Abstract
The ability of transient immunosuppression with a combination of a nondepleting anti-CD4 (NDCD4) antibody and Cyclosporine (CyA) to abrogate immune reactivity to both adeno-associated virus vector (AAV) and its transgene product was evaluated. This combination of immunosuppressants resulted in a 20-fold reduction in the resulting anti-AAV8 antibody titres, to levels in naïve mice, following intravenous administration of 2×1012 AAV8 vector particles/kg to immunocompetent mice. This allowed efficient transduction upon secondary challenge with vector pseudotyped with the same capsid. Persistent tolerance did not result, however, as an anti-AAV8 antibody response was elicited upon rechallenge with AAV8 without immunosuppression. The route of vector administration, vector dose, AAV serotype or the concomitant administration of adenoviral vector appeared to have little impact on the ability of the NDCD4 antibody and CyA combination to moderate the primary humoral response to AAV capsid proteins. The combination of NDCD4 and CyA also abrogated the humoral response to the transgene product, that otherwise invariably would occur, following intramuscular injection of AAV5, leading to stable transgene expression. These observations could significantly improve the prospects of using rAAV vectors for chronic disorders by allowing for repeated vector administration and avoiding the development of antibodies to the transgene product.
Keywords: AAV, nondepeleting CD4 antibody, inhibitors
Introduction
Recombinant adeno-associated viral vectors (AAV) show great promise for gene therapy of a variety of different genetic disorders including haemophilia B, lysosomal storage disorders, inherited retinal degeneration, α–1 antitrypsin and lipoprotein lipase deficiency.1–7 In addition to their excellent safety profile, the greatest attribute of these vectors is their ability to mediate persistent therapeutic transgene expression following a single administration of vector in a variety of animal models.1, 2, 8–10 The majority of the transgenic protein is expressed by episomally retained double stranded genomes that persist in post-mitotic tissues in a variety of forms including concatemers by hitherto unknown mechanisms.11–13 This has favourable safety implications with respect to insertional mutagenesis but raises the possibility that transgene expression will decline over time with the natural turn-over of the transduced cells. Indeed, we have observed gradual decline in transgene expression in a proportion of rhesus macaques at approximately 3–5 years after liver targeted delivery of AAV.14
Therefore, for chronic disorders such as haemophilia B, repeat administration of rAAV vectors may become necessary to maintain expression of human FIX (hFIX) within therapeutic levels. However, serotype specific antibodies directed towards the viral capsid proteins, generated following primary vector administration, will prevent efficient gene transfer with rAAV of the same serotype.14–18 Although, we have demonstrated the feasibility of using vector particles of different serotypes for vector readministration, this strategy does not permit the use of “vector of choice” more than once because of the generation of a humoral immune response. This is of concern for life-long disorders as there are a limited number of serotypes that are suitable for optimal transduction of target tissues such as the liver, each with serotype specific differences in tropism, seroprevalence rates and production methodology14, 16, 19
An alternative, potentially more practical approach that may allow for effective re-administration of rAAV of the same serotype involves the use of transient immunosuppression at the time of vector administration. The goal with this strategy would be to attenuate the development of serotype specific neutralizing rAAV antibodies without inducing permanent tolerance. A large body of data suggests that T cells play a central role in the development of a humoral response by B lymphocytes following AAV mediated gene transfer.20–23 However, moderation of T cell function with conventional immunosuppressive agents such as the calcineurin inhibitor cyclosporine (CyA) cannot avert a humoral response to rAAV.17 Hence, the focus has turned to more refined targets such as the CD40–CD40 ligand and CD28–CD80/86 costimulatory pathways.22, 23 Indeed, cytotoxic T-lymphocyte-associated antigen-4 immunoglobulin (CTLA4Ig), which blocks the binding of CD80/86 to CD28 on T-lymphocytes when combined with a neutralising antibody against the CD40 ligand (CD40L), impaired the ability of mice to mount a humoral response to AAV delivered to the lungs, allowing effective re-administration of vector of the same serotype.17 Although lungs are an immunologically distinct compartment, it is likely that this type of approach will be effective following systemic administration of AAV, based on the success with adenoviral vectors in murine and nonhuman primate models.24, 25 However, the clinical use of anti-CD40L antibody, a critical component of this immunosuppressive combination, is limited by its thrombogenic properties.26
The CD4+ T cell population is another key target for mediating immunological hyporesponsiveness because of its interactions with both B and cytotoxic T lymphocytes. Depleting CD4 antibodies have been shown to induce tolerance in the setting of allogeneic transplant and inhibit primary humoral responses to viral capsid proteins in animal models.21 However, their use in the clinic has been restricted by the occurrence of prolonged immunosuppression as a result of CD4 T-cell depletion. In contrast, nondepleting CD4 antibodies (NDCD4ab) induce long-term antigen specific tolerance to foreign antigens without causing depletion of CD4 lymphocytes by resetting the immunological balance through the deletion of alloreactive T-cells whilst facilitating the selective expansion or survival of regulatory T-cells (T-regs).27 Therefore, transient CD4 receptor blockade at the time of vector administration may offer several advantages for gene therapy of haemophilia with rAAV vectors, including: (1) dampening of the humoral response to viral capsid, and (2) prevention of inhibitor formation after gene transfer, which is a serious complication of protein concentrate therapy. However, CD4 co-receptor blockade has not been extensively evaluated in the context of AAV mediated gene transfer for gene therapy of haemophilia B.
In this study, we show that CyA and NDCD4 antibody, when co-administered with rAAV vectors prevent a primary humoral response to capsid proteins, enabling successful gene transfer upon re-administration of vectors based on the same serotype without causing tolerance to viral proteins. This combination of immunosuppressive agents also facilitated the development of immunological tolerance to the hFIX transgene following intramuscular delivery of AAV, resulting in stable long term expression of hFIX. Importantly, immunosuppression was only required transiently at the time of vector administration and did not cause lymphodepletion, which is an important safety consideration for patients with chronic disorders such as haemophilia.
Results
Attenuation of humoral response against AAV8 following transient immunosuppression
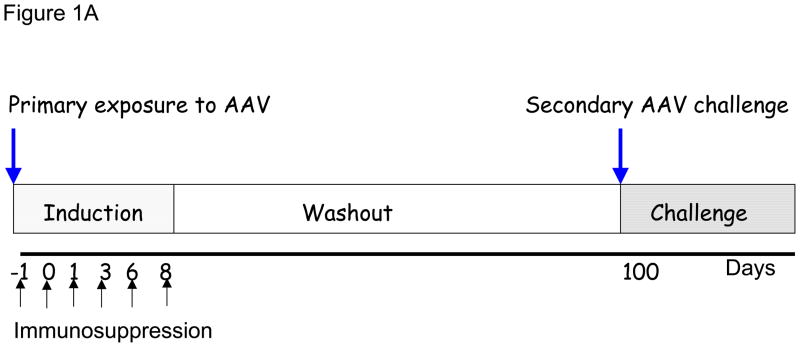
In our initial experiments we determined if the humoral response to a primary exposure of AAV8 could be modulated as this serotype shows great promise for liver targeted gene therapy. We used our previously described self complementary AAV vector which contains the hFIX cDNA under the control of a liver specific promoter (scAAV2/8-LP1-hFIXco) pseudotyped with AAV8 capsid protein.16 As described in the experimental schema in Figure 1A, six groups of 6–8 weeks old male C57Bl/6 immunocompetent mice received a single tail vein administration of 2×1012vg/kg of scAAV2/8-LP1-hFIXco concomitantly with NDCD4 antibody (20mg/kg/mouse intraperitoneally [IP]) alone or in combination with either CyA (25mg/kg/mouse, IP), or MMF (mycophenolate mofetil, 20mg/kg/mouse, IP) on days −1, 0, +1, +3, +6, and +8 with respect to vector administration on day 0. For comparison, cohorts of scAAV transduced mice were treated with CyA or MMF alone along the same schedule. The positive control group consisted of mice transduced with scAAV in the absence of immunosuppression. The negative control group consisted of mice that had not been exposed to scAAV vector or immunosuppression. The dose of AAV selected for these studies is sufficient to mediate hFIX expression in mice and nonhuman primates at levels that would be therapeutically relevant in haemophilia B patients (>5% of normal levels).3, 16, 28
Figure 1. Successful attenuation of humoral response following transient immunosuppression.
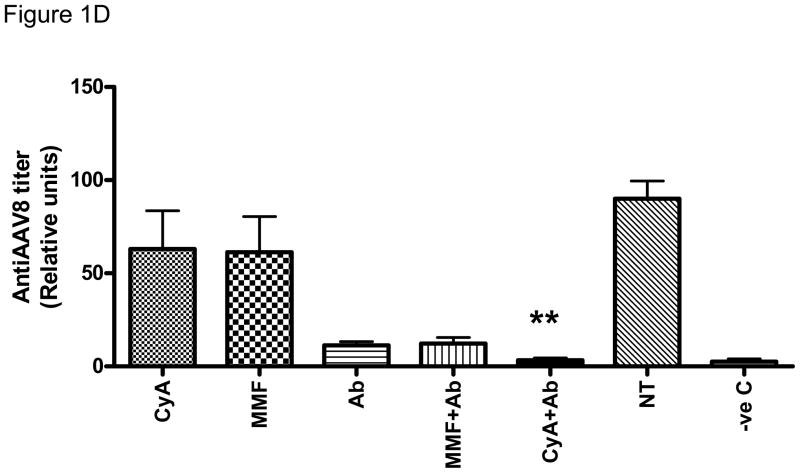
(A) Schema of immunosuppression. C57Bl/6 mice (N=7–8/group) received immunosuppression on days −1, 0, 1, 3, 6, 8 and 2×1012/kg AAV8 LP1 hFIX on day 0. (B) Normal levels of CD4+ T cells in the peripheral blood of mice CyA/NDC4 when compared to untreated animals (n=2 in each group) as shown by specific staining for CD3 (PE) and CD4 (FITC). (C) Transduction efficiency is unaffected by concurrent administration of immunosuppression. hFIX levels were measured by ELISA on day 98 (n=6) mean ±SEM (D) Attenuation of humoral response to capsid by immunosuppression. Anti-AAV antibody was measure by ELISA on day 98 (n=6). Results shown as mean ±SEM.
Immunosuppression was well tolerated with no overt evidence of toxicity. In agreement with previously published data in rodents, nonhuman primates and humans, the NDCD4 antibody did not induce lymphopenia as illustrated by flow cytometric analysis of CD3 and CD4 levels using antibodies that target epitopes that are distinct from that used by the NDCD4 antibody (Figure 1B)..29–32 CD4+CD25+FoxP3+ T cells numbers in the spleen of NDCD4 antibody treated cohort were equivalent to the numbers seen in naïve animals. The kinetic of hFIX expression was similar in each of the scAAV2/8-LP1-hFIXco transduced cohorts with mean steady state plasma levels of approximately 40% of normal (Figure 1C). This level of transgene expression is consistent with that previously reported with this vector dose, suggesting that scAAV mediated transduction in C57BL/6 mice is not influenced by concurrent administration of immunosuppressive agents.16 The magnitude of the anti-AAV8 humoral immune response in the cohorts treated with single agent CyA (63±21 relative units [RU]/ml) and MMF (61±19 RU/ml) as determined by an AAV8 specific immunocapture assay was lower but not statistically different (p= 0.31 and 0.27 respectively) from that in the positive control group (90±10 RU/ml), which was transduced with scAAV in the absence of immunosuppression. In contrast, the anti-AAV8 antibody titres were significantly reduced in mice transduced with scAAV2/8-LP1-hFIXco (11±2 RU/ml) in the presence of NDCD4 antibody alone (p=0.007). Combining MMF with NDCD4 antibody did not cause a further reduction in the humoral response (12±3 RU/ml) to the AAV capsid protein. However, the lowest anti-AAV8 antibody titres were observed in the cohort of mice treated with a combination of CyA and NDCD4 antibody with mean levels of 3±1 RU/ml. These levels were comparable to background anti-AAV8 antibody titres (2.5±1 RU/ml) in negative control animals which had not been exposed to AAV (Figure 1D). A surprising aspect of these results was that an additive effect was observed when NDCD4 antibody was combined with CyA but not MMF especially since the latter, through the inhibition of inosine monophosphate dehydrogenase, affects both B and T lymphocyte function.33, 34 These observations were not vector stock or mouse strain specific as they were reproducible in the Balb/c immunocompetent mice using a different stock of vector (data not shown).
Transient immunosuppression allows effective transduction upon re-administration of vectors pseudotyped with the same capsid protein
Three months after primary vector exposure, the mice were challenged with a second AAV8 vector (dose = 2×1012vg/kg) containing the human interferon beta (hIFNβ) gene under a constitutively active promoter (rAAV2/8-CAGG-hIFNβ) without immunosuppression. Importantly, hIFNβ does not cross-react with the murine IFN receptor and in the context of these experiments simply served as a secretable marker of transduction.35
As shown in Figure 2A, efficient transduction with rAAV8-CAGG-hIFNβ was observed only in the cohort of mice treated with the combination of NDCD4 antibody and CyA at the time of primary vector administration. The level of hIFNβ in these animals was 13±2 μg/ml and comparable to that observed in mice whose primary exposure to AAV consisted of rAAV8-CAGG-hIFNβ (16±1μg/ml). Successful gene transfer with the second AAV vector (rAAV2/8-CAGG-hIFNβ) did not affect expression from the first vector as the expression of hFIX remained at approximately 40% (2237±49ng/ml) on day 110 following administration of scAAV2/8-LP1-hFIXco.
Figure 2. Re-administration of AAV8.
(A) Successful transduction with the same serotype of AAV. 2×1012/kg AAV8 CAGG IFNβ was given on day 100, 20 days later IFNβ levels were measured by ELISA (n=7, mean ±SEM). (B) Semi-quantitative PCR confirming selective transduction. 250ng of liver DNA from (n=2) CyA+Ab, NT (no immunosuppression), Naïve and IFN (hIFNβ only) cohorts were analysed with primers against hFIX, hIFNβ and βactin (C) Mice are not tolerised to capsid protein following transient immunosuppression. Anti-AAV8 abs were determined by ELISA on day 60 following 2nd administration n=6 mean ±SEM. (D) Mice were challenged with AAV8 LP1 hFX 5×1011/kg on day 296, 21 days later mice were bleed and FX levels were determined by ELISA n=3
Liver is preferentially transduced after tail vein administration of AAV vectors.1 Therefore, transduction of this organ following challenge with rAAV8-CAGG-hIFNβ was assessed at a molecular level using a semi-quantitative PCR assay.35, 36 As shown in Figure 2B, the hFIX provirus was detectable in the liver of animals exposed to scAAV2/8-LP1-hFIXco with a rough correlation between the transgene copy number and plasma hFIX level in individual animals. Amongst the animals that received rAAV2/8-CAGG-hIFNβ as a secondary challenge, the hIFNβ transgene was only detected in the NDCD4 antibody+CyA cohort. The hIFNβ transgene copy number in these animals was similar (mean 2.2×10−3 copies/cell) to that observed in mice whose only exposure to AAV8 was rAAV8-CAGG-hIFNβ (3.7×10−3 copies/cell).
In all cohorts, the total anti-AAV8 antibody levels increased following administration of rAAV2/8-CAGG-hIFNβ (Figure 2C). The level of anti-AAV8 antibody titre in the NDCD4 antibody+CyA cohort was similar to that in mice exposed to rAAV2/8-CAGG-hIFNβ in the absence of immunosuppression. Therefore, these data suggests that transient immunosuppression with NDCD4 antibody+CyA combination at the time of primary exposure to AAV8 significantly attenuates humoral immunity to levels that allow efficient transduction upon re-exposure to vectors based on the same serotype (secondary challenge) in the absence of immunosuppression. Importantly, transient immunosuppression at the time of primary exposure does not lead to permanent tolerance to AAV capsid protein as illustrated by an increase in anti-AAV8 antibody titres upon secondary challenge. Indeed, when these mice where challenged again with AAV8 vector, this time encoding the human coagulation factor X (5×1011vg/kg scAAV2/8-LP1-hFX) gene, transduction was completely blocked as illustrated by an absence of detectable human FX (hFX) in plasma of animals transduced with (Figure 2D). Transgene expression mediated by the first (scAAV2/8-LP1-hFIX) and second (rAAV2/8-CAGG-hIFNβ) AAV vectors was, however, unaffected by the administration of the third vector in the NDCD4 antibody+CyA cohort of mice with steady state hFIX and hIFNβ levels of (2098±83ng/ml) and (11±4μg/ml) respectively.
Immunosuppression can block humoral immune response to wild type AAV2
In the next set of experiments the immunomodulatory properties of the NDCD4 antibody and CyA were further assessed in a more stringent assay; in the context of wt-AAV2 infection administered with adenovirus, to facilitate replication of wt-AAV as well as serving as an immunological adjuvant. 4×1012vg/kg/mouse of wt-AAV2 was administered into the tail vein of 6–8 week old, male, immunocompetent Balb/C mice either alone or in combination with an E1-E3 deleted adenoviral vector encoding GFP (rAd5-CMVGFP, 4×1012vg/kg/mouse). These animals were treated concomitantly with a combination of NDCD4 antibody and CyA along the schedule described before. As shown in Figure 3, the relative anti-AAV2 antibody titers were at least two fold higher in the cohort that received wt-AAV2 together with rAd5-CMVGFP when compared with mice that received wt-AAV2 alone. The concurrent administration of NDCD4 antibody and CyA at the time of primary infection with wt-AAV2 and rAd5-CMVGFP resulted in a 15 fold reduction in anti-AAV2 titer to values observed in negative control animals that had not been exposed to AAV2 or adenovirus. The anti-AAV2 capsid antibody titres determined by serotype specific ELISA correlated directly with the results of neutralizing antibody assay (data not shown). Hence, the combination of NDCD4 antibody and CyA also appears to be effective at attenuating humoral immunity to AAV capsid proteins in an environment which may facilitate replication of wt-AAV2 in addition to being associated with inflammation that invariable follows administration of early generation adenoviral vectors, neither of which occurs when using recombinant AAV alone.
Figure 3. Attenuation of immune response after wild type AAV infection.
Balb/c mice were treated with 4×1012/kg wt-AAV2 alone or with 4×1012/kg Ad GFP. 4 weeks latter anti-AAV2 Abs were measured by ELISA n=3 mean ±SEM.
Effect of NDCD4 antibody and Cyclosporine on humoral response to the transgene
We and others have previously shown that intramuscular administration of AAV2 or AAV5 vectors encoding hFIX is associated with a humoral but not a cytotoxic T cell immune response to the transgene.1,37,38 This has not been clearly shown with AAV8 vectors, which have distinct biological properties.14 Therefore, in the next set of experiments, 4×1011vg/kg of AAV vectors pseudotyped with serotype 5 encoding hFIX under the control of the constitutively active CAGG enhancer-promoter element (CAGG-hFIX) was administered into the hind limb muscle of Balb/C mice together with NDCD4 antibody and CyA along the schedule described in Figure 1. The positive control group consisted of animals transduced with AAV in the absence of immunosuppression. Within 4 weeks of intramuscular administration, animals transduced with AAV2/5-CAGG-hFIX in the absence of immunosuppression developed anti-hFIX antibodies (Figure 4C), which abrogated transgene expression (Figure 4A). These animals also had high titres of anti-AAV5 antibodies (115±4 RU/ml). In contrast, transient immunosuppression with a combination of NDCD4 antibody and CyA at the time of intramuscular administration of AAV2/5-CAGG-hFIX averted the generation of a humoral immune response to the hFIX transgene as well as the capsid protein (Figure 4B and 4C). Stable expression of hFIX at an average of 2% (111±9ng/ml) of normal levels was observed in this group of animals for the duration of the study (6 months). Since immune-competent mice are not tolerant to the non–species-specific hF.IX antigen we injected recombinant human FIX (Benefix 2500 IU/kg) into the muscle at 3 month after gene transfer to determine if peripheral tolerance can be broken. The anti-hFIX antibody titres in animals treated with rAAV2/5-CAGG-hFIX increased from 9.89±3 to 36±1 RU but remained unchanged in mice conditioned with a combination of NDCD4 antibody and CyA at the time of intramuscular administration of rAAV2/5-CAGG-hFIX (1.5±0.3 and 1.8±0.7 pre and post Benefix respectively). As described before, anti-hFIX antibodies were detectable in naive animals not treated with AAV.57 RT-qPCR analysis confirmed that the transcription of the transgene was limited to the muscle as the hFIX mRNA could not be detected in any other tissue including the liver. As with the intravenous route, repeat intramuscular administration of 4×1011vg/kg of rAAV-CAGG-hFIX vector pseudotyped with serotype 5 capsid protein at two months after primary exposure but this time without immunosuppression resulted in efficient transduction and a rise in plasma hFIX to an average of 3.6% (180±12ng/ml) normal level in the cohort of mice that received serotype 5 capsid pseudotyped rAAV concurrently with NDCD4 antibody and CyA but not in mice previously transduced with rAAV2/5-CAGG-hFIX vector alone.
Figure 4. Prevention of humoral response to transgene and capsid following intra muscular administration.
(A) Attenuation of humoral immunity to transgene after intra muscular delivery of 4×1011vg/kg rAAV2/5 CAGG hFIX. Transgene levels measured by ELISA at day 21 n=3 mean ±SEM. (B) Attenuation of humoral response to capsid after intra muscular administration of vector. Anti-AAV5 antibodies detected by ELISA at day 21 n=3 mean ±SEM. (C) Anti –hFIX antibody response is prevented with the use of transient immunosupression. hFIX antibody was measured by ELISA on day 21 n=3 mean ±SEM.
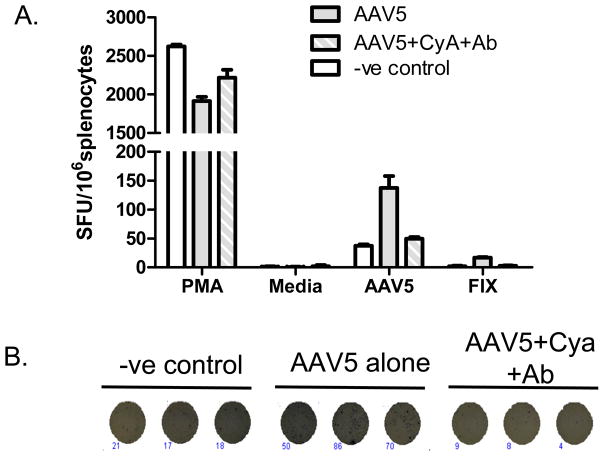
Importantly, there was no evidence of T cell infiltration in the muscle of any of the mice treated with rAAV2/5 CAGG-FIX, irrespective of whether they received immunosuppression. We next determined the frequency of AAV capsid-specific T cells in splenocytes using an IFN-γ Enzyme-linked immunosorbent spot (ELISPOT) assay following stimulation with whole AAV5 capsid for 24 hours. As a positive control, the cells were incubated with phorbol myristate acetate (PMA) whilst the negative control involved incubation of the splenocytes with medium alone. AAV capsid–specific T cells were not detected in mice that had not been exposed to AAV5 vector. In contrast, T cells reacting to AAV5 capsid were detectable at 137±20 SFU/106 lymphocytes following administration of AAV2/5-CAGG-hFIX. Transient immunosuppression with a combination of NDCD4 antibody and CyA at the time of intramuscular administration of AAV2/5-CAGG-hFIX substantially reduced the frequency of AAV capsid–specific T cells following AAV administration to levels observed in untransduced mice (Figure 5). As with AAV capsid, protein, hFIX reactive T cells were detectable above background levels in mice transduced with AAV5 without immunosuppression.
Figure 5. AAV capsid or hFIX specific T cells at 6 months after intramuscular delivery of rAAV2/5 CAGG hFIX.
A. Capsid (AAV5) or hFIX specific IFN-γ ELISpot; results are expressed in spot forming units (SFU)/106 splenocytes as average (± SD) of 3 replicates. Media = negative control; and PMA, positive control. B A representative ELISpot assay showing difference in the number of IFN-γ expressing AAV5 capsid specific T cells in splenocytes obtained from AAV untreated (-ve control) or AAV5 transduced animals with (AAV5+CyA+Ab) or without immunosuppression (AAV5 alone).
Discussion
Modulation of the immune response at the time of primary exposure to AAV has significant appeal for gene therapy of chronic disorders such as haemophilia. Our study demonstrates that a short exposure to NDCD4 antibody and CyA, at the time of vector administration, efficiently inhibited a primary humoral response to the AAV capsid proteins without any toxicity. This enabled efficient transduction following secondary challenge with vectors based on the same serotype to the level observed in mice not previously exposed to AAV8. Blockade of the humoral response, following transient immunosuppression, was independent of the route of vector administration (observed with both systemic and intramuscular route), AAV serotype, mouse strain or the concomitant administration of an early generation adenoviral vector capable of serving as an immunological adjuvant. Although further validation of these results in a context relevant to humans is essential, to our knowledge this is first illustration of efficient transduction of the liver in immunocompetent hosts following sequential administration of rAAV vectors based on the same serotype. This is highly relevant for somatic gene therapy with AAV of chronic disorders affecting the liver, such as haemophilia, as it offers an opportunity to improve expression with repeat administration of the vector of choice in the event that transgene expression declines over time due to the loss of the episomally retained rAAV genome as a result of the natural turnover of hepatocytes.
It is unknown based on our data if a short course of NDCD4 antibody and CyA would be sufficient to dampen the re-call cytotoxic T-cell response of the type recently observed in haemophilia B patients following liver targeted delivery of AAV-2.3 This phenomenon has not been satisfactorily reproduced in animal models making it difficult to critically evaluate the effectiveness of immunosuppressive strategy. However, our study shows that co-receptor blockade can reduce the number of capsid specific T cells present in the spleen and lymph node compartment. Furthermore, transient immunosuppression with NDCD4 antibody and CyA prevents the formation of neutralizing anti-hFIX antibodies (or inhibitors) following intramuscular delivery of rAAV. The development of neutralizing antibodies to the transgene following AAV mediated gene transfer in patients with severe haemophilia B would represent a serious adverse event that would render these subjects unresponsive to recombinant protein replacement therapy. Whilst subjects recruited to our ongoing Phase I/II gene therapy trials with AAV vectors will have attained a degree of tolerance to hFIX through years of exposure to recombinant protein, there is still a theoretical risk of provoking a neutralizing hFIX antibody response following gene transfer because endogenously expressed transgene product is processed in an immunological distinct manner when compared to infused protein concentrates.
This dual property of NDCD4 antibody and CyA combination gives it an important advantage over previous regimens containing Cyclophosphamide which block inhibitor formation without altering the immunological response to capsid proteins.39 Elegant studies by Miao and colleagues have shown that transient blockade of the inducible CD28/CTLA4 with a combination of CTLA4-Ig and an anti-murine CD40L antibody or single agent anti-inducible co-stimulator (ICOS) antibody resulted in long-term tolerance to human factor VIII after nonviral gene transfer into haemophilia A mice.40, 41 Since blockade of the co-stimulatory pathway has allowed successful readministration of adenoviral vectors, we would speculate that ICOS alone may be sufficient to dampen down the humoral response to capsid and transgenic proteins, but this needs to be formally established.25 Recently, Wang et al. demonstrated long-term expression of dystrophin in a canine model of muscular dystrophy when AAV-6 vector encoding the canine micro-dystrophin gene was administered intramuscularly together with 16 weeks of immunosuppression with a combination of anti-thymocyte globulin (ATG) CyA and MF. The effects of this protracted regimen on capsid mediated immune response are unclear but withdrawal of immunosuppression was followed by patchy T cell infiltration of the muscle.42 ATG has become an important component of transplant conditioning in humans but it causes prolonged lymphopenia, which is associated with an increased risk of infection. Liver targeted delivery of rAAV concurrent with a combination of MMF and sirolimus in nonhuman primates resulted in partial reduction in anti-AAV2 capsid antibody titre and prevention of inhibitors to hFIX.43 However, when daclizumab was added to this regimen the magnitude of the humoral response to the AAV2 capsid and hFIX proteins increased dramatically to levels that were higher than the cohort of macaques that received vector without immunosuppression due to depletion of the CD4+CD25+FoxP3+ regulatory T cells (Tregs). This indicates that careful selection of immunosuppressive agents is necessary.
The studies described in this report were designed to establish proof-of-concept that CD4 receptor blockade can result in a hyporesponsive/tolerant state to the viral capsid and transgenic proteins. The immunological mechanisms by which NDCD4 antibody exerts its effects have already been extensively studied by our group in a variety of different settings. 27, 29, 44–46 Based on these published data, we hypothesise that NDCD4 antibody mediated receptor blockade leads to induction of antigen-specific CD4+ regulatory T cells (T-regs) following rAAV-hFIX mediated gene transfer. Continuous expression of hFIX within the muscle bed allows the persistence of these T-regs and down-modulation of the activity of effector T cells resulting in tolerance to hFIX protein. In contrast, viral capsid proteins are present for a brief period after vector administration thereby reducing the scope for long-term tolerance through antigen mediated persistence of AAV capsid specific T-regs. This result is in fact the desirable outcome since long-term tolerance to an AAV vector would render patients unable to mount an appropriate immune response to infection by wt-AAV which is endemic amongst humans. An alternative explanation, suggested by recent studies, is that functional tolerance induced by antigens expressed in the muscle may result from up-regulation of the programmed death-1 molecule that leads to ignorance of CD4+ T cells and blockade of the cytotoxic function of antigen specific CD8+ T cells.47,48 Further studies are required to fully understand the mechanisms by which NDCD4 antibody coreceptors blockade achieves the hyporesponsive/tolerant state but these should be conducted in a context relevant to humans. NDCD4 antibody has been shown to be safe in healthy human volunteers but needs to be tested further in combination with CyA in relevant nonhuman primate models prior to use in the clinic.32 Our previous studies suggest that the chimpanzee and baboon models may be most suitable for these studies as they are the only non-human primate species to show binding affinity of NDCD4 antibody that is comparable to humans.31 These studies are warranted as our data in mice suggest that NDCD4 antibody combined with CyA is capable of attenuating immunological response to the vector capsid and transgenic proteins, which are currently the two major obstacles to stable transgene expression following AAV mediated gene transfer to the liver.
Materials and methods
AAV and adenoviral vector production and purification
The CAGG-FIX, CAGG-tsFlk and LP1-hFIXco plasmids have been described before.16, 35, 36 rAAV-hX consisted of the full-length 1.5kb human FX cDNA under the control of the LP1 promoter and flanked by AAV2 ITRs. The production of rAAV and scAAV vectors pseudotyped with capsid from alternative serotypes has been previously described. 14, 16 In brief, an adenoviral helper plasmid and chimeric AAV2 Rep-2Cap, -5Cap, or -8Cap packaging plasmids were used to generate AAV-2, -5, and -8, pseudotyped vector particles.49–51 The vector stocks were purified using column chromatography as previously described. 1, 52 Vector genome (vg) titres were determined by qPCR method using linear plasmid DNA as standards. The purified vector stocks were consistently free of contamination with wild type AAV and cellular and adenoviral proteins as judged by our previously described methods.52,1 Adenoviral vector rAd5-CMVGFP was prepared as described previously.53
Animal studies and Bio-assays
All procedures were performed under the authority of the UK Home Office Project and Personal Licenses regulations and in compliancewith the guidelines of the University College London ethical review committee. C57Bl/6 and Balb/c immunocompetent mice were obtained from Charles Rivers or Harlan Laboratories. Tail vein administration of 1AAV and adenoviral vector particles was performed as described before.14, 16 Blood samples were collected at regular intervals and assayed for the appropriate transgene (hFIX hFX or ts-Flk-1) by the ELISA. Methods for detection of hFIX and ts-Flk-1 have been described before.1, 54 Human FX levels in mouse plasma were assessed using a complement pair of goat anti-human FX (Quadratech, Epsom, UK). For intramuscular injections, the mice were anaesthetised with isoflurane and then AAV was injected at a maximum volume of 30μL/site directly into the quadriceps and tibialis anterior muscle, which were accessed through a 1cm incision in the hind limb. MMF (mycophenolate mofetil, Roche, Welwyn Garden City, UK) 20mg/kg/mouse cyclosporine (Sandimmune, Sandoz, Holzkirchen, Germany) 25mg/kg/mouse, human CTLA4-Ig 5mg/kg/mouse (Bristol-Myers Squibb, Princeton, NJ) and NDCD4 (YTS 177.9.6.3.3F4) 20mg/kg/mouse were injected intraperitoneally on days −1, 0, +1, +3, +6 and +8 with respect to AAV administration.46
Effect of immunosuppression on cell surface markers was evaluated using flow cytometry. In brief, citrated blood was treated with 4 volumes of cold lysis buffer (155mM ammonium chloride, 10mM sodium bicarbonate, 0.1mM EDTA) to lyse erythrocytes. The leucocytes were then washed in PBS and then stained with anti-mouse CD3 PE (clone 145-2C11 BD Bioscience) and CD4-FITC (YTS 3.1) for 1 hour before washing and subsequent analysis on the Epics-Elite flow cytometer (Beckman-Coulter, High Wycombe).
To determine rAAV transduction at a molecular level, 4 weeks after the 2nd AAV administration 2 mice were culled from each cohort. DNA was extracted from the liver. 250ng of DNA was subject to PCR for FIX (forward 5′ TTTCCTGATGTGGACTATGT reverse 5′TCATGGAAGCCAGCACAGAACATG), IFNβ (forward 5′CTGTTGTGCTTCTCCACTACAG reverse 5′GCCTTCAGGTAATGCAGAATCC) and β-actin (forward 5′TGACGGGGTCACCCACACTGTGCCCATCTA reverse 5′CTAGAAGCATTTGCGGTGGACGATGCAGGG).
An immunocapture assay was used to detect anti-AAV specific antibodies in murine plasma as described before.1 Results were expressed as the end-point titre, defined as the reciprocal of the interpolated dilution with an absorbance value equal to five times the mean absorbance background value. Neutralizing antibody titres were assessed by determining the ability of murine serum to inhibit transduction of 293T cells by pseudotyped scAAV vector containing the enhanced green fluorescent protein (GFP) cDNA under the control of the CMV promoter (scAAV CMV-GFP) as previously described.2,55 The neutralizing antibody titre is expressed as the dilution that inhibited transduction of 293T cells by 50%. hFIX antibodies were determined as described before. 1 The frequency of AAV capsid- or hFIX specific T cells in splenocytes was determined using an IFN-γ Enzyme-linked immunosorbent spot (ELISPOT) assay (R&D systems) according to the manufacturer’s instructions In brief, plates were blocked with media prior to loading between 1–5×105 splenocytes/well and then stimulated with rAAV2/5 CAGG-FIX vector at an MOI of 2×103, FIX 5μg/mL or PMA 1ng/mL for 24hrs at 37°C. Spots were counted with an Elispot reader (AID, Strassberg, Germany) with intensity >10, size>20 and gradient>5.
The probability of statistical differences between experimental groups was determined by one-way ANOVA and paired student t test using GraphPad Prizm version 4.0 software (GraphPad, San Diego, CA).
Acknowledgments
This work was supported by The Katharine Dormandy Trust, U.K., Medical Research Council, U.K., Department of Health’s NIHR Biomedical Research Centres funding award to UCLH/UCL, U.K, The ASSISI Foundation of Memphis, the American Lebanese Syrian Associated Charities (ALSAC), National Heart, Lung, and Blood Institute(NHLBI) grant HL073838
Footnotes
Conflicts/Disclosures
Hermann Waldmann and Stephen Cobbold are shareholders in TolerRx Inc. The other authors have no financial conflicts of interest to declare.
Reference List
- 1.Nathwani AC, Davidoff A, Hanawa H, Zhuo F, Vanin EF, Nienhuis AW. Factors influencing in-vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001 Mar;97(5):1258–1265. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- 2.Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver- targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002 Sep 1;100(5):1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- 3.Manno CS, Arruda VR, Pierce GF, Glader B, Ragni M, Rasko J, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006 Feb 12; doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 4.Flotte TR, Brantly ML, Spencer LT, Byrne BJ, Spencer CT, Baker DJ, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004 Jan;15(1):93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- 5.Ross CJ, Twisk J, Bakker AC, Miao F, Verbart D, Rip J, et al. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum Gene Ther. 2006 May;17(5):487–499. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- 6.Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008 May 22;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 8.Ali RR, Reichel MB, De Alwis M, Kanuga N, Kinnon C, Levinsky RJ, et al. Adeno-associated virus gene transfer to mouse retina. Hum Gene Ther. 1998 Jan 1;9(1):81–86. doi: 10.1089/hum.1998.9.1-81. [DOI] [PubMed] [Google Scholar]
- 9.Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors [see comments] Nat Med. 1999 Jan;5(1):64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 10.Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector [see comments] Nat Med. 1999 Jan;5(1):56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 11.Nakai H, Yant SR, Storm TA, Fuess S, Meuse L, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001 Aug;75(15):6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakai H, Wu X, Fuess S, Storm TA, Munroe D, Montini E, et al. Large-scale molecular characterization of adeno-associated virus vector integration in mouse liver. J Virol. 2005 Mar;79(6):3606–3614. doi: 10.1128/JVI.79.6.3606-3614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inagaki K, Lewis SM, Wu X, Ma C, Munroe DJ, Fuess S, et al. DNA palindromes with a modest arm length of greater, similar 20 base pairs are a significant target for recombinant adeno-associated virus vector integration in the liver, muscles, and heart in mice. J Virol. 2007 Oct;81(20):11290–11303. doi: 10.1128/JVI.00963-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y, et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5 and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. 11. 2005. pp. 875–888. [DOI] [PubMed] [Google Scholar]
- 15.Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Lozier JN, Hoffer F, et al. Sustained high level expression of human FIX following liver targeted delivery of recombinant adeno-associated virus encoding the human FIX gene in rhesus macaques. Blood. 2001;98(11):782a(Abstr.). [Google Scholar]
- 16.Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, et al. Self complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006 Apr 1;107(7):2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halbert CL, Standaert TA, Wilson CB, Miller AD. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998 Dec;72(12):9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther. 2010 Jan;18(1):126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathwani AC, Cochrane M, McIntosh J, Ng CY, Zhou J, Gray JT, et al. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2008 Aug 14; doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999 Sep;6(9):1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 21.Xiao W, Chirmule N, Schnell MA, Tazelaar J, Hughes JV, Wilson JM. Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors [In Process Citation] Mol Ther. 2000 Apr;1(4):323–329. doi: 10.1006/mthe.2000.0045. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Chirmule N, Gao G, Wilson J. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno- associated virus vectors In vivo: role of immature dendritic cells [In Process Citation] J Virol. 2000 Sep;74(17):8003–8010. doi: 10.1128/jvi.74.17.8003-8010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, et al. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol. 2000 May;74(5):2420–2425. doi: 10.1128/jvi.74.5.2420-2425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziller C, Stoeckel F, Boon L, Haegel-Kronenberger H. Transient blocking of both B7.1 (CD80) and B7.2 (CD86) in addition to CD40-CD40L interaction fully abrogates the immune response following systemic injection of adenovirus vector. Gene Ther. 2002 May;9(9):537–546. doi: 10.1038/sj.gt.3301684. [DOI] [PubMed] [Google Scholar]
- 25.Haegel-Kronenberger H, Haanstra K, Ziller-Remy C, Ortiz Buijsse AP, Vermeiren J, Stoeckel F, et al. Inhibition of costimulation allows for repeated systemic administration of adenoviral vector in rhesus monkeys. Gene Ther. 2004 Feb;11(3):241–252. doi: 10.1038/sj.gt.3302152. [DOI] [PubMed] [Google Scholar]
- 26.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003 Mar;48(3):719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 27.Waldmann H, Adams E, Fairchild P, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev. 2006 Aug;212:301–313. doi: 10.1111/j.0105-2896.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 28.Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007 Feb 15;109(4):1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. “Infectious” transplantation tolerance. Science. 1993 Feb 12;259(5097):974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 30.Bemelman F, Honey K, Adams E, Cobbold S, Waldmann H. Bone marrow transplantation induces either clonal deletion or infectious tolerance depending on the dose. J Immunol. 1998 Mar 15;160(6):2645–2648. [PubMed] [Google Scholar]
- 31.Winsor-Hines D, Merrill C, O’Mahony M, Rao PE, Cobbold SP, Waldmann H, et al. Induction of immunological tolerance/hyporesponsiveness in baboons with a nondepleting CD4 antibody. J Immunol. 2004 Oct 1;173(7):4715–4723. doi: 10.4049/jimmunol.173.7.4715. [DOI] [PubMed] [Google Scholar]
- 32.Ng CM, Stefanich E, Anand BS, Fielder PJ, Vaickus L. Pharmacokinetics/pharmacodynamics of nondepleting anti-CD4 monoclonal antibody (TRX1) in healthy human volunteers. Pharm Res. 2006 Jan;23(1):95–103. doi: 10.1007/s11095-005-8814-3. [DOI] [PubMed] [Google Scholar]
- 33.Zmonarski SC, Boratynska M, Madziarska K, Klinger M, Kusztel M, Patrzalek D, et al. Mycophenolate mofetil severely depresses antibody response to CMV infection in early posttransplant period. Transplant Proc. 2003 Sep;35(6):2205–2206. doi: 10.1016/s0041-1345(03)00764-4. [DOI] [PubMed] [Google Scholar]
- 34.Shaddy RE, Fuller TC, Anderson JB, Lambert LM, Brinkman MK, Profaizer T, et al. Mycophenolic mofetil reduces the HLA antibody response of children to valved allograft implantation. Ann Thorac Surg. 2004 May;77(5):1734–1739. doi: 10.1016/j.athoracsur.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin R, Khwaja A, Singh N, McIntosh J, Meager A, Wadhwa M, et al. Continuous delivery of human type I interferons (alpha/beta) has significant activity against acute myeloid leukemia cells in vitro and in a xenograft model. Blood. 2007 Feb 1;109(3):1244–1247. doi: 10.1182/blood-2006-02-002915. [DOI] [PubMed] [Google Scholar]
- 36.Streck CJ, Dickson PV, Ng CY, Zhou J, Gray JT, Nathwani AC, et al. Adeno-associated virus vector-mediated systemic delivery of IFN-beta combined with low-dose cyclophosphamide affects tumor regression in murine neuroblastoma models. Clin Cancer Res. 2005 Aug 15;11(16):6020–6029. doi: 10.1158/1078-0432.CCR-05-0502. [DOI] [PubMed] [Google Scholar]
- 37.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001 Sep;4(3):192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 38.Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang L, Herzog RW. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fields PA, Arruda VR, Armstrong E, Chu K, Mingozzi F, Hagstrom JN, et al. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther. 2001 Sep;4(3):201–210. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 40.Peng B, Ye P, Blazar BR, Freeman GJ, Rawlings DJ, Ochs HD, et al. Transient blockade of the inducible costimulator pathway generates long-term tolerance to factor VIII after nonviral gene transfer into hemophilia A mice. Blood. 2008 Sep 1;112(5):1662–1672. doi: 10.1182/blood-2008-01-128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao CH, Ye P, Thompson AR, Rawlings DJ, Ochs HD. Immunomodulation of transgene responses following naked DNA transfer of human factor VIII into hemophilia A mice. Blood. 2006 Jul 1;108(1):19–27. doi: 10.1182/blood-2005-11-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007 Jun;15(6):1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- 43.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007 Oct 1;110(7):2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamin RJ, Waldmann H. Induction of tolerance by monoclonal antibody therapy. Nature. 1986 Apr 3;320(6061):449–451. doi: 10.1038/320449a0. [DOI] [PubMed] [Google Scholar]
- 45.Qin SX, Wise M, Cobbold SP, Leong L, Kong YC, Parnes JR, et al. Induction of tolerance in peripheral T cells with monoclonal antibodies. Eur J Immunol. 1990 Dec;20(12):2737–2745. doi: 10.1002/eji.1830201231. [DOI] [PubMed] [Google Scholar]
- 46.Cobbold SP, Adams E, Marshall SE, Davies JD, Waldmann H. Mechanisms of peripheral tolerance and suppression induced by monoclonal antibodies to CD4 and CD8. Immunol Rev. 1996 Feb;149:5–33. doi: 10.1111/j.1600-065x.1996.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 47.Calbo S, Delagreverie H, Arnoult C, Authier FJ, Tron F, Boyer O. Functional tolerance of CD8+ T cells induced by muscle-specific antigen expression. J Immunol. 2008 Jul 1;181(1):408–417. doi: 10.4049/jimmunol.181.1.408. [DOI] [PubMed] [Google Scholar]
- 48.Lin SW, Hensley SE, Tatsis N, Lasaro MO, Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007 Dec;117(12):3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004 Jun;78(12):6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J, et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther. 2006 Jan;13(1):67–76. doi: 10.1016/j.ymthe.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Davidoff AM, Ng CY, Sleep S, McIntosh J, Azam S, Zhao Y, et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. 121. 2004. pp. 209–215. [DOI] [PubMed] [Google Scholar]
- 53.Nathwani AC, Persons DA, Stevenson SC, Frare P, McClelland A, Nienhuis AW, et al. Adenovirus-mediated expresssion of the murine ecotropic receptor facilitates transduction of human hematopoietic cells with an ecotropic retroviral vector. Gene Ther. 1999 Aug;6(8):1456–1468. doi: 10.1038/sj.gt.3300974. [DOI] [PubMed] [Google Scholar]
- 54.Davidoff AM, Ng CY, Zhang Y, Streck CJ, Mabry SJ, Barton SH, et al. Careful Decoy Receptor Titering is Required to Inhibit Tumor Angiogenesis While Avoiding Adversely Altering VEGF Bioavailability. Mol Ther. 2005 Feb;11(2):300–310. doi: 10.1016/j.ymthe.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Nathwani AC, Hanawa H, Vandergriff J, Kelly P, Vanin EF, Nienhuis AW. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38- subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Ther. 2000 Feb;7(3):183–195. doi: 10.1038/sj.gt.3301068. [DOI] [PubMed] [Google Scholar]
- 56.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, Arruda VR, High KA, Herzog RW. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003 May;111(9):1347–56. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]