Abstract
BACKGROUND/OBJECTIVES
Significant controversy exists as to the meaning of a low glomerular filtration rate (GFR) in the elderly. The goal of the study was to evaluate whether elderly patients with low GFR are at risk for anemia, hyperkalemia, acidosis, and hyperphosphatemia.
DESIGN
Retrospective study
SETTING
Veterans Affairs Medical Center
PARTICIPANTS
All patients over 65 years of age with chronic kidney disease (CKD) and a GFR between 15 and 60 mL/min/1.73m2.
MEASUREMENTS
Anemia was defined as a hemoglobin <10g/dL, hyperkalemia as a potassium >5.5mEq/L, acidosis as a bicarbonate <21mEq/L, and hyperphosphatemia as a phosphorus >4.6mg/dL. Multivariable logistic regression was used to evaluate whether age modifies the effect of low GFR on metabolic complications by including an interaction term between age and GFR in each model.
RESULTS
13874 veterans were included in the study. The average age was 79, the average GFR was 46.5; 3.1% had anemia, 2.5% hyperkalemia, 2.3% acidosis, and 4.4% had hyperphosphatemia. Lower GFR was associated with increased rates of metabolic complications across all age groups (odds ratio per 5mL/min/1.73m2 decrease in GFR in multivariable models was 1.21 for anemia, 1.26 for hyperkalemia, 1.45 for acidosis, and 1.72 for hyperphosphatemia). There was no significant interaction between age and GFR in models including only age and GFR or in multivariable models (p values for the age X GFR interaction term: 0.66 for anemia, 0.19 for hyperkalemia, 0.54 for acidosis, and 0.22 for hyperphosphatemia).
CONCLUSION
Elderly patients with CKD are at risk for anemia, hyperkalemia, acidosis, and hyperphosphatemia; age does not modify the relationship between GFR and development of metabolic complications. Elderly patients with low GFR should be monitored for metabolic complications, regardless of age.
Keywords: chronic renal insufficiency, aging, anemia, hyperkalemia, acidosis, hyperphosphatemia
INTRODUCTION
The prevalence of chronic kidney disease (CKD) is very high in the elderly population; more than 35% of patients over 70 years of age have stage 3 CKD or higher.1 In addition, the increasing prevalence of CKD in the US is driven largely by growth in elderly subjects with CKD.1 These estimates of CKD prevalence are based on the current K/DOQI definition of CKD (presence of a glomerular filtration rate (GFR) less than 60 ml/min/1.73m2 and/or proteinuria).2 There has been significant controversy as to the meaning of a low GFR in the elderly. Some investigators argue that gradual decline in GFR is an expected consequence of aging, and that rigid cut-offs of GFR result in arbitrary “labeling” of patients with a disease, especially at levels of GFR considered to be mild CKD (GFR 45–59 ml/min/1.73m2).3
CKD increases the risk of end-stage renal disease (ESRD) among the elderly, although the magnitude of risk is significantly lower compared to younger patients with CKD. The decreased magnitude of risk is presumably due to elderly patients’ increased risk for all-cause mortality and possibly a slower rate of decline in renal function.4–6 In addition, the increased cardiovascular risk conferred by CKD is seen, perhaps even accentuated, in the elderly with CKD.7, 8
Another approach to evaluate the significance of a low GFR in older patients is to assess whether lower GFR is associated with the presence of metabolic abnormalities typically seen in CKD such as anemia, hyperkalemia, acidosis, and hyperphosphatemia. Recent reports from the KEEP and NHANES studies indicate that metabolic abnormalities often accompany the diagnosis of CKD among patients over 65 years of age.9 However, whether the association between low GFR and metabolic complications is consistent across advanced age groups has not been evaluated. A robust association of the presence of metabolic abnormalities with low GFR regardless of age would argue against the benign prognosis implied by the “aging related decline in GFR” theory and reinforce the importance of the diagnosis of CKD using current cut-offs of GFR. On the other hand, if low GFR in older patients is less likely to be associated with the presence of metabolic abnormalities of CKD, perhaps a lower GFR threshold should be established for diagnosis of CKD in older patients.
The objective of the present study was to a) report the prevalence of anemia, hyperkalemia, acidosis, and hyperphosphatemia in a cohort of older patients with CKD and b) determine whether the association between GFR and anemia, hyperkalemia, acidosis, or hyperphosphatemia is modified by advanced age.
METHODS
We performed a retrospective cross-sectional study of patients in Veterans Affairs Integrated Service Network (VISN) 10. The study was approved by the Louis Stokes Cleveland VA Medical Center’s Institutional Review Board. The following data were collected from the VISN 10 data warehouse: demographic information, laboratory data, medications, and ICD-9 codes. Inclusion criteria were a) age 65 years or greater, b) an index outpatient GFR (calculated using the 4 variable MDRD equation) in 2008 between 15 and 60 ml/min/1.73m2, and c) an outpatient GFR less than or equal to 60 ml/min/1.73m2 between 90 and 365 days prior to the index GFR.10 Subjects on dialysis or who had received a kidney transplant were excluded. The most recent laboratory values simultaneous with or in the six months prior to the index GFR were used to assess for the metabolic complications of anemia, hyperphosphatemia, hyperkalemia, and acidosis. Greater than 99% of the potassium and bicarbonate levels were concurrent with the index GFR while 84% of the hemoglobin and 73% of the phosphorus levels were concurrent with the index GFR. For the minority of subjects with a metabolic complication laboratory value prior to the index GFR, the average time from the laboratory evaluation to the index GFR was 129 days for potassium, 112 days for bicarbonate, 103 days for hemoglobin, and 100 days for phosphorus.
Anemia was defined as a hemoglobin less than 10 g/dL.11 Hyperkalemia was defined as a potassium greater than 5.5 mEq/L, a level above which KDOQI guidelines recommend discontinuing angiotensin converting enzyme inhibitors/angiotensin receptor blockers (ACE-I/ARBs).12 Acidosis was defined by a serum bicarbonate level less than 21 mEq/L and hyperphosphatemia as a phosphorus level greater than 4.6 mg/dL according to the KDOQI clinical practice guidelines for nutrition and bone metabolism and disease.13, 14 Secondary analyses were conducted evaluating the most extreme laboratory value concurrent with or in the year prior to the index GFR (lowest hemoglobin, highest potassium, lowest bicarbonate, and highest phosphorus).
Diabetes was defined by 2 or more ICD-9 codes (250–250.xx) or receipt of 2 or more medications for diabetes prior to the index GFR. Hypertension was defined by two or more ICD-9 codes (401–405.xx) prior to the index GFR or 2 or more clinic blood pressures greater than 130/80 mmHg in the 2 years prior to the index GFR. Cancer was defined by 2 or more ICD-9 codes (140–208, 230–234, V10) prior to the index GFR. VA administrative ICD-9 codes for patient comorbidities have been demonstrated to have greater than 90–95% sensitivity and specificity.15
The subjects were stratified by age and baseline characteristics were described using mean and standard deviation for continuous variables and percent for categorical variables. Differences between groups were evaluated using ANOVA for continuous variables and Pearson’s chi-square test for categorical variables. Multivariable logistic regression models were used to evaluate the association between GFR and anemia, hyperphosphatemia, hyperkalemia, and acidosis adjusting for age, race, diabetes, hypertension, cancer, and use of phosphate binders, ACE-I/ARB, erythropoietin, and iron. To evaluate whether age modifies the effect of low GFR on metabolic complications, an interaction term including age and GFR was included in each model. The assumed linear association between GFR and log odds of metabolic complications was evaluated. Analyses were conducted using R version 2.12.2 (http://www.r-project.org).
RESULTS
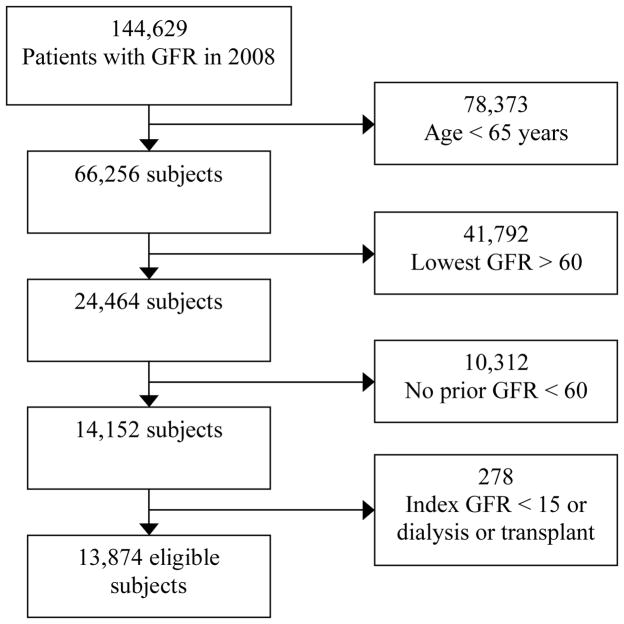
There were 144,629 patients who had a GFR checked in calendar year 2008. Of these, 13,874 met the inclusion and exclusion criteria and were included in the present analyses (see Figure 1 for details). Of the 13,874 eligible subjects, 13,848 had a potassium, 13,773 had a bicarbonate, 3,432 had a phosphorus, and 11,200 had a hemoglobin value concurrent with or in the 6 months prior to the index GFR.
Figure 1. CONSORT Diagram.
Abbreviations: GFR, glomerular filtration rate.
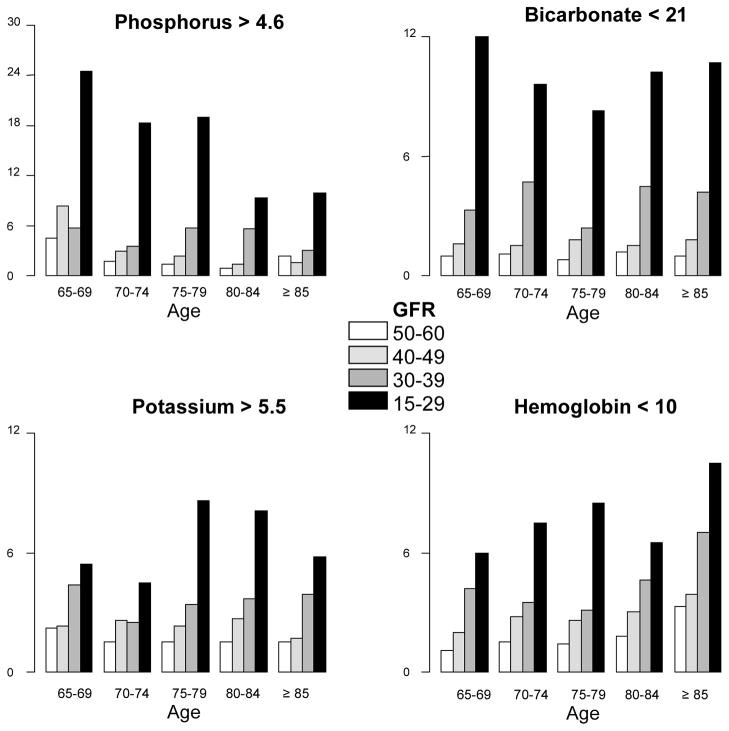
Baseline characteristics by age group are shown in Table 1. Elderly subjects were less likely to be black and had lower rates of hypertension and diabetes but higher rates of cancer. Average bicarbonate levels were similar across the groups but older age was associated with lower hemoglobin levels (see Table 1). The rates of hyperphosphatemia, acidosis, hyperkalemia, and anemia at any time concurrent with or in the 6 months prior to the index GFR by age and GFR categories are displayed in Figure 2 for descriptive purposes only (all statistical analyses are conducted with age and GFR as continuous variables).
Table 1.
Baseline Characteristics by Age Category
| Variable | Age group | P value | ||||
|---|---|---|---|---|---|---|
| 65–69 yr | 70–74 yr | 75–79 yr | 80–84 yr | ≥85 yr | ||
| N | 1574 | 2448 | 3713 | 3673 | 2466 | |
| Gender (% male) | 97.0 | 97.9 | 99.1 | 98.0 | 96.3 | < 0.001 |
| Race (% black) | 7.9 | 7.4 | 6.2 | 5.2 | 5.6 | < 0.001 |
| eGFR (mean (± SD)) | 48.5 (9.8) | 47.8 (9.5) | 46.8 (9.7) | 45.8 (9.4) | 44.6 (9.5) | < 0.001 |
| eGFR (column %) | < 0.001 | |||||
| <50–60 ml/min/1.73m2 | 49.9 | 47.6 | 45.9 | 42.0 | 37.8 | |
| <40–49.9 | 32.7 | 31.3 | 30.8 | 33.9 | 34.3 | |
| <30–39.9 | 11.6 | 14.7 | 16.1 | 17.1 | 19.6 | |
| 15–29.9 | 5.8 | 6.4 | 7.2 | 7.1 | 8.4 | |
| Past Medical History (%) | ||||||
| Hypertension | 89.1 | 87.3 | 85.0 | 84.3 | 85.0 | < 0.001 |
| Diabetes | 50.3 | 46.7 | 42.4 | 34.3 | 24.8 | < 0.001 |
| Cancer | 20.0 | 22.0 | 24.7 | 25.6 | 26.7 | < 0.001 |
| Laboratory (mean (± SD)) | ||||||
| Hemoglobin (g/dL) | 13.6 (1.8) | 13.4 (1.7) | 13.2 (1.7) | 13.0 (1.6) | 12.7 (1.6) | < 0.001 |
| Potassium (mEq/L) | 4.46 (0.5) | 4.46 (0.5) | 4.48 (0.5) | 4.50 (0.5) | 4.50 (0.5) | 0.002 |
| Bicarbonate (mEq/L) | 27.1 (3.2) | 26.9 (3.1) | 27.0 (3.1) | 26.9 (3.1) | 26.8 (3.2) | 0.11 |
| Phosphorus (mg/dL) | 3.63 (0.7) | 3.51 (0.6) | 3.57 (0.7) | 3.54 (0.6) | 3.55 (0.6) | 0.04 |
| Medications (%) | ||||||
| ACEI/ARB | 68.9 | 66.4 | 63.5 | 59.3 | 51.7 | < 0.001 |
| Phosphate binder | 1.5 | 1.3 | 1.0 | 1.2 | 1.1 | 0.50 |
| Iron supplements | 7.1 | 5.6 | 6.8 | 6.4 | 8.2 | 0.009 |
| Erythropoetin | 1.8 | 1.9 | 1.7 | 1.6 | 2.0 | 0.75 |
Figure 2. Metabolic complications by age and GFR category (%).
Abbreviations: GFR, glomerular filtration rate.
Logistic regression models were constructed with metabolic complications in the 6 months prior to the index GFR as the dependent variables. None of the interaction terms between age and GFR were significant. This was true for models that included only age and GFR and for multivariable models (P values for the age × GFR interaction term: 0.66 with anemia as the outcome, 0.19 with hyperkalemia, 0.54 with acidosis, and 0.22 with hyperphosphatemia). A decrease in GFR was associated with increased odds for all metabolic complications in models including only age and GFR as well as multivariable models (adjusting for age, GFR, race, diabetes, hypertension, cancer, and use of phosphate binders, ACE-I/ARBs, erythropoietin, and iron). The adjusted odds ratios (95% confidence interval) for a decrease in GFR from 35 to 30 ml/min/1.73m2 were 1.21 (1.15 to 1.28) for anemia, 1.26 (1.19 to 1.32) for hyperkalemia, 1.45 (1.37 to 1.52) for acidosis, and 1.72 (1.50 to 1.96) for hyperphosphatemia (all P values ≤ 0.001). There was no significant colinearity between age and GFR; the variance inflation factors for age and GFR were less than 2 in all multivariable models. Additionally, there was no evidence of a non-linear relationship between GFR or age and any of the outcomes with the exception of GFR and hyperphosphatemia. However, the relationship between GFR and hyperphosphatemia was linear when analyses were limited to subjects with a GFR less than 45 ml/min/1.73m2. Secondary analyses with lowest hemoglobin, lowest bicarbonate, highest potassium, and highest phosphorus in the 12 months prior to the index GFR as dependent variables produced similar results.
Increasing age was significantly associated with increased odds for anemia in the model including only age and GFR (odds ratio (OR) per 5 year increase: 1.18 (95% CI: 1.09 to 1.29) and the fully adjusted model (OR: 1.23; 95% CI: 1.13 to 1.35). Conversely, increasing age was significantly associated with decreased odds for hyperphosphatemia after adjustment for GFR (OR: 0.79; 95% CI: 0.69 to 0.89) as well as in multivariable adjusted models (OR: 0.82; 95% CI: 0.72 to 0.94). There was no association between age and either hyperkalemia or acidosis.
DISCUSSION
This study demonstrates that, among older patients with CKD, lower GFR is associated with the presence of metabolic complications of CKD such as anemia, hyperkalemia, acidosis, and hyperphosphatemia, regardless of age. K/DOQI guidelines recommend monitoring for metabolic complications in all patients with CKD; based on our results, these recommendations should be applied to all age groups.2
Previous research has demonstrated significant changes in renal physiology and function with aging. Over 50 years ago, Davies et al demonstrated that both renal plasma flow and GFR decrease with advanced age.16 Additionally, the renin angiotensin system is suppressed in the elderly, both at baseline and in response to a potassium load.17, 18 More recently, elderly kidney donors have been found to have increased nephrosclerosis on biopsy which is independent of multiple risk factors including measured GFR, hypertension, urine albumin excretion, and nighttime blood pressure.19 Observational studies have found an association between aging and increased risk for acute kidney injury.20, 21 Finally, the elderly are at increased risk for anemia. In NHANES, both decreased GFR and old age were associated with anemia.22 Whether age modified the relationship between GFR and anemia is unknown as no test for interaction was performed.22
Furthermore, altered renal tubular function in the elderly may result in electrolyte abnormalities. Aging is associated with decreased tubular reabsorption of phosphorus which is manifested clinically by lower phosphorus levels in the elderly.23, 24 Studies have revealed decreased net acid excretion in response to ammonium chloride loading among the elderly as well as an association between advanced age and metabolic acidosis which is independent of GFR.25, 26 Finally, old rats had increased potassium after a potassium load compared to young rats and were unable to increase potassium excretion in response to a high potassium diet.27
Our study indicates that aging by itself, is not associated with hyperkalemia or acidosis. This implies that any decline in renal function that occurs as a consequence of aging is modest, and may not sufficiently impair glomerular and distal tubular function to result in these metabolic consequences. However, increasing age was associated with decreased risk for hyperphosphatemia, possibly an indication of impaired proximal tubular function. In addition, aging was independently associated with anemia, a finding that has been reported in other cohorts.22 It is possible that factors other than kidney function may contribute to anemia in older adults.28
On the other hand, lower GFR is independently associated with the presence of metabolic abnormalities irrespective of age. This is an important finding that informs the debate about the significance of lower GFR in older adults. Recent studies have shown that there is an “epidemic” of chronic kidney disease driven primarily by growth in the older population.1 This has stimulated the development of a large number of clinical and research programs targeted at CKD.29, 30 Some authors have been critical of this approach, arguing that diagnosis of CKD based on modest reductions in GFR, especially in the elderly, artificially inflates the estimates of chronic kidney disease.3 In this context, our data indicate that the increased risk for metabolic complications with low GFR is not modified by advanced age, and that elderly patients with reduced GFR are likely to have metabolic consequences such as anemia, hyperkalemia, acidosis, and hyperphosphatemia, all of which have diagnostic and therapeutic implications. It is worth noting that rates of acidosis, anemia, and hyperkalemia increase linearly with decreasing renal function whereas hyperphosphatemia is seen mainly at more advanced stages of CKD.
Other studies have shown that elderly with CKD are at increased risk for clinical outcomes of cardiovascular disease, AKI, and ESRD.5, 7, 8 Therefore, the data support that elderly patients with low GFR have increased risk across the spectrum of biochemical and clinical outcomes and argue against the benign prognosis implied by “age related decline in GFR”. Furthermore, these data reinforce the importance of the diagnosis of advanced CKD using current cut-offs of GFR. Clinically, our results support recommendations for monitoring patients with advanced CKD for metabolic complications, regardless of age. The majority of the growing elderly CKD population is not seen in nephrology clinics; therefore, geriatricians should be aware of the need to monitor for metabolic complications in their patients with CKD.
Strengths of our study include the large sample size. Additionally, our assessment of metabolic complications is based on laboratory data, as opposed to billing or administrative data. We evaluated multiple metabolic complications and found consistent results for the most recent laboratory values prior to the index GFR as well as the most extreme values in the year prior to the index GFR. Finally, we were able to adjust for multiple potential confounders including comorbidities, medications, and laboratory values. However, our study has a few limitations that need to be considered. The study relies on laboratory values obtained in the course of clinical care which could introduce bias. Our assessment of metabolic complications was concurrent with our assessment of renal function in a majority of patients, but not all. The cohort is predominantly male, typical of the elderly VA population. While the majority of patients receive medications from the VA pharmacy, we are unable to account for medications filled at outside pharmacies. Our study does not include middle aged subjects. In addition, residual confounding may exist despite adjustment for known covariates. Finally, tests for interaction may be underpowered.
CONCLUSION
In conclusion, we demonstrate that, similar to younger patients with CKD, elderly patients with low GFR are at risk for anemia, hyperkalemia, acidosis, and hyperphosphatemia. Based on these results, it is important to monitor elderly patients with low GFR for these metabolic complications. Whether the benefit, if any, of treating these metabolic complications is consistent across age groups is unknown.
Acknowledgments
The project described was supported in part by Award Number K23DK087919 (P.E.D.) from the National Institute Of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health. The study results were presented at the 2010 American Society of Nephrology meeting in Denver, CO.
Sponsor’s Role: None.
Footnotes
Author Contributions:
Study concept and design: Drawz, Babineau, Rahman
Acquisition of Data: Drawz
Analysis and interpretation of data: Drawz, Babineau, Rahman
Preparation of Manuscript: Drawz
Final editing of Manuscript: Drawz, Babineau, Rahman
Conflicts of interest: Drs Drawz and Rahman report receiving grant support from NIH. Dr Babineau report no conflicts of interest.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 3.Glassock RJ, Winearls C. An epidemic of chronic kidney disease: Fact or fiction? Nephrol Dial Transplant. 2008;23:1117–1121. doi: 10.1093/ndt/gfn086. [DOI] [PubMed] [Google Scholar]
- 4.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 5.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmelgarn BR, Zhang J, Manns BJ, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69:2155–2161. doi: 10.1038/sj.ki.5000270. [DOI] [PubMed] [Google Scholar]
- 7.Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 8.Hallan S, Astor B, Romundstad S, et al. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med. 2007;167:2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 9.Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2010;55:S23–33. doi: 10.1053/j.ajkd.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Greene T, Kusek J, et al. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828. [Google Scholar]
- 11.Goldsmith D. 2009: A requiem for rHuEPOs--but should we nail down the coffin in 2010? Clin J Am Soc Nephrol. 2010;5:929–935. doi: 10.2215/CJN.09131209. [DOI] [PubMed] [Google Scholar]
- 12.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–290. [PubMed] [Google Scholar]
- 13.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35:S1–140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 14.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. [PubMed] [Google Scholar]
- 15.Borzecki AM, Wong AT, Hickey EC, et al. Identifying hypertension-related comorbidities from administrative data: What’s the optimal approach? Am J Med Qual. 2004;19:201–206. doi: 10.1177/106286060401900504. [DOI] [PubMed] [Google Scholar]
- 16.Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950;29:496–507. doi: 10.1172/JCI102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulkerrin E, Epstein FH, Clark BA. Aldosterone responses to hyperkalemia in healthy elderly humans. J Am Soc Nephrol. 1995;6:1459–1462. doi: 10.1681/ASN.V651459. [DOI] [PubMed] [Google Scholar]
- 18.Weidmann P, De Myttenaere-Bursztein S, Maxwell MH, et al. Effect on aging on plasma renin and aldosterone in normal man. Kidney Int. 1975;8:325–333. doi: 10.1038/ki.1975.120. [DOI] [PubMed] [Google Scholar]
- 19.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson S, Eldadah B, Halter JB, et al. Acute kidney injury in older adults. J Am Soc Nephrol. 2011;22:28–38. doi: 10.1681/ASN.2010090934. [DOI] [PubMed] [Google Scholar]
- 21.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 22.Astor BC, Muntner P, Levin A, et al. Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2002;162:1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 23.Minisola S, Pacitti MT, Scarda A, et al. Serum ionized calcium, parathyroid hormone and related variables: effect of age and sex. Bone Miner. 1993;23:183–193. doi: 10.1016/s0169-6009(08)80095-5. [DOI] [PubMed] [Google Scholar]
- 24.Mulroney SE, Woda C, Haramati A. Changes in renal phosphate reabsorption in the aged rat. Proc Soc Exp Biol Med. 1998;218:62–67. doi: 10.3181/00379727-218-44268. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal BN, Cabebe FG. Renal acidification in elderly subjects. Nephron. 1980;26:291–295. doi: 10.1159/000182004. [DOI] [PubMed] [Google Scholar]
- 26.Frassetto LA, Morris RC, Jr, Sebastian A. Effect of age on blood acid-base composition in adult humans: Role of age-related renal functional decline. Am J Physiol. 1996;271:F1114–1122. doi: 10.1152/ajprenal.1996.271.6.F1114. [DOI] [PubMed] [Google Scholar]
- 27.Bengele HH, Mathias R, Perkins JH, et al. Impaired renal and extrarenal potassium adaptation in old rats. Kidney Int. 1983;23:684–690. doi: 10.1038/ki.1983.79. [DOI] [PubMed] [Google Scholar]
- 28.Balducci L. Anemia, fatigue and aging. Transfus Clin Biol. 2010;17:375–381. doi: 10.1016/j.tracli.2010.09.169. [DOI] [PubMed] [Google Scholar]
- 29.Hostetter TH, Lising M. National kidney disease education program. J Am Soc Nephrol. 2003;14:S114–116. doi: 10.1097/01.asn.0000070156.78824.c7. [DOI] [PubMed] [Google Scholar]
- 30.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]