Abstract
OBJECTIVES
The purpose of this study was to determine the total propofol dose (mg/kg) for non-emergent pediatric procedural sedation and evaluate dosing differences with regard to a patient's sex, age, and body mass index. Adverse events were recorded and evaluated to determine whether certain patient groups were at a higher risk than others.
METHODS
This study was a retrospective observational pilot study including patients 0 to 18 years of age admitted between January 2008 and November 2009 for non-emergent gastrointestinal endoscopic procedures or radiologic imaging, who received propofol for procedural sedation. Data gathered included sex, age, height, weight, chronic medical conditions and medication use, concomitant anesthetic gas, preprocedure midazolam, procedure length, propofol dose in mg/kg, other medications administered during procedure, and adverse events that occurred. Comparisons between adverse event groups and categories of baseline characteristics were made using the Wilcoxon signed-rank, Kruskal-Wallis nonparametric and Pearson's chisquare tests, as appropriate.
RESULTS
A total of 101 patients met inclusion criteria and were included in the analysis. The mean dose of propofol required for female patients was 3.7 mg/kg versus 3.4 mg/kg for males (p=0.3). The mean dose of propofol for patients ≤9 years, 10 to 12 years, and >12 years was 3.2, 3.9, and 3.9 mg/kg, respectively (p=0.25). The mean dose of propofol for underweight, healthy weight, overweight, and obese patients was 4.2, 3.9, 3.6, and 2.6 mg/kg, respectively (p=0.38). Hypotension occurred in 42.6% of patients, and bradycardia occurred in 13.9% of patients.
CONCLUSIONS
There were no differences in dose requirements based on sex or age. The difference in dosing between different body weight categories was not statistically significant. The dose of propofol was higher in patients that experienced bradycardia and hypotension, but there was no statistical significance. Given the above, future studies with larger sample sizes should be conducted to establish if statistical significance exists.
INDEX TERMS: computed tomography, endoscopy, gastrointestinal, magnetic resonance imaging, pediatrics, propofol
INTRODUCTION
Propofol is an intravenous sedative-hypnotic commonly used for induction and maintenance of anesthesia in children and adults. Propofol is approved by the US Food and Drug Administration (FDA) for the induction of general anesthesia in children older than 3 years of age at a dose of 2.5 to 3.5 mg/kg. It is indicated for the maintenance of general anesthesia in children older than 2 months at a dose of 7.5 to 18 mg/ kg/hour.1 Propofol is no longer recommended in pediatric patients for continuous sedation, following case reports of propofol infusion syndrome, characterized by metabolic acidosis, bradyarrhythmias, and ultimate cardiac death.2,3 It is not FDA approved for procedural sedation in pediatric patients; therefore, no manufacturer dosing recommendations exist. However, it is commonly used for this indication, and previous studies have demonstrated its safety and efficacy in this setting.4–6
The purpose of this study was to determine the total propofol dose (mg/kg) for non-emergent procedural sedation and to evaluate dosing differences with regard to a patient's sex, age, and body mass index (BMI). Types of procedures included colonoscopies, esophagogastroduodenoscopies (EGD), computed tomography (CT), and magnetic resonance imaging (MRI), which differed in duration and use of concomitant medications or anesthetic gas. Adverse events were recorded and evaluated to determine if there was a higher risk associated with certain patient groups.
MATERIALS AND METHODS
Study Design
This study was a retrospective observational pilot study approved by the institutional review board at the research institution. All pediatric patients aged 0 to 18 years admitted between January 2008 and November 2009 for a non-emergent EGD, colonoscopy, MRI, or CT, who received propofol for procedural sedation were included. Data were collected from the sedation record, which was scanned into the patient's medical record. Patients were excluded if an incomplete medical record prevented complete data collection.
All decisions regarding medication, dose, route, and airway maintenance were at the discretion of the anesthesia provider. The patient's telemetry and pulse oximetry were continuously monitored, and oxygen was applied as needed to maintain oxygen saturations greater than 90%.
Data Collection
Patients were identified using the International Classification of Diseases, Ninth Revision (ICD-9) coded diagnoses for the procedures included in pediatric patients. Data gathered included sex, age, height, weight, chronic medical conditions and medication use, preprocedure midazolam, concomitant anesthetic gas, fentanyl, and mid-azolam, procedure length, dose of propofol in milligrams per kilogram (mg/kg) and adverse events that occurred.
BMI was calculated and then plotted on a BMI- for-age curve for either males or females and reported as a percentile comparing individual BMI to others of the same age and sex. A BMI less than the fifth percentile was considered underweight. A BMI in the 5th to 84th percentile was considered a healthy weight. A BMI between the 85th and 95th percentile was considered overweight, and obese was a BMI above the 95th percentile.7 The 8 patients who did not have a calculated BMI were younger than 2 years of age. In that age group, BMI is not validated, and a patient instead is considered overweight if their weight-for-height value exceeds the 95th percentile for their age.
An adverse event was recorded if a patient's blood pressure, respiratory rate, or heart rate fell below the bottom percentile of what is considered normal for the patient's age and was also a 20% change from baseline.8 All serious adverse events were documented including those in which an intervention was necessary to treat the event.
Statistical Analysis
Data are presented as means and standard deviations for continuous variables and as frequency and percentage for categorical variables. Comparisons between the adverse event groups and categories of baseline characteristics were made using the Wilcoxon signed-rank, Kruskal-Wallis nonparametric, and Pearson chi-square tests, as appropriate. A p value of <0.05 was considered statistically significant.
RESULTS
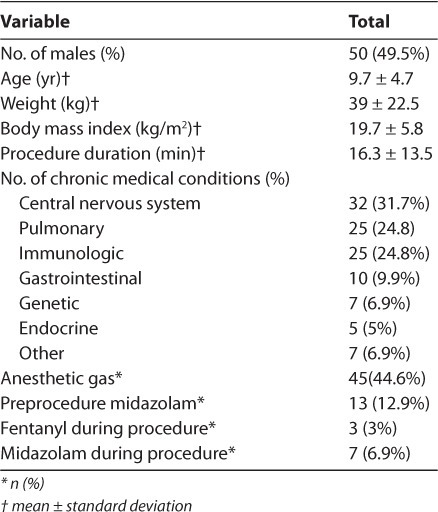
Initially, 3460 procedures were identified by ICD-9 diagnoses. After chart review, 76 gastro-intestinal endoscopy patients and 30 radiological imaging patients received propofol for procedural sedation. Two patients from the first group and 3 from the second were excluded because insufficient data was available. Characteristics of the patients included are presented in Table 1.
Table 1.
Patient Characteristics
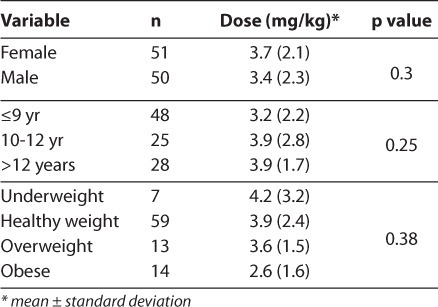
Results comparing dosing requirements for sex, age, and BMI are in Table 2. There were no differences in dosing requirements between male and female pediatric patients or between different age groups. There was a numerical but non-statistically significant difference in dosing requirements between different BMI categories.
Table 2.
Dose
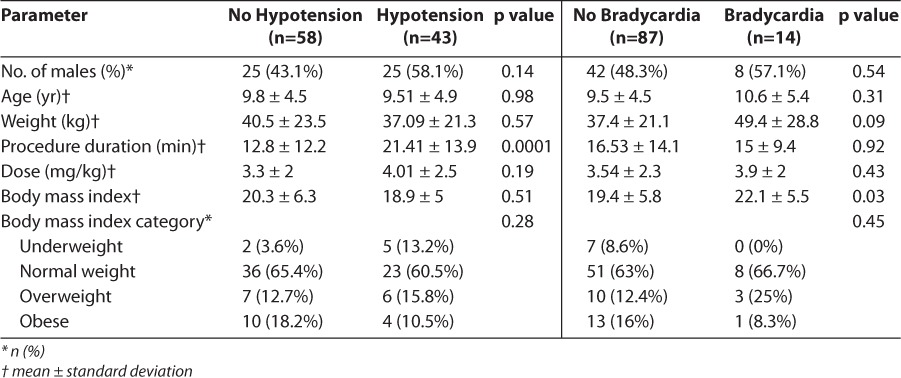
Each adverse event was evaluated to determine whether there was an increased risk due to sex, age, weight, procedure duration, dose, and BMI. The comparison of patients who experienced hypotension (42.6%) and bradycardia (13.9%) is presented in Table 3. For both, the dose was larger in patients who experienced an adverse drug event; however, the difference was not significant. There was a statistically significant difference in procedure duration when comparing patients who experienced hypotension versus those who did not (21.41 minutes compared to 12.8 minutes, respectively, p=0.0001). When comparing patient characteristics for those who experienced bradycardia versus those who did not, there was a statistically significant difference in BMI (22.1 vs 19.4, respectively, p=0.03).
Table 3.
Adverse Events Based on Patient Parameters
There were three adverse reactions in 2 patients with documented interventions; however, all procedures were completed, and no patient required overnight hospitalization. One patient experienced laryngospasm and seizure that required succinylcholine and levalbuterol to facilitate bag valve mask ventilation. The seizure resolved without intervention. This patient did have a prior seizure disorder and was also diagnosed with asthma. The patient received propofol, 5.7 mg/kg, fentanyl, and midazolam during the procedure. The second patient experienced bradypnea that did not self-resolve and required bag valve mask ventilation. The patient received propofol, 0.4 mg/kg, fentanyl, and midazolam during the procedure.
DISCUSSION
Dose
According to the manufacturer, no pharmacokinetic gender differences have been observed. Other studies in adults have demonstrated that women recover more quickly than men following sedation with propofol.9,10 In a study of 20 adult laparoscopic cholecystectomy patients, time to response to verbal stimuli from the time propofol infusion was stopped was shorter in females than in males, with no differences in plasma concentrations.11 A study of propofol pharmacokinetics in elderly patients found that blood concentrations of propofol were approximately 10% lower in females than in males during infusions running at the same milligram per kilogram rate.12
While these studies demonstrate that sex differences of propofol pharmacokinetics and pharmacodynamics may exist, this has not been tested in the pediatric population. This study showed no differences between the required doses of propofol for male and those for female pediatric patients.
Studies in adults that analyzed the influence of obesity on propofol pharmacokinetics have resulted in conflicting data, and the optimal dosing scheme remains controversial. Additionally, most studies examined the pharmacokinetics of bolus dosing followed by a continuous infusion as opposed to bolus dosing alone. A study by Servin et al13 found no difference in initial volume of distribution when comparing obese patients to lean adults.
Despite the growing epidemic of childhood obesity, clinical studies examining pharmacokinetic alterations in this population are lacking.14 Propofol is a highly lipophilic medication, and it would be a reasonable assumption that it would accumulate in the adipose tissue of obese patients; however, as fat mass increases, the amount of blood flow supplying the adipose tissue remains the same.15 This may result in decreased distribution of propofol into adipose tissue, supporting the use of ideal or adjusted body weight for dosing. A recent study by Olutoye et al16 found the effective dose of propofol that caused loss of consciousness in 95% of patients was 1.99 mg/kg (confidence interval (CI), 1.745–2.183) in obese children versus 3.183 mg/kg (CI, 2.681–3.225) in non-obese children. In our study, obese patients received an average dose of 2.6 mg/kg (CI 1.7–3.6) versus 3.9 mg/kg (CI, 3.3–4.5) in children considered to be at a healthy weight. Although not statistically significant, there is a tendency toward a decreased dose requirement in obese patients.
Significant changes in body fat and muscle mass occur during puberty, and these may also change dosing requirements.17 This study considered the effect that puberty may have had on dosing requirements. To do this ideally, the doses for the pediatric patients in the prepubescent phase through full sexual maturity would have been compared based on the Tanner score, a sexual maturity score that correlates to stages of puberty. As this information was not available, age was used as a surrogate marker. The age group 10 to 12 was chosen because this is when the onset of puberty generally begins. As seen in Table 2, this study found no statistically significant differences between children in the age groups ≤9, 10 to 12, or >12 years.
Adverse Drug Events
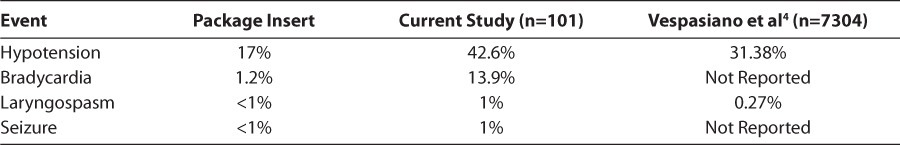
Table 4 compares the rate of adverse drug events in the current study to those reported in the prescribing information and the results of a large prospective, observational study. The incidence of hypotension and bradycardia are high in the current study compared to the prescribing information, however, the authors of the package insert do not clearly define what qualified as an adverse event. The incidence of hypotension in this study was much more comparable to the findings of Vespasiano et al.4 They documented hypotension when a change of ≥25 mm Hg from baseline occurred. If systolic blood pressure (SBP) seemed elevated as a result of stress and/or anxiety, the normal SBP for age was substituted as the baseline value.4
Table 4.
Adverse Drug Events Comparison
Limitations
There are a number of limitations to the current study. The sample size was small and may have been insufficient to establish statistical significance. As it was a retrospective study, some information that may have influenced study results but was not able to be collected include time to awakening, level of consciousness achieved, and Tanner score. Additionally, dosing was at the discretion of the provider, so all dosing schemes were different including number and frequency of boluses. Finally, information about how chronic medications may have influenced sedation requirements was not included.
CONCLUSIONS
Adverse reactions were common, but few required intervention, and no procedures were aborted due to complications. There were no differences in dose requirements based on sex or age. The differences in dosing among underweight, healthy weight, overweight, and obese pediatric patients were not statistically significant. The dose of propofol was higher in patients who experienced bradycardia and hypotension, but there was no statistical significance. Given the above results, future studies should be conducted with larger sample sizes to establish whether statistical significance exists.
ACKNOWLEDGMENTS
The Introduction and Materials and Methods were presented in poster form at the American Society of Health-System Pharmacists Midyear Clinical Meeting, Las Vegas, NV, December 8, 2009. The study was presented in poster form at the Annual Meeting of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition, New Orleans, LA, October 23, 2010, and in platform presentation form at the Eastern States Residency Conference, Hershey, PA, April 30, 2010.
ABBREVIATIONS
- BMI
body mass index
- CT
computed tomography
- EGD
esophagogastroduodenoscopy
- ICD-9
International Classification of Diseases Ninth Revision
- MRI
magnetic resonance imaging
- SBP
systolic blood pressure
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Irvine, CA: Teva Parenteral Medicines, Inc.; 2008. Propofol [package insert] [Google Scholar]
- 2.Bray RJ. Propofol infusion syndrome in children. Paediatr Anaesth. 1998;8(6):491–499. doi: 10.1046/j.1460-9592.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 3.Parke TJ, Stevens JE, Rice ASC et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ. 1992;305(6854):613–616. doi: 10.1136/bmj.305.6854.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vespasiano M, Finkelstein M, Kurachek S. Propofol sedation: intensivists' experience with 7304 cases in a children's hospital. Pediatrics. 2007;120(6):e1411–e1417. doi: 10.1542/peds.2007-0145. [DOI] [PubMed] [Google Scholar]
- 5.Larsen R, Galloway D, Wadera S et al. Safety of propofol sedation for pediatric outpatient procedures. Clin Pediatr. 2009;48(8):819–823. doi: 10.1177/0009922809337529. [DOI] [PubMed] [Google Scholar]
- 6.Cravero JP, Beach ML, Blike GT et al. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108(3):795–804. doi: 10.1213/ane.0b013e31818fc334. [DOI] [PubMed] [Google Scholar]
- 7.Barlow SE, Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 8.Kliegman RM, editor. Nelson Textbook of Pediatrics. 18th ed. Philadelphia: WB Saunders Elsevier; 2007. Charts for heart rate, respirations, and blood pressure; p. 389. et al, eds. [Google Scholar]
- 9.Apfelbaum JL, Grasela TH, Hug CCJ et al. The initial clinical experience of 1819 physicians in maintaining anesthesia with propofol: characteristics associated with prolonged time to awakening. Anesth Analg. 1993;77(4 suppl):S10–S4. [PubMed] [Google Scholar]
- 10.Myles PS, McLeod AD, Hunt JO, Fletcher H. Sex differences in speed of emergence and quality of recovery after anaesthesia: cohort study. BMJ. 2001;322(7288):710–711. doi: 10.1136/bmj.322.7288.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoymork SC, Raeder J, Grismsmo B, Steen PA. Bispectral index, predicted and measured drug levels of target-controlled infusions of remifentanil and propofol during laparoscopic cholecystectomy and emergence. Acta Anaesthesiol Scand. 2000;44(9):1138–1144. doi: 10.1034/j.1399-6576.2000.440918.x. [DOI] [PubMed] [Google Scholar]
- 12.Vuyk J, Oostwouder CJ, Vletter AA et al. Gender differences in the pharmacokinetics of propofol in elderly patients during and after continuous infusion. Br J Anaesth. 2001;86(2):183–188. doi: 10.1093/bja/86.2.183. [DOI] [PubMed] [Google Scholar]
- 13.Servin FS, Farinotti R, Haberer JP, Desmonts JM. Propofol infusion for maintenance of anesthesia in morbidly obese patients receiving nitrous oxide. Anesthesiology. 1993;778(4):657–665. doi: 10.1097/00000542-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Mulla H, Johnson TN. Dosing dilemmas in obese children. Arch Dis Child Educ Pract Ed. 2010;95(4):112–117. doi: 10.1136/adc.2009.163055. [DOI] [PubMed] [Google Scholar]
- 15.Lesser GT, Deutsch S. Measurement of adipose tissue blood flow and perfusion in man by uptake of 85Kr. J Appl Physiol. 1967;23(5):621–630. doi: 10.1152/jappl.1967.23.5.621. [DOI] [PubMed] [Google Scholar]
- 16.Vlessides M. Obese children require less propofol. Anesthesiology News. February 2012;38(2) http://www.anesthesiologynews.com/ViewArticle.aspx?d=Clinical+Anesthesiology&d_id=1&i=February+2012&i_id=812&a_id=20127. Accessed February 25, 2012. [Google Scholar]
- 17.Stang J, Story M. Adolescent growth and development. In: Stang J, Story M, editors. Guidelines for Adolescent Nutrition Services. Minneapolis, MN: Center for Leadership, Education and Training in Maternal and Child Nutrition, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota; 2005. pp. 1–8. [Google Scholar]


