Abstract
OBJECTIVES
Reintubation and subsequent mechanical ventilation (MV) in preterm infants after surfactant replacement therapy are associated with excess morbidity and mortality and likely increase in-hospital costs. Specific surfactant therapy selection for prevention of respiratory distress syndrome (RDS) in preterm infants receiving conventional MV may impact not only clinical outcomes but also pharmacoeconomic outcomes.
METHODS
We conducted a pharmacoeconomic analysis of the impact of surfactant selection and reintubation and subsequent MV of preterm infants on health care resource utilization. Rates of reintubation and duration of MV after reintubation were determined from 1546 preterm infants enrolled in two surfactant trials comparing lucinactant to beractant and poractant alfa. Hospital costs were obtained from a 2010 US database from 1564 preterm infants with RDS, with a direct cost of $2637 per day for MV in the neonatal intensive care unit. Cost of reintubation by study and treatment was estimated as the incidence of reintubation multiplied by days on MV therapy after reintubation multiplied by cost per day for direct MV costs, standardized per 100 surfactant-treated infants.
RESULTS
There were no differences between studies or treatment groups in the overall extubation rate. Average MV duration following reintubation was similar between groups in both trials; however, reintubation rates were significantly lower (p<0 05) for infants treated with lucinactant than for those receiving beractant or poractant alfa. The observed differences in reintubation rates resulted in a projected cost saving of $160,013 to $252,203 per 100 infants treated with lucinactant versus animal-derived surfactants.
CONCLUSIONS
In this analysis, higher reintubation rates following successful extubation in preterm infants receiving animal-derived surfactant preparations significantly increased estimated in-hospital costs, primarily due to excess costs associated with MV. This analysis suggests that surfactant selection may have a significant pharmacoeconomic impact on cost of patient care. Additional cost assessment of potential reduction in reintubation-associated morbidity is warranted.
INDEX TERMS: cost analysis, lucinactant, mechanical ventilation, respiratory distress syndrome, surfactant
INTRODUCTION
Respiratory distress syndrome (RDS) is the most common respiratory disorder among preterm infants and is an important cause of infant mortality.1 Exogenous surfactant administration has significantly reduced mortality and morbidity in premature newborns with RDS,2 and intratracheal instillation of surfactant has become the standard of care in this population.3 Until recently, exogenous surfactants approved for use in the United States for the treatment and prevention of RDS were of animal origin. Lucinactant (Surfaxin; Discovery Laboratories, Inc., Warrington, PA), a non-animal-derived, synthetic surfactant, has recently been approved by the United States Food and Drug Administration for the prevention of RDS in infants at high risk for RDS following successful completion of two phase 3 clinical trials. Lucinactant contains phospholipids and sinapultide (KL4), a 21-amino acid synthetic peptide consisting of lysines (K) and leucines (L) arranged in the sequence KLLLLKLLLLKLLLLK, which mimics the action of human surfactant protein B (SP-B).4,5 Of the four known SPs, the hydrophobic SP-B and SP-C proteins are known to act in a critical manner to stabilize the phospholipid monolayer and enhance the ability of phospholipids to lower surface tension. Of the two, SP-B appears to play the main role, as infants who are congenitally deficient in SP-B develop a fatal form of respiratory failure shortly after birth,6,7 whereas those deficient in SP-C tend to develop chronic lung disease in early adulthood.8 Lucinactant has been studied in multiple clinical trials, including two phase 3 studies in infants at risk for RDS9,10 and phase 2 studies in preterm infants with bronchopulmonary dysplasia (BPD)11 and in adults with acute respiratory distress syndrome.12
Although appropriate administration of surfactant replacement therapy (SRT) for the prevention and treatment of RDS has improved outcomes and reduced mortality, preterm infants who receive surfactant commonly fail to maintain adequate gas exchange following extubation and may require endotracheal reintubation and mechanical ventilation (MV). A recently published study showed that reintubation with MV was administered in 35% to 47% of preterm infants treated with exogenous surfactant and appears to be an independent risk factor predictive of major morbidity and mortality.13 Several acute and long-term complications have been associated with endotracheal intubation and extended placement of an endotracheal tube, including oxygen desaturation, bradycardia, airway trauma, subglottic stenosis, and tracheomalacia, and morbidities associated with MV, such as air leak, pneumonia, sepsis, and BPD.14,15
Reintubation and subsequent MV also has the potential to increase in-hospital costs and consumption of resources available in the neonatal intensive care unit (NICU), including additional nursing and respiratory care hours, as well as increased radiology, laboratory, pharmacy, and other in-hospital costs.
Several studies have evaluated the cost of treating RDS and the cost effectiveness of surfactant replacement,16–25 but the economic consequences of reintubation in preterm infants have not been previously evaluated. The objectives of this study were to estimate the economic impact and health care resource utilization of reintubation and conventional MV strategies in surviving preterm infants weighing 600 to 1250 grams treated with surfactant for the prevention of RDS. Other strategies, such as endotracheal intubation for surfactant administration with early extubation and continuous positive airway pressure (CPAP) initiation (InSurE method)26,27 and early initiation of CPAP were not assessed.28,29
METHODS AND DESIGN
Model and Data Sources
A pharmacoeconomic analysis was conducted to estimate direct and indirect costs of reintubation by study and treatment. Model inputs for calculation of direct costs included 1) incidence of reintubation, 2) days of MV after reintubation, and 3) cost per day of MV in the NICU. Rates of reintubation and average number of days of MV after reintubation per infant were based on data from two multicenter, masked, randomized, controlled comparative surfactant trials: the Safety and Effectiveness of Lucinactant vs. Exosurf in a Clinical Trial (SELECT) and Surfaxin Therapy Against RDS (STAR) trial. Methods, study design, and maternal and neonatal demographics, as well as results for both RDS prevention trials, have been described in detail.9,10 Briefly, in SELECT, infants at risk for RDS from North and Central America (Unite States, Mexico, Panama), Europe (Poland, Russia, Hungary), and South America (Brazil, Chile, Ecuador, Uruguay), weighing between 600 and 1250 grams at birth were randomized to receive lucinactant (n=527), colfosceril palmitate (n=509; Exosurf; GlaxoSmithKline, Brentford, UK), or beractant (n=258; Survanta; Abbott Laboratories, Columbus, OH) in a 2:2:1 ratio (study conducted from July 2001 to December 2003). In STAR, infants from North America (United States, Canada) and Europe (France, Hungary, Poland, Portugal, Spain, United Kingdom) were randomized to receive lucinactant (n=124) or poractant alfa (n=128; Curosurf, Chiesi Farmaceutici, Parma, Italy) in a 1:1 ratio (study conducted from August 2001 to May 2003). The average number of surfactant doses ranged from 1.3 to 1.9 doses, and the study populations were generally similar between infants enrolled in the two trials.30
From birth to 36 weeks postmenstrual age (PMA), rates of initial extubation and rates of reintubation for infants extubated at least once through 36 weeks PMA were calculated. Together, the trials enrolled a total of 1546 infants, of whom 1272 were extubated at least once through 36 weeks PMA (>80% in all treatment groups), of whom 33% to 47% required reintubation.
The economic input, cost per day for direct ventilation costs, was obtained from a hospital-based data set (Premiere Hospital Database, 2010; Charlotte, NC), which included 1564 preterm infants with RDS with a birth weight of 500 to 1249 grams from over 500 hospitals in the United States and was based on an average direct cost of $2637 per day of MV in the NICU. Cost inputs for indirect in-hospital charges associated with reintubation included room and board, laboratory, pharmacy, respiratory care, and radiology were also obtained using this database. Costs included in this analysis may not be inclusive of all services received during the days of MV, and pharmacy costs were not assumed to include surfactant costs.
Study Design and Methods
The primary outcome, direct cost of reintubation and subsequent MV, was defined as the actual costs associated with MV in the NICU based on data from the Premier Hospital Database. Total direct cost of reintubation by study and treatment was estimated as the incidence of reintubation multiplied by days of MV after reintubation multiplied by cost per day for direct MV costs, standardized for 100 surfactant-treated infants.
Indirect costs associated with reintubation, including hospital charges related to monitoring and support needed for clinical care of infants receiving MV, were also estimated. Indirect costs were obtained from average in-hospital costs derived with the Premiere Hospital Database. Unlike direct costs of reintubation, it was not possible to calculate total indirect costs of reintubation by study and treatment as not every hospital department charge would occur every day. Therefore, we identified per-patient costs for infants receiving or not receiving MV by hospital reporting department, without considering other costs associated with potentially different morbidity profiles across groups.
RESULTS
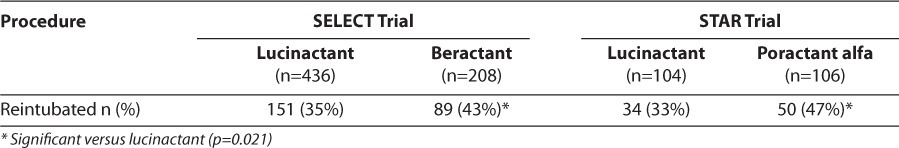
Initial extubation rates were similar among patients receiving surfactant treatments in both trials [80%–84%; p=not significant (NS)]. The reintubation rate following initial extubation was significantly lower for infants treated with lucinactant (range, 33%–35%) than that for infants receiving animal-derived surfactants (range, 43%–47%; p=0.021).13 Table 1 displays rates of reintubation by study and surfactant preparation.
Table 1.
Reintubation Rates for Infants Extubated At Least Once Through 36 Weeks Post Menstrual Age, by Study and Surfactant Preparation
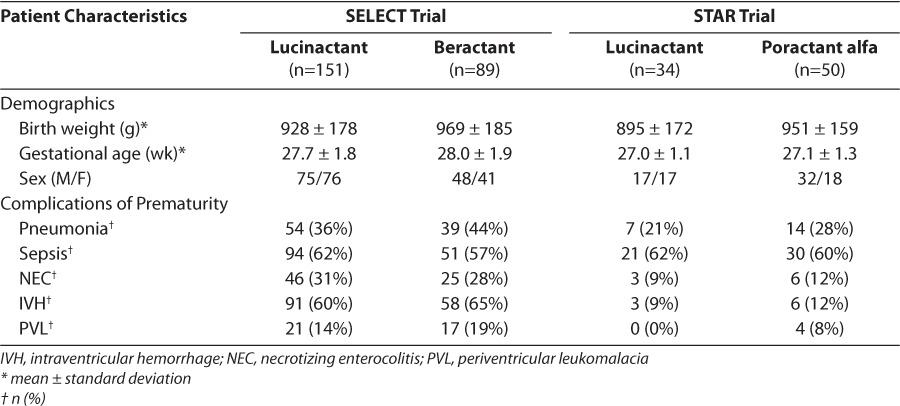
Patient characteristics (birth weight, gender, gestational age) and comorbidities (incidences of sepsis, pneumonia, necrotizing enterocolitis, intraventricular hemorrhage, periventricular leukomalacia) for reintubated infants are presented in Table 2. Generally, patient characteristics were similar among the different surfactant treatment groups; however, more male than female infants were reintubated in the poractant alfa group. In addition, comorbidities were similar between treatment groups in both trials.
Table 2.
Demographics and Complications of Prematurity for Reintubated Infants by Study and Surfactant Preparation
Average duration of days of MV per infant following reintubation was similar between treatment groups in each trial; however, as reintubation rates were significantly lower for lucinactant-treated infants, this analysis resulted in fewer total days of MV after reintubation. Table 3 displays the average duration of MV following reintubation per infant and the total duration of MV after reintubation per study and treatment group standardized per 100 infants.
Table 3.
Cost Component and Cost Comparison
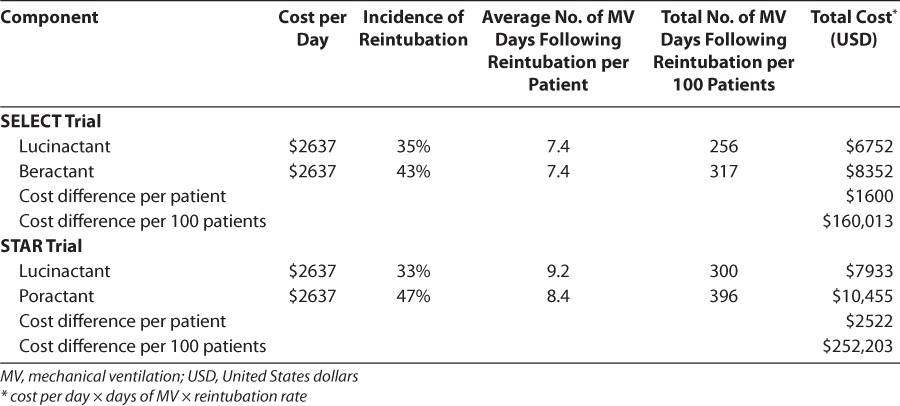
The estimated direct costs of reintubation per infant were approximately $6700 to $7900 for lucinactant and between $8400 and $10,500 for animal-derived surfactants in the SELECT and the STAR trial, respectively, suggesting a potential savings per infant ranging from $1600 to $2500 per infant for infants treated with lucinactant. When standardized per 100 reintubated infants, the lower reintubation rate and fewer overall days of MV suggest a potential savings of approximately $160,000 to $252,000 for infants treated with lucinactant (Table 3).
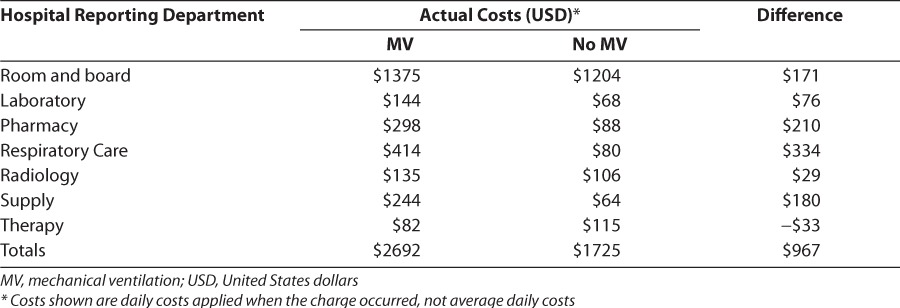
Table 4 displays the average per-patient indirect costs for infants receiving or not receiving MV by hospital department. In general, MV was associated with higher radiology, laboratory, and other indirect costs, reflecting a greater impact on medical resource use.
Table 4.
Per Patient Cost by Hospital Department
DISCUSSION
We conducted a pharmacoeconomic analysis of the impact of surfactant selection and reintubation and subsequent MV of preterm infants on health care resource use. As a follow-up to our published observations of increased morbidity and mortality associated with reintubation and MV in preterm infants receiving surfactant preparations,13 this analysis evaluated the pharmacoeconomic impact of reintubation and MV by examining costs associated with in-patient health care resource use in this population, which is becoming an increasingly important concept in the practice of neonatology.31 This study demonstrates the potential financial impact of therapeutic choices and also adds increasing evidence of the need to evaluate the impact of therapies designed to prevent RDS in preterm infants.
Reintubation has been associated with increased mortality and morbidity in adults with acute distress respiratory syndrome32,33 but has not been previously evaluated in preterm infants. In preterm infants at risk for RDS, who were treated with surfactant, we previously reported that reintubation was a predictive independent risk factor for death. Similarly, major complications of prematurity such as BPD, air leakage, sepsis, necrotizing enterocolitis, and intraventricular hemorrhage were also significantly higher in pre-term infants who required reintubation, showing a strong association between reintubation and poor clinical outcomes.13
More than 80% of infants in the STAR trial and SELECT were successfully extubated during the early hospital course following surfactant administration, reflecting a clinical improvement in pulmonary function following surfactant administration. However, between 35% and 47% of those infants required reintubation for multiple causes, including poor respiratory effort, apnea, and acidosis, among others. Although initial extubation rates were similar across treatment groups in both trials, rates of subsequent reintubation were significantly lower for infants treated with synthetic lucinactant than for those treated with the commercially available animal-derived surfactants beractant and poractant alfa.13 There are several potential factors that may have led to the relatively lower rate of reintubation observed in infants treated with lucinactant. Compared with the amount of SP-B protein found in animal-derived surfactants, the concentration of sinapultide in lucinactant is consistently much higher than the SP-B concentration in beractant.34 In addition, lucinactant appears to be more resistant to inactivation by plasma proteins and oxidant species than other exogenous surfactants35–38 and has also been shown to reduce plasma protein and neutrophil influx into the alveolar space in vivo and to modulate the inflammatory processes in vitro.39,40 These factors may have resulted in improved overall lung function, potentially leading to lower rates of reintubation.
Preterm infants at risk for RDS who survive typically require substantially more resources over the course of their hospitalization.25,41 We hypothesized that the need for reintubation would increase in-patient hospital costs. Consistent with what has been observed in other critical care populations,33,42 our analysis suggests that higher reintubation rates following successful extubation in preterm infants receiving surfactant preparations significantly increases in-hospital costs primarily because of excess costs associated with MV. The use of MV is associated with direct costs, consisting of expenses directly related to the use of MV, and indirect costs, which are those charges associated with additional medical, imaging, laboratory analyses, supplies, and other miscellaneous costs needed for an adequate clinical management of patients on MV. This analysis provides an assessment of otherwise unavailable data describing the economic impact and increased resource utilization of reintubation and MV.
There are a few potential limitations pertaining to this analysis. First, the clinical inputs used for this analysis are derived from two phase 3 clinical trials conducted between 2001 and 2003 in study sites in various regions of the world, whereas the cost data used for the analysis is derived from a recent US-based hospital cost database. While services and care may differ between different global regions, the clinical trial protocols followed by all sites included guidelines on the respiratory care and treatment of the preterm infants that reflected standards of care practiced in the United States. Moreover, these standards of care have remained fairly constant over the last 10 years. It is therefore unlikely that the services and care delivered during the clinical trials differs significantly from that practiced in the United States at present. Notably, new respiratory support strategies, such as endotracheal intubation for surfactant administration with early extubation and continuous positive airway pressure (CPAP) initiation (InSurE method)26,27 or early initiation of CPAP,28,29 are now used in some care centers in addition to those used in clinical trials, and these new strategies may have health benefits not evaluated in this study. However, these support strategies are not defined as part of the current standard of care and are themselves experimental.
It should be noted that the primary comparison in SELECT was lucinactant compared with colfosceril palmitate, a surfactant that is no longer commercially available in the United States, with beractant serving only as a reference agent. Furthermore, the STAR trial, which compared lucinactant with poractant alfa in a noninferiority manner, was terminated prior to achieving its enrollment goal. While these are limitations in the clinical studies, the endpoint of reintubation, a prespecified endpoint, was evaluated across a large number of infants included in this analysis, with over 650 preterm infants treated with lucinactant and nearly 400 preterm infants treated with beractant and poractant alfa. This large sample size is sufficient to provide reliable estimates.
Another potential limitation of this analysis is the self-selecting nature of the Premiere Hospital Database dataset that was used to estimate current costs in the United States associated with preterm infants treated in the NICU. However, the large dataset of 1564 infants that was captured in the Premiere Hospital Database represents information from over 500 NICUs in the United States and is, therefore, likely to be a good overall estimate of current costs associated with treating this population in the NICU. Future studies should be conducted to validate the results that we found, inclusive of a more comprehensive assessment of the costs associated with potential differences in morbidity profiles (such as differences in rates of BPD) when selecting a particular surfactant preparation.
CONCLUSIONS
Among preterm infants receiving surfactant therapy for the prevention of RDS, the need for reintubation after successful extubation appears to be a frequent event. Pharmacoeconomic modeling suggests that higher rates of reintubation result in higher direct and indirect costs associated with MV, which in turn leads to higher inhospital patient cost. In this analysis, the lower reintubation rates observed in infants treated with lucinactant, relative to infants treated with animal-derived surfactants, potentially resulted in lower estimated in-hospital costs. Selection of a specific surfactant for the prevention of RDS may have a significant pharmacoeconomic impact. Additional cost assessment of potential reduction in reintubation-associated morbidity is warranted.
ABBREVIATIONS
- BPD
bronchopulmonary dysplasia
- CPAP
continuous positive airway pressure
- IVH
intraventricular hemorrhage
- MV
mechanical ventilation
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- ns
not significant
- n
number
- PMA
post menstrual age
- PVL
periventricular leukomalacia
- RDS
respiratory distress syndrome
- SP
surfactant protein
- SRT
surfactant replacement therapy
- US
United States
- SELECT
Safety and Effectiveness of Lucinactant vs. Exosurf in a Clinical Trial
- STAR
Surfaxin Therapy Against RDS Trial
Footnotes
DISCLOSURE This analysis was funded by Discovery Laboratories, Inc. Warrington, PA. Drs Moya and Sinha have served as members of scientific advisory boards for Discovery Laboratories, Inc., and were compensated for their time and effort for their scientific contribution, analysis, and interpretation of the data and critical review of the manuscript. Dr Guardia is a consultant to Discovery Laboratories, Inc., and is a former company employee; Dr Segal and Mr. Simmons are employees of Discovery Laboratories, Inc. Dr Greenspan declares no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Dr Guardia had full access to all data in the study and takes responsability for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Kochanek KD, Xu JQ, Murphy SL Natl Vital Stat Rep. 4. Vol. 59. Hyattsville, MD: National Center for Health Statistics; 2011. Deaths: preliminary data for 2009. [PubMed] [Google Scholar]
- 2.Soll RF, Hallman M, Merritt TA. Surfactant in the prevention and treatment of respiratory distress syndrome. In: Boynton BR, editor. New Therapies for Neonatal Respiratory Failure. Cambridge, UK: Cambridge University Press; 1994. pp. 49–80. [Google Scholar]
- 3.Halliday HL. History of surfactant from 1980. Biol Neonate. 2005;87(4):317–322. doi: 10.1159/000084879. [DOI] [PubMed] [Google Scholar]
- 4.Revak SD, Merritt TA, Hallman M et al. The use of synthetic peptides in the formation of biophysically and biologically active pulmonary surfactants. Pediatr Res. 1991;29(5):460–465. doi: 10.1203/00006450-199105010-00010. [DOI] [PubMed] [Google Scholar]
- 5.Cochrane CG, Revak SD. Pulmonary surfactant protein B (SP-B): Structure-function relationships. Science. 1991;254(5031):566–568. doi: 10.1126/science.1948032. [DOI] [PubMed] [Google Scholar]
- 6.Nogee LM, de Mello DE, Dehner LP et al. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993;328(6):406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- 7.Clark JC, Wert SE, Bachurski CJ et al. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis causing respiratory failure in newborn mice. Proc Natl Acad Sci U S A. 1995;92(17):7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole FS, Hamvas A, Nogee LM. Genetic disorders of neonatal respiratory function. Pediatr Res. 2001;50(2):157–162. doi: 10.1203/00006450-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Moya FR, Gadzinowski J, Bancalari E, et al. for the International Surfaxin Collaborative Study Group. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics. 2005;115(4):1018–1029. doi: 10.1542/peds.2004-2183. [DOI] [PubMed] [Google Scholar]
- 10.Sinha SK, Lacaze-Masmonteil T, Valls i Soler A, et al. A multicenter, randomized, controlled trial of lucinactant versus poractant alfa among very preterm infants at high risk for respiratory distress syndrome. Pediatrics. 2005;115(4):1030–1038. doi: 10.1542/peds.2004-2231. for the Surfaxin Therapy Against Respiratory Distress Syndrome Collaborative Group. [DOI] [PubMed] [Google Scholar]
- 11.Laughon M, Bose C, Moya F et al. A pilot, randomized, controlled trial of later treatment with a peptide-containing, synthetic surfactant for the prevention of bronchopulmonary dysplasia. Pediatrics. 2009;123(1):89–96. doi: 10.1542/peds.2007-2680. [DOI] [PubMed] [Google Scholar]
- 12.Wiswell TE, Smith RM, Katz LB et al. Bronchopulmonary segmental lavage with Surfaxin (KL4-Surfactant) for acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(4):1188–1195. doi: 10.1164/ajrccm.160.4.9808118. [DOI] [PubMed] [Google Scholar]
- 13.Guardia CG, Moya FR, Sinha S et al. Reintubation and risk of morbidity and mortality in preterm infants after surfactant therapy. J Neonatal Perinatal Med. 2011;4(2):101–109. [Google Scholar]
- 14.Korones S. Complications of assisted ventilation. In: Goldsmith J, Karotkin E, editors. Assisted Ventilation of the Neonate. Philadelphia, PA: WB Saunders; 2003. pp. 345–377. [Google Scholar]
- 15.Carlo W, Martin R, Fanaroff A. Assisted ventilation and complications of respiratory distress. In: Martin R, Fanaroff A, editors. Neonatal-Perinatal Medicine. St. Louis, MO: Mosby; 1997. pp. 1028–1040. [Google Scholar]
- 16.Gdovin JM, Moya FR, Vishalpura T et al. A Comparative pharmacoeconomic assessment of two surfactants for the prevention of respiratory distress syndrome. J Pediatr Pharmacol Ther. 2006;11(1):43–54. doi: 10.5863/1551-6776-11.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egberts J. Estimated costs of different treatments of the respiratory distress syndrome in a large cohort of preterm infants less than 30 weeks of gestation. Biol Neonate. 1992;61(suppl 1):S59–S65. doi: 10.1159/000243846. [DOI] [PubMed] [Google Scholar]
- 18.Eidelman AI. Economic consequences of surfactant therapy. J Perinatol. 1993;13(2):137–139. [PubMed] [Google Scholar]
- 19.Merritt TA, Hallman M, Vaucher Y et al. Impact of surfactant treatment on cost of neonatal intensive care: a cost benefit analysis. J Perinatol. 1990;10(4):416–419. [PubMed] [Google Scholar]
- 20.Mugford M, Piercy J, Chalmers I. Cost implications of different approaches to the prevention of respiratory distress syndrome. Arch Dis Child. 1991;66(spec no. 7):757–764. doi: 10.1136/adc.66.7_spec_no.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mugford M, Howard S. Cost effectiveness of surfactant replacement in preterm babies. Pharmacoeconomics. 1993;3(5):362–373. doi: 10.2165/00019053-199303050-00004. [DOI] [PubMed] [Google Scholar]
- 22.Phibbs CS, Phibbs RH, Wakeley A et al. Cost effects of surfactant therapy for neonatal respiratory distress syndrome. J Pediatr. 1993;123(6):953–962. doi: 10.1016/s0022-3476(05)80394-4. [DOI] [PubMed] [Google Scholar]
- 23.Soll RF, Jacobs J, Pashko S et al. Cost effectiveness of beractant in the prevention of respiratory distress syndrome. Pharmacoeconomics. 1993;4(4):278–286. doi: 10.2165/00019053-199304040-00006. [DOI] [PubMed] [Google Scholar]
- 24.Tubman TRJ, Haliday HL, Normand C. Cost of surfactant replacement treatment for severe neonatal respiratory distress syndrome: a randomized controlled trial. BMJ. 1990;301(6756):842–845. doi: 10.1136/bmj.301.6756.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniscalco WM, Kending JW, Shapiro DL. Surfactant replacement therapy: impact on hospital charges for premature infants with respiratory distress syndrome. Pediatrics. 1989;83(1):1–6. [PubMed] [Google Scholar]
- 26.Dani C, Corsini I, Bertini G et al. The INSURE method in preterm infants of less than 30 weeks' gestation. J Matern Fetal Neonatal Med. 2010;23(9):1024–1029. doi: 10.3109/14767050903572174. [DOI] [PubMed] [Google Scholar]
- 27.Dani C, Bertini G, Pezzati M et al. Early extubation and nasal continuous positive airway pressure after surfactant treatment for respiratory distress syndrome among preterm infants <30 weeks' gestation. Pediatrics. 2004;113(6):e560–e563. doi: 10.1542/peds.113.6.e560. [DOI] [PubMed] [Google Scholar]
- 28.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970–1979. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booth C, Premkumar MH, Yannoulis A et al. Sustainable use of continuous positive airway pressure in extremely preterm infants during the first week after delivery. Arch Dis Child Fetal Neonatal Ed. 2006;91(6):F398–F404. doi: 10.1136/adc.2005.092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moya FR, Sinha SK, Gadzinowski J et al. One-year follow-up of very preterm infants who received lucinactant for prevention of respiratory distress syndrome: results from 2 multicenter randomized, controlled trials. Pediatrics. 2007;119(6):e1361–e1370. doi: 10.1542/peds.2006-0149. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics. Hyattsville, MD: 2005. Health, United States, 2005 with Chartbook on Trends in the Health of Americans. [PubMed] [Google Scholar]
- 32.Seymour CW, Martinez A, Christie JD, Fuchs BD. The outcome of extubation failure in a community hospital intensive care unit: a cohort study. Crit Care. 2004;8(5):R322–R327. doi: 10.1186/cc2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112(1):186–192. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- 34.Cochrane CG, Revak SD, Merritt TA et al. The efficacy and safety of KL4-surfactant in preterm infants with respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153(1):404–410. doi: 10.1164/ajrccm.153.1.8542150. [DOI] [PubMed] [Google Scholar]
- 35.Merritt TA, Amirkhanian JD, Helbock H et al. Reduction of the surface-tension-lowering ability of surfactant after exposure to hypochlorous acid. Biochem J. 1993;295(pt 1):19–22. doi: 10.1042/bj2950019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson S, Kheiter A, Merritt TA. Oxidative inactivation of surfactants. Lung. 1999;177(3):179–189. doi: 10.1007/pl00007639. [DOI] [PubMed] [Google Scholar]
- 37.Amirkhanian JD, Merritt TA. Inhibitory effects of oxyradicals on surfactant function: utilizing in vitro Fenton reaction. Lung. 1998;176(1):63–72. doi: 10.1007/pl00007592. [DOI] [PubMed] [Google Scholar]
- 38.Manalo E, Merritt TA, Kheiter A et al. Comparative effects of some serum components and proteolytic products of fibrinogen on surface tension-lowering abilities of beractant and a synthetic peptide containing surfactant KL4. Pediatr Res. 1996;39(6):947–952. doi: 10.1203/00006450-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Kinniry P, Pick J, Stephens S et al. KL(4)-surfactant prevents hyperoxic and LPS-induced lung injury in mice. Pediatr Pulmonol. 2006;41(10):916–928. doi: 10.1002/ppul.20468. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Miller TL, Chidekel AC et al. KL4-surfactant (Surfaxin) protects human airway epithelium from hyperoxia. Pediatr Res. 2008;64(2):154–158. doi: 10.1203/PDR.0b013e318175dd14. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz RM, Luby AM, Scanlon JW et al. Effects of surfactant on morbidity, mortality, and resource use in newborn infants weighing 500 to 1500 g. N Eng J Med. 1994;330(21):1476–1480. doi: 10.1056/NEJM199405263302102. [DOI] [PubMed] [Google Scholar]
- 42.Seymour CW, Martinez A, Christie JD et al. The outcome of extubation failure in a community hospital intensive care unit: a cohort study. Crit Care. 2004;8(5):R322–R327. doi: 10.1186/cc2913. [DOI] [PMC free article] [PubMed] [Google Scholar]


