Abstract
Background and Purpose:
Tinnitus is associated with an increased activity in central auditory system as demonstrated by neuroimaging studies. Brain perfusion scanning using single photon emission computed tomography (SPECT) was done to understand the pattern of brain blood perfusion of tinnitus subjects and find the areas which are mostly abnormal in these patients.
Materials and Methods:
A number of 122 patients with tinnitus were enrolled to this cross-sectional study. They underwent SPECT and magnetic resonance imaging (MRI) of brain, and the images were fused to find the regions with abnormal perfusion.
Results:
SPECT scan results were abnormal in 101 patients (83%). Most patients had bilateral abnormal perfusion (N = 65, 53.3%), and most subjects had abnormality in middle-temporal gyrus (N = 83, 68%) and temporoparietal cortex (N = 46, 37.7%). Patients with multifocal involvement had the least mean age than other 2 groups (patients with no abnormality and unifocal abnormality) (P value = 0.045).
Conclusions:
Brain blood perfusion pattern differs in patient with tinnitus than others. These patients have brain perfusion abnormality, mostly in auditory gyrus (middle temporal) and associative cortex (temporoparietal cortex). Multifocal abnormalities might be due to more cognitive and emotional brain centers involvement due to tinnitus or more stress and anxiety of tinnitus in the young patients.
Keywords: Magnetic resonance imaging, neuroimaging, perfusion, single photon emission computed tomography, tinnitus
INTRODUCTION
Tinnitus is defined as an aberrant auditory sensation unrelated to an external source. It is produced in the central auditory pathway.[1–5] This symptom is a common phenomenon disturbing millions of individuals worldwide. Study of tinnitus has resulted in a number of hypothetical mechanisms and suspected origins in the auditory pathways. Most hypotheses postulate that the generation of tinnitus is related to cochlear or acoustic nerve disorders, or central auditory cortices dysfunction and their interactions.[3–9] Surgical destruction of the cochlea or the cochlear nerve has been ineffective; therefore, tinnitus is described as an auditory phantom perception.[6,10] The perception of tinnitus sensation is thought to trace back to the central mechanism in most cases.[3,11,12] It has been speculated that the tinnitus may be a consequence of maladaptive cortical reorganization after an injury in the periphery, analogous to the pathophysiologic model of chronic pain.[3,13] Numerous investigations showed that tinnitus is associated with an increased activity in central auditory system as demonstrated by electrophysiology and neuroimaging studies and with vascular pathogenic changes detected by ultrasonography.[2,4,14,15]
Single photon emission computed tomography (SPECT) scanning of the brain reflects regional cerebral blood perfusion.[16] Brain blood perfusion scanning has a role in diagnosis, therapeutic management, and follow-up of patients and in researches. SPECT scanning of the brain with the radioisotope technetium 99m (TC-99m) is a nuclear imaging technique providing an objective analytical detection of regional cerebral blood perfusion of the brain. The cerebral blood flow has correlation with its metabolic function.[17] In 1989, SPECT was introduced into the medical audiologic tinnitus patient diagnostic protocol (MATPP) firstly, as an investigative tool to objectify tinnitus.[18] SPECT imaging of brain in patients with disabling tinnitus has revealed responsible loci by indicating perfusion asymmetries in cortical areas. In some studies, medial temporal system has been involved in more than 90% of the patients.[5,17,19,20] Adjacent perfusion asymmetries involving the frontal, temporal and parietal lobes have suggested an inter-neural network resulting in the transition of the sense to the affected components of tinnitus.[1,4] There are few studies with low sample size, which have evaluated the brain blood perfusion findings using SPECT scanning of tinnitus patients and information about the blood flow in the brain, and diagnostic accuracy of this technique in tinnitus patients is extremely scarce.[1,21,22] Brain perfusion scanning using SPECT was done to understand the pattern of brain blood perfusion of tinnitus subjects and find the areas which are mostly abnormal in these patients.
METHODS
Subjects
From January 2006 to May 2008, 122 patients with subjective idiopathic tinnitus and 9 healthy controls were enrolled to this cross sectional study. The subjects were selected from patients who were referred to ENT and Head and Neck Research Center of Rasoul-e-Akram hospital (Tehran, Iran) to evaluate and treat their tinnitus.
The inclusion criteria were moderate to severe unilateral or bilateral tinnitus (tinnitus questionnaire score of 44 or more) which permanently lasted for more than 3 months.[23] All subjects had to be healthy, had no ear, mental, and brain disorders and history of invasive therapeutic brain procedures and brain trauma. They had to have no medical disease and medication. The pregnant and breastfeeding women and those who had decision to be pregnant, the subjects with history of tinnitus treatment in the last 3 months and alcohol/drug abuse in the last 6 months were not included into the study.
All subjects gave written informed consent according to the declaration of Helsinki, National Committee of Ethics in Medical Research (Technology and Research Deputy of Health Ministry) and the Committee on Ethics at the ENT and Head and Neck Research Center of Tehran University of Medical Sciences, radiation safety and radioactive drug research committee prior to participating in the study.
Tinnitus assessment
Pitch and loudness matching of tinnitus were identified for the affected ear to an external tone presented to the contralateral ear. This task was accomplished using Tinnitus Evaluation Device (TinED®), which includes 6 channels to reconstruct the most troublesome tinnitus with a similar frequency and intensity. An accuracy of the calibrating equipment shall be sufficient to determine that, the TinED® is within the tolerances permitted by American Standard Specification for Audiometers, S3.6-2004. The subjects had to have loudness matching of tinnitus more than 6-decibel sensation level (dB SL) to be included in this study.
Using tinnitus questionnaire (TQ), the severity of tinnitus in subjects was rated to 6 scales.[24] We used Persian version of TQ, which was translated and validated.[25] Subjects with TQ score of 44 or more were considered to have moderate to severe tinnitus.
Single photon emission computerized tomography imaging (SPECT imaging)
The patients were placed in a quiet, dimly lit room and instructed to keep the eyes open (or use a mask) and the ears unplugged. They were also instructed not to speak, read, or move during 5 min prior to and 5 min post injection. They were asked to think about their tinnitus during the test.
A commercial ethyl cysteinate dimer (ECD) preparation was used. After approximately 30 min, each subject received a 15 mCi intravenous injection of tracer while they were still lying down. No sedation was used.
One hour after intravenous injection of 15 mCi 99mTc-ECD, SPECT scanning was acquired (120 projections; 40 projections per head; 25 sec/projection). Scanning was performed using a dual head SMV gamma camera, equipped with a pair of low energy, high resolution collimators.
Planar and processed SPECT images were visually assessed by a nuclear medicine physician twice, who was blinded to all other clinical and imaging information. The images were visually graded as normal for no appreciable abnormal activity and abnormal for hyper activity. Semi-quantitative evaluation of planar images over the lesion and background areas on the anterior, was also performed by drawing regions of interest (ROI) posterior or contralateral hemisphere images without any lesion. Geometric means of the contraleteral, posterior and anterior ROI values were used for the calculation of lesion-to-background ratio. Transverse views with the best visualization of the lesions were selected for ROI drawing on the SPECT images. Lesion to background ratio was calculated accordingly for all sets of images. All activity ratios were classified to determine an intensity of activity as: 1 > normal, 1 < hyperactivity. If the ratio was <1, the visually suspected sites considered normal, and if >1, those were classified as abnormal sites or lesions.[26]
Magnetic resonance imaging
MRIs were obtained using tool marked Siemens, 1.5 Tesla, Avanto 18 channels. The participants were kept about 8 minutes without any movement. The images were stored in Dicom format, to be applied in Brain Anatomical Functional Images Co-registration Software (Brain AFICS®).
Image fusion
SPECT is inherently a perfusion modality; therefore, it is sometimes difficult to exactly define the anatomical area with disturbed function. Therefore, it is helpful to correlate the relatively coarse SPECT images to high-resolution anatomic MR images.
Brain Anatomical Functional Images Co-registration Software (BrainAFICS®), a software system designed in ENT and Head and Neck research Center of TUMS, is capable of registering and fusing unsynchronized positron emission tomography (PET) and SPECT images with MR images with different dimensions in a subject [Figure 1].
Figure 1.
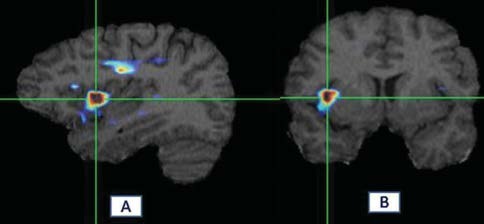
Fusion of SPECT and MRI in tinnitus-related brain abnormalities in one subject. ROIs were used for semi-quantitative analysis of hyperactivity. (a, b) Abnormalities in inferotemporal gyrus. Hyperactive areas are shown red with BrainAFICS software. Hyperactivity of brain was defined visually and semi-quantitatively analysis. Activity ratio was calculated as follow; for unilaterally involved subjects; after localized abnormal area visually, ROIs were drawn and the count per voxel were compared with the ROIs in the other side or beside. In bilaterally involved subjects; count per voxel of the abnormal areas were compared with similar ROIs on cerebellum (as reference)
After fusion, cerebral zones were categorized in 8 regions; middle temporal, inferotemporal, medial temporal, superior temporal, temporoparietal, frontal, frontoparietal and parietal.
Validation
After final fusion of images, each image was evaluated and reported by an expert twice. Almost all images were reported similarly in both times, but if the assessment was not similar, images were interpreted for third time and the repeated report was considered as main result. The Kendall′s Tau-b correlation confirmed validation of reports (P value < 0.001, R = 0.7). The nuclear medicine specialist was blinded to the patients and controls when she was interpreting the images.
Statistical analysis
For finding the association of sides and sites of SPECT abnormality with tinnitus and difference between groups, Chi-square and Fisher′s exact test were used. Comparison of quantitative variable was done by T-test between two independent groups and analysis of variance (ANOVA) between more than two groups. Probability value <0.05 was considered significant. Summary data were presented as mean ± standard deviation (SD). All analyses were done using SPSS V.16 (Chicago, United States).
RESULTS
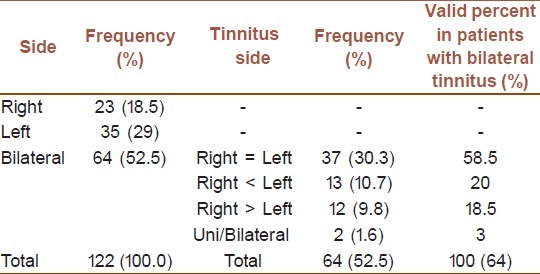
Subjects consisted of 89 (73%) males and 33 (27%) females undergone evaluation. All subjects completed the study, and no adverse effect was seen. Tinnitus was present in left ear of 35 (28.7%) subjects, right ear of 23 (18.9%) subjects, and both ears of 64 (52.5%) subjects [Table 1].
Table 1.
Side of tinnitus in patients
The time interval between the SPECT scanning and the tinnitus symptom was 77.17 ± 78.7 months for the left ear (from 3 to 300 months) and 81.8 ± 76.6 months for the right ear (from 3 to 396 months). Mean recorded pitching of the tinnitus was 7.76 ± 3.71 dB, based on subjective assessment.
Abnormalities (defined as hyperperfusion areas detected by brain SPECT scanning) were seen in 101 tinnitus patients (83%) and 4 controls (44%). Most tinnitus subjects (74 [60%]) had multifocal abnormalities from which 27 subjects (36.5%) had more than one abnormalities in each hemisphere. There was no significant association between the side of the tinnitus and number of brain abnormal foci (P value > 0.05). Patients with unifocal and multifocal brain abnormalities did not show different tinnitus severity (P value > 0.05). The subjects with multifocal brain involvement had the lowest mean age than the patients with unifocal or no abnormal area. (45.62 ± 13.98 years vs. 52.75 ± 12.83 years vs. 52.22 ± 9.11 years for multifocal, unifocal and no lesion, respectively; P value = 0.045). In controls, 3 persons (33.3%) had multifocal abnormalities, and more than one abnormality was seen in 1 hemisphere of 2 (22%) persons.
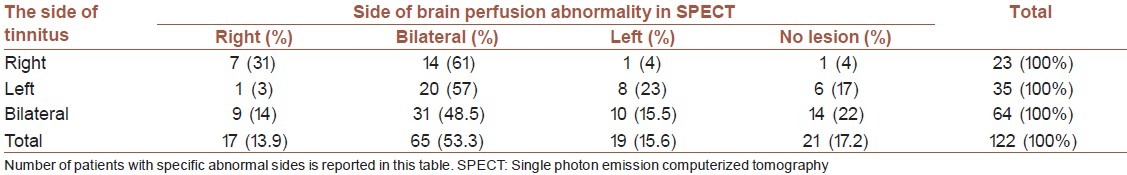
The most common abnormal areas were middle temporal gyrus (30.3% in the right and 37.7% in the left hemispheres) and temporoparietal cortex (24.6% in the right and 13.1% in the left hemispheres) [Table 2]. From all evaluated tinnitus subjects, 19 (15.6%) had left sided brain perfusion abnormality, 17 (13.9%) had right sided brain perfusion abnormality, and 65 (53.3%) had brain perfusion abnormalities in both hemispheres. Statistically significant relation was found between the side of the brain perfusion abnormalities and the side of the tinnitus (P value = 0.027) [Table 3]. The patients with right hemisphere lesion in SPECT scanning had the most severe sensation of tinnitus, while the least severity was seen in the patients with left hemisphere involvement (right sided brain abnormality: 12.8 ± 6.1 dB SL Vs. left sided brain abnormality: 5.8 ± 2.2 dB SL Vs. bilateral brain abnormality: 7.3 ± 2.7 dB SL Vs. no brain perfusion abnormality: 7.2 ± 3.03 dB SL, P value = 0.006 using ANOVA).
Table 2.
Site of abnormalities in brain SPECT scanning
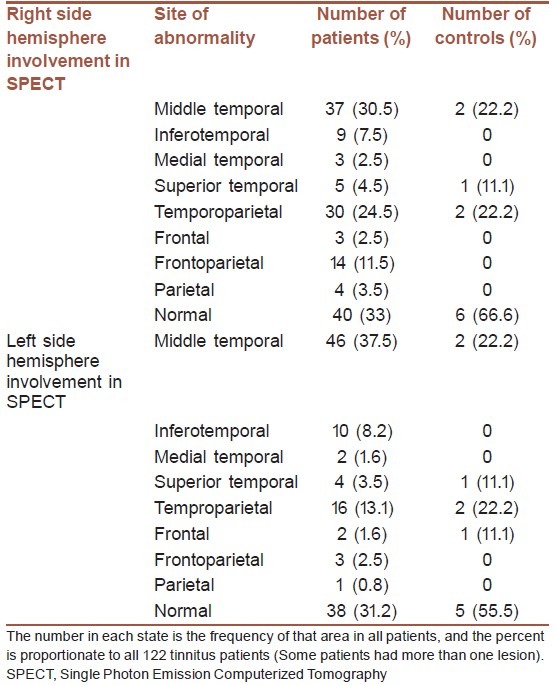
Table 3.
Results of brain SPECT scanning in patients with different side of tinnitus. (P value: 0.027)
DISCUSSION
Tinnitus has an association with a wide variety of auditory system disorders. Generating peripherally or centrally, tinnitus is believed to be associated with activity in specific cortical regions as shown by PET[9,27,28] and SPECT[1,9,20] and Functional MRI[3,9,29] and with vascularity changes.[15]
Unilaterally increased metabolic activity in the secondary auditory cortex represents a robust finding in tinnitus patients.[30] There is controversy in different studies whether more abnormal side is the left or the right hemisphere. Some studies have reported the right hemisphere,[27,31] but others found the left hemisphere more involved in imaging studies, no investigation has shown bilateral brain involvement in the tinnitus patients.[21,22,32,33]
All studies declare that there is no association between the side of tinnitus and dominant side of brain perfusion/function abnormality. In the present study, bilateral brain abnormalities detected by SPECT scanning were seen in most tinnitus patients, and in contrast to the previous studies, it was associated to the side of tinnitus. Right hemisphere was more abnormal in patients with sensation of tinnitus in the right ear, and the patients with left ear tinnitus had more lesions in the left hemisphere, and in patients with bilateral tinnitus, bilateral brain involvement in SPECT scanning was the most finding.
Superior and transverse temporal gyrus, middle frontal gyrus, middle temporal gyrus, lateral and medial posterior sites, temporofrontal, paralimbic, anterior middle temporal gyrus and hippocampus are represented as the most common involved areas in different studies.[21,27,31,33] But, based on the results of the present study, the most common involved area was middle temporal (Brodmann area 21) which is a secondary auditory cortex, and temporoparietal cortex (Brodmann area 22) was the second most common involved part in the brain that is an associative cortex.
Patients with more severe tinnitus had right hemisphere perfusion abnormality more frequently than the left hemisphere. Considering association between tinnitus severity with side of brain abnormalities, it could be concluded that the tinnitus severity may be an important predictor of brain abnormality side with unknown mechanisms, or the pathologic brain perfusion in the right hemisphere cause more severe tinnitus, and vice versa.
Patients′ age seems to play an important role in the brain perfusion abnormality, and older patients should be expected to have unifocal abnormality more while younger patients have multifocal brain perfusion abnormalities. Relationship between number of brain lesions with age may be due to the negative feedback at the result of more stress in young person about tinnitus as a life threatening condition that leads to more brain involvement and multifocal abnormality. Another hypothesis is more plasticity in cortical neurons of young patients that lead to involvement of more areas in younger patients. As other hypothesis, this could be the atrophic effect of tinnitus that results in brain atrophy in older patients with longer duration of tinnitus and actually leads to less brain hyperperfused foci.[34] Finally, auditory processing is shifted from the phylogenetically non-classical system, towards the phylogenetically classical auditory system that performs the finer analysis of sounds. The efficiency of synapses that connect auditory input to the non-classical pathways decreases during ontogeny and these synapses become ineffective at the time of adulthood.[35]
In the study performed by Farhadi M. et al., 5 mCi F18-FDG (fluorodeoxyglucose) was used for metabolic function assessment of 55 tinnitus patients. Similar to the present study, the most involved brain regions were reported there to be secondary auditory cortex and associative cortex; middle temporal gyrus (Brodmann area 21) and temporoparietal cortex (Brodmann area 22), respectively. In that study, the brain metabolic function was evaluated using radiotracer F18-FDG, while herein, we evaluated the brain perfusion of the subjects using technetium-99m. Brain perfusion in each area may be compatible with function of that area, but these may be differently patterned in tinnitus patients and results in different findings as could be seen herein, in contrast to the findings of the present study, there was no significant association between the side of tinnitus and side of brain metabolic function abnormalities, and between age and metabolic lesions numbers, and also between the tinnitus severity and brain lesion side in the that study. It has to be considered that, these differences may be due to smaller sample size in the mentioned study.[28]
Study limitations
Although it caused interpretation of tinnitus origin and mechanism unbiased, not considering hearing loss made us unable to categorize the findings on the basis of tinnitus type. Intermittent or constant, evoking and inhibiting with and without some sensory-motor pathways may have some association with brain findings but were not involved in the interpretation of the results. Small number of controls limits us to do comparative analyses between subjects and controls.
CONCLUSION
Abnormalities of the cerebral blood flow are seen in the patients who complain of tinnitus. The most common abnormal foci are middle temporal gyrus (Brodmann area 21) and temporoparietal cortex (Brodmann area 22), that means the secondary auditory cortex and associative cortex are mostly associated with tinnitus, and the pathophysiology of tinnitus could be there. Higher number of abnormal foci in younger patients could be explained with more cognitive and emotional brain centers involvement due to tinnitus or stress, and anxiety of tinnitus in the young patients or atrophic effect of tinnitus or developing process by aging.
ACKNOWLEDGMENT
This investigation was supported by Iran National Science Foundation
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shulman A. A final common pathway for tinnitus - The medial temporal lobe system. Int Tinnitus J. 1995;1:115–26. [PubMed] [Google Scholar]
- 2.Cacace AT, Cousins JP, Parnes SM, Semenoff D, Holmes T, McFarland DJ, et al. Cutaneous-evoked tinnitus. I. Phenomenology, psychophysics and functional imaging. Audiol Neurootol. 1999;4:247–57. doi: 10.1159/000013848. [DOI] [PubMed] [Google Scholar]
- 3.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Cacace AT. Expanding the biological basis of tinnitus: Crossmodal origins and the role of neuroplasticity. Hear Res. 2003;175:112–32. doi: 10.1016/s0378-5955(02)00717-7. [DOI] [PubMed] [Google Scholar]
- 5.Shulman A, Goldstein B, Strashun AM. Final common pathway for tinnitus: Theoretical and clinical implications of neuroanatomical substrates. Int Tinnitus J. 2009;15:5–50. [PubMed] [Google Scholar]
- 6.Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): Clinical presentation. Neurosurgery. 1997;40:1–9. doi: 10.1097/00006123-199701000-00001. discussion 9-10. [DOI] [PubMed] [Google Scholar]
- 7.Zenner HP, Ernst A. Cochlear-motor, transduction and signal-transfer tinnitus: Models for three types of cochlear tinnitus. Eur Arch Otorhinolaryngol. 1993;249:447–54. doi: 10.1007/BF00168852. [DOI] [PubMed] [Google Scholar]
- 8.Coles RR. Tinnitus. In: Stephens D, editor. Adult audiology. 1 ed. Oxford: Butterworth-Heinemann; 1997. pp. 1–34. [Google Scholar]
- 9.Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: The neuroscience of tinnitus. J Neurosci. 2010;30:14972–9. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jastreboff PJ. Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neurosci Res. 1990;8:221–54. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- 11.Hazell JW, Jastreboff PJ. Tinnitus. I: Auditory mechanisms: A model for tinnitus and hearing impairment. J Otolaryngol. 1990;19:1–5. [PubMed] [Google Scholar]
- 12.Bauer CA, Brozoski TJ, Holder TM, Caspary DM. Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear Res. 2000;147:175–82. doi: 10.1016/s0378-5955(00)00130-1. [DOI] [PubMed] [Google Scholar]
- 13.Moller AR. Similarities between severe tinnitus and chronic pain. J Am Acad Audiol. 2000;11:115–24. [PubMed] [Google Scholar]
- 14.Kaltenbach JA. Neurophysiologic mechanisms of tinnitus. J Am Acad Audiol. 2000;11:125–37. [PubMed] [Google Scholar]
- 15.Fukatsu M, Yamada T, Suzuki S, Yoneyama A, Joh T. Tinnitus is associated with increase in the intima-media thickness of carotid arteries. Am J Med Sci. 342:2–4. doi: 10.1097/MAJ.0b013e31820ab3bb. [DOI] [PubMed] [Google Scholar]
- 16.Juni JE, Waxman AD, Devous MD, Sr, Tikofsky RS, Ichise M, Van Heertum RL, et al. Procedure guideline for brain perfusion SPECT using (99m)Tc radiopharmaceuticals 3.0. J Nucl Med Technol. 2009;37:191–5. doi: 10.2967/jnmt.109.067850. [DOI] [PubMed] [Google Scholar]
- 17.Crick F, Koch C. The problem of consciousness. Sci Am. 1992;267:152–9. doi: 10.1038/scientificamerican0992-152. [DOI] [PubMed] [Google Scholar]
- 18.Shulman A, Strashun AM, Goldstein B, Afiiyie MO. Tinnitus. Proceedings of the Fourth International Tinnitus Seminar ed. Amsterdam: Kugler Publications; 1992. Neurospect cerebral blood flow studies in patients with a central type tinnitus; pp. 211–6. [Google Scholar]
- 19.Shulman A, Strashun A. SPECT imaging of brain and tinnitus. Case reports. In: Heertum RV, Tikofsky A, editors. Cerebral spect imaging. 2nd ed. New York: Raven Press; 1995. pp. 210–2. [Google Scholar]
- 20.Shulman A, Strashun AM, Afriyie M, Aronson F, Abel W, Goldstein B. SPECT Imaging of Brain and Tinnitus-Neurotologic/Neurologic Implications. Int Tinnitus J. 1995;1:13–29. [PubMed] [Google Scholar]
- 21.Sataloff RT, Mandel S, Muscal E, Park CH, Rosen DC, Kim SM, et al. Single-photon-emission computed tomography (SPECT) in neurotologic assessment: A preliminary report. Am J Otol. 1996;17:909–16. [PubMed] [Google Scholar]
- 22.Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: A PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec. 1996;58:195–9. doi: 10.1159/000276835. [DOI] [PubMed] [Google Scholar]
- 23.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122:143–8. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 24.Hallam RS, Jakes SC, Hinchcliffe R. Cognitive variables in tinnitus annoyance. Br J Clin Psychol. 1988;27(Pt 3):213–22. doi: 10.1111/j.2044-8260.1988.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 25.Farhadi M, Mahmoudian S, Yazdanparasti V, Daneshi A. Effects of Auditory Electrical Stimulation (AES) on Tinnitus Improvement and Associated Complaints by Using Persian Tinnitus Questionnaire (PTQ) Hakim. 2005;8:1–8. [Google Scholar]
- 26.Costa DC, Ell PJ. Brain blood flow in neurology and psychiatry: Clinician′s guide to nuclear medicine; London: Churchill Livingstone; 1991. [Google Scholar]
- 27.Mirz F, Pedersen B, Ishizu K, Johannsen P, Ovesen T, Stodkilde-Jorgensen H, et al. Positron emission tomography of cortical centers of tinnitus. Hear Res. 1999;134:133–44. doi: 10.1016/s0378-5955(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 28.Farhadi M, Mahmoudian S, Saddadi F, Karimian AR, Mirzaee M, Ahmadizadeh M, et al. Functional brain abnormalities localized in 55 chronic tinnitus patients: Fusion of SPECT coincidence imaging and MRI. J Cereb Blood Flow Metab. 2010;30:864–70. doi: 10.1038/jcbfm.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smits M, Kovacs S, de Ridder D, Peeters RR, van Hecke P, Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007;49:669–79. doi: 10.1007/s00234-007-0231-3. [DOI] [PubMed] [Google Scholar]
- 30.Langguth B, Eichhammer P, Kreutzer A, Maenner P, Marienhagen J, Kleinjung T, et al. The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus--first results from a PET study. Acta Otolaryngol Suppl. 2006:84–8. doi: 10.1080/03655230600895317. [DOI] [PubMed] [Google Scholar]
- 31.Mirz F, Gjedde A, Ishizu K, Pedersen CB. Cortical networks subserving the perception of tinnitus--a PET study. Acta Otolaryngol Suppl. 2000;543:241–3. doi: 10.1080/000164800454503. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Tian J, Yin D, Jiang S, Yang W, Han D, et al. Regional glucose metabolic increases in left auditory cortex in tinnitus patients: A preliminary study with positron emission tomography. Chin Med J (Engl) 2001;114:848–51. [PubMed] [Google Scholar]
- 33.Wang H, Tian J, Yin D. Positron emission tomography of tinnitus-related brain areas. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2000;35:420–4. [PubMed] [Google Scholar]
- 34.Jastreboff PJ, Hazell JW. A neurophysiological approach to tinnitus: Clinical implications. Br J Audiol. 1993;27:7–l7. doi: 10.3109/03005369309077884. [DOI] [PubMed] [Google Scholar]
- 35.Moller AR, Rollins PR. The non-classical auditory pathways are involved in hearing in children but not in adults. Neurosci Lett. 2002;319:41–4. doi: 10.1016/s0304-3940(01)02516-2. [DOI] [PubMed] [Google Scholar]


