Abstract
Background
Controllers are under prescribed for children with asthma and when they are, adherence is sub-optimal.
Objectives
To test whether an interactive website grounded in social cognitive theory can improve the dispensing of controller medications and adherence with them where indicated.
Design
Randomized Controlled Trial
Methods
Parents of eligible patients were randomized to be prompted to assess their child’s asthma each month for six months and receive tailored feedback on controller use and adherence strategies. For the next six months, participation in the site was optional. Outcomes were assessed at 6 and 12 months.
Results
603 families were enrolled. At baseline, 176 (29%) children had mild to severe persistent asthma, while 71% of children met criteria for mild intermittent asthma. Among patients who should have been on controllers at baseline but were not, there was no statistically significant increase in controller prescriptions at 6 months (OR=2.85 [0.63, 14.04], p=0.17). There was a trend to improved adherence with controllers among users at 6 months (OR=1.54, [0.90, 2.63], p=0.10). Among patients who used controller medicine at both baseline and at 6 month, users in the intervention arm had significantly higher adherence than those in control arm at six months (OR=1.92[1.05, 3.55], p=0.02). For patients with persistent asthma at baseline and who were on controller medicine at both time points, patients in the intervention arm had significantly better adherence than those in the control arm at 6 months (OR=3.33[1.20, 10.07], p=0.01). However, there were no discernible differences at the 12 month assessment.
Conclusion
A tailored interactive website shows some benefit in improving controller medication adherence during a period of active intervention.
Keywords: Asthma, quality of care, adherence, randomized controlled trial, Children's Hospital and Regional Medical Center
Background
Asthma is the most common chronic illness in adolescents and children, affecting an estimated 7 million U.S. children.1 The burden of asthma on children is substantial: children with asthma have a three-fold greater risk of school absence than children without asthma2 and almost 30% of children with asthma have some limitation in activity, compared to 5% of children without asthma.3 The NIH expert panel guideline have consistently recommended the use of inhaled anti-inflammatory medication (“controllers”) for children with persistent asthma symptoms in lieu of continual reliance on relief of symptoms provided by beta agonists (“relievers”).4 Although use of controller medication has increased over the past decade and a half, frequency and duration of use fall short of recommendations.5–9 Numerous surveys in varied populations10 report between 40% and 60% of children with persistent asthma are not receiving controller medications.11,12 Even for children who are appropriately prescribed controller medication, adherence with regimens that have been shown to be efficacious remains a significant problem. Only about 30–50% of patients take medication as prescribed.12–17
The inability of practice guidelines alone to make a substantial impact has been demonstrated in a number of conditions,18 including asthma.19,20 Reasons include that physicians are often unaware of practice guidelines,21 perceive them as unhelpful,21 and feel skeptical about the recommendations or lack the systems to effectively carry them out.22 Academic detailing, a strategy whereby a respected source makes tailored recommendations to providers, has proven effective, though it is labor and resource intensive to perform and maintain.18,23 In contrast, attempts to motivate provider change by targeting patients and families have been effective.24 Using the patient as a vector of information to providers is increasingly common. Pharmaceutical companies now routinely target patients via direct-to-consumer advertising, and consumers themselves frequently search the Internet for medical information which they then discuss with their providers.25,26
Computerized decision-support systems can improve medication prescribing patterns and adherence to preventive care guidelines.27 A recent systematic review found that a majority of clinical decision support systems improve the quality of care. For asthma specifically, of the five identified trials, two demonstrated benefit but published evaluations with long-term follow-up are rare.28,29 Finally, no study to our knowledge has employed a tailored, evidence-based decision support system accessed over the internet that assists parents in asthma management for their children and encourages parents to deliver the information to their child’s health care provider. We therefore designed a study to determine whether an asthma management web site could improve the quality of asthma care focusing on two processes: provision of controller medications where appropriate and adherence to controller prescriptions.
Methods
Study overview
We conducted a randomized controlled trial in which parents of children with asthma were randomly assigned using a computer algorithm to an asthma treatment arm or an active control arm in which a media reduction intervention was used in place of asthma intervention. For purposes of this report, the media reduction intervention is considered the control arm. The study internet site was used both for delivering the intervention and for gathering data for analysis. Baseline to month 6 was considered the active intervention period. Outcomes were assessed at the conclusion of the 6-month intervention and again at 12 months. Enrollment was completed between June and December 2008.
Patients
Patients for this study were recruited from two clinical settings, Group Health Cooperative (GHC), a large not-for-profit HMO in Seattle and an academic medical center-based primary care practice network, the University of Washington Physician Network (UWPN), a network of 5 clinical practices in Western WA. The following recruitment strategy was employed. First, administrative databases at both clinical sites were reviewed to derive a list of potentially eligible patients. Potential eligibility was based on being between the ages of 2–10 years old and having had at least one clinical encounter (clinic visit, emergency room, or inpatient admission) with an ICD-9 diagnosis code for asthma or two prescription fills for bronchodilators in the last year. The low end of the age range was selected to have greater certainty that subjects did have asthma as wheezing is common in the first two years of life and the high end was selected since the intervention was targeting parents and older children would need to be subjects themselves. Parents of potentially eligible patients were then sent a card briefly describing the study and informing them that if they did not decline to be contacted, someone would be calling them to describe the study further and offer them a chance to participate. A survey research group then contacted families that did not opt out and invited them to participate, determined final eligibility, and completed a phone informed consent. To be eligible, families had to have self reported “convenient” access to an internet-enabled computer, speak English at home, confirm that their child had asthma/breathing problems and still be a patient at a participating clinic
Both the University of Washington and Seattle Children’s Institutional Review Boards approved the study protocol.
Intervention Overview
We created a web-based tailored intervention, called My Child's Asthma, which relies on social cognitive theory for its theoretical framework. Social cognitive theory postulates that behavior is a function of the dynamic and reciprocal relationship between personal factors (including cognitions), behavior and environmental influences.30 Expectations about the outcomes of a behavior, such as positive and negative outcome expectations as well as the expectation that one can successfully perform a behavior (self-efficacy beliefs) influence the likelihood that a new behavior will be tried and adopted. My Child’s Asthma web-site content was developed to increase positive beliefs about asthma management. My Child’s Asthma aimed to optimize asthma care by (1) increasing provider-prescribed controllers for children with persistent asthma and (2) promoting controller adherence among children on controllers. The website gathers information from parents and applied algorithms to determine asthma severity, home care practices (including controller use and adherence), functional status and three parental beliefs related to administration of controller on a daily basis (positive and negative outcomes expectations and self-efficacy). In addition, the website also gave parents feedback on their child’s asthma symptoms at each successive visit. Finally, the intervention allowed participants to set goals for themselves from pre-determined selections that pertained to their situation. For example, if their child was on controller medications but not taking them regularly they could select “I will give my child her controller as directed by her doctor” or “I will make an appointment to discuss my son’s controller usage with his doctor”.
Study Procedure
Once baseline questionnaires were completed and consent had been obtained, patients’ names were stored on a secure server. All questionnaires were completed online. The “baseline” timeframe included two surveys. The first survey included an online consent process as well as questions regarding contact information, child and family demographics, parent literacy and comfort using technology and the internet, attitudes and beliefs about both parenting and asthma care, the child’s asthma history, and child sleep problems. The second baseline survey was administered two weeks after the first, but before the intervention began, and included the same questions regarding asthma symptoms, medication use, quality of life, and parent attitudes and beliefs about asthma treatment. For each family, the participating parent was assigned a username and password. For the first six months, the “active intervention period,” they received monthly email reminders with personal links to log on. If they failed to log on based on the first email within two weeks, they received two subsequent ones. During active intervention, parents were invited to complete an asthma assessment every month as described above for the first six months of the study. We developed an attention control intervention aimed to reduce TV viewing. Control parents were invited to complete a media usage survey each month, designed to take equivalent time and using similar feedback mechanisms. Both groups had the opportunity to set goals for themselves at each visit (e.g. “I will give my child their inhaler daily or I will reduce my child’s TV viewing) and both received recommendations and access to information. For each session that parents completed during the first six months, they were allotted $5 towards a Toys R Us gift card that they received after completing their six month assessment.
From six to twelve months, use of the system was no longer incentivized; rather, intervention parents were given the choice to opt-in to receiving monthly reminder emails. Parents who completed asthma assessments during this time received the same type of personalized feedback as during the active intervention period.
My Child’s Asthma included an assessment of asthma severity (based on EPR-2 guidelines which were the latest available when it was implemented), determination of parental self-efficacy and positive and negative outcome expectations regarding daily controller use. The algorithm in this study was based on the EPR-2 guidelines and utilized all components that could be ascertained via a web-based assessment -- daytime symptoms, nocturnal symptoms, and quick-reliever use. (Algorithms are available from author upon request) Each session concluded with recommendations regarding controller medications use and written care plans. Because the system was not designed or intended to replace medical care, when the algorithm determined that a child might benefit from beginning controller medication usage, it advised parents to talk to their child’s doctor about initiating them. It also allowed them to print out materials including an assessment of how the determination to start controllers was made. For children who were on controller medication, My Child’s Asthma targeted promoting adherence by encouraging parents to set goals regarding controller use and other aspects of asthma care by selecting from lists of suggested goals tailoring recommendations according to current adherence, and the parent’s outcome expectations for controller use and self-efficacy for administering controller medication to their child daily. For example, if parents reported that they did not believe controllers worked as the primary reason they weren’t using them, they were presented with information about their efficacy. If they believed they worked but could not remember to use them, they were provided with tips on how to remember to dispense them more consistently. Written care plan recommendations were tailored solely on parent report of possession of a care plan at home and at school and whether it had been updated within the past year. My Child’s Asthma also kept records of previous severity and symptoms as well as goals established at prior visits and provided summaries of how each child (and each parent) was doing with respect to these each month. Figure 1 presents some representative screen shots.
Figure 1.
Sample Asthmanet Screen Shots Goal Setting Summary Report
Measures
Outcomes
Our primary outcomes were appropriate controller usage (i.e. self reported controller use for patients determined to need them based on expert panel report guidelines) and adherence with prescribed controllers (for those who reported being prescribed them).31 Parents were asked what controller medication(s) their child was currently using at each of the assessment timepoints; the question asked separately about inhaled and oral controllers. We assessed controller usage by asking parents if their child was taking a controller medicine and if so, which one(s). If parents were not sure, the question was asked again using visual prompts illustrating all commonly used controller medications. Adherence was measured by asking parents: “During a recent typical week, on how many days has your child used a given controller medicine (Name)” There were five options for answers: 0 days per week, 1–2 days per week, 3–4 days per week, 5–6 days per week, and 7 days per week. We defined being adherent as taking controller medications at least once per day for 5 or more days per week as this has been used in prior studies and the benefits of regular usage are discernible at that level of adherence.32 Adherence was only measured when parents reported that a child was currently using a controller medication. Those who were no longer using a controller are included in neither the numerator nor the denominator for adherence.
We assessed these outcomes at 6 months and again at 1 year. In addition, we determined participant satisfaction with the intervention at 6 months, defined as parental enthusiasm for the idea that the child’s health insurer should provide access to website like My Child’s Asthma after the end of the study.
Parent beliefs regarding controller medication
We also assessed three social cognitive theory (SCT) variables related to outcome expectations and parental self-efficacy with respect to daily controller use. (Table 2) We asked parents to respond to the statements that “taking a controller medicine every day helps make my child’s asthma better” (positive outcome expectation) and “I am concerned about the side effects of controller medicines” (negative outcome expectation).We assessed self-efficacy by asking parents to rate their agreement with the statement, “If I decided to give my child a controller medicine every day, I am confident that I could do it” (for parents who reported not giving controller medicines) or “I am confident that I can continue to give my child a controller medicine everyday” (for parents who were already giving controller medicines). Responses were on a Likert scale (disagree strongly, disagree somewhat, I don’t know, agree somewhat and agree strongly). Both the question wording and the answer options differed based on whether or not the child currently used a controller. These variables are summarized in Table 2. In subsequent analyses, we dichotomized the responses to these SCT variables into high (agree somewhat or strongly) and low (disagree somewhat or strongly).
Table 2.
Social cognitive theory domains at baseline
| SCT domains | Overall | N | Control | Intervention | P-value |
|---|---|---|---|---|---|
|
Positive outcome expectation – “Taking a controller medicine everyday helps make my child’s asthma better” Disagree strongly Disagree somewhat I don’t know Agree somewhat Agree strongly |
74 (13%) 72 (13%) 157 (28%) 119 (21%) 144 (25%) |
566 |
39 (13%) 42 (14%) 74 (25%) 65 (22%) 80 (27%) |
35 (13%) 30 (11%) 83 (31%) 54 (20%) 64 (24%) |
0.48 |
|
Negative outcome expectation – “I am concerned about the side effects of controller medicine” Disagree strongly Disagree somewhat I don’t know Agree somewhat Agree strongly |
34 (6%) 54 (10%) 110 (19%) 204 (36%) 164 (29%) |
566 |
21 (7%) 22 (7%) 49 (16%) 113 (38%) 95 (32%) |
13 (5%) 32 (12%) 61 (23%) 91 (34%) 69 (26%) |
0.05 |
|
Self efficacy – “If I decided to give my child a controller medicine everyday, I am confident that I could do it” Disagree strongly Disagree somewhat I don’t know Agree somewhat Agree strongly |
15 (3%) 37 (7%) 81 (14%) 141 (25%) 292 (52%) |
566 |
11 (4%) 19 (6%) 45 (15%) 74 (25%) 151 (50%) |
4 (2%) 18 (7%) 36 (14%) 67 (25%) 141 (53%) |
0.57 |
Other variables
Demographic data was collected at baseline. Asthma symptoms and severity were collected at baseline and then at six and twelve months for both groups.
Statistical Analysis
All analyses were conducted using the R statistical software (Vienna, Austria; version 2.12.0). We report means and standard deviations on continuous variables, and proportions on categorical variables. We used Student t-test to compare continuous variables across study groups, and chi-square test to compare binary or categorical variables across study groups. For controller use and controller adherence, we further conducted multivariable logistic regression to study the effect of intervention on controller usage and adherence while controlling for unbalanced confounding factors including baseline asthma severity, race/ethnicity, and parental education.
A priori power calculations were based on demonstrating improvements in the provision of controllers and adherence. We based our power calculations on the assumption that only 50% of patients who needed controllers would be on them and that adherence would also be less than 50%. Based on those assumptions, we estimated we had 80% power to detect a difference of 10% given our sample size.
Results
Baseline characteristics
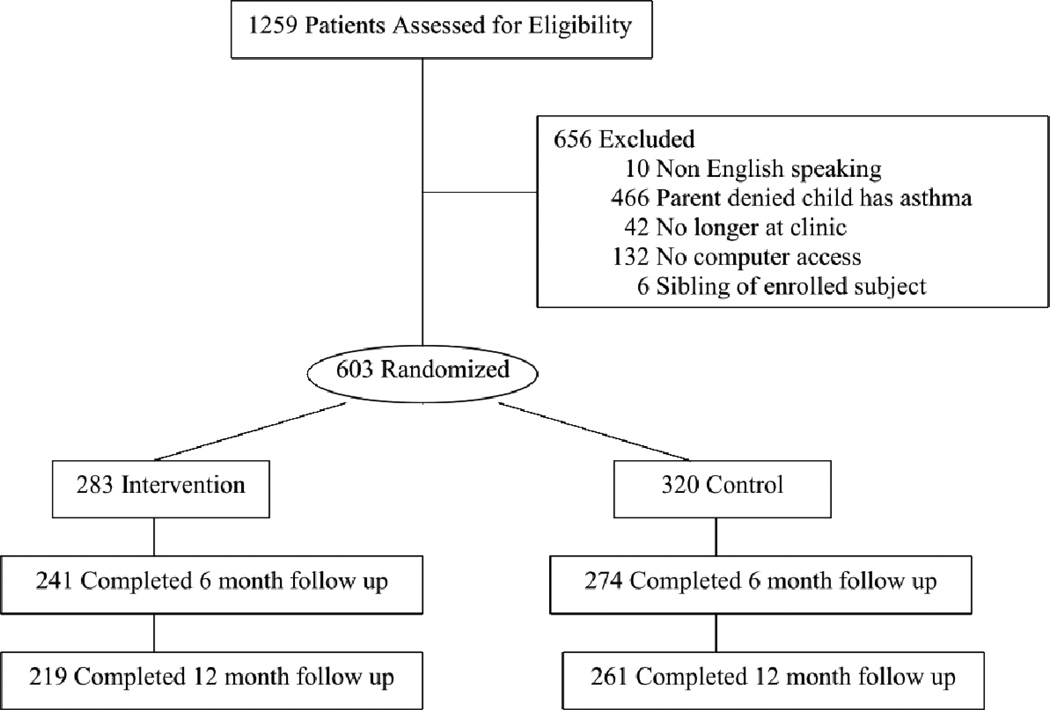
A total of 1851 potentially eligible patients were contacted and 1259 agreed to participate (participation rate 68%). Of those that agreed to participate, 656 were excluded prior to enrollment (see figure) and 603 enrolled and provided valid baseline information. Among them, 320 (53%) were randomized to control arm and 283 (47%) to intervention arm. The primary reason for exclusion was that the parents reported that their child did not have asthma at the initial phone contact. (Figure 2). Of enrolled patients, 95 (16%) patients were enrolled from the UWPN network and 508 (84%) patients were enrolled from the GHC population. There were no differences with respect to child age, child gender, child race and other demographics between the two sites and data from these two sites were combined for all subsequent analyses. The baseline characteristics of the sample are presented in Table 1. At baseline, 176 (29%) children had mild to severe persistent asthma, while 71% of children met criteria for mild intermittent asthma. Because severity was determined regardless of controller use, the intermittent group included children whose low level of symptoms may have been due to the effect of their controllers. Overall, 54% of patients were on at least one controller medication and of these, 199 (61%) took their controller 5 or more days per week. Among controller users, 60% were adherent in control arm vs. 61% in intervention arm. Four domains of social cognitive theory, positive outcome expectation, negative outcome expectation, self-efficacy and stage of change were assessed at baseline. There were no significant differences in these variables between control and intervention arms (Table 2).
Figure 2.
Patient Flow
Table 1.
Baseline characteristics on the entire sample
| Variable | Overall | N | Control | Intervention | P-value |
|---|---|---|---|---|---|
| 603 | 320 (53%) | 283 (47%) | |||
| Child age (years) | 6.15 (2.57) | 603 | 6.08 (2.63) | 6.22 (2.51) | 0.49 |
|
Child Gender Male Female |
402 (67%) 201 (33%) |
603 |
220 (69%) 100 (31%) |
182 (64%) 101 (36%) |
0.25 |
|
Child Race/Ethnicity Asian Black Hawaiian Hispanic Multiracial Native American White |
41 (7%) 45 (7%) 1 (0%) 10 (2%) 103 (17%) 1 (0%) 402 (67%) |
603 |
22 (7%) 26 (8%) 0 (0%) 6 (2%) 62 (19%) 0 (0%) 204 (64%) |
19 (7%) 19 (7%) 1 (0%) 4 (1%) 41 (14%) 1 (0%) 198 (70%) |
0.44 |
|
Asthma severity Mild intermittent Mild persistent Moderate persistent Severe persistent |
427 (71%) 124 (21%) 38 (6%) 14 (2%) |
603 |
215 (67%) 77 (24%) 20 (6%) 8 (2%) |
212 (75%) 47 (17%) 18 (6%) 6 (2%) |
0.14 |
|
# of asthma related sick visits past 3 months 0 1 2 3 ≥4 |
394 (69%) 79 (14%) 60 (11%) 25 (4%) 12 (2%) |
570 |
202 (67%) 41 (14%) 36 (12%) 13 (4%) 10 (3%) |
192 (72%) 38 (14%) 24 (9%) 12 (4%) 2 (1%) |
0.21 |
|
# of hospitalization past year 0 1 2+ |
517 (91%) 43 (8%) 10 (2%) |
570 |
267 (88%) 30 (10%) 5 (2%) |
250 (93%) 13 (5%) 5 (2%) |
0.26 |
|
Study site GHC UWPN |
508 (84%) 95 (16%) |
603 |
275 (86%) 45 (14%) |
233 (82%) 50 (18%) |
0.23 |
|
Caregiver Enrolled Mother Father Other |
525 (87%) 68 (11%) 10 (2%) |
603 |
276 (86%) 40 (13%) 4 (1%) |
249 (88%) 28 (10%) 6 (2%) |
0.18 |
|
Caregiver age <18 18–25 26–35 36–45 46–55 56–65 |
1 (0%) 13 (2%) 212 (35%) 309 (52%) 52 (9%) 11 (2%) |
598 |
0 (0%) 8 (3%) 116 (37%) 164 (52%) 23 (7%) 5 (2%) |
1 (0%) 5 (2%) 96 (34%) 145 (51%) 29 (10%) 6 (2%) |
0.61 |
|
Caregiver education No college Some college Bachelor degree Graduate degree |
90 (15%) 214 (36%) 155 (26%) 140 (23%) |
599 |
41 (13%) 115 (36%) 86 (27%) 76 (24%) |
49 (17%) 99 (35%) 69 (25%) 64 (23%) |
0.47 |
|
Quick relief medication No Yes |
54 (9%) 518 (91%) |
572 |
27 (9%) 276 (91%) |
27 (10%) 242 (90%) |
0.65 |
|
Any controller use No Yes |
237 (42%) 328 (58%) |
565 |
123 (41%) 176 (59%) |
114 (43%) 152 (57%) |
0.68 |
|
Controller use among children with persistent asthma symptoms No Yes |
57 (33%) 118 (67%) |
175 |
36 (35%) 68 (65%) |
21 (30%) 50 (70%) |
0.48 |
|
Frequency of controller use 0 days/week 1–2 days/week 3–4 days/week 5–6 days/week 7 days/week |
310 (55%) 38 (7%) 18 (3%) 25 (4%) 174 (31%) |
565 |
161 (54%) 20 (7%) 12 (4%) 16 (5%) 90 (30%) |
149 (56%) 18 (7%) 6 (2%) 9 (3%) 84 (32%) |
0.59 |
|
Frequency of controller use among controller users 0 days/week 1–2 days/week 3–4 days/week 5–6 days/week 7 days/week |
73 (22%) 38 (12%) 18 (6%) 25 (8%) 174 (53%) |
328 |
38 (22%) 20 (11%) 12 (7%) 16 (9%) 90 (51%) |
35 (23%) 18 (12%) 6 (4%) 9 (6%) 84 (55%) |
0.62 |
|
Adherent to controller (controller users) No Yes |
129 (39%) 199 (61%) |
328 |
70 (40%) 106 (60%) |
59 (39%) 93 (61%) |
0.86 |
Overall engagement with My Child Asthma over the study period is reported elsewhere.33 Briefly, 61% of control families and 57% of intervention ones made all required visits during the 6 month active period.
Controller use at six months
515 (85%) subjects completed the six month survey. 15.69% non-users converted to controller use in the control arm, while 15.79% non-users converted to controller users in intervention arm (p=0.98). Among the 49 patients who should have been on controllers (i.e., with persistent asthma) at baseline but were not, 5 out of 30 (16.7%) were on controller medicine at 6 months in control arm vs. 7 out of 19 (36.84%) in intervention arm (OR=2.85, 95% CI=(0.63, 14.04), p=0.17). Among 100 patients with persistent asthma who were on controllers at baseline, 3 out of 58 (5%) in the control arm discontinued controllers at 6 month vs. 6 out of 42 (14%) in intervention arm (OR=0.33, 95% CI=(0.05, 1.67), p=0.16). (Table 3).
Table 3.
Results at Six Months
| Variable | Overall | N | Control | Intervention | P-value |
|---|---|---|---|---|---|
|
Any controller use No Yes |
223 (44%) 289 (56%) |
512 |
118 (43%) 154 (57%) |
105 (44%) 135 (56%) |
0.93 |
|
Controller use among children who had persistent asthma at baseline No Yes |
46 (31%) 103 (69%) |
149 |
28 (32%) 60 (68%) |
18 (30%) 43 (70%) |
0.86 |
|
Controller use among children who had persistent asthma and were on controller at baseline No Yes |
9 (9%) 91(91%) |
100 |
3 (5%) 55 (95%) |
6 (14%) 36 (86%) |
0.16 |
|
Frequency of controller use 0 days/week 1–2 days/week 3–4 days/week 5–6 days/week 7 days/week |
275 (54%) 31 (6%) 11 (2%) 27 (5%) 161 (32%) |
505 |
150 (56%) 21 (8%) 4 (1%) 14 (5%) 80 (30%) |
125 (53%) 10 (4%) 7 (3%) 13 (6%) 81 (34%) |
0.30 |
|
Adherent to controller (controller users) No Yes |
94 (33%) 188 (67%) |
282 |
57 (38%) 94 (62%) |
37 (28%) 94 (72%) |
.10 |
|
Adherent to controller (controller users with persistent asthma) No Yes |
35 (39%) 54 (61%) |
89 |
27 (50%) 27 (50%) |
8 (23%) 27 (77%) |
0.01 |
|
Adherent to controllers (controller users who were not adherent at baseline) No Yes |
49 (66%) 25 (34%) |
74 |
31 (78%) 9 (22%) |
18 (53%) 16 (47%) |
0.03 |
|
Positive outcome expectation Low High |
269 (52%) 246 (48%) |
515 |
152 (56%) 122 (44%) |
117 (49%) 124 (51%) |
0.12 |
|
Negative outcome expectation Low High |
180 (35%) 335 (65%) |
515 |
84 (31%) 190 (69%) |
96 (40%) 145 (60%) |
0.03 |
|
Self efficacy Low High |
80 (16%) 435 (84%) |
515 |
56 (20%) 218 (80%) |
24 (10%) 217 (90%) |
0.001 |
Controller adherence at six months
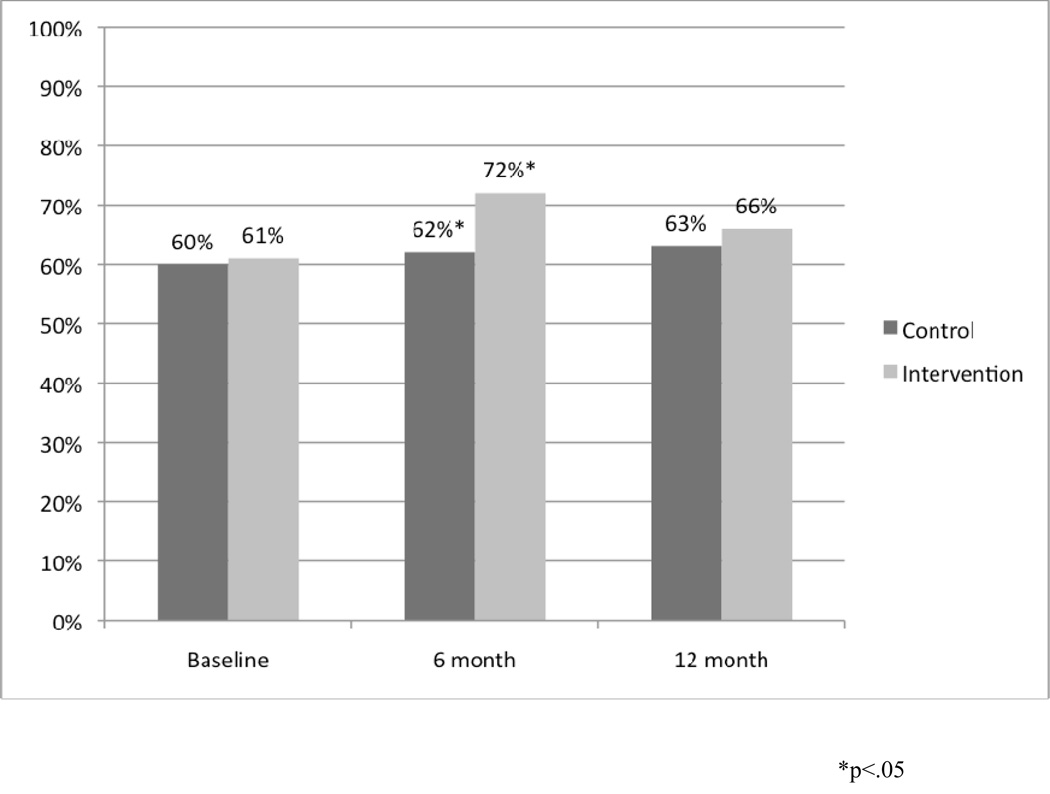
Overall adherence to controllers among users at 6 months was improved with a trend toward significance in the intervention group compared to the control with 72% reporting usage 5 or more days per week vs. 62% (OR=1.54, 95% CI=(0.90, 2.63), p=0.10). Among the 242 patients who used controller medicine at both baseline and at 6 month, despite identical adherence rates at baseline (69% in both groups), users in the intervention arm had significantly higher adherence than those in control arm at six months (76.6% vs. 63%, OR=1.92, 95% CI=(1.05, 3.55), p=0.02). We also examined adherence among 89 subjects who had persistent asthma at baseline and were on controllers at both baseline and 6 months. For this persistent asthma group, despite no significant difference in adherence between intervention and control arms at baseline (69.4% vs. 67.3%), patients in the intervention arm had significantly better adherence than those in the control arm at 6 months (77% vs. 50%, OR=3.33, 95% CI=(1.20, 10.07), p=0.01). (Table 3)
Social cognitive theory domains at six months
At six months, there were no significant differences in positive outcome expectation regarding controller use between control and intervention arms. The proportion of parents with high negative outcome expectation was significantly lower in intervention arm (60% vs. 69%, p=0.03). There were significant differences in self efficacy to administer controller medication at 6 months, with 90% of intervention parents “somewhat or strongly agreeing” that they can give their child controller medication daily vs. 80% in the control arm (p=0.001). (Table 3) Notable as well was the fact that at six months 45% of intervention parents reported that the system made it easier to talk to their child’s doctor about his/her asthma.
Asthma symptoms at six months
At baseline, and the 6 and 12 month assessment, a series (n=22) of questions about asthma symptoms were asked. These questions include “during a recent typical week, on how many days has your child used quick-relief medicine?”; “during a recent typical week, how often has your child had chest tightness, cough, shortness of breath, or wheezing”, and so forth. We did look at changes in symptoms within children. At both 6 and 12 months, the proportions of children with stable or improved symptoms were not significantly different between intervention and control arms. There were also no differences in asthma related quality of life measures between groups at both points (data not shown).
Results at 12 months
At 12 months, there were no differences in the proportion of children on controllers between control and intervention arms (57% vs 50%; p=0.17). Of those who met severity criteria for controller therapy at baseline (N=135), 34 out of 53 (64%) in the intervention arm vs 50 out of 82 (60%) in the control arm were on them at 12 months (p=0.86). At 12 months, adherence rates among controller users were similar between the two groups with 88 out of 140 (63%) reporting administering controllers 5 or more days per week in control arm vs. 69 out of 105 (66% in intervention arm (p=0.69). Figure 3 displays controller adherence among controller users at all three time points.
Figure 3.
Controller Adherence at All Three Time Points
Study retention and perceived value
613 parents completed baseline survey, 515 (85%) completed the 6-month assessment, and 481 (80%) completed the 12-month assessment. At 6 months, parents in the intervention arm were surveyed regarding to their satisfaction with the intervention, 45% of intervention parents felt more comfortable managing their children’s asthma than 6 months ago, 74% of parents felt the intervention was helpful for them to track their children’s asthma symptoms overtime, 68% of intervention parents felt the intervention was helpful in providing them information to care for their children’s asthma, and 72% felt that it was somewhat to very important for their insurance to provide access to a website like My Child’s Asthma.
Discussion
We found that an interactive, tailored web site that relies on social cognitive theory could improve adherence with asthma controller medications during a period of active intervention although those benefits did not persist once the system no longer actively engaged families. We did not find that it improved the provision of controller medications to patients who might benefit from them nor that symptoms were improved. The benefits that were achieved disappeared after active engagement with the system was no longer incentivized.
Significant benefits to this approach are the interactivity and scaleability of the intervention. My Child’s Asthma queried parents automatically without health care staff time making it both effective and low cost. Although we did not conduct a formal cost-effectiveness analysis of the intervention, given this scaleability even modest effect sizes may prove beneficial to insurers as improved adherence with controller medication has been shown to reduce asthma hospitalizations. Website development represents a largely fixed cost that can have considerable reach and is inexpensive to maintain. The number needed to treat at the end of the active engagement period in order to improve adherence among children with persistent asthma who were not adherent with medication is 4. It should be noted of course that this was only achievable during active engagement.
The fact that we did not improve the provision of controller medications is disappointing. This may reflect the fact that mere knowledge of the necessity of controllers is inadequate to improve uptake. It may also be due in part to the higher than expected use of controllers at baseline. National estimates are that fewer than 50% of children who should be on them are and our baseline rate was 58%. The relatively high percentage of children reported to be on controllers at baseline may be due to the relatively high socioeconomic status of our insured sample (as reflected by parental education). The failure to increase the proportion of children using controllers may also account for the fact that no significant differences in quality of life were found. Further study of similar interventions in other populations is therefore warranted.
The modest impact on adherence and lack of effect on controller use may be due to lack of a direct interaction between My Child’s Asthma’s and primary care providers, and related to this, the parents’ perception that program was not connected to their source of health care. That is, intervening only through parents without directly involving health care providers may have a limited impact. However, integrating external website such as My Child’s Asthma into proprietary systems and even into work flow has proven challenging.34 Further, given that only about 50% of subjects completed all required visits during the active intervention period, future research should explore ways of increasing on line engagement. There are several limitations of this study which warrant mention. First, our study was conducted within two networks of practices and the extent to which it can be generalized is questionable. Notably, our study population’s racial and ethnic make-up is consistent with the greater Seattle metropolitan area. However, given the relatively high socioeconomic status of our sample, the extent to which it could be applied to other, less advantaged populations is unclear. Second, we measured adherence based on self report. It is possible that social desirability in the intervention arm lead to higher reported adherence. Measuring adherence is inherently complicated and difficult, but patient self report using questionnaire data as we did has been shown to have moderate to high concordance with direct measurement.35 Third, the modest improvement in adherence may not translate into clinical improvement. Although prior studies have reported that consistent and regular use results in improved function, recent studies suggest that even intermittent usage is adequate.36 Finally, we used the EPR-2 recommendations rather than the EPR-3 recommendations as those were what was available at the time the study was initiated. The two guidelines have considerable overlap and there is no reason to assume that the same approach would not work for the updated ones.
In spite of these limitations, this study has some important implications. An interactive asthma management web site has the potential to improve controller adherence and thereby improve the quality of asthma care. Our results however, suggest that the system must continue to be active for there to be benefits. Future studies in different populations should assess controller prescribing and determine how long active engagement can be maintained. The modest benefit demonstrated in this study, while encouraging, suggests that there is ample additional work to be done to leverage the potential of internet based chronic disease management.
What’s new.
We found that a tailored, interactive web site can improve children’s adherence with asthma controller medications during a six month period when visits were incentivized but that adherence waned in the following six months when incentives ceased.
Acknowledgements
This study was supported by a grant from the NHLBI (1 R01 HL079402-01) to DAC. We are grateful to the families and the providers that participated in this study.
Abbreviations
- NHLBI
National Heart, Lung and Blood Institute
- UWPN
University of Washington Practice Network
- GHC
Group Health Cooperative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflicts of interest to declare.
References
- 1.Akinbami LJ, Moorman JE, Liu X. Summary Health Statistics for US Children: National Health Interview Survey 2010. 2009 [Google Scholar]
- 2.Fowler MG, Davenport MG, Garg R. School functioning of US children with asthma. Pediatrics. 1992;90(6):939–944. [PubMed] [Google Scholar]
- 3.Taylor WR, Newacheck PW. Impact of childhood asthma on health. Pediatrics. 1992;90(5):657–662. [PubMed] [Google Scholar]
- 4.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Diette GB, Wu AW, Skinner EA, et al. Treatment patterns among adult patients with asthma: factors associated with overuse of inhaled beta-agonists and underuse of inhaled corticosteroids. Arch Intern Med. 1999;159(22):2697–2704. doi: 10.1001/archinte.159.22.2697. [DOI] [PubMed] [Google Scholar]
- 6.Eggleston PA, Malveaux FJ, Butz AM, et al. Medications used by children with asthma living in the inner city. Pediatrics. 1998;101(3 Pt 1):349–354. doi: 10.1542/peds.101.3.349. [DOI] [PubMed] [Google Scholar]
- 7.Jatulis DE, Meng YY, Elashoff RM, et al. Preventive pharmacologic therapy among asthmatics: five years after publication of guidelines. Ann Allergy Asthma Immunol. 1998;81(1):82–88. doi: 10.1016/S1081-1206(10)63113-4. [DOI] [PubMed] [Google Scholar]
- 8.Legorreta AP, Christian-Herman J, O'Connor RD, Hasan MM, Evans R, Leung KM. Compliance with national asthma management guidelines and specialty care: a health maintenance organization experience. Arch Intern Med. 1998;158(5):457–464. doi: 10.1001/archinte.158.5.457. [see comments]. [DOI] [PubMed] [Google Scholar]
- 9.Meng YY, Leung KM, Berkbigler D, Halbert RJ, Legorreta AP. Compliance with US asthma management guidelines and specialty care: a regional variation or national concern? J Eval Clin Pract. 1999;5(2):213–221. doi: 10.1046/j.1365-2753.1999.00177.x. [see comments]. [DOI] [PubMed] [Google Scholar]
- 10.Finkelstein JA, Barton MB, Donahue JG, Algatt-Bergstrom P, Markson LE, Platt R. Comparing asthma care for Medicaid and non-Medicaid children in a health maintenance organization. Arch Pediatr Adolesc Med. 2000;154(6):563–568. doi: 10.1001/archpedi.154.6.563. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein JA, Lozano P, Shulruff R, et al. Self-reported physician practices for children with asthma: are national guidelines followed? Pediatrics. 2000;106(4 Suppl):886–896. [PubMed] [Google Scholar]
- 12.Celano MP, Linzer JF, Demi A, et al. Treatment adherence among low-income, African American children with persistent asthma. J Asthma. 2010 Apr;47(3):317–322. doi: 10.3109/02770900903580850. [DOI] [PubMed] [Google Scholar]
- 13.Feldman R, Bacher M, Campbell N, Drover A, Chockalingam A. Adherence to pharmacologic management of hypertension. Canadian Journal of Public Health. Revue Canadienne de Sante Publique. 1998;89(5):I16–I18. doi: 10.1007/BF03404494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein JA, Lozano P, Farber HJ, Miroshnik I, Lieu TA. Underuse of controller medications among Medicaid-insured children with asthma. Arch Pediatr Adolesc Med. 2002;156(6):562–567. doi: 10.1001/archpedi.156.6.562. [DOI] [PubMed] [Google Scholar]
- 15.Jay S, Litt IF, Durant RH. Compliance with therapeutic regimens. Journal of Adolescent Health Care. 1984;5(2):124–136. doi: 10.1016/s0197-0070(84)80012-1. [DOI] [PubMed] [Google Scholar]
- 16.Mallion JM, Baguet JP, Siche JP, Tremel F, de Gaudemaris R. Compliance, electronic monitoring and antihypertensive drugs. Journal of Hypertension - Supplement. 1998;16(1):S75–S79. [PubMed] [Google Scholar]
- 17.Lozano P, Finkelstein JA, Hecht J, Shulruff R, Weiss KB. Asthma medication use and disease burden in children in a primary care population. Arch Pediatr Adolesc Med. 2003 Jan;157(1):81–88. doi: 10.1001/archpedi.157.1.81. [DOI] [PubMed] [Google Scholar]
- 18.Lomas J, Anderson GM, Domnick-Pierre K, Vayda E, Enkin MW, Hannah WJ. Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med. 1989;321(19):1306–1311. doi: 10.1056/NEJM198911093211906. [DOI] [PubMed] [Google Scholar]
- 19.Bailey R, Weingarten S, Lewis M, Mohsenifar Z. Impact of clinical pathways and practice guidelines on the management of acute exacerbations of bronchial asthma. Chest. 1998;113(1):28–33. doi: 10.1378/chest.113.1.28. [DOI] [PubMed] [Google Scholar]
- 20.Kwan-Gett TS, Lozano P, Mullin K, Marcuse EK. One-year experience with an inpatient asthma clinical pathway. Arch Pediatr Adolesc Med. 1997;151(7):684–689. doi: 10.1001/archpedi.1997.02170440046008. [DOI] [PubMed] [Google Scholar]
- 21.Christakis DA, Rivara FP. Pediatricians' awareness of and attitudes about four clinical practice guidelines. Pediatrics. 1998;101(5):825–830. doi: 10.1542/peds.101.5.825. [DOI] [PubMed] [Google Scholar]
- 22.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [see comments]. [DOI] [PubMed] [Google Scholar]
- 23.Avorn J, Soumerai SB. Improving drug-therapy decisions through educational outreach. A randomized controlled trial of academically based "detailing". N Engl J Med. 1983 Jun 16;308(24):1457–1463. doi: 10.1056/NEJM198306163082406. [DOI] [PubMed] [Google Scholar]
- 24.Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA. 1995 Sep 6;274(9):700–705. doi: 10.1001/jama.274.9.700. [DOI] [PubMed] [Google Scholar]
- 25.Morris CA, Avorn J. Internet marketing of herbal products. JAMA. 2003 Sep 17;290(11):1505–1509. doi: 10.1001/jama.290.11.1505. [DOI] [PubMed] [Google Scholar]
- 26.Baker L, Wagner TH, Singer S, Bundorf MK. Use of the Internet and e-mail for health care information: results from a national survey. Jama. 2003;289(18):2400–2406. doi: 10.1001/jama.289.18.2400. [DOI] [PubMed] [Google Scholar]
- 27.Ellrodt G, Cook DJ, Lee J, Cho M, Hunt D, Weingarten S. Evidence-based disease management. JAMA. 1997 Nov 26;278(20):1687–1692. 1997. [PubMed] [Google Scholar]
- 28.Roshanov P, Misra S, Gerstein H, et al. Computerized clinical decision support systems for chronic disease management: A decision-maker-researcher partnership systematic review. Implementation Science. 2011;6(1):92. doi: 10.1186/1748-5908-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brug J, Glanz K, Van Assema P, Kok G, van Breukelen GJ. The impact of computer-tailored feedback and iterative feedback on fat, fruit, and vegetable intake. Health Educ Behav. 1998 Aug;25(4):517–531. doi: 10.1177/109019819802500409. 1998. [DOI] [PubMed] [Google Scholar]
- 30.Bandura A. Health promotion by Social Cognitive Mean. Health Education and Behavior. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 31.National Heart, L and Blood Institute. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. Bethesday, MD: U.S. Department of Health and Human Services; 1997. NIH Publication No. 97-4051. [Google Scholar]
- 32.Farber HJ, Chi FW, Capra A, et al. Use of asthma medication dispensing patterns to predict risk of adverse health outcomes: a study of Medicaid-insured children in managed care programs. Ann Allergy Asthma Immunol. 2004 Mar;92(3):319–328. doi: 10.1016/S1081-1206(10)61569-4. [DOI] [PubMed] [Google Scholar]
- 33.Meischke H, Lozano P, Zhou C, Garrison MM, Christakis D. Engagement in "My Child's Asthma", an interactive web-based pediatric asthma management intervention. Int J Med Inform. 2011 Nov;80(11):765–774. doi: 10.1016/j.ijmedinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christakis DA, Zimmerman FJ, Wright JA, Garrison MM, Rivara FP, Davis RL. A randomized controlled trial of point-of-care evidence to improve the antibiotic prescribing practices for otitis media in children. Pediatrics. 2001;107(2):E15. doi: 10.1542/peds.107.2.e15. [DOI] [PubMed] [Google Scholar]
- 35.Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care. 2004 Jul;42(7):649–652. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- 36.Martinez FD, Chinchilli VM, Morgan WJ, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011 Feb 19;377(9766):650–657. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]