Abstract
We report a benzonatate overdose in a teenager resulting in life-threatening toxicity to increase awareness of this overdose, and discuss recent pediatric warnings and labeling information provided by the US Food and Drug Administration (FDA). After an overdose of benzonatate, a 13-yr-old female presented to our emergency department with coma, seizures, hypotension, prolonged QT interval on electrocardiogram, and metabolic acidosis. Benzonatate is an antitussive medication with sodium channel-blocking properties and local anesthetic effects on the respiratory stretch receptors due to a tetracaine-like metabolite. Overdose is reported to cause coma, seizures, hypotension, tachycardia, ventricular dysrhythmias, and cardiac arrest. The FDA recently issued a Drug Safety Communication warning that accidental benzonatate ingestion in children younger than 10 years of age have increased risk of death and added the new information to the Warnings and Precautions section of benzonatate's label.
INDEX TERMS: altered mental status, benzonatate, metabolic acidosis, pediatrics, seizures
ABBREVIATIONS: AERS, Adverse Event Reporting System; ECG, electrocardiogram; ED, emergency department; EMS, emergency medical services; FDA, Food and Drug Administration
INTRODUCTION
We report a case of a teenager with coma, seizures, and metabolic acidosis after an intentional overdose of benzonatate. Benzonatate is a unique non-narcotic antitussive medication with sodium channel-blocking properties and anesthetic effects on the respiratory stretch receptors related to its structural similarity to tetracaine and the ester-linked class of local anesthetic agents.1 Benzonatate in overdose has been reported to cause altered mental status, coma, seizures, hypotension, tachycardia, ventricular dysrhythmias, and cardiac arrest.2 The emergency care provider should consider benzonatate toxicity in the differential diagnosis when a patient presents in this fashion after a suspected medication overdose. Prescribing providers and dispensing pharmacists should be familiar with the pharmacology and toxicology of this medication, as well as the new pediatric warnings and labeling information issued by the US Food and Drug Administration (FDA).3
CASE REPORT
A 13-year-old Hispanic female, with no significant past medical history and currently on no medications, was transferred by emergency medical services (EMS) from her home to a children's hospital emergency department (ED) with altered mental status. The patient's mother called 911 because she found the patient unresponsive and seizing with shaking of her extremities, choking, and urinary incontinence. The patient was last seen with normal behavior 3 hours earlier. When EMS arrived on the scene, they described the patient as having a Glasgow Coma Scale of 3 with spontaneous respirations. Blood and an unknown yellow substance were found in the patient's mouth. The patient's respirations were supported with a nasal trumpet and supplemental oxygen. Intravenous naloxone 2 mg was given with no response. Her serum glucose was 191 mg/dL.
Upon arrival to the ED 20 minutes later, the patient was comatose, diaphoretic, hypotensive, and tachycardic. Her blood pressure was 110/49 mm Hg, which decreased to 96/42 within an hour; heart rate, 129 beats per minute; temperature, 36.4°C; respiratory rate, 26 breaths per minute; oxygen saturation, 98% on 100% O2 via nonrebreather face mask; weight, 85 kg; and body mass index of 33. Physical examination demonstrated an unresponsive obese teenage female with spontaneous respirations and coarse rales bilaterally. A yellow capsule was discovered adhered to her back and identified as benzonatate 100 mg. Her electrocardiogram (ECG) from 1 hour after arrival to the ED demonstrated a normal sinus rhythm with heart rate of 127 beats per minute, QRS, 96 ms; and QTc, 499 ms. She was initially unresponsive to painful stimuli but 1 hr after ED arrival began to localize to pain and move spontaneously. No further seizure activity was noted. Computed tomography of the brain was normal. Laboratory studies at the time of ED presentation revealed: pH, 7.0; Pco2, 32 mm Hg; Hco3, 8 mEq/L; base excess, −23 mmol/L; and serum lactate, 1.9 mmol/L. Serum glucose, electrolytes, including calcium, phosphorus, and magnesium, were normal except for a CO2 of 12 mmol/L with an anion gap of 28 mEq/L. Serum creatinine was 1.3 mg/dL, and osmolar gap was 20 mOsm/kg. A comprehensive urine toxicology screen (Quest Diagnostics Laboratory, Dallas, Texas) was positive only for chlorpheniramine. However, it is not capable of detecting benzonatate or dextromethorphan. The urine drugs screen was negative for amphetamine, barbiturate, benzodiazepine, cocaine, methylenedioxymethamphetamine, opiate, phencyclidine, and cannabinoid. Upon transfer to the intensive care unit, 2 hours after ED arrival, the patient was awake enough to admit to ingestion of approximately 40 capsules of 100 mg benzonatate and 5 tablets of 4 mg chlorpheniramine/30 mg dextromethorphan. Within 12 hours of arrival to the hospital, the tachycardia and hypotension resolved with maintenance intravenous fluids, and heart rate and blood pressure were 98 beats per minute and 112/72 mm Hg, respectively. Her laboratory studies, ECG, and mental status returned to normal by the next day. The patient was transferred to the pediatric floor after she was observed overnight in the intensive care unit. She was evaluated by psychiatry and was referred to outpatient psychiatric counseling. She was discharged home on hospital day 3.
DISCUSSION
The FDA approved benzonatate in 1958 as a prescription treatment for the symptomatic relief of cough in patients older than 10 years of age. Benzonatate is available in the United States as 100-mg and 200-mg yellow liquid-filled spherical capsules under the brand name Tessalon Perles (Tessalon Perles, Forest Pharmaceuticals, Inc, St. Louis, MO) and also as generic preparations. Benzonatate, also known as 4-(butylamino) benzoic acid, is structurally related to tetracaine (Figure) and the ester-linked class of local anesthetics.4 This structural resemblance results in similarities in mechanism of action and toxicity between benzonatate and local anesthetic agents. After oral ingestion and systemic absorption via the gastrointestinal tract, benzonatate acts peripherally by directly anesthetizing the vagal stretch receptors in the respiratory passages, lungs, and pleura.1 Overdose results in neurologic and cardiovascular toxicity related to sodium channel blockade.
Figure.
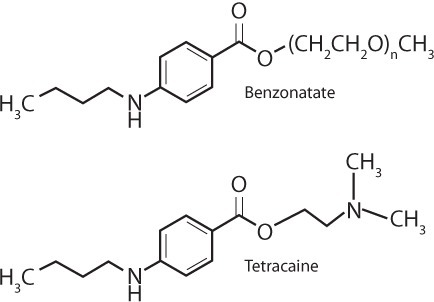
Benzonatate and Tetracaine Structures
Onset of symptoms is rapid after overdose, often within 15 to 20 min.5 Toxicity may have severe morbidities and can also result in death. A 7-yr retrospective review of the National Poison Center Database System from 2000 to 2006 reported 2,173 patients with ingestion of benzonatate alone, of which the mean age was 20 years and 30% of patients were younger than 6 years of age.6 Serious outcomes occurred in 5% of patients with clinically significant effects including agitation, seizure, coma, hypotension, tachycardia, ventricular dysrhythmia, cardiac arrest, and asystole.
To date, 5 cases of benzonatate ingestion with significant toxicity have been reported in peer-reviewed medical publications.6–8 These case reports include 3 fatalities (2 children and 1 adult). The fourth patient was a 39-year-old male who suffered a seizure and unstable ventricular tachycardia that responded to cardioversion. The fifth patient was a 17-year-old female who survived cardiac arrest, recurrent dysrhythmias, but had residual blindness. Another case published in abstract form only reports a child with seizures after benzonatate ingestion.9 Additionally, there are 2 reports of intravenous administration of benzonatate in adults resulting in ventricular fibrillation. One of these patients died, while the other survived after defibrillation.10,11
A search of the FDA's Adverse Event Reporting System (AERS) database through May 19, 2010, identified 31 cases of overdose associated with benzonatate.3 The median age was 18 years (range, 1–66 years). Common adverse events included cardiac arrest, coma, and convulsion. The quantities ingested ranged from 1 to 30 benzonatate capsules. Among 6 overdose cases that included a specific time frame of events following the overdose, all cases developed symptoms within 1 hour of ingestion.
Of the 31 overdose cases reported in AERS, 7 cases involved accidental ingestions, all in children younger than 10 years. Five of the 7 accidental ingestions that occurred in children ages 2 years and younger resulted in death. Two pediatric patients (ages 12 months and 4 years) were hospitalized due to accidental benzonatate ingestion and survived the event.
As a result of these and other reports, the FDA recently issued a Drug Safety Communication warning of the risk of death from accidental ingestion of benzonatate in children younger than 10 years.3 The FDA is concerned that the yellow liquid-filled capsules may be attractive to young children, and ingestion of just 1 or 2 capsules has been reported to cause toxicity in this age group. Additionally, young children are more likely to suck or chew on the capsules, releasing benzonatate in the mouth. This may cause local anesthesia of the pharynx and upper airway structures, resulting in an increased risk of choking and pulmonary aspiration. The FDA added new information to the Warnings and Precautions section of labeling for benzonatate products to make patients, caregivers, and healthcare professionals including pharmacists aware of these safety issues.
Management of benzonatate toxicity begins with supportive care and continuous monitoring of neurologic and cardiovascular status. Seizures and cardiac arrhythmias should be anticipated and treated in standard fashion with airway management and use of Pediatric Advanced Life Support and Advanced Cardiac Life Support guidelines. Due to rapid onset of toxicity, patients are likely to be symptomatic by the time of arrival to a healthcare facility. As a result, gastrointestinal decontamination procedures are unlikely to be of benefit and may be contraindicated due to increased risk of pulmonary aspiration. Because the structure and toxicity of benzonatate are similar to local anesthetic agents, intravenous lipid emulsion therapy may be considered for patients with life-threatening cardiovascular collapse.7 Animal studies and human case reports suggest that intravenous lipid emulsion therapy may be life saving in patients with severe local anesthetic overdose.12,13 Although optimal dosing has not been established, the suggested intravenous dose of 20% lipid emulsion is 1.5 mL/kg over 1 minute, repeated as needed, followed by a continuous infusion of 0.25 to 0.50 mL/kg/min for 30 to 60 minutes if there is evidence of recovery.14 There are no reports on the use of lipid emulsion therapy for benzonatate toxicity. If the patient does not respond rapidly, cardiopulmonary by-pass and extracorporeal membrane oxygenation may be considered.12,13,15
A limitation of this case report is lack of confirmation of benzonatate in the patient's blood or urine, although rapid laboratory determination of plasma benzonatate concentration is not available to the clinician. Plasma benzonatate concentrations can be measured using high-pressure liquid chromatography with tandem mass spectrometry if the sample is sent out to a reference laboratory.8 Our patient's clinical presentation was consistent with prior descriptions of benzonatate toxicity, a benzonatate capsule was found on the patient's body, and she subsequently admitted to ingestion of a large quantity of benzonatate. It is unlikely that chlorpheniramine or dextromethorphan played a predominant role in her toxicity based on her clinical presentation and the quantity reported as ingested.
CONCLUSIONS
Benzonatate is a unique antitussive medication with sodium channel-blocking properties that may cause severe, life-threatening toxicity in overdose, including coma, seizures, ventricular dysrhythmias, and cardiac arrest. Prescribing healthcare workers, dispensing pharmacists, patients, and caregivers should be aware of this toxicity and the new FDA warnings and precautions for benzonatate. The emergency care provider should consider benzonatate toxicity in the differential diagnosis of patients presenting with seizures and ventricular dysrhythmias.
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Homsi J, Walsh D, Nelson KA. Important drugs for cough in advanced cancer. Support Care Cancer. 2001;9(8):565–574. doi: 10.1007/s005200100252. [DOI] [PubMed] [Google Scholar]
- 2.Winger ML, Spiller HA, Griffith JR. Benzonatate ingestion reported to the National Poison Center Database System (NPDS) J Med Toxicol. 2010;6(4):398–402. doi: 10.1007/s13181-010-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. FDA Drug Safety Communication: death resulting from overdose after accidental ingestion of Tessalon (benzonatate) by children under 10 years of age. http://www.fda.gov/drugs/drugsafety/ucm236651.htm. Accessed March 26, 2012.
- 4.Dicpinigaitis PV, Gayle YE, Solomon G et al. Inhibition of cough-reflex sensitivity by benzonatate and guaifenesin in acute viral cough. Respir Med. 2009;103(6):902–906. doi: 10.1016/j.rmed.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Aschenbrenner D. Drug watch: possible death from cough suppressant. Am J Nurs. 2011;111(11):68. [Google Scholar]
- 6.Cohan JA, Manning TJ, Lukash L et al. Two fatalities resulting from Tessalon (benzonatate) Vet Hum Toxicol. 1986;28(6):543–544. [PubMed] [Google Scholar]
- 7.Cohen V, Jellinek SP, Stansfield L et al. Cardiac arrest with residual blindness after overdose of Tessalon (benzonatate) perles. J Emerg Med. 2011;41(2):166–171. doi: 10.1016/j.jemermed.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Crouch BI, Knick KA, Crouch DJ et al. Benzonatate overdose associated with seizures and arrhythmias. J Toxicol Clin Toxicol. 1998;36(7):713–718. doi: 10.3109/15563659809162620. [DOI] [PubMed] [Google Scholar]
- 9.Sheen S OK, Osterhoudt J, Birenbaum D. Seizures in a toddler associated with benzonatate ingestion (abstract 31) J Toxicol Clin Toxicol. 1997;35(5):493. [Google Scholar]
- 10.Shropshire A, Clifton J, Aks S. Death from intentional IV administration of benzonatate (abstract 169) J Toxicol Clin Toxicol. 1999;37(5):652. [Google Scholar]
- 11.Weber J, Tabba M, Thompson M. Survival after recreational IV administration of benzonatate (abstract) J Toxicol Clin Toxicol. 2003;41(5):745–746. [Google Scholar]
- 12.Weinberg GL, Di Gregorio G, Ripper R et al. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108(5):907–913. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblatt MA, Abel M, Fischer GW et al. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006;105(1):217–218. doi: 10.1097/00000542-200607000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Lipid Rescue. http:/lipidrescue.org/. Accessed March 26, 2012.
- 15.Long WB, Rosenblum S, Grady IP. Successful resuscitation of bupivacaine-induced cardiac arrest using cardiopulmonary bypass. Anesth Analg. 1989;69(3):403–406. [PubMed] [Google Scholar]


