Abstract
Accumulating evidence indicates that T cells play an important role in the pathogenesis of hypertension. Here we review the investigations that have shown that T cells are infiltrating the kidney in hypertension. Interstitial accumulation of immune cells is associated with increments in oxidative stress and renal angiotensin II activity that result in the impairment in pressure natriuresis. The severity of salt-sensitive hypertension is directly correlated with the intensity of immune cell infiltration in the kidney. Reducing the renal infiltration of T cells prevents or ameliorates hypertension and the induction of tubulointerstitial inflammation results in salt-sensitive hypertension. The potential participation of autoimmune mechanisms in the renal infiltration of immune competent cells is discussed.
Keywords: renal angiotensin II activity, salt-sensitive hypertension, T cells, tubulointerstial nephritis
Introduction
The association of dysfunction of the immune system and arterial hypertension has been known for some time. The majority of early reports indicated increased immune reactivity in hypertension and the findings included the detection of anti-thymocyte antibodies in the spontaneously hypertensive rats (SHRs) [1] and amelioration of hypertension with the administration of immunosuppressive drugs such as 6-mercaptopurine [2] and cyclophosphamide [3]. Pioneer investigations by Svendsen showed that the nude (athymic) mice as well as thymectomy prevented hypertension in several experimental models and, furthermore, that the capacity to develop hypertension in these models was restored by thymic grafts [4]. These studies led him to suggest that ‘lymphocyte-dependent arteriolar lesions’ were critical for the development of hypertension. Concordant with this hypothesis were studies showing that anti-thymocytic serum ameliorated hypertension [5] and reports that transferring spleen cells of rats with deoxycorticosterone-salt hypertension to normotensive rats induced hypertension [6]. However, other studies suggested that hypertension was associated with a reduction in immune reactivity, since the SHR was found to have suppressed antibody formation and decreased delayed-type hypersensitivity [7] and injections of Interleukin-2 and thymic grafts from normotensive Wistar Kyoto rats corrected hypertension [8].
In 1981, the immune reactivity in hypertension was interpreted as an adaptive response aimed at reducing the otherwise life-threatening increments in blood pressure [5]. Subsequently, the interest in the relationship between immunity and hypertension was lost and a scholarly editorial a decade later noted with regret that immune dysfunction was then rarely mentioned in discussions on arterial hypertension [9]. The interest in the role of T cells in the pathogenesis of hypertension was revived by the demonstration that immunosuppressive treatment improved or prevented hypertension in experimental models of acquired and genetic models of salt-sensitive hypertension as well as in spontaneously hypertensive rats [reviewed in 10]. More recently, elegant studies using angiotensin II infusions have shown that T cells are necessary for the development of hypertension [11] and T regulatory cells suppress the hypertensive response [12].
Infiltration of T cells in hypertension: where, when and how much?
Renal infiltration of T cells is a constant finding in experimental models of hypertension [10]. In addition, lymphocytes have been found infiltrating the aortic wall in angiotensin II-induced hypertension [13]. Data in humans are available only from the kidneys obtained at autopsy and from renal biopsies taken from hypertensive patients during surgery, when lumbar sympathectomies were used in the treatment of hypertension nearly 6 decades ago. There are three such studies that aimed to clarify whether arteriosclerosis in the kidney was the cause or the consequence of essential hypertension. Relevant to the present discussion, all three studies noted the existence of interstitial infiltrates of immune cells in the kidneys of hypertensive patients. Heptinstall [14] found renal infiltrates of lymphocytes in 16 of 37 patients, the majority of which had only minor arteriosclerotic vascular changes. Gareau and Cartier [15] noted interstitial inflammation in 7 of 55 patients and noted that ‘it is impossible to determine by the histological examination alone if interstitial nephritis influences the evolution of the nephrosclerosis or if it induced hypertension’. The third and more extensive report was given by Sommers et al. [16] who evaluated kidney biopsies from 1350 patients who underwent sympathectomies for hypertension and found that 20% of the biopsies with normal arterioles or minor vascular lesions and 50% of the biopsies with more advanced vascular disease showed interstitial collections of lymphocytes which they termed ‘aggregates’. More recent data in humans come from Hughson et al. [17] who examined autopsy material to determine whether hypertensive individuals had a reduced nephron number. They found no significant differences in nephron numbers in hypertensive and normotensive individuals but their data showed that the number infiltrating immune-competent cells (CD68 positive) in tubulointerstitial areas of the kidneys was significantly higher in hypertensive patients.
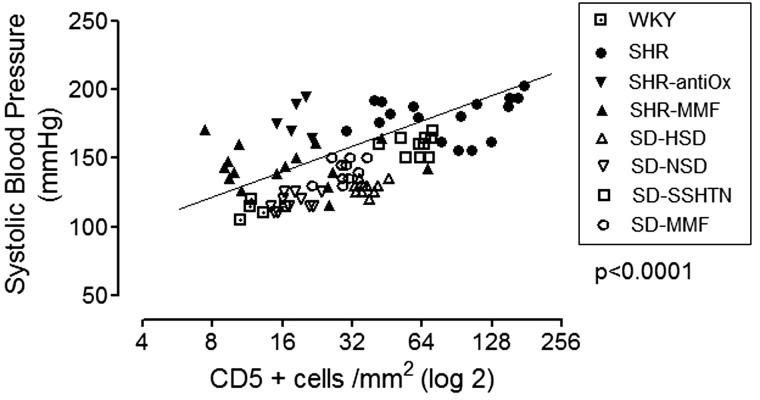
Given the likely importance of T cells in the pathogenesis of hypertension, it is important to consider whether the immune cell infiltration precedes or follows hypertension and if there is a relationship between the severity of hypertension and the number of infiltrating cells. At least in the SHRs, it has been shown that increased numbers of immune-competent cells are present as early as 3–4 weeks of age before the hypertension develops, and their accumulation increases concurrently with the progressive increment in blood pressure [18]. In relation to the association between the severity of hypertension and the tubulointerstitial immune cell infiltration, studies have found a correlation between the number of infiltrating immune cells and the blood pressure levels [19–21]. Figure 1 shows the data from these studies.
Fig. 1.
Relationship between tubulointerstitial lymphocyte infiltration (CD5+ cells) and blood pressure. Data were obtained from refs. [19–21]. WKY, Wistar Kyoto rats; SHR, spontaneously hypertensive rats; SHR-Antiox, SHR receiving antioxidant diet; SHR-MMF, SHR treated with 20 mg daily of mycophenolate mofetil (MMF); SD-HSD, Sprague–Dawley rats on a high salt diet; SD-NSD, SD rats on a normal salt diet; SD-SSHTN, SD rats with salt-sensitive hypertension induced with a high salt diet administered after 2 weeks of subcutaneous angiotensin II infusion; SD-MMF, rats treated as the SD-SSHTN group with the addition of MMF.
Amelioration and prevention of hypertension by immune suppression
A number of studies have shown that a variety of strategies addressed to suppress immune reactivity and to reduce immune cell infiltration improve hypertension. These studies have been reviewed elsewhere [10]. Of particular interest are studies of the rag−/− mice, which lack lymphocytes, as these mice do not have an appropriate blood pressure increment to angiotensin II administration [13]. Furthermore, the adoptive transfer of T lymphocytes from the wild-type mice can restore the hypertensive response [13]. Subsequent work from the same research group demonstrated the importance of the immune co-stimulatory axis in the development of hypertension and showed that amelioration of angiotensin II-induced hypertension results from third ventricular lesions that cause suppression of both early activation and tissue homing capacity of lymphocytes [reviewed in 11]. Concordant with the animal data, humans with essential hypertension treated with mycophenolate mofetil (MMF) for rheumatoid arthritis or psoriasis had an improvement in blood pressure during the period in which they received MMF treatment [22].
Induction of salt-sensitive hypertension by renal tubulointerstitial inflammation
A commonly held assumption is that interstitial nephritis is not associated with hypertension. Yet, critical review of the data does not support this assumption. To be sure, if the renal damage is such that mechanisms of sodium reabsorption are severely disrupted, a salt wasting nephropathy may result, but hypertension is present in a significant number of cases of interstitial nephritis. Recent studies of drug-induced interstitial nephritis found that hypertension was present in five of eight patients [23]; in interstitial nephritis due to proton pump inhibitors, hypertension was present in 6 of 23 patients [24]; in HIV-associated interstitial nephritis, hypertension was present in 52% of 29 patients [25] and in aristolochic acid nephropathy hypertension was present in 59.3% of 300 patients [26]. Experimentally, the notion that interstitial nephritis may cause hypertension is supported by studies that show that the induction of interstitial inflammation by protein overload or cellophane wrapping of the kidney result in salt-sensitive hypertension [27, 28]. Protein overload in rats results in heavy proteinuria and intense infiltration of immune cells in the renal interstitium. In this model, the administration of a high salt diet caused hypertension and the administration of MMF corrected the renal inflammation and hypertension [27]. Similar findings were reported by Vanegas et al. [28] in the experimental model of hypertension resulting from cellophane wrapping of the kidneys that is associated with intense tubulointerstitial infiltration of the kidney, increased renal angiotensin II activity and progressive hypertension. Also in this model, all features were corrected with MMF treatment.
How does renal infiltration of immune competent cells induce hypertension?
In 1963, Borst and Borst-De Geus [29] postulated that ‘hypertension is part of a homeostatic reaction to deficient renal sodium output’ and Guyton et al. [30] in classical studies described the pressure natriuresis relationship and how its impairment results in fluid and sodium retention that requires an adaptive increment in blood pressure to restore physiologic balance. Renal inflammation is constantly associated with oxidative stress and increased intrarenal angiotensin II and, in combination, they act to stimulate sodium retention and impairment of the pressure natriuresis relationship [10]. Glomerular vasoconstriction, reduced GFR, remodeling of glomerular arterioles and transforming growth factor-β-induced loss of glomerular hemodynamic autoregulation [31] are all consequences of renal inflammation. In the tubulointerstial areas of the kidney, the pressure natriuresis is further impaired by the loss of the peritubular capillary network, development of fibrosis and increased angiotensin II activity and oxidative stress that stimulates sodium reabsorption. The net result of these effects is that the pressure natriuresis relationship is shifted to the right. The impairment of the pressure natriuresis relationship resulting from interstitial inflammation was shown in studies in the salt-sensitive hypertension that follows transient inhibition of nitric oxide synthase. In these studies, renal sodium excretion was studied at 90, 110 and 130 mmHg of renal arterial pressure and the urinary sodium excretion rate and the fractional sodium excretion were essentially unchanged in the rats with interstitial inflammation while the control rats increased three to four times the urinary sodium excretion. The administration of mycophenolate mofetil corrected the interstitial inflammation, prevented salt-sensitive hypertension and increased the pressure induced natriuresis. [Franco M et al. Abstract TH-OR109, Annual Meeting ASN. 2011].
The improvement in salt-sensitive hypertension induced by strategies that reduce tubulointerstitial inflammation suggest that immune-competent cells play a role in the development of salt-sensitive hypertension; yet, studies by Titze et al. [32] have demonstrated that macrophages in the skin protect from sodium-induced blood pressure increment. Tonicity responsive enhancer-binding protein (TonEBP) in skin macrophages is stimulated by hypertonic accumulation of sodium proteoglycans. In turn, TonEBP binds to the promoter of the gene of vascular endothelial growth factor-C which stimulates lymphangiogenesis and the resulting increment in the subcutaneous lymphatic network ameliorates the increment in blood pressure that follows as a consequence of a high salt diet. Machnik et al. [33] have further shown that the depletion of the mononuclear phagocyte system induces salt-sensitive hypertension. These elegant studies clearly show that skin macrophages play a role in adaptation to sodium retention but no studies have defined whether renal macrophages also play a similar role. Hypertonicity is a physiologic condition in the renal medulla and it is unknown if TonEBP is activated in renal macrophages or what effects, if any, this would have in the pressure natriuresis relationship and hypertension.
Is autoimmunity involved in hypertension?
Despite the wealth of evidence that supports the involvement of T cells in the pathogenesis of hypertension, it remains to be defined whether the infiltration of T cells in the kidney and vascular walls is due to non-specific chemokine cues or whether the T cells are reacting with an as yet undefined antigen(s). We have suggested that heat shock protein 70 (HSP70), which is over-expressed in the kidney in models of salt-sensitive hypertension, could act as an antigen to drive an autoimmune response and proliferative responses of lymphocytes in these models [10]. Further studies are needed to clarify what role, if any, autoimmunity may play in the pathogenesis of hypertension.
Conflict of interest statement
R.J.J. is listed as inventor on a patent application from the University of Colorado related to developing isoform-specific fructokinase inhibitors in the treatment of disorders associated with obesity and insulin resistance. He has consulted for Ardea, Astellas, Danone and Novartis, and is on the scientific board of Amway.
Acknowledgements
B.R.-I. research has received grants from the National Science and Technology Fund (FONACIT), Venezuela and from the Asociación de Amigos del Riñón, Maracaibo, Venezuela. M.F. research is funded by CONACYT grant 79661. R.J.J. received grants from the NIH and from Amway, Cardero, Danone, Questcor and the Sugar Foundation and NIH grant HL-68607.
References
- 1.Takeichi N, Ba D, Kobayashi H. Natural cytotoxic autoantibody against thymocytes in spontaneously hypertensive rats. Cell Immunol. 1981;60:181–190. doi: 10.1016/0008-8749(81)90258-6. doi:10.1016/0008-8749(81)90258-6. [DOI] [PubMed] [Google Scholar]
- 2.Dzielak DJ. Immune mechanisms in experimental and essential hypertension. Am J Physiol. 1991;260:R459–R467. doi: 10.1152/ajpregu.1991.260.3.R459. doi: [DOI] [PubMed] [Google Scholar]
- 3.Bataillard A, Vincent M, Sassard J, et al. Antihypertensive effect of an immunosuppressive agent, cyclophosphamide, in genetically hypertensive rats of the Lyon strain. Int J Immunopharmacol. 1989;11:377–384. doi: 10.1016/0192-0561(89)90084-2. doi:10.1016/0192-0561(89)90084-2. [DOI] [PubMed] [Google Scholar]
- 4.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand A. 1976;84:523–528. doi: 10.1111/j.1699-0463.1976.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 5.Bendich A, Belisle EH, Strausser HR. Immune system modulation and its effect on the blood pressure of the spontaneously hypertensive male and female rat. Biochem Biophys Res Commun. 1981;31:600–607. doi: 10.1016/0006-291x(81)91787-3. doi:10.1016/0006-291X(81)91787-3. [DOI] [PubMed] [Google Scholar]
- 6.Olsen F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol Microbiol Scand C. 1980;88:1–5. doi: 10.1111/j.1699-0463.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 7.Takeichi N, Suzuki K, Okayasu T, et al. Immunological depression in spontaneously hypertensive rats. Clin Exp Immunol. 1980;40:120–126. [PMC free article] [PubMed] [Google Scholar]
- 8.Tuttle RS, Boppana DP. Antihypertensive effect of interleukin-2. Hypertension. 1990;15:89–94. doi: 10.1161/01.hyp.15.1.89. doi:10.1161/01.HYP.15.1.89. [DOI] [PubMed] [Google Scholar]
- 9.Dzielak DJ. AIDS, lupus, rheumatoid arthritis-hypertension? Hypertension. 1990;15:95–96. doi: 10.1161/01.hyp.15.1.95. doi:10.1161/01.HYP.15.1.95. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Iturbe B, Franco M, Tapia E, et al. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2012;39:96–103. doi: 10.1111/j.1440-1681.2011.05482.x. doi:10.1111/j.1440-1681.2011.05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison DG, Guzik TJ, Lob HE, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. doi:10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barhoumi T, Kasal DA, Li MW. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. doi:10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 13.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. doi:10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heptinstall RH. Renal biopsies in hypertension. Br Heart J. 1954;16:133–141. doi: 10.1136/hrt.16.2.133. doi:10.1136/hrt.16.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gareau RJ, Cartier GE. Histological study of 55 renal biopsies in hypertensive patients. Union Med Can. 1955;84:1134–1142. [PubMed] [Google Scholar]
- 16.Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol. 1958;34:685–715. [PMC free article] [PubMed] [Google Scholar]
- 17.Hughson MD, Gobe GC, Hoy WE, et al. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis. 2008;52:18–28. doi: 10.1053/j.ajkd.2008.03.023. doi:10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Iturbe B, Quiroz Y, Ferrebuz A, et al. Evolution of renal interstitial inflammation and NF-kappaB activation in spontaneously hypertensive rats. Am J Nephrol. 2004;24:587–594. doi: 10.1159/000082313. doi:10.1159/000082313. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Iturbe B, Quiroz Y, Nava M, et al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Iturbe B, Zhan CD, Quiroz Y, et al. Antioxidant-rich diet relieves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension. 2003;41:341–346. doi: 10.1161/01.hyp.0000052833.20759.64. doi:10.1161/01.HYP.0000052833.20759.64. [DOI] [PubMed] [Google Scholar]
- 21.Franco M, Martínez F, Quiroz Y, et al. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293:R251–R256. doi: 10.1152/ajpregu.00645.2006. doi:10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 22.Herrera J, Ferrebuz A, MacGregor EG, et al. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–S225. doi: 10.1681/ASN.2006080918. doi:10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 23.Preddie DC, Markowitz GS, Radhakrishnan J. Mycophenolate mofetil for the treatment of interstitial nephritis. Clin J Am Soc Nephrol. 2006;1:718–722. doi: 10.2215/CJN.01711105. doi:10.2215/CJN.01711105. [DOI] [PubMed] [Google Scholar]
- 24.Torpey N, Barker T, Ross C. Drug-induced tubulo-interstitial nephritis secondary to proton pump inhibitors: experience from a single UK renal unit. Nephrol Dial Transplant. 2004;19:1441–1446. doi: 10.1093/ndt/gfh137. doi:10.1093/ndt/gfh137. [DOI] [PubMed] [Google Scholar]
- 25.Parkhie SM, Fine DM, Lucas GM, et al. Characteristics of patients with HIV and biopsy-proven acute interstitial nephritis. Clin J Am Soc Nephrol. 2010;5:798–804. doi: 10.2215/CJN.08211109. doi:10.2215/CJN.08211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Su T, Li XM, et al. Aristolochi acid nephropathy: variation in presentation and prognosis. Nephrol Dial Transplat. 2011;27:292–298. doi: 10.1093/ndt/gfr291. doi:10.1093/ndt/gfr291. [DOI] [PubMed] [Google Scholar]
- 27.Álvarez V, Quiroz Y, Nava M, et al. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol. 2002;283:F1132–F1141. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 28.Vanegas V, Ferrebuz A, Quiroz Y, et al. Hypertension in Page (cellophane-wrapped) kidney is due to interstitial nephritis. Kidney Int. 2005;68:1161–1170. doi: 10.1111/j.1523-1755.2005.00508.x. doi:10.1111/j.1523-1755.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 29.Borst JG, Borst-De Geus A. Hypertension explained by Starling's theory of circulatory homoeostasis. Lancet. 1963;1:677–682. doi: 10.1016/s0140-6736(63)91443-0. doi:10.1016/S0140-6736(63)91443-0. [DOI] [PubMed] [Google Scholar]
- 30.Guyton AC, Coleman TG, Cowley AV, Jr, et al. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. doi:10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 31.Sharma K, Cook A, Smith M. TGF-beta impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol. 2005;288:F1069–F1077. doi: 10.1152/ajprenal.00345.2004. doi:10.1152/ajprenal.00345.2004. [DOI] [PubMed] [Google Scholar]
- 32.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C dependent buffering mechanism. Nature Med. 2009;15:545–552. doi: 10.1038/nm.1960. doi:10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 33.Machnik A, Dhalmann A, Kopp C, et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhyancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55:755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339. doi:10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]