Abstract
Guidelines recommend restricting simultaneous liver-kidney (SLK) transplant to candidates with prolonged dialysis or estimated glomerular filtration rate (eGFR) <30 ml/min/1.73m2 for 90 days. However, few studies exist to support the latter recommendation. Using SRTR and Medicare dialysis data, we assembled a cohort of 4997 liver transplant recipients from 2/27/2002–1/1/2008. Serial eGFRs were calculated from serum creatinines submitted with MELD reports. We categorized recipients by eGFR patterns in the 90 days pre-transplant: Group 1 (eGFR always >30), Group 2 (eGFR fluctuated), Group 3 (eGFR always <30) and Group 4 (short-term dialysis). For Group 2, we characterized fluctuations in renal function using time-weighted mean eGFR. Among liver-alone recipients in Group 3, the rate of end-stage renal disease (ESRD) by 3 years was 31%, versus <10% for other groups (p<0.001). In multivariable Cox regression, eGFR Group, diabetes (HR 2.65, p<0.001) and black race (HR 1.83, p=0.02) were associated with ESRD. Among liver-alone recipients in Group 2, only diabetics with time-weighted mean eGFR<30 had a substantial ESRD risk (25.6%). In summary, among liver transplant candidates not on prolonged dialysis, SLK should be considered for those whose eGFR is always <30 and diabetic candidates whose weighted mean eGFR is <30 for 90 days.
Keywords: liver transplantation, end-stage renal disease, chronic kidney disease, diabetes complicat1ions
Introduction
End-stage renal disease (ESRD) after liver transplantation is associated with a markedly elevated mortality risk.(1–3) Although implementation of the Model for End-Stage Liver Disease (MELD) score-based allocation for liver transplants has led to decreased waitlist mortality, the priority assigned to candidates with renal dysfunction has led a rising prevalence of liver transplant recipients who later develop ESRD.(2, 3) Provision of a simultaneous liver-kidney (SLK) transplant can protect a recipient from developing ESRD, but the shortage of kidney allografts creates an ethical imperative to perform SLKs only when the risk of ESRD with a liver transplant alone (LTA) is high. Due to a lack of data on outcomes for liver transplant candidates with sustained renal dysfunction that is not severe enough for dialysis, the decision about SLK transplantation is particularly challenging.
In 2008, the American Society of Transplant Surgeons, the American Society of Transplantation, the United Network for Organ Sharing (UNOS), and the American Society of Nephrology convened a consensus conference to devise guidelines for SLK transplantation. The conference recommended SLK for patients with: A) ESRD with cirrhosis and portal hypertension; B) acute kidney injury with creatinine ≥2.0 mg/dL and dialysis ≥8 weeks; C) end-stage liver disease and chronic kidney disease (CKD) with a kidney biopsy showing >30% glomerulosclerosis or 30% fibrosis; and D) end-stage liver disease and CKD with a glomerular filtration rate (GFR) ≤30 mL/min/1.73m2 for ≥12 weeks.(4)
Selecting liver transplant candidates who are not on dialysis but have evidence of CKD (the focus of recommendations C and D) remains difficult because the consensus recommendations do not address the common scenario of renal function that fluctuates above and below the 30 mL/min/1.73m2 cutpoint.(5) Notably, the guidelines’ cutoff of GFR <30 mL/min/1.73m2 for ≥12 weeks relied chiefly upon single-center data, with small sample sizes.(6–9) Assessing renal prognosis among patients with advanced liver disease is hampered by equations that often underestimate actual GFR, the risks of renal biopsy, and the lack of availability of direct GFR measurement (e.g., with iothalamate) in clinical practice.(10) Although diabetes and hepatitis C might predict renal prognosis after liver transplant, the guidelines also do not address how to use these attributes to identify candidates for SLK.
Validation of the guidelines about SLK transplant for patients with sustained renal dysfunction would require a large cohort. We recognized that longitudinal data on MELD score (which includes serum creatinine) provided to the Organ Procurement and Transplantation Network (OPTN) for wait-listed patients would enable the creation of a national cohort with estimated GFR (eGFR) at multiple time points. Therefore, our goals were to: 1) assess ESRD risk after liver transplantation for a cohort of recipients with likely CKD, stratified by severity and duration of renal function; 2) examine whether diabetes or other attributes predict ESRD after liver transplantation; 3) identify subgroups that commonly receive LTA for whom the risk of post-transplant ESRD is high enough to warrant consideration of an SLK transplant.
Methods
Data source
This study used a linked dataset from the Scientific Registry of Transplant Recipients (SRTR) and the Center for Medicare and Medicaid Services (CMS). The SRTR includes outcomes of death determined through center reports and through the Social Security Death Master File. The SRTR includes ESRD outcomes determined through kidney transplantation reported to OPTN. We additionally ascertained ESRD outcomes by linking to CMS claims for chronic dialysis. The Institutional Review Board at the University of Pennsylvania deemed this study exempt under provisions of the Code of Federal Regulations 45 CFR 46.101, category 4.
Study subjects
We assembled a cohort of adults (≥18 years) who underwent liver transplantation from 2/27/2002 (when the MELD system was implemented) to 1/1/2008. The end date was chosen so that all recipients had ≥3 years of follow-up. Subjects were on the liver transplant waiting list for at least 90 days and had ≥ two serum creatinine values reported to UNOS during that period. We chose 90 days as the minimal duration of observation to compare patterns of renal dysfunction to the SLK Consensus Guidelines and to National Kidney Foundation guidelines for CKD.(4, 11) We excluded recipients whose eGFR at transplant was >60 ml/min/1.73m2 because they were unlikely to be eligible for SLK transplant at any center (0/8,848 received an SLK during this period), and to be consistent with consensus guidelines defining CKD in cirrhotic patients.(12) Subjects with known ESRD (defined as dialysis for ≥ 3 months or kidney transplant before liver transplant) were excluded. We also excluded liver recipients with human immunodeficiency virus because their workup and treatment were likely to be substantially different from other recipients.
End-stage renal disease and death
The primary outcomes were ESRD or death after LTA. ESRD after liver transplant was defined as 1) initiation of chronic dialysis with submission of a 2728 form to the United States Renal Data Systems or 2) kidney transplant. We also reported unadjusted rates of ESRD and death by one and three years after transplantation. These time-points were chosen for two main reasons. First, one and three year mortality are quality benchmarks for transplant centers and made available to the public by the SRTR.(13) Second, if we found that a large proportion of patients developed ESRD within these time frames, a strong argument could be made to increase the rate of SLK transplants.
Primary exposure: Pre-transplant renal function
We calculated eGFR according to the Modification of Diet in Renal Disease Study (MDRD). We created a longitudinal dataset by calculating eGFR from the MELD scores (which include serum creatinine) submitted prior to transplant. In order to have a full 90 days of eGFR data for the entire cohort, we assigned each patient an eGFR at 90 days before transplant that was calculated using the most recent MELD score submitted prior to the 90-day period. For example, a patient with a creatinine submitted to UNOS at 100 days before transplantation would have the eGFR calculated from this value, and assigned as the day 90 eGFR. The rationale was that UNOS allocates liver transplants on the basis of the most recently-submitted MELD score; therefore, wait-listed patients can be considered to have lab values similar to their last MELD.
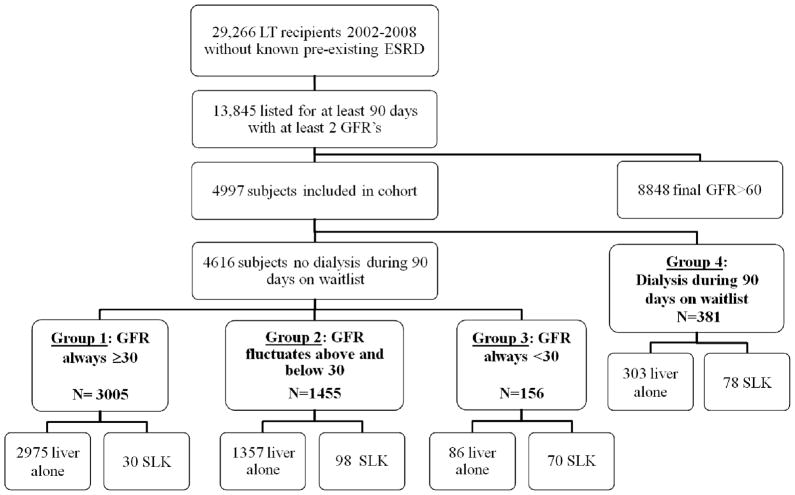
As in Figure 1, cohort members were divided into three main groups with eGFR cutpoints guided by the SLK Consensus Group recommendations. Group 1 recipients had eGFRs that always remained ≥30 ml/min/1.73m2 for the 90 days; Group 2 recipients had eGFRs that fluctuated above and below 30 ml/min/m2; Group 3 recipients had eGFR always <30 ml/min/1.73m2. For comparison, we created a fourth group of recipients who received any temporary dialysis during the 90 days prior to transplant.
Figure 1.
Cohort generation
For recipients with fluctuating renal function (Group 2), we performed additional analyses by subdividing the group according to time-weighted mean eGFR. The time-weighting provided a quantitative method to distinguish between individuals with changes in eGFR of different magnitudes and duration.(14, 15) For example, a patient with an eGFR of 50 ml/min/1.73m2 on day 90, 45 on day 60, and 28 at transplantation would have a weighted mean eGFR of 46.5 ml/min/1.73 m2, reflective of 30 days with an eGFR of 50 (on days 90 through 61), 60 days with an eGFR of 45 (on days 60 through the day before transplantation), and 1 day with an eGFR of 28 ml/min/1.73m2 (the day of transplantation). Appendix 1 provides more detailed examples.
Appendix 1.
Example of time-weighted estimated glomerular filtration rate (eGFR) calculations
| eGFR number | eGFRa in ml/min/1.73 m2 | Days before transplant | Days between MELD updates (days before transplant - days until next creatinine submitted with MELD) | eGFR weight (eGFR * days with eGFR) |
|---|---|---|---|---|
| 1 | 50 | 90 | 90 – 60=30 | 50 * 30=1500 |
| 2 | 45 | 60 | 60 – 50=10 | 45 * 10=450 |
| 3 | 40 | 50 | 50 – 20=30 | 40 * 30=1200 |
| 4 | 30 | 20 | 20 – 0=20 | 30 * 20=600 |
| 5b | 20 | 0 | 1 | 20 * 1=20 |
| Time-weighed eGFR=(1500+450+1200+600+20)/ 91 days=41.4 ml/min/1.73m2 | ||||
| The individual was categorized as belonging to Group 2 (fluctuating eGFR). | ||||
eGFR calculated from serum creatinine submitted with MELD score
The eGFR value calculated on the day of transplantation was given a weight of 1 day, since the patient had that lab value only on the day of transplantation.
We weighted a patient’s eGFR over the 90 days prior to transplantation. (Note: since patients have an eGFR value on the day of transplantation, this value was assigned a weight of 1 day, as it contributes 1 day of follow-up. The data points were thus weighted over 91 days). The example shown in Supplementary Table A reflects five eGFR calculations obtained with sequential MELD updates in the interval prior to liver transplant (eGFR numbers 1 through 5). In this example, the eGFR of 50 ml/min/m2 at 90 days prior to transplantation contributes 30 days to the eGFR weight, since the next eGFR is updated on day 60 before transplant. Without any intervening eGFR values reported through a MELD score, it is assumed that the patient’s eGFR remains at 50 ml/min/m2 for those 30 days. The subsequent eGFR of 45 ml/min/m2 contributes 10 days to the eGFR weight, since the next update occurs on day 50 before transplant.
The time-weighting approach has important advantages over other approaches, such as categorizing eGFR as a simple arithmetic mean of eGFR values. Two further examples of liver-alone transplant candidates in Group 2 illustrate the point. For example, if patient A has an eGFR of 60 for forty-five days and 20 for forty-five days, the arithmetic mean of the two eGFR data points is 40 and the weighted mean is 40 ml/min/1.73 m2 (since each eGFR value contributes to 50% of the follow-up time of 90 days). On the other hand, if patient B has an eGFR of 60 for thirty days and 20 for sixty days, the arithmetic mean of the two eGFR data points is 40, while the weighted mean equals 33.3 ml/min/1.73 m2 (the eGFR is 60 for 1/3 of the time, and 20 for 2/3 of the time). The weighted mean reveals that patient B’s renal dysfunction has a longer duration and may be more likely to persist after transplantation.
Statistical Analysis
Analyses were conducted using Stata 12.0. Outcomes were ascertained from the date of liver transplantation until ESRD, death, or March 1, 2011, whichever occurred first. P-values are two-sided.
For unadjusted comparisons of continuous variables between eGFR groups, we used the ANOVA test for normally distributed variables and the Kruskal-Wallis test for non-normally distributed variables. The chi-square test was used to compare categorical variables. We fit separate multivariable Cox regression models for the outcomes of mortality and ESRD. We inspected graphical displays to confirm the proportional hazards assumption for the eGFR groups. On the basis of prior studies about clinical risks for ESRD and clinical judgment, we identified independent variables for these models.(2, 16, 17) In addition to eGFR group, recipient variables were assessed at transplant and included: age (categorized as <40, ≥40 and <50, ≥50 and <60, and ≥60 years), gender, race (black or non-black), diabetes, hypertension, primary cause of liver disease (hepatitis C, hepatitis B, alcohol, non-alcoholic steatohepatitis, cholestatic, autoimmune, hepatocellular carcinoma, cryptogenic, and other), international normalized ratio of prothrombin time (categorized as <1.4, >1.4 and <1.7, >1.7 ), total bilirubin (categorized as <2.2, ≥2.2 and ≤4.4, >4.4 mg/dL) serum albumin (<2.7, ≥2.7 and ≤3.1, >3.1 g/dL), and serum sodium (<134, 134–138, >138 mEq/l).
Secondary analyses
No subjects lacked creatinine data, but some lacked data on other variables, including diabetes (n=112, 2%). To estimate the maximum effect of missing data on outcomes, we performed sensitivity analyses in which extreme values were assigned to individuals with missing data. In the example of diabetes, we categorized individuals for whom diabetes status was missing as not having diabetes in our primary analysis, and then categorized these individuals as having diabetes in a sensitivity analysis. Results were similar to the primary analysis and not shown.
For Group 2, we undertook a secondary analysis in which we calculated the percentage of time that each recipient had eGFR <30 ml/min/1.73 m2. Findings were similar to our primary approach of calculating weighted mean eGFR and not shown.
Results
A total of 29,266 adults without pre-existing ESRD underwent liver transplantation between 2/27/2002 and 1/1/2008, among whom 13,845 were on the waiting list for at least 90 days and had ≥ 2 serum creatinines reported. We excluded 8,848 recipients with a final eGFR >60 ml/min/1.73m2. As in Figure 1, the overall cohort comprised 4,997 liver recipients at 111 transplant centers.
The mean number of creatinine values per individual used to assess eGFR patterns in the 90 days prior to transplant was 7.9 (SD 4.8). Group 1 comprised 3,005 (60%) recipients with eGFR always >30 ml/min/1.73m2. Group 2 comprised 1,455 (29%) individuals with eGFR fluctuating above and below 30 ml/min/1.73m2. Group 3 comprised 156 (3%) individuals with eGFR always <30 ml/min/1.73m2. Group 4 comprised 381 recipients (8%) who received any dialysis during the 90 days before transplant.
Subject characteristics
Table 1 shows subject characteristics. Group 3 recipients were less likely to be male, and more likely to have diabetes and hypertension than Groups 2 and 1 recipients (p<0.001 for each comparison). Cause of end-stage liver disease also differed between groups; in particular, a lower percentage of Group 3 recipients had liver disease attributed to hepatitis C (26%) compared to Group 2 (42%) and Group 1 (39%).
Table 1.
Baseline Subject Characteristics and Serologic Data for Liver Transplant Recipients, by GFR Category (n=4997)
| Baseline attributes | Group 1 (n=3005) | Group 2 (n=1455) | Group 3 (n=156) | Dialysis (n=381) | P |
|---|---|---|---|---|---|
| Age, years (IQR) | 56 (50, 61) | 55 (51, 61) | 57 (51, 62) | 53 (48, 59) | <0.001 |
| Male, n (%) | 1826 (61) | 792 (54) | 61 (39) | 242 (64) | <0.001 |
| Race, n (%) | <0.001 | ||||
| White | 2326 (77) | 1074 (74) | 118 (76) | 257 (67) | |
| Black | 169 (6) | 92 (6) | 8 (5) | 18 (5) | |
| Asian | 106 (4) | 22 (2) | 7 (4) | 15 (4) | |
| Hispanic ethnicity | 376 (13) | 252 (17) | 22 (14) | 85 (22) | |
| Other or Multiracial | 28 (1) | 15 (1) | 1 (1) | 6 (2) | |
| Cause of Liver Disease, n (%) | <0.001 | ||||
| Hepatitis C | 1168 (39) | 606 (42) | 41 (26) | 169 (44) | |
| Alcohol | 384 (13) | 197 (14) | 14 (9) | 49 (13) | |
| Cryptogenic cirrhosis | 297 (10) | 178 (12) | 30 (19) | 36 (9) | |
| Cholestatic | 282 (9) | 141 (10) | 6 (4) | 41 (11) | |
| Hepatocellular carcinoma | 245 (8) | 37 (3) | 5 (3) | 17 (4) | |
| Non-alcoholic Steatohepatitis | 144 (5) | 74 (5) | 14 (9) | 11 (3) | |
| Autoimmune | 129 (4) | 77 (5) | 12 (8) | 20 (5) | |
| Hepatitis B | 79 (3) | 21 (1) | 7 (4) | 11 (3) | |
| Other | 277 (9) | 124 (9) | 27 (17) | 27 (7) | |
| Diabetes | 718 (24) | 410 (28) | 82 (53) | 92 (24) | <0.001 |
| Hypertension | 612 (20) | 288 (20) | 64 (41) | 51 (13) | <0.001 |
| MELD Score at transplant (IQR) | 18 (15, 23) | 27 (21, 33) | 22 (18, 25) | 37 (32, 40) | <0.001 |
| Last eGFR before transplant, mL/min/1.73 m2 | 48 (41, 55) | 28 (20, 36) | 20 (15, 25) | N/A | <0.001 |
| INR, INR units | 1.5 (1.3, 1.8) | 1.8 (1.5, 2.2) | 1.3 (1.1, 1.5) | 2.1 (1.7, 2.6) | <0.001 |
| Total bilirubin, mg/dL | 2.9 (1.7, 5.4) | 4.7 (2.4, 12.7) | 1.2 (0.7, 2.4) | 13.1 (5.0, 30.5) | <0.001 |
| Serum albumin, g/dL | 2.8 (2.4, 3.3) | 2.8 (2.3, 3.3) | 3 (2.5, 3.5) | 2.9 (2.4, 3.5) | <0.001 |
| Serum sodium, mEq/L | 136 (132, 138) | 135 (131, 139) | 138 (135, 141) | 137 (134, 140) | <0.001 |
| Donor Type, n (%) | <0.001 | ||||
| Deceased donor | 2835 (94) | 1415 (97) | 150 (96) | 379 (99) | |
| Live donor | 170 (6) | 40 (3) | 6 (4) | 2 (1) | |
| Simultaneous Liver- | 30 (1) | 98 (7) | 70 (45) | 78 (20) | <0.001 |
| Kidney transplant, n (%) | |||||
| Weeks on dialysis during 90 days on waitlist | N/A | N/A | N/A | N/A | |
| ≤ 1 week | 227 (60) | ||||
| 1–4 weeks | 125 (33) | ||||
| ≥ 4 weeks | 29 (8) |
276 subjects (6%) underwent SLK transplantation. The percentage receiving an SLK was 1% in Group 1, 7% in Group 2, 45% in Group 3, and 20% in Group 4 (p<0.001).
End-stage renal disease and death among recipients of liver-alone transplants
For ESRD and death, we focused analyses on LTA recipients (n=4,721, 94% of the overall cohort). Table 2 shows that the rates of death far exceeded the rates of ESRD in each group except Group 3. The rates of death by 3 years were 21%, 25%, 37%, and 32% among Groups 1, 2, 3, and 4, respectively. By contrast, the rates of ESRD by 3 years were 5%, 6%, 31%, and 6% for Groups 1, 2, 3, and 4 respectively.
Table 2.
Cumulative incidence of ESRD and death by 1 and 3 years after liver-alone transplant
| Group 1 (n=2975) | Group 2 (n=1357) | Group 3 (n=86) | Dialysis (n=303) | P | |
|---|---|---|---|---|---|
| ESRD by 1 year | 61 (2.1%) | 53 (3.9%) | 22 (25.6%) | 13 (4.3%) | <0.001 |
| ESRD by 3 years | 148 (5.0%) | 87 (6.4%) | 27 (31.4%) | 18 (5.9%) | <0.001 |
| Death by 1 year | 385 (12.9%) | 224 (16.5%) | 19 (22.1%) | 71 (23.4%) | <0.001 |
| Death by 3 years | 635 (21.3%) | 344 (25.4%) | 32 (37.2%) | 97 (32.0%) | <0.001 |
Multivariable Cox regression analyses of ESRD and death for liver-transplant-alone recipients
In multivariable regression, lower pre-transplant eGFR was strongly associated with a significantly increased risk of ESRD (HR=7.23 for Group 3 vs. Group 1, p<0.001; HR=1.66 for Group 2 vs. Group 1; p=0.003). By comparison, the HR associated with ESRD was 2.69 for the group receiving any dialysis compared to Group 1. Diabetes (HR=2.65, p<0.001), black race (HR=1.83, p=0.02) and male gender (HR=1.51, p=0.009) were also independently associated with reaching ESRD after liver transplant. Notably, as shown in Table 3, neither age, nor hypertension, nor hepatitis C was significantly associated with the outcome.
Table 3.
Multivariable Cox Regression Analysis of End-Stage Renal Disease after Liver-alone Transplant, Censored for Death*
| Characteristic | Hazard Ratio | Confidence Interval | P |
|---|---|---|---|
| eGFR category | |||
| Group 1: eGFR always ≥30 ml/min/m2 | Reference | Reference | Reference |
| Group 2: eGFR fluctuated above and below 30 ml/min/m2 | 1.66 | (1.19, 2.32) | 0.003 |
| Group 3: eGFR always <30 ml/min/m2 | 7.23 | (4.08, 12.8) | <0.001 |
| Group 4: Any Dialysis | 2.69 | (1.55, 4.68) | <0.001 |
| Pre-transplant diabetes | 2.65 | (1.97, 3.59) | <0.001 |
| Black race versus other | 1.83 | (1.09, 3.06) | 0.021 |
| Male | 1.51 | (1.11, 2.06) | 0.009 |
| Total bilirubin lowest tertile (<2.2 mg/dL) | Reference | Reference | Reference |
| Total bilirubin middle tertile (2.2–4.4 mg/dL) | 0.73 | (0.52, 1.02) | 0.066 |
| Total bilirubin highest tertile (>4.4 mg/dL) | 0.40 | (0.25, 0.63) | <0.001 |
| Serum albumin lowest tertile (<2.7 g/dL) | Reference | Reference | Reference |
| Serum albumin middle tertile (2.7–3.1 g/dL) | 0.72 | (0.51, 1.02) | 0.066 |
| Serum albumin highest tertile (>3.1 g/dL) | 0.61 | (0.43, 0.88) | 0.007 |
| Serum sodium lowest tertile (<134 mEq/L) | Reference | Reference | Reference |
| Serum sodium middle tertile (134–138 mEq/L) | 1.39 | (0.96, 2.01) | 0.085 |
| Serum sodium highest tertile (>138 mEq/L ) | 2.40 | (1.64, 3.52) | <0.001 |
Model also adjusted for age-strata, pre-transplant hypertension, cause of liver disease, and international normalized ratio of prothrombin time at transplant. None of these variables were significantly associated with the outcome.
The pattern of renal dysfunction was also associated with mortality. Compared to Group 1, the HR associated with death for Group 2 was 1.36 (CI 1.15 – 1.60, p=0.001) and for Group 4, the HR was 1.95 (CI 1.49–2.56, p<0.001). The HR for death associated with Group 3, the group with the lowest number of patients, was 1.35 (CI 0.80–2.28, p=0.27) and did not reach statistical significance.
ESRD among recipients with eGFR fluctuating above and below 30ml/min/1.73m2 (Group 2)
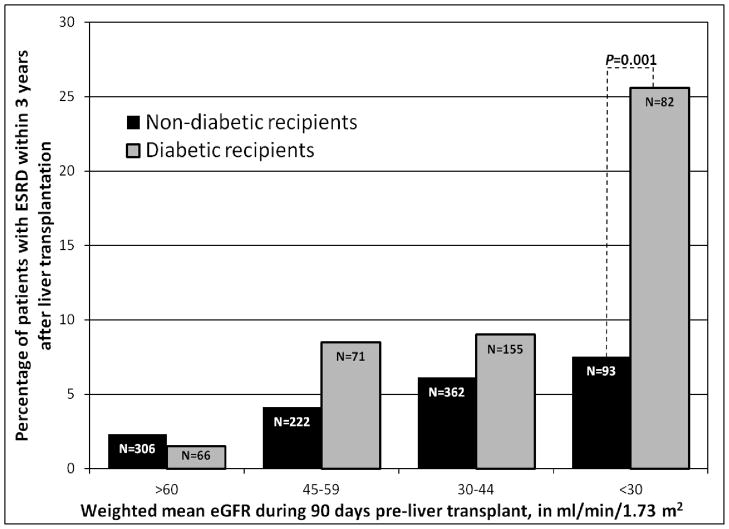
As shown in Figure 2, the 1,357 LTA recipients in Group 2 were categorized by weighted mean eGFR: >60 (Group 2a), 45 – 59 (Group 2b), 30–44 (Group 2c), and <30 ml/min/1.73m2 (Group 2d). Among recipients with a weighted mean eGFR ≥30 ml/min/1.73m2, the rate of ESRD by three years was <10%, with no statistically significant difference between diabetic and non-diabetic recipients.
Figure 2.
Risk of end-stage renal disease by three years after liver-alone transplant among recipients whose eGFR fluctuated prior to transplant (Group 2)
However, in subgroup 2d, the rate of ESRD by three years was 7.5% (7/93) in non-diabetics versus 25.6% (21/82) among diabetic recipients (p<0.001). Of note, a substantial minority of individuals was excluded from Group 2d because they received an SLK transplant: 20/113 (17.7%) of non-diabetic and 18/100 (18.0%) of diabetic recipients with a weighted mean eGFR <30 ml/min/1.73m2.
We also fit a multivariable Cox regression model for the outcome of death-censored ESRD among Group 2 recipients receiving LTA. This analysis confirmed that time-weighted mean eGFR <30 ml/min/1.73m2 (HR 5.28 versus reference mean eGFR >60 ml/min/1.73m2, p=0.002) and diabetes (HR 2.14, p=0.007) were independently associated with ESRD. Table 4 shows these results.
Table 4.
Multivariable Cox Regression Analysis of End-Stage Renal Disease among 1357 liver-alone recipients in Group 2 (eGFR fluctuates above and below 30 ml/min/m2) *
| Characteristic | Hazard Ratio | Confidence Interval | P |
|---|---|---|---|
| Time-weighted mean eGFR | |||
| >60 ml/min/m2 | Reference | Reference | Reference |
| 45–59 ml/min/m2 | 1.53 | (0.53, 4.48) | 0.43 |
| 30–44 ml/min/m2 | 2.35 | (0.90, 6.15) | 0.08 |
| <30 ml/min/m2 | 5.28 | (1.83, 15.3) | 0.002 |
| Pre-transplant diabetes | 2.14 | (1.24, 3.72) | 0.007 |
Model also adjusted for age-strata, race, gender, pre-transplant hypertension, cause of liver disease, as well as albumin, serum sodium, serum bilirubin and international normalized ratio of prothrombin time at transplant. None of these variables were significantly associated with the outcome.
Discussion
In a national cohort of liver transplant recipients, severity and duration of pre-transplant renal dysfunction were strongly associated with an increased risk of post-transplant ESRD. This risk was most pronounced in recipients with sustained eGFR <30 ml/min/1.73m2 for the 90 days before transplantation. These findings validate the multi-society recommendations to consider SLK transplant for this group.(4) We also found that diabetic LTA recipients with a weighted mean eGFR <30 ml/min/1.73m2 face an elevated risk of post-transplant ESRD. Transplant centers caring for similar patients should consider listing these candidates for SLK. Our novel approach to categorizing pre-transplant renal dysfunction could be used to refine future consensus guidelines.
Decisions about SLK impact both SLK recipients as well as patients waiting for a kidney transplant. Approximately 30,000 adults were added to the kidney transplant waitlist in 2011, but only 9,700 received a deceased donor transplant, while 6,500 others were removed for death or being “too sick to transplant.”(18) Given the scarcity of kidney allografts, there is an ethical imperative that SLKs only be performed in those for whom inadequate renal recovery is expected after LTA. Additionally, SLK recipients not on dialysis may not gain a survival benefit.(19)
A recent decision analysis examined the net benefit of allocating a kidney allograft as part of an SLK versus allocating the kidney separately to a renal transplant candidate. The analysis demonstrated that while “combined” allocation of a kidney with an SLK transplant yields more quality-adjusted life years in many scenarios, this net benefit is not seen when the liver recipient has a high likelihood of being dialysis-free after LTA.(20) In these cases, split allocation of the liver and kidney yields significantly more life-years. The threshold favoring split allocation of a kidney was a >50% chance of renal recovery after LTA. While the specific results of a decision analysis (in this case, a 50% chance of renal recovery) should not be considered a definitive answer to a clinical problem, the study suggests that SLK transplant will provide net benefit compared to a kidney-alone transplant only when the risk of post-transplant ESRD after LTA is substantial.
As in Figure 2, the risk of ESRD among individuals with weighted mean eGFR <30 ml/min/1.73m2 (Group 2d) was greatly increased by the presence of diabetes. Twenty-six percent of diabetic patients in subgroup 2d reached ESRD by three years. The effect of diabetes may have impaired recovery from acute kidney injury among these patients, or these patients may have had pre-existing diabetic nephropathy that became evident while these patients suffered clinical deterioration on the waiting list. Notably, among diabetic patients in Group 2 with weighted mean eGFR <30 ml/min/1.73m2, approximately 18% received an SLK. If one makes the assumption that these 18% were at higher risk for ESRD (had they received an LTA) than recipients who actually did receive an LTA, then the 25% risk of ESRD shown in Figure 2 may understate the overall risk of ESRD after LTA in this group. For this reason, we believe that carefully-selected Group 2d patients merit consideration for SLK.
It is important to recognize that the post-transplant mortality rate far outstrips the rate of ESRD for patients in Group 1 and most patients in Group 2. Given this increased hazard of post-transplant mortality relative to ESRD, the overriding goal in managing these patients on the waiting list should be to obtain the optimal liver to minimize mortality risk. For these patients, the decision to take the offer of a LTA versus waiting for an SLK may also depend on the patient’s age and the likely waiting time for a combined organ offer.
Black race and lower albumin at transplantation were associated with a greater hazard of post-transplant ESRD. Black LTA recipients may develop ESRD at a more rapid rate than others due to recently-described genetic factors as well as social reasons such as less education and less access to medical care.(21, 22) Recipients with lower albumin concentrations may be more chronically ill, thus having an eGFR that overestimates the true GFR due to diminished muscle mass. Lower albumin may also be a sign of proteinuria.
Similar to prior research, our analyses showed that higher pre-transplant bilirubin was associated with a lower risk of ESRD, even after adjusting for the degree of renal dysfunction and primary diagnosis.(2) Interestingly, patients in the highest tertile of bilirubin had the highest creatinine concentrations at transplantation (data not shown). We hypothesize that the renal dysfunction among patients with the highest bilirubin levels was more likely to be driven by acute illness and therefore, more likely to reverse after transplantation. However, future research should explore other physiologic explanations. Interestingly, we did not find that hepatitis C was associated with post-transplant ESRD, an association that has been inconsistently reported.(2, 23) It is possible that the lack of association between hepatitis C and ESRD in our study is because we limited the cohort to individuals with evidence of pre-transplant renal disease.(2)
Our approach to categorizing LTA recipients by eGFR patterns should be validated in other populations, such as multi-center prospective cohorts. If validated, the results have important implications for clinical practice. Ideally, the findings would influence clinicians, when considering liver transplant candidates who were not on dialysis, to limit SLK transplant to those with either eGFR always <30 ml/min/1.73 m2 or carefully-selected diabetic candidates with a weighted mean eGFR <30 ml/min/1.73m2. Only a small proportion of LTA recipients in our cohort meet these criteria. Specifically, even if all of Group 3 (86 patients) and all diabetic recipients in Group 2d (82 patients) instead received SLK, the burden (168 over 6 years; 3.4% of 4997 patients in the whole cohort) on the pool of deceased donor kidneys would be moderate. However, in listing patients for SLK, transplant staff should use the full range of available clinical information, as well as the pattern of renal dysfunction, to select only those liver transplant candidates who have the most evidence of severe and durable kidney injury.
Our study has multiple limitations. First, our inclusion criteria required a minimum of 90 days on the waitlist to allow for calculation of longitudinal eGFR patterns. This criterion allowed us to validate consensus guidelines. Additionally, our results may prove useful to clinicians evaluating candidates with 90 days of data on pre-transplant renal function, regardless of waiting list duration. However, it is possible that our findings may not be generalizable to otherwise similar liver transplant recipients listed for <90 days, who often have greater severity of illness. A second limitation is that eGFR may overestimate actual GFR in liver transplant candidates due to decreased muscle mass.(1, 10) However, eGFRs are likely to represent the highest GFR these patients could have; therefore, recipients would likely have a “true” GFR <60 ml/min/m2 at transplant. Further, using the MDRD equation to estimate GFR conforms to mainstream clinical practice and the MDRD eGFR was a major point of reference in the 2008 Consensus Guidelines.(4) We also used this equation to ascertain changes in renal function within each patient over time.(24). A third limitation is that the SRTR dataset does not include proteinuria, urine sodium concentration, or inflammatory biomarkers such as neutrophil gelatinase-associated lipoprotein (NGAL); these values might be useful predictors of renal prognosis. Prospective studies of liver transplant candidates should be performed that compare a range of injury biomarkers to relevant renal outcomes including biopsy and renal recovery. A fourth limitation is that our results will not provide much guidance to clinicians caring for liver transplant candidates needing prolonged dialysis. However, liver transplant recipients requiring dialysis have been studied extensively.(2, 16, 19) The novelty of this analysis lies in the creation of a national cohort of liver recipients with pre-transplant, non-dialysis-dependent CKD.
Our outcomes were limited to ESRD and death. We acknowledge that CKD after transplant is also associated with mortality and merits study in other settings.(25) On the other hand, the SRTR-CMS database offers advantages. It allowed us to create a cohort at over 100 centers. Also, the SRTR accurately ascertains ESRD outcomes because all U.S. renal transplants are reported to the SRTR, while ESRD defined as chronic dialysis is captured by CMS. Lastly, it is impossible to know the potential renal outcomes had the SLK recipients received a LTA and thus, we are not able to confidently identify cases of SLK that were not warranted. This dataset only allows us to characterize subgroups that received LTA in practice and were at highest risk for ESRD.
In summary, we have demonstrated that the rate of post-transplant ESRD is significantly greater in liver recipients with evidence of advanced CKD for at least 90 days before transplant. Our results support consensus guidelines that liver transplant candidates with eGFR always <30 ml/min/1.73 m2 –corresponding to stage 4 or 5 CKD – should receive SLK. We provide new data showing that diabetic liver candidates with a time-weighted mean eGFR <30 ml/min/1.73 m2 for at least 90 days before transplant should be considered for SLK. Subsequent studies should examine other potential pre-transplant risk factors, such as proteinuria or other biomarkers, to better determine which patients with end-stage liver disease and renal dysfunction derive the most benefit from dual organ transplants.
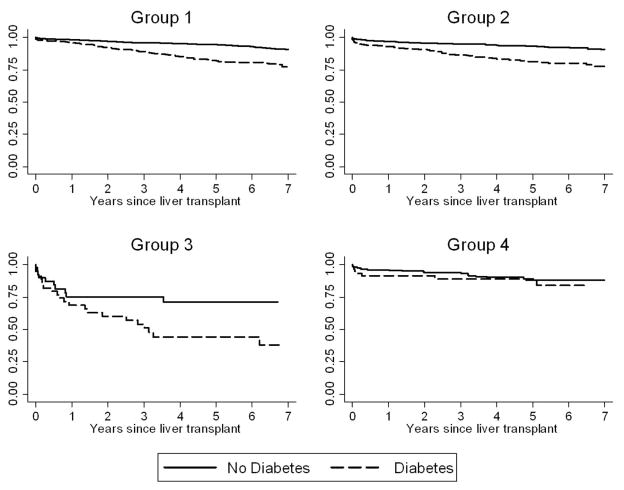
Appendix 2.
Risk of ESRD among liver transplant recipients, stratified by presence of diabetes
Acknowledgments
Funding sources:
Dr. Ruebner is supported by NIH grant T32-HD064567
Dr. Goldberg is supported by NIH grant F32-DK089694-01
Dr. Levine is supported by NIH grant K08-DK092282-01
Dr. Reese is supported by NIH grant K23-DK078688-01
The SRTR comprises information on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of OPTN, and has been described elsewhere.(2) The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This work was supported in part by HRSA contract HHSH250201000018C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- SLK
Simultaneous Liver-Kidney transplant
- eGFR
Estimated Glomerular Filtration Rate
- ESRD
End-Stage Renal Disease
- MELD
Model for End-Stage Liver Disease
- LTA
Liver Transplant Alone
- GFR
Glomerular Filtration Rate
- CKD
Chronic Kidney Disease
- OPTN
Organ Procurement and Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients
- CMS
Center for Medicare and Medicaid Services
- UNOS
United Network for Organ Sharing
- MDRD
Modification of Diet in Renal Disease Study
- NGAL
Neutrophil Gelatinase-Associated Lipoprotein
Footnotes
Conflicts of interest: The authors have no financial conflicts of interest to disclose as defined by the American Journal of Transplantation.
References
- 1.Bloom RD, Reese PP. Chronic kidney disease after nonrenal solid-organ transplantation. J Am Soc Nephrol. 2007;18(12):3031–3041. doi: 10.1681/ASN.2007040394. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11(11):2372–2378. doi: 10.1111/j.1600-6143.2011.03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6(11):2651–2659. doi: 10.1111/j.1600-6143.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 4.Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK) Am J Transplant. 2008;8(11):2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 5.Davis CL, Feng S, Sung R, Wong F, Goodrich NP, Melton LB, et al. Simultaneous liver-kidney transplantation: evaluation to decision making. Am J Transplant. 2007;7(7):1702–1709. doi: 10.1111/j.1600-6143.2007.01856.x. [DOI] [PubMed] [Google Scholar]
- 6.Bahirwani R, Reddy KR. Outcomes after liver transplantation: chronic kidney disease. Liver Transpl. 2009;15 (Suppl 2):S70–74. doi: 10.1002/lt.21900. [DOI] [PubMed] [Google Scholar]
- 7.Campbell MS, Kotlyar DS, Brensinger CM, Lewis JD, Shetty K, Bloom RD, et al. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005;11(9):1048–1055. doi: 10.1002/lt.20445. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez EQ, Gonwa TA, Levy MF, Goldstein RM, Mai ML, Hays SR, et al. Preoperative and perioperative predictors of the need for renal replacement therapy after orthotopic liver transplantation. Transplantation. 2004;78(7):1048–1054. doi: 10.1097/01.tp.0000137176.95730.5b. [DOI] [PubMed] [Google Scholar]
- 9.Cantarovich M, Tchervenkov J, Paraskevas S, Ghali P, Wong P, Deschenes M, et al. Early Changes in Kidney Function Predict Long-Term Chronic Kidney Disease and Mortality in Patients After Liver Transplantation. Transplantation. 2011 doi: 10.1097/TP.0b013e3182384aff. [DOI] [PubMed] [Google Scholar]
- 10.Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10(2):301–309. doi: 10.1002/lt.20017. [DOI] [PubMed] [Google Scholar]
- 11.National Kidney Foundation. KDOQI Guidelines. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Part 4. Definition and Classification of Stages of Chronic Kidney Disease. 2000 URL: http://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm.
- 12.Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60(5):702–709. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 13.Scientific Registry of Transplant Recipients. Guide to the Program-Specific Reports v 13.5. 2011 Jun; URL: http://www.srtr.org/csr/current/all_csr_documentation.pdf.
- 14.Rothman K, Greenland S, Lash T. Modern Epidemiology. 3. Philadelphia: WoltersKluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 15.Checkoway H, Rice CH. Time-weighted averages, peaks, and other indices of exposure in occupational epidemiology. Am J Ind Med. 1992;21(1):25–33. doi: 10.1002/ajim.4700210106. [DOI] [PubMed] [Google Scholar]
- 16.Dellon ES, Galanko JA, Medapalli RK, Russo MW. Impact of dialysis and older age on survival after liver transplantation. Am J Transplant. 2006;6(9):2183–2190. doi: 10.1111/j.1600-6143.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Welch K, Eikstadt R, Marrero JA, Fontana RJ, Lok AS. Renal outcomes after liver transplantation in the model for end-stage liver diseaseera. Liver Transpl. 2009;15(9):1142–1148. doi: 10.1002/lt.21821. [DOI] [PubMed] [Google Scholar]
- 18.OPTN Data Report on Removal Reasons by Year for Kidney Transplant Candidates. Based on OPTN data as of January 6th, 2011. URL: http://optn.transplant.hrsa.gov/latestData/rptData.asp.
- 19.Locke JE, Warren DS, Singer AL, Segev DL, Simpkins CE, Maley WR, et al. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation. 2008;85(7):935–942. doi: 10.1097/TP.0b013e318168476d. [DOI] [PubMed] [Google Scholar]
- 20.Kiberd B, Skedgel C, Alwayn I, Peltekian K. Simultaneous liver kidney transplantation: a medical decision analysis. Transplantation. 2011;91(1):121–127. doi: 10.1097/tp.0b013e3181fcc943. [DOI] [PubMed] [Google Scholar]
- 21.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343(21):1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 23.Bahirwani R, Shaked O, Kurd S, Bloom R, Reddy KR. Chronic kidney disease after orthotopic liver transplantation: impact of hepatitis C infection. Transplantation. 2011;91(11):1245–1249. doi: 10.1097/TP.0b013e318218d5bd. [DOI] [PubMed] [Google Scholar]
- 24.See TC, Thompson BC, Howie AJ, Karamshi M, Papadopoulou AM, Davies N, et al. Transjugular renal biopsy: our experience and technical considerations. Cardiovasc Intervent Radiol. 2008;31(5):906–918. doi: 10.1007/s00270-008-9308-6. [DOI] [PubMed] [Google Scholar]
- 25.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]