Abstract
OBJECTIVE
To compare the efficacy and safety of dexmedetomidine and fentanyl for sedation in mechanically ventilated premature neonates.
METHODS
This was a retrospective, observational case-control study in a level III neonatal intensive care unit. Forty-eight premature neonates requiring mechanical ventilation were included. Patients received fentanyl (n=24) or dexmedetomidine (n=24) for pain or sedation. Each group also received fentanyl and lorazepam boluses as needed for agitation. The primary outcomes were efficacy and frequency of acute adverse events associated with each drug. Days on mechanical ventilation, stooling patterns, feeding tolerance, and neurologic outcomes were also evaluated.
RESULTS
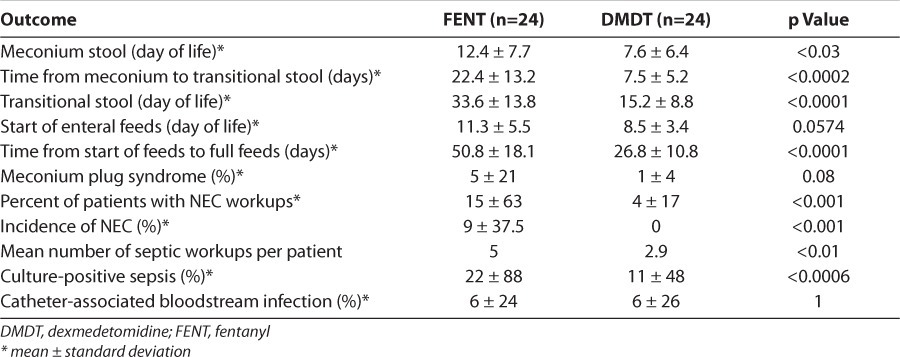
There were no significant differences in baseline demographics between the dexmedetomidine and fentanyl patients. Patients in the dexmedetomidine group required less adjunctive sedation and had more days free of additional sedation in comparison to fentanyl (54.1% vs. 16.5%, p<0.0001). There were no differences in hemodynamic parameters between the 2 groups. Duration of mechanical ventilation was shorter in the dexmedetomidine group (14.4 vs. 28.4 days, p<0.001). Meconium passage (7.5 vs. 22.4 days, p<0.0002) and time from initiation to achievement of full enteral feeds (26.8 vs. 50.8 days, p<0.0001) were shorter in the dexmedetomidine group. Incidence of culture-positive sepsis was lower in the dexmedetomidine group (48% vs. 88%). The incidence of either severe intraventricular hemorrhage or periventricular leukomalacia was not statistically significantly reduced (2% vs. 7%).
CONCLUSIONS
Dexmedetomidine was safe and effective for sedation in the premature neonates included in this study. Prospective randomized-controlled trials are needed before routine use of dexmedetomidine can be recommended.
INDEX TERMS: dexmedetomidine, fentanyl, mechanical ventilation, neonate, sedation
INTRODUCTION
Sedation in mechanically ventilated neonates presents a unique therapeutic dilemma in which the provider must balance patient comfort with the potentially negative consequences of drug exposure to the developing brain. Standard therapy for sedation in these patients generally involves a combination of a narcotic and a benzodiazepine.1 Common adverse effects include respiratory depression, gastrointestinal complications, and neurologic dysfunction, all of which can lead to additional morbidity in the premature infant.2,3 Respiratory depression may lengthen the duration of mechanical ventilation, increasing the risk of chronic lung disease.4 Decreased gastrointestinal motility can lead to the development of abnormal stooling patterns, meconium plug syndrome, and delayed introduction or advancement of enteral feeds.2,5 In the hemodynamically unstable neonate, narcotic use has been linked to increased risk of intraventricular hemorrhage (IVH).6 Narcotic- and benzodiazepine-mediated neuronal apoptosis has been implicated as a potential cause of long-term neurologic complications in premature neonates.7,8
Dexmedetomidine is a centrally acting α2 receptor agonist that has both sedative and analgesic properties.9 In contrast to a narcotic, dexmedetomidine does not have an appreciable effect on respiratory drive or gastric motility. Animal models suggest a potentially neuroprotective effect of dexmedetomidine, especially in the hypoxic-ischemic environment.10 There is also some evidence to suggest that dexmedetomidine diminishes inflammatory processes, particularly in sepsis and the mechanically ventilated lung.11,12 While it is not approved by the U.S. Food and Drug Administration for use in children or neonates, the adult and pediatric literature has been mostly in favor of its use for mechanically ventilated patients. In these populations, dexmedetomidine infusion is associated with decreases in the duration of mechanical ventilation, decreased use of narcotics/adjunctive sedation, and reduced incidence of intensive care unit (ICU) delirium/organic brain dysfunction and sepsis.13–21 Published literature describing the use of dexmedetomidine infusion in premature neonates is lacking. We detailed our initial experience with dexmedetomidine in a premature infant in a previously published case report.22 The patient was a 23-week gestational age infant in whom adequate sedation was not obtained with fentanyl infusion, scheduled lorazepam and phenobarbital, and as-needed boluses of lorazepam, midazolam, and fentanyl. After dexmedetomidine was initiated, the patient showed a dramatic response to therapy, allowing for ventilator synchrony and overall improved respiratory status. The impressive response in that case and the relative benefits with dexmedetomidine in other study populations compelled us to implement dexmedetomidine as our first-line sedative in many of our neonates. We subsequently decided to compare our clinical outcomes with those experienced when we used more traditional sedation approaches as a means of assuring quality improvement with this approach. This study describes our use of dexmedetomidine in mechanically-ventilated premature infants in a neonatal intensive care unit (NICU), and compares them to matching cases treated with fentanyl infusion as the primary sedative. Since this reflected retrospective case review only, the study was exempted from informed consent by our institutional review board.
MATERIALS AND METHODS
This retrospective historical case control study includes premature neonates born at Women's Hospital of Greensboro between January 2005 and May 2010. Infants were eligible for study inclusion if they were <36 weeks gestation at birth, less than 2 weeks of life at study entry, and receiving mechanical ventilation. The fentanyl historical control patients were selected from the NICU database when sufficiently matched to a dexmedetomidine-treated neonate based on baseline demographics described in Table 1. Particular focus for matching cohort pairs was on gestational ages being within one week of each other since many of the long-term patient outcomes in the neonatal population are gestational age-dependent. Fentanyl patients were eligible for inclusion if they received fentanyl via continuous infusion or scheduled intravenous (IV) boluses for sedation within the first 48 hours of life and were born between January 2005 and May 2010. Patients in the dexmedetomidine group were born between September 2008 and May 2010. These date ranges were chosen to minimize risk of practice variability in the NICU between the two groups. Dexmedetomidine was initiated within 48 hours of life, either empirically or in those who met predefined criteria for continuous infusion sedation (patients who required at least 5 doses of fentanyl 1 mcg/kg, lorazepam 0.1 mg/kg, or any combination of the 2 agents in a 24-hour period). Infants were excluded from either group if they had major congenital anomalies considered incompatible with life.
Table 1.
Baseline Demographics
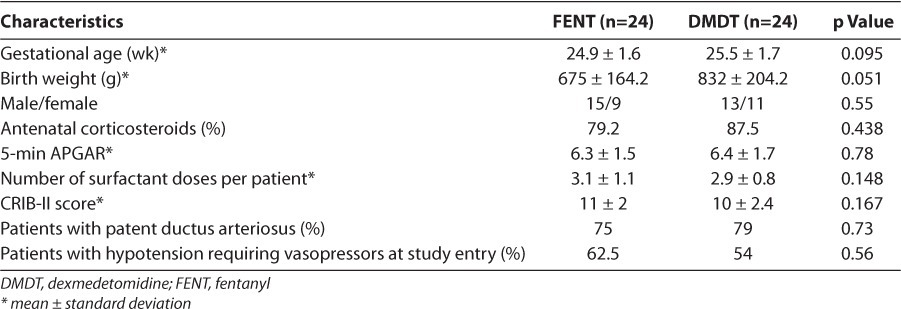
Data collection included patient demographics and indices of treatment efficacy and toxicity. Mean daily doses/infusion rates of either fentanyl or dexmedetomidine were obtained for each patient. The treatment period for the primary outcome began when the patient was started on the study drug and ended after the study drug was discontinued, either because there was no continuing need for sedation or on account of hospital transfer/death. Data were collected for several secondary outcomes beyond the actual infusion period to better assess the safety outcomes. The primary efficacy outcome was the need for adjunctive analgesia or sedation during the treatment period. Adjunctive sedation was defined as any bolus dose(s) of fentanyl (1 mcg/kg) or lorazepam (0.1 mg/kg) given in addition to the continuous infusion or scheduled boluses of the treatment drug. Total fentanyl and lorazepam requirements were collected for each patient. The percent of time during which the infant required no additional sedation and the mean number of doses per day of adjunctive sedation during the study period were calculated. Since dexmedetomidine is thought to have minimal inhibitory effects on respiratory drive and gastrointestinal function, several variables were collected to evaluate these endpoints and compare them to infants treated with fentanyl, including duration of mechanical ventilation, stool pattern, and feeding tolerance. Respiratory function was evaluated by recording the duration of mechanical ventilation from birth to sustained extubation (at least 48 hours without requiring reintubuation). The mean number of chest x-rays in each group was also calculated. Gastrointestinal function was measured using multiple markers. Time to first meconium stool and duration of meconium passage (determined by days until transitional stools) were used as markers of gastric motility. Feeding tolerance was explored by determining time to start enteral feeds and time to achieve full enteral feeds (150 mL/kg/day).
Since little published literature exists describing how to use dexmedetomidine in premature neonates, a comparison of dosing strategies was included in the study. Loading dose, mean effective dose, and duration of drug infusion were evaluated.
Weaning of fentanyl and dexmedetomidine was done cautiously to prevent withdrawal. Fentanyl was weaned by 1 mcg/kg/day as tolerated. Weaning was to be halted if any infant displayed signs of withdrawal. Dexmedetomidine weaning was similar, except infusion rates were decreased by 0.1 mcg/kg every 12 to 24 hours as tolerated.
To assess drug toxicity, hemodynamic parameters (heart rate and systolic, diastolic, and mean arterial blood pressures) were recorded hourly for both groups. Dosage adjustments were to be held for any patient who showed >20% change in baseline heart rate or blood pressure or required a volume bolus to maintain blood pressure goals. Cranial ultrasound results (at 7-10 days and at 30 days) to evaluate IVH and periventricular leukomalacia (PVL) and rates of culture-positive sepsis were included as available. Data are presented as means ± standard deviations or percentages. Median data are presented in the instance of significantly skewed data. Student's t-test and chi-square test were used as appropriate for statistical analysis of selected demographic data and outcomes. A p-value of <0.05 was considered statistically significant.
RESULTS
A total of 48 patients were included in the analysis. The baseline demographics of each group are described in Table 1. None of the demographic data represented statistically significant differences, although the difference in mean birth weight was 157 grams. The mean gestational age was 25 weeks, the mean age at study entry was 27 hours, and the severity of illness in fentanyl and dexmedetomidine groups, as measured by CRIB-II scores, was similar. The primary underlying diagnoses at study entry were respiratory distress syndrome, respiratory failure secondary to prematurity (all patients were intubated immediately following delivery), sepsis, and hypotension. Echocardiogram-confirmed patent ductus arteriosus was present in approximately 80% of the patients in each group. All patients who had a patent ductus arteriosus were treated with indomethacin. The primary indication for the use of fentanyl or dexmedetomidine was as part of a sedation treatment regimen for mechanical ventilation.
Dexmedetomidine Dosing
Nearly half of the dexmedetomidine patients received a 0.5-mcg/kg bolus at the start of therapy. Every patient was started at a maintenance infusion rate of 0.3 mcg/kg/hr. Increases in infusion rate by 0.1 mcg/kg/hr were made up to twice daily based on patient response (i.e., elevated sedation scores with a need for >3 doses of adjunctive sedation during a 12-hour nursing shift). Hemodynamic parameters (systolic blood pressure, diastolic blood pressure, mean arterial pressure, and heart rate) were monitored hourly to assess toxicity.
Dexmedetomidine was infused via peripheral or central venous access on a syringe pump to allow for the smaller infusion rates required in the extremely low birth weight population. The standard manufacturer-recommended 4-mcg/mL dilution was used (reconstituted in normal saline). For infants whose calculated initial bolus was less than 0.1 mL (the smallest allowable bolus volume to be infused on the syringe pump), 0.1 mL was given over an extended infusion period to prevent hypotension or fluctuations in blood pressure. A visual compatibility test was done to assess the compatibility of dexmedetomidine with total parenteral nutrition. We found it to display no visible particles, so dexmedetomidine was infused with total parenteral nutrition when intravenous access was limited.
The mean dexmedetomidine infusion rate was 0.6 mcg/kg/hr (range, 0.3–1.2 mcg/kg/hr) and the mean duration was 12 days. Dexmedetomidine was weaned by decreasing the infusion by 0.1 mcg/kg/hr every 12 to 24 hours, as tolerated. No patients displayed signs of withdrawal (hypertension, tachycardia, increased agitation) during dexmedetomidine weaning. It was not felt that any patient developed tolerance over the course of therapy, since the highest effective dose was maintained for several days without need for up-titration secondary to decreased response. If an infant was on extended infusions, the drip was weight-adjusted as appropriate.
Sedation/Efficacy Outcomes
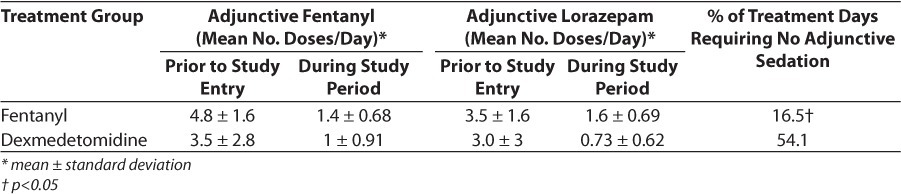
Sedation use in each group is summarized in Table 2. The mean treatment durations for fentanyl and dexmedetomidine were 20 and 12.4 days, respectively. Dexmedetomidine patients had similar requirements to fentanyl patients prior to the start of dexmedetomidine infusion. Mean number of sedative boluses per day during the treatment period was similar as well (combined fentanyl + lorazepam boluses in fentanyl group = 3.0, vs. 1.73 in dexmedetomidine group). However, dexmedetomidine patients had a greater percentage of treatment days requiring no additional sedation compared to fentanyl patients (54.1% and 16.5%, respectively). The mean total fentanyl and lorazepam exposures in the dexmedetomidine group were 20.6 mcg/kg and 2.7 mg/kg, respectively. Fentanyl patients received 398 mcg/kg of fentanyl and 6.8 mg/kg of lorazepam.
Table 2.
Sedation requirements
Hemodynamic Outcomes
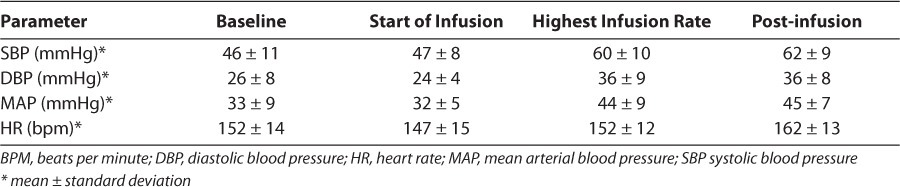
In the fentanyl group, there were no significant changes from baseline hemodynamic parameters (Table 3). No patients required intervention for hypotension as defined earlier. A primary shortterm safety concern with dexmedetomidine use is its effect on hemodynamic parameters such as blood pressure and heart rate. While hypertension, hypotension, and bradycardia are potential adverse effects associated with dexmedetomidine, no patient in the dexmedetomidine group experienced any appreciable effect on either blood pressure or heart rate per the hourly measurements obtained (Table 4). Dexmedetomidine titration was done slowly and in small increments to minimize potential fluctuations in hemodynamic parameters. Likewise, weaning was completed slowly to allow the patients to acclimate before discontinuation of the drug.
Table 3.
Hemodynamic profile associated with fentanyl

Table 4.
Hemodynamic profile associated with Dexmedetomidine
Respiratory Outcomes
Patients in the dexmedetomidine group had a significantly shorter duration of mechanical ventilation (14.4 days) than patients in the fentanyl group (28.4 days; Table 5). Since dexmedetomidine has minimal appreciable effect on respiratory drive, extubation while receiving the drug, regardless of the infusion rate, is permissible. Clinicians are often reluctant to extubate patients who are still receiving narcotic infusions. Eighty-three percent of the patients in the dexmedetomidine group were receiving dexmedetomidine when they were extubated, most at rates above 0.3 mcg/kg/hr (Figure). This allowed the provider to extubate the patient when appropriate from a respiratory standpoint, rather than waiting until the sedation was light enough to prevent respiratory compromise. None of the patients in the fentanyl group were receiving continuous infusion of fentanyl at the time of extubation. Significantly fewer dexmedetomidine patients required dexamethasone for facilitation of extubation compared to fentanyl patients (18.2% vs. 45.8%, respectively; Table 5). At our institution, eligibility for dexamethasone use is limited to infants requiring mechanical ventilation for >3 weeks, who are unable to tolerate ventilator weaning to settings compatible with extubation, and who exhibit radiographic changes consistent with severe lung disease. Since the mean age at the time of extubation in the dexmedetomidine group was 14 days, few of these patients required dexamethasone to facilitate extubation. Dexmedetomidine patients underwent an average of 28 chest x-rays during their hospital stay versus 49 chest x-rays in the fentanyl population (Table 5). This allows for less radiation exposure to the infant, as well as cost containment, and is most likely a result of fewer ventilator days and fewer sepsis evaluations.
Table 5.
Respiratory outcomes
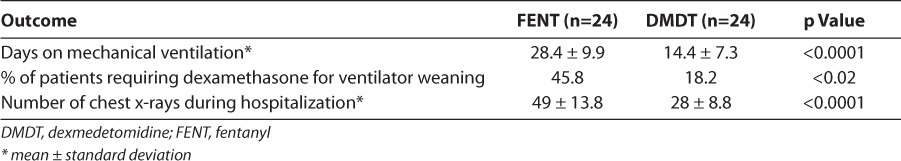
Figure.
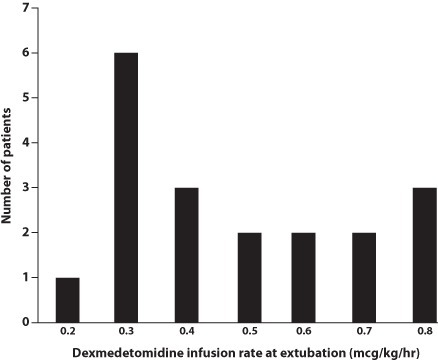
Dexmedetomidine infusion rates upon patient extubation.
Gastrointestinal Outcomes
Since dexmedetomidine does not impact gastrointestinal motility as strongly as opioids, we examined possible clinical benefits to this pharmacologic difference. There were statistically significant differences favoring dexmedetomidine in outcomes reflecting gastrointestinal function. This includes considerable differences in stooling and feeding. The main outcomes describing gastrointestinal function are outlined in Table 6 and show numerous statistically significant benefits to dexmedetomidine. Time to first meconium stool was shorter in dexmedetomidine patients than in fentanyl patients (7.6 vs. 12.4 days, respectively). The duration of meconium passage was also reduced in the dexmedetomidine group compared to the fentanyl group (7.5 vs. 22.4 days, respectively). The dexmedetomidine patients started to have transitional stools around day 15 of life, compared to day 33 of life in the fentanyl patients. There was not a statistically significant difference in the incidence of meconium plug syndrome between dexmedetomidine and fentanyl patients (4% vs. 21%, respectively), although the only patient in the dexmedetomidine group who experienced a meconium plug had received a large quantity of narcotic boluses in the days preceding the initiation of dexmedetomidine. In conjunction with delayed stooling, fentanyl patients took longer to initiate and achieve full enteral feeds compared to dexmedetomidine patients. Dexmedetomidine patients were started on trophic enteral feeds approximately 2 days earlier than fentanyl patients. Feeding tolerance was improved in dexmedetomidine patients, who, on average, were maintained on full enteral feeds 24 days earlier than the fentanyl patients. No patient in the dexmedetomidine group was diagnosed with confirmed necrotizing enterocolitis (NEC) during the study period, compared to 9% of patients in the fentanyl group.
Table 6.
Gastrointestinal and Infection outcomes
Other Outcomes
No patient in the dexmedetomidine group showed signs of withdrawal during the weaning period. Approximately half the patients in the fentanyl group required slower weaning of the fentanyl drip because of signs of withdrawal. No adjunctive medications were started to treat withdrawal in the fentanyl group; instead, the frequency of weaning was decreased to every 48 hours. Patients in the dexmedetomidine group experienced an insignificant decrease in the combined outcomes of severe IVH (grades III-IV) and PVL compared to the fentanyl patients (2% vs. 7%, respectively). There was a lower incidence of culture-positive sepsis in the dexmedetomidine group (combined blood, sputum, and urine), as well as sepsis and NEC workups, during their hospital stay. There was no difference between the 2 groups in the incidence of catheter-associated bloodstream infections. These results are summarized in Table 7.
Table 7.
Other outcomes

DISCUSSION
Meta-analyses of narcotic and benzodiazepine use in mechanically ventilated neonates show increased time to achieve full enteral feeds, prolonged duration of mechanical ventilation, higher incidence of adverse neurologic events (death, severe IVH, PVL), and increased length of hospitalization.1–3 Failure to provide sedation in premature neonates has negative consequences as well, including acute physiologic and behavioral responses to painful stimuli that create a catabolic state, which can also lead to increased risk of IVH and PVL.23 Additional consequences related to untreated pain in the neonate include ventilator dyssynchrony leading to periods of hypoxemia, blood pressure fluctuations, and hyperalgesia. The neonatal provider is thus forced to choose between the negative impacts of unaddressed pain/agitation in the neonate and the negative adverse effects of the drugs currently used to treat it.
Dexmedetomidine presents a novel option for the management of pain and sedation in pre-mature neonates. This study demonstrated the achievement of adequate sedation without the development of hemodynamic instability when neonates were given a continuous infusion of dexmedetomidine. Patients in the dexmedetomidine group required less adjunctive analgesic and sedation medications, suggesting that dexmedetomidine effectively provides both anti-nociception and hypnosis in premature infants. Animal models suggest that the hypnotic effect of dexmedetomidine may actually be stronger in the premature patient, while its analgesic effect is consistent across developmental ages.24
An absence of appreciable respiratory suppression with dexmedetomidine was demonstrated in the premature neonate population in this study. Despite having similar baseline respiratory disease, as evidenced by the number of surfactant doses and CRIB-II scores, patients in the dexmedetomidine group were extubated approximately 2 weeks earlier than patients in the fentanyl group. This outcome is most likely not entirely the result of decreased respiratory drive suppression with dexmedetomidine. Interleukin (IL)-1β and cyclooxygenase-2/prostaglandin E2 released in response to pulmonary inflammation are important mediators of ventilator-induced lung injury.11 Dexmedetomidine-induced reductions in IL-1β and cyclooxygenase-2 in rat models of ventilator-induced lung injury have mitigated pulmonary inflammatory responses and led to reduction in lung tissue damage. The role of inflammation in the development of neonatal chronic lung disease is not well understood, but mechanical ventilation–induced trauma to the premature lung is a known risk factor.25 It is possible that reduced pulmonary inflammatory mediators in the dexmedetomidine group contributed to less severe lung disease, allowing for earlier extubation. Finally, the reluctance of neonatologists to extubate from higher fentanyl infusion rates may also prolong intubation time, thus worsening ventilator-induced lung damage. As can be seen in the Figure, dexmedetomidine infusion rates at extubation could be fairly high, and no such reluctance to extubate neonates while still on dexmedetomidine infusion is necessary. Further studies are needed to better characterize the effect of dexmedetomidine on the premature lung.
The lower incidence of culture-positive sepsis seen in this study is consistent with the Safety and Efficacy of Dexmedetomidine Compared with Midazolam (SEDCOM) trial, which also showed a 50% lower rate of sepsis in the dexmedetomidine group.13 The SEDCOM trial evaluated the use of dexmedetomidine versus midazolam for sedation of critically ill adult patients, focusing particularly on those with expected duration of mechanical ventilation >24 hours and incidence of delirium. A lower incidence of sepsis was an incidental finding in the study. Where benzodiazepines inhibit macrophage function, alpha agonists such as dexmedetomidine are thought to promote macrophage activity and exhibit some independent antimicrobial effects.26 Several animal models suggest that dexmedetomidine has protective immunomodulation effects in the environment of sepsis, particularly with reduction in inflammatory mediators such as tissue necrosis factor-alpha (TNF-α), IL-1β, and interleukin-6.12,26 Animal models of endotoxemia demonstrate that dexmedetomidine-treated subjects have lower mortality rates, lower inflammatory markers, and better overall outcomes.12,27–29 The Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) study evaluated the effect of sepsis on patients randomized to dexmedetomidine or lorazepam as an a priori sub-analysis and found shorter duration of mechanical ventilation, more coma-/delirium-free days, and a 70% reduction in 28-day mortality for patients who received dexmedetomidine.30 The dexmedetomidine group in our study may have had a reduced incidence of culture-positive sepsis for a variety of reasons independent of the immunomodulatory effects dexmedetomidine, including shorter duration of mechanical ventilation, earlier enteral feeding, and fewer days with central intravenous access.
In a study comparing dexmedetomidine to propofol in 40 adult ICU patients with severe sepsis, the dexmedetomidine patients showed significant reductions in TNF-α, IL-1, IL-6, and intra-abdominal pressure (IAP).31 Although information is scarce characterizing the influence of IAP in neonatal sepsis, elevated IAP in children and adults is associated with development of abdominal compartment syndrome, which carries a mortality rate of 50%-60%.32 Some data suggest that elevated IAP in the setting of neonatal NEC is a predictor of mortality.33 Dexmedetomidine may play a beneficial role in this population. Since the pathophysiology of NEC remains unclear, the authors are unsure as to what part dexmedetomidine may have played in the decreased incidence of NEC in the dexmedetomidine group.
Narcotic-induced gastrointestinal dysfunction is an important source of morbidity in the neonatal patient. Narcotics have been shown to delay enteral feeds and meconium passage.2,34 Delayed enteral nutrition is associated with increased risk of infection, delayed intestinal maturation, slower growth, cholestasis, and adverse neurologic outcomes.35 Feeding tolerance may be impacted by meconium passage. Some studies have shown that infants with earlier evacuation of meconium achieve full feeds more rapidly.36,37 Opioid exposure in premature neonates represents a significant risk factor for the development of meconium plug syndrome, which can lead to intestinal perforation and a need for surgical intervention.38 Per the hospital feeding protocol, once infants were deemed eligible for feeds (normotensive/no use of vasopressors, no indomethacin within 48 hours), trophic enteral feeds at 20 mL/kg/day were initiated for 5 days and advanced as tolerated up to 150 mL/kg/day. If patients were designated as unable to take anything orally, it was at the discretion of the provider to determine at what rate to resume feeds when appropriate. Since dexmedetomidine does not alter gastrointestinal function like fentanyl, patients in the dexmedetomidine group were expected to achieve enteral feeds and meconium passage more quickly compared to the fentanyl group, and this was indeed the case.
Patients in the dexmedetomidine group had an insignificantly lower incidence of severe IVH (grades III-IV) and PVL than the fentanyl group. Hypotension prior to starting narcotic infusion has been linked to development of severe IVH.23 There was no statistically significant difference in baseline use of vasopressors for hypotension in the 2 groups. Other risk factors for IVH or PVL, such as antenatal corticosteroid use, early sepsis, maternal chorioamnionitis, and incidence of respiratory distress syndrome, were also not statistically significant.39 Neonatal benzodiazepine use has been linked to increased mortality, severe IVH, and PVL.3 Overall benzodiazepine use was higher in the fentanyl group, but it is not known whether this played an important role in the neurologic outcomes in the study.
Animal neonatal models with dexmedetomidine have demonstrated significant neuroprotective effects with its use, particularly in the hypoxic-ischemic neonatal brain.40–42 Several theories regarding alpha agonist–mediated neuroprotection abound, including anti-apoptosis in perinatal excitotoxic brain injury and reduced ischemic volume in cerebral ischemia. Neonatal rats exposed to dexmedetomidine prior to asphyxia demonstrated improved neurologic functional deficit and dose-dependent reduction of white matter loss compared to placebo. It is not known at this time what the long-term neurologic implications for premature neonates will be following dexmedetomidine infusion.
One of the primary limitations of this study is its retrospective case-control design. Although historical controls were used for the fentanyl patients, there was overlap in the dates of included patients with the dexmedetomidine group. Patients born before 2005 were not included in the study in an effort to ensure that there were no major changes in standards of care in the unit that would significantly impact outcomes in either group. Another weakness of the study is the small sample size. Larger numbers of patients will be needed to determine whether the findings in this study are consistent when evaluated prospectively with a greater number of participants. Also, the differences in birth weight cannot be ignored entirely. Although gestational ages were similar, the Ballard score used to estimate gestation is accurate to within 2 weeks, and it is possible that differences in maturity explain important parts of our results. However, we do not feel this is the case, given the similarities in severity of other diseases.
Another study weakness is that long-term neurologic outcomes in the patient groups are not available at this time. While the short-term safety of dexmedetomidine in premature neonates seems to be favorable based on our findings, long-term follow-up is warranted and needed before widespread use of dexmedetomidine can be supported.
Our impetus for changing to dexmedetomidine from routine narcotic infusions was a combination of negative outcomes routinely reported with the standard sedative approach of narcotics and benzodiazepines. It seemed unwise to continue to practice in such a knowingly harmful manner. Alternatively, the human and animal studies with dexmedetomidine seemed to point to this agent as effective, relatively safe, and possibly neuroprotective. This study seems to support our approach in our institution. We now have a large population that we have treated with dexmedetomidine as our primary agent and we continue to have impressive results.
CONCLUSIONS
Dexmedetomidine demonstrated efficacy and short-term safety when used for sedation in the premature neonates included in this study. Potential benefits for dexmedetomidine use over fentanyl include shorter duration of mechanical ventilation, fewer days to achieve full enteral feeds, more rapid passage of meconium, and lower infection rates. While many of the outcomes were positive in this retrospective case-control study, it is reasonable for clinicians to await prospective randomized controlled trials before dexmedetomidine is used routinely in premature neonates.
ACKNOWLEDGMENT
This research was presented at the 19th Pediatric Pharmacy Conference, October 9, 2010, in St Louis, Missouri.
ABBREVIATIONS
- IAP
intra-abdominal pressure
- ICU
intensive care unit
- IL
interleukin
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- PVL
periventricular leukomalacia
- TNF
tissue necrosis factor
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Aranda JV, Carlo W, Hummel P et al. Analgesia and sedation during mechanical ventilation in neonates. Clin Ther. 2005;27(6):877–899. doi: 10.1016/j.clinthera.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Bellù R, de Waal K, Zanini R. Opioids for neonates receiving mechanical ventilation. Arch Dis Child Fetal Neonatal Ed. 2010;95(4):F241–251. doi: 10.1136/adc.2008.150318. [DOI] [PubMed] [Google Scholar]
- 3.Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev. 2003;(1):CD002052. doi: 10.1002/14651858.CD002052. [DOI] [PubMed] [Google Scholar]
- 4.Doyle LW, Anderson PJ. Pulmonary and neurological follow-up of extremely pre-term infants. Neonatology. 2010;97(4):388–394. doi: 10.1159/000297771. Epub 2010 Jun 10. [DOI] [PubMed] [Google Scholar]
- 5.Härtel C, Haase B, Browning-Carmo K et al. Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants? J Pediatr Gastroenterol Nutr. 2009;48(4):464–470. doi: 10.1097/MPG.0b013e31818c5fc3. [DOI] [PubMed] [Google Scholar]
- 6.Hall RW, Kronsberg SS, Barton BA. Morphine, hypotension, and adverse outcomes among preterm neonates: who's to blame? Secondary results from the NEOPAIN trial. Pediatrics. 2005;115(5):1351–1359. doi: 10.1542/peds.2004-1398. et al. NEOPAIN Trial Investigators Group. [DOI] [PubMed] [Google Scholar]
- 7.Fredriksson A, Pontén E, Gordh T et al. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107(3):427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 8.Mao J, Sung B, Ji RR et al. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002;22(17):7650–7661. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol. 2008;4(5):619–627. doi: 10.1517/17425255.4.5.619. [DOI] [PubMed] [Google Scholar]
- 10.Ma D, Hossain M, Rajakumaraswamy N et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoreceptor subtype. Eur J Pharmacol. 2004;502(1–2):87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Yang CL, Tsai PS, Huang CJ. Effects of dexmedetomidine on regulating pulmonary inflammation in a rat model of ventilator-induced lung injury. Acta Anaesthesiol Taiwan. 2008;46(4):151–159. doi: 10.1016/S1875-4597(09)60002-3. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi T, Kurita A, Kobayashi K et al. Dose- and time-related effects of dexmedetomidine on mortality and inflammation responses to endotoxin-induced shock in rats. Crit Care. 2010;14:R38. doi: 10.1007/s00540-008-0611-9. Epub 2010 Mar 16. [DOI] [PubMed] [Google Scholar]
- 13.Riker RR, Shehabi Y, Bokesch PM et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 14.Pandharipande PP, Pun BT, Herr DL et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 15.Shehabi Y, Grant P, Wolfenden H et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009;111(5):1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 16.Tobias JD, Berkenbosch JW. Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. South Med J. 2004;97(5):451–455. doi: 10.1097/00007611-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Reiter PD, Pietras M, Dobyns EL. Prolonged dexmedetomidine infusions in critically ill infants and children. Indian Pediatr. 2009;46(9):767–773. [PubMed] [Google Scholar]
- 18.Bejian S, Valasek C, Nigro JJ et al. Prolonged use of dexmedetomidine in the paediatric cardiothoracic intensive care unit. Cardiol Young. 2009;19(1):98–104. doi: 10.1017/S1047951109003515. [DOI] [PubMed] [Google Scholar]
- 19.Carroll CL, Krieger D, Campbell M et al. Use of dexmedetomidine for sedation of children hospitalized in the intensive care unit. J Hosp Med. 2008;3(2):142–147. doi: 10.1002/jhm.282. [DOI] [PubMed] [Google Scholar]
- 20.Buck ML, Willson DF. Use of dexmedetomidine in the pediatric intensive care unit. Pharmacotherapy. 2008;28(1):51–57. doi: 10.1592/phco.28.1.51. [DOI] [PubMed] [Google Scholar]
- 21.Walker J, Maccallum M, Fischer C et al. Sedation using dexmedetomidine in pediatric burn patients. J Burn Care Res. 2006;27(2):206–210. doi: 10.1097/01.BCR.0000200910.76019.CF. [DOI] [PubMed] [Google Scholar]
- 22.O'Mara K, Gal P, Ransom JL et al. Successful use of dexmedetomidine for sedation in a 24 week gestational age neonate. Ann Pharmacother. 2009;43(10):1707–1713. doi: 10.1345/aph.1M245. [DOI] [PubMed] [Google Scholar]
- 23.Anand KJ, Hall RW, Desai N et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363(9422):1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 24.Sanders RD, Giombini M, Ma D et al. Dexmedetomidine exerts dose-dependent age-independent antinociception but age-dependent hypnosis in Fischer rats. Anesth Analg. 2005;100(5):1295–1302. doi: 10.1213/01.ANE.0000149595.41576.B3. [DOI] [PubMed] [Google Scholar]
- 25.Thome U, Ambalavanan N. Permissive hypercapnia to decrease lung injury in ventilated preterm neonates. Sem Fetal and Neonatal Med. 2009;14(1):21–27. doi: 10.1016/j.siny.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Ayoglu H, Kulah C, Turan I. Antimicrobial effects of two anaesthetic agents: dexmedetomidine and midazolam. Anaesth Intensive Care. 2008;36(6):681–684. doi: 10.1177/0310057X0803600508. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi T, Kidani Y, Kanakura H et al. Effects of dexmedetomidine on mortality rate and inflammatory response to endotoxin-induced shock in rats. Crit Care Med. 2004;32(6):1322–1326. doi: 10.1097/01.ccm.0000128579.84228.2a. [DOI] [PubMed] [Google Scholar]
- 28.Sanders RD, Hussell T, Maze M. Sedation and immunomodulation. Crit Care Clin. 2009;25(3):551–570. doi: 10.1016/j.ccc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Lai YC, Tsai PS, Huang CJ. Effects of dexmedetomidine on regulating endotoxin-induced up-regulation of inflammatory molecules in murine macrophages. J Surg Res. 2009;154(2):212–219. doi: 10.1016/j.jss.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Pandharipande PP, Sanders RD, Girard TD et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori–designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38. doi: 10.1186/cc8916. E pub Mar 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tasdogan M, Memis D, Sut N et al. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth. 2009;21(6):394–400. doi: 10.1016/j.jclinane.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Carlotti AP, Carvalho WB. Abdominal compartment syndrome: a review. Pediatr Crit Care Med. 2009;10(1):115–120. doi: 10.1097/PCC.0b013e31819371b2. [DOI] [PubMed] [Google Scholar]
- 33.Bonnard A, Carricaburu E, Alberti C et al. Is intraabdominal pressure a good predictor of mortality in necrotizing enterocolitis? Intensive Care Med. 2010;36(3):551–552. doi: 10.1007/s00134-009-1732-9. [DOI] [PubMed] [Google Scholar]
- 34.Bekkali N, Hamers SL, Schipperus MR et al. Duration of meconium passage in pre-term and term infants. Arch Dis Child Fetal Neonatal Ed. 2008;93(5):F376–379. doi: 10.1136/adc.2008.138024. [DOI] [PubMed] [Google Scholar]
- 35.Härtel C, Haase B, Browning-Carmo K et al. Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants? J Pediatr Gastroenterol Nutr. 2009;48(4):464–470. doi: 10.1097/MPG.0b013e31818c5fc3. [DOI] [PubMed] [Google Scholar]
- 36.Shim SY, Kim HS, Kim DH et al. Induction of early meconium evacuation promotes feeding tolerance in very low birth weight infants. Neonatology. 2007;92(1):67–72. doi: 10.1159/000100804. [DOI] [PubMed] [Google Scholar]
- 37.Mihatsch WA, Franz AR, Lindner W et al. Meconium passage in extremely low birthweight infants and its relation to very early enteral nutrition. Acta Paediatr. 2001;90(4):409–411. [PubMed] [Google Scholar]
- 38.Emil S, Nguyen T, Sills J et al. Meconium obstruction in extremely low birth weight neonates: guidelines for diagnosis and management. J Pediatr Surg. 2004;39(5):731–737. doi: 10.1016/j.jpedsurg.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 39.Bassan H. Intracranial hemorrhage in the preterm infant: understanding it, preventing it. Clin Perinatol. 2009;36(4):737–762. doi: 10.1016/j.clp.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Paris A, Mantz J, Tonner PH et al. The effects of dexmedetomidine on the perinatal excitotoxic brain injury are mediated by the alpha 2A adrenoreceptor subtype. Anesth Analg. 2006;102(2):456–461. doi: 10.1213/01.ane.0000194301.79118.e9. [DOI] [PubMed] [Google Scholar]
- 41.Engelhard K, Werner C, Eberspächer E et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96(2):524–531. doi: 10.1097/00000539-200302000-00041. [DOI] [PubMed] [Google Scholar]
- 42.Jolkkonen J, Puurunen K, Koistinaho J et al. Neuroprotection by the alpha2-adrenoceptor agonist, dexmedetomidine, in rat focal cerebral ischemia. Eur J Pharmacol. 1999;372(1):31–36. doi: 10.1016/s0014-2999(99)00186-7. [DOI] [PubMed] [Google Scholar]


