Abstract
The endothelium plays a pivotal role in vascular homeostasis, regulating the tone of the vascular wall, and its interaction with circulating blood elements. Alterations in endothelial functions facilitate the infiltration of inflammatory cells and permit vascular smooth muscle proliferation and platelet aggregation. Therefore, endothelial dysfunction is an early event in disease processes including atherosclerosis, and because of its critical role in vascular health the endothelium is worthy of the intense focus it has received. However, there are limitations to studying human endothelial function in vivo, or human vascular segments ex vivo. Thus, methods for endothelial cell culture have been developed and refined. More recently, methods to derive endothelial cells from pluripotent cells have extended the scientific range of human endothelial cell studies. Pluripotent stem cells may be generated, expanded and then differentiated into endothelial cells for in vitro studies. Constructs for molecular imaging can also be employed to facilitate tracking these cells in vivo. Furthermore, one can generate patient-specific endothelial cells to study the effects of genetic or epigenetic alterations on endothelial behavior. Finally, there is the opportunity to apply these cells for vascular therapy. This review focuses on the generation of endothelial cells from stem cells; their characterization by genetic, histological and functional studies; and their translational applications.
Keywords: Endothelial cell, nuclear reprogramming, pluripotent stem cell, vascular regeneration, therapeutics development
Introduction
The endothelium is a delicate monolayer of cells lining the lumen of vessels. Apposed between the circulating blood elements and the vessel wall, it exerts substantial control over the cardiovascular system. The endothelium regulates immune cell entry into the vessel and the surrounding parenchyma by its expression of adhesion molecules and chemokines. Vasoactive factors secreted by the endothelium modulate vessel tone and blood flow. The proliferation and migration of vascular smooth muscle cells is also tightly regulated by the endothelium. Thus, the endothelium plays a critical role in vascular homeostasis. Metabolic, hemodynamic, inflammatory or age-related derangements of endothelial function initiate vascular disease, and permit its progression toward cardiovascular morbidity and mortality. Accordingly, there has been intense interest in understanding endothelial biology, ever since the recognition that it was more than a “mere cellophane wrapper lining the vessel wall”. 1, 2
Studies of human endothelial cell function in living human subjects are limited by the difficulty in directly accessing or imaging the cells. Nevertheless, useful biological and prognostic information has been obtained by innovative clinical investigators. For example, the endothelium synthesizes vasodilator factors, which are known to be released by increases in shear stress, to induce flow-mediated vasodilation 3, 4 Flow-mediated vasodilation can be visualized by ultrasonography or angiography;5, 6 is impaired by hypercholesterolemia and other metabolic perturbations; 7, 8 and is predictive of cardiovascular morbidity and mortality9, 10. Alternatively, one may measure biomarkers of endothelial cell function in the circulating blood (such as soluble adhesion molecules, which are increased with vascular inflammation and endothelial activation). Circulating endothelial cells and “endothelial progenitor cells” (EPCs) may be detected by fluorescent activated cell sorting (FACS).11, 12 The number of EPCs is reduced in patients with cardiovascular risk factors, and appears to be an independent predictor for mortality.13, 14 More recently, direct “endothelial biopsy” has permitted immunohistochemical and genetic techniques for single cell analyses.15 However, these approaches have severe limitations with respect to gaining molecular insights into endothelial biology, therefore methods for culturing endothelial cells in vitro have been developed and refined.
The development of endothelial cell culture 30 years ago dramatically accelerated our understanding of endothelial biology. This methodology facilitated molecular insights into the response of the endothelium to hemodynamic and humoral factors,4,16 as well as its activation by inflammatory cytokines and lipids.17, 18 Cell culture studies have revealed important insights into the endothelial regulation of vascular tone and structure; blood fluidity; vascular permeability; and angiogenesis. More recently, the availability of methods to derive endothelial cells from human pluripotent cells has extended the scientific range of human endothelial cell studies. One may genetically engineer the parental pluripotent stem cells i.e. either embryonic stem cells (ESC) or induced pluripotent stem cells (iPSCs), expand them, and differentiate them to endothelial cells for in vitro studies (i.e. ESC-ECs- or iPSC-ECs). Constructs for molecular imaging can be employed to facilitate tracking the cells in vivo. Furthermore, one can also generate patient-specific endothelial cells to study the effects of genetic or epigenetic alterations on endothelial behavior. Ultimately these cells may be utilized for therapeutic cell delivery applications. This review focuses on the generation of endothelial cells from stem cells; their characterization by genetic, histological and functional studies; and their possible applications.
Endothelial cell culture methodology
The quality of the science depends upon the quality of the cells. Accordingly, one must adhere meticulously to good cell culture practice (GCCP). The details of GCCP are discussed in depth elsewhere,19 but the general tenets as they apply to endothelial cell culture are worthy of brief review.
Authenticity
It is essential to regularly confirm the authenticity, as well as the stability, of the endothelial cell lines. Endothelial cells (ECs) should be regularly interrogated for the expression of characteristic functions, surface markers, and gene expression (described below). Careful recording of these features, and recording of growth conditions and population doublings, should be used to establish the optimal passage numbers and growth conditions for experimental protocols.
The derivation of the cells should be known, and their phenotypic and genotypic characteristics confirmed. It is of particular importance in the generation of new endothelial cell lines from pluripotent cells that the parental cells be well characterized. For those cells derived from patient materials, one should record key patient characteristics (e.g. age, gender, disorders, medications) and the tissue origin of the cells. Furthermore, the methods by which the parental cells were reprogrammed and differentiated to endothelial cells should be recorded in detail.
Standardizing culture conditions
The media and other reagents used in the cell culture should be well characterized, and standardized so as to reduce variability. However, although serum-free medias are available (e.g. Cell Applications, Genlantis) endothelial media usually contains 10% fetal calf serum. Because of lot-to-lot variability and incomplete characterization of serum and other animal products, it is important to evaluate each new batch in parallel with the existing batch. Additionally, commercial endothelial growth media are typically supplemented with defined growth factors to promote proliferation and maintenance of phenotype [i.e. vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1)] or undefined growth factors derived from bovine neural tissue. The media may also contain other chemical factors (e.g. ascorbic acid, heparin, hydrocortisone).
Passaging endothelial cells
Even under pristine conditions, cells undergo phenotypic and genotypic changes in culture. Primary cell lines have a limited number of population doublings (usually no more than 50–60 doublings but often less), a phenomenon known as the “Hayflick limit”. At the Hayflick limit, cells enter a senescence phase where the cells have lost the ability to divide because their telomeres have shortened to a critical length. However, the signs of senescence are often present before this limit is reached. Endothelial cells during senescence will lose their cobblestone morphology and assume a “fried egg” morphology, replicate more slowly, have greater expression of oxidant-sensitive genes such as adhesion molecules and chemokines, and have reduced endothelial function (i.e. reduced expression of endothelial nitric oxide synthase, eNOS).20 The investigator must be familiar with these changes over time, and restrict their studies to endothelial cells in a range of passage numbers (more specifically, population doublings) where the experimental protocol will be unaffected by senescence.
The phenotypic alterations occurring over time in cultured cells are in part due to the artificiality of the culture system. Cells grown on polystyrene (even that coated with a thin layer of matrix proteins) experience a substrate rigidity that is many orders greater than that of tissue (i.e.. polystyrene has a Young’s Modulus of 106 kPa, in comparison to physiological soft tissues which are 2–50 kPa). Culturing cells on soft hydrogels, which possess biomimetic physicochemical properties has been shown to maintain some phenotypic properties, e.g. muscle stem cells retain their ability for asymmetric division and replication, and undergo less cell death.21 Preliminary studies from our laboratory indicate that more tissue-like substrate rigidity can favorably influence the maintenance of endothelial phenotype (data unpublished).
Immortalized cell lines can be sub-cultured almost indefinitely. However, such cells are often derived from malignancies; from viral transformation; from treatment with mutagens; or by genetic modification (e.g. with SV40 large T-antigen or telomerase). For example, the HMEC-1 line is a line of human microvascular dermal endothelial cells that was immortalized by transfection with a region of the simian virus 40 gene product, large T antigen. These cells can be passaged for at least 95 passages without undergoing senescence, where as normal microvascular dermal endothelial cells enter senescence after only 8–10 passages.22 However, because of their genetic and epigenetic alterations, the behavior of immortalized cell lines may be less representative of cells in vivo, and new scientific insights using these cells should be confirmed with primary cell lines.
Care and handling of ECs
Standard operating procedures for cell culture should be established. Care should be taken not to expose the cells or tissues to inappropriate conditions (e.g. excessive time out of the incubator). The optimal culture temperature and oxygenation should be established. Lower temperatures (less than 34°C) may slow growth, but higher temperatures (greater than 38°C) may favor apoptosis. Traditionally, endothelial cell cultures have been carried out in 95%O2/5%CO2, but a more appropriate oxygen tension maybe in the physiologic range of 5–20%.23 The optimal pH range (~pH 7.4) for endothelial cell culture is narrow.
To obtain single-cell suspensions of endothelial cells we have found that the use of TrypLE Express (Invitrogen), a recombinant fungal trypsin-like protease that is temperature-stable, induces less cell death than other approaches.
Cryopreservation permits endothelial cell lines to be stored for prolonged periods, but care must be taken during the processes of freezing, storage and recovery. We have good results when we harvest cells for storage during exponential growth stage, and use 10% v/v DMSO in 10% fetal bovine serum as a cryoprotectant. Commercial freezing media contains proprietary components but are designed to increase the recovery of cells upon thawing. Cells should be gradually exposed to the freezing media to minimize osmotic shock. Upon reconstitution in freezing media, the cells should be cooled at 1 degree per minute in cell freezing containers stored at −80°C. After 4–24h, the vials can be transferred to liquid nitrogen. During transfer, care should be taken to avoid exposing the frozen vial to room temperature, as the vial’s temperature may change rapidly even within only 1 minute.
The cells should be free of biological contamination (other cells, pathogens such as mycoplasma, or endotoxins). Commercially purchased cells are commonly prescreened for biological contamination, but primary isolated cells are at greater risk for biological contamination. Endotoxin levels can be quantified using a limulus amebocyte lysate colorimetric assay. Myocoplasma levels can be quantified using commercial kits that detect mycoplasma RNA or DNA. Mycoplasma is a common contaminant. This bacterium from the class of Mollicutes competes with eukaryotic cells for nutrients. Mycoplasma contamination can alter cellular function such as proliferation rates, and NO signaling, and cans introduce genetic aberrations.24 Once mycoplasma is detected, it can be eliminated by treatment of anti-mycoplasma reagents or antibiotics. However, it may be more prudent to simply discard the infected cells, and carefully decontaminate the incubator and other equipment that has been in contact with the cells.
Genetic, histological and Functional Confirmation of endothelial lineage
Immunohistochemical and genetic characterization
EC characterization by immunocytochemistry
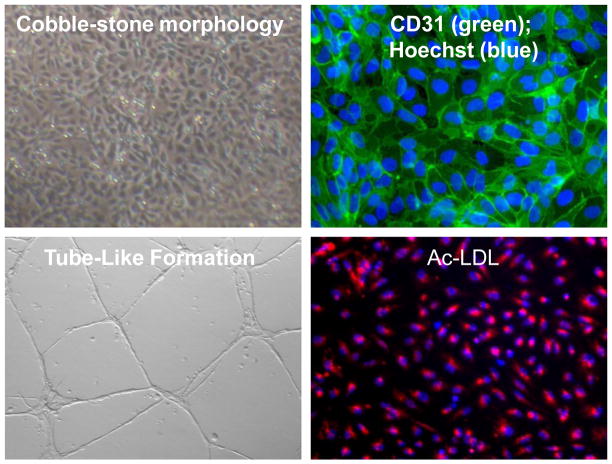
To document endothelial lineage, no one immunohistochemical marker is definitive. Rather, one should utilize a set of endothelial markers such as PECAM-1 (CD31, Figure 1), VE-cadherin (CD144), endothelial nitric oxide synthase (eNOS), von Willebrand factor (vWF) and fetal liver kinase-1 (Flk-1, also known as kinase insert domain receptor, KDR, or vascular endothelial growth factor receptor 2, VEGFR-2). Briefly, the cells are fixed with paraformaldehyde (4%), permeabilized with Triton X-100 (0.1%) and blocked with either normal goat or donkey serum (1%) for 30 minutes, followed by overnight incubation with the primary antibodies at 4°C. The cells are washed with 1× PBS and incubated with secondary antibodies (i.e. Alexa Fluor-488 or -594) for 1 hour at room temperature. Nuclei may be stained with Hoechst 33342 dye and the cells examined by fluorescent microscopy. However, since none of the histological markers alone are specific to ECs, functional assays are required to confirm EC lineage.
Fig 1.
Endothelial cell characteristics. Human induced pluripotent stem cell derived endothelial cells (iPSC-ECs) have a characteristic endothelial cell cobble-stone morphology, express the endothelial marker CD31, form tube-like structures in Matrigel and incorporate acetylated LDL (ac-LDL).
Functional assays
ECs Synthesize and Release NO
ECs regulate blood flow and blood pressure by releasing the endothelium-derived relaxing factors including nitric oxide (NO) and prostacyclin. These endothelium-derived factors are critical for maintaining vascular homeostasis.25 The detection of NO radical in biological samples has been done using a porphyrin electrode, or by spin trapping of NO combined with electron paramagnetic resonance (EPR).26 These are technically difficult due to the short half-life and low concentration of these radicals. The oxidized degradation products, nitrite and nitrate, are stable and can be measured by chemiluminescence after reacting the sample in a reduction chamber to regenerate NO and react it with ozone.26, 27 However, the most popular and simple method, albeit less sensitive, is to use the fluorimetric Griess reaction assay to measure total nitrates and nitrites.28, 29 In addition, there are NO fluorescence dye indicators including the 4,5-diaminofluorescein (DAF-2) dye, but these are not satisfactory due to non-specific reactions, including with ascorbyl radical in the culture medium.30–32 Improved NO probes for intracellular detection are under development, but are not yet commercially available.33 As an alternative indicator of bioactive NO production, the amount of cyclic Guanosine Monophosphate (cGMP), a secondary messenger of NO, may be measured.
EC Activation
Endothelial cells are ‘activated’ by inflammatory cytokines such as TNF-alpha in vitro, which induces the transcription of cell adhesion molecules (such as ICAM-1) involved in the recruitment of leukocytes.34, 35 This activation process may also be involved in the homing of highly proliferative endothelial progenitor cells and thus is important in both neovascularization in regions of ischemia or re-endothelialization of areas where the endothelium is impaired.34, 35 A monocyte-endothelial cell adhesion assay can be used to quantify EC activation. Briefly, ECs are washed with Hanks balanced salt solution (HBSS) containing 2 mmol/L Ca2+, 2 mmol/L Mg2+ and 20 mmol/L HEPES. Human monocytoid cells (THP-1) are washed and added to the EC monolayer at a final concentration of 2×106 cells/mL. The dish with ECs and monocytes is placed on a rocking platform and rotated frequently to ensure even distribution of monocytes. After 30 minutes, the medium is removed and ECs are washed with fresh HBSS to remove nonadherent monocytes. Cells are fixed (2% glutaraldehyde in HBSS), stained (CD14 antibody is used to visualize the monocytes) and adherent monocytes are counted by microscopy.20, 36
Ac-LDL Uptake by EC
Normal ECs can take up acetylated low-density lipoprotein (ac-LDL, Figure 1). The uptake of ac-LDL serves as a commonly used marker for the identification of EC. For acetylated LDL uptake assay, cells are incubated with Dil-labeled Ac-LDL (10 μg/ml; Invitrogen) for 4 hours at 37°C. After incubation, the cells are visualized and photographed under a fluorescence microscope.
Angiogenesis and Vasculogenesis assays
New blood vessels arise by two general mechanisms during development, vasculogenesis and angiogenesis. Vasculogenesis involves the generation of new vascular networks by incorporation of circulating progenitor cells. Angiogenesis is the formation of new vessels by sprouting from existing vessels. Both angiogenesis and vasculogenesis require EC proliferation and migration. The ability of EC to form tube-like networks in vitro has been employed widely to identify ECs, and to assess vasculogenic and angiogenic potential (Figure 1). Notably, other cell types can form networks in matrigel, but only endothelial cells are capable of forming tubes with lumens. For the in vitro Matrigel assay, cells (2.5 × 105) are seeded on 24-well plates pre-coated with growth factor-reduced Matrigel and incubated for 24 hours at 37°C to induced tubular network formation. In order to determine if these ECs form functional blood vessels in vivo, a Matrigel plug assay is performed using immunodeficient NOD SCID mice, if using human ECs. Matrigel is mixed with bFGF (50 ng/ml) and ECs (5×105). The mixture is subcutaneously injected into the mice. After 14 days, the Matrigel plugs are removed, paraffin-embedded, sectioned and stained with CD31. Capillaries can be quantified using fluorescent microscopy; if human ECs were used human and mouse specific EC antibodies help to determine the contribution of the human ECs in the matrigel network.
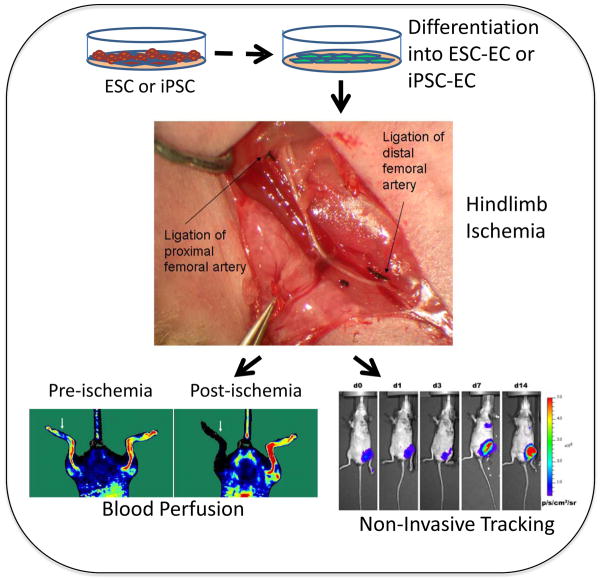
Furthermore, we also assess the functionality of pluripotent stem cell derived endothelial cells (ESC-ECs and iPSC-ECs) using the mouse hindlimb ischemia model (Figure 2). Unilateral hindlimb ischemia is induced by ligating the femoral artery of (4–6 months) male NOD SCID mice.34, 35 The cells are then delivered, for example by intramuscular (IM) injections into the ischemic limb, and perfusion of the ischemic and non-ischemic hindlimb is assessed over the next 4 weeks using laser Doppler.34, 35
Fig. 2.
Assessing the functionality of pluripotent stem cell derived endothelial cells using the mouse hindlimb ischemia model. Unilateral hindlimb ischemia is induced by ligating the femoral artery. Cells are injected intramuscular and perfusion in the ischemic and non-ischemic hindlimb is assessed using laser Doppler. Additionally, Non-invasive tracking may be utilized to investigate homing of the cells to the ischemic tissue. (Images are reproduced with permission from Niiyama et al, J Vis Exp, 2009).
EC Gene Expression Profiles
There is limited information about the gene expression profiles of the ECs derived from stem cells and the native ECs. Li et al compared the gene profiles of human iPSC-ECs or ESC-ECs, and human umbilical vein endothelial cells (HUVECs) using whole genome microarray.37 The results suggested that gene expression variation of iPSC-ECs and ESC-ECs may contribute to biological differences between iPSC-ECs and ESC-ECs as compared to HUVECs. For iPSC-ECs, it is important to note that somatic memory does exist38–40 which may be useful to explain why iPSC-ECs have slower growth rate and faster loss of endothelial phenotype then ESC-ECs or native ECs. Nevertheless, a better understanding of the gene expression profiles that guide the development and differentiation of iPSC would facilitate methods to improve the derivation of high fidelity iPSC-ECs.
Deriving Endothelial Cells from Pluripotent stem cells
Rationale
Successful derivation of endothelial cells from human pluripotent stem cells has opened up new opportunities for understanding endothelial biology. Researchers are now able to genetically engineer the parental cells into pluripotent stem cells, expand them, and differentiate them to endothelial cells for in vitro studies. These cells can be tracked during in vivo studies by employing constructs for molecular imaging. Similarly, patient-specific endothelial cells can be generated to study the effects of genetic or epigenetic alterations on endothelial behavior. More importantly, the ability to derive large numbers of endothelial cells from parental pluripotential stem cells increases the feasibility of cell therapy applications.
Types of stem cells
Stem cells are capable of self-renewal and directed differentiation. Before the introduction of induced pluripotent stem cells (iPSCs) in 2006, two broad categories of stem cells had been classified: embryonic stem cells (ESCs) and adult stem cells. ESCs are pluripotent stem cells derived from the inner cell mass of the fetal blastocysts. They are able to differentiate into all derivatives of the three germ layers: ectoderm, endoderm and mesoderm. In contrast to ESCs, adult stem cells are already partially committed to a lineage, and can thus produce only a limited number of cell types. For example, hematopoietic stem cells are adult stem cells that are multipotent and can give rise to all types of blood cells but are not able to produce cells of endodermal or ectodermal lineage. However, by comparison to adult differentiated cells, adult stem cells have greater capacity for proliferation and ability to repopulate or repair tissue.
Deriving ECs from Embryonic Stem Cells
ESCs are pluripotent cells derived from the inner cell mass of the blastocyst. Human and murine ESCs characteristically express pluripotency markers such as Oct3/4 and Nanog transcriptional factors. However, human and murine ESCs express species-specific markers as well. For example, murine ESCs express epithelial cadherin (E-cadherin) and stage-specific extracellular antigen-1 (SSEA-1), whereas human ESCs express SSEA-3/4 and TRA-1-60. Human stem cell lines were first established by Thomson and colleagues in 1998.41 Since that time, intensive efforts have been made at methods for directed differentiation and isolation to obtain pure cell populations without contamination of undifferentiated cells, which can give rise to teratomas or tumors consisting of tissue derived from the ectoderm, endoderm and mesoderm germ layers.
General Considerations of Microenvironmental Conditions
Soluble factors represent the most conventional method to induce endothelial differentiation of pluripotent stem cells. Among the variety of cytokines and growth factors, VEGF appears to play a critical role during the differentiation of ESCs into ECs. VEGF at 50 ng/ml greatly increases the yield of CD31+ cells from mouse ESC-derived embryoid bodies (EBs).42 VEGF is required for the proliferation of mouse ESC-derived vascular endothelial growth factor receptor 2 positive progenitor cells.43 VEGF also dose-dependently enhances the formation of EC colonies and plays a critical role in the determination of arterial and/or venous fate of the ECs.43,44 During the differentiation of Flk1+ sorted-cells, high VEGF concentrations can promote up-regulation of arterial markers while lower concentrations of VEGF induce venous marker expression.44
Apart from growth factor- or cytokine- mediated endothelial differentiation, extracellular matrix (ECM) plays a key role in angiogenesis and vasculogenesis through supporting adhesion, migration, proliferation, and survival of ECs.45 Among those ECM molecules, gelatin, fibronectin, and collagen type I and IV have been demonstrated to support ESC differentiation into mesodermal Flk1+ cells which have the potential to become hematopoietic cells and ECs.46 These Flk1+ sorted cells were able to give rise to ECs that could generate endothelial type tube-like formation.47 In addition, laminar shear stress can influence the differentiation of ESC into ECs through the PI3K-Akt pathway.48 Stimulation of murine ESCs with laminar shear stress (15 dyn/cm2) enhanced their differentiation into Flk1 expressing cell. 49 Furthermore, exposure of Flk1+ cells to shear stress (5 dyn/cm2) promotes differentiation into CD31+ ECs.50
Murine ESCs to ECs differentiation
Mouse ESCs spontaneously differentiate into endodermal, mesodermal, and ectodermal lineage upon removal of leukemia inhibitory factor (LIF) from culture.51 Additionally, mouse ESCs can spontaneously differentiate to form three-dimensional clusters of cells called embryoid bodies (EBs) when they are cultured in suspension. The hemangioblasts are a precursor population of cells within the EBs that can give rise to hematopoietic cells and ECs.52–54 Thus, the ESC can be directed to differentiate into vascular endothelial cells (ESC-derived ECs).55–57
EB approach to generating ECs
We have previously demonstrated that murine ESCs can be differentiated into endothelial lineage by dissociating the ESC colonies into single cells using TrypLE Express and then plating the cells onto ultra-low adhesion dishes in a differentiation media consisting of Alpha Minimum Eagle’s Media, 10% serum and β-mercaptoethanol.35 In suspension culture, the murine ESCs form floating aggregates known as EBs. After 4 days of suspension culture, the EBs are then allowed to attach onto 0.2% gelatin-coated dishes and cultured for another 10 days in differentiation media. Flk1 is expressed at day 3 of differentiation while late-stage endothelial markers VE-cadherin and tyrosine kinase receptor 1 (Tie1) are detected after day 5 of differentiation.42 Once the EBs are seeded on the 2-dimensional tissue culture plates, EC formation can be observed at the borders of the EB colonies.53 After 3 weeks of differentiation, the murine ESC-derived ECs can be purified by FACS using their expression of vascular endothelial cadherin (VE-cadherin). We prefer to use VE-cadherin rather than CD31 for the purification of murine ECs, because murine ESCs are known to express low levels of platelet-endothelial cell adhesion molecules (CD31) even in the pluripotent state.58
2D culture of mESCs to derive ECs
ECs can be differentiated in the absence of EB formation by using two-dimensional culture systems. Yamashita reported the differentiation of murine ESCs into ECs using VEGF and collagen IV-coated dishes.47 Murine ESCs were differentiated in differentiation media consisting of α-Minimum Eagle’s Medium, FBS (10%), and β-mercaptoethanol (0.05 mmol/L) on collagen IV-coated dishes for 4 days before purification of Flk1+ and E-cadherin- mesodermal progenitors. The sorted cells were then grown on collagen IV-coated dishes in differentiation media supplemented by VEGF. Using the same differentiation media, Narazaki et al. differentiated murine iPSCs on collagen IV-coated dishes for 4 days before FACS-sorting vascular progenitor cells using Flk1+.59 Upon purification, the Flk1+ cells were then cultured on collagen IV-dishes in differentiation media supplemented with VEGF (100 ng/ml) and 8-bromoadenosine-3′:5′-cyclic monophosphate (8-Br-cAMP, 0.5 mmol/L) for an additional 3 days before further characterizing for endothelial phenotypic markers. McCloskey et al. reported a modification of this procedure in which murine ESCs were first differentiated on collagen IV-dishes in the same differentiation media.60 After 4 days the cells were purified for Flk1+ expression by FACS and plated onto collagen IV-coated dishes in differentiation media supplemented by VEGF for one week, producing ECs with cobblestone morphology. However in our experience, a differentiation period of 3 weeks yielded higher efficiency of ECs when compared to shorter time periods of differentiation.
An important consideration when designing this type of differentiation protocol is that cell density appears to influence EC differentiation. For instance, when Flk1+ progenitor cells from mouse ESCs were isolated and plated at a low density, the cells failed to differentiate into ECs.43 We have also observed that EC differentiation is dependent on the cell density, suggesting that cell-cell interaction plays an important role in differentiation. Based on these findings, a defined cell seeding density of 10,000 cells/cm2 is used to induce endothelial differentiation after the selection of Flk1+ progenitor cells from mouse ESCs.47
Differentiation to EC in semi-solid culture or on stromal cells
Another differentiation approach involves semisolid culture. Using this method, murine ESCs are cultured in differentiation media composed of Iscove modified Dulbecco Medium (IMDM), 1% methylcellulose, 15% FBS, 2 mmol/l glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin, 100 μM monothioglycerol, 1 × BIT (BSA, insulin, transferrin). When cultured in this semisolid environment for 11 days, the ESCs can form aggregates that can be directly transferred into collagen gels to facilitate sprouting of vascular networks. Furthermore, the aggregates can be dissociated for purification by FACS, or studies by conventional qPCR or immunocytochemical staining.
Coculture of murine ESCs with OP9 stromal cells have also been shown to promote endothelial differentiation. OP9 stromal cells are derived from murine bone marrow. These cells have been immunophenotyped to be positive for antigens associated with mesenchymal stem cells such as CD29 and CD44, while being negative in hematopoietic markers CD34 and CD45.61 When murine ESCs or iPSCs are cocultured with OP9 in the presence of an angiopoietin-1 variant, the pluripotent cells efficiently differentiate into Flk1+ mesodermal precursors.62
Differentiation efficiencies
The reported efficiency of differentiation protocols varies considerably. However it is difficult to compare the efficiencies of the various differentiation protocols due to the fact that different starting cells and markers are employed at different time points using different technologies. That being said, the reported efficiencies for yielding ECs with the EB formation method are 16% in mESCs35, and range from 10–50% in hESCs63–65. The OP9 co-culture method yields 35% ECs from mESCs62, 7–13% from hESCs66, 67, and 16% from hiPSCs67. The collagen IV method yielded 15–39% in mESCs47, 59, and 14% in miPSC-ECs59.
Characterization of ESC derived endothelial cells
These ESC-derived ECs (ESC-ECs) are validated to be of EC lineage based on the multiple surface markers, gene expression and functional studies as described above.42 Subsequently, the cells can be further characterized in vivo, by their ability to incorporate into the vasculature of the ischemic limb or myocardium.68–70 We tracked the fate and function of transplanted ESC-ECs in the ischemic murine myocardium.35, 71 Murine ESCs were first transduced with a construct that encoded luciferase (for bioluminescence imaging) and red fluorescent protein (for histological tracking). After the ESCs differentiated into ECs, the ESC-ECs or vehicle were injected into the ischemic area of the left ventricle after ligation of the left anterior descending coronary artery. Bioluminescence imaging showed that the ESC-ECs survived up to 8 weeks, and echocardiography revealed improved systolic function in the hearts injected with ESC-ECs. Histological studies revealed increased myocardial capillary density in the hearts treated with cell therapy. Additionally, we have shown the ESC-ECs engraft into ischemic hindlimbs (a murine model for peripheral artery disease, Figure 2) and restore perfusion in the leg.35 These studies suggest that ESC-EC may have a therapeutic effect in the treatment of ischemic vascular diseases.
Human ESCs to ECs Differentiation
Human ESCs exhibit a number of differences from murine ESCs, such as their independence from LIF and dependency on basic fibroblast growth factor (bFGF) for maintenance of self-renewal. However, Levenberg et al. reported human ESCs can be differentiated spontaneously through EB formation in the absence of bFGF.72 After 10–15 days of differentiation, these human EBs gave rise to various lineages including CD31+ ECs. When the CD31+ cells were purified from the EBs by FACS, about 80% purity as achieved.72 As a modification of this protocol, human ESCs were grown in suspension as EBs in the presence of Iscove’s modified Dulbecco’s medium, 20% defined FBS and 450 mmol/l monothioglycerol.63 After 4 days, the EBs were reattached to gelatin-coated dishes in the presence of endothelial cell growth medium-2 that contained numerous growth factors including VEGF and epidermal growth factor (EGF). After 12 days of differentiation, the EB outgrowths were FACS purified based on CD31 expression. This method yielded 12% CD31+ cells, and upon expansion and repurification, the cells were 97% CD31+.
Using a more directed approach mimicking the growth factor induction signals during cardiovascular development, Yang et al. demonstrated a stepwise differentiation protocol yielding endothelial, cardiac, and smooth muscle lineages from the same precursor stem cell.65 The human ESCs were differentiated in the presence of bone morphogenetic protein (BMP4) on day 0; BMP4, bFGF, and activin during days 1–4; Wnt inhibitor DKK1 and VEGF from days 4–8, and VEGF, DKK1, and bFGF afterwards.
The derivation of endothelial cells from hESC via different culture conditions include hESC-induced EB formation grown on gelatin-coated plates64, 65, 72–75, hESC grown on bone marrow stromal OP9 co-culture 66, 67, 76, hESC grown on collagen IV- coated plates77 and hESC-induced EBs grown on methylcellulose78. Although the differentiation of human ESCs to endothelial cells have been achieved using a variety of differentiation protocols, methodologies involving cytokines/ growth factors and ECM environments need to be further explored and refined.
Induced Pluripotent Stem Cells as a source for ECs
Generation of induced pluripotent stem cells (iPSC)
In 2006, Yamanaka and colleagues79 demonstrated that mouse fibroblasts could be reprogrammed into iPSCs by introduction of four transcriptional factors including octamer-binding transcription factor-3/4 (Oct 3/4), SRY-related high-mobility-group (HMG)-box protein-2 (Sox2), Krüppel-like factor 4 (Klf4) and c-Myc. 79, 80 The induced expression of these four transcriptional factors activates a network of genes required for pluripotency, and gradually transforms the somatic cells into pluripotential stem cells. These cells exhibit similar morphological and growth patterns compared to ESCs and they have the potential to differentiate into lineages of the three germ layers81–84. In 2007, Thomson and colleagues85 used Oct 3/4 and Sox2 together with Nanog and Lin28 as reprogramming factors to generate iPSCs. The development of iPSCs has opened up a new avenue for cardiac and vascular regeneration as well as the opportunity to understand the differentiation of endothelial cells from pluripotent stem cells.13,86,87
Differentiating iPSCs to ECs
iPSCs are capable of differentiating into all cardiovascular cells including ECs, vascular mural cells, smooth muscle cells,88 and cardiomyocytes.55, 59. Generally, approaches for differentiation of human or murine ESCs can be applied also in the differentiation of human iPSCs (Figure 3). We differentiate human iPSCs after dissociating the colonies (with type IV collagenase for 10 min) and then transferring iPSCs to ultra low attachment dishes containing differentiation media for 4 days to form EBs. The differentiation media consisted of α-Minimum Eagle’s Medium, FBS (20%), β-mercaptoethanol (0.05 mmol/L), non-essential amino acids (1%), BMP4 (50 ng/ml) and VEGF-A. The 4-day EBs were then seeded on 0.2% gelatin-coated dishes and cultured for another 10 days in differentiation media in the absence of BMP-4. Differentiation media was changed every 2 days. To purify the pluripotent stem cell derived ECs, single cell suspensions were obtained using accutase for 20 minutes at 37°C to dissociate differentiated cells, which were then washed with 1× PBS containing 5% BSA, passed through a 70-μm cell strainer and incubated with PE-conjugated anti-human CD31 antibody and/or anti-VE-cadherin antibody for 30 minutes. Isotype-matched antibody served as negative control and 1% propidium iodide was used to stain the non-viable cells. Then FACS purified ECs were expanded in EGM-2MV. Currently, our methodology yields 10–20% VE-cadherin+/CD31+ cells, which can be purified to 75–90% with a second FACS.64
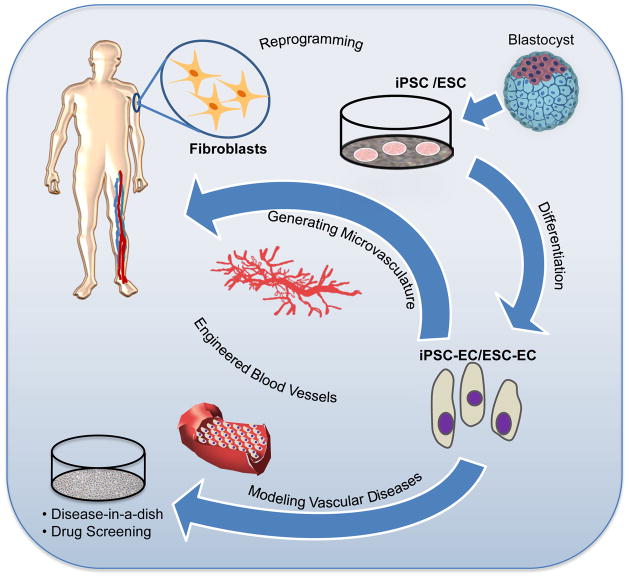
Fig. 3.
Potential therapeutic applications of endothelial cell derivation. An immediate application of iPSC technology is to derive vascular cells from patients with vascular disease, which may be useful for drug screening for vascular disease therapeutics. ECs derived from pluripotent stem cells may be used in the development of vascular grafts and engineered blood vessels. Additionally, in the future iPSC-ECs may be used for vascular regeneration though the injection of these cells directly into ischemic tissue.
Direct reprogramming as a source for ECs
The ability to generate iPSCs has already had practical applications in the development of disease-specific cells for understanding mechanisms of disease, and in the production of iPSC derived cell lines for drug screening. Although there is promise for the application of iPSC-derived cells for therapy, there are major limitations, including the risk of teratoma, the difficulty in differentiating high-fidelity therapeutic cells, and the prolonged process which currently takes months. Direct reprogramming, from one somatic cell type to another desired somatic cell type, is thus an attractive alternative approach. For example, fibroblasts have been converted to neurons89, and to cardiomyocytes90. Typically, a handful of genes, typically known to be involved in development of the desired cell type, are overexpressed in the fibroblast for transdifferentiation. A recent paper by Margariti et al91 converted fibroblasts to ECs using the Yamanaka factors and culture conditions favoring endothelial cell growth.
Heterogeneity of the endothelial cells derived from nuclear reprogramming
There is heterogeneity of endothelial structure and function in vivo, that is not generally appreciated, nor fully maintained in cell culture. In vivo, the phenotype of the endothelial cell is influenced by the developmental program (eg. lymphatic, venous or arterial), the surrounding parenchyma and its metabolic activity (eg. the elaboration of angiogenic cytokines by tumor cells), as well as hemodynamic and humoral factors. Endothelial heterogeneity is necessary to support specialized tissue function. For example, the endothelium of the cerebral microvasculature forms tight cell junctions and is far less permeable than the lymphatic endothelium which is fenestrated. In the former case, the endothelium protects the brain from shifts in interstitial fluid that could adversely effect intracranial pressure, whereas in the latter case, the increased permeability of the lymphatic endothelium subserves transport of interstitial protein back to the systemic circulation. One manifestation of endothelial heterogeneity is the elaboration of different surface proteins, that have been termed vascular “zip codes”. The remarkable heterogeneity of endothelial surface markers have been well demonstrated by phage display studies.92 The functional relevance of this heterogeneity is illustrated by the expression of specific adhesion molecules that facilitate monocyte entry at sites of disturbed flow in the aorta whereas different adhesion molecules regulate the trafficking of immune cells through the venous endothelium of the lymph nodes.93, 94
The meticulous investigator is cognizant of endothelial heterogeneity. At the very least, one should confirm by immunohistochemistry or FACS that the endothelial cells one is using experimentally are of venous (eg. EPHB), arterial (Ephrin B2) or lymphatic (podoplanin or Lyve 1) subtype. We have found that our iPSC-derived endothelial cells are heterogenous, with all three subtypes represented. Furthermore, the culture conditions can influence the percentage of each subtype. Higher doses of VEGF-A (50ng/ml) and 8Br-cAMP in the differentiation medium increase the prevalence of the arterial phenotype, for example (unpublished results). One should be aware that in the absence of their normal milieu, endothelial cells may lose some of their in vivo characteristics, or acquire new properties, which could affect experimental results, and the relevance of the cell culture model.
MiRNAs and endothelial lineage
Recent work has highlighted the role of miRNA in regulation of endothelial cell development and phenotype. Pivotal studies by Yang et al95 showed that knockdown of miRNA processing enzyme, Dicer, disrupted angiogenesis. Since then, a score of miRNA has been identified to modulate EC genes including miR-126, miR-19a and miR-21.96, 97 However, little is known about the role of miRNA in the EC differentiation. In a recent paper, Kane et al98 found that overexpression of miR-99b, -181a, and -181b levels increased the expression of EC markers and function. In addition, long noncoding RNA (lncRNA) regulates vascular development. Alterations in the ratio of tie-1 mRNA to the tie-1 antisense lncRNA, has been implicated in human vascular anomalies.99 It is likely that with a greater understanding of the regulation of their roles in endothelial development, miRNA and lncRNA may be manipulated for the purpose of EC differentiation or directed reprogramming.
Potential therapeutic applications of endothelial cell derivation
Modeling vascular disease
An immediate application of iPSC technology is to derive vascular cells from patients with vascular diseases. In particular, one would focus on vascular diseases that are believed to have a strong genetic component; the pathophysiology of which is not well understood; and/or do not have good animal models. Fibroblasts may be harvested from a patient with such a vascular disease. The fibroblasts can be induced to the pluripotential state using reprogramming factors, and then induced to form endothelial or vascular smooth muscle cells. Once their vascular phenotype has been confirmed by the characterization studies described above, the iPSC-derived vascular cell may be further studied to characterize abnormal cell functions (Figure 3). For example, patient-specific iPSC derived cardiomyocytes recapitulated the morphological and functional phenotypes for familial dilated cardiomyopathy and these cells may serve as a useful model to explore disease mechanisms and therapeutics.100 Similarly, once the “disease-in-a-dish” model is created, and the model is shown to faithfully recapitulate the vascular disease of the patient, then it may also be a useful tool for drug or small molecule screening for vascular disease. Such an approach may uncover therapeutic avenues that may merit further development.
ECs in Engineered Blood Vessels
Vascular regeneration refers to the restoration of normal vascular function and structure, the reversal of vascular senescence, and the development of vascular networks. ECs are essential components in vascular regeneration, and in many different tissue engineering applications such as vascular grafts. Vascular tissue engineering may involve the in vitro engineering of biological conduits for revascularization of various tissues (Figure 3). An early report described a vascular graft made with ECs, vascular smooth muscle cells (VSMCs), fibroblast, and collagen gel medium on a Dacron mesh.101 Since then, a variety of tissue-engineered grafts have utilized different scaffolds to enhance cell viability, maintain vascular phenotype, and provide for the mechanical strength to withstand hemodynamic forces. Vascular cells can be seeded onto various materials including collagen,101 biodegradable scaffolds,102 or vessels made of decellularized matrices. 103 ECs and SMCs may be obtained from the patient’s own vein segments, then cultured in vitro to get sufficient cell number for a graft. The ECs can form a barrier layer and are able to secrete vWF and prostacyclin in in vitro condition.
The major disadvantages of using adult cells from blood vessels are their limited proliferation capacity and susceptibility to cellular senescence.104 Furthermore, patient-specific deficiencies of endothelial function, or even tissue-specific differences in endothelial function, may reduce the applicability of autologous adult endothelial cells.105 However, the use of stem or progenitor cells to derive ECs may surmount these difficulties.
So-called ‘endothelial progenitor cells’ or EPCs may be isolated from the patient’s blood or bone marrow (using FACS and surface markers such as CD34, Flk-1 and VEGFR2), and expanded in ex vivo culture.11, 12 Parenthetically, the widely used term “EPC” is a misnomer. The surface markers that are commonly used for identification of human EPCs include markers that are not specific for endothelial lineage, such as CD133 and KDR (kinase insert domain receptor).12 In addition, methods for harvesting, purifying, and culturing EPCs are not standardized. Thus, semantic confusion is compounded by methodological variation. Casual readers of the literature may not recognize that EPCs are a mixed population of progenitor cells of different lineages. Within this population of cells, there are true endothelial progenitors that can incorporate into the vascular network, whereas hematopoietic progenitors may contribute by secreting angiogenic cytokines. In cell culture, EPCs may form early-outgrowth and late-outgrowth colonies. Cells derived from the former colonies are clearly not of endothelial origin, expressing markers of hematopoietic lineage, and are morphologically distinct from late-outgrowth cells, which grow in a cobblestone pattern reminiscent of endothelial cells (ECs). At present, there are no surface markers that clearly distinguish early endothelial progenitors; the best approach currently is to define endothelial lineage morphologically (ie, with tubulogenesis assays). The formation of a tubular network in Matrigel is a defining feature of endothelial progenitors, which can also incorporate into existing tubular networks formed by differentiated ECs. Kaushal and colleagues demonstrated that EPC-seeded de-cellularized porcine iliac arteries implanted into sheep were structurally, functionally and morphologically similar to a native vessel with a high flow patency.106 EPCs have also been grown on a decellularized vessel, and successfully transplanted in an interposition graft in the carotid artery. 107 However, this approach may generate an endothelial monolayer with some differences from in vivo ECs, with lower expressions of NO synthase, prostacyclin, and tissue plasminogen activator (tPA).108 It should be noted also that EPC decrease in number with aging, which might complicate the use of these cells for clinical conditions.109
Pluripotent stem cell- derived ECs may have several advantages over ECs from patient vein segments or EPCs including that they can potentially generate larger quantities of ECs. In one study, mouse ESCs were added to a microporous tube made of polyurethane, and differentiated into ECs and SMCs in vitro.110 ECs may also be genetically engineered, for example to reduce senescence. ECs derived from mouse ESCs were transduced with human telomerase reverse transcriptase to immortalize the ECs.111 These genetically modified ECs were used to endothelialize hybrid grafts made of a SMC layer on polyglycolic acid scaffolds which were then subcutaneously implanted into hairless SCID (nude) mice. A typical blood vessel structure with EC-lining and a vessel wall composed of SMC and collagen was generated using this approach.
ECs for generating the microvasculature (Figure 3)
ECs have the ability to form capillary-like networks in vitro.112–114 HUVECs, keratinocytes, and fibroblasts were seeded together in a collagen sponge to form spontaneous capillary-like structures in vitro, followed by implantation in mice.115 The capillary-like tubes formed within the engineered skin tissue connected with the host vessels within 4 days of implantation. To produce larger and more complex tissue constructs, homogeneous distribution of micro-vessels throughout the tissue needs to be achieved for transport of nutrients and oxygen.116 Also, grafts seeded with CD34+ bone marrow derived EPCs showed significantly improved endothelialization and increased microvessels within the neointima when these grafts were implanted into the thoracic aortae of adult dogs.117–119 In human, vascular regeneration has been attempted with autologous BMNCs or granulocyte colony-stimulating factor–expanded peripheral blood mononuclear cells in patients with coronary as well as peripheral arterial disease (PAD). Phase I and II trials have been completed in patients with acute myocardial infarction,120 as well as in subjects with chronic ischemic heart disease.121–123 Thus far, the majority of these early trials have suggested that modest improvements may be gained in some cardiac end points such as global and regional contractility with little risk for these cell-based therapies.120 Additionally, since the first study in 2002 there have been at least 30 reported therapeutic cell trials in patients with PAD, some of which have shown promise in improving perfusion, healing ulcers, and increasing walking distance.124, 125 However, most of these trials have been small observational studies, and need confirmation in larger randomized clinical trials.
Concluding Remarks
“One is only as old as one’s endothelium”.126 Cardiovascular health begins with restoration of endothelial homeostasis. Accordingly, studies of endothelial biology will continue to lead to new insights of clinical relevance and therapeutic promise. The generation of disease-specific iPSC derived vascular cells provides an opportunity to delve deeper into the mechanisms of disease. Such cells can be used to understand the early events in the pathogenesis of diseases that are now only recognized in their late stages. Furthermore, adult and pluripotential stem cells are under investigation to determine their utility for ischemic syndromes affecting the coronary and peripheral circulations. Although it remains to be seen whether adult stem cells promote vascular regeneration and relieve ischemic syndromes, the early studies have been promising, albeit the effect sizes modest. Because they have greater proliferative capacity, pluripotential stem cells appear to hold greater promise for the future of vascular regeneration. The fidelity and safety of iPSC/ESC derived endothelial cells needs to be confirmed prior to clinical trials. Furthermore, methods for their derivation need to be improved. Still to be determined are important clinical issues such as dosing, duration and methods of delivery of therapeutic cells. Under development are bioengineering solutions, in which the cells are administered within a decellularized or synthetic matrix. These exciting developments in translational endothelial biology will open new vistas for exploration and novel therapeutic avenues for development.
Methodological Reviews Text Box.
Methodological Reviews discuss methods that are of broad interest to the community of cardiovascular investigators and that enable a better understanding of cardiovascular biology, particularly recent technologies in which the methods are still in flux and/or not widely known. It is hoped that these articles, written by recognized experts, will be useful to all investigators, but especially to early-career investigators.
Acknowledgments
The authors gratefully acknowledge Julieanna Qi for assistance in preparing the figures.
Sources of Funding
This work was supported by grants to John P. Cooke from the National Institutes of Health (U01HL100397, UM1HL113456, R01EY020609, 1K12HL087746). Wing Tak Wong was supported by a postdoctoral fellowship from the American Heart Association (12POST8830020). Ngan F. Huang was supported by a grant from the National Institutes of Health (HL098688). Nazish Sayed was supported by NRSA grant (HL098049-01A1).
Non-standard abbreviations
- ac-LDL
Acetylated low-density lipoprotein
- bFGF
Basic fibroblast growth factor
- BMP4
Bone morphogenetic protein
- CD31
Platelet-endothelial cell adhesion molecules
- cGMP
Cyclic Guanosine Monophosphate
- E-caherin
Epithelial cadherin
- EBs
Embryoid bodies
- ECM
Extracellular matrix
- ECs
Endothelial cells
- EGF
Epidermal growth factor
- eNOS
Endothelial nitric oxide synthase
- EPCs
Endothelial progenitor cells
- ESCs
Embryonic stem cells
- FACS
Fluorescent activated cell sorting
- Flk-1
Fetal liver kinase-1
- GCCP
Good cell culture practice
- HUVECs
Human umbilical vein endothelial cells
- IGF-1
Insulin-like growth factor-1
- iPSCs
Induced pluripotent stem cells
- KDR
Kinase insert domain receptor
- Klf4
Krüppel-like factor 4
- LIF
Leukemia inhibitory factor
- lncRNA
Long noncoding RNA
- NO
Nitric oxide
- Oct3/4
Octamer-binding transcription factor-3/4
- PAD
Peripheral arterial disease
- Sox2
SRY-related high-mobility-group (HMG)-box protein-2
- SSEA-1
Stage-specific extracellular antigen-1
- Tie1
Tyrosine kinase receptor 1
- VEGF
Vascular endothelial growth factor
- VSMCs
Vascular smooth muscle cells
- vWF
von Willebrand factor
Footnotes
Disclosures
None
References
- 1.Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991;88:1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida K, Harrison DG, Navas JP, Fisher AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW, Murphy TJ. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 4.Busse R, Fleming I. Vascular endothelium and blood flow. Handb Exp Pharmacol. 2006:43–78. doi: 10.1007/3-540-36028-x_2. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 6.Lundman P, Eriksson MJ, Stuhlinger M, Cooke JP, Hamsten A, Tornvall P. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol. 2001;38:111–116. doi: 10.1016/s0735-1097(01)01318-3. [DOI] [PubMed] [Google Scholar]
- 7.Tsao PS, Wang B, Buitrago R, Shyy JY, Cooke JP. Nitric oxide regulates monocyte chemotactic protein-1. Circulation. 1997;96:934–940. doi: 10.1161/01.cir.96.3.934. [DOI] [PubMed] [Google Scholar]
- 8.Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, Dzau VJ. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. The Journal of clinical investigation. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niebauer J, Cooke JP. Cardiovascular effects of exercise: Role of endothelial shear stress. J Am Coll Cardiol. 1996;28:1652–1660. doi: 10.1016/S0735-1097(96)00393-2. [DOI] [PubMed] [Google Scholar]
- 10.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 11.Mund JA, Case J. The ontogeny of endothelial progenitor cells through flow cytometry. Curr Opin Hematol. 2011;18:166–170. doi: 10.1097/MOH.0b013e328345a16a. [DOI] [PubMed] [Google Scholar]
- 12.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: Adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122:517–526. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 15.Lehle K, Straub RH, Morawietz H, Kunz-Schughart LA. Relevance of disease-and organ-specific endothelial cells for in vitro research. Cell Biol Int. 2010;34:1231–1238. doi: 10.1042/CBI20100531. [DOI] [PubMed] [Google Scholar]
- 16.Cooke JP. The endothelium: A new target for therapy. Vasc Med. 2000;5:49–53. doi: 10.1177/1358836X0000500108. [DOI] [PubMed] [Google Scholar]
- 17.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 18.Rajavashisth TB, Liao JK, Galis ZS, Tripathi S, Laufs U, Tripathi J, Chai NN, Xu XP, Jovinge S, Shah PK, Libby P. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999;274:11924–11929. doi: 10.1074/jbc.274.17.11924. [DOI] [PubMed] [Google Scholar]
- 19.Coecke S, Balls M, Bowe G, Davis J, Gstraunthaler G, Hartung T, Hay R, Merten OW, Price A, Schechtman L, Stacey G, Stokes W. Guidance on good cell culture practice. A report of the second ecvam task force on good cell culture practice. Altern Lab Anim. 2005;33:261–287. doi: 10.1177/026119290503300313. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. Enos activity is reduced in senescent human endothelial cells: Preservation by htert immortalization. Circ Res. 2001;89:793–798. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. Hmec-1: Establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 23.Akeo K, Nagasaki K, Tanaka Y, Curran SA, Dorey CK. Comparison of effects of oxygen and antioxidative enzymes on cell growth between retinal pigment epithelial cells and vascular endothelial cells in vitro. Ophthalmic Res. 1992;24:357–364. doi: 10.1159/000267194. [DOI] [PubMed] [Google Scholar]
- 24.McGarrity GJ, Vanaman V, Sarama J. Cytogenetic effects of mycoplasmal infection of cell cultures: A review. In Vitro. 1984;20:1–18. doi: 10.1007/BF02633326. [DOI] [PubMed] [Google Scholar]
- 25.Cooke JP. Flow, no, and atherogenesis. Proc Natl Acad Sci U S A. 2003;100:768–770. doi: 10.1073/pnas.0430082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archer S. Measurement of nitric oxide in biological models. Faseb J. 1993;7:349–360. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- 27.Cox RD, Frank CW. Determination of nitrate and nitrite in blood and urine by chemiluminescence. J Anal Toxicol. 1982;6:148–152. doi: 10.1093/jat/6.3.148. [DOI] [PubMed] [Google Scholar]
- 28.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 29.Scuteri A, Stuehlinger MC, Cooke JP, Wright JG, Lakatta EG, Anderson DE, Fleg JL. Nitric oxide inhibition as a mechanism for blood pressure increase during salt loading in normotensive postmenopausal women. J Hypertens. 2003;21:1339–1346. doi: 10.1097/00004872-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 31.Broillet M, Randin O, Chatton J. Photoactivation and calcium sensitivity of the fluorescent no indicator 4,5-diaminofluorescein (daf-2): Implications for cellular no imaging. FEBS Lett. 2001;491:227–232. doi: 10.1016/s0014-5793(01)02206-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, He P. Improved measurements of intracellular nitric oxide in intact microvessels using 4,5-diaminofluorescein diacetate. Am J Physiol Heart Circ Physiol. 2011;301:H108–114. doi: 10.1152/ajpheart.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Seidlits SK, Adams MM, Lynch VM, Schmidt CE, Anslyn EV, Shear JB. A highly selective low-background fluorescent imaging agent for nitric oxide. J Am Chem Soc. 2010;132:13114–13116. doi: 10.1021/ja1040013. [DOI] [PubMed] [Google Scholar]
- 34.Rufaihah AJ, Huang NF, Jame S, Lee J, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, Gambhir SS, Cooke JP. Endothelial cells derived from human ipscs increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, Cooke JP. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol. 2010;30:984–991. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boger RH, Bode-Boger SM, Tsao PS, Lin PS, Chan JR, Cooke JP. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J Am Coll Cardiol. 2000;36:2287–2295. doi: 10.1016/s0735-1097(00)01013-5. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Hu S, Ghosh Z, Han Z, Wu JC. Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells. Stem Cells Dev. 2011;20:1701–1710. doi: 10.1089/scd.2010.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh Z, Wilson KD, Wu Y, Hu S, Quertermous T, Wu JC. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One. 2010;5:e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qf1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential modeling of fragile × syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6:407–411. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 42.Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–3431. [PubMed] [Google Scholar]
- 43.Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi N. Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood. 1999;93:1253–1263. [PubMed] [Google Scholar]
- 44.Lanner F, Sohl M, Farnebo F. Functional arterial and venous fate is determined by graded vegf signaling and notch status during embryonic stem cell differentiation. Arterioscler Thromb Vasc Biol. 2007;27:487–493. doi: 10.1161/01.ATV.0000255990.91805.6d. [DOI] [PubMed] [Google Scholar]
- 45.Davis GE, Senger DR. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 46.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies flk1+ve-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 48.Zeng L, Xiao Q, Margariti A, Zhang Z, Zampetaki A, Patel S, Capogrossi MC, Hu Y, Xu Q. Hdac3 is crucial in shear- and vegf-induced stem cell differentiation toward endothelial cells. J Cell Biol. 2006;174:1059–1069. doi: 10.1083/jcb.200605113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahsan T, Nerem RM. Fluid shear stress promotes an endothelial-like phenotype during the early differentiation of embryonic stem cells. Tissue Eng Part A. 2010;16:3547–3553. doi: 10.1089/ten.tea.2010.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915–1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 51.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 52.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development. 1988;102:471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 53.Bautch VL, Stanford WL, Rapoport R, Russell S, Byrum RS, Futch TA. Blood island formation in attached cultures of murine embryonic stem cells. Dev Dyn. 1996;205:1–12. doi: 10.1002/(SICI)1097-0177(199601)205:1<1::AID-AJA1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 54.Wiles MV, Keller G. Multiple hematopoietic lineages develop from embryonic stem (es) cells in culture. Development. 1991;111:259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- 55.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 56.Adams B, Xiao Q, Xu Q. Stem cell therapy for vascular disease. Trends Cardiovasc Med. 2007;17:246–251. doi: 10.1016/j.tcm.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Tateishi K, Takehara N, Matsubara H, Oh H. Stemming heart failure with cardiac- or reprogrammed-stem cells. J Cell Mol Med. 2008;12:2217–2232. doi: 10.1111/j.1582-4934.2008.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li ZJ, Wang ZZ, Zheng YZ, Xu B, Yang RC, Scadden DT, Han ZC. Kinetic expression of platelet endothelial cell adhesion molecule-1 (pecam-1/cd31) during embryonic stem cell differentiation. J Cell Biochem. 2005;95:559–570. doi: 10.1002/jcb.20436. [DOI] [PubMed] [Google Scholar]
- 59.Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 60.McCloskey KE, Smith DA, Jo H, Nerem RM. Embryonic stem cell-derived endothelial cells may lack complete functional maturation in vitro. J Vasc Res. 2006;43:411–421. doi: 10.1159/000094791. [DOI] [PubMed] [Google Scholar]
- 61.Gao J, Yan XL, Li R, Liu Y, He W, Sun S, Zhang Y, Liu B, Xiong J, Mao N. Characterization of op9 as authentic mesenchymal stem cell line. J Genet Genomics. 2010;37:475–482. doi: 10.1016/S1673-8527(09)60067-9. [DOI] [PubMed] [Google Scholar]
- 62.Joo HJ, Kim H, Park SW, Cho HJ, Kim HS, Lim DS, Chung HM, Kim I, Han YM, Koh GY. Angiopoietin-1 promotes endothelial differentiation from embryonic stem cells and induced pluripotent stem cells. Blood. 2011;118:2094–2104. doi: 10.1182/blood-2010-12-323907. [DOI] [PubMed] [Google Scholar]
- 63.Yu J, Huang NF, Wilson KD, Velotta JB, Huang M, Li Z, Lee A, Robbins RC, Cooke JP, Wu JC. Nachrs mediate human embryonic stem cell-derived endothelial cells: Proliferation, apoptosis, and angiogenesis. PLoS One. 2009;4:e7040. doi: 10.1371/journal.pone.0007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rufaihah AJ, Huang NF, Jame S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, Gambhir SS, Cooke JP. Endothelial cells derived from human ipscs increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a kdr+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 66.Vodyanik MA, Slukvin II. Hematoendothelial differentiation of human embryonic stem cells. Curr Protoc Cell Biol. 2007;Chapter 23(Unit 23):26. doi: 10.1002/0471143030.cb2306s36. [DOI] [PubMed] [Google Scholar]
- 67.Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, Slukvin I. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 69.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 70.Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, Kamp TJ. Transplantation of embryonic stem cells into the infarcted mouse heart: Formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Z, Wilson KD, Smith B, Kraft DL, Jia F, Huang M, Xie X, Robbins RC, Gambhir SS, Weissman IL, Wu JC. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS One. 2009;4:e8443. doi: 10.1371/journal.pone.0008443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, Studer L, Lee G, Lyden D, Benezra R, Zaninovic N, Rosenwaks Z, Rabbany SY, Rafii S. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by tgfbeta inhibition is id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Li L, Shojaei F, Levac K, Cerdan C, Menendez P, Martin T, Rouleau A, Bhatia M. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerecht-Nir S, Ziskind A, Cohen S, Itskovitz-Eldor J. Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab Invest. 2003;83:1811–1820. doi: 10.1097/01.lab.0000106502.41391.f0. [DOI] [PubMed] [Google Scholar]
- 78.Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 81.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 83.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 84.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 85.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 86.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kennedy D. Breakthrough of the year. Science. 2007;318:1833. doi: 10.1126/science.1154158. [DOI] [PubMed] [Google Scholar]
- 88.Xie CQ, Huang H, Wei S, Song LS, Zhang J, Ritchie RP, Chen L, Zhang M, Chen YE. A comparison of murine smooth muscle cells generated from embryonic versus induced pluripotent stem cells. Stem Cells Dev. 2009;18:741–748. doi: 10.1089/scd.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, Ragoussis J, Huang Y, Han JD, Zeng L, Hu Y, Xu Q. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U S A. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, Chen L, Flamm A, Valtanen H, Weavind LM, Hicks ME, Pollock RE, Botz GH, Bucana CD, Koivunen E, Cahill D, Troncoso P, Baggerly KA, Pentz RD, Do KA, Logothetis CJ, Pasqualini R. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 93.Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: Dogmas and enigmas. Nat Rev Immunol. 2004;4:360–370. doi: 10.1038/nri1354. [DOI] [PubMed] [Google Scholar]
- 94.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 95.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 96.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. Microrna-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YS, Chien S, Wang N. Microrna-19a mediates the suppressive effect of laminar flow on cyclin d1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107:3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kane NM, Howard L, Descamps B, Meloni M, McClure J, Lu R, McCahill A, Breen C, Mackenzie RM, Delles C, Mountford JC, Milligan G, Emanueli C, Baker AH. Role of micrornas 99b, 181a, and 181b in the differentiation of human embryonic stem cells to vascular endothelial cells. Stem Cells. 2012;30:643–654. doi: 10.1002/stem.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li K, Blum Y, Verma A, Liu Z, Pramanik K, Leigh NR, Chun CZ, Samant GV, Zhao B, Garnaas MK, Horswill MA, Stanhope SA, North PE, Miao RQ, Wilkinson GA, Affolter M, Ramchandran R. A noncoding antisense rna in tie-1 locus regulates tie-1 function in vivo. Blood. 2010;115:133–139. doi: 10.1182/blood-2009-09-242180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4:130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 102.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 103.Shimizu K, Ito A, Arinobe M, Murase Y, Iwata Y, Narita Y, Kagami H, Ueda M, Honda H. Effective cell-seeding technique using magnetite nanoparticles and magnetic force onto decellularized blood vessels for vascular tissue engineering. J Biosci Bioeng. 2007;103:472–478. doi: 10.1263/jbb.103.472. [DOI] [PubMed] [Google Scholar]
- 104.Alsberg E, von Recum HA, Mahoney MJ. Environmental cues to guide stem cell fate decision for tissue engineering applications. Expert Opin Biol Ther. 2006;6:847–866. doi: 10.1517/14712598.6.9.847. [DOI] [PubMed] [Google Scholar]
- 105.Aird WC. Phenotypic heterogeneity of the endothelium: Ii. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 106.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boehm TD, Werner A, Radtke S, Mueller T, Kirschner S, Gohlke F. The effect of suture materials and techniques on the outcome of repair of the rotator cuff: A prospective, randomised study. J Bone Joint Surg Br. 2005;87:819–823. doi: 10.1302/0301-620X.87B6.15638. [DOI] [PubMed] [Google Scholar]
- 108.Shirota T, He H, Yasui H, Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: Proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9:127–136. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 109.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 110.Huang H, Nakayama Y, Qin K, Yamamoto K, Ando J, Yamashita J, Itoh H, Kanda K, Yaku H, Okamoto Y, Nemoto Y. Differentiation from embryonic stem cells to vascular wall cells under in vitro pulsatile flow loading. J Artif Organs. 2005;8:110–118. doi: 10.1007/s10047-005-0291-2. [DOI] [PubMed] [Google Scholar]
- 111.Shen G, Tsung HC, Wu CF, Liu XY, Wang XY, Liu W, Cui L, Cao YL. Tissue engineering of blood vessels with endothelial cells differentiated from mouse embryonic stem cells. Cell Res. 2003;13:335–341. doi: 10.1038/sj.cr.7290178. [DOI] [PubMed] [Google Scholar]
- 112.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 113.Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, Sierra-Honigmann MR, Lorber MI, Tellides G, Kashgarian M, Bothwell AL, Pober JS. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci U S A. 2000;97:9191–9196. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Black AF, Berthod F, L’Heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. Faseb J. 1998;12:1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 115.Tremblay PL, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am J Transplant. 2005;5:1002–1010. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 116.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 117.Bhattacharya V, McSweeney PA, Shi Q, Bruno B, Ishida A, Nash R, Storb RF, Sauvage LR, Hammond WP, Wu MH. Enhanced endothelialization and microvessel formation in polyester grafts seeded with cd34(+) bone marrow cells. Blood. 2000;95:581–585. [PubMed] [Google Scholar]
- 118.Shi Q, Bhattacharya V, Hong-De Wu M, Sauvage LR. Utilizing granulocyte colony-stimulating factor to enhance vascular graft endothelialization from circulating blood cells. Ann Vasc Surg. 2002;16:314–320. doi: 10.1007/s10016-001-0238-x. [DOI] [PubMed] [Google Scholar]
- 119.Shi Q, Wu MH, Fujita Y, Ishida A, Wijelath ES, Hammond WP, Wechezak AR, Yu C, Storb RF, Sauvage LR. Genetic tracing of arterial graft flow surface endothelialization in allogeneic marrow transplanted dogs. Cardiovasc Surg. 1999;7:98–105. doi: 10.1016/s0967-2109(98)00027-1. [DOI] [PubMed] [Google Scholar]
- 120.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 121.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: Part ii: Cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 122.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 123.Tse HF, Thambar S, Kwong YL, Rowlings P, Bellamy G, McCrohon J, Thomas P, Bastian B, Chan JK, Lo G, Ho CL, Chan WS, Kwong RY, Parker A, Hauser TH, Chan J, Fong DY, Lau CP. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (protect-cad trial) Eur Heart J. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 124.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 125.Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, Yamamoto Y, Onodera R, Teramukai S, Fukushima M, Matsubara H. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (therapeutic angiogenesis by cell transplantation [tact] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 126.Altschul R. Endothelium, its development, morphology, function, and pathology. New york, NY: Macmillan; 1954. p. vii. [Google Scholar]