Abstract
One characteristic abnormality of lesional skin in psoriasis is the excessive production of antimicrobial peptides and proteins (AMPs). AMPs typically are small (12–50 amino acids), have positive charge and amphipathic structure, and are found in all living organisms including mammals, insects, plants and invertebrates. These peptides are best known for their integral role in killing pathogenic microorganisms; however, in vertebrates, they are also capable of modifying host inflammatory responses by a variety of mechanisms. In psoriatic lesions, many AMPs are highly expressed, and especially the associations between psoriasis and cathelicidin, β-defensins or S100 proteins have been well studied. Among them, a cathelicidin peptide, LL-37, has been highlighted as a modulator of psoriasis development in recent years. AMPs had been thought to worsen psoriatic lesions but recent evidence has also suggested the possibility that the induction of AMPs expression might improve aspects of the disease. Further investigations are needed to uncover a previously under-appreciated role for AMPs in modulating the immune response in psoriasis, and to improve disease without the risks of systemic immunosuppressive approaches.
Keywords: antimicrobial peptides, cathelicidin, LL-37, psoriasis, Toll-like receptor
PSORIASIS AND ANTIMICROBIAL PEPTIDES
Psoriasis vulgaris is a common inflammatory skin disease with characteristic histological changes including abnormal epidermal proliferation and a cellular infiltrate including neutrophils and T cells.1 For a long time, psoriasis had been thought to be an epidermal disease because psoriatic keratinocytes have an increase in cell proliferation rate and a shortening of the duration of the cell cycle.2,3 However, since the effectiveness of T-cell suppressive approaches for psoriasis treatment and animal experiments with severe combined immunodeficiency mice indicated that CD4+ cells induce psoriatic eruptions, T cells have been thought to be central players in the pathogenesis of psoriasis.4–7 Although the pathogenesis of psoriasis is still unclear, abnormal T-cell-mediated immune responses are critical to manifestation of the disease.1 This process includes the contribution of both T-helper (Th)1 and Th17 cell subsets to inflammation by producing cytokines including tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-12, IL-17A, IL-22 and IL-23.1 Clinical observations of exacerbations of disease and benefits realized by targeting in these cytokines have strongly supported the role for these molecules in psoriasis and other autoimmune inflammatory diseases.8–19
Despite the current focus on T cells in the pathogenesis of psoriasis, the keratinocytes within the epidermis of psoriatic plaques are clearly abnormal in many respects and likely influence immunocytes by production of inflammatory cytokines and chemokines.20 Among the abnormalities in function of psoriatic keratinocytes is the excessive production of antimicrobial peptides and proteins (AMPs). AMPs typically are small (12–50 amino acids residues), have positive charge and amphipathic structure, and are found in all living organisms including mammals, insects, plants and invertebrates.21 The structures of AMPs are very similar between different species and divided into several classes such as α-helix, β-sheet, extended or loop structure. These antimicrobial molecules are best known for their integral role in killing pathogenic microorganisms such as Gram-positive and Gram-negative bacteria, protozoa, fungi as well as some viruses; however, in vertebrates, AMPs are also suspected of modifying host inflammatory responses by a variety of mechanisms including action as chemotactic agents, angiogenic factors and regulators of cell proliferation.21
Antimicrobial peptides and proteins are thought to have several mechanisms to activate mammalian cells.21 First, AMPs directly bind to specific receptors, leading to the initiation of receptor signaling. Second, AMPs alter the membrane microdomain of receptors and may modify functions without a ligand, or make the receptors insensitive. Third, AMPs stimulate the release of membrane-bound growth factors that consequently bind to high affinity receptors such as epidermal growth factor receptors (EGFR).
Today, more than 1800 AMPs have been identified (Antimicrobial Peptides Database: http://aps.unmc.edu/AP/main.php), and more than 20 AMPs have been found in skin.21 In psoriatic lesions, many of them are highly expressed such as cathelicidin, β-defensins, S100 proteins, RNase 7, lysozyme, elafin, neutrophil gelatinase-associated lipocalin, and so forth.22,23 Especially, many studies have focused on the association between psoriasis and cathelicidin, β-defensins or S100 proteins. Among them, a cathelicidin peptide, LL-37, has been highlighted as a modulator of psoriasis development in recent years.
DEFENSINS
Defensins are one type of cationic microbial peptide and contain six conserved cystein residues that form three pairs of intramolecular disulfide bonds.21,22 These peptides are separated into α-, β- and θ-defensins by amino acid sequences of cystein residues and disulfide bonds alignment. In humans, six α-defensins (human neutrophil peptide [HNP]1–6) have been identified and they are expressed by neutrophils (HNP1–4) and Paneth cells (HNP5, 6). Of α-defensins, HNP1, HNP2 and HNP3 have been identified from lesional psoriatic scale extracts.23 Four human β-defensins (HBD1–4) have broad-spectrum antimicrobial activity and immune-modulating functions and are expressed in epithelia and peripheral blood cells. HBD1 is constitutively expressed in epithelia but HBD2–4 are only induced by stimulation with pro-inflammatory cytokines and microbial products. 21 HBD2 and HBD3 have been isolated from psoriatic scales by Harder et al.24,25 in 1997 and 2001, respectively. Individual β-defensin copy numbers have been shown to be associated with psoriasis.26 TNF-α and IFN-γ, which are highly expressed in psoriatic lesions, induce HBD2 and HBD3 expression in keratinocytes.25,27 In addition, Th17 cytokines, IL-17A and IL-22 are also inducers of HBD2.28 The mechanism of β-defensins’ expression induction has been studied by many groups but the role for these peptides in the pathogenesis of psoriasis is not fully understood, although AMPs are thought to contribute to a low rate of infection in psoriatic lesions.29
S-100 PROTEINS
S-100 proteins are a family of low molecular weight (9–13-kDa) proteins characterized by the presence of two calcium binding sites of helix–loop–helix motifs.30 Twenty-one different types of S-100 proteins have been identified and most S-100 protein genes are located in the epidermal differentiation complex on chromosome 1q21. Among the 21 S-100 proteins, 13 S-100 proteins are expressed in normal or diseased epidermis. S-100 proteins exist in cells as anti-parallel homo- or heterodimers consisting of the monomers held together by non-covalent bonds. S-100 proteins are involved in regulation of protein phosphorylation, transcription factors, intracellular Ca2+ signaling, cytoskeletal membrane interaction, enzyme activities, cell cycle progression, differentiation and the inflammatory response. In addition, S100A7 (psoriasin), S100A8 (calgranulin A), S100A9 (calgranulin B), S100A12 (calgranulin C) and S100A15 have antimicrobial activity.22 These are all abundantly expressed in psoriatic lesions or are elevated in serum from psoriatic patients. Especially S100A7, psoriasin, has been well studied because this protein was first discovered in psoriatic skin lesions as a new Ca2+-binding S-100 protein of unknown biological function in 1991.31 This molecule is induced by calcium, vitamin D, retinoic acid, microbial products, TNF-α, IL-17A and IL-22,28,30,32–34 and is thought to have a chemotactic role in psoriasis.35
CATHELICIDIN
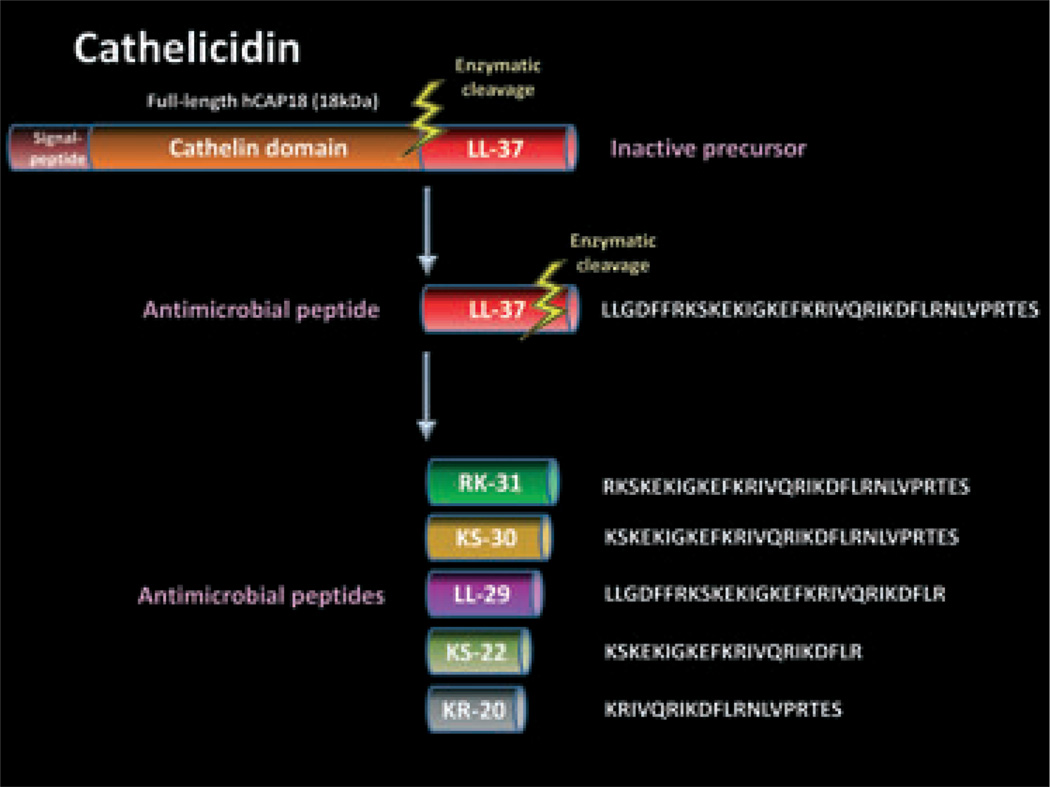
Cathelicidins are one of the major AMPs expressed in both invertebrates and vertebrates.21 In humans, only one cathelicidin gene, CAMP, has been identified as a coding region for the 18-kDa preproprotein hCAP18 although some mammals have multiple cathelicidin genes. “Cathelicidin” is named from the highly conserved “cathelin” domain of the precursor proteins. The precursor contains a signal peptide domain on its N-terminus. LL-37 is the C-terminal peptide fragment derived from hCAP18 (Fig. 1). LL-37 is named from a 37-residue peptide starting with a pair of leucines. This peptide is expressed by various types of cells such as epidermal keratinocytes, intestine cells, respiratory epithelial cells, neutrophils, T cells, natural killer cells, monocytes and mast cells.21,36 It is detectable in skin, trachea, esophagus, intestine, stomach, liver, spleen and bone marrow. Sweat, saliva, wound fluid and seminal plasma also contain this peptide. LL-37 needs to be released by enzymatic cleavage such as kallikreins in keratinocytes and protease 3 in neutrophils (Fig. 1).21 Similarly to other AMPs, LL-37 has a positive charge, and forms an amphipathic and α-helical structure. LL-37 not only has the capacity to kill a wide variety of microbes, but also can modify host immune and growth responses. The host responses to cathelicidin are complex and dependent on the disease. Observations of LL-37 action have included those demonstrating pro-inflammatory activity,37,38 anti-inflammatory activity,39–41 promotion of chemotaxis,42 angiogenesis43 and enhancing wound repair.44 LL-37 activates host cells through formyl-peptide receptor-like 1 or G protein-coupled receptors, leading to intracellular signaling pathways. EGFR transactivation by LL-37 is also one important mechanism to activate the cells. LL-37 is further cleaved into smaller peptides such as RK-31, KS- 30, LL-29, KS-22, KR-20, and so forth45 (Fig. 1). Different peptide forms have different antimicrobial activities and pro-inflammatory functions.37
Figure 1.
Structure of human cathelicidin. Human cathelicidin precursor protein hCAP18 contains a signal peptide domain on its N-terminus. LL-37 is the C-terminal peptide fragment derived from hCAP18, and needs to be released by enzymatic cleavage such as kallikreins in keratinocytes and protease 3 in neutrophils. LL-37 is further cleaved into smaller peptides such as RK-31, KS-30, LL-29, KS-22 and KR-20.
LL-37 IN THE PATHOGENESIS OF PSORIASIS
In 1997, Frohm et al.46 first reported that cathelicidin/LL-37 expression is upregulated in psoriatic epidermis as well as other skin inflammatory disorders, and suggested that this induction enhances the antimicrobial defense capacity of the disrupted barrier in the lesions. Our group has demonstrated that LL-37 was the exclusive detectable form of the peptide in psoriasis by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry analysis. 47 The expression and processing of the cathelicidin precursor protein is somewhat specific for the cell, tissue and disease state. Cathelicidin is found at low levels in normal skin and is not in the form of LL-37.38 In contrast, excess cathelicidin is seen in the inflammatory skin disease rosacea, but in contrast to normal skin these cathelicidin peptides are found in several unique forms.38 In this way, psoriasis differs significantly with rosacea where many cathelicidin peptide forms other than LL-37 can be detected or normal uninflamed skin where LL-37 is absent. The significance of observing different processed forms of the cathelicidin precursor protein is that different peptide forms have different antimicrobial activities and pro-inflammatory functions.37 Thus, although psoriasis and rosacea both have elevated cathelicidin expression, the balance of peptides with different and potentially competitive actions may partially explain differences in phenotype.
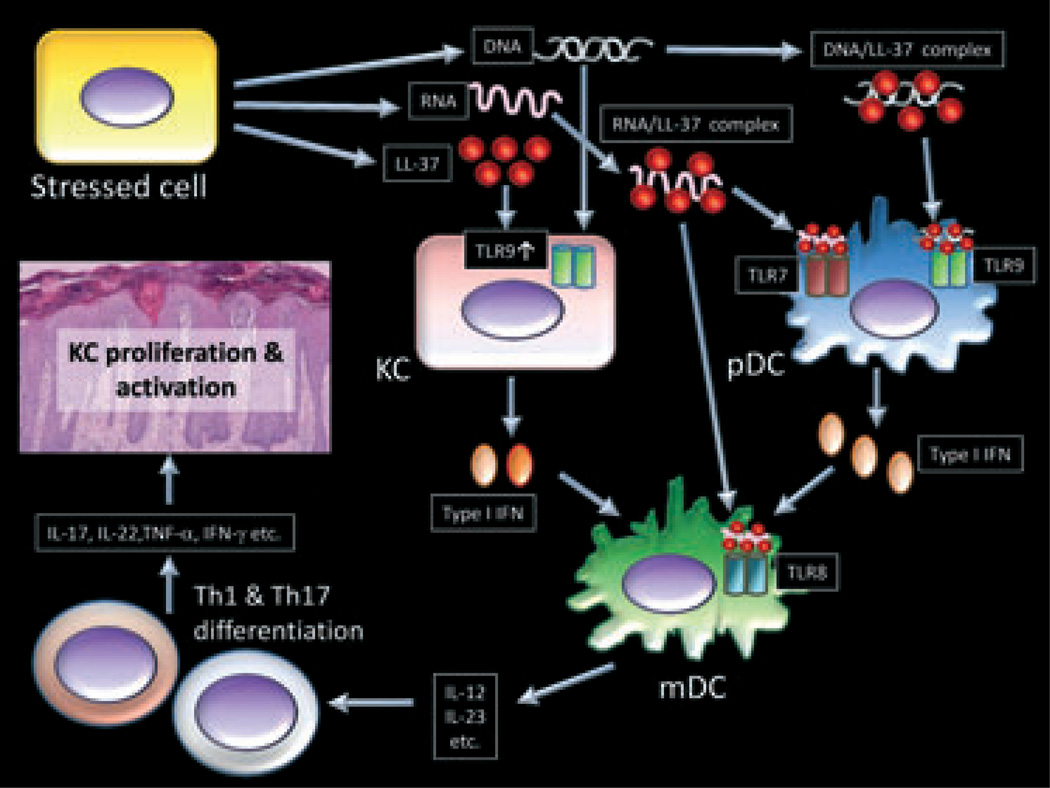
In 2002, Ong et al.29 showed that the excess production of antimicrobial peptides including LL-37 and HBD2 in psoriatic lesions is associated with a low rate of infection, and that atopic dermatitis patients are susceptible to bacterial and viral infection because the expression of AMPs are not induced sufficiently. In 2007, Lande et al.48 have shown another important immune-modulatory function of LL-37 in psoriasis. The cathelicidin antimicrobial peptide LL-37 has been hypothesized to drive inflammation in psoriasis through its capacity to enable plasmacytoid dendritic cells (pDCs) to recognize self-DNA through Toll-like receptor 9 (TLR9) (Fig. 2). This response is in contrast to the classical concept that TLR9 recognizes unmethylated DNA sequences (CpG dinucleotides) found in microbial DNA,49 and in turn serves as an innate warning system against infection. A complex between LL-37 and DNA has been proposed to be necessary for activation of TLR9 in pDCs.48 This activation induces a large amount of type I IFN production, leading to myeloid dendritic cell (mDC) activation, Th1 /Th17 differentiation and keratinocytes activation in turn (Fig. 2).1
Figure 2.
Proposed role for LL-37 in the pathogenesis of psoriasis. Stressed cells stimulated by trauma or bacterial products release LL-37, self-RNA and self-DNA. Self-RNA and self-DNA form complexes with LL-37 and the complexes are then recognized by Toll-like receptor (TLR)7 or TLR9 in plasmacytoid dendritic cells (pDCs) or TLR8 in myeloid dendritic cells (mDCs). On the other hand, LL-37 induces TLR9 expression in keratinocytes (KCs) and greatly enhances type I IFN production induced by TLR9 signaling. Stimulated mDCs induce the differentiation of naïve T cells into Th1 cells or Th17 cells. Interleukin (IL)-17A, IL-17F and IL-22 are produced by Th17 cells and IFN-γ and tumor necrosis factor (TNF)-α are produced by Th1 cells. These mediators act on keratinocytes, leading to the activation, proliferation and production of antimicrobial peptides and proteins (AMPs) or chemokines.
Recently, we have observed that the activation of keratinocytes by LL-37 and DNA also greatly increases type I IFN through TLR9 (Fig. 2).47 TLR9 expression is upregulated in psoriatic keratinocytes and cultured keratinocytes express it in a weakly functional form.50,51 However, TLR9 expression and function is greatly enhanced on keratinocytes exposed to LL-37.47 Because the complex between LL-37 and DNA did not induce type I IFN in keratinocytes, the synergistic induction of type I IFN by LL-37 and DNA in keratinocytes is different to the mechanism of action previously reported in pDCs, and could act either by enabling access of nucleic acid to TLR9 in the endosome, and /or by enhancing the sensitivity of TLR9. The involvement of type I IFN in the pathogenesis of psoriasis has been suggested by several studies,52–64 and this highlights the importance of our observations that keratinocytes exposed to LL-37 and DNA will increase type I IFN production. For example, type I IFN signaling is activated in psoriasis,53,61–63 and psoriasis can be exacerbated by IFN-α52,54,56,58,60 or IFN-β treatment.57,59,64 Moreover, mice deficient for IFN regulatory factor-2, a transcriptional repressor of IFN-α /β signaling, develop psoriasis-like skin disease.55 In addition, type I IFN have been reported to activate autoimmune T cells through the maturation of dendritic cells.65 pDCs are currently thought to be major type I IFN-producing cells, and produce a large amount of IFN-α in psoriasis.66 However, LL-37 can enable keratinocytes to also enhance production of type I IFN, an observation likely to have significance in modulating skin inflammatory phenomena. These findings suggest that the relative contribution of keratinocytes compared to pDCs in the inflammatory milieu of the psoriatic epidermis needs to be reexamined. This conclusion is particularly true in light of the much greater abundance of keratinocytes in the skin compared to pDCs, and the location of keratinocytes placing them in a more superficial site with greater direct contact to genomic DNA and other external stimuli known to exacerbate inflammation. The observation that minor superficial injury of the epidermis leads to exacerbation of disease at that site, a process known as the Köbner phenomenon, further supports this conclusion that epidermal cells also play a critical role in this disease.
Furthermore, LL-37 has been reported to form a complex with self-RNA, leading to the activation of TLR7 in pDCs and TLR8 in mDCs in psoriasis (Fig. 2).67 Self-RNA–LL37 complexes have been shown to be present in psoriatic skin lesions and be associated with mature mDCs in vivo. Recently, human slan DCs, a rich source of TNF-α, also have been reported to respond to complexes formed of LL-37 and self-RNA in psoriasis.68 They have shown that slan DCs are more powerful in programming Th1 /Th17 cells that produce IL-17, IL-22, TNF-α and IFN-γ compared to classic CD1c+ DCs, and LL-37–self-RNA complexes activate slan DCs via TLR7 signaling.
Peric et al.17 have shown that IL-17A enhances cathelicidin expression induced by vitamin D3 in keratinocytes. They have demonstrated that IL-17RA expression is upregulated in psoriatic lesions and increased IL-17A in psoriatic skin increases cathelicidin through a vitamin D3, Act1- and MEK–ERK-dependent mechanism. Chamorro et al.69 have reported that LL-37 suppresses apoptosis in keratinocytes via a Cox-2-dependent mechanism including IAP-2, suggesting that this peptide might contribute to the resistance to apoptosis of dermal endothelial cells in psoriasis. Kanda et al.70 have focused on the serum LL-37 level in psoriasis and shown that it was higher than in a normal control, and was reduced by cyclosporin A treatment.
LL-37 AND VITAMIN D3
Vitamin D is an essential hormone for the maintenance of calcium homeostasis and bone health.71 The active form of vitamin D, 1α,25-dihydroxyvitamin D3 (1,25[OH]2D3), is synthesized in the skin, liver and kidneys in turn.72 In the skin, 7-dehydrocholesterol, a derivative of cholesterol is produced by ultraviolet (UV)-B irradiation. This previtamin D3 is converted to 25-hydroxyvitamin D3 (25[OH]D3) by CYP27A1 in the liver, and then 25(OH)D3 is converted to 1,25(OH)2D3 by CYP27B1 in the kidneys. Because keratinocytes express both CYP27A1 and CYP27B1, they can activate vitamin D3 independently of renal and hepatic hydroxylation steps.73 Nowadays, many studies have demonstrated additional roles for vitamin D3 in the immune system, cardiovascular system and cancer prevention.74
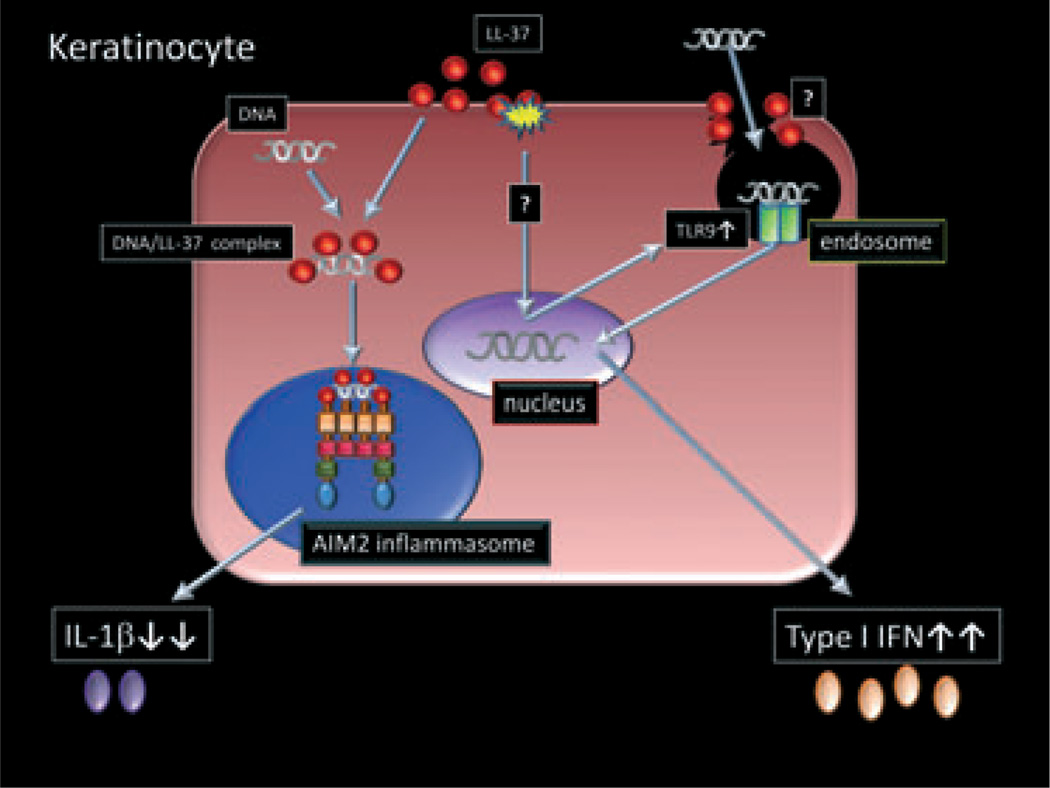
Vitamin D analogs have been used in the topical treatment of psoriasis for a long time. They are known to show clinical improvement on psoriatic plaques, and have been speculated to act through the stimulation of cellular differentiation and inhibition of proliferation. 75 Topical calcipotriol has been reported to suppress the expressions of HBD2, HBD3, IL-17A, IL-17F and IL-8 in psoriatic plaques.33 In addition, a recent study has demonstrated that CD4+CD25+ regulatory T cells are induced by both topical calcipotriol treatment.76 These immunosuppressive functions are also thought to lead to decreased inflammation and clinical improvement. On the other hand, Vitamin D3 is only a strong inducer of cathelicidin expression in keratinocytes and monocytes.77 Because LL-37 had been considered to trigger the development of psoriasis, this paradoxical phenomenon remains to be clarified. However, Dombrowski et al.78 have recently reported that LL-37 also serves as an anti-inflammatory agent by blocking activation of the DNA-sensing inflammasomes, which might be a mechanistic explanation why vitamin D3 is effective for psoriasis. They have shown that intracellular or cytosolic LL-37 blocks the DNA-triggered formation of AIM2 inflammasomes in keratinocytes, inhibiting IL-1β release (Fig. 3). Because our recent study has shown that extracellular or endosomal LL-37 enhances TLR9 expression and function in keratinocytes, leading to increased IFN-α/β production (Fig. 3), the location of LL-37 and DNA might be crucial for their pro- or anti-inflammatory effect.47 Thus, LL-37–DNA complex formation may be important because it is required for the anti-IL-1β effect but sequential exposure leads to a pro-IFN-α/β effect in keratinocytes.
Figure 3.
Different interactions between LL-37 and DNA in keratinocytes. In keratinocytes, extracellular or endosomal LL-37 enhances DNA recognition by Toll-like receptor (TLR)9, leading to type I interferon (IFN) production. The mechanism of TLR9 induction by LL-37 is unclear. Another mechanism may enhance TLR9 sensitivity rather than the upregulation of TLR9 expression and complex formation in keratinocytes. Intracellular or cytosolic LL-37 binds to DNA and inhibits interleukin (IL)-1β production through AIM2 inflammasome.
Several studies have focused on the associations between cathelicidin /LL-37 and other therapies of psoriasis.33,79,80 For example, Vahavihu et al.80 have reported that narrowband UV-B treatment induces cathelicidin expression by correcting vitamin D insufficiency in psoriasis. In addition, Gambichler et al.79 have shown LL-37 expression is downregulated by etanercept, a biologic which blocks the pro-inflammatory cytokine TNF-α, in psoriasis, suggesting that this mechanism might be associated with the clinical improvement.
CONCLUSION
Herein, we reviewed the roles hypothesized for AMPs in the pathogenesis of psoriasis. Many AMPs are highly upregulated in psoriatic lesions and most of them have not only antimicrobial activities but also immune-modulatory functions. AMPs had been thought to worsen psoriatic lesions but recent evidence has also suggested the possibility that the induction of AMPs expression might improve the aspects of the disease. Thus, further investigations are needed to uncover the role for AMPs in modulating the immune response in psoriasis, and to improve disease without the risks of systemic immunosuppressive approaches.
Footnotes
Conflict of interest: the authors state no conflict of interests.
REFERENCES
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein GD, McCullough JL, Ross PA. Cell kinetic basis for pathophysiology of psoriasis. J Invest Dermatol. 1985;85:579–583. doi: 10.1111/1523-1747.ep12283594. [DOI] [PubMed] [Google Scholar]
- 3.Lebwoh lM. Psoriasis. Lancet. 2003;361:1197–1204. doi: 10.1016/S0140-6736(03)12954-6. [DOI] [PubMed] [Google Scholar]
- 4.Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–1887. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths CE, Voorhees JJ. Cyclosporine A in the treatment of psoriasis: a clinical and mechanistic perspective. J Invest Dermatol. 1990;95:53S–55S. doi: 10.1111/1523-1747.ep12505786. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths CE, Powles AV, Leonard JN, Fry L, Baker BS, Valdimarsson H. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed) 1986;293:731–732. doi: 10.1136/bmj.293.6549.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110–3116. [PubMed] [Google Scholar]
- 8.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin-23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 12.Toichi E, Torres G, McCormick TS, et al. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol. 2006;177:4917–4926. doi: 10.4049/jimmunol.177.7.4917. [DOI] [PubMed] [Google Scholar]
- 13.Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 14.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 16.Ma HL, Liang S, Li J, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peric M, Koglin S, Kim SM, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181:8504–8512. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Beelen AJ, Teunissen MB, Kapsenberg ML, de Jong EC. Interleukin-17 in inflammatory skin disorders. Curr Opin Allergy Clin Immunol. 2007;7:374–381. doi: 10.1097/ACI.0b013e3282ef869e. [DOI] [PubMed] [Google Scholar]
- 19.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albanesi C, De Pita O, Girolomoni G. Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin Dermatol. 2007;25:581–588. doi: 10.1016/j.clindermatol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchau AS, Gallo RL. Innate immunity and antimicrobial defense systems in psoriasis. Clin Dermatol. 2007;25:616–624. doi: 10.1016/j.clindermatol.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harder J, Schroder JM. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol. 2005;77:476–486. doi: 10.1189/jlb.0704409. [DOI] [PubMed] [Google Scholar]
- 24.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 25.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 26.Hollox EJ, Huffmeier U, Zeeuwen PL, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 28.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 30.Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. J Invest Dermatol. 2004;123:23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]
- 31.Madsen P, Rasmussen HH, Leffers H, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J Invest Dermatol. 1991;97:701–712. doi: 10.1111/1523-1747.ep12484041. [DOI] [PubMed] [Google Scholar]
- 32.Glaser R, Meyer-Hoffert U, Harder J, et al. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J Invest Dermatol. 2009;129:641–649. doi: 10.1038/jid.2008.268. [DOI] [PubMed] [Google Scholar]
- 33.Peric M, Koglin S, Dombrowski Y, et al. Vitamin D analogs differentially control antimicrobial peptide/“alarmin” expression in psoriasis. PLoS One. 2009;4:e6340. doi: 10.1371/journal.pone.0006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Anti-microbial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 35.Jinquan T, Vorum H, Larsen CG, et al. Psoriasin: a novel chemotactic protein. J Invest Dermatol. 1996;107:5–10. doi: 10.1111/1523-1747.ep12294284. [DOI] [PubMed] [Google Scholar]
- 36.Mendez-Samperio P. The human cathelicidin hCAP18/LL-37: a multi-functional peptide involved in mycobacterial infections. Peptides. 2010;31:1791–1798. doi: 10.1016/j.peptides.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Braff MH, Hawkins MA, Di Nardo A, et al. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005;174:4271–4278. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 39.Mookherjee N, Brown KL, Bowdish DM, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 40.Mookherjee N, Wilson HL, Doria S, et al. Bovine and human cathelicidin cationic host defense peptides similarly suppress transcriptional responses to bacterial lipopolysaccharide. J Leukoc Biol. 2006;80:1563–1574. doi: 10.1189/jlb.0106048. [DOI] [PubMed] [Google Scholar]
- 41.Morioka Y, Yamasaki K, Leung D, Gallo RL. Cathelicidin antimicrobial peptides inhibit hyaluronan-induced cytokine release and modulate chronic allergic dermatitis. J Immunol. 2008;181:3915–3922. doi: 10.4049/jimmunol.181.6.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule-and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tokumaru S, Sayama K, Shirakata Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 45.Yamasaki K, Schauber J, Coda A, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 46.Frohm M, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 47.Morizane S, Yamasaki K, Muhleisen B, et al. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J Invest Dermatol. 2012;132:135–143. doi: 10.1038/jid.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 49.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 50.Lebre MC, van der Aar AM, van Baarsen L, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 51.Miller LS, Sorensen OE, Liu PT, et al. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. JImmunol. 2005;174:6137–6143. doi: 10.4049/jimmunol.174.10.6137. [DOI] [PubMed] [Google Scholar]
- 52.Downs AM, Dunnill MG. Exacerbation of psoriasis by interferon-alpha therapy for hepatitis C. Clin Exp Dermatol. 2000;25:351–352. doi: 10.1046/j.1365-2230.2000.00655-4.x. [DOI] [PubMed] [Google Scholar]
- 53.Fah J, Pavlovic J, Burg G. Expression of MxAprotein in inflammatory dermatoses. J Histochem Cytochem. 1995;43:47–52. doi: 10.1177/43.1.7822763. [DOI] [PubMed] [Google Scholar]
- 54.Funk J, Langeland T, Schrumpf E, Hanssen LE. Psoriasis induced by interferon-alpha. Br J Dermatol. 1991;125:463–465. doi: 10.1111/j.1365-2133.1991.tb14774.x. [DOI] [PubMed] [Google Scholar]
- 55.Hida S, Ogasawara K, Sato K, et al. CD8(+) T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-alpha/beta signaling. Immunity. 2000;13:643–655. doi: 10.1016/s1074-7613(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 56.Ketikoglou I, Karatapanis S, Elefsiniotis I, Kafiri G, Moulakakis A. Extensive psoriasis induced by pegylated interferon alpha-2b treatment for chronic hepatitis B. Eur J Dermatol. 2005;15:107–109. [PubMed] [Google Scholar]
- 57.Kowalzick L. Psoriasis flare caused by recombinant interferon beta injections. J Am Acad Dermatol. 1997;36:501. doi: 10.1016/s0190-9622(97)80248-4. [DOI] [PubMed] [Google Scholar]
- 58.Ladoyanni E, Nambi R. Psoriasis exacerbated by interferon-alpha in a patient with chronic myeloid leukemia. J Drugs Dermatol. 2005;4:221–222. [PubMed] [Google Scholar]
- 59.Lopez-Lerma I, Iranzo P, Herrero C. New-onset psoriasis in a patient treated with interferon beta-1a. Br J Dermatol. 2009;160:716–717. doi: 10.1111/j.1365-2133.2008.09005.x. [DOI] [PubMed] [Google Scholar]
- 60.Pauluzzi P, Kokelj F, Perkan V, Pozzato G, Moretti M. Psoriasis exacerbation induced by interferon-alpha. Report of two cases. Acta Derm Venereol. 1993;73:395. doi: 10.2340/0001555573395. [DOI] [PubMed] [Google Scholar]
- 61.Schmid P, Itin P, Cox D, McMaster GK, Horisberger MA. The type I interferon system is locally activated in psoriatic lesions. J Interferon Res. 1994;14:229–234. doi: 10.1089/jir.1994.14.229. [DOI] [PubMed] [Google Scholar]
- 62.Suomela S, Cao L, Bowcock A, Saarialho-Kere U. Interferon alpha-inducible protein 27 (IFI27) is upregulated in psoriatic skin and certain epithelial cancers. J Invest Dermatol. 2004;122:717–721. doi: 10.1111/j.0022-202X.2004.22322.x. [DOI] [PubMed] [Google Scholar]
- 63.van der Fits L, van der Wel LI, Laman JD, Prens EP, Verschuren MC. In psoriasis lesional skin the type I interferon signaling pathway is activated, whereas interferon-alpha sensitivity is unaltered. J Invest Dermatol. 2004;122:51–60. doi: 10.1046/j.0022-202X.2003.22113.x. [DOI] [PubMed] [Google Scholar]
- 64.Webster GF, Knobler RL, Lublin FD, Kramer EM, Hochman LR. Cutaneous ulcerations and pustular psoriasis flare caused by recombinant interferon beta injections in patients with multiple sclerosis. J Am Acad Dermatol. 1996;34:365–367. doi: 10.1016/s0190-9622(07)80010-7. [DOI] [PubMed] [Google Scholar]
- 65.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–550. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 66.Nestle FO, Conrad C, Tun-Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganguly D, Chamilos G, Lande R, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hansel A, Gunther C, Ingwersen J, et al. Human slan (6-sulfo LacNAc)dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responses. J Allergy Clin Immunol. 2011;127:787–794. doi: 10.1016/j.jaci.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Chamorro CI, Weber G, Gronberg A, Pivarcsi A, Stahle M. The human antimicrobial peptide LL-37 suppresses apoptosis in keratinocytes. J Invest Dermatol. 2009;129:937–944. doi: 10.1038/jid.2008.321. [DOI] [PubMed] [Google Scholar]
- 70.Kanda N, Ishikawa T, Kamata M, Tada Y, Watanabe S. Increased serum leucine, leucine-37 levels in psoriasis: positive and negative feedback loops of leucine, leucine-37 and pro- or anti-inflammatory cytokines. Hum Immunol. 2010;71:1161–1171. doi: 10.1016/j.humimm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Holick MF. Vitamin D and bone health. J Nutr. 1996;126:1159S–1164S. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- 72.Dombrowski Y, Peric M, Koglin S, Ruzicka T, Schauber J. Control of cutaneous antimicrobial peptides by vitamin D3. Arch Dermatol Res. 2010;302:401–408. doi: 10.1007/s00403-010-1045-4. [DOI] [PubMed] [Google Scholar]
- 73.Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller J, Gallo RL. Vitamin D and innate immunity. Dermatol Ther. 2010;23:13–22. doi: 10.1111/j.1529-8019.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- 75.Fogh K, Kragballe K. Recent developments in vitamin D analogs. Curr Pharm Des. 2000;6:961–972. doi: 10.2174/1381612003400128. [DOI] [PubMed] [Google Scholar]
- 76.Gorman S, Kuritzky LA, Judge MA, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+ CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 77.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 78.Dombrowski Y, Peric M, Koglin S, et al. Cytosolic DNA triggers inflamma-some activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002001. 82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gambichler T, Kobus S, Kobus A, et al. Expression of antimicrobial peptides and proteins in etanercept-treated psoriasis patients. Regul Pept. 2011;167:163–166. doi: 10.1016/j.regpep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321–328. doi: 10.1111/j.1365-2133.2010.09767.x. [DOI] [PubMed] [Google Scholar]