Abstract
Background:
H. pylori is a urease positive organism, and this activity in a gastric biopsy could be considered as a proof of the presence of H. pylori. For the reasons of high price and difficult accessibility to the commercial CLO-test in Iran, we designed an affordable equivalent test with high specificity, accuracy and availability.
Methods:
Biopsy samples from 80 symptomatic patients with gastrointestinal problems were included in this study. The results of our in-house made rapid urease kit were compared with the commercial CLO-test up to 3 hours and 24 hours after inoculation of the biopsy samples. Culture results and gram staining were proposed as gold standard.
Results:
Helicobacter pylori was isolated from 36 patients (45.0%) after cultivation of biopsy samples. After 3 hours, 33 (91.6%) cases of positive samples for H. pylori, showed urease positive reaction using both, in-house made and CLO-test kits. However, 2 (5.5%) cases showed urease reaction at 24 hours using both the kits. The specificity of 100% was determined for both, in-house made and commercial CLO-test kits after 3 hours. The sensitivity for both the kits was estimated at 97.1% after 3hours. However, after 24 hours, sensitivity and specificity of 97.1% and 88.64% was estimated for the in-house and 97.2 % and 95.4% for the commercial CLO-test kits, respectively.
Conclusion:
Specificity and sensitivity of 100% and 97.1 % for up to 3 hours follow biopsy sampling, could be considered as an advantage for our in-house rapid urease kit. Moreover, the rapid urease agar media designed in our lab is cost-effective with adequate sensitivity and specificity levels for the detection of H. pylori, compared with the commercial CLO-test.
Keywords: Helicobacter pylori, Rapid urease test, CLO-test, gastritis
INTRODUCTION
H. pylori is a spiral, motile gram negative bacillus that is able to live in human gastric mucosa and cause clinical manifestations including peptic ulcer and gastric cancer.[1–4]
There are some invasive and non-invasive techniques for H. pylori infection diagnosis such as urea breath test (UBT), H. pylori IgG antibodies, H. pylori stool antigen (HpSA) and molecular methods.[5–8] Most of them require an endoscopy and biopsy, e.g histological examination to ensure the presence of bacteria with curved and spiral forms, culturing on solid specific media and rapid urease test. An endoscopy with biopsy has been recommended as the only reliable method for the diagnosis of H. pylori infection.[9–11] The gastric biopsies provided by endoscopy are used for the isolation of H. pylori by culture, histological investigation of bacteria and rapid urease tests.[12–16] Among these tests, positive culture can be used as the gold standard for the diagnosis of H. pylori with 100% specificity.[14,15] But, this method is time consuming and not easily available, and requires skilled persons to perform it with highest sensitivity. Consequently, a rapid and simple test that is able to accurately identify H. pylori infection, could expedite therapeutic decisions. H. pylori is a urease positive organism, and therefore, the presence of this activity in a gastric biopsy could be considered as a proof of the presence of H. pylori.[17] Therefore, such a test is rapid, economical and easy to be used by laboratory staff. The most common commercial rapid urease test is CLO- test, whose manipulation does not need high experience of laboratory staff as needed in histological examinations and culture.[18] CLO-test has been found to be highly accurate when read in 3 hours by 100% specificity and high sensitivity (different percentage was reported 70% to 90%).[19]
An important value of commercial CLO-test as H. pylori infection diagnostic tool for the patients referred to the endoscopy ward is evident, but due to the high price and difficult accessibility in Iran, we designed an affordable equivalent test in our center with high specificity, accuracy and longer expiry date.
Our in-house results were compared with commercial CLO-test up to 3 hours and 24 hours after the inoculation of biopsy samples of the patients. Culture results and gram staining were proposed as gold standard.
MATERIALS AND METHODS
Patient groups
80 symptomatic patients with gastrointestinal problems, aged (>18) years during the period of March-November 2009, referred to the endoscopy ward of Motahhary Clinic in Shiraz- Iran, were enrolled in this study. Exclusion criteria for patients’ recruitment were previous attempts to eradicate H. pylori and use of antibiotics or proton pump inhibitors within the last 2 weeks prior to endoscopy, and previous gastric surgery. The study was approved by the ethical committee in our center, and the written consents were obtained from all the participating patients. The sample size was determined according to statistical analysis software for providing sensitivity and specificity above 90%.
H. pylori detection
4 gastric biopsy samples were taken from each patient by a sterile needle for: commercial CLO-test (ASAN pharm. Co., Seoul, Korea), rapid urease agar media designed in our lab, culture and gram staining. Having placed gastric mucosa biopsies from each patient in a commercial labeled CLO-test cartridge and in our in-house made rapid urease agar, we read positive or negative reaction on the basis of changing in color from yellow to red, at room temperature after 3 and 24 hours.
Biopsy samples were cultured on Colombia agar base medium (Merck, Germany), supplemented with 10% lysed horse blood, 7% fetal calf serum, 0.25% yeast extract and antibiotics of amphotericin B (5 μg/l), trimetoprime (5 μg/l) and vancomycin (10 μg/l). The plates were kept in a microaerophilic atmosphere (7% O2, 7.1% CO2, 7.1% H2 and 79.8% N2) provided by Anoxomate (Mark II, Mart Microbiology BV, Netherlands) at 37°C for 48-72 hours. Translucid, small size colonies were examined by oxidase, catalase, rapid urease tests and modified Gram staining in our lab.
Biopsy samples obtained from each patient were gently homogenized and crushed between two sterile slides. After fixation, the presence of curved and spiral shape bacteria was evaluated by modified Gram staining (carbolfuchsin was used instead of safranin).
Quality Control of our in-house made rapid urease test based on sensitivity and expiry date For quality control of our rapid urease test medium, H. pylori ATCC 26695 was used to estimate the minimal amount of cfu/ml to obtain the positive reaction. Moreover, to find the best expiry date, we used the media after 15-20 months with the same protocol for inoculating and culturing the biopsy samples.
Statistical Analysis
Statistical analysis was performed using SPSS software for Windows, version 11.5 (SPSS). Student T-test, Chi-square and logistic regression were also done for the evaluation of variables correlation. P value < 0.05 was considered as significant.
RESULTS
Over a period of 8 month study, a total of 80 patients consisting of 39 males (48.8%) and 41 females (51.2%), aged >18 years with upper abdominal dyspeptic complaints, gastroesophagal reflux or abdominal pain referred to the endoscopy ward of Motahhary clinic in Shiraz, Iran, were studied.
Helicobacter pylori was isolated from 36 patients (45.0%) after the cultivation of biopsy samples on specific media. 42 samples (52.5%) were negative and 2 samples were contaminated, which were considered as negative.
The result of rapid urease tests were read after 3 hours and 24 hours for both, in-house and CLO-test kits. After 3 hours, 33 (91.6%) cases of positive samples for H. pylori showed urease positive reaction using both, in-house and CLO-test kits. However, 2 (5.5%) cases showed urease reaction at 24 hours using both the kits.
Among the negative biopsy samples for H. pylori, none of them had urease reaction and only 5 (11.3%) samples showed positive reaction after 24 hours using in-house made kit. Of the 44 negative biopsies, 43 (97.7%) samples had negative CLO-test result and 1 (2.3%) sample revealed positive reaction within 3 hours. Only 2 (4.5%) samples were positive and 42 (95.5%) remained negative for urease reaction at 24 hours. A good correlation was found between CLO-test results and negative cultures. Cross tabulation results of the CLO-test for positive cultures were almost the same as the results of ours [Table 1].
Table 1.
The rapid urease tests results after incubation of the gastric biopsy samples up to 3 and 24 hours
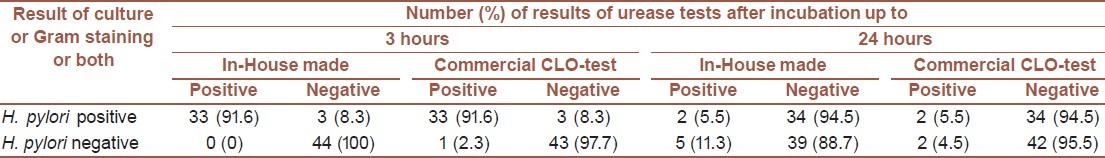
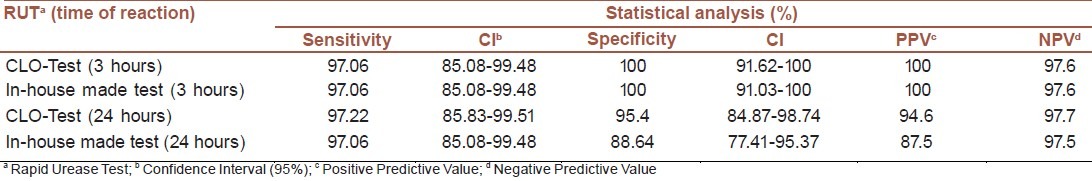
Statistical analysis of the above mentioned results with confidence interval 95% revealed 100% specificity for both, in-house and commercial CLO-test kits, when the results were read after 3 hours. The sensitivity for both the kits was estimated at 97.1%. However, after 24 hours, the sensitivity and specificity for in-house kit were estimated as 97.1% and 88.6%, while for commercial CLO-test kit as 97.2% and 95.5%, respectively. No statistical significant differences were seen among these results.
Positive predictive values (PPV) and negative predictive values (NPV) after 3 hours were 100% and 97.6%, respectively, for both the kits. After 24 hrs, PPV was 87.5% and 94.6% for in- house made and CLO-test kits, respectively, while NPV was 97.5% and 97.7 % for in-house made and CLO-test, respectively [Table 2].
Table 2.
Statistical analysis of the results for the in-house made rapid urease test and CLO-test kit
Minimal concentration of H. pylori to show positive urease reaction in our in-house kit was determined to be 10000-12000 cfu/ml, while it has been estimated to be 10000 cfu/ml for the commercial CLO-test kit. Moreover, when we used our kit after 15-20 months with the same protocol for inoculating and culturing the biopsy samples, the same results were obtained up to 20 months.
DISCUSSION
H. pylori infection is diagnosed by non-invasive and invasive techniques that need an endoscopy followed by biopsy. On the basis of some guidelines, an endoscopy followed by biopsy is the only method which could be used reliably for the diagnosis of the infections caused by H. pylori.[20–22] This indicates that, in spite of the current commercial non-invasive techniques with adequate sensitivity and specificity for reporting the existence or absence of H. pylori, an endoscopy along with histopathology conserve as the only method to confirm the presence of H. pylori and lesions associated with infections. According to most studies, among the tissue based methods, rapid urease tests have been reported to be more sensitive than the histology, used to detect H. pylori. However, histology is necessary for the observation of pathologic manifestations associated with infections including gastritis, intestinal metaplasia and other pathogenic conditions.[23] Rapid urease test is based on the detection of urease activity of H. pylori. After urea hydrolyzing, ammonium ions are released and raise the PH of media and change the color of indicator (phenol red) from yellow to magenta.[24]
To an endoscopist, rapid urease gel test has more advantages than the other diagnostic tests. It is easy to be handled and can facilitate H. pylori recognition before the patient leaves the clinic.[25,26]
The main purpose of the present study was to invent an in-house made rapid urease test kit with high sensitivity and specificity, for the detection of urease enzyme produced by Helicobacter pylori in biopsy samples. To do so, we evaluated the sensitivity and specificity of our kit in comparison with commercial CLO-test after 3 hours and 24 hours. In this regard, positive H. pylori cultures along with positive modified Gram stain slides were selected as gold standard.
Although culture method has 100% specificity, different levels of sensitivity have been reported for that.[23,27,28]
One of the most important reasons for these variations could be the experience of the laboratory technicians and the protocols used for this technique.[16,29–32] To solve these problems, we established a high confident cultivating method for biopsy samples, published in our previous study.[33] In this method, H. pylori pure colonies grew 24-48 hours after the cultivation of biopsy samples.
Rapid urease tests showed that, our in-house kit sensitivity results in 3 hours and 24 hours after inoculation were concordant with those of the commercial CLO-test, 97.1%, 97.2%, respectively. The specificity of our method was estimated to be 100% and 88.6% at 3 hours and 24 hours, respectively. No significant difference was found with commercial CLO-test that revealed 100% and 95.5% specificity at 3 hours and 24 hours, respectively. The data showed that, after 24 hours, the specificity dropped for both tests. No false positive results were seen for both tests by 3 hours. The false positive results of our kit and CLO-test kit at 24 hours (5 and 2, respectively), might have been caused by the instability of the reagent over time, by chemical reactions from autolysis of the tissue or by contamination of the sample with organisms that have urease activities lower than H. pylori.[34] Same as CLO-test, there are important warnings for positive rapid urease agar reports, such as using sterile needle for inoculation of tissues to the agar. It is also notable that, if blood or alkaline bile is present in the biopsy samples, slight red tinge appears in agar, but doesn’t diffuse in media. Only when red area is deepening in color and expanding and spread in the size, then the test will be considered as positive.[35] By using H. pylori ATCC 26695, minimal concentration of organism needed to show a positive urease reaction in our kit was 10000-12000 cfu/ml. It has been determined to be 10000 cfu/ml for CLO-test, that is compatible with our results.[36] It shows that urease positivity for both the kits depends on higher grades of bacterial density, which results in abundance of urease enzyme, as reported by others.[18,37,38]
In conclusion, specificity and sensitivity of 100% and 97.1%, for up to 3 hours after biopsy sampling and long expiry date up to 20 months, could be taken as an advantage for our in-house made rapid urease kit. Consequently, clinicians could rely on its results to get more information to institute quick therapeutic modalities before the patients leave the endoscopy room. Moreover, the rapid urease agar media designed in our center has economic benefits and adequate sensitivity and specificity for the detection of H. pylori, and it can compete with similar existing commercial CLO-test.
ACKNOWLEDGMENT
This study was funded in full by Prof. alborzi Clinical Microbiology Research Center, Shiraz University of Medical Sciences, grant #88-7. The authors wish to thank Dr Hassan Khajehei for his critical editorial assistance. We also thank Marziyeh Hosseini in Prof. Alborzi Clinical Microbiology Research Centre for her great technical assistances.
Footnotes
Source of Support: Prof. Alborzi Clinical Microbiology Research Center, Shiraz University of Medical Sciences; grant#88-7.
Conflict of Interest: None declared.
REFERENCES
- 1.Benjamin D. Helicobacter pylori. In: Long SS, Pickering LK, Prober CG, editors. Long: Principles and Practice of Pediatric Infectious Diseases. Philadelphia: Churchill Livingstone; 2008. p. 174. [Google Scholar]
- 2.Elviss NC, Owen RJ, Xerry J, Walker AM, Davies K. Helicobacter pylori antibiotic resistance patterns and genotypes in adult dyspeptic patients from a regional population in North Wales. J Antimicrob Chemother. 2004;54:435–40. doi: 10.1093/jac/dkh343. [DOI] [PubMed] [Google Scholar]
- 3.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med. 2009;12:576–83. [PubMed] [Google Scholar]
- 4.Mohagheghi MA, Mosavi-Jarrahi A, Malekzadeh R, Parkin M. Cancer incidence in Tehran metropolis: The first report from the Tehran. Arch Iran Med. 2009;12:15–23. [PubMed] [Google Scholar]
- 5.Vaira D, Vakil N. Blood, urine, stool, breath, money, and Helicobacter pylori. Gut. 2001;48:287–9. doi: 10.1136/gut.48.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohil R, Hassall E. Peptic ulcer disease in children. Best Pract Res Clin Gastroenterol. 2000;14:53–73. doi: 10.1053/bega.1999.0059. [DOI] [PubMed] [Google Scholar]
- 7.Majumdar D, Atherton J. Peptic ulcers and their complications. Surgery (Oxford) 2006;24:110–4. [Google Scholar]
- 8.Ricci C, Holton J, Vaira D. Diagnosis of Helicobacter pylori: Invasive and non-invasive tests. Best Pract Res Clin Gastroenterol. 2007;21:299–313. doi: 10.1016/j.bpg.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, et al. A new method of diagnosing gastric intestinal metaplasia: Narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819–24. doi: 10.1055/s-2006-944632. [DOI] [PubMed] [Google Scholar]
- 10.Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Confocal laser endomicroscopy. Gastrointest Endosc Clin North Am. 2005;15:715–31. doi: 10.1016/j.giec.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Yanez P, la Garza AM, Pérez-Pérez G, Cabrera L, Munoz O, Torres J. Comparison of invasive and noninvasive methods for the diagnosis and evaluation of eradication of Helicobacter pylori infection in children. Arch Med Res. 2000;31:415–21. doi: 10.1016/s0188-4409(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 12.Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–5. [PubMed] [Google Scholar]
- 13.Farshad S, Japoni A, Alborzi A. Helicobacter pylori and Extradigestive Disorders in the Past 10 Years. IRMJ. 2009;11:123–32. [Google Scholar]
- 14.Van der Hulst RW, Verheul SB, Weel JFL, Gerrits Y, Ten Kate FJ, Dankert J, et al. Effect of specimen collection techniques, transport media, and incubation of cultures on the detection rate of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1996;15:211–5. doi: 10.1007/BF01591356. [DOI] [PubMed] [Google Scholar]
- 15.Pelerito A, Oleastro M, Lopes AI, Ramalho P, Cabral J, Monteiro L. Evaluation of rapid test Assure® Helicobacter pylori for diagnosis of H.pylori in pediatric population. J Microbial Methods. 2006;66:331–5. doi: 10.1016/j.mimet.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Ogata SK, Kawakami E, Patricio FR, Pedroso MZ, Santos AM. Evaluation of invasive and non-invasive methods for the diagnosis of Helicobacter pylori infection in symptomatic children and adolescents. Sao Paulo Med J. 2001;119:67–71. doi: 10.1590/S1516-31802001000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yousfi MM, El-Zimaity HM, Cole RA, Genta RM, Graham DY. Comparison of agar gel (CLOtest) or reagent strip (PyloriTek) rapid urease tests for detection of Helicobacter pylori infection. Am J Gastroenterol. 1997;92:997–9. [PubMed] [Google Scholar]
- 18.Roma-Giannikou E, Roubani A, Sgouras DN, Panayiotou J, Van-Vliet C, Polyzos A, et al. Endoscopic tests for the diagnosis of Helicobacter pylori infection in children: Validation of rapid urease test. Helicobacter. 2010;15:227–32. doi: 10.1111/j.1523-5378.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- 19.Graham DY. Helicobacter pylori and the endoscopist: Whether to diagnose. Gastrointest Endosc. 1991;37(5):577–9. doi: 10.1016/s0016-5107(91)70838-9. [DOI] [PubMed] [Google Scholar]
- 20.Bourke B, Ceponis P, Chiba N, Czinn S, Ferraro R, Fischbach L, et al. Canadian Helicobacter Study Group Consensus Conference: Update on the approach to Helicobacter pylori infection in children and adolescents-an evidence-based evaluation. Can J Gastroenterol. 2005;19:399–408. [PubMed] [Google Scholar]
- 21.Drumm B, Koletzko S, Oderda G. Helicobacter pylori infection in children: a consensus statement. J Pediatr Gastroenterol Nutr. 2000;30:207–13. doi: 10.1097/00005176-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Gold BD, Colletti RB, Abbott M, Czinn SJ, Elitsur Y, Hassall E, et al. Helicobacter pylori infection in children: Recommendations for diagnosis and treatment. J pediatr Gastroenterol Nutr. 2000;31:490–7. doi: 10.1097/00005176-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Guarner J, Kalach N, Elitsur Y, Koletzko S. Helicobacter pylori diagnostic tests in children: review of the literature from 1999 to 2009. Eur J Pediatr. 2010;169:15–25. doi: 10.1007/s00431-009-1033-x. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin S. Detection of H. pylori infection by biopsy urease, histology, and culture. Methods Mol Med. 1997;8:7–18. doi: 10.1385/0-89603-381-3:7. [DOI] [PubMed] [Google Scholar]
- 25.Prince MI, Osborne JS, Ingoe L, Jones DE, Cobden I, Barton JR. The CLO test in the UK: inappropriate reading and missed results. Eur J Gastroenterol Hepatol. 1999;11:1251–4. doi: 10.1097/00042737-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Binek J, Fantin AC, Meyenberger C. Attitude to Helicobacter pylori infection among Swiss gastroenterologists. Schweiz Med Wochenschr. 1999;129:441–5. [PubMed] [Google Scholar]
- 27.Morris A, Ali MR, Brown P, Lane M, Patton K. Campylobacter pylori infection in biopsy specimens of gastric antrum: Laboratory diagnosis and estimation of sampling error. Br Med J. 1989;42:727–32. doi: 10.1136/jcp.42.7.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Megraud F. Advantages and disadvantages of current diagnostic tests for the detection of Helicobacter pylori. Scand J Gastroenterol. 1996;215:57–62. doi: 10.3109/00365529609094536. [DOI] [PubMed] [Google Scholar]
- 29.Ozçay F, Koçak N, Temizel IN, Demir H, Özen H, Yüce A, et al. Helicobacter pylori infection in Turkish children: Comparison of diagnostic tests, evaluation of eradication rate, and changes in symptoms after eradication. Helicobacter. 2004;9:242–8. doi: 10.1111/j.1083-4389.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- 30.Ni YH, Lin JT, Huang SF, Yang JC, Chang MH. Accurate diagnosis Helicobacter pylori infection by stool antigen test and 6 other currently available tests in children. J Pediatr. 2000;136:823–7. [PubMed] [Google Scholar]
- 31.Leong RW, Lee CC, Ling TK, Leung WK, Sung JJ. Evaluation of the string test for the detection of Helicobacter pylori. World J Gastroenterol. 2003;9:309–11. doi: 10.3748/wjg.v9.i2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goel N, Sherwal BL, Patwari AK, Bajaj P, Choudhury M. Evaluation of invasive and non-invasive diagnostic modalities for Helicobacter pylori infection in children. Indian Pediatr. 2003;40:141–6. [PubMed] [Google Scholar]
- 33.Farshad S, Alborzi A, Japoni A, Ranjbar R, Hosseini Asl K, Badiee P, et al. Antimicrobial susceptibility of Helicobacter pylori strains isolated from patients in Shiraz, Southern Iran. World J Gastroenterol. 2010;16:5746–51. doi: 10.3748/wjg.v16.i45.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westblom TU, Madan E, Kemp J, Subik MA. Evaluation of a rapid urease test to detect Campylobacter pylori infection. J Clin Microbiol. 1988;26:1393–4. doi: 10.1128/jcm.26.7.1393-1394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waived C. CLO Test Detection of H. pylori from gastric biopsy. Policy. 2009;1:25–35. [Google Scholar]
- 36.Deltenre M, Glupczynski Y, Prez CD, Nyst JF, Burette A, Labbé M, et al. The reliability of urease tests, histology and culture in the diagnosis of Campylobacter pylori infection. Scand J Gastroenterol. 1989;24:19–24. doi: 10.3109/00365528909091730. [DOI] [PubMed] [Google Scholar]
- 37.Madani S, Rabah R, Tolia V. Diagnosis of Helicobacter pylori infection from antral biopsies in pediatric patients: Is urease test that reliable? Dig Dis Sci. 2000;45:1233–7. doi: 10.1023/a:1005574608074. [DOI] [PubMed] [Google Scholar]
- 38.Drumm B. Helicobacter pylori in the pediatric patient. Gastroenterol Clin North Am. 1993;22:169–82. [PubMed] [Google Scholar]


