Abstract
The most recently discovered serotonin (5-HT) receptor subtype, 5-HT7, is considered to be associated with several CNS disorders. Noninvasive in vivo positron emission tomography (PET) studies of cerebral 5-HT7 receptors could provide a significant advance in the understanding of the neurobiology and eventual dysfunctions of the 5-HT7 receptor. To date, no appropriate 5-HT7 receptor PET ligand has been developed. Here, we modified known 5-HT7 selective phenylpiperazinyl-butyloxindole derivatives so that they may be labeled either with carbon-11 or fluorine-18. A set of potential 5-HT7 ligands for PET molecular imaging was successfully synthesized. Two compounds (10 and 14) were tested against a range of targets. Both compounds display a promising in vitro profile with respect to PET imaging of the 5-HT7 receptor in thalamic regions.
Keywords: Oxindole, 5-HT7 receptor distribution, PET
The relatively recently discovered G-protein coupled 5-HT7 receptor has been implicated in various central nervous system (CNS) disorders such as schizophrenia, depression, epilepsy, migraine, and in the control of circadian rhythm.1 For example, the atypical antipsychotic drug amisulpride has antidepressant effects,2,3 and a study in 5-HT7 receptor knockout mice supports that the 5-HT7 receptor antagonism of amisulpride alleviates depression symptoms.4 Other atypical antipsychotics also have relatively high affinity for the 5-HT7 receptor, but their involvement in alleviating depressive symptoms through blocking the 5-HT7 receptor remains to be investigated.5,6
In vivo studies of cerebral 5-HT7 receptor binding in humans would thus provide a significant advance in the understanding of the above-mentioned physiology and pathophysiology. Positron emission tomography (PET) is used to quantify neuroreceptor binding in vivo, and the availability of an appropriate PET radiotracer for the 5-HT7 receptor would be of particular interest.
Previous attempts of other groups to develop a 5-HT7 receptor selective PET tracer have not been convincingly successful.7,8 Most recently, 18F-labeled SB-269970 derivatives were synthesized and evaluated in vivo in cats,9,10 but in the absence of a validated reference region or an arterial input function it was not possible to fully evaluate the validity of those radiolabeled compounds.11
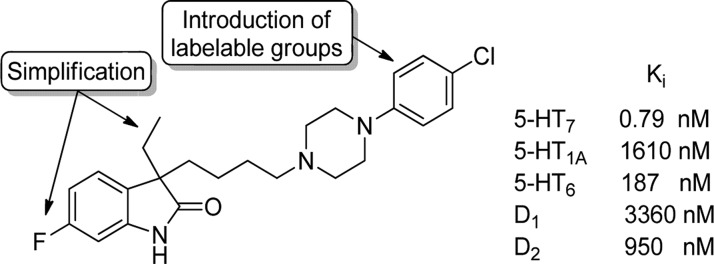
Several lead structures of 5-HT7 receptor ligands have been identified within various structural classes.12 Among these structures, phenylpiperazinyl-butyloxindoles display an interesting selectivity profile (Figure 1).13,14 Some oxindoles showed inhibition constants (Ki) 2000-fold lower for the 5-HT7 receptor than for the 5-HT1A receptor. This large difference in Ki is necessary because of low brain tissue 5-HT7 receptor density (Bmax) compared to 5-HT1A receptor values in, e.g., hippocampus and cortical areas.15,16
Figure 1.
Proposed structural modifications of a known 5-HT7 receptor selective phenylpiperazinyl-butyloxindole derivative and its binding affinities.
Therefore, the aim of this study was to synthesize, simplify, and fine-tune phenylpiperazinyl-butyloxindole derivatives in the search of radioligands for 5-HT7 receptor PET imaging (Figure 1). Thereby, we aimed to develop selective reference compounds suitable either for 11C- or for 18F-labeling. Whereas 11C with its half-life (20.4 min) is limited to short experimental time frames, it concurrently allows for test–retest experiments within the same day. In contrast, the longer half-life of 18F (110 min) provides the possibility of a longer experimental setup or even the shipment of the tracer to other facilities. Further, its lower β+-traveling energy should in principle lead to a higher resolution in PET experiments.
Results and Discussion
A convenient synthetic route to phenylpiperazinyl-butyloxindole derivatives has been described by Volk and co-workers.13,17 The method is based on a reductive alkylation of isatins,17 which circumvents the usual problem of N-alkylation and C(3)-dialkylation of oxindoles.18−20 However, the described one-pot reductive alkylation to the corresponding 3-alkyloxindoles made use of high hydrogen pressure (15 bar) at 180 °C. Recently, two different approaches using oxindoles as starting material were published to synthesize C(3)-monoalkylated oxindole derivatives by reductive alkylation of alcohols using either [Cp·IrCl2]221 or Raney nickel22 as the reductive agent without high hydrogen pressure.
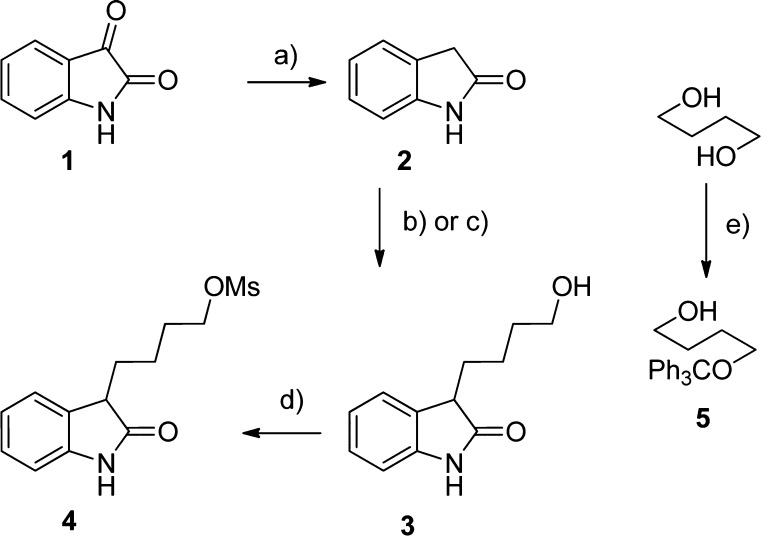
Scheme 1 illustrates the applied approach to synthesize C(3)-monoalkylated oxindole derivatives starting from isatin (1), which is first reduced under Wolff–Kishner conditions to oxindole (2). In contrast to reported procedures, hydroxyalkylation of 2 was performed under microwave (MW) conditions either resulting quantitatively in 3 in the presence of Raney nickel or yielding 60% using [Cp·IrCl2]2. In both cases, the reaction time could be minimized from 12 h to 20 min–4 h using MW irradiation.
Scheme 1. Synthesis of C(3)-Monoalkylated Oxindole Derivatives via Reductive Alkylation.
(a) N2H4·H2O, 130 °C, 30 min, 70%; (b) 1,4-butanediol, Ra–Ni, MW, 200 °C, 4 h, 95%; (c) (1) Compound 5, [Cp·IrCl2]2, KOH, toluene, MW, 110 °C, 20 min, 37%; (2) 1 M HCl, THF, RT, 20 min, 60%; (d) MsCl, Et3N, THF, −78 °C, 1 h, 83%; (e) TrCl, pyridine, CH2Cl2, RT, 90 min, 83%.
However, the iridium catalyzed C(3)-alkylation is hampered not only by lower yields but also by increased synthetic efforts as butane-1,4-diol could not directly be applied to the reductive alkylation. Many side-products were detected using this strategy. Finally, the last synthetic step, that is, mesylation of 3 proceeded uneventfully.
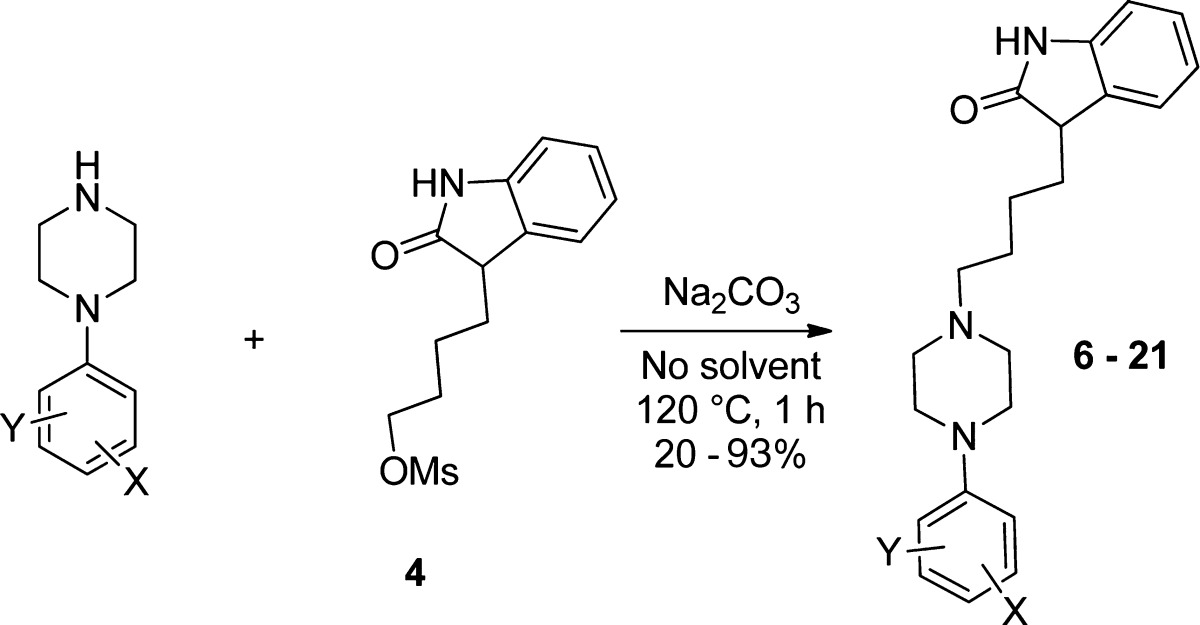
The synthesis of the desired phenylpiperazinyl-butyloxindole derivatives proceeded in reasonable yields when the corresponding arylpiperazine and 4 were heated under neat reaction conditions (Table 1). Interestingly, treatment of 4 with various arylpiperazines using DMF and Na2CO3 did not lead to the desired products (for further data, see the Supporting Information). The arylpiperazine derivatives applied were either commercially available or synthesized using previously published methods.23,24
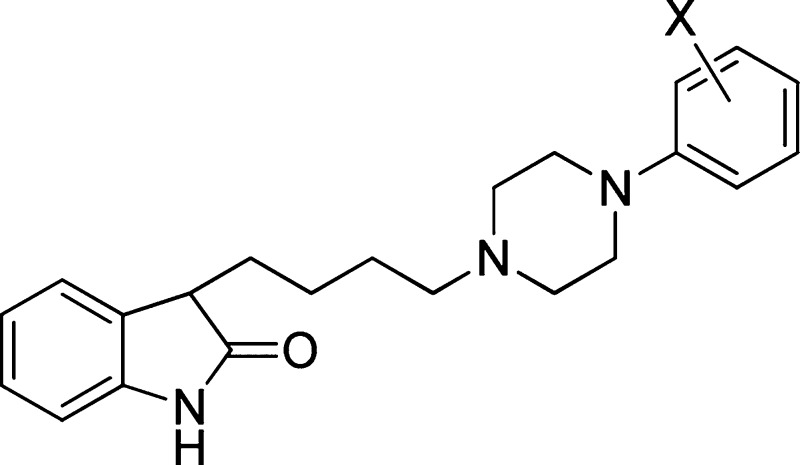
Table 1. Chemical yield of the condensation reaction between the mesylate (4) and the phenylpiperazine derivatives, human recombinant 5-HT7 receptor affinity and lipophilicity of compounds 6–21.
| compd | X | Y | yield (%) | 5-HT7Ki (nM)a | log D7.4 |
|---|---|---|---|---|---|
| 6 | H | H | 58 | 2.116,b | ndc |
| 7 | 4-Cl | H | 71 | 7.07,b | 5.56 |
| 8 | 4-F | H | 72 | 1.15,b | 4.65 |
| 9 | 2-Me | H | 72 | 6.3 | 5.97 |
| 10 | 4-Me | H | 76 | 1.1 | 5.19 |
| 11 | 2-OCH2CH2F | H | 20 | 3.5 | 4.15 |
| 12 | 3-OCH2CH2F | H | 27 | 8.5 | 4.66 |
| 13 | 2-OMe | H | 93 | 7.6 | 4.15 |
| 14 | 3-OMe | H | 32 | 2.6 | 4.73 |
| 15 | 2-OH | H | 52 | 7.4 | 3.91 |
| 16 | 3-OH | H | 60 | 27.5 | 3.40 |
| 17 | 4-OH | H | 34 | 6.8 | 3.33 |
| 18 | 2-SMe | H | 64 | 6.7 | 5.89 |
| 19 | 2-NMe2 | H | 83 | 2.0 | 3.58 |
| 20 | 2-CN | H | 73 | 2.0 | 4.67 |
| 21 | 4-F | 3-NO2 | 78 | 25.0 | 4.90 |
The development of a successful in vivo PET probe for neuroreceptor imaging requires a range of properties such as high selectivity for the target, the ability to cross the blood-brain barrier (BBB), and low relative nonspecific binding. To guide the selection of suitable candidates for in vivo PET, the lipophilicity and in vitro affinity for the 5-HT7 receptor was determined for all compounds.
Lipophilicities were determined using the HPLC method, according to Krass et al.25 Extrapolated log D7.4 values are displayed in Tables 1 and 2. As expected, hydroxyl derivatives showed lower log D7.4 values (between 3 and 4) compared to the less polar compounds (log D7.4 between 4 and 6). In general, all log D7.4 values appear relatively high considering that Rowley et al. suggested the ideal interval for small molecules to penetrate the BBB to be 2–3.26 However, with our setup, other known CNS PET ligands (e.g., MDL 100907, altanserin, or WAY 100635) show similarly high values.11 This suggests that our ligands may also have good properties for molecular imaging.
Table 2. Ki (nM) of Selected Receptors for 10 and 14ab.
| compd |
||||
|---|---|---|---|---|
|
10 |
14 |
|||
| receptors | Ki | selectivity (receptor/5-HT7) | Ki | selectivity (receptor/5-HT7) |
| 5-HT7 | 1.1 | 1 | 2.6 | 1 |
| 5-HT1A | 2410 | 2191 | 261 | 100 |
| 5-HT2B | 121 | 110 | 192 | 74 |
| 5-HT2A | 113 | 103 | 132 | 51 |
| 5-HT2C | 676 | 515 | 3297 | 1268 |
| α1 | 53 | 48 | 47 | 18 |
Receptors and radioligands used in binding assays and data analysis; see Methods.
Ki values are based on at least two independent experiments.
Consequently, we investigated the influence of various substitution patterns on the affinity toward the 5-HT7 receptor. Thereby, we concentrated on structure–activity relationships at the arylpiperazine moiety (Table 1).
Interestingly, substitution of the 4-position of the phenylpiperazine by a fluorine atom and a methyl group (8 and 10) showed a slight improvement in binding profile toward the 5-HT7 receptor compared to its 4-Cl and 4-unsubstituted analogues. Both compounds (8, 10) represent possible reference structures for PET tracers with nanomolar affinity which can in principle be labeled either by 11C-cross-coupling reactions27,28 or by a 18F-nucleophilic substitution followed by decarbonylation.29 Surprisingly, no general trend of the substitution pattern in 2- and 3-position was observed. Whereas the 2-OMe moiety (13) resulted in lower affinity compared to its 3-OMe analogue (14), 2-(2-fluoroethoxy) and 3-(2-fluoroethoxy) derivatives (11 and 12, respectively) showed contrary findings. However, all ether compounds (11–14) demonstrated nanomolar affinity and could in theory be labeled with either [11C]CH3I or [18F]FETos.30 In addition, their corresponding precursors showed at least 3 times lower affinity.
Furthermore, the influence of three different possible labeling moieties at the 2-position were explored. 11C-Labeling of thioethers, amines or cyano compounds is well described.31 All three compounds (18–20) showed reasonable affinities, and thus, they proved to be promising PET ligands. Finally, a possibility to introduce 18F via a direct one-step fluorination would be desirable. Compound 21 could be labeled with this approach using a trimethylammonium leaving group, which is the preferred site of attack of [18F]fluoride compared to the nitro-group.32 Unfortunately, this compound displayed lower affinity.
Encouraged by the promising results regarding lipophilicity and affinity, 10 and 14 were submitted to a commercial screening package for their selectivity profile on 37 receptors. Compound 14 was found to be over 100-fold selective against a total of 33, whereas 10 against 34 receptors, enzymes, or ion channels (see the Supporting Information). However, both compounds showed some affinity toward a small set of receptors (Table 2).
To avoid significant contributions from targets other than 5-HT7 receptors to the PET imaging signal, selectivity versus the Bmax value of the target has to be taken into account. The selectivity toward the 5-HT1A and α1 receptor, because of the high density (Bmax) of these two receptors in certain brain regions, is of particular concern. Since the displaceable PET-signal consists of density multiplied by affinity, a high Bmax and high Kd will give a large PET signal compared to the corresponding signal from a relatively low abundance of the 5-HT7 receptor. For example, a ∼350-fold Kd difference over the 5-HT1A receptor is necessary in order to avoid more than 10% PET signal interference from the 5-HT1A receptors in cortical regions, whereas in the thalamus only a 35-fold selectivity must be achieved. For the α1 receptor a ∼2000-fold selectivity is necessary in cortical regions, but only a 100–200-fold selectivity in thalamic regions.
In contrast to 14, compound 10 fulfils the requirement for selectively imaging the 5-HT7 receptor against the 5-HT1A receptor in thalamic and cortical regions, whereas 14 is predicted to represent 5-HT7 receptors binding only in the thalamus. Unfortunately, both compounds show a limited specificity against the α1 receptor. They should image the α1 receptor rather than the 5-HT7 receptor in cortical regions. But in thalamic regions a major PET signal belonging to the 5-HT7 receptor is predicted for both compounds, and with a slightly higher selectivity for 10.
However, 10 also displays affinity toward the 5-HT2A receptor. Therefore, the PET images would be anticipated to be composed of a 5-HT2A signal in cortex regions, but requirements for imaging thalamic regions should be fulfilled. In addition, both compounds show a 70–80% inhibition of the H1 receptor at a concentration of 10–7 M and 10 displays some affinity toward the SERT. But in both cases (for the H1 and also for the SERT), an interference of the PET signal is not expected due to the low Bmax number in the relevant brain regions. Finally, both compounds showed inhibition on σ-receptors. σ-Receptors are abundant in cortex regions and could cause decomposition during PET imaging.
The discussion is based on human binding data determined by autoradiography and human brain membranes (Table 3).33−40
Table 3. Receptor Distribution of Colocalized Targets.
| 5-HT7(fmol/mg tissue) | 5-HT1A(fmol/mg tissue) | 5-HT2A(fmol/mg tissue) | α1(fmol/mg protein) | H1(fmol/mg tissue) | σ fmol/mg protein | SERT (fmol/mg tissue) | |
|---|---|---|---|---|---|---|---|
| frontal cortex | 2.3 | 73 | 80 | 283 | 19.1 | 101 | 4.3 |
| temporal cortex | 2.3 | 78.6 | 467 | 23.5 | 3.5 | ||
| parietal cortex | 78 | 75 | 434 | 16.6 | |||
| occipital cortex | 1.2 | 30 | 75 | 13.2 | 106 | 3.8 | |
| cingulate cortex | 75 | 381 | 22.3 | ||||
| hippocampus | 5.7 | 76 | 25 | 568 | 73 | 2.6 | |
| thalamus | 12 | 4.16 | 299 | 4.3 | 58 | 6.7 | |
| caudate | 23 | 277 | 5.3 | 84 | 17 | ||
| putamen | 10 | 215 | 4.4 | 9.8 | |||
| amygdala | 4.2 | 18 | 25 | 393 | 3 |
| ratios (receptor/5-HT7) | 5-HT1A | 5-HT2A | α1 | H1 | σ | SERT |
|---|---|---|---|---|---|---|
| frontal cortex | 32 | 35 | 123 | 8.3 | 44 | 1.86 |
| temporal cortex | 34 | 203 | 10 | 1.5 | ||
| occipital cortex | 25 | 33 | 88 | 3.2 | ||
| hippocampus | 13 | 4.4 | 99 | 13 | 0.46 | |
| thalamus | 0.35 | 25 | 0.36 | 5 | 0.56 | |
| amygdala | 4.3 | 6.0 | 94 | 0.71 |
Conclusion
A set of potential 5-HT7 receptor reference ligands for PET molecular imaging were successfully synthesized. Compounds 10 and 14 were tested toward a broader range of receptors. Both compounds display a promising in vitro profile for PET imaging of the 5-HT7 receptor in thalamic regions. Based on in vitro data, it is possible that they may not provide target-specific imaging as signals from the α1 receptor may contaminate the signal detected from the 5-HT7 receptor. However, we believe that those compounds display an interesting starting point for developing 5-HT7 selective PET ligands with even higher selectivity and affinity.
Methods
General
The syntheses of selected compounds are described below. The general chemistry, experimental information, spectral data of all new compounds, and determination of lipophilicities and Ki values are supplied in the Supporting Information. Purity of all final compounds was determined by HPLC or GC analysis and is >96%.
General Procedure to Couple 3-[4-(Methanesulfonyloxy)butyl]oxindole 4 with Substituted 4-Phenylpiperazines
The melt of the secondary amine (12 mmol) was heated to 120 °C under slow stirring. The appropriate 3-[4-(methanesulfonyloxy)butyl]oxindole (4, 12 mmol) and sodium carbonate (1.36 g, 12 mmol) were added. After 1 h reaction time, the brown melt was cooled to ambient temperature. EtOAc and water were added, and the layers were separated. The organic layer was dried over MgSO4 and evaporated. The residual oil or solid was purified by column chromatography using EtOAc as eluent.
3-{4-[4-(4-Methylphenyl)piperazine-1-yl]butyl}-1,3-dihydro-2H-indol-2-one (10)
Compound 10 was prepared using the aboved mentioned procedure. 1-(4-Methylphenyl)piperazine (0.704 g, 3.98 mmol), sodium carbonate (0.452 g, 4.0 mmol), and 4 (1.14 g, 4.0 mmol) yield in 10 (1.09 g, 3.0 mmol, 76%) as a white solid. Mp 109–110 °C. 1H NMR (CDCl3, 400 MHz): δ 8.98 (1H, s), 7.23–7.17 (2H, m), 7.07–6.99 (3H, m), 6.89–6.80 (3H, m), 3.48 (1H, t, J = 6.0 Hz), 3.14 (4H, t, J = 6.0 Hz), 2.58 (4H, t, J = 6.0 Hz), 2.37 (2H, t, J = 7.5 Hz), 2.28 (3H, s), 2.04–1.96 (2H, m), 1.61–1.51 (2H, m), 1.49–1.37 (2H, m). 13C NMR (CDCl3, 75 MHz): δ 181.0, 149.76, 141.87, 130.07, 130.0, 129.61, 128.51, 122.63, 116.75, 109.99, 58.72, 53.67, 50.03, 46.00, 30.81, 27.18, 24.19, 20.87. MS (FD) m/z (% rel. int.): 363.41 (100.0 [M]+); 364.41 (27.74 [M+1]+); 365.44 (3.14 [M+2]+). LC-MS (ESI): RT: 4.83 min, m/z: 364.2 [M+H]+ at 210 and 254 nm. Rf: 0.2 (EtOAc). HRMS (ESI) [MH+] calcd for C23H30N3O, 364.2383; found, 364.2386.
3-{4-[4-(3-Methoxyphenyl)piperazine-1-yl]butyl}-1,3-dihydro-2H-indol-2-one (14)
Compound 14 was prepared using the above-mentioned procedure. 1-(3-Methoxyphenyl)piperazine (0.674 g, 3.51 mmol), sodium carbonate (0.40 g, 3.51 mmol) and 4 (1.0 g, 3.51 mmol) yield in 14 (0.42 g, 1.10 mmol, 32%) as a colorless oil. 1H NMR (300 MHz, CDCl3): δ 8.25 (1H, s), 7.17–7.04 (3H, m), 6.93 (1H, t, J = 6.0 Hz), 6.79 (1H, d, J = 1.5 Hz), 6.46–6.42 (1H, dt, J1 = 3.0 Hz, J2 = 6.0 Hz), 6.46–6.31 (2H, m), 3.70 (3H, s), 3.40 (1H, t, J = 6.0 Hz), 3.10 (4H, t, J = 4.5 Hz), 2.49 (4H, t, J = 4.5 Hz), 2.30 (2H, t, J = 7.5 Hz), 1.92–1.88 (2H, m), 1.54–1.41 (2H, m), 1.40–1.29 (2H, m). 13C NMR (75 MHz, CDCl3): δ 180.60, 160.68, 152.81, 141.77, 129.90, 129.83, 128.02, 124.28, 122.42, 109.86, 108.99, 104.56, 102.60, 102.60, 58.45, 55.35, 53.30, 49.12, 46.16, 30.54, 26.90, 23.93. MS (FD) m/z (% rel. int.): 379.41 (100.0 [M]+); 380.41 (18.26 [M+1]+). LC-MS (ESI): RT: 4.99 min, m/z: 380.4 [M+H]+ at 210 and 254 nm. Rf: 0.1 (EtOAc). HRMS (ESI) [MH+] calcd for C23H30N3O2, 380.2338; found, 380.2340.
Acknowledgments
The authors wish to thank Hanne D. Hansen, Valdemar Lykke Andersen, and Anders Ettrup for fruitful discussions.
Supporting Information Available
Experimental procedures, spectroscopic data for selected compounds, receptor distribution, and detailed in vitro profiles. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
M.M.H., G.M.K., and J.L.K. conceived the project. M.M.H., J.L.K., B.V., and F.A. designed the experiments. M.M.H. performed all chemical syntheses. B.V. provided M.H.H. with intermediates. K.P. and F.A. carried out all affinity measurements. Compounds were analyzed by M.M.H. HRMS data were provided by B.V. The project was coordinated by M.H.H., G.M.K., and J.L.K. M.H.H. wrote the manuscript with the help of B.V., G.M.K., and J.L.K. L.K.B. did the log D measurements.
This work was supported by the Intra European Fellowship (MC-IEF-275329). The Faculty of Health and Medical Sciences, University of Copenhagen, and the Lundbeck Foundation (Cimbi) is gratefully acknowledged.
The authors declare no competing financial interest.
Supplementary Material
References
- Matthys A.; Haegeman G.; Van Craenenbroeck K.; Vanhoenacker P. (2011) Role of the 5-HT7 receptor in the central nervous system: from current status to future perspectives. Mol. Neurobiol. 43, 228–253. [DOI] [PubMed] [Google Scholar]
- Cassano G. B.; Jori M. C. (2002) Efficacy and safety of amisulpride 50 mg versus paroxetine 20 mg in major depression: a randomized, double-blind, parallel group study. Int. Clin. Psychopharmacol. 17, 27–32. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y.; Boyer P.; Turjanski S.; Rein W. (1997) Amisulpride versus imipramine and placebo in dysthymia and major depression. Amisulpride Study Group. J. Affective Disord. 43, 95–103. [DOI] [PubMed] [Google Scholar]
- Abbas A. I.; Hedlund P. B.; Huang X. P.; Tran T. B.; Meltzer H. Y.; Roth B. L. (2009) Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berlin, Ger.) 205, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T.; Horisawa T.; Tokuda K.; Ishiyama T.; Ogasa M.; Tagashira R.; Matsumoto K.; Nishikawa H.; Ueda Y.; Toma S.; Oki H.; Tanno N.; Saji I.; Ito A.; Ohno Y.; Nakamura M. (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J. Pharmacol. Exp. Ther. 334, 171–181. [DOI] [PubMed] [Google Scholar]
- Smith C.; Rahman T.; Toohey N.; Mazurkiewicz J.; Herrick-Davis K.; Teitler M. (2006) Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol. Pharmacol. 70, 1264–1270. [DOI] [PubMed] [Google Scholar]
- Zhang M. R.; Haradahira T.; Maeda J.; Okauchi T.; Kida T.; Obayashi S. (2002) Synthesis and preliminary PET study of the 5-HT7 receptor antagonist [11C]DR4446. J. Labelled Compd. Radiopharm. 45, 857–866. [Google Scholar]
- Andries J.; Lemoine L.; Mouchel-Blaisot A.; Tang S.; Verdurand M.; Le B. D.; Zimmer L.; Billard T. (2010) Looking for a 5-HT7 radiotracer for positron emission tomography. Bioorg. Med. Chem. Lett. 20, 3730–3733. [DOI] [PubMed] [Google Scholar]
- Lemoine L.; Andries J.; Le Bars D.; Billard T.; Zimmer L. (2011) Comparison of 4 radiolabeled antagonists for serotonin 5-HT7 receptor neuroimaging: toward the first PET radiotracer. J. Nucl. Med. 52, 1811–1818. [DOI] [PubMed] [Google Scholar]
- Andries J.; Lemoine L.; Le Bars D.; Zimmer L.; Billard T. (2011) Synthesis and biological evaluation of potential 5-HT7 receptor PET radiotracers. Eur. J. Med. Chem. 46, 3455–3461. [DOI] [PubMed] [Google Scholar]
- Herth M. M.; Hansen H. D.; Ettrup A.; Dyssegaard A.; Lehel S.; Kristensen J.; Knudsen G. M. (2012) Synthesis and evaluation of [11C]Cimbi-806 as a potential PET ligand for 5-HT7 receptor imaging. Bioorg. Med. Chem. 20, 4574–4581. [DOI] [PubMed] [Google Scholar]
- Leopoldo M.; Lacivita E.; Berardi F.; Perrone R.; Hedlund P. B. (2011) Serotonin 5-HT7 receptor agents: Structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol. Ther. 129, 120–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk B.; Barkoczy J.; Hegedus E.; Udvari S.; Gacsalyi I.; Mezei T.; Pallagi K.; Kompagne H.; Levay G.; Egyed A.; Harsing L. G.; Spedding M.; Simig G. (2008) Phenylpiperazinyl-butyl)oxindoles as selective 5-HT7 receptor antagonists. J. Med. Chem. 51, 2522–2532. [DOI] [PubMed] [Google Scholar]
- Volk B.; Gacsalyi I.; Pallagi K.; Poszavacz L.; Gyonos I.; Szabo E.; Bako T.; Spedding M.; Simig G.; Szenasi G. (2011) Optimization of (arylpiperazinylbutyl)oxindoles exhibiting selective 5-HT7 receptor antagonist activity. J. Med. Chem. 54, 6657–6669. [DOI] [PubMed] [Google Scholar]
- Hall H.; Lundkvist C.; Halldin C.; Farde L.; Pike V. W.; McCarron J. A.; Fletcher A.; Cliffe I. A.; Barf T.; Wikstrom H.; Sedvall G. (1997) Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]WAY 100635. Brain Res. 745, 96–108. [DOI] [PubMed] [Google Scholar]
- Varnas K.; Thomas D. R.; Tupala E.; Tiihonen J.; Hall H. (2004) Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci. Lett. 367, 313–316. [DOI] [PubMed] [Google Scholar]
- Volk B.; Simig G. (2003) New one-pot synthesis of 3-alkyl- and 3-(ω-hydroxyalkyl)oxindoles from isatins. Eur. J. Org. Chem. 3991–3996. [Google Scholar]
- Gruda I. (1972) Formation of N-substituted and C-substituted derivatives during alkylation of indol-2(3H)-one. Can. J. Chem. 50, 18–23. [Google Scholar]
- Reisch J.; Muller M.; Labitzke H. (1984) Synthetic pathways for 3-substituted indol-2-ones. Arch. Pharm. 317, 639–646. [Google Scholar]
- Kende A. S.; Hodges J. C. (1982) Regioselective C-3 alkylations of oxindole dianion. Synth. Commun. 12, 1–10. [Google Scholar]
- Grigg R.; Whitney S.; Sridharan V.; Keep A.; Derrick A. (2009) Iridium catalysed C-3 alkylation of oxindole with alcohols under solvent free thermal or microwave conditions. Tetrahedron 65, 4375–4383. [Google Scholar]
- Volk B.; Mezei T.; Simig G. (2002) Raney nickel-induced 3-alkylation of oxindole with alcohols and diols. Synthesis-Stuttgart 595–597. [Google Scholar]
- Herth M.; Kramer V.; Roesch F. (2009) Synthesis of novel WAY 100635 derivatives containing a norbornene group and radiofluoroination of [18F]AH1.MZ. J. Labelled Compd. Radiopharm. 52, 201–207. [Google Scholar]
- Takahashi T.; Sakuraba A.; Hirohashi T.; Shibata T.; Hirose M.; Haga Y.; Nonoshita K.; Kanno T.; Ito J.; Iwaasa H.; Kanatani A.; Fukami T.; Sato N. (2006) Novel potent neuropeptide YY5 receptor antagonists: Synthesis and structure-activity relationships of phenylpiperazine derivatives. Bioorg. Med. Chem. 14, 7501–7511. [DOI] [PubMed] [Google Scholar]
- Krass J. D.; Jastorff B.; Genieser H. G. (1997) Determination of lipophilicity by gradient elution high-performance liquid chromatography. Anal. Chem. 69, 2575–2581. [DOI] [PubMed] [Google Scholar]
- Rowley M.; Kulagowski J. J.; Watt A. P.; Rathbone D.; Stevenson G. I.; Carling R. W.; Baker R.; Marshall G. R.; Kemp J. A.; Foster A. C.; Grimwood S.; Hargreaves R.; Hurley C.; Saywell K. L.; Tricklebank M. D.; Leeson P. D. (1997) Effect of plasma protein binding on in vivo activity and brain penetration of glycine/NMDA receptor antagonists. J. Med. Chem. 40, 4053–4068. [DOI] [PubMed] [Google Scholar]
- Koyama H.; Siqin; Zhang Z.; Sumi K.; Hatta Y.; Nagata H.; Doi H.; Suzuki M. (2011) Highly efficient syntheses of [methyl-11C]thymidine and its analogue 4′-[methyl-11C]thiothymidine as nucleoside PET probes for cancer cell proliferation by Pd(0)-mediated rapid C-[11C]methylation. Org Biomol. Chem. 9, 4287–4294. [DOI] [PubMed] [Google Scholar]
- Kihlberg T.; Karimi F.; Langstrom B. (2002) [C-11]Carbon monoxide in selenium-mediated synthesis of C-11-carbamoyl compounds. J. Org. Chem. 67, 3687–3692. [DOI] [PubMed] [Google Scholar]
- Shen B.; Loffler D.; Zeller K. P.; Ubele M.; Reischl G.; Machulla H. J. (2007) Decarbonylation of multi-substituted 18F-benzaldehydes for modelling syntheses of F-18-labelled aromatic amino acids. Appl. Radiat. Isot. 65, 1227–1231. [DOI] [PubMed] [Google Scholar]
- Ametamey S. M.; Honer M.; Schubiger P. A. (2008) Molecular imaging with PET. Chem. Rev. 108, 1501–1516. [DOI] [PubMed] [Google Scholar]
- Allard M.; Fouquet E.; James D.; Szlosek-Pinaud M. (2008) State of art in C-11 labelled radiotracers synthesis. Curr. Med. Chem. 15, 235–277. [DOI] [PubMed] [Google Scholar]
- Angelini G.; Speranza M.; Wolf A. P.; Shiue C. Y. (1985) Nucleophilic aromatic-substitution of activated cationic groups by F-18-labeled fluoride - a useful route to no-carrier-added (NCA) F-18-labeled aryl fluorides. J. Fluorine Chem. 27, 177–191. [Google Scholar]
- Hall H.; Farde L.; Halldin C.; Lundkvist C.; Sedvall G. (2000) Autoradiographic localization of 5-HT2A receptors in the human brain using [3H]M100907 and [11C]M100907. Synapse 38, 421–431. [DOI] [PubMed] [Google Scholar]
- Weissman A. D.; Su T. P.; Hedreen J. C.; London E. D. (1988) Sigma-receptors in post-mortem human brains. J. Pharmacol. Exp. Ther. 247, 29–33. [PubMed] [Google Scholar]
- Varnas K.; Halldin C.; Hall H. (2004) Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Human Brain Mapping 22, 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnas K.; Thomas D. R.; Tupala E.; Tiihonen J.; Hall H. (2004) Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci. Lett. 367, 313–316. [DOI] [PubMed] [Google Scholar]
- Hall H.; Lundkvist C.; Halldin C.; Farde L.; Pike V. W.; McCarron J. A.; Fletcher A.; Cliffe I. A.; Barf T.; Wikstrom H.; Sedvall G. (1997) Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Res. 745, 96–108. [DOI] [PubMed] [Google Scholar]
- Grossisseroff R.; Dillon K. A.; Fieldust S. J.; Biegon A. (1990) Autoradiographic analysis of alpha-1-noradrenergic receptors in the human brain postmortem - Effect of suicide. Arch. Gen. Psychiatry 47, 1049–1053. [DOI] [PubMed] [Google Scholar]
- Thomas D. R.; Atkinson P. J.; Hastie P. G.; Roberts J. C.; Middlemiss D. N.; Price G. W. (2002) [3H]SB-269970 radiolabels 5-HT7 receptors in rodent, pig and primate brain tissues. Neuropharmacology 42, 74–81. [DOI] [PubMed] [Google Scholar]
- Kanaba S.; Richelson E. (1984) Histamine H1 receptors in human brain labeled with [3H]Doxepin. Brain Research 304, 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.