Abstract
X-linked inhibitor of apoptosis protein (XIAP)-associated factor 1 (XAF1) has been implicated as a novel tumor suppressor, which was proposed to exert pro-apoptotic effect by antagonizing the anticaspase activity of XIAP. Here, we delineated the domain architecture of XAF1 by applying limited proteolysis and peptide mass fingerprinting analysis. Our results indicated that XAF1 has a distinct domain organization, with a highly compact N-terminal domain (XAF1NTD) followed by a middle domain (XAF1MD), a 42-residue unstructured linker and a C-terminal domain (XAF1CTD). The search of XIAP binding region within XAF1 revealed that a modest affinity XIAPRING binding site (dissociation constant, Kd, ∼18 μM) is located at the C-terminal portion of XAF1. This C-terminal region, embracing XAF1CTD and a flexible tail at C-terminus (residue Thr251-Ser301), is functionally identified as XIAPRING-binding domain of XAF1 (XAF1RBD) in the present study. We have also mapped the interaction sites for XAF1RBD on XIAPRING by using NMR spectroscopy. By applying in vitro ubiquitination assay, we observed that XAF1RBD/XIAP interaction is essential for the ubiquitination of GST-XAF1RBD fusion protein. In addition, the C-terminal XAF1 fragment harboring XAF1RBD was found to be substantially ubiquitinated by XIAPRING. Base on these observations, we speculate a possible role of XAF1RBD in targeting XAF1 for XIAP-mediated ubiquitination.
Keywords: XAF1, XIAP, RING, ubiquitination, limited proteolysis, domain organization, apoptosis, protein-protein interaction, NMR spectroscopy
Statement
This work describes the systematic delineation of the domain architecture of XIAP-associated factor 1 (XAF1), which is a novel tumor suppressor and known to be a negative regulator of XIAP. We have also identified and characterized the interacting domains between XAF1 and XIAP by using GST pull-down assay, NMR spectroscopy, isothermal titration calorimetry, and in vitro ubiquitination assay. Based on these results, we proposed the possible roles of XAF1 in XIAPRING-mediated ubiquitination and apoptosis.
Introduction
X-linked inhibitor of apoptosis protein (XIAP)-associated factor 1 (XAF1) is a 34 kDa pro-apoptotic zinc-finger rich protein, which has been implicated as a putative tumor suppressor.1 The xaf1 is an interferon (INF)-stimulated gene, and XAF1 is ubiquitously expressed in normal adult and fetal tissue. However, XAF1 mRNA expression is epigenetically down-regulated in various cancer cell lines by promoter hypermethylation.2–4 Although the precise epigenetic regulating mechanisms remain largely undetermined, the restoring of XAF1 level in the tumor, either by epigenetic therapy or application of recombinant protein, represents a promising novel therapeutic approach in treatment of cancer.
At present, the exact mechanisms of XAF1 in affecting apoptosis remain unclear. Initially, it was proposed that XAF1 exerted its pro-apoptotic effect by directly antagonizing the anticaspase activity of XIAP, and triggering the nuclear sequestration of XIAP.1 Subsequent studies demonstrated that XAF1 played multiple roles in apoptosis, such as induction of Bax expression, activation of mitochondrial pathway, and degradation of XIAP.5 Although the apoptotic significances of XIAP-XAF1 interaction remain to be elucidated, the pro-apoptotic mechanisms of XAF1 are clearly more versatile than previously expected.
The current knowledge of the domain architecture and modular function of XAF1 is putative in nature. It is derived chiefly from multiple sequence alignment1,2 and the identification studies of truncated splice variants of XAF1.6,7 As a leading member of the IAP family of proteins, XIAP has been well characterized and extensively studied. XIAP has been shown to consist of three baculoviral IAP repeat (BIR) domains (XIAPBIR1-3),8 an evolutionarily conserved ubiquitin-associated domain (XIAPUBA)9 and a RING-finger domain (XIAPRING) conferring ubiquitin protein ligase (E3) activity.10 However, the molecular determinants involved in XIAP-XAF1 interaction remains undetermined.
In this report, limited proteolysis has been applied to identify three distinct domains in XAF1. Subsequently, nuclear magnetic resonance (NMR) spectroscopy, isothermal titration calorimetry (ITC), and GST pull-down experiments have been used to locate the XIAPRING-binding domain in XAF1 (XAF1RBD), which has been found to serve as the sole molecular determinant to mediate XAF1′s interaction with XIAP. In addition, we have used NMR chemical shift perturbation (CSP) to map the XAF1 binding site on XIAPRING. Unexpectedly, we observed that a C-terminal fragment of XAF1 harboring the XAF1RBD is a target for XIAPRING-mediated ubiquitination. Based on these results, we will discuss the possible roles of XAF1RBD in XIAPRING-mediated ubiquitination and apoptosis.
Results
Domain organization of human XAF1
The domain architecture of full-length human XAF1 (residues Met1-Ser301, Swiss-Prot entry Q6GPH4) has not been clearly defined. Previous protein sequence analysis on XAF1 predicted that it contained seven zinc finger (Znf) motifs1 [Fig. 1(C)]. The N-terminal region, embracing the first five Znf motifs, is a homologous counterpart (∼41% identity) of the TRAF-typed zinc finger domain in human FLN29 protein.1
Figure 1.
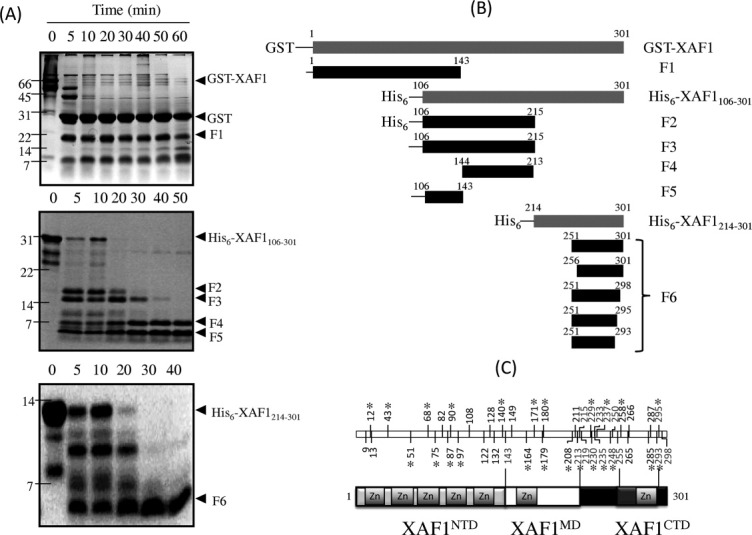
Domain organization of XAF1. (A) Time-course of limited proteolysis of XAF1 and its fragment. GST-XAF1 (top panel), His6-XAF1106–301 (middle panel) and His6-XAF1214–301(bottom panel) was subjected to proteolysis by trypsin and visualized by Coomassie blue stained SDS-PAGE, respectively. Major proteolytic fragments marked with black arrowheads were identified by MALDI-TOF MS and are shown schematically in (B). Endpoint numbering follows full-length XAF1 numbering scheme. The mass spectrometric data used to identify fragment endpoints is available as Supporting Information. (C) (Top panel) Summary of the trypsin-sensitive (light gray) and trypsin-resistant (black) cleavage sites of XAF1 identified in the present study. Asterisk indicates lysine residue. (Bottom panel) Schematic domain organization of full-length XAF1. Structural domain regions are color coded: Light gray, XAF1NTD; white: XAF1MD; dark gray: XAF1CTD. Flexible regions are colored as black. ZN donates zinc finger motif.
To investigate the domain organization of XAF1 experimentally, we performed limited proteolysis using trypsin digestion enzyme. Proteolytic stable products were separated by gel electrophoresis, followed by in-gel digestion and peptide mass fingerprinting (PMF) using matrix-assisted laser desorption/ionization time of flight mass spectroscopy (MALDI-TOF MS) [Fig. 1(A) and Supporting Information Table S1). Partially purified GST-XAF1 protein, when subjected to proteolytic treatment with trypsin, gave two intense bands (F0 and F1) above the 14 kDa marker position on a Coomassie-stained SDS-PAGE [Fig. 1(A), top panel]. A database search and manual verification of PMF data of F0 and F1 showed that the peptide bonds of the arginine residue at the linker region (LVPRGSPEF) of GST-XAF1 expression construct and at sequence position 143 were cleaved, resulting in the release of the GST moiety (F0) and a trypsin-resistant XAF1 fragment (F1, residues Met1-Arg143), respectively [Fig. 1(B)].
In order to provide further support for the existence of a structural domain within this region of residues Met1-Arg143, we performed circular dichroism (CD) and two-dimensional 1H-15N Heteronuclear Single Quantum Coherence (HSQC) NMR spectroscopy on a purified XAF1 fragment corresponding to this region. The far-UV CD spectrum showed two negative bands of different magnitudes at around 207 and 222 nm, characteristic of α-helical and β-sheets structures [Fig. 2(A), top panel]. The result indicated that this XAF1 fragment exhibits a well-defined secondary structure. The NMR data also supported that it has a well-folded protein core. The amide cross-peaks of the 1H-15N HSQC spectrum were well-dispersed [Fig. 2(B)]. Furthermore, the CD-monitored denaturation curves were sigmoidal in shape, indicating the denaturation is a cooperative process, thereby strongly suggesting the existence of a compact domain in this N-terminal region of XAF1 [Fig. 2(A), bottom panel]. Therefore, residues Met1-Arg143 of XAF1 is defined as an N-terminal domain, XAF1NTD, in the present study.
Figure 2.
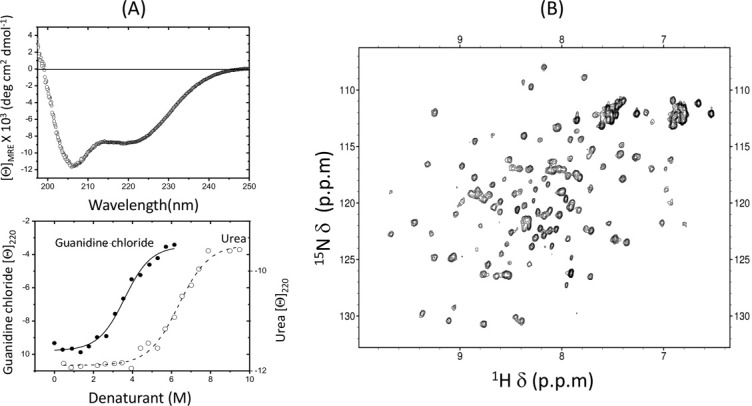
Structural characterization of XAF1NTD. (A) (Top panel) The far-UV CD spectrum of XAF1NTD. (Bottom panel) Urea-induced (open circle) and guanidine chloride-induced (close circle) equilibrium denaturation curves measured as ellipticity of XAF1NTD at 222 nm. (B) 1H-15N HSQC spectrum of XAF1NTD.
To explore the domain architecture of C-terminal region of XAF1, we generated a His6-tagged XAF1 fragment (His6-XAF1106–301, residues Gly106-Ser301) and subjected it to limited proteolysis using trypsin enzyme digestion. Proteolysis of His6-XAF1106-301 gave rise to two moderately stable fragments in gel bands F2 and F3 immediately after the addition of protease. Prolonged incubation with trypsin yielded two proteolytic stable fragments in bands F4 and F5 [Fig. 1(A), middle panel]. The PMF analysis along with the mass measurements (Supporting Information Tables S1 and S2) indicated that bands F2 and F3 correspond to a fragment encompassing the same amino acid sequence (residues Gly106-Arg215), except that F3 lacks the N-terminal His6-tag [Fig. 1(B)]. The XAF1 fragment in band F3 was further cleaved at the residues Arg143 and Lys213, resulting in peptide bands F4 (residues Ile144-Lys213) and F5 (residues Gly106-Arg143, containing the C-terminus Znf motif of XAF1NTD), respectively [Fig. 1(B), middle panel]. These results established that residues Ile144-Lys213 represent a newly identified middle domain (XAF1MD), which is consecutively connected to the XAF1NTD at residue Arg143.
For the remaining C-terminal portion of the XAF1, we have conducted similar digestion studies on a purified His6-tagged XAF1 fragment, His6-XAF1214-301, corresponding to residues Thr214-Ser301. A third protected fragment corresponding to gel band F6 [Fig. 1(A), bottom panel] was located at the C-terminal end of the XAF1 protein. According to the MS data, band F6 contained a mixture of peptides, sharing a common segment, residues Gly256-Lys293, at the interior region of His6-XAF1214–301 [Fig. 1(B), Supporting Information Table S2). This XAF1 segment is denoted as C-terminal domain (XAF1CTD), which is separated from the XAF1MD by a 42-residue spanning linker (residues Thr214-Arg255). Lysine and arginine residues within this linker were all susceptible to proteolysis, suggesting that the linker is structurally disordered. The XAF1CTD is followed by a short C-terminal flexible tail covering residues Leu294-Ser301.
Based on these limited proteolysis data, we concluded that human XAF1 possesses distinct domain architecture with well-defined domain boundaries [Fig. 1(C)]: Met1-Arg143 (XAF1NTD), Ile144-Lys213 (XAF1MD), and Gly256-Lys293 (XAF1CTD). A spanning unstructured 42-residue linker connects the XAF1MD and XAF1CTD, which was readily accessible to trypsin digestion. Residues within the XAF1NTD were predicted to form a TRAF-like zinc finger domain.1 Our CD and NMR data provided further support for the existence of a compact structural moiety within this N-terminal part of human XAF1.
Identification of XIAPRING binding domain in XAF1
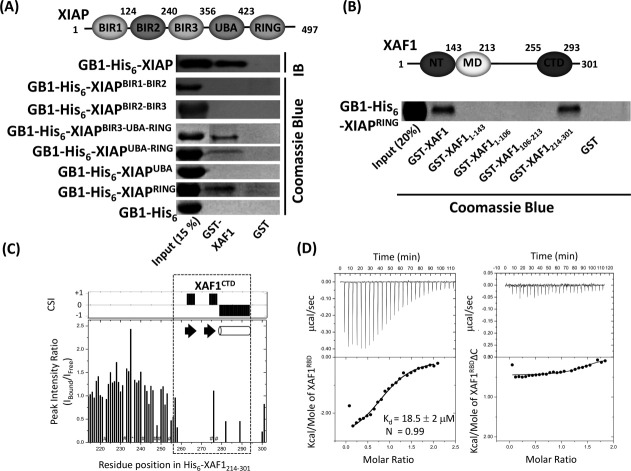
In an attempt to identify the molecular determinant(s) of XAF1 that mediates the interaction with XIAP, in vitro GST pull-down assay was conducted to evaluate the interaction of GST-XAF1 with a panel of GB1-His6 tagged full-length XIAP (GB1-His6-XIAP) and its domain fragments. A band at the molecular mass of GB1-His6-XIAP, which immune-reacted with anti-XIAP antibody, was detected with GST-XAF1 but not with GST alone [Fig. 3(A)]. As shown in Figure 3(A), all XIAPRING-bearing fragments, as well as the XIAPRING alone, showed interactions with GST-XAF1. Our result is in agreement with previous report that XAF1 directly interacted with all RING-bearing IAPs.11 In a subsequent series of pull-down assays using various truncated GST-XAF1 fragments as baits, we found that XAF1 was solely associating with XIAPRING via its C-terminal portion (residues Thr214-Ser301), including the XAF1CTD [Fig. 3(B), lane 6].
Figure 3.
Identification of XAF1RBD in XAF1. (A) (Top panel) Schematic diagram showing the domain organization of XIAP. The domain boundaries of the protein constructs, used in this study, are indicated. (Bottom panel) A GST pull-down assay showing that the GST-tagged XAF1 specifically binds to XIAPRING. IB: Immunoblot analysis (anti-XIAP antibody). (B) (Top panel) Schematic representation of the domain organization of XAF1 with the domain boundaries identified in this study. A GST pull-down assay indicates that the C-terminal region of XAF1 binds to XIAPRING. (C) The calculated peak intensity ratio of the C-terminal XAF1 fragment (His6-XAF1214–301) after the addition of XIAPRING. Chemical shift index (CSI) plot is indicated on top. Proline and the residues that exhibited peak broadening are marked by asterisks and numbered sign, respectively. The region of XAF1CTD is indicated by dashed-line rectangle. (D) ITC titrations of XIAPRING with XAF1RBD (left panel) or XAF1RBDΔC (right panel). Deletion of C-terminal flexible tail of XAF1 abolished the binding.
To further refine the XIAPRING-binding region of XAF1 within residues Thr214-Ser301, we performed 1H-15N HSQC titration experiments of 15N-His6-XAF1214–301 with unlabeled XIAPRING. Upon XIAPRING binding, various resonances of the 15N-His6-XAF1214–301 disappeared, suggesting that this interaction falls into the intermediate exchange regime on the NMR chemical shift time scale (Supporting Information Fig. S1). At intermediate exchange, NMR resonances of the residues involved in intermolecular interaction will be broadened, leading to the weakening and/or disappearing of their signals.12 For a quantitative evaluation, the peak intensity ratios between free and bound 15N-His6-XAF1214-301 were evaluated using a molar ratio of 1:1 sample (Supporting Information Fig. S1 and Fig. 3C). A majority of peaks corresponding to the backbone amide signals within residues Ala259-Ser301 disappeared. This suggested that the segment corresponding to residues Ala259-Ser301 forms the binding region of XIAPRING. Therefore, we have designated the region covering residues Thr251-Ser301, which includes the XAF1CTD and the flexible C-terminus tail of XAF1, as XIAPRING-binding domain (XAF1RBD) in the present study.
To quantify the affinity between XAF1RBD and XIAPRING, isothermal titration calorimetry (ITC) experiment was performed using purified XAF1RBD and XIAPRING [Fig. 3(D), left panel]. The dissociation constant (Kd) was determined to be 18.5 ± 2 μM. The binding stoichiometry was measured to be around 0.9. This result showed that XAF1RBD contains a single moderate-affinity binding site for the XIAPRING. The C-terminal deletion mutant of XAF1RBD, XAF1RBDΔC (residues Thr251-Ser292), showed no detectable binding by ITC [Fig. 3(D), right panel]. This result suggested that the flexible C-terminus tail of XAF1 (residues Lys293-Ser301) is essential for interaction with XIAPRING. The binding affinity observed between XAF1RBD and XIAPRING is highly comparable with those reported for the association of ubiquitin conjugating enzyme UbcH5B (E2) with the RING domain of cIAP-2 (cIAP-2RING) (Kd = 19–43 μM, as determined by ITC) and with XIAPRING (as determined by GST pull-down experiment).13
Mapping of XAF1RBD-binding surface on XIAPRING
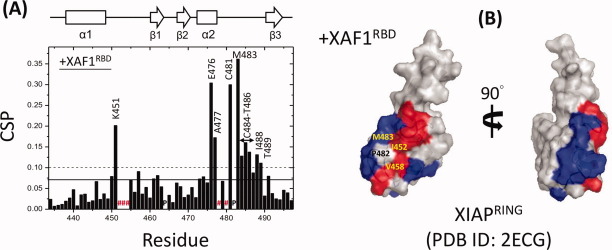
To localize the XAF1RBD binding site on XIAPRING, NMR chemical shift perturbation (CSP) experiments were performed by monitoring the 1H-15N TROSY-HSQC spectrum of 15N-XIAPRING upon the addition of increasing amounts of unlabeled XAF1RBD. During the course of titration, we observed both significant chemical shift perturbations and cross-peak intensity attenuations in certain backbone amide resonances, indicating that these residues are likely located at or close to the binding interface. Mapping the locations of these significantly changed residues onto the structure of XIAPRING (PDB ID: 2ECG) revealed that residues in the stretches of Lys451-Met454 (α1-β1 loop) and Glu476-Thr489 (C-terminal portion of α2 helix and α2-β3 loop) contributed to a specific XAF1RBD-binding pocket on XIAPRING [Supporting Information Fig. S2 and Fig. 4(A,B)].
Figure 4.
Mapping of XAF1RBD binding interface on XIAPRING. (A) The residue-wise chemical shift perturbation (CSP) in XIAPRING upon the addition of XAF1RBD. The average (solid line) and the average plus one standard deviation (SD) (dotted line) of CSP values are indicated. Residues that exhibited peak broadening are marked by red numbered sign. Prolines are marked by “P.” The secondary structures along the sequence are marked at the top. (B) Surface map of significantly perturbed residues in XIAPRING upon XAF1RBD binding. Residues with a CSP value higher than one SD above the average was considered as significantly perturbed and are colored blue. Peaks broadening residues are colored red.
The RING domain is known to function as an E3 ligase, which confers specificity to ubiquitination by serving as a scaffold to recruit ubiquitin-charged E2 (Ub-E2).14,15 However, the molecular details of how the RING domain, in the ternary complex, mediating the transfer of ubiquitin from E2 to target substrate is still largely unknown.15 Previous studies demonstrated that the RING domains of IAP family members associated with overlapping but partially distinct subset of E2s.16 XIAP and cIAP-2 could interact with UbcH7 and all of the UbcH5 family members (i.e., UbcH5A-C). Recently, an X-ray crystallographic study revealed that interaction between cIAP-2RING and UbcH5B involves the cIAP-2RING residues in the helix α1, α1-β1 loop, and α2-β3 loop.13 It is intriguing to note that the XIAPRING residues perturbed upon XAF1RBD binding are located near to the primary sequence positions [Met483, Ile452, and Val458, Fig. 4(B)] where homologous residues of cIAP-2RING are involved in the interaction with E2.13 Therefore, XAF1 may act as either a competitive inhibitor of XIAPRING-dependent ubiquitination cascade by displacing E2 from XIAPRING, or simply as a substrate for ubiquitination.
GST-XAF1RBD is a target for ubiquitination through interaction with XIAPRING
Based on the present ITC measurement and previously reported studies,13 XIAPRING possesses a comparable affinity for binding of XAF1RBD and UbcH5b. To test whether XAF1RBD could act as a competitive inhibitor of XIAPRING-dependent ubiquitination cascade by displacing E2 from XIAPRING, in vitro E3 ligase activities of XIAPRING in the presence and absence of XAF1RBD were measured. The in vitro E3 ligase activity of XIAPRING, as shown by its autoubiquitination, remained unchanged even in the presence of 10-fold molar excess of XAF1RBD. This result implied that XAF1RBD cannot effectively displace E2 and to inhibit the ligase activity of XIAPRING (Supporting Information Fig. S3).
Unexpectedly, ubiquitinated GST-XAF1RBD was detected by using anti-GST antibody during western blotting of a standard in vitro ubiquitination assay using GST-XAF1RBD as the substrate and GB1-His6-XIAP as the E3 ligase. It should be noted that the ubiquitination could also be observed even using GST-XAF1RBD (NO-K) [Fig. 5(B)], which is a GST-XAF1RBD mutant where all lysine residues in the XAF1RBD have been replaced by arginine (K258R, K285R, K293R, K295R) thereby impeding ubiquitin conjugation on the XAF1RBD moiety. In contrast, when GST alone or GST-tagged XAF1RBDΔC was included in the ubiquitination assay, ubiquitinated adducts of GST or GST-XAF1RBDΔC were not observed [Fig. 5(B)]. Given that the XIAP binding affinity for the GST-XAF1RBD (NO-K) is not significantly different from that of the wild type GST-XAF1RBD [Fig. 5(A)], our result indicated that XAF1RBD/XIAP interaction induces the ubiquitination of GST moiety in the GST-XAF1RBD via the E3 ligase activity of XIAPRING in GB1-His6-XIAP. Disruption of the interaction between XAF1RBD and XIAPRING by deleting the C-terminal tail of XAF1RBD will totally abolish this effect [Figs. 3(D) and 5(A) lane 4 and 5(B) lane 5). These results suggested that XAF1RBD may function as a promoter of XIAPRING-mediated ubiquitination.
Figure 5.
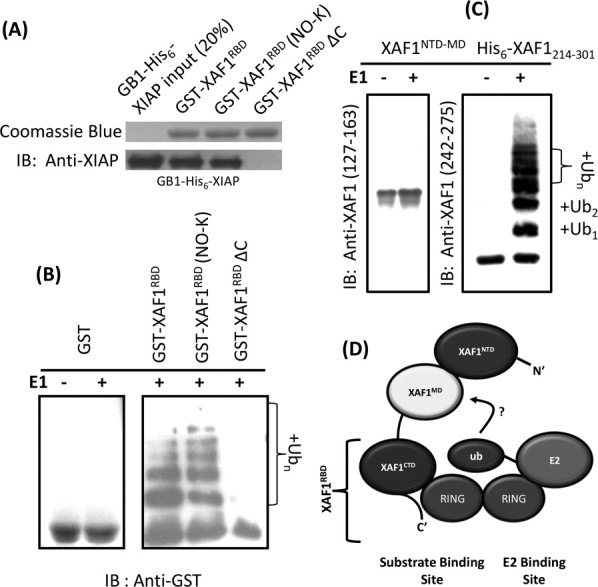
Protein harboring XAF1RBD is a substrate of XIAPRING-mediated ubiquitination. (A) GST pull-down assays show that GB1-His6-XIAP interacts with GST- XAF1RBD and GST-XAF1RBD (NO-K) but not with GST-XAF1RBDΔC. (B) In vitro XIAP-mediated ubiquitination assay of GST-XAF1RBD and its mutant. Binding of XAFRBD and XIAP induces ubiquitination of GST moiety at N-terminal. (C) In vitro XIAPRING-mediated ubiquitination assay of XAF1 fragments. XAF1 fragment containing XAF1RBD is ubiquitinated. IB: Immunoblot analysis (anti-GST antibody for panel B, anti-XAF1 (127–163), and anti-XAF1 (242–275) antibodies for panel C). (D) Proposed mechanism of XIAPRING for its dual role in XAF1 recognition and ubiquitination. The ubiquitin is transferred from E2-Ub to XAF1 in an unknown manner.
The C-terminal XAF1 fragment is ubiquitinated by XIAPRING
We speculate that the interaction between XIAPRING and XAF1RBD at the C-terminal end of full-length XAF1 may somehow induce the XIAPRING-mediated ubiquitination of the N-terminal portion of a XAF1 fragment containing the XAF1RBD. Indeed, the N-terminal truncated XAF1 fragment (His6-XAF1214–301), harboring the XAF1RBD and the unstructured XAF1 linker, was found to be significantly ubiquitinated by XIAPRING. On the other hand, the C-terminal truncation XAF1 mutant (residues Met1-Lys213, XAF1NTD-MD), composed of XAF1NTD and XAF1MD only, showed no ubiquitination by XIAPRING [Fig. 5(C)].
Discussion
XIAP-associated factor 1 (XAF1) is a 301-amino acids interferon (INF)-inducible pro-apoptotic protein. It is found to have extremely low level in numerous cancer cells,2 thereby implicating XAF1 as a putative tumor suppressor.1 The molecular mechanisms underlying the pro-apoptotic activity of XAF1 remain unclear. XAF1 was initially proposed to antagonize the anticaspase activity of XIAP by nuclear sequestration of XIAP-XAF1 complex.1 It was later reported that XAF1 can translocate into mitochondria and activates the intrinsic pathway of apoptosis.5
The molecular determinant involved in XIAP-XAF1 interaction remains largely unknown except it has been reported that XAF1 can interact with all RING-bearing IAPs including XIAP.11 Here, we have extended this interaction study with the identification of the XAF1RBD domain responsible for the interaction with XIAP. We have accidentally discovered that this XAF1RBD could induce the ubiquitination of the GST fusion tag at its N-terminal via its interaction with the RING domain of XIAP. Following up on this discovery, the C-terminal XAF1 fragment containing XAF1RBD was found to be substantially ubiquitinated by XIAPRINGin vitro. It should be noted that the C-terminal region of XAF1 has been suggested to be crucial for its pro-apoptotic activity in the previous studies.6,17 An artificially C-terminal truncated form of XAF1 (residues 1–178) has also been reported to act in a dominant negative manner, blocking the ability of INF-β to sensitize cell to TRAIL-induced apoptosis.17 In addition, a previous study showed that the mRNA expression ratio of XAF1A (full-length) and XAF1C (splice variant contains only the XAF1NTD) differs among cancer cell lines derived from a variety of tissue types.6 Our results implied the possibility that the ubiquitination of putative tumor suppressor XAF1, presumably via association between XAF1RBD and XIAPRING, may be involved in regulating apoptosis.
The role of XIAP in the ubiquitin-mediated regulation of apoptosis is emerging.10 XIAPRING has been demonstrated to be required for the ubiquitination and proteasomal degradation of several XIAP-associated apoptosis-related proteins, such as caspase-318 and direct inhibitor of apoptosis-binding protein with low pI (DIABLO).19 In this sense, XIAPRING may also regulate the cellular level of XAF1 through the ubiquitin-proteasome pathway. In particular, it is known that the cellular expression ratio of XIAP and XAF1 could control the cell fate.20,21 Furthermore, it is possible that XAF1 may act as a promoter of the XIAP-mediated ubiquitination. It should be noted that XIAP-mediated ubiquitination of XAF1 has been reported to be involved in the proteasomal degradation of a ternary complex of XAF1-XIAP-Survivin.11 In this ternary complex, XAF1 may either be itself ubiquitinated or promote the XIAP-mediated ubiquitination of Survivin in the vicinity of XAF1-XIAP, in a manner similar to the ubiquitination of GST moiety in GST-XAF1RBD observed in the present work. Although the same study also demonstrated that XAF1 can associate with all RING-bearing IAPs,11 we cannot exclude the possibility that ubiquitination of XAF1, mediated by the RING domain of XIAP or other IAPs, may serve nonproteasomal regulatory functions such as signal transduction22,23 and intracellular trafficking among compartments and organelles.24 It was reported that cIAP-1 and cIAP-2 can mediate K6-linked,25 K11-linked,26 and monoubiquitination27 of substrates for nonproteasomal functions. In future experiments, it will be interesting to fully characterize the physiological ubiquitination function of XAF1 in apoptosis.
It has been reported that the RING domains in IAPs belong to the dimeric RING finger family.28 By gel filtration analysis, the estimated molecular mass of purified XIAPRING protein, in the present study, was 15,512 Da, which is consistent with a dimeric form of XIAPRING (Supporting Information Fig. S4). During ubiquitination, the RING domain dimer serves as a scaffold to bind Ub-E2 and mediates the transfer of ubiquitin to the target substrate, which interacts with the substrate binding domain in conjunction with either protomer RING units.15 To catalyze the ubiquitination, IAP protein possesses BIR domain for substrate recognition and capture.28,29 Several XIAP-associated proteins, including DIABLO, caspase-3, and apoptosis-inducing factor (AIF),30 contain a canonical IAP binding motif (IBM) which interacts with the IBM-interaction groove locating on the BIR domain of XIAP,8 and thereby presumably enabling them to be ubiquitinated by the RING domain of XIAP.29 Our result showed that XAF1 do not interact with any BIR domains of XIAP [Fig. 3(A)], which is consistent with the fact that XAF1 does not contain any IBM motif. Instead, the C-terminal XAF1 fragment (residues Thr214-Ser301) containing XAF1RBD could serve as an ubiquitination substrate of XIAPRING alone. The XAF1/XIAP interaction seems to be mediated primarily, if not solely, by the specific interaction between XAF1RBD and XIAPRING. It should also be noted that although the XAF1RBD-binding site on XIAPRING was shown to be overlapping partially with the regions required for UbcH5-binding, 10-fold molar excess of XAF1RBD was not able to inhibit the action of UbcH5b in the autoubiquitination experiment of GB1-His6-XIAPRING. Therefore, we speculate that both XAF1RBD and E2-Ub simultaneously bind to a XIAPRING dimer. One protomer RING unit serves as a substrate-recognition site to bind XAF1RBD while the binding of E2-Ub occurs at the second protomer RING unit. This will catalyze the formation of isopeptide bond between the ubiquitin and the lysine residue of the C-terminal XAF1 fragment anchoring on the complementary protomer [Fig. 5(D)]. Therefore, XAF1RBD/XIAPRING interaction appears to be important for the substrate-recognition for XIAP-mediated ubiquintation. However, at present, we cannot discern whether XAF1RBD will promote ubiquintation on other XIAP-associated proteins, such as DIABLO, caspase-3, AIF, and Survivin. Further studies will be needed to fully characterize the functional role of XAF1RBD in the context of apoptosis regulation through XIAP-mediated ubiquitination.
Materials and Methods
Protein expression and purification
The genes encoding full-length human XIAP (Met1-S497) and its domain containing fragments (Met1-Glu240 (XIAPBIR1-BIR2), Glu240-Thr356 (XIAPBIR2-BIR3), Glu240-Ser497 (XIAPBIR3-UBA-RING), Thr356-Ser497 (XIAPUBA-RING), Thr356-Gln433 (XIAPUBA), and Glu434-Ser497 (XIAPRING)) were ligated into pGEX-4T-1 (GE-healthcare) and pGB1-HIS vectors. Likewise, the DNA sequences encoding the full-length human XAF1 (Met1-Ser301) and its fragments, (Met1-Gly106, Met1-Arg143(XAF1NTD), Met1-Lys213(XAF1NTD-MD), Gly106-S301, Gly106-Lys213, Thr214-Ser301, Thr251-Ser301(XAF1RBD), and Thr251-Ser292 (XAF1RBDΔC)), were all subcloned into pGEX-4T-1, pET-H, and pGB1-HIS,9 yielding fusion proteins with an N-terminal GST, His6, and GB1-His6 tags, respectively. XAF1RBD mutants were prepared by site-directed mutagenesis of the wild-type GST-tagged expression construct using a QuickChange Site-Directed Mutagenesis Kit (Stratagene). For recombinant protein expressions, the DNA constructs were transformed into Escherichia coli host BL21 (DE3) (Novagen). Unlabeled protein was prepared from cells grown in Luria-Bertani (LB) media supplemented with 50 μM ZnSO4. Uniformly 15N-labeled proteins were obtained from cells grown in M9 minimal media incorporating [U-15N]-ammonium chloride (Cambridge Isotopes Laboratories, CIL) as the sole nitrogen source. The expression and purification of His6-, GB1-His6-, and GST-tagged XAF1 and XIAP proteins were essentially the same as previously described.9 Briefly, XAF1NTD, XAF1NTD-MD, XAF1RBD, and XIAPRING protein domains were purified by GST affinity chromatograph, on column thrombin digestion at 25°C for 12 h, and gel filtration on a Superdex 75 prep grade column (GE healthcare). The His6- and GST-tagged E2s (UbcH5A-C) were expressed and purified as previously described.31 All protein samples were prepared in buffer HB (20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM DTT).
Limited proteolysis and MALDI-TOF MS analysis
All proteins were concentrated to 1 mg/mL before limited proteolysis experiment. Limited proteolyses by trypsin (Fluka, TCPK-treated) were carried out at room temperature in a protein to protease ratio of 3000: 1. After the incubation has started, samples were collected at 0, 5, 10, 20, 30, 40, 50, and 60 min. PMSF (2 mM) was added to terminate the reaction. The samples were divided into two equal portions. One half of the sample was mixed with 3× Laemmli buffer, followed by SDS-PAGE electrophoresis and Coomassie blue gel staining. Gel bands were excised and submitted to the Research Genome Center core services at The University of Hong Kong for MALDI-TOF PMF (Supporting Information Table S1). Another half of the sample was treated with 0.1% TFA. Thereafter, molecular masses of the proteolytic products were analyzed by Voyager linear MALDI-TOF spectrometer (PerSeptive Biosystems) (Supporting Information Table S2).
Circular dichroism (CD) spectroscopy
All the far-UV (195–250 nm) CD spectra were recorded on a JASCO model 810 instrument at 25°C using 1 mm path-length sample cell. About 0.5 mg/mL of protein were prepared in buffer HB for the experiment. For the denaturant-induced unfolding, stock solutions of guanidine chloride (8M) and urea (9.8M) were added to 200 μL of 0.2 mg/mL protein at various concentrations (0–8M). The mixture was incubated at 25°C for at least 1 h before far-UV CD measurement.
In vitro GST-pull down assay and ubiquitination assay
Proteins used in GST pull-down assay were purified as described in the previous section. Purified GST or GST-tagged XAF1 fragments (200 μg each) were incubated with GB1-His6-XIAP domains (100 μg each) at 4°C for 2 h in the presence of 0.1% BSA. The complex was immobilized on Glutathione-Sepharose 4B beads (GE Healthcare), followed by extensively washing in PBS. The bound materials were eluted with 3× Laemmli buffer and analyzed by SDS-PAGE followed by Coomassie Blue staining or ImmunoBlot (IB) analyses with an anti-XIAP antibody (Thermo Scientific).
For in vitro ubiquitination assay of GST-XAF1RBD, substrates including GST, GST-XAF1RBD, and mutated GST-XAF1RBD were incubated, respectively with Glutathione-Sepharose 4B resin, followed by extensive washing with buffer U (10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 3 mM MgCl2, 2 mM DTT, 5 mM ATP). The resin was then resuspended in 30 μL of a reaction mixture which is named buffer U, containing 50 ng human E1 (Boston Biochem), 0.4 μg human E2 UbcH5B (Boston Biochem), 100 μg ubiquitin (Boston Biochem), and 3 μg E3 (GB1-His6-XIAP). Reactions were carried out at 30°C for 1 h. The resin was washed again by buffer U followed by boiling in 50 μL 1× Laemmli's buffer. Ubiquitinated GST-tagged proteins were separated on SDS-PAGE and detected by Anti-GST (Santa Cruz).
For ubiquitination assays of XAF1NT-MD and His6-XAF1214–301, 100 μg of substrate (XAF1NT-MD or His6-XAF1214–301) was contained in the above reaction mixture. Reactions were carried out at 37°C for 45 min, and terminated by boiling in 1× Laemmli's buffer. Ubiquitinated XAF1 proteins were separated on SDS-PAGE and detected by Anti-XAF1 (127–163) and Anti-XAF1 (215–301) (Santa Cruz), which specifically recognizes epitopes between residues 127–163 and 215–301 of XAF1, respectively.
Isothermal titration calorimetry
ITC experiments were performed with a VP-ITC instrument (MicroCal). Typically, 1 mM of XAF1RBD or XAF1RBDΔC was titrated into a 100 μM solution of XIAPRING. Experiments were carried out at 25°C in a buffer containing 10 mM HEPES, pH 7.4, 100 mM NaCl. The data were analyzed using Origin 7. The binding constant was obtained by fitting the thermogram into a one site binding model.
NMR spectroscopy and titration studies
NMR studies were performed at 298 K using a Bruker Advance 700 MHz NMR spectrometer equipped with a TCI cyroprobe. Data were acquired and processed using TopSpin 2.1 (Bruker) and SPARKY. All samples were buffer-exchanged into buffer BT (20 mM BisTris-HCl, pH 6.7, 150 mM NaCl, 5 mM d10-DTT, 1 mM PMSF, 90% H2O/10% D2O) at a protein concentration of 0.5 to 0.8 mM for all NMR studies. The sequential backbone resonance assignments of His6-XAF1214–301 and XIAPRING were obtained using two-dimensional 1H–15N HSQC, three-dimensional HNCO, HCACO, HNCACB and HN(CO) CACB spectra. The chemical shift index (CSI) was calculated from the chemical shifts of backbone carbonyl, Cα and Cβ atoms according to Wishart.32 The CSI values were used to predict the secondary structure.32
For titration studies of XIAPRING and XAF1RBD, the XAF1RBD binding surfaces on XIAPRING were mapped using the CSP approach. A series of two-dimensional 1H-15N TROSY-HSQC spectra of a 15N-XIAPRING (0.5 mM) were recorded as a function of the increasing amount of unlabeled XAF1RBD. The NMR titration experiments were performed until the molar ratios of [XAF1RBD]/[15N-XIAPRING] reached the values of 1: 1.4. Binding event was monitored by changes in the cross-peaks positions of the 1H-15N TROSY-HSQC spectra. These changes of cross-peak chemical shifts were quantified using combined amide CSP calculated as Δσ = [(ΔσH)2 + (ΔσN/5)2]1/2, where ΔσH and ΔσN are the observed chemical shift changes for 1H and 15N dimensions, respectively. The titration experiment of 15N-His6-XAF1214-301 with XIAPRING was performed essentially the same as described in the above paragraph. The effects of line broadenings were estimated by measuring the ratios of the intensities for each peak in 1H-15N HSQC spectra, in the absence of XIAPRING and the presence of a 1:1 complex.
Supplementary material
Additional Supporting Information may be found in the online version of this article.
References
- 1.Liston P, Fong WG, Kelly NL, Toji S, Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW, Korneluk RG. Identification of XAF1 as an antagonist of XIAP anti-caspase activity. Nat Cell Biol. 2001;3:128–133. doi: 10.1038/35055027. [DOI] [PubMed] [Google Scholar]
- 2.Fong WG, Liston P, Rajcan-Separovic E, St Jean M, Craig C, Korneluk RG. Expression and genetic analysis of XIAP-associated factor 1 (XAF1) in cancer cell lines. Genomics. 2000;70:113–122. doi: 10.1006/geno.2000.6364. [DOI] [PubMed] [Google Scholar]
- 3.Ng KC, Campos EI, Martinka M, Li G. XAF1 expression is significantly reduced in human melanoma. J Invest Dermatol. 2004;123:1127–1134. doi: 10.1111/j.0022-202X.2004.23467.x. [DOI] [PubMed] [Google Scholar]
- 4.Zou B, Chim CS, Zeng H, Leung SY, Yang Y, Tu SP, Lin MC, Wang J, He H, Jiang SH, Sun YW, Yu LF, Yuen ST, Kung HF, Wong BC. Correlation between the single-site CpG methylation and expression silencing of the XAF1 gene in human gastric and colon cancers. Gastroenterology. 2006;131:1835–1843. doi: 10.1053/j.gastro.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 5.Straszewski-Chavez SL, Visintin IP, Karassina N, Los G, Liston P, Halaban R, Fadiel A, Mor G. XAF1 mediates tumor necrosis factor-alpha-induced apoptosis and X-linked inhibitor of apoptosis cleavage by acting through the mitochondrial pathway. J Biol Chem. 2007;282:13059–13072. doi: 10.1074/jbc.M609038200. [DOI] [PubMed] [Google Scholar]
- 6.Yin W, Cheepala S, Clifford JL. Identification of a novel splice variant of X-linked inhibitor of apoptosis-associated factor 1. Biochem Biophys Res Commun. 2006;339:1148–1154. doi: 10.1016/j.bbrc.2005.11.128. [DOI] [PubMed] [Google Scholar]
- 7.Fang X, Liu Z, Fan Y, Zheng C, Nilson S, Egevad L, Ekman P, Xu D. Switch to full-length of XAF1 mRNA expression in prostate cancer cells by the DNA methylation inhibitor. Int J Cancer. 2006;118:2485–2489. doi: 10.1002/ijc.21636. [DOI] [PubMed] [Google Scholar]
- 8.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tse MK, Hui SK, Yang Y, Yin ST, Hu HY, Zou B, Wong BC, Sze KH. Structural analysis of the UBA domain of X-linked inhibitor of apoptosis protein reveals different surfaces for ubiquitin-binding and self-association. PLoS One. 2011;6:e28511. doi: 10.1371/journal.pone.0028511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galban S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 2010;17:54–60. doi: 10.1038/cdd.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora V, Cheung HH, Plenchette S, Micali OC, Liston P, Korneluk RG. Degradation of survivin by the X-linked inhibitor of apoptosis (XIAP)-XAF1 complex. J Biol Chem. 2007;282:26202–26209. doi: 10.1074/jbc.M700776200. [DOI] [PubMed] [Google Scholar]
- 12.Reibarkh M, Malia TJ, Wagner G. NMR distinction of single- and multiple-mode binding of small-molecule protein ligands. J Am Chem Soc. 2006;128:2160–2161. doi: 10.1021/ja055971z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mace PD, Linke K, Feltham R, Schumacher FR, Smith CA, Vaux DL, Silke J, Day CL. Structures of the cIAP2 RING domain reveal conformational changes associated with ubiquitin-conjugating enzyme (E2) recruitment. J Biol Chem. 2008;283:31633–31640. doi: 10.1074/jbc.M804753200. [DOI] [PubMed] [Google Scholar]
- 14.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 15.Plechanovova A, Jaffray EG, McMahon SA, Johnson KA, Navratilova I, Naismith JH, Hay RT. Mechanism of ubiquitylation by dimeric RING ligase RNF4. Nat Struct Mol Biol. 2011;18:1052–1059. doi: 10.1038/nsmb.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S, Yang X. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J Biol Chem. 2003;278:10055–10060. doi: 10.1074/jbc.M207197200. [DOI] [PubMed] [Google Scholar]
- 17.Leaman DW, Chawla-Sarkar M, Vyas K, Reheman M, Tamai K, Toji S, Borden EC. Identification of X-linked inhibitor of apoptosis-associated factor-1 as an interferon-stimulated gene that augments TRAIL Apo2L-induced apoptosis. J Biol Chem. 2002;277:28504–28511. doi: 10.1074/jbc.M204851200. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacFarlane M, Merrison W, Bratton SB, Cohen GM. Proteasome-mediated degradation of Smac during apoptosis: XIAP promotes Smac ubiquitination in vitro. J Biol Chem. 2002;277:36611–36616. doi: 10.1074/jbc.M200317200. [DOI] [PubMed] [Google Scholar]
- 20.Shibata T, Noguchi T, Takeno S, Gabbert HE, Ramp U, Kawahara K. Disturbed XIAP and XAF1 expression balance is an independent prognostic factor in gastric adenocarcinomas. Ann Surg Oncol. 2008;15:3579–3587. doi: 10.1245/s10434-008-0062-4. [DOI] [PubMed] [Google Scholar]
- 21.Perrelet D, Perrin FE, Liston P, Korneluk RG, MacKenzie A, Ferrer-Alcon M, Kato AC. Motoneuron resistance to apoptotic cell death in vivo correlates with the ratio between X-linked inhibitor of apoptosis proteins (XIAPs) and its inhibitor, XIAP-associated factor 1. J Neurosci. 2004;24:3777–3785. doi: 10.1523/JNEUROSCI.0413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Chen ZJ. The novel functions of ubiquitination in signaling. Curr Opin Cell Biol. 2004;16:119–126. doi: 10.1016/j.ceb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 24.Di Fiore PP, Polo S, Hofmann K. When ubiquitin meets ubiquitin receptors: a signalling connection. Nat Rev Mol Cell Biol. 2003;4:491–497. doi: 10.1038/nrm1124. [DOI] [PubMed] [Google Scholar]
- 25.Tang ED, Wang CY, Xiong Y, Guan KL. A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. J Biol Chem. 2003;278:37297–37305. doi: 10.1074/jbc.M303389200. [DOI] [PubMed] [Google Scholar]
- 26.Dynek JN, Goncharov T, Dueber EC, Fedorova AV, Izrael-Tomasevic A, Phu L, Helgason E, Fairbrother WJ, Deshayes K, Kirkpatrick DS, Vucic D. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 2010;29:4198–4209. doi: 10.1038/emboj.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H, Joazeiro CA, Bonfoco E, Kamada S, Leverson JD, Hunter T. The inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J Biol Chem. 2000;275:26661–26664. doi: 10.1074/jbc.C000199200. [DOI] [PubMed] [Google Scholar]
- 28.Vaux DL, Silke J. IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol. 2005;6:287–297. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- 29.Lipkowitz S, Weissman AM. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat Rev Cancer. 2011;11:629–643. doi: 10.1038/nrc3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson JC, Wilkinson AS, Galban S, Csomos RA, Duckett CS. Apoptosis-inducing factor is a target for ubiquitination through interaction with XIAP. Mol Cell Biol. 2008;28:237–247. doi: 10.1128/MCB.01065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorick KL, Jensen JP, Weissman AM. Expression, purification, and properties of the Ubc4/5 family of E2 enzymes. In: Raymond JD, editor. Methods in enzymology. Vol. 398. San Diego, California, USA: Elsevier Academic Press; 2005. pp. 54–68. [DOI] [PubMed] [Google Scholar]
- 32.Wishart DS. Interpreting protein chemical shift data. Prog Nucl Magn Reson Spectrosc. 2011;58:62–87. doi: 10.1016/j.pnmrs.2010.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.