Abstract
Bilateral vestibular loss (BVL) may present with or without vertigo and hearing loss. Amongst the causes of BVL are vestibulotoxic antibiotics, autoimmune ear diseases, Menière’s disease and meningitis. Clinical diagnosis of BVL is based on the result of three simple bedside tests: a positive head impulse test, reduced dynamic visual acuity and a positive Romberg test on foam rubber. With these signs, diagnosis of severe BVL is usually straightforward to establish.
Keywords: bilateral vestibular loss, dynamic visual acuity, head impulse test, vertigo
Introduction
Patients with bilateral vestibular loss (BVL) may present with or without vertigo and hearing loss. They usually complain about oscillopsia during head movements and about unsteadiness, especially while walking in the dark [Dandy, 1941; Crawford, 1952]. Common causes of BVL are vestibulotoxic antibiotics (especially gentamicin), even after short periods of administration, autoimmune ear diseases such as Cogan’s syndrome, Menière’s disease and meningitis [Zingler et al. 2007]. BVL may also be associated with multiple system atrophy, neurofibromatosis type 2, hereditary ataxias such as Friedreich’s ataxia [Fahey et al. 2008], spinocerebellar ataxia [Gordon et al. 2003] and episodic ataxia type 2. A combination of vestibular loss, peripheral neuropathy and bilateral vestibular areflexia is known as CANVAS syndrome [Szmulewicz et al. 2011].
Clinical diagnosis of BVL is based on the result of three simple bedside tests: a positive head impulse test (HIT) [Halmagyi and Curthoys, 1988], reduced dynamic visual acuity (DVA) during head shaking [Demer et al. 1994] and a positive Romberg test on foam rubber [Halmagyi et al. 1994]. With these three clinical signs, diagnosis of severe BVL is usually straightforward to establish but mild BVL remains a diagnostic challenge, as clinical tests might not be conclusive and data from laboratory tests, including caloric irrigation, rotational chair and video head impulse testing, show considerable overlap between patients and normal subjects [Weber et al. 2009]. Vestibular outcome seems to be independent of age, gender, time course of manifestation and severity of BVL [Zingler et al. 2008]. Roughly 80% of patients with BVL do not improve and, thus, the prognosis seems less favourable than assumed previously [Zingler et al. 2008].
Head impulse test
Owing to its short latency (about 10 ms), the vestibulo-ocular reflex (VOR) keeps the eyes on target during head movements with equal eye rotations opposite to the direction of head rotations [Aw et al. 1996]. The image is efficiently stabilized on the retina as long as eye and head velocities are equal. In BVL, the VOR is impaired or absent bilaterally and gaze is only stabilized by visual reflexes, which react with considerable delay, i.e. a latency of 100 ms or more [Viirre and Demer, 1996].
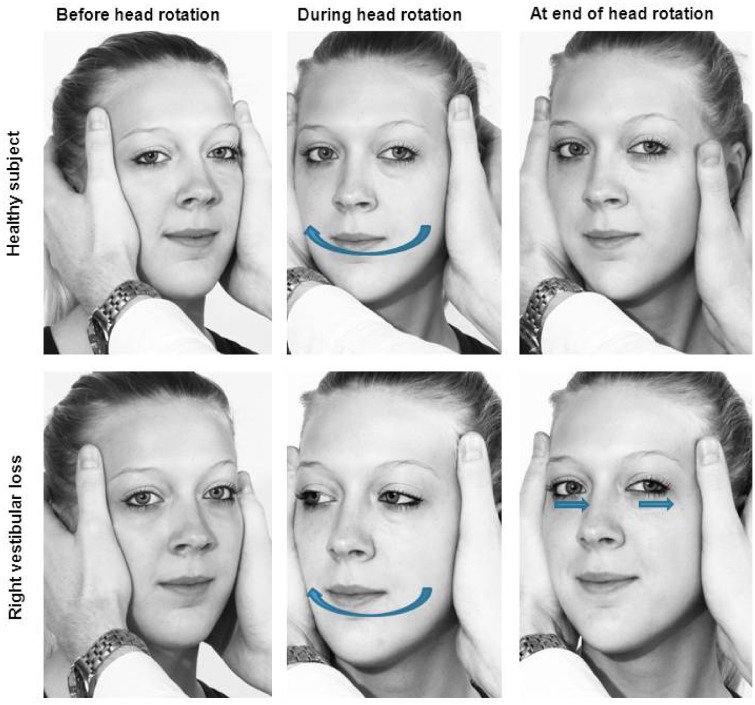
To perform a HIT, the examiner stands in front of the patient seated at the bedside or on a chair. The examiner asks the patient to focus on a target (e.g. the examiner’s tip of the nose). The examiner then manually delivers brisk, passive horizontal head rotations of approximately 10–20° amplitude (Figure 1). Normally, the patient’s eyes keep focusing on the examiner’s nose. Instead, in a patient with BVL, where the VOR is impaired or absent, the eyes drift off the target to the side to which the head is rotated, so that the patient has to make a catch-up saccade to move them back to focus on the examiner’s nose. Overt saccades after head rotation are the telltale sign of vestibular loss, whereas covert saccades during head rotation remain imperceptible to the naked eye [Weber et al. 2008]. While catch-up saccades appear with head impulses to either side in BVL, they indicate the affected side in patients with unilateral vestibular loss (UVL) whereas the patient’s eyes keep focusing on the examiner’s nose when the head is rotated to the healthy side.
Figure 1.
Head impulse test. While the patient is asked to fixate on a target, the examiner briskly rotates the patient’s head to the right or left and observes their eye movements. Top row: In a healthy subject, the vestibulo-ocular reflex will keep the eyes on the target. Bottom row: In a patient with bilateral vestibular loss (re-enacted scene), the eyes will move with the head during head impulses to both sides, so that the patient will have to make catch-up saccades after the head movements to bring the eyes back on target.
Sensitivity of bedside HIT is adequate and therefore clinically useful in the hands of both neuro-otological experts and nonexperts [Jorns-Haderli et al. 2007]. In a group of subjects with UVL and BVL, its sensitivity was significantly lower (63% versus 72%) and its specificity was significantly higher for experts than nonexperts (78% versus 64%) when a quantitative HIT measurement with scleral search coils was used as a reference [Jorns-Haderli et al. 2007]. In other words, neuro-otological experts were inclined to trade off sensitivity for specificity of the HIT, in order to avoid false-positive results. In another study, the accuracy of the bedside HIT for identifying BVL was comparable with 84% sensitivity and 82% specificity [Schubert et al. 2004].
The bedside HIT can identify patients with severe and moderate BVL. However, identification of mild BVL remains a challenge and covert saccades may conceal BVL even in patients with total vestibular loss [Weber et al. 2009]. In cases of an inconclusive bedside test, it has now become practical to measure HIT with high-speed video goggles in order to quantify the deficit of the VOR and detect covert saccades [MacDougall et al. 2009].
Dynamic visual acuity
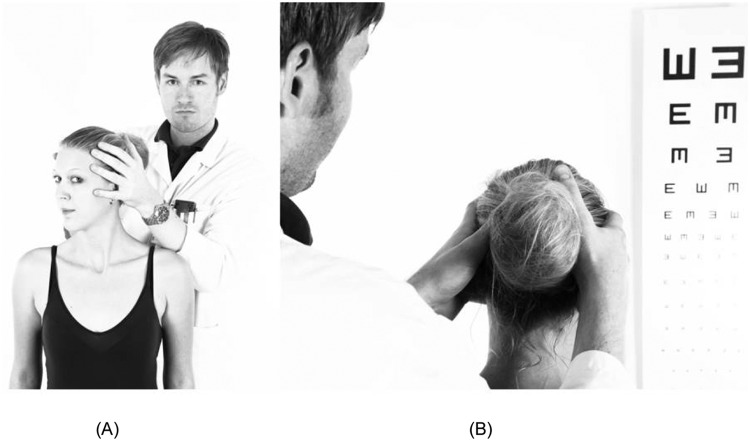
DVA assesses a subject’s ability to perceive objects accurately while the head is moved passively. To measure DVA clinically (Figure 2), the examiner oscillates the patient’s head horizontally or vertically at 0.5–2 Hz and asks the patient to read optotypes on a visual acuity chart [Demer et al. 1994; Longridge and Mallinson, 1984]. In computerized DVA, optotypes are usually displayed during directional head impulses above a velocity threshold of 120–150°/s [Herdman et al. 1998; Vital et al. 2010]. In patients with BVL, the VOR is no longer able to stabilize gaze, while the movements are too fast for the smooth pursuit system to keep the eyes on target. As a consequence, visual acuity decreases compared with what the patient is able to read when the head remains still. In healthy subjects, visual acuity may decline by one or two lines on the optotype chart. A decline of more than two lines is considered abnormal [Fife et al. 2000; Sargent et al. 1997], but severely affected BVL patients may show a decline of five or more lines. Subjects with UVL may also have abnormal DVA, especially at higher head oscillation frequencies [Dannenbaum, 2009]. DVA based on directional head impulses also indicate the side of the lesion in UVL [Herdman et al. 1998; Vital et al. 2010]. DVA might display false-negative results when other mechanisms such as augmented cervico-ocular and visual reflexes compensate at least partially for the retinal instability during head movements [Chambers et al. 1985; Vital et al. 2010]. However, computerized DVA testing has good sensitivity (94.5%) and specificity (95.2%) in subjects with unilateral and bilateral vestibular loss [Herdman et al. 1998].
Figure 2.
Dynamic visual acuity. (A) The examiner oscillates the patient’s head at about 2 Hz in the horizontal or vertical plane. (B) The patient is asked to read the optotypes on a visual acuity chart while the head is moving. A patient with bilateral vestibular loss will typically lose three or more lines compared with static visual acuity.
Romberg test on rubber foam
Postural control depends on visual, proprioceptive and vestibular input. In patients with BVL, postural control is impaired due to a loss of vestibulo-spinal reflexes [Horak et al. 2002]. A simple way of diagnosing ataxia is the Romberg test [Khasnis and Gokula, 2003]: the examiner observes postural stability with the patient placing his feet together, initially with eyes open and then with the eyes closed (Figure 3). The Romberg test is positive when a patient is able to stand with feet together and eyes open, but sways or falls with eyes closed [Lanska and Goetz, 2000]. However, this test is not specific for vestibular loss and does not help to distinguish between UVL and BVL, but also detects cerebellar and proprioceptive impairment. The test’s sensitivity can be increased by having the patient stand on foam rubber, which disrupts proprioceptive inputs [Lanska and Goetz, 2000; Shumway-Cook and Horak, 1986]. Foam posturography with eyes closed proved to be very sensitive (up to 79%) and specific (up to 80%) to detect patients with both UVL and BVL [Fujimoto et al. 2009].
Figure 3.

Romberg test on rubber foam. The patient is asked to stand on a mat of rubber foam with their feet together and eyes closed. Without visual and disrupted proprioceptive input, a patient with total bilateral vestibular loss will fall off the mat. The examiner must be ready to prevent the fall.
Footnotes
Funding: This research was supported by the Betty and David Koetser Foundation for Brain Research.
Conflict of interest statement: K. P. Weber acts as an unpaid consultant and has received funding for travel from GN Otometrics.
Contributor Information
Jens A. Petersen, Department of Neurology, University Hospital Zurich, Switzerland
Dominik Straumann, Department of Neurology, University Hospital Zurich, Switzerland.
Konrad P. Weber, Departments of Neurology and Ophthalmology, University Hospital Zurich, Frauenklinikstrasse 26, 8091 Zurich, Switzerland
References
- Aw S., Haslwanter T., Halmagyi G., Curthoys I., Yavor R., Todd M. (1996) Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations. I. Responses in normal subjects. J Neurophysiol 76: 4009–4020 [DOI] [PubMed] [Google Scholar]
- Chambers B., Mai M., Barber H. (1985) Bilateral vestibular loss, oscillopsia, and the cervico-ocular reflex. Otolaryngol Head Neck Surg 93: 403–407 [DOI] [PubMed] [Google Scholar]
- Crawford J. (1952) Living without a balancing mechanism. N Engl J Med 246: 458–460 [DOI] [PubMed] [Google Scholar]
- Dandy W. (1941) The surgical treatment of Ménière’s disease. Surg Gynecol Obstet 72: 421–425 [Google Scholar]
- Dannenbaum E., Paquet N., Chilingaryan G., Fung J. (2009) Clinical evaluation of dynamic visual acuity in subjects with unilateral vestibular hypofunction. Otol Neurotol 30: 368–372 [DOI] [PubMed] [Google Scholar]
- Demer J., Honrubia V., Baloh R. (1994) Dynamic visual acuity: a test for oscillopsia and vestibulo-ocular reflex function. Am J Otol 15: 340–347 [PubMed] [Google Scholar]
- Fahey M., Cremer P., Aw S., Millist L., Todd M., White O., et al. (2008) Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain 131: 1035–1045 [DOI] [PubMed] [Google Scholar]
- Fife T., Tusa R., Furman J., Zee D., Frohman E., Baloh R., et al. (2000) Assessment: vestibular testing techniques in adults and children: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 55: 1431–1441 [DOI] [PubMed] [Google Scholar]
- Fujimoto C., Murofushi T., Chihara Y., Ushio M., Sugasawa K., Yamaguchi T., et al. (2009) Assessment of diagnostic accuracy of foam posturography for peripheral vestibular disorders: analysis of parameters related to visual and somatosensory dependence. Clin Neurophysiol 120: 1408–1414 [DOI] [PubMed] [Google Scholar]
- Gordon C., Joffe V., Vainstein G., Gadoth N. (2003) Vestibulo-ocular arreflexia in families with spinocerebellar ataxia type 3 (Machado-Joseph disease). J Neurol Neurosurg Psychiatry 74: 1403–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmagyi G., Curthoys I. (1988) A clinical sign of canal paresis. Arch Neurol 45: 737–739 [DOI] [PubMed] [Google Scholar]
- Halmagyi G., Fattore C., Curthoys I., Wade S. (1994) Gentamicin vestibulotoxicity. Otolaryngol Head Neck Surg 111: 571–574 [DOI] [PubMed] [Google Scholar]
- Herdman S., Tusa R., Blatt P., Suzuki A., Venuto P., Roberts D. (1998) Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am J Otol 19: 790–796 [PubMed] [Google Scholar]
- Horak F., Buchanan J., Creath R., Jeka J. (2002) Vestibulospinal control of posture. Adv Exp Med Biol 508: 139–145 [DOI] [PubMed] [Google Scholar]
- Jorns-Haderli M., Straumann D., Palla A. (2007) Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J Neurol Neurosurg Psychiatry 78: 1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasnis A., Gokula R. (2003) Romberg’s test. J Postgrad Med 49: 169–172 [PubMed] [Google Scholar]
- Lanska D., Goetz C. (2000) Romberg’s sign: development, adoption, and adaptation in the 19th century. Neurology 55: 1201–1206 [DOI] [PubMed] [Google Scholar]
- Longridge N., Mallinson A. (1984) A discussion of the dynamic illegible "E" test: a new method of screening for aminoglycoside vestibulotoxicity. Otolaryngol Head Neck Surg 92: 671–677 [DOI] [PubMed] [Google Scholar]
- MacDougall H., Weber K., McGarvie L., Halmagyi G., Curthoys I. (2009) The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology 73: 1134–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent E., Goebel J., Hanson J., Beck D. (1997) Idiopathic bilateral vestibular loss. Otolaryngol Head Neck Surg 116: 157–162 [DOI] [PubMed] [Google Scholar]
- Schubert M., Tusa R., Grine L., Herdman S. (2004) Optimizing the sensitivity of the head thrust test for identifying vestibular hypofunction. Phys Ther 84: 151–158 [PubMed] [Google Scholar]
- Shumway-Cook A., Horak F. (1986) Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther 66: 1548–1550 [DOI] [PubMed] [Google Scholar]
- Szmulewicz D., Waterston J., MacDougall H., Mossman S., Chancellor A., McLean C., et al. (2011) Cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS): a review of the clinical features and video-oculographic diagnosis. Ann N Y Acad Sci 1233: 139–147 [DOI] [PubMed] [Google Scholar]
- Viirre E., Demer J. (1996) The human vertical vestibulo-ocular reflex during combined linear and angular acceleration with near-target fixation. Exp Brain Res 112: 313–324 [DOI] [PubMed] [Google Scholar]
- Vital D., Hegemann S., Straumann D., Bergamin O., Bockisch C., Angehrn D., et al. (2010) A new dynamic visual acuity test to assess peripheral vestibular function. Arch Otolaryngol Head Neck Surg 136: 686–691 [DOI] [PubMed] [Google Scholar]
- Weber K., Aw S., Todd M., McGarvie L., Curthoys I., Halmagyi G. (2008) Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology 70: 454–463 [DOI] [PubMed] [Google Scholar]
- Weber K., Aw S., Todd M., McGarvie L., Curthoys I., Halmagyi G. (2009) Horizontal head impulse test detects gentamicin vestibulotoxicity. Neurology 72: 1417–1424 [DOI] [PubMed] [Google Scholar]
- Zingler V., Cnyrim C., Jahn K., Weintz E., Fernbacher J., Frenzel C., et al. (2007) Causative factors and epidemiology of bilateral vestibulopathy in 255 patients. Ann Neurol 61: 524–532 [DOI] [PubMed] [Google Scholar]
- Zingler V., Weintz E., Jahn K., Mike A., Huppert D., Rettinger N., et al. (2008) Follow-up of vestibular function in bilateral vestibulopathy. J Neurol Neurosurg Psychiatry 79: 284–288 [DOI] [PubMed] [Google Scholar]