Abstract
Bariatric surgery induces a mean weight loss of 15–30% of initial body weight (depending on the procedure), as well as a 45–95% rate of diabetes remission. Procedures that induce greater weight loss are associated with higher rates of diabetes remission. Improvements in glucose homeostasis after bariatric surgery are likely mediated by a combination of caloric restriction (followed by weight loss) and the effects of altered gut anatomy on the secretion of glucoregulatory gut hormones.
Obesity, which afflicts 35.9% of U.S. adults, dramatically increases the risk of type 2 diabetes.1,2 More than two-thirds of the 23 million U.S. adults who have type 2 diabetes are obese.3,4 Nutrition therapy, exercise, and medical management remain the cornerstones of both obesity and diabetes treatment, but the long-term success of lifestyle modification has been modest.5
Bariatric surgery is currently the most effective therapy for producing mean long-term (10-year) weight losses of ≥ 15% of initial body weight, and such weight losses are associated with significant reductions in the incidence of comorbid conditions such as type 2 diabetes.5 In 2009, > 220,000 bariatric surgery procedures were performed in the United States alone.6 Given its dramatic effects on glucose homeostasis, bariatric surgery has garnered increasing interest as a potential treatment for type 2 diabetes.7-9
Types of Bariatric Procedures
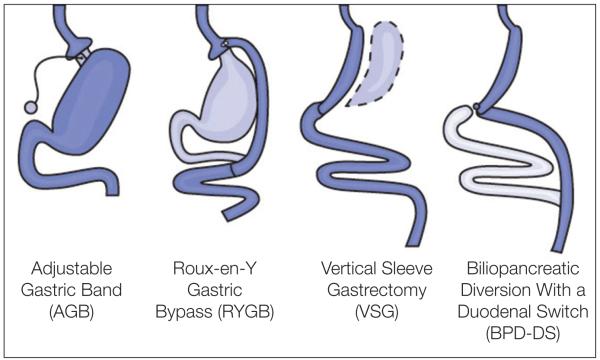
As shown in Figure 1, several bariatric procedures are currently available.9 These procedures were initially classified as restrictive, malabsorptive, or combined (based on their purported mechanism of weight loss), but recent evidence suggests that the mechanisms for each type are less clear than originally thought and likely involve multiple pathways.10 Bariatric surgeries are now more broadly classified as: 1) restrictive procedures, which dramatically reduce the volume of the stomach to limit gastric capacity and promote early satiety but do not alter intestinal anatomy; or 2) gastrointestinal (GI) diversionary procedures, which bypass segments of the small bowel.11
Figure 1.
Bariatric and metabolic surgical operations. Reproduced from the National Institute of Diabetes and Digestive and Kidney Diseases Web site (ref. 9).
Restrictive procedures
Adjustable gastric banding (AGB), in which an inflatable silicone band is placed around the fundus of the stomach, is believed to be the most commonly performed restrictive procedure worldwide.12 In this procedure, the GI anatomy remains intact, and the rate of gastric emptying is not altered.13
A s recently as 20 09, AGB accounted for 40% of bariatric procedures performed in the United States.14 However, AGB is rapidly being supplanted by vertical sleeve gastrectomy (VSG), a newer restrictive procedure that removes 75% of the stomach, including virtually all of the hormonally rich gastric fundus.5 Although VSG is conceptually a restrictive procedure, the removal of endocrine-rich gastric tissue and the accelerated rate of gastric emptying15,16 caused by this procedure is hypothesized to have significant physiological implications and may account for its superior efficacy relative to other restrictive procedures.
GI diversionary procedures
Roux-en-Y gastric bypass (RYGB), the most commonly performed GI diversionary procedure, restricts gastric size and bypasses the entire duodenum and the proximal jejunum.17 RYGB accounts for ~ 50% of bariatric surgeries performed worldwide.12 Biliopancreatic diversion (BPD) is a more extensive surgical procedure that includes a partial gastrectomy and bypasses a longer segment of the small bowel to induce significant malabsorption.17 BPD accounts for only 5% of bariatric procedures performed in the United States and is usually reserved for patients who have a BMI > 50 kg/m2.17 Detailed descriptions of these procedures are provided in Table 1.5
Table 1.
Description of Bariatric Surgery Procedures Currently in Use5
| Procedure | Description | Common Complications | Early Operative Mortality Rate (%) |
Effect on Gastric Emptying |
|---|---|---|---|---|
| Restrictive procedures | ||||
| AGB | Inflatable silicone band placed around the gastric fundus to create a 30-cc pouch; gastric anatomy remains intact |
Band slippage or band erosion, gastric reflux, esophageal dilatations, and vomiting |
0.1* | No change |
| VSG | 75% of the stomach is removed, including virtually all of the fundus; no rearrangement of small bowel |
Leakage, vomiting due to overeating |
NA | Accelerated |
| Gastrointestinal diversionary procedures | ||||
| BPD | Partial gastrectomy performed to create a 150- to 200-cc gastric sleeve, which is anastomosed to the small intestine. The excluded portion of the small intestine that serves as the conduit for bile and pancreatic juices is attached 100 cm proximal to the ileocecal valve. In the duodenal switch variation, the gastric antrum, pylorus, and a short portion of the duodenum are left intact. |
Anastomotic leakage and ulceration, chronic diarrhea, protein malnutrition, vitamin deficiencies, anemia |
1.1 | Accelerated |
| RYGB | Gastrectomy performed to create a 30-cc pouch, to which the distal jejunum is anastomosed. The proximal jejunum is reattached 75–150 cm below the gastro-jejunal anastomosis. |
Anastomotic leaks, failure of the staple partition, acute gastric dilatation, vomiting, ulceration, wound hernias, intestinal obstruction, dumping syndrome |
0.5 | Accelerated |
Rate includes estimates from laparoscopic adjustable gastric band and vertical banded gastroplasty, a restrictive procedure that is now rarely performed.
Effect of the Procedures on Weight Loss
Typically, procedures that induce greater weight loss are associated with higher rates of diabetes remission.18 However, comparison of bariatric data has been difficult because of a lack of standardization in reporting weight loss.7,19-22 In the surgical literature, weight loss is typically expressed as a percentage of excess weight lost (EWL), in which excess weight is defined as total preoperative weight minus ideal weight.18 The percentage of EWL is thus defined as weight loss/excess weight × 100. The percentage change in total weight and percentage change in BMI after bariatric surgery have also been reported.18-22
In a meta-analysis, Buchwald et al.22 evaluated the effect of bariatric surgery on weight loss and obesity-related comorbidities in 135,246 patients. An overall EWL of 55.9% was reported for all procedures, whereas individual rates associated with each procedure varied (Table 2). On average, bariatric surgery has been found to result in a BMI reduction of 10-15 kg/m2 and a weight loss of 30-50 kg.18,19
Table 2.
| AGB | RYGB | BPD | |
|---|---|---|---|
| EWL (%) | 46.2 | 59.7 | 63.6 |
| Remission of type 2 diabetes (%) | 56.7 | 80.3 | 95.1 |
VSG was not included in this meta-analysis.
Two randomized, controlled trials reported greater weight loss with RYGB than with AGB.23,24 A recent trial that included 250 participants randomly assigned to RYGB or AGB reported a mean percentage of EWL of 68.4 and 45.4%, respectively, 4 years after surgery.24 These results were similar to those of an earlier, smaller, randomized trial that provided 5 years of follow-up, in which the mean EWL was 66.6% in participants who underwent RYGB and 47.5% in those who underwent AGB.23
Although VSG and AGB are both classified as restrictive procedures, VSG appears to induce superior weight loss compared to AGB. One randomized trial that compared the magnitude of weight loss between the two procedures at 1 and 3 years found that AGB resulted in an EWL of 41.4 and 48%, respectively, versus 57.5 and 66%, respectively, for VSG.25 Two randomized trials reported comparable short-term weight loss between VSG and RYGB at 3 months26 and 12 months.27 In a randomized trial that compared the long-term efficacy of VSG and RYGB on weight outcomes, Kehagias et al.28 also reported comparable weight loss between the two procedures at 3 years (68 vs. 62% of EWL, respectively).
Maximal weight loss is typically achieved 12-18 months after all procedures,21 although evidence suggests that some patients regain a significant amount of weight several years after surgery.29 Ten-year follow-up from the Swedish Obese Subjects (SOS) study, a prospective cohort study that included 4,047 obese participants who underwent a variety of bariatric surgery procedures or nonsurgical conventional management, found that each procedure was associated with significant weight regain.29 Mean weight loss decreased in the RYGB group from 32% at 1 year to 25% at 10 years, in the gastric banding group from 20 to 13%, and in the group treated with vertical banded gastroplasy (a restrictive procedure now rarely performed) from 25 to 18%. For all procedures, substantial weight loss was nonethe-less sustained at a greater level than that achieved with nonsurgical weight loss interventions.
Definitions of Diabetes Remission
There is considerable heterogeneity among definitions of diabetes remission, which hinders comparison of remission rates among various surgical studies. Diabetes remission is often broadly defined as “normal” fasting blood glucose and A1C values without the use of antidiabetic medications.18 However, this definition does not include specific criteria for A1C levels (cut-offs have varied from < 6.0 to 7.0% in the surgical literature), a quantifiable timeframe for which antidiabetic medications must be discontinued, or more rigorous measures of assessment (e.g., oral glucose tolerance testing).
In response to a growing demand for a standardized definition of diabetes remission, a consensus group consisting of experts in pediatric and adult endocrinology, bariatric/metabolic surgery, transplantation, metabolism, and hematology-oncology was convened by the American Diabetes Association (ADA) in 2009.30 “Partial remission” was defined as mild hyperglycemia (fasting glucose levels of 100-125 mg/dl and A1C levels of < 6.5%) for at least 1 year in the absence of active pharmacological therapy or ongoing procedures. “Complete remission” was defined as a return to normal measures of glucose metabolism (fasting glucose levels of < 100 mg/dl and A1C levels < 6.0%) for at least 1 year in the absence of active pharmacological therapy or ongoing surgical procedures such as repeated replacement of endoluminal devices. “Prolonged remission” was defined as complete remission for at least 5 years’ duration. The group was unable to reach a consensus on the value of oral glucose tolerance testing in defining remission.
Evidence Regarding Diabetes Remission from Bariatric Surgery
Observational evidence suggests that bariatric surgery is associated with a 45-95% rate of diabetes remission, depending on the type of procedure.18,22,31 In the largest meta-analysis to date,22 which included 3,188 patients with type 2 diabetes who underwent bariatric surgery, the disease resolved in 78% and resolved or improved in 87%. Weight loss and diabetes remission were greatest for BPD, followed by RYGB, and then AGB (Table 2). VSG was not included in this meta-analysis because it was not a commonly performed bariatric procedure at the time.
Among studies that included exclusively diabetic patients, the rate of clinical and biochemical remission of diabetes in the first 2 years after surgery was 82%. Sixty-two percent remained free of diabetes > 2 years after surgery. However, many of the studies in this meta-analysis had significant methodological limitations. Therefore, cautious interpretation of conclusions about bariatric surgery and diabetes remission is advised. Few of the studies were randomized, controlled trials, and many were uncontrolled case series with significant amounts of missing data. Participants were not always enrolled consecutively, and heterogeneous outcomes were used to define diabetes remission.
Using the more stringent definition recommended by the ADA consensus group, Pournaras et al.32 retrospectively compared rates of diabetes remission in 209 subjects with type 2 diabetes who underwent RYGB, VSG, and AGB at three bariatric centers in the United Kingdom. Mean follow-up was 23 months (range 12-75 months). A total of 72 (34.4%) had complete remission of diabetes according to the new definition. The remission rates were 40.6% after RYGB, 26% after VSG, and 7% after AGB (P < 0.001 between groups). The remission rate for RYGB was significantly lower with the new definition than with the previously used definition (40.6 vs. 57.5%, P = 0.003).
Trials Comparing Diabetes Remission Rates From Bariatic Surgery and Intensive Medical Management
In response to increasing demand for high-quality studies comparing the diabetes remission rates of bariatric surgery and medical management, several randomized, controlled trials have recently been completed.
Dixon et al.33 randomized 60 obese participants with BMIs of 30-40 kg/m2 and a recent diagnosis of type 2 diabetes (< 2 years) to either AGB or conventional medical therapy. The primary endpoint was the rate of diabetes remission at 2 years, defined as a fasting glucose of < 126 mg/dl and an A1C of < 6.2% in the absence of anti-diabetic medications. Conventional medical therapy consisted of visits every 6 weeks with at least one member of the medical team, which included a general physician, nurse, diabetes educator, and dietitian, for the duration of the trial. Pharmacological therapy was determined on an individual basis by a diabetologist, and all participants in this group received individual counseling about lifestyle modification. Fifty-five participants (92%) completed follow-up at 2 years. Twenty-two of 30 participants (73%) in the surgical group achieved the primary endpoint compared to 4 of 30 (13%) in the medical therapy group. Surgical and conventional therapy groups lost a mean of 20.7 and 1.7% of initial body weight, respectively.
In the recently comple ted STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial,Schauer et al. 34 randomly assigned 150 obese participants with poorly controlled type 2 diabetes (A1C > 7.0%) to one of three interventions: 1) intensive medical management alone, 2) RYGB, or 3) VSG, with intensive medical therapy as an adjunct to both surgical procedures. The primary endpoint was defined as the proportion of participants with an A1C ≤ 6.0% at 12 months. All participants received intensive medical therapy per ADA guidelines, including lifestyle counseling, weight management, frequent self-monitoring of blood glucose, and use of newer glycemic therapies such as incretin mimetics.
Of the 150 participants, 140 (93%) completed follow-up at 12 months. Twenty-one of 50 participants (42%) in the RYGB group achieved the primary endpoint, compared to 18 of 49 (37%) in the VSG group, and 5 of 41 (12%) in the medical management group. All participants in the RYGB group achieved the target A1C without antidiabetic medications, whereas 5 of 18 (28%) in the VSG required one or more glucose-lowering drugs. In contrast, the use of antidiabetic medications increased in the medical management group. The mean percentage of weight loss among participants undergoing either RYGB or VSG (25.7 and 24.7%, respectively) was significantly greater than for those undergoing medical therapy (5.2%, P < 0.001 for both comparisons).
Mingrone et al.35 randomly assigned 60 participants with a BMI ≥ 35 kg/m2 and a history of type 2 diabetes for ≥ 5 years to undergo RYGB, BPD, or standard medical therapy. The primary endpoint was the rate of diabetes remission at 2 years (defined as a fasting glucose of < 100 mg/dl and an A1C of < 6.5% without antidiabetic medications). Standard medical therapy consisted of visits (at baseline and months 1, 3, 6, 9, 12, and 24) with a multidisciplinary team that included a diabetologist, dietitian, and nurse. Pharmacological therapy was optimized on an individual basis, and a lifestyle modification program was provided, although the specific nature of this program was not described.
Fifty-six participants (93%) completed the 2-year follow-up. Remission was achieved by 75% in the RYGB group and 95% in the BPD group. No participants in the medical therapy group achieved remission. At 2 years, participants in the two surgical groups had significantly greater percentage reductions in mean body weight than those in the medical therapy group (33.3% for RYGB and 33.8% for BPD, compared to 4.7% for the control group; P < 0.001 for both comparisons).
Importantly, all three of these studies used intensive medical management rather than routine care as the control. The weight loss that was achieved in the medical management groups in these studies was comparable to that reported in three recent randomized trials in which intensive weight loss interventions were delivered within primary care practices.36-38 At 2 years, weight loss in the intensive treatment arms in these studies ranged from 1.7 to 5.2%.
Predictors of Diabetes Remission
If bariatric surgery is to be considered a potential treatment for type 2 diabetes, it is crucial to identify preoperative predictors of remission to help determine which patients will benefit the most from surgery. After adjusting for BMI, sex, and preoperative A1C level, Torquati et al.39 found that preoperative treatment with oral antidiabetic agents (as opposed to insulin) and smaller preoperative waist circumference predicted diabetes remission. Two studies reported that patients with longstanding diabetes (> 10 years), preoperative insulin use, and poor preoperative glycemic control were less likely to achieve diabetes remission.40,41 However, these studies did not control for important confounding factors that may also influence diabetes remission, including age, sex, preoperative BMI, and preoperative A1C level. Eating behaviors and eating habits have been associated with weight loss after bariatric surgery.42 However, it is unknown whether these variables also may be associated with diabetes remission, particularly over long periods of time.
Potential Mechanisms for Improved Glycemic Control After Bariatric Surgery
Caloric restriction
All bariatric procedures induce significant reductions in caloric intake in the early postoperative period, and the beneficial effect of caloric restriction on glycemia is well established.43 To determine whether caloric restriction ipso facto accounts for the immediate improvement in glycemic parameters within the first week of RYGB (before weight loss), Isbell et al.44 compared the metabolic response to a mixed-nutrient meal in nine participants before and after RYGB (an average of 4 days postoperatively) and nine matched obese control subjects before and after 4 days of the postoperative diet. Half of the participants in each group had type 2 diabetes.
Participants who underwent caloric restriction comparable to those in the RYGB group displayed similar changes in meal-stimulated glucose and insulin sensitivity after 4 days on an isocaloric diet. The potent insulin secretagogue glucagon-like peptide 1 (GLP-1) increased significantly after RYGB but remained unchanged in the calorie-restricted group.
Because a comparable improvement in glucose tolerance and insulin sensitivity was observed after caloric restriction without alterations in the incretin response, these data suggest that decreased caloric intake alone accounts for the rapid improvement in glycemia after surgery. However, it is important to note that the groups were clinically (although not statistically) different at baseline with respect to weight (153.2 ± 32.2 kg in the RYGB group versus 127.0 ± 36.5 kg in the diet group), and the confounding effects of type 2 diabetes and weight were inadequately adjusted for given the small sample size.45 Furthermore, use of insulin and other antidiabetic medications was not reported, which limits the generalizability of these findings.45
Weight loss
Weight loss is one of the predominant mechanisms by which bariatric surgery induces diabetes remission, as demonstrated in multiple studies that have shown higher rates of diabetes remission with greater weight loss.17,22,29,33 Studies of AGB provide, perhaps, the strongest evidence for the role of weight loss in diabetes remission because improvements in glucose homeostasis occur independently of changes in the secretion of glucoregulatory gut hormones. In the landmark study by Dixon et al.33 described above, remission of type 2 diabetes was strongly related to weight loss.
Changes in the enteroinsular axis
Significant anatomical differences exist between AGB, VSG, and procedures that bypass segments of the gut (RYGB and BPD), resulting in distinctly different effects on the secretion of the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), the satiety factor peptide YY (PYY), and the orexigenic hormone ghrelin. These neuroendocrine factors are secreted by the gut mucosa in response to ingestion of nutrients, neural signals, and nutrient sensing.46 Many of these peptides influence glycemic control by modulating insulin secretion and possibly insulin sensitivity. The incretin effect is blunted in obese individuals with type 2 diabetes, which may contribute to impaired glucose homeostasis.46
Because AGB leaves the GI tract intact and does not significantly alter the rate of gastric emptying,13 changes in the secretion of gut hormones occur in response to weight loss only and are less pronounced than changes after malabsorptive or combined procedures.10 RYGB excludes the proximal gut and thus expedites nutrient delivery to the distal ileum, resulting in altered secretion of the enteroendocrine factors described in more detail below.17 Similar changes in the secretion of gut peptides are observed after BPD15 and VSG26,45 because gastric emptying is accelerated after both procedures.15,16 Table 3 offers a summary of the effect of the various bariatric procedures on glucoregulatory gut peptides. Table 4 summarizes the roles these hormones play in influencing appetite and satiety.
Table 3.
Overview of Altered Secretion of Gut Peptides in Response to Different Bariatric Procedures
| AGB | VSG | RYGB | BPD | |
|---|---|---|---|---|
| Ghrelin | Increase | Decrease | Increase/decrease (variable) |
Decrease |
| GLP-1 | No change | Increase | Increase | Increase |
| GIP | No change | Unknown | Increase/decrease (variable, but most studies report reduced levels) |
Decrease |
| PYY | No change | Increase | Increase | Increase |
Table 4.
Roles of Hormones Influencing Appetite and Satiety
| Primary Action | Additional Effects on Glucose Homeostasis |
Released by | Target | |
|---|---|---|---|---|
| Ghrelin | Stimulates appetite | Inhibits insulin secretion, inhibits adiponectin (an insulin- sensitizing hormone) |
Primarily secreted by the gastric fundus, but small concentrations also released by the proximal small intestine, liver, pancreas, kidney, lung, and other organs |
Hypothalamus, pancreatic islet cells |
| GLP-1 | Stimulates postprandial insulin release |
Suppresses glucagon secretion, suppresses endogenous glucose production, delays gastric emptying to attenuate postprandial hyperglycemia, and induces satiety |
L cells of distal ileum (in response to nutrients) |
Multiple organs (pancreatic islet cells, liver, heart, lungs, and hypothalamus) |
| GIP | Stimulates postprandial insulin release (but is less potent than GLP-1) |
Stimulates glucagon secretion |
K cells of proximal gut (in response to nutrients) |
Pancreatic islet cells |
| PYY | Induces satiety | Delays gastric emptying to attenuate postpran dial hyperglycemia |
Produced mainly by the L cells of distal ileum (in response to nutrients), but can also be secreted by other endocrine cells and neurons of the central nervous system |
Melanocortin neurons in the hypothalamus |
GLP-1
This incretin hormone, a potent satiety signal and insulin secretagogue, is secreted by the L cells of the distal ileum in response to nutrients and neural signals arising from the proximal gut.46 GLP-1 acts directly on pancreatic β-cells to enhance glucose-dependent insulin secretion. It also suppresses glucagon secretion, although the mechanism for this has not been clearly elucidated.46 GLP-1 attenuates postprandial glycemia by slowing gastric emptying and exerts additional effects on the central nervous system to induce satiety and decrease food intake.46
Basal levels and the postprandial response of GLP-1 are reduced in obese individuals with type 2 diabetes.47 Despite the reduced concentration, GLP-1 function remains intact in patients with diabetes when levels are restored.48
Multiple studies of RYGB and BPD have demonstrated a markedly enhanced GLP-1 response after these two procedures.49-54 Such a response appears to be independent of weight loss itself. Laferrère et al.52 demonstrated a sixfold increase in the postprandial GLP-1 response in obese, diabetic participants who had undergone RYGB, whereas the GLP-1 response was unchanged in matched obese, diabetic control subjects who had lost an equivalent amount of weight on a hypocaloric diet. Moreover, GLP-1 levels remain persistently elevated after RYGB, with levels increased at least 1 year after surgery.50
Because rapid gastric emptying occurs after VSG, the GLP-1 response is also enhanced. One randomized trial that compared the effects of RYGB versus VSG on glucose metabolism reported significant increases in postprandial GLP-1 levels at 1 week and 3 months after both procedures. However, this increased response for GLP-1 was greater with RYGB than with VSG at both time points, as measured by the area under the curve.55 In contrast, GLP-1 levels are not altered by AGB, in part because the rate of gastric emptying is not altered. Korner et al.51 showed that postprandial GLP-1 levels increased threefold after RYGB but remained unchanged after gastric banding.
The physiological activity of GLP-1 has been extensively investigated through blockade of its receptor by exendin 9-39-amide (Ex-9).56-60 Ex-9 completely eliminates the effect of endogenous GLP-1 on postprandial insulin release but has no effect on other hormones that enhance insulin secretion.58-60 Kindel et al.61 found that diabetic rats that underwent duodenal-jejunal exclusion (a procedure that simulates the GI diversion of RYGB but does not induce weight loss) had significant improvement in glucose tolerance compared to sham-operated rats. However, infusion of Ex-9 attenuated the improvement in glucose tolerance, suggesting that GLP-1 directly improves glucose homeostasis in rodents after a gastric bypass-like procedure.
Extending these findings to human subjects, Salehi et al.62 used Ex-9 to determine whether RYGB-associated hyperinsulinism is mediated by enhanced GLP-1 action and whether this accounts for greater β-cell stimulation in subjects with postsurgical hypoglycemia.62 The administration of Ex-9 reduced postprandial insulin secretion rates by 33% in individuals who had undergone RYGB, compared to 16% in BMI-matched control subjects, demonstrating that GLP-1 has a heightened effect on postprandial insulin release after RYGB.
GIP
This incretin hormone is secreted by the K cells of the proximal gut in response to the ingestion of carbohydrates and lipids. Although less potent than GLP-1, GIP also acts on pancreatic β-cells to augment postprandial insulin secretion.63 Unlike GLP-1, GIP does not have any effect on the rate of gastric emptying or satiety. Data from studies examining GIP levels in individuals with type 2 diabetes remain discrepant, with some investigators reporting unchanged64 or even increased levels of this hormone.65 However, multiple studies have consistently demonstrated that the insulinotropic activity of GIP is impaired in type 2 diabetes.66-68
The response of GIP to bariatric surgery has been more variable than that of GLP-1. Several studies52,69 have reported that GIP levels are reduced after RYGB, possibly as a result of reduced stimulation of the K cells in the proximal gut as a consequence of the bypass. In contrast, Laferrère et al.50 reported a transient elevation in GIP levels 1 month after RYGB, but this response was not sustained. Näslund et al.70 reported persistent elevations in GIP levels up to 20 years after jejunoileal bypass. Like RYGB, this procedure causes early delivery of nutrient-rich chyme to the distal small intestine.
Few studies have evaluated the effect of restrictive procedures on GIP concentrations. Two observational studies51,71 reported no change in GIP levels 6, 12, and 24 months after AGB. To date, no studies have examined the effect of VSG on the GIP response.
PYY
This peptide is a satiety signal that is cosecreted with GLP-1 from the L cells of the distal ileum in response to nutrients.72 In addition to decreasing appetite through central mechanisms,73 PYY indirectly affects glucose homeostasis through activation of melanocortin neurons in the hypothalamus that affect insulin sensitivity.74
Procedures such as RYGB that expedite nutrient delivery to the distal ileum result in an exaggerated PYY response to nutrient ingestion.75,76 Korner et al.75 reported a tenfold increase in postprandial PYY concentrations following RYGB, compared to the response observed in lean and obese nonsurgical control subjects. There have also been reports that PYY levels increase after VSG, but the response appears to be transient. In a randomized trial, Karamanakos et al.55 found that VSG and RYGB resulted in comparable increases in PYY levels for the first 6 months after surgery. However, the secretion of PYY was significantly reduced by 12 months in the VSG group, whereas the response was maintained in the RGYB group. The authors speculated that reduced PYY secretion was the result of physiological adaptation of the gastric remnant over time. Because AGB does not alter the rate of gastric emptying, the PYY response remains unchanged after this procedure.75
Ghrelin
This orexigenic hormone is primarily secreted by the gastric fundus and proximal small intestine and acts on the hypothalamus to stimulate appetite.10 This hormone and its receptor are also expressed in pancreatic islet cells.10 Ghrelin is known to inhibit insulin secretion, possibly through an autocrine or paracrine mechanism that has yet to be elucidated.77
The role of ghrelin in diabetes remission after bariatric surgery remains controversial. Ghrelin contributes to the marked loss of appetite and reduction in food intake that mediates weight loss after certain procedures and is known to enhance insulin sensitivity. Because ghrelin inhibits insulin secretion, suppresses the insulin-sensitizing hormone adiponectin, and stimulates the release of counterregulatory hormones,78 a reduction in ghrelin secretion may have beneficial effects on glucose homeostasis.
The effects of gastric bypass on ghrelin levels are inconsistent. Some reports79,80 have demonstrated reduced levels of ghrelin after RYGB, whereas others have shown no change81 or increased levels.82 Sundbom et al.83 reported a transient reduction in ghrelin secretion in the immediate and early postoperative period after RYGB, followed by a gradual increase in ghrelin secretion as weight loss ensued.
Variations in surgical techniques may account for these discrepant findings.10 If a small amount of gastric ghrelin-producing tissue is left intact, the residual cells can compensate to maintain normal ghrelin secretion. Ghrelin secretion is also affected by the vagus nerve, which is sometimes intentionally severed during RYGB. Ghrelin levels are typically higher after procedures such as AGB that maintain vagal integrity.
Because a significant amount of the gastric fundus is removed in VSG, ghrelin levels are significantly reduced after this procedure.26 In a randomized, controlled trial, fasting and postprandial ghrelin levels were found to be lower in participants who underwent VSG than in those who underwent RYGB.26
Hindgut versus foregut hypotheses
Two possible theories have been proposed to explain the rapid improvement in glucose homeostasis after gastric bypass. The hindgut hypothesis suggests that the expedited delivery of nutrients to the distal ileum (as a result of the shortened length of the small bowel) improves glycemia through the enhanced secretion of gut peptides such as GLP-1, which augments glucose-dependent insulin secretion.84 In contrast, the foregut hypothesis suggests that the exclusion of the proximal bowel (as a consequence of GI rearrangement) prevents the secretion of an unidentified “putative signal” that promotes insulin resistance and type 2 diabetes.84 Existing data from both animal and human studies lend support to both the foregut85-88 and hindgut theories,89-92 but the predominant mechanism of improved glucose metabolism has yet to be elucidated.
Effects of Bariatric Surgery on Insulin Sensitivity
Bariatric surgery affects both insulin secretion and insulin sensitivity, which are related in a hyperbolic manner such that compensatory increase in one induces a subsequent reduction in the other.93 Although it is well established that insulin sensitivity substantially improves in response to weight loss,42 some of the procedures may also enhance insulin sensitivity independent of weight loss.
The mechanisms underlying improved glucose homeostasis after bariatric surgery have been most intensively studied in participants who have undergone BPD.94-96 Mari et al.95 reported a significant increase in insulin sensitivity, as measured by the gold standard hyperinsulinemic-euglycemic clamp methodology, within days of BPD in a group of obese patients with glucose tolerance levels ranging from normal to frank diabetes. Insulin sensitivity did not improve in a matched obese control group with a similar metabolic profile who had undergone abdominal surgery for reasons other than BPD (mainly cholecystectomy and abdominal hernia repair), despite equivalent caloric restriction. This suggests that the improved glucose homeostasis after BPD occurred independent of weight loss and reduced food intake. Other studies have shown normalization of insulin-mediated, whole-body glucose uptake after BPD (often in excess of normal values) when compared to lean individuals, suggesting that this procedure confers supranormal insulin sensitivity.96
The short-term effects of RYGB on insulin sensitivity are less clear. Several studies97-99 that used the hyperinsulinemic-euglycemic clamp methodology found no improvement in insulin sensitivity within the first 4 weeks after RYGB. However, these studies were limited by the fact that participants were either nondiabetic or included a mixture of normoglycemic to frankly diabetic participants. In contrast, Kashyap et al.100 reported enhanced insulin sensitivity as assessed by a hyperglycemic clamp as early as 1 and 4 weeks after RYGB in an exclusively diabetic study population. Insulin sensitivity was unchanged at both time points in participants who underwent gastric restrictive surgery (AGB or VSG), despite weight losses equivalent to those in the RYGB group.
Because diabetic and nondiabetic individuals may respond differently to the various surgeries, further study using rigorous methodology is needed to better elucidate the short-term effects of bariatric surgery on insulin sensitivity.
Use of Bariatric Surgery for Diabetes Remission in Overweight to Mildly Obese Patients
Given the remarkable rate of diabetes remission with bariatric surgery, there is considerable interest in offering this intervention to diabetic patients with a BMI of < 35 kg/m2. Several case series and nonrandomized prospective trials have evaluated the efficacy of various bariatric surgery procedures on diabetes remission in less obese individuals.27,101,102 However, most of these studies have been limited by significant methodological limitations, including small sample size, poor follow-up, and heterogeneous criteria for diabetes remission. In a nonrandomized study that included 20 patients with poorly controlled diabetes and a mean BMI of 31 kg/m2 who underwent VSG, Lee et al.27 reported a 50% diabetes remission rate 52 weeks after surgery. Similar results were reported in a study of AGB performed in diabetic patients with a mean BMI of 33 kg/m2.101 In a case series that included 37 obese, diabetic patients with a mean BMI of 32.5 kg/m2 who underwent RYGB, Cohen et al.102 reported that diabetes was resolved in all participants after a follow-up of 6-48 months. However, participants in this series had mild diabetes (a mean fasting glucose level of 146 mg/dl on oral medications only), and only nine participants returned for follow-up at 48 months.
A 2009 study103 found that, among patients with a BMI of 32-34 kg/m2, only the most severe category of diabetes (A1C level of > 9% on maximal medical therapy) was deemed “appropriate” for bariatric surgery. The expert panel (consisting of 11 bariatric surgeons, two internists, and one endocrinologist) who developed these criteria felt that the current evidence does not support bariatric surgery in individuals with a BMI of < 32 kg/m2.
A recent survey study104 also found guarded enthusiasm among endocrinologists and primary care physicians for the use of bariatric surgery in people with type 2 diabetes and a BMI < 35 kg/m2. Only 20.8% of respondents indicated that they would be likely to refer their patients with type 2 diabetes and a BMI of 30-34.9 kg/m2 to a randomized research trial of bariatric surgery.
Diabetes Relapse Rate
Evidence from the SOS study suggests that improvement in glycemia and cardiovascular benefits persist for years after bariatric surgery.105 However, emerging evidence suggests that diabetes does recur in a significant number of patients.106,107
One small study106 that included 42 patients who had previously undergone RYGB and had ≥ 3 years of follow-up found that 10 patients (24%) experienced a relapse of diabetes. In that study, diabetes recurrence was defined as an A1C > 6.0% and a fasting glucose level > 125 mg/dl and/or a requirement for glycemic medication in patients who had previously been in remission. Long-term studies suggest an even higher rate of diabetes relapse. In a case series that followed 157 patients who had previously undergone RYGB and had experienced initial remission of diabetes, 68 (43.1%) experienced a recurrence.107 Follow-up ranged from 5 to 16 years, and diabetes status was determined by patient interview and evaluation of antidiabetic medications. Both studies found that less initial weight loss and greater weight regain were associated with a greater likelihood of diabetes relapse.106,107
The criteria for diabetes relapse have not been clearly defined.108 Postprandial hypoglycemia (typically asymptomatic) is relatively common in patients after RYGB. This may have the cumulative effect of falsely lowering A1C levels and masking the early stages of type 2 diabetes relapse.108 Further long-term studies are needed to assess the durability of diabetes remission and more accurately quantify the rate of relapse.
The issue of diabetes relapse is of particular concern when the use of bariatric surgery is considered for younger adults or adolescents. Consistent with many major academic medical centers in urban areas, the mean age of our patients is 44 years. However, we have recently observed a growing number of individuals in their 30s, many with type 2 diabetes and others with a strong family history of the disease, who are presenting for bariatric surgery with the primary objective of treating or preventing type 2 diabetes. Although surgical interventions may be particularly efficacious in the short term (within the first decade), relapse subsequently may occur and warrant further treatment.
Conclusion
Bariatric surgery induces significant improvements in glucose homeostasis through a number of mechanisms. Diabetes remission rates are highest for procedures that induce the greatest weight loss. Based on meta-analyses, BPD confers the highest rates of remission, followed by RYGB and AGB. Emerging evidence suggests that VSG induces rates of remission that are intermediate between RYGB and AGB.
All bariatric surgery procedures initially induce caloric restriction in the early postoperative period, but the various procedures appear to have differential effects on the secretion of glucoregulatory gut hormones. AGB does not alter the integrity of the GI tract or nutrient transit time and is not associated with changes in the secretion of gut peptides that are known to enhance insulin action. In contrast, VSG, RYGB, and BPD are associated with the enhanced secretion of the incretin hormones, reduced secretion of ghrelin, and greater weight loss. In conjunction, these changes result in reduced hyperinsulinemia and improved insulin sensitivity.
With increasing numbers of diabetic patients undergoing bariatric surgery, long-term randomized clinical trials comparing the effectiveness of surgical and medical therapies for type 2 diabetes are urgently needed. Studies are underway to evaluate the risk-benefit ratio of surgery in individuals with diabetes who meet the present criteria for bariatric surgery, as well as for less obese (BMI 30-35 kg/m2) populations.
Acknowledgment
This study was supported by grant 1RC1DK086132 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Note of disclosure: Dr. Sarwer has served as a paid consultant for Allergan, BAROnova, EnteroMedics, and Ethicon Endo-Surgery, which are manufacturers of products for bariatric surgery. He is also on the board of directors of the Surgical Review Corporation, which created the International Center of Excellence for Bariatric Surgery program to evaluate bariatric surgeons and hospitals around the world and also manages these bariatric Center of Excellence programs on behalf of several bariatric surgery professional societies.
Contributor Information
Marion L. Vetter, Division of Endocrinology, Diabetes, and Metabolism and medical director at the Center for Weight and Eating Disorders, Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
Scott Ritter, Center for Weight and Eating Disorders, Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
Thomas A. Wadden, Department of Psychiatry and Director of the Center for Weight and Eating Disorders, Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
David B. Sarwer, Department of Psychiatry and Surgery and director of clinical services at the Center for Weight and Eating Disorders, Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Geiss LS, Pan L, Cadwell B, Gregg EW, Benjamin SM, Engelgau MM. Changes in incidence of diabetes in U.S. adults, 1997-2003. Am J Prev Med. 2006;30:371–377. doi: 10.1016/j.amepre.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Kramer H, Cao G, Dugas L, Luke A, Cooper R, Durazo-Arvizu R. Increasing BMI and waist circumference and prevalence of obesity among adults with type 2 diabetes: the National Health and Nutrition Examination Surveys. J Diabetes Complications. 2010;24:368–374. doi: 10.1016/j.jdiacomp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention: National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, Ga: 2011. [Google Scholar]
- 5.Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009;2:CD003641. doi: 10.1002/14651858.CD003641.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Taylor K. [Accessed 3 April 2012];Metabolic and bariatric surgery fact sheet [article online] Available from www.asmbs.org/asmbs-press-kit. [Google Scholar]
- 7.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, Dohm L. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetter ML, Cardillo S, Rickels MR, Iqbal N. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009;150:94–103. doi: 10.7326/0003-4819-150-2-200901200-00007. [DOI] [PubMed] [Google Scholar]
- 9.NIDDK Weight Information Network [Accessed 3 April 2012];Bariatric surgery for severe obesity [article online] Available from www.win.niddk.nih. gov/publications/gastric.htm.
- 10.Cummings DE, Overduin J, Shannon MH, Foster-Schubert KE. Hormonal mechanisms of weight loss and diabetes resolution after bariatric surgery. Surg Obes Rel Dis. 2005;1:358–368. doi: 10.1016/j.soard.2005.03.208. [DOI] [PubMed] [Google Scholar]
- 11.Perugini RA, Malkani S. Remission of type diabetes following bariatric surgery: review of mechanisms and concept of ‘reversibility. Curr Opin Endocrinol Diabetes Obes. 2011;18:119–128. doi: 10.1097/MED.0b013e3283446c1f. [DOI] [PubMed] [Google Scholar]
- 12.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2008;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 13.de Jong JR, van Ramshorst B, Gooszen HG, Smout AJ, Tiel-Van Buul MM. Weight loss after laparoscopic adjustable gastric banding is not caused by altered gastric emptying. Obes Surg. 2009;19:287–292. doi: 10.1007/s11695-008-9746-x. [DOI] [PubMed] [Google Scholar]
- 14.Data Laparoscopy Research Team (iData Research) US market for laparoscopic devices. iData Research Inc.; Vancouver, B.C.: 2009. [Google Scholar]
- 15.Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, Gonzalez P, Papapietro K. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19:1515–1521. doi: 10.1007/s11695-009-9954-z. [DOI] [PubMed] [Google Scholar]
- 16.Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, Karkavitsas N. Sleeve gastrectomy: a restrictive procedure? Obes Surg. 2007;17:57–62. doi: 10.1007/s11695-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 17.Rubino F. Bariatric surgery: effects on glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2006;9:497–507. doi: 10.1097/01.mco.0000232914.14978.c5. [DOI] [PubMed] [Google Scholar]
- 18.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 19.Ferchak CV, Meneghini LF. Obesity, bariatric surgery, and type 2 diabetes: a systematic review. Diabetes Metab Res Rev. 2004;20:438–445. doi: 10.1002/dmrr.507. [DOI] [PubMed] [Google Scholar]
- 20.Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L, Rhodes S, Morton SC, Shekelle PG. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 21.Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–4231. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 22.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 23.Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3:127–132. doi: 10.1016/j.soard.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen NT, Slone JA, Nguyen XM, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg. 2009;250:631–641. doi: 10.1097/SLA.0b013e3181b92480. [DOI] [PubMed] [Google Scholar]
- 25.Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16:1450–1456. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- 26.Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flüe M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 27.Lee WJ, Ser KH, Chong K, Lee YC, Chen SC, Tsou JJ, Chen JC, Chen CM. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change in insulin secretion. Surgery. 2010;147:664–669. doi: 10.1016/j.surg.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 28.Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg. 2011;21:1650–1656. doi: 10.1007/s11695-011-0479-x. [DOI] [PubMed] [Google Scholar]
- 29.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 30.Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, McLaughlin S, Phillips GL, 2nd, Robertson RP, Rubino F, Kahn R, Kirkman MS. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–2135. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dixon JB, Pories WJ, O’Brien PE, Schauer PR, Zimmet P. Surgery as an effective early intervention for diabesity: why the reluctance? Diabetes Care. 2005;28:472–474. doi: 10.2337/diacare.28.2.472. [DOI] [PubMed] [Google Scholar]
- 32.Pournaras DJ, Aasheim ET, Søvik TT, Andrews R, Mahon D, Welbourn R, Olbers T, le Roux CW. Effect of definition of type II diabetes remission in the evaluation for bariatric surgery for metabolic disorders. Br J Surg. 2012;99:100–103. doi: 10.1002/bjs.7704. [DOI] [PubMed] [Google Scholar]
- 33.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 34.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 36.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, Kumanyika S, Schmitz KH, Diewald LK, Barg R, Chittams J, Moore RH. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, Miller ER, 3rd, Dalcin A, Jerome GJ, Geller S, Noronha G, Pozefsky T, Charleston J, Reynolds JB, Durkin N, Rubin RR, Louis TA, Brancati FL. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, Emmons KM, Rosner BA, Colditz GA. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172:565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torquati A, Lutfi R, Abumrad N, Richards WO. Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg. 2005;9:1112–1116. doi: 10.1016/j.gassur.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D. Effect of lapaoroscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg. 2003;237:751–756. doi: 10.1097/01.SLA.0000071560.76194.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarwer DB, Wadden TA, Moore RH, Baker AW, Gibbons LM, Raper SE, Williams NN. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4:640–646. doi: 10.1016/j.soard.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77:1287–1293. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 44.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozer K, Abdelnour S, Alva AS. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric by-pass surgery: comment on Isbell et al. Diabetes Care. 2010;33:e176. doi: 10.2337/dc10-1586. [DOI] [PubMed] [Google Scholar]
- 46.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 47.Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric empyting, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25:781–792. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- 48.Legakis IN, Tzioras C, Phenekos C. Decreased glucagon-like peptide 1 fasting levels in type 2 diabetes. Diabetes Care. 2003;26:252. doi: 10.2337/diacare.26.1.252. [DOI] [PubMed] [Google Scholar]
- 49.Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laferrère B, Tran H, Egger JR, Teixeira J, McGinty J, Yap K, Bawa B, Olivan B. The increase in GLP-1 levels and incretin effect after Roux-en-Y gastric bypass surgery (RYGBP) persists up to 1 year in patients with type 2 diabetes mellitus (T2DM) [Abstract] Obesity. 2007;15:7. [Google Scholar]
- 51.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide 1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Rouxen-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mingrone G, Nolfe G, Gissey GC, Iaconelli A, Leccesi L, Guidone C, Nanni G, Holst JJ. Circadian rhythms of GIP and GLP-1 in glucose tolerant and in type 2 diabetics after biliopancreatic diversion. Diabetologia. 2009;52:873–881. doi: 10.1007/s00125-009-1288-9. [DOI] [PubMed] [Google Scholar]
- 54.Salinary S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion and restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–380. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes and in fasting and postprandial ghrelin and peptide-YY after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 56.Raufman JP, Singh L, Eng J. Exendin-3, a novel peptide from Heloderma horridum venom, interacts with vasoactive intestinal peptide receptors and a newly described receptor on dispersed acini from guinea pig pancreas: description of exendin-3(9-39) amide, a specific exendin receptor antagonist. J Biol Chem. 1991;266:2897–2902. [PubMed] [Google Scholar]
- 57.Eng J, Kleiman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. J Biol Chem. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 58.Wang Z, Wang RM, Owji AA, Smith DM, Ghatel MA, Bloom SR. Glucagon-like peptide-1 is a physiological incretin in rat. J Clin Invest. 1995;95:417–421. doi: 10.1172/JCI117671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Alessio DA, Vogel R, Prigeon R, Laschansky E, Koerker D, Eng J, Ensinck JW. Elimination of the action of glucagon-like peptide 1 causes an impairment of glucose tolerance after nutrient ingestion by healthy baboons. J Clin Invest. 1996;97:133–138. doi: 10.1172/JCI118380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin (9-39) amide is an antagonist of glucagon-like peptide-1 (7-36) amide in humans. J Clin Invest. 1998;101:1421–1430. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kindel TL, Yoder SM, Seeley RJ, D’Alessio DA, Tso P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg. 2009;13:1762–1772. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 62.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide-1 stimulated postprandial insulin secretion in humans. Diabetes. 2011;60:2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meier JJ, Nauck MA, Schmidt WE, Gallwitz B. Gastric inhibitory polypeptide: the neglected incretin revisited. Regul Pept. 2002;107:1–13. doi: 10.1016/s0167-0115(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 64.Krarup T. Immunoreactive gastric inhibitory polypeptide. Endocr Rev. 1988;9:122–134. doi: 10.1210/edrv-9-1-122. [DOI] [PubMed] [Google Scholar]
- 65.Ross SA, Brown JC, Dupré J. Hypersecretion of gastric inhibitory polypeptide following oral glucose in diabetes mellitus. Diabetes. 1977;26:525–529. doi: 10.2337/diab.26.6.525. [DOI] [PubMed] [Google Scholar]
- 66.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 67.Vilsbøll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45:1111–1119. doi: 10.1007/s00125-002-0878-6. [DOI] [PubMed] [Google Scholar]
- 68.Vilsbøll T, Knop FK, Krarup T, Johansen A, Madsbad S, Larsen S, Hansen T, Pedersen O, Holst JJ. The pathophysiology of diabetes involves a defective amplification of late phase insulin response to glucose by glucose-dependent insulkinotropic polypeptide-regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88:4897–4903. doi: 10.1210/jc.2003-030738. [DOI] [PubMed] [Google Scholar]
- 69.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en-Y gastric bypass for morbid obesity and the control of type 2 diabetes mellitus. Am Surg. 2004;70:1–4. [PubMed] [Google Scholar]
- 70.Näslund E, Backman L, Holst JJ, Theodorsson E, Hellström PM. Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg. 1998;8:253–260. doi: 10.1381/096089298765554449. [DOI] [PubMed] [Google Scholar]
- 71.Shak JR, Roper J, Perez-Perez GI, Tseng CH, Francois F, Gamagaris Z, Patterson C, Weinshel E, Fielding GA, Ren C, Blaser MJ. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes Surg. 2008;18:1089–1096. doi: 10.1007/s11695-008-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ballantyne GH, Peptide YY. (1-36) and peptide YY (3-36): Part I: distribution, release, and actions. Obes Surg. 2006;16:651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 73.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36N. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 74.Korner J, Leibel RL. To eat or not to eat: how the gut talks to the brain. N Engl J Med. 2003;349:926–928. doi: 10.1056/NEJMp038114. [DOI] [PubMed] [Google Scholar]
- 75.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, Restuccia NL, Bessler M. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity. 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 76.Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, Delgado S, Casamitjana R, Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 77.Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, Hosoda H, Kangawa K, Yada T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55:3486–3493. doi: 10.2337/db06-0878. [DOI] [PubMed] [Google Scholar]
- 78.Cummings DE, Foster-Schubert KE, Overduin J. Ghrelin and energy balance: focus on current controversies. Curr Drug Targets. 2005;6:153–169. doi: 10.2174/1389450053174569. [DOI] [PubMed] [Google Scholar]
- 79.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 80.Geloneze B, Tambascia MA, Pilla VF, Geloneze SR, Repetto EM, Pareja JC. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg. 2003;13:17–22. doi: 10.1381/096089203321136539. [DOI] [PubMed] [Google Scholar]
- 81.Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 82.Holdstock C, Engström BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–3183. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 83.Sundbom M, Holdstock C, m BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 84.Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–559. doi: 10.1097/00000658-200211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramos AC, Galvão Neto MP, de Souza YM, Galvão M, Murakami AH, Silva AC, Canseco EG, Santamaría R, Zambrano TA. Laparoscopic duodenal-jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI < 30 kg/m2 (LBMI) Obes Surg. 2009;19:307–312. doi: 10.1007/s11695-008-9759-5. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez-Grunert L, Galvao Neto MP, Alamo M, Ramos AC, Baez PB, Tarnoff M. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis. 2008;4:55–59. doi: 10.1016/j.soard.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 89.Koopmans HS, Ferri GL, Sarson DL, Polak JM, Bloom SR. The effects of ileal transposition and ileal bypass on food intake and GI hormone levels in rats. Physiol Behav. 1984;33:601–609. doi: 10.1016/0031-9384(84)90378-0. [DOI] [PubMed] [Google Scholar]
- 90.Ohtani N, Sasaki I, Naito H, Shibata C, Tsuchiya T, Matsuno S. Effect of ileojejunal transposition of gastrointestinal motility, gastric emptying, and small intestinal transit in dogs. J Gastrointest Surg. 1999;3:516–523. doi: 10.1016/s1091-255x(99)80106-1. [DOI] [PubMed] [Google Scholar]
- 91.Sarson DL, Scopinaro N, Bloom SR. Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes. 1981;5:471–480. [PubMed] [Google Scholar]
- 92.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 93.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes. 1994;43:587–592. doi: 10.2337/diab.43.4.587. [DOI] [PubMed] [Google Scholar]
- 94.Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, Nanni G, Castagneto M, Calvani M, Mingrone G. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 95.Mari A, Manco M, Guidone C, Nanni G, Castagneto M, Mingrone G, Ferrannini E. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia. 2006;49:2136–2143. doi: 10.1007/s00125-006-0337-x. [DOI] [PubMed] [Google Scholar]
- 96.Muscelli E, Mingrone G, Camastra S, Manco M, Pereira JA, Pareja JC, Ferrannini E. Differential effect of weight loss on insulin resistance in surgically treated obese patients. Am J Med. 2005;118:51–57. doi: 10.1016/j.amjmed.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 97.Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S, Baldi S, Nannipieri M, Rebelos E, Anselmino M, Muscelli E, Ferrannini E. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54:2093–2102. doi: 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 98.Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lima MM, Pareja JC, Alegre SM, Geloneze SR, Kahn SE, Astiarraga BD, Chaim EA, Geloneze B. Acute effect of Roux-en-Y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3871–3875. doi: 10.1210/jc.2010-0085. [DOI] [PubMed] [Google Scholar]
- 100.Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, Kirwan JP, Schauer PR. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes. 2010;34:462–471. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sultan S, Parikh M, Youn H, Kurian M, Fielding G, Ren C. Early U.S. outcomes afer laparoscopic adjustable gastric banding in patients with a body mass índex less than 35 kg/m2. Surg Endosc. 2009;23:1569–1573. doi: 10.1007/s00464-009-0341-6. [DOI] [PubMed] [Google Scholar]
- 102.Cohen R, Pinheiro JS, Correa JL, Schiavon CA. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m2: a tailored approach. Surg Obes Relat Dis. 2006;2:401–404. doi: 10.1016/j.soard.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Yermilov I, McGory ML, Shekelle PW, Ko CY, Maggard MA. Appropriateness criteria for bariatric surgery: beyond the NIH guidelines. Obesity. 2009;17:1521–1527. doi: 10.1038/oby.2009.78. [DOI] [PubMed] [Google Scholar]
- 104.Sarwer DB, Ritter S, Wadden TA, Spitzer JC, Vetter ML, Moore RH. Physicians’ attitudes about referring their type 2 diabetes patients for bariatric surgery. Surg Obes Relat Dis. doi: 10.1016/j.soard.2011.12.013. Electronically Published ahead of print on 30 January 2012 (doi: 10.1016/j. soard.2011.12.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 106.DiGiorgi M, Rosen DJ, Choi JJ, Milone L, Schrope B, Olivero-Rivera L, Restuccia N, Yuen S, Fisk M, Inabnet WB, Bessler M. Re-emergence of diabetes after gastric by-pass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6:249–253. doi: 10.1016/j.soard.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 107.Chikunguwo SM, Wolfe LG, Dodson P, Meador JG, Baugh N, Clore JN, Kellum JM, Maher JW. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric by-pass. Surg Obes Relat Dis. 2010;6:254–259. doi: 10.1016/j.soard.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 108.Laferrère B. Do we really know why diabetes remits after gastric bypass surgery? Endocrine. 2011;40:162–167. doi: 10.1007/s12020-011-9514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]