters who are more likely to suffer from HOA and related night vision disturbances after LASIK.20 Indeed, Chan and Manche39 found that large pupil size does not positively correlate with any postoperative visual symptoms 12 months after wavefront-guided LASIK surgery. One potential limitation for the aberrometric minimization programmed with the ablation profile is the cyclotorsion in the supine position that can lead even to the induction of aberrations.40 The IR technology has been recently developed with the aim of compensating for these cyclotorsional movements during surgery by means of axial registration, while considering the iris periphery as a reference for centration. The aim of the current study was to investigate the efficacy of wavefront-guided LASIK treatments using the IR technology for the correction of refractive errors in patients with large pupils.
In the current series, a significant improvement in UDVA was found, which was consistent with a significant reduction of the spherocylindrical error. The efficacy index was of 1.06, an excellent value comparable to or even better than those reported by other authors with other wavefront-guided platforms.23–33 In addition, no significant changes in CDVA were observed. This reveals that an optimum visual quality was maintained after surgery. Indeed, the mean safety index was 1.05, with only losses of lines of CDVA observed in two eyes (3.85%). These results are also consistent with those reported in other previous series evaluating wavefront-guided LASIK procedures.23–33 Gains of lines of CDVA were detected in a total of seven eyes (13.46%). It should be noted that the improvement in CDVA should not be the main goal of the wavefront-guided procedures considering the neural limitations as well as other optical limitations, such as scattering, that cannot be controlled with the surgical procedure.41 The predictability of refractive correction was excellent, with 96.15% and 92.31% of eyes having a spherical equivalent within ±1.00 D and within ±0.50 D of emmetropia, respectively. These outcomes were similar and even better than those reported by other authors using other wavefront-guided platforms.23–33
Mesopic CS was maintained after surgery for the highest spatial frequencies evaluated, with a minimal but statistically significant improvement for three cycles/degree. This confirms that no deterioration of visual quality was induced with the surgery. Some contradictory findings have been reported in the peer reviewed literature concerning this issue. Some authors have found improvements in CS after wavefront-guided LASIK,26 whereas others have found no change,24,29 or even a small worsening.42 Several factors may have accounted for this significant variability among studies, such as the laser platform or the microkeratome used, the clinical procedure used for testing the CS, the optical zone programmed, and the specific clinical characteristics of the samples evaluated in each study. Furthermore, no significant correlations between postoperative CS and mesopic pupil diameter were found in our series. This reveals that pupil size did not compromise the postoperative visual quality. A similar finding was reported by Tuan and Liang43 in a study evaluating the CS outcomes after wavefront-guided procedures using the WaveScan aberrometer (Abbott Medical Optics Inc) and the Star S4 excimer laser system (Abbott Medical Optics Inc) in a sample of 274 myopic astigmatic eyes.
Regarding HOA, no significant changes were detected in the magnitude of primary coma and trefoil; however, a mean change of 0.18 μm in primary spherical aberration was found, which was statistically significant. Therefore, the wavefront-guided ablation pattern was able to prevent the induction of some kind of aberration except the primary spherical aberration. It should be considered that a myopic ablation of 2 D or higher was programmed in 71% of eyes, and a correlation between the change in M and the change in primary spherical aberration was present. Specifically, the more negative the value of M, the more positive the postoperative spherical aberration. In any case, the levels of this aberration were within the normal range as defined for the healthy population.44 In addition, the magnitude of this induction was of small magnitude and was also much lower than those induced by standard ablation profiles.2–11 For this reason, no significant correlations were found between the postoperative magnitude of this aberration and CDVA or CS.
It should be mentioned that lower levels of the induction of primary spherical aberration have been reported with optimized non-wavefront guided ablation profiles (aspheric) for similar amounts of myopia corrections.45–47 One factor that may have accounted for this fact is the selection of a smaller optical zone than the scotopic pupil size.48 Roberts and Koester49 estimated the effect of the optical zone for entrance pupils in the range between 2 mm and 8 mm using an optical analysis computer program. These authors concluded that the optical zone diameter in corneal refractive surgery must be at least as large as the entrance pupil diameter to preclude glare at the fovea, and larger than the entrance pupil to preclude parafoveal glare. A similar conclusion was reached by Klonos et al50 using a computer model. However, when scotopic pupil size is larger than 7 mm, the ablation diameter often cannot be as large as the pupil size, as recommended by Roberts and Koester49 and Klonos et al50 due to pachymetric limitations. In the current study, we used an optical zone of 6.5 mm and a total ablation zone of 8.5 mm in all cases. These diameters, as well as the customized blend zone, were thought to be enough to avoid the potential night vision disturbances after LASIK in these patients with large pupil sizes. Macsai et al51 found that the use of a peripheral transition zone 1.0 mm larger than the pupil diameter under scotopic conditions resulted in a low incidence of glare and halos postoperatively, and did not adversely affect the visual acuity. In our study, there was no negative effect of the selected optical zone on CDVA or CS. In addition, no significant correlations were detected between postoperative CS and the magnitude of the HOA analyzed.
This study has limitations such as that we fixed the optical and transition zones, and did not change them according to the mesopic pupil diameter. This was due to the limitations of the measured wavefront diameter with the used aberrometer which could not reach >8.0 mm. Also, the study did not include a comparison to the conventional ablation of Munnerlyn’s formula. Conventional ablation is comprehensively studied and has been proven to be unsatisfactory, so we felt no need to compare this in cases with large pupils.
In summary, ocular wavefront-guided LASIK using the VISX STAR S4 IR excimer laser platform is an effective and predictable procedure for the correction of low to moderate myopia and/or myopic astigmatism in large pupil patients, maintaining an excellent level of visual quality. Future studies with longer follow-ups are necessary to confirm the stability of the outcomes reported here. In addition, other potentially influencing factors on the postoperative visual quality in these types of patients after wavefront-guided LASIK procedures, such as the creation of the flap or the ablation pattern design, should be investigated further.
Acknowledgment
We would like to acknowledge IPASS (Investigación Personalizada al Servicio de la Salud, Alicante, Spain) for their collaboration in this study.
Footnotes
Disclosure
This study was supported by an unrestricted educational grant by Abbott Medical Optics Inc, Santa Ana, CA. This study was presented at ESCRS, Vienna, Austria in September 2011; at AAO, Orlando, Florida in October 2011; and at ASCRS, Chicago, IL, USA in April 2012. The authors report no other conflicts of interest in this work.
References
- 1.Sugar A, Rapuano CJ, Culberston WW, et al. Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109(1):175–187. doi: 10.1016/s0161-6420(01)00966-6. [DOI] [PubMed] [Google Scholar]
- 2.Pesudovs K. Wavefront aberration outcomes of LASIK for high myopia and high hyperopia. J Refract Surg. 2005;21(5):S508–S512. doi: 10.3928/1081-597X-20050901-18. [DOI] [PubMed] [Google Scholar]
- 3.Kohnen T, Mahmoud K, Bühren J. Comparison of corneal higher-order aberrations induced by myopic and hyperopic LASIK. Ophthalmology. 2005;112(10):1692. doi: 10.1016/j.ophtha.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.McCormick GJ, Porter J, Cox IG, MacRae S. Higher-order aberrations in eyes with irregular corneas after laser refractive surgery. Ophthalmology. 2005;112(10):1699–1708. doi: 10.1016/j.ophtha.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Llorente L, Barbero S, Merayo J, Marcos S. Total and corneal optical aberrations induced by laser in situ keratomileusis for hyperopia. J Refract Surg. 2004;20(3):203–216. doi: 10.3928/1081-597X-20040501-03. [DOI] [PubMed] [Google Scholar]
- 6.Yamane N, Miyata K, Samejima T, et al. Ocular higher-order aberrations and contrast sensitivity after conventional laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2004;45(11):3986–3990. doi: 10.1167/iovs.04-0629. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Koch DD. Anterior corneal optical aberrations induced by laser in situ keratomileusis for hyperopia. J Cataract Refract Surg. 2003;29(9):1702–1708. doi: 10.1016/s0886-3350(03)00576-5. [DOI] [PubMed] [Google Scholar]
- 8.Oliver KM, O’Brart DP, Stephenson CG, et al. Anterior corneal optical aberrations induced by photorefractive keratectomy for hyperopia. J Refract Surg. 2001;17(4):406–413. doi: 10.3928/1081-597X-20010701-01. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Barriuso E, Lloves JM, Marcos S, Navarro R, Llorente L, Barbero S. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Invest Ophthalmol Vis Sci. 2001;42(6):1396–1403. [PubMed] [Google Scholar]
- 10.Mrochen M, Kaemmerer M, Mierdel P, Seiler T. Increased higher-order optical aberrations after laser refractive surgery: a problem of subclinical decentration. J Cataract Refract Surg. 2001;27(3):362–369. doi: 10.1016/s0886-3350(00)00806-3. [DOI] [PubMed] [Google Scholar]
- 11.Oshika T, Klyce SD, Applegate RA, Howland HC, El Danasoury MA. Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 1999;127(1):1–7. doi: 10.1016/s0002-9394(98)00288-8. [DOI] [PubMed] [Google Scholar]
- 12.Gatinel D, Malet J, Hoang-Xuan T, Azar DT. Corneal asphericity change after excimer laser hyperopic surgery: theoretical effects on corneal profiles and corresponding Zernike expansions. Invest Ophthalmol Vis Sci. 2004;45(5):1349–1359. doi: 10.1167/iovs.03-0753. [DOI] [PubMed] [Google Scholar]
- 13.Cano D, Barbero S, Marcos S. Comparison of real and computer-simulated outcomes of LASIK refractive surgery. J Opt Soc Am A Opt Image Sci Vis. 2004;21(6):926–936. doi: 10.1364/josaa.21.000926. [DOI] [PubMed] [Google Scholar]
- 14.Hersh PS, Fry K, Blaker JW. Spherical aberration after laser in situ keratomileusis and photorefractive keratectomy. Clinical results and theoretical models of etiology. J Cataract Refract Surg. 2003;29(11):2096–2104. doi: 10.1016/j.jcrs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Mihashi T. Higher-order wavefront aberrations induced by small ablation area and sub-clinical decentration in simulated corneal refractive surgery using a perturbed schematic eye model. Semin Ophthalmol. 2003;18(1):41–47. doi: 10.1076/soph.18.1.41.14071. [DOI] [PubMed] [Google Scholar]
- 16.Marcos S, Cano D, Barbero S. Increase in corneal asphericity after standard laser in situ keratomileusis for myopia is not inherent to the Munnerlyn algorithm. J Refract Surg. 2003;19(5):S592–S596. doi: 10.3928/1081-597X-20030901-17. [DOI] [PubMed] [Google Scholar]
- 17.Pallikaris IG, Kymionis GD, Panagopoulou SI, Siganos CS, Theodorakris MA, Pallikaris AI. Induced optical aberrations following formation of a laser in situ keratomileusis flap. J Cataract Refract Surg. 2002;28(10):1737–1741. doi: 10.1016/s0886-3350(02)01507-9. [DOI] [PubMed] [Google Scholar]
- 18.Villa C, Gutiérrez R, Jiménez JR, González-Méijome JM. Night vision disturbances after successful LASIK surgery. Br J Ophthalmol. 2007;91(8):1031–1037. doi: 10.1136/bjo.2006.110874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalita MR, Xu M, Krueger RR. Correlation of aberrations with visual symptoms using wavefront analysis in eyes after laser in situ keratomileusis. J Refract Surg. 2003;19(6):S682–S686. doi: 10.3928/1081-597X-20031101-13. [DOI] [PubMed] [Google Scholar]
- 20.Salz JJ, Trattler W. Pupil size and corneal laser surgery. Curr Opin Ophthalmol. 2006;17(4):373–379. doi: 10.1097/01.icu.0000233958.96133.02. [DOI] [PubMed] [Google Scholar]
- 21.Hamam H. A quick method for analyzing Hartmann-Shack patterns: application to refractive surgery. J Refract Surg. 2000;16(5):S636–S642. doi: 10.3928/1081-597X-20000901-30. [DOI] [PubMed] [Google Scholar]
- 22.Seiler T, Dastjerdi MH. Customized corneal ablation. Curr Opin Ophthalmol. 2002;13(4):256–260. doi: 10.1097/00055735-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Straziota CE, Randleman JB, Stulting RD. Visual acuity and higher-order aberrations with wavefront-guided and wavefront-optimized laser in situ keratomileusis. J Cataract Refract Surg. 2010;36(3):437–441. doi: 10.1016/j.jcrs.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Moshirfar M, Schliesser JA, Chang JC, et al. Visual outcomes after wavefront-guided photorefractive keratectomy and wavefront-guided laser in situ keratomileusis: Prospective comparison. J Cataract Refract Surg. 2010;36(8):1336–1343. doi: 10.1016/j.jcrs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed EM, Muftuoglu O, Bowman W, et al. Wavefront-guided ablation retreatment using Iris registration. Eye Contact Lens. 2010;36(1):54–59. doi: 10.1097/ICL.0b013e3181c89130. [DOI] [PubMed] [Google Scholar]
- 26.Keir NJ, Simpson T, Jones LW, Fonn D. Wavefront-guided LASIK for myopia: effect on visual acuity, contrast sensitivity, and higher order aberrations. J Refract Surg. 2009;25(6):524–533. doi: 10.3928/1081597X-20090512-06. [DOI] [PubMed] [Google Scholar]
- 27.Schallhorn SC, Venter JA. One-month outcomes of wavefront-guided LASIK for low to moderate myopia with the VISX STAR S4 laser in 32,569 eyes. J Refract Surg. 2009;25(Suppl 7):S634–S641. doi: 10.3928/1081597X-20090611-02. [DOI] [PubMed] [Google Scholar]
- 28.Awwad ST, Bowman RW, Cavanagh HD, McCulley JP. Wavefront-guided LASIK for myopia using the LADAR CustomCornea and the VISX CustomVue. J Refract Surg. 2007;23(1):26–38. doi: 10.3928/1081-597X-20070101-06. [DOI] [PubMed] [Google Scholar]
- 29.Alió JL, Montés-Mico R. Wavefront-guided versus standard LASIK enhancement for residual refractive errors. Ophthalmology. 2006;113(2):191–197. doi: 10.1016/j.ophtha.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Jabbur NS, Kraff C for Visx Wavefront Study Group. Wavefront-guided laser in situ keratomileusis using the WaveScan system for correction of low to moderate myopia with astigmatism: 6-month results in 277 eyes. J Cataract Refract Surg. 2005;31(8):1493–1501. doi: 10.1016/j.jcrs.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 31.Kanjani N, Jacob S, Agarwal A, et al. Wavefront- and topography-guided ablation in myopic eyes using Zyoptix. J Cataract Refract Surg. 2004;30(2):398–402. doi: 10.1016/j.jcrs.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Kim TI, Yang SJ, Tchah H. Bilateral comparison of wavefront-guided versus conventional laser in situ keratomileusis with Bausch and Lomb Zyoptix. J Refract Surg. 2004;20(5):432–438. doi: 10.3928/1081-597X-20040901-04. [DOI] [PubMed] [Google Scholar]
- 33.Kohnen T, Bühren J, Kühne C, Mirshahi A. Wavefront-guided LASIK with the Zyoptix 3.1 system for the correction of myopia and compound myopic astigmatism with 1-year follow-up: clinical outcome and change in higher order aberrations. Ophthalmology. 2004;111(12):2175–2185. doi: 10.1016/j.ophtha.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 34.Khalifa M, El-Kateb M, Shaheen MS. Iris registration in wavefront-guided LASIK to correct mixed astigmatism. J Cataract Refract Surg. 2009;35(3):433–437. doi: 10.1016/j.jcrs.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Scheffel M, Kuehne C, Kohnen T. Comparison of monocular and binocular infrared pupillometers under mesopic lighting conditions. J Cataract Refract Surg. 2010;36(4):625–630. doi: 10.1016/j.jcrs.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Tsai YY, Lin JM. Ablation centration after active eye-tracker-assisted photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000;26(1):28–34. doi: 10.1016/s0886-3350(99)00328-4. [DOI] [PubMed] [Google Scholar]
- 37.Thibos LN, Horner D. Power vector analysis of the optical outcomes of refractive surgery. J Cataract Refract Surg. 2001;27(1):80–85. doi: 10.1016/s0886-3350(00)00797-5. [DOI] [PubMed] [Google Scholar]
- 38.Waring GO., 3rd Standard graphs for reporting refractive surgery. J Refract Surg. 2000;16(4):459–466. [PubMed] [Google Scholar]
- 39.Chan A, Manche EE. Effect of preoperative pupil size on quality of vision after wavefront-guided LASIK. Ophthalmology. 2011;118(4):736–741. doi: 10.1016/j.ophtha.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 40.Arba-Mosquera S, Merayo-Lloves J, de Ortueta D. Clinical effects of pure cyclotorsional errors during refractive surgery. Invest Ophthalmol Vis Sci. 2008;49(11):4828–4836. doi: 10.1167/iovs.08-1766. [DOI] [PubMed] [Google Scholar]
- 41.Schwiegerling J. Theoretical limits to visual performance. Surv Ophthalmol. 2000;45(2):139–146. doi: 10.1016/s0039-6257(00)00145-4. [DOI] [PubMed] [Google Scholar]
- 42.Karimian F, Feizi S, Jafarinasab MR. Conventional versus custom ablation in photorefractive keratectomy: randomized clinical trial. J Cataract Refract Surg. 2010;36(4):637–643. doi: 10.1016/j.jcrs.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 43.Tuan KM, Liang J. Improved contrast sensitivity and visual acuity after wavefront-guided laser in situ keratomileusis: in-depth statistical analysis. J Cataract Refract Surg. 2006;32(2):215–220. doi: 10.1016/j.jcrs.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 44.Netto MV, Ambrósio R, Jr, Shen TT, Wilson SE. Wavefront analysis in normal refractive surgery candidates. J Refract Surg. 2005;21(4):332–338. doi: 10.3928/1081-597X-20050701-06. [DOI] [PubMed] [Google Scholar]
- 45.Arbelaez MC, Vidal C, Jabri BA, Arba Mosquera S. LASIK for myopia with Aspheric “aberration neutral” ablations using the ESIRIS laser system. J Refract Surg. 2009;25(11):991–999. doi: 10.3928/1081597X-20091016-04. [DOI] [PubMed] [Google Scholar]
- 46.Kosaki R, Maeda N, Hayashi H, Fujikado T, Okamoto S. Effect of NIDEK optimized aspheric transition zone ablation profile on higher order aberrations during LASIK for myopia. J Refract Surg. 2009;25(4):331–338. doi: 10.3928/1081597X-20090401-06. [DOI] [PubMed] [Google Scholar]
- 47.El Danasoury AM. NIDEK optimized prolate ablation for the treatment of myopia with and without astigmatism. J Refract Surg. 2009;25(Suppl 1):S136–S141. doi: 10.3928/1081597X-20090115-11. [DOI] [PubMed] [Google Scholar]
- 48.Bühren J, Kühne C, Kohnen T. Influence of pupil and optical zone diameter on higher-order aberrations after wavefront-guided myopic LASIK. J Cataract Refract Surg. 2005;31(12):2272–2280. doi: 10.1016/j.jcrs.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Roberts CW, Koester CJ. Optical zone diameters for photorefractive corneal surgery. Invest Ophthalmol Vis Sci. 1993;34(7):2275–2281. [PubMed] [Google Scholar]
- 50.Klonos GG, Pallikaris J, Fitzke FW. A computer model for predicting image quality after photorefractive keratectomy. J Refract Surg. 1996;12(2):S280–S284. doi: 10.3928/1081-597X-19960201-18. [DOI] [PubMed] [Google Scholar]
- 51.Macsai MS, Stubbe K, Beck AP, Ravage ZB. Effect of expanding the treatment zone of the Nidek EC-5000 laser on laser in situ keratomileusis outcomes. J Cataract Refract Surg. 2004;30(11):2336–2343. doi: 10.1016/j.jcrs.2004.05.015. [DOI] [PubMed] [Google Scholar]