Abstract
OBJECTIVES
The American Academy of Pediatrics and the Society of Critical Care Medicine have documented the importance of pharmacist involvement in pediatric care. Numerous studies have reported the impact of clinical pharmacy interventions in various adult care settings. However, in the pediatric critical care setting, the impact has not been well documented. The purpose of this study was to describe clinical pharmacy faculty interventions in a pediatric intensive care unit (PICU).
METHODS
A pediatric clinical pharmacy faculty member performed and documented clinical interventions in a level I, 18-bed, tertiary care PICU. Information gathered included medication name, specific intervention performed, basic patient demographics, and length of stay from May to December 2009.
RESULTS
During the study period, there were 893 interventions performed on 159 patients over 66 days of service. (Average of 5.5 interventions/patient, and 34 interventions/100 patient PICU days.) Dosing recommendations and pharmacokinetics were the most common type of intervention (28.8% and 21.4%, respectively). Antibiotics and sedatives/analgesia were the most common drug classes in which interventions were made (34.4% and 20.3%, respectively). Ninety-eight percent of all interventions were accepted by the medical staff. The estimated annual cost savings from these interventions was $119,700.
CONCLUSIONS
The average number of interventions per patient in this study was higher than that reported in the literature to date. Dosing recommendations and pharmacokinetics were the most commonly recommended interventions documented. Although this study showed considerable cost savings by a pharmacy clinical faculty member, further study of economic benefits is needed.
INDEX TERMS: pediatric clinical pharmacist, pediatric critical care, pharmacoeconomics, pharmacy faculty, pharmacy interventions
INTRODUCTION
Clinical pharmacists have been shown to enhance outcomes and lower costs in adult critical care units.1,2 In 1988, the Society of Critical Care Medicine released guidelines outlining the important role of pharmacists in the care of critically ill patients.3 In addition, The American Academy of Pediatrics proposed that pharmacist inclusion in the multidisciplinary care team can play an integral part in the reduction of medication errors.4 Yet, while numerous studies have reported the impact of clinical pharmacy interventions in various adult inpatient settings,5–7 the impact of pharmacy interventions in the pediatric critical care setting has not been extensively documented.
The primary objective of this study was to determine the number of pharmacy interventions performed on patients in our tertiary care pediatric intensive care unit (PICU). Secondary objectives of the study were to delineate the specific classes of medications, the types of interventions performed, and to estimate potential medical cost savings.
METHODS
Study Design
A postgraduate dual residency trained pediatric clinical pharmacy specialist participated in patient care rounds in a level I, 18-bed, tertiary care, medical-surgical PICU located in a free standing children's hospital. The academic clinical faculty member was fully funded through the university and provided services and interventions through a contractual agreement to patients in the PICU. On average, the faculty member is available for patient care rounds and follow-up in the afternoon (8 hours each day) approximately 12 to 15 days each month (25 hours each week). This is a retrospective review of the interventions from May 1 to December 31, 2009.
All patients admitted to the PICU during this timeframe and on whom interventions were performed were eligible for inclusion in the study. Exclusion criteria were limited to patients admitted into the PICU who did not receive clinical pharmacy interventions. Basic patient demographics were collected including age, sex, Pediatric Risk of Mortality II (PRISM II) score,8 hospital length of stay, PICU length of stay, and mortality. Specific recorded information about the interventions performed included medication class, type of intervention, and acceptance of intervention by the medical staff.
Institutional review boards of each author's institutions as well as the hospital approved the study. Written informed consent was deemed unnecessary.
Intervention Descriptions
The interventions were divided into multiple broad categories. “Dosing Recommendations” included both increases and decreases of dose or frequency to optimize therapy and/or minimize side effects. “Pharmacokinetic” recommendations included discontinuation of unnecessary drug levels, dose adjustments based on drug levels, obtaining drug levels, and additional monitoring parameters. The pharmacist's recommendations were further broken down into the most common drugs where therapeutic drug monitoring was performed (vancomycin, gentamicin, enoxaparin, and phenobarbital).
“Antibiotic Recommendations” made included a step-up or step-down of antibiotic spectrum, as well as the addition or discontinuation of antibiotics. “Intravenous to Oral” conversions reflected therapies that could be given by the oral route, thereby reducing medication cost and risk of infection. Clinically significant “Drug Interactions” requiring modification in therapy or additional monitoring were recorded. “Discontinuation of Medications” was recommended for therapies no longer indicated. Due to the nature of the PICU and long-term sedation requirements, weaning off of intravenous “Sedative and Analgesic agents” were recorded and typically included oral lorazepam and methadone tapering.
Recommendations including dose or agent changes based on a patient's renal function and estimated creatinine clearance were listed as “Renal Recommendations.” “Laboratory Evaluations” included additional monitoring parameters for nonpharmacokinetic interventions. Questions relating to compatibility and proper administration of medications mainly came from the nursing staff and commonly involved compatibility with parenteral nutrition. Those interventions were labeled as “Compatibility/Administration.” Because the clinical faculty member was not generally involved with pharmacy dispensing, order clarifications were not documented. Any intervention performed by the clinical faculty member that could not be categorized into one of the specified categories was classified as “Other.” This included activities such as participation in cardiac arrests and intubations.
Cost savings were calculated based on Pharmacy One Source Quantify information and literature (Pharmacy One Source, Bellevue, Washington; www.pharmacyonesource.com).9–11
RESULTS
There were 537 patients admitted into the PICU during the study period. One hundred fifty-nine were identified for inclusion in the study. Table 1 notes the demographics for the study population. Three patients were admitted to the PICU twice during the study period for 162 patient admissions. Overall mortality rate was 9.6% for the patients in the study.
Table 1.
Patient Demographics and Outcomes
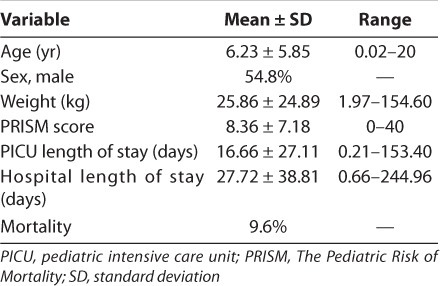
There were a total of 893 interventions performed during the study on the 159 patients. This represents 29.6% of the PICU population. There was an average of 5.5 interventions per patient admission. There were 66 total days in which the pharmacy faculty member performed these interventions for an average of 13.5 interventions per day.
The mean hospital length of stay was 27.7 days, and the mean PICU length of stay was 16.7 days (Table 1). The mean intervention per patient PICU day was 0.34 and per hospital day was 0.23. There were on average 34 interventions per 100 patient PICU days. The medical staff accepted 98% of all suggested interventions.
Breaking down the interventions by intervention class, “Dosing Recommendations” for various classes of medications and changes based on “Pharmacokinetics” were the most common type of interventions performed (28.8% and 21.4%, respectively). Approximately 10% of the interventions did not fall under a specified category and were categorized as “Other.” Antibiotic, sedative/analgesic agents, and gastrointestinal agents were the most common classes of medications (22.2%, 18.7%, and 18.7%, respectively) in which dosing interventions were performed. Table 2 describes the number and percentage of interventions by the individual drug class.
Table 2.
Interventions by Drug Class (cont.)
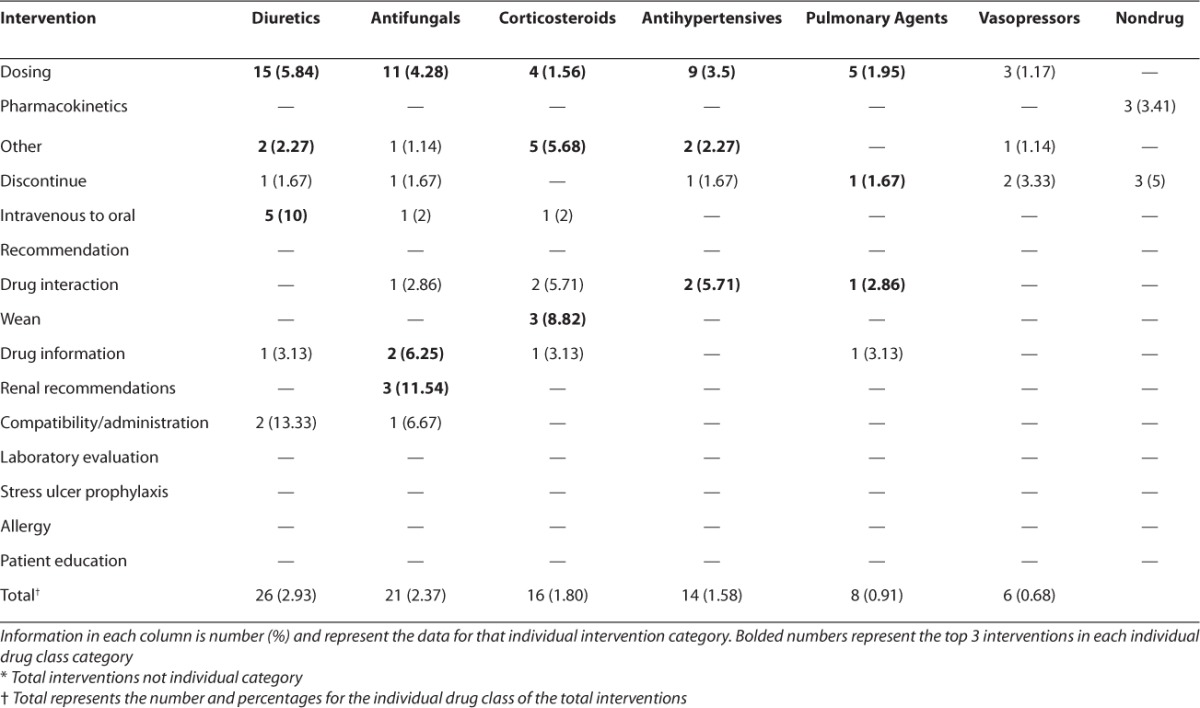
Table 2.
Interventions by Drug Class
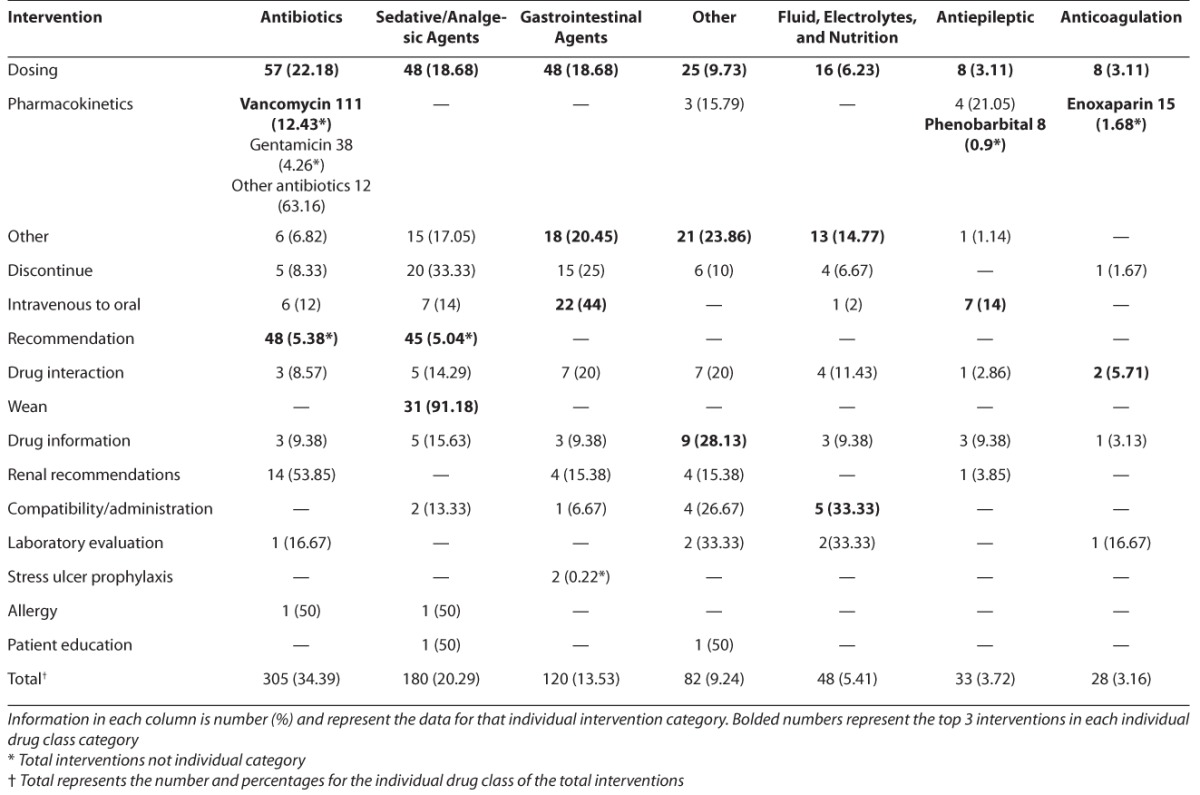
The interventions were further broken down using the specific drug classification grouping. Antibiotic, sedative and analgesic agents, and gastrointestinal agents were the most common drug classes in which interventions of any type were made (34.4%, 20.3%, and 13.5%, respectively). Approximately 10% of all interventions involved drugs not included in the prespecified drug class categories, and were thus recorded as other. For the antibiotics class of medications, “Pharmacokinetics,” “Dosing,” and “Agent Recommendations” were the most common interventions (52.8%, 18.7%, and 15.7%, respectively). Within the sedation class of medications, “Dosing,”, “Agent Selection,” and “Weaning” were the most common types of interventions (26.7%, 25.0%, and 17.2%, respectively). “Dosing” (40%) and “Intravenous to Oral” conversion (18.3%) were the most common intervention for gastrointestinal medications.
Ninety-eight of all recommended interventions were accepted by the medical staff. The estimated cost savings for the 8-mo study period was $79,800 or an estimated annual cost savings of $119,700.
DISCUSSION
There are limited reports of clinical pharmacist interventions in pediatric settings. There is only 1 report found in the literature describing academic pharmacy faculty, residents, and students in a variety of pediatric settings.12 Krupicka et al13 presented a study of 201 PICU patients, of which 77 of them had clinical pharmacy interventions. The most common interventions in that study were dosing recommendations.
The average number of interventions per patient in our study was higher than that reported in the literature to date.12,13 Due to a few chronic patients, the patient length of stay was longer in our study resulting in similar number of interventions per 100 patient days as previously described.13 Approximately 30% of the patients admitted to the PICU had clinical pharmacy interventions performed. This percentage is affected by other obligations of the clinical faculty member limiting the days in the PICU. Additionally, this percentage includes patients who did not require clinical interventions or who were only admitted to the PICU for a short time. It would be expected that a full-time clinical specialist would be able to intervene on more patients. Consistent with the report by Krupicka et al,13 dosing recommendations were the most common interventions in our study. The individualized drug therapy for pediatric patients and the variety of ways to dose many of the medications in pediatrics likely contributed to dosing recommendations being the most influential intervention class.
Antibiotics were the class of medications with the most frequent interventions (34%). About half of the interventions in this class were pharmacokinetic evaluations to optimize drug levels and minimize toxicities. The majority of the dosing recommendations for antibiotics were to increase dosing to enhance penetration of the antibiotic into the specific site of the infection. Interventions were also frequently recommended to adjust for rapidly changing volumes of distribution and fluctuating renal function in this critically ill population. Most antibiotic agent recommendations occurred at the onset of therapy in order to choose optimal empiric agents based on likely etiology of infection. These antibiotics commonly included ampicillin, acyclovir, gentamicin, vancomycin, piperacillin/tazobactam, meropenem, ceftriaxone, and ceftazidime. Agent recommendations accounted for 15% of antibiotic interventions, which included de-escalation of therapy as part of antimicrobial stewardship.
These data show that an academic clinical pharmacy faculty member had significant input into the drug therapy for patients in the PICU, as 98% of the recommended interventions were implemented by the medical faculty. The small percentage of interventions not accepted by the PICU team mainly included consult team denial of recommendation and stylistic differences between the attending physician and the clinical faculty member. For example, the clinical faculty member may have suggested to wean sedation and the medical team preferred to postpone the wean. During the patient's PICU stay, there were on average 5.5 interventions performed for each patient admission. This was higher than previously reported in the literature.12,13 Of note, our clinical pharmacist attended rounds only part time each week. It is possible that full time attendance at rounds would have changed the number or nature of interventions significantly. Although pharmacy students did accompany the faculty member on rounds, they did not contribute to the interventions in this study.
Estimated cost savings associated with the interventions performed were considerable. The cost savings calculations included both hard and soft costs based on the Pharmacy One Source Quantify information and literature.9–11 Hard costs represented the direct cost of the medication. Soft costs included indirect cost such as nursing care and laboratory testing. Exclusively looking at drug costs would miss additional potential hospital savings. For example, if a patient had a pseudomonal pneumonia requiring mechanical ventilation and the piperacillin/tazobactam dose was increased to attain higher pulmonary penetration, this would result in increased drug costs. However, this dose change potentially decreased hospital and nursing costs related to patient ventilator days, PICU days, or overall hospital days. It is important to note that the majority of cost information for this computer program comes from the adult population, which could significantly affect our results. However, the authors are unaware of the existence of any pediatric specific programs.
The limitations of this study included a relatively small sample size, no true pharmacoeconomic data, short study duration, and lack of a comparison group. The relatively small sample size was due to multiple factors but most importantly the short study duration. This study duration, however, was longer than previous PICU specific studies.13 A true pharmacoeconomic study was beyond the scope and design of this project.
This study demonstrated and attempted to quantify some of the benefits to having an academic clinical pharmacy faculty member in a PICU. In addition to the education of students, the faculty member assisted the medical staff in providing patient care, and likely helped decrease medical costs. Further larger comparative studies are needed to elucidate the true impact on clinical outcomes and medical economics of clinical pharmacy interventions in the pediatric critical care setting.
ACKNOWLEDGMENTS
This project was supported by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), Department of Health and Human Services (DHHS) under grant number D34HP00006, Centers of Excellence for $2,771,566. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by the BHPr, HRSA, DHHS, or the US Government. This project was presented in poster form at the Pediatric Pharmacy Advocacy Group Annual Meeting, Memphis, Tennessee, March 2011. A variation of this project was presented at the Southern Society of Pediatric Research Meeting, New Orleans, Louisiana, February 2011, and the abstract was published in the Journal of Investigative Medicine. 2011;59(2):422–423.
ABBREVIATIONS
- BHPr
Bureau of Health Professions
- DHHS
Department of Health and Human Services
- HRSA
Health Resources and Services Administration
- PICU
Pediatric Intensive Care Unit
- PRISM II score
Pediatric Risk of Mortality II Score
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The corresponding author (LaRochelle) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Kane SL, Weber RJ, Dasta JF. The impact of critical care pharmacists on enhancing patient outcomes. Intensive Care Med. 2003;29(5):691–698. doi: 10.1007/s00134-003-1705-3. [DOI] [PubMed] [Google Scholar]
- 2.Chuang LC, Sutton JD, Henderson GT. Impact of a clinical pharmacist on cost saving and cost avoidance in drug therapy in an intensive care unit. Hosp Pharm. 1994;29(3):215–218. [PubMed] [Google Scholar]
- 3.Task Force on Guidelines, Society of Critical Care Medicine. Recommendations for services and personnel for delivery of care in a critical care setting. Crit Care Med. 1988;16(8):809–811. doi: 10.1097/00003246-198808000-00016. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics, Committee on Drugs and Committee on Hospital Care. Prevention of medication errors in the pediatric inpatient setting. Pediatrics. 2003;112(2):431–436. doi: 10.1542/peds.112.2.431. [DOI] [PubMed] [Google Scholar]
- 5.Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals. Pharmacotherapy. 2006;26(6):735–747. doi: 10.1592/phco.26.6.735. [DOI] [PubMed] [Google Scholar]
- 6.Leape LL, Cullen DJ, Clapp MD et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282(3):267–270. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- 7.Marshall J, Finn CA, Theodore AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008;36(2):427–433. doi: 10.1097/01.CCM.0000300275.63811.B3. [DOI] [PubMed] [Google Scholar]
- 8.Pollack MM, Ruttimann UK, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16(11):1110–1116. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Bellevue, WA: Pharmacy One Source Quantify Clinical Intervention Documentation. http://www.pharmacyonesource.com. Accessed May 10, 2011. [Google Scholar]
- 10.Hatoum HT, Hutchinson RA, Witte KW, Newby GP. Evaluation of the contribution of clinical pharmacists: in-patient care and cost reduction. Drug Intell Clin Pharm. 1988;22(3):252–259. doi: 10.1177/106002808802200318. [DOI] [PubMed] [Google Scholar]
- 11.Bates DW, Spell N, Cullen DJ et al. The costs of adverse drug events in hospitalized patients. JAMA. 1997;227(4):307–311. [PubMed] [Google Scholar]
- 12.Condren ME, Haase MR, Luedtke SA, Gaylor AS. Clinical activities of an academic pediatric pharmacy team. Ann Pharmacother. 2004;4(38):574–578. doi: 10.1345/aph.1D384. [DOI] [PubMed] [Google Scholar]
- 13.Krupicka MI, Bratton SL, Sonnenthal K, Goldstein B. Impact of pediatric clinical pharmacist in the pediatric intensive care unit. Crit Care Med. 2002;30(4):919–921. doi: 10.1097/00003246-200204000-00035. [DOI] [PubMed] [Google Scholar]


