Abstract
Background:
This study aimed to compare different body mass index (BMI) categories in individuals with diabetes, prediabetes and normal glucose tolerance among the first degree relatives of type 2 diabetic patients.
Materials and Methods:
A cross-sectional study was conducted during 2005-2007 in Isfahan, Iran. It evaluated 3323 first-degree relatives of diabetic patients selected by consecutive convenient sampling method. Participants were classified as diabetic, prediabetic, and normal glucose tolerance test groups according to the results of 75 g oral glucose tolerance test (OGTT). The analysis of variance (ANOVA) was used for comparison of quantitative variables, and chi square test for comparison of categorical parameters.
Results:
The study population consisted of 3323 individuals including 306 diabetics (98 males and 208 females), 1309 prediabetics (337 males and 972 females), and 1708 normal subjects (430 males and 1278 females). Among diabetic patients, the prevalence of obesity was 48.5% in women and 27.6% in men. Among prediabetics, the corresponding figures were 45.6% and 27.3%, respectively.
Conclusions:
Our findings suggest that men are diagnosed with T2DM at lower BMI than women. Moreover, the alarming high prevalence of overweight and obesity among females necessitates preventing and controlling this underlying problem among females.
Keywords: Diabetes Mellitus, Family History, Glucose Tolerance Test, Obesity, Prevention
INTRODUCTION
The role of obesogenic environments, with high-calorie intake and sedentary lifestyle, on the development of obesity and ultimately diabetes is well documented. The causal relationship of obesity and diabetes is strong enough for the term diabesity to be used in experimental and human studies.[1–3] However, genetic background increases this risk of diabetes mellitus.[4–6]
Epidemiological studies have suggested that genetic factors as well as obesity, measured by body mass index (BMI), and abdominal obesity, measured by waist circumference are major risk factors for the development of type 2 diabetes mellitus (T2DM).[7] Positive family history of diabetes can be used as a potential public health tool and an indicator of genetic background.[8,9] It has been demonstrated that having a parent with T2DM increases by two- to four- folds an offspring's risk of developing this disorder.[10–14] However, not all obese individuals are affected by disorders in glucose metabolism and diabetes.[15,16] There are even reports from normal metabolic profile of morbidly obese persons.[17–20]
Middle Eastern countries are expected to have the world's highest increases in the absolute burden of diabetes in the next two decades.[21] National studies have shown high prevalence of obesity,[22] metabolic syndrome,[23] and diabetes in Iran.[24] These high prevalence rates and the aforementioned importance of family history mandate conducting studies in relatives of T2DM patients to determine and to control their risk factors as a high-risk approach for preventing diabetes. This study thus aimed to compare different BMI categories in individuals with diabetes, prediabetes, and normal glucose tolerance among the first degree relatives of T2DM patients.[25]
MATERIALS AND METHODS
This study was a part of the Isfahan Diabetes Prevention Program Study, conducted in 2005-2008 among first degree relatives of T2DM patients.[26] It was carried out by Isfahan Endocrine and Metabolism Research Center (IEMRC) affiliated to Isfahan University of Medical Sciences (IUMS), Isfahan, Iran. Participants were selected by consecutive convenient sampling from among 3700 individuals aged 35-60 years who were the first degree relatives of T2DM patients. All participants underwent an oral glucose tolerance test after 10-12 hours overnight fasting. Individuals with diabetes, prediabetes, and normal glucose tolerance test, according to the 2005 American Diabetes Association criteria[25] were enrolled in the current study. Those with unknown glucose tolerance state and pregnant women were not recruited.
The study protocol was approved by the Ethics Committee of Isfahan University of Medical Sciences and the local scientific ethical committee according to the Declaration of Helsinki. All patients gave informed consents prior to the study.
Anthropometric parameters were measured under standard protocol and by using calibrated instruments. Height was measured as the vertical distance from the vertex point to the base of the heels. The reading was recorded to the nearest 0.1 cm. Weight was measured in kilograms by making the subject stand on a weighing machine with minimal clothing without shoes. Weight was recorded with an allowance deducted for clothing. BMI was calculated as weight (in kilograms) divided by height (in meters) squared. A BMI of 18-25 kg/m2 was considered as normal, 25-29.9 kg/m2 as overweight, and ≥ 30 kg/m2 as obese.
Waist circumference was measured at level of maximal point at right iliac crest in mid axillary line in normal respiration.
Blood pressure (BP) was measured twice in a sitting position after 5 minutes resting.
Statistical Analysis:
Statistical analysis was performed by SPSS16.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Data is expressed as mean and standard deviation (SD). The analysis of variance (ANOVA) was used for comparison of quantitative variables. Post-hoc and chi-square tests were used for comparison of categorical parameters. P values less than 0.05 were considered as statistically significant.
RESULTS
The study population consisted of 3323 individuals including 306 with diabetes (98 males and 208 females), 1309 with prediabetes (337 males and 972 females), and 1708 with normal glucose tolerance test (430 males and 1278 females). Table 1 presents the demographic, anthropometric and clinical characteristics of all participants. Table 2 shows the results of post hoc tests for continuous variables among participants with diabetes, prediabetes and normal glucose tolerance test. Demographic, anthropometric and clinical characteristics of all participants and the results of post hoc tests for continuous variables among the studied groups based on gender are presented in Tables 3 and 4. In diabetic patients, no significant difference was present between male and female subgroups with regard to age (p = 0.87). The subgroups were also identical in mean systolic and diastolic blood pressures (p = 0.635 and p = 0.293, respectively). However, mean values of weight, BMI, and waist circumference were significantly different between males and females (p < 0.001 for all three variables).
Table 1.
Demographic, anthropometric and clinical characteristics of diabetic, prediabetic and normal participants
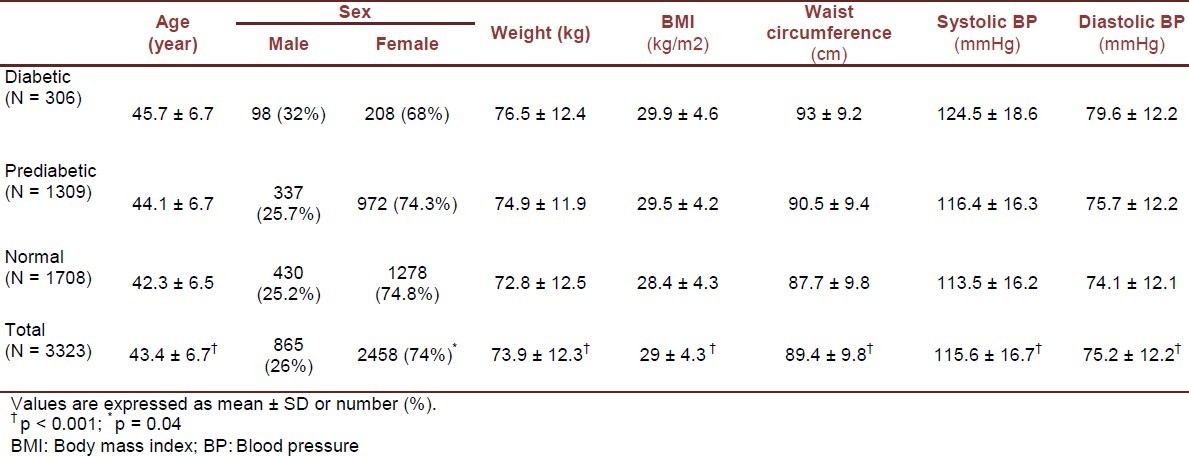
Table 2.
Results of post hoc tests (p values) for continuous variables among diabetic (D), prediabetic (Pre) and normal (N) groups
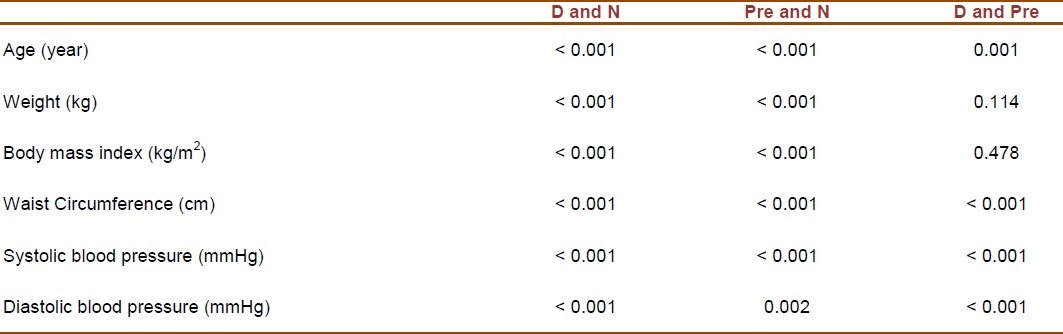
Table 3.
Demographic, anthropometric and clinical characteristics of diabetic, prediabetic and normal participants by gender
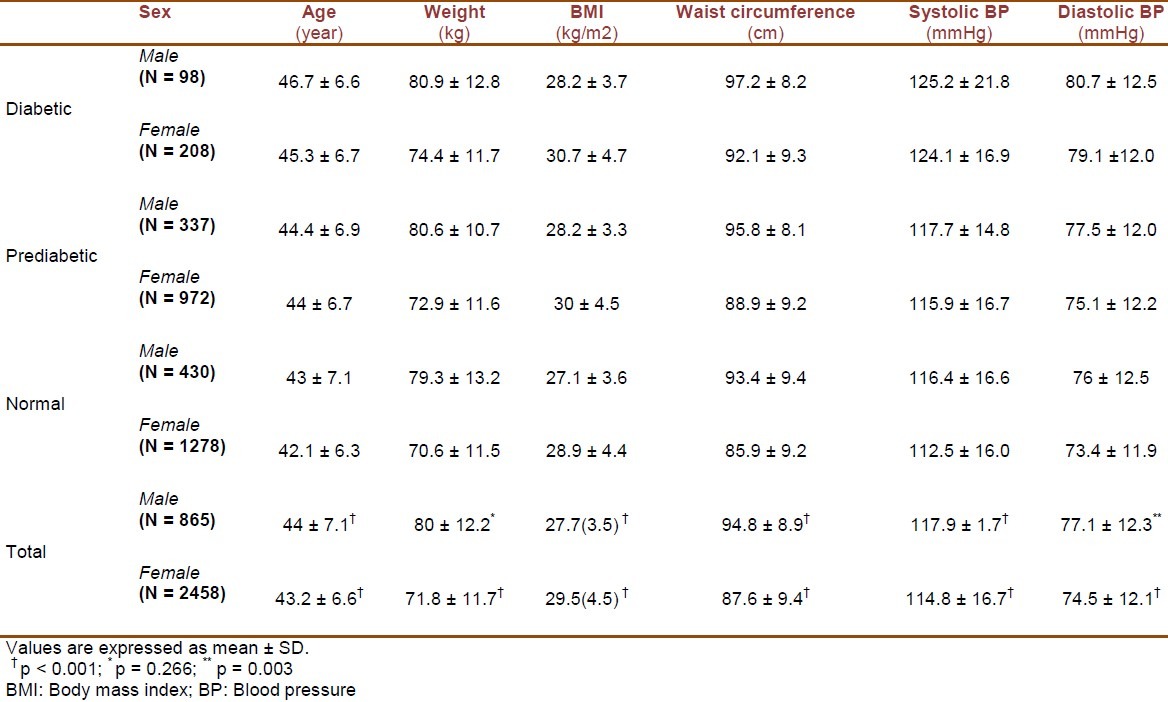
Table 4.
Results of post hoc tests (p values) for continuous variables among diabetic (D), prediabetic (Pre) and normal (N) groups by gender
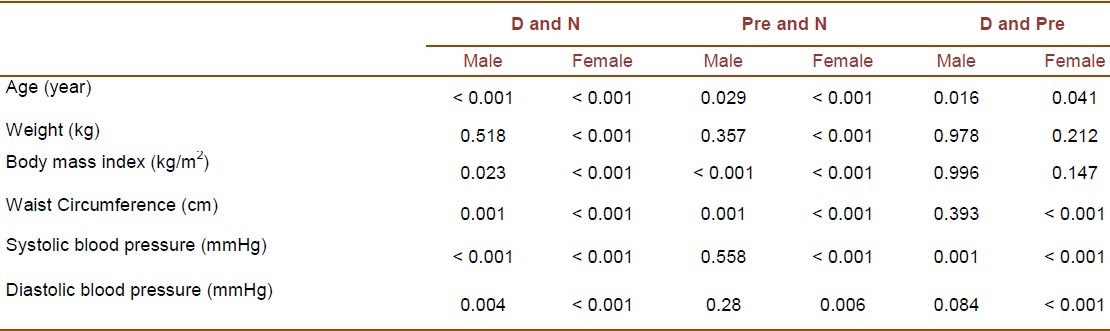
In prediabetic subjects, the male and female subgroups were identical regarding mean age and systolic blood pressure (p = 0.424 and p = 0.075, respectively). However, significant differences were observed in mean values of weight, BMI, waist circumference and diastolic blood pressure between the two subgroups (p < 0.00.1 for weight, BMI and waist circumference; p = 0.002 for diastolic blood pressure). Among participants with normal glucose tolerance test results, the existing differences in age, weight, BMI, waist circumference, systolic and diastolic blood pressures between males and females were all significant (p = 0.021 for age; p < 0.001 for other variables).
Table 5 shows the prevalence of normal weight, overweight and obesity in the three studied groups by sex. In diabetic patients, the prevalence of obesity was 48.6% in women and 27.6% in men (p = 0.001). Among prediabetics, the corresponding figures were 45.6% and 27.3%, respectively (p < 0.001).
Table 5.
The prevalence of normal weight, overweight and obesity in diabetic, prediabetic and normal groups by gender
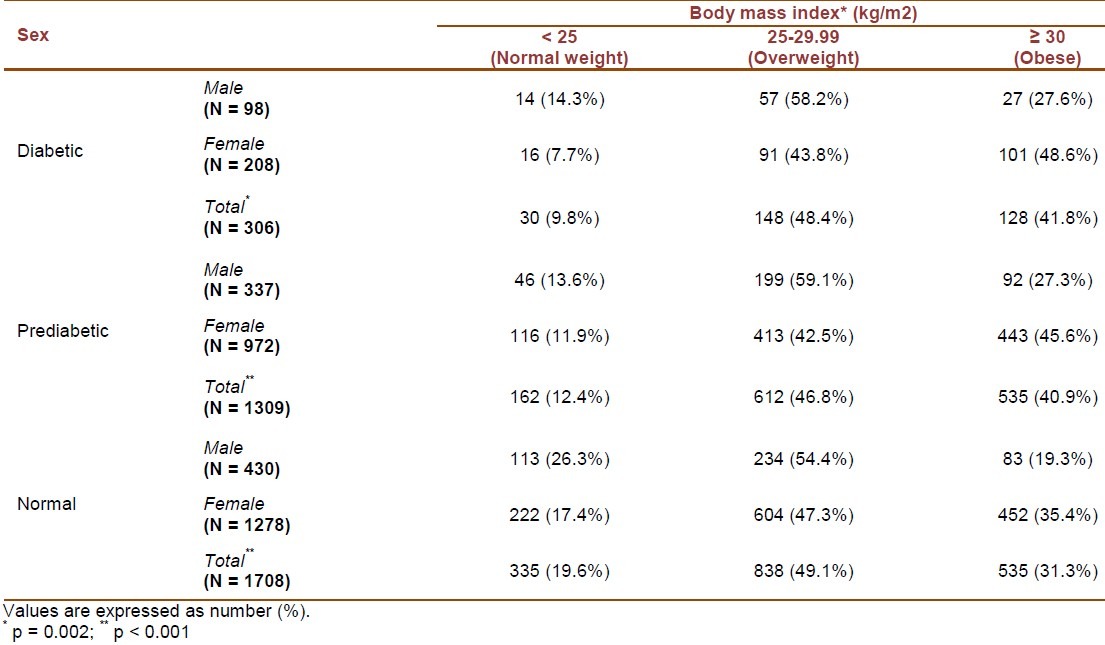
In diabetic patients, the BMI of 92.4% of females and 85.8% of males was greater than 25 kg/m2 (p = 0.098). In prediabetic individuals, 88.1% of females and 86.4% of males had a BMI of > 25 kg/m2 (p = 0.442). In participants with normal glucose tolerance, the corresponding figures were 82.7% and 73.7%, respectively (p < 0.001).
DISCUSSION
Our study on first degree relatives of T2DM patients revealed a considerably high prevalence of overweight and obesity among prediabetic and diabetic individuals. Of special concern was the significantly higher prevalence of weight disorders in women than in men.
Although the group studied in the current survey were genetically prone to diabetes, but a high prevalence of overweight and obesity was documented among individuals affected with prediabetes and diabetes. This finding underscores the importance of prevention and early control of excess weight in populations at risk for T2DM. Obesity and T2DM are multifactorial health conditions caused by a complex genetic-environment interaction. Nevertheless, the role of modifiable lifestyle factors on weight status is well documented.[27,28] Therefore, the fundamental role of lifestyle factors in the development of diabetes should be considered in preventive strategies. Two strategies have been proposed in this regard. The population strategy seeks to eliminate the causes of disease in populations as a whole, whereas the high-risk strategy aims to recognize persons at increased risk, and to consider individual interventions. However, controversies exist about the cost-effectiveness of the high-risk approach. Our findings are consistent with a recent review highlighting the role of lifestyle factors in the development of diabetes. This review clarified that most of the genes identified to date, have modest effect on disease risk, and suggested that obesity and diabetes are not likely to develop without exposure to obesogenic environment. Thus, irrespective of genetic susceptibility, healthy lifestyle may be helpful in preventing diabesity. However, due to individual response to a lifestyle intervention program, individualized interventions according to genotype may be considered in the future.[29]
The national estimates of overweight and obesity in Iran are 28.6% and 14.2%, respectively. Excess weight is mainly reported among women with a prevalence of 48% for overweight and 43.4% for abdominal obesity.[22,23] Given that in our community, sedentary lifestyle is known as the major contributing factor for the emergence of this public health problem,[22] increasing public awareness in this regard may have long-term health impacts.
We found that among diabetic patients, the mean BMI was lower in men than in women. This finding is consistent with a study in Scotland which showed T2DM men to have lower BMI than women. The study considered this gender difference as one of the main reasons of the high prevalence of T2DM among middle-aged European men.
It thus suggested studying this pattern in other ethnic groups.[30] Our findings may serve as a confirmatory evidence of this pattern in a Middle-Eastern population.
Study limitations and strengths:
The main limitation of our study was its cross-sectional nature which made the determination of causative effects impossible. A follow-up study of the same population will be of help in this regard. The main strength of this study was enrollment of a large sample size of the first-degree relatives of diabetic patients, as a group at-risk for disorders of glucose metabolism.
CONCLUSION
Excess weight has a fundamental role in the development of dysglycemia and diabetes among the first degree relatives of diabetic patients. Our findings suggest that men are diagnosed with T2DM at lower BMI than women. Moreover, the alarming high prevalence of overweight and obesity among females necessitates preventing and controlling this underlying problem among females. Relatives of T2DM patients shall be considered as the primary target population for diabetes prevention programs.
ACKNOWLEDGMENTS
This study was funded by Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Astrup A, Finer N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes Rev. 2000;1(2):57–9. doi: 10.1046/j.1467-789x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Reifsnyder PC, Leiter EH. Deconstructing and reconstructing obesity-induced diabetes (diabesity) in mice. Diabetes. 2002;51(3):825–32. doi: 10.2337/diabetes.51.3.825. [DOI] [PubMed] [Google Scholar]
- 4.Permutt MA, Wasson J, Cox N. Genetic epidemiology of diabetes. J Clin Invest. 2005;115(6):1431–9. doi: 10.1172/JCI24758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weedon MN, McCarthy MI, Hitman G, Walker M, Groves CJ, Zeggini E, et al. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med. 2006;3(10):e374. doi: 10.1371/journal.pmed.0030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 7.Hussain A, Vaaler S, Sayeed MA, Mahtab H, Ali SM, Khan AK. Type 2 diabetes and impaired fasting blood glucose in rural Bangladesh: a population-based study. Eur J Public Health. 2007;17(3):291–6. doi: 10.1093/eurpub/ckl235. [DOI] [PubMed] [Google Scholar]
- 8.Yoon PW, Scheuner MT, Peterson-Oehlke KL, Gwinn M, Faucett A, Khoury MJ. Can family history be used as a tool for public health and preventive medicine? Genet Med. 2002;4(4):304–10. doi: 10.1097/00125817-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Harrison TA, Hindorff LA, Kim H, Wines RC, Bowen DJ, McGrath BB, et al. Family history of diabetes as a potential public health tool. Am J Prev Med. 2003;24(2):152–9. doi: 10.1016/s0749-3797(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 10.Pierce M, Keen H, Bradley C. Risk of diabetes in offspring of parents with non-insulin-dependent diabetes. Diabet Med. 1995;12(1):6–13. doi: 10.1111/j.1464-5491.1995.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 11.Elbagir MN, Eltom MA, Elmahadi EM, Kadam IM, Berne C. A population-based study of the prevalence of diabetes and impaired glucose tolerance in adults in northern Sudan. Diabetes Care. 1996;19(10):1126–8. doi: 10.2337/diacare.19.10.1126. [DOI] [PubMed] [Google Scholar]
- 12.Pajunen P, Kotronen A, Korpi-Hyovalti E, Keinanen-Kiukaanniemi S, Oksa H, Niskanen L, et al. Metabolically healthy and unhealthy obesity phenotypes in the general population: the FIN-D2D Survey. BMC Public Health. 2011;11:754. doi: 10.1186/1471-2458-11-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SH, Ha HS, Park YJ, Lee JH, Yim HW, Yoon KH, et al. Identifying metabolically obese but normal-weight (MONW) individuals in a nondiabetic Korean population: the Chungju Metabolic disease Cohort (CMC) study. Clin Endocrinol (Oxf ) 2011;75(4):475–81. doi: 10.1111/j.1365-2265.2011.04085.x. [DOI] [PubMed] [Google Scholar]
- 14.Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34(1):210–5. doi: 10.2337/dc10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol. 2010;21(1):38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- 16.Soverini V, Moscatiello S, Villanova N, Ragni E, Di DS, Marchesini G. Metabolic syndrome and insulin resistance in subjects with morbid obesity. Obes Surg. 2010;20(3):295–301. doi: 10.1007/s11695-009-9999-z. [DOI] [PubMed] [Google Scholar]
- 17.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168(15):1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 18.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud’homme D, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90(7):4145–50. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 19.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86(3):1020–5. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 20.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–16. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 21.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 22.Kelishadi R, Alikhani S, Delavari A, Alaedini F, Safaie A, Hojatzadeh E. Obesity and associated lifestyle behaviours in Iran: findings from the First National Non-communicable Disease Risk Factor Surveillance Survey. Public Health Nutr. 2008;11(3):246–51. doi: 10.1017/S1368980007000262. [DOI] [PubMed] [Google Scholar]
- 23.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care. 2009;32(6):1092–7. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteghamati A, Meysamie A, Khalilzadeh O, Rashidi A, Haghazali M, Asgari F, et al. Third national Surveillance of Risk Factors of Non-Communicable Diseases (SuRFNCD-2007) in Iran: methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health. 2009;9:167. doi: 10.1186/1471-2458-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janghorbani M, Amini M. Comparison of fasting glucose with post-load glucose values and glycated hemoglobin for prediction of type 2 diabetes: the Isfahan diabetes prevention study. Rev Diabet Stud. 2009;6(2):117–23. doi: 10.1900/RDS.2009.6.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supplement 1. American Diabetes Association: clinical practice recommendations 2000. Diabetes Care. 2000;23(Suppl 1):S1–116. [PubMed] [Google Scholar]
- 27.Djousse L, Driver JA, Gaziano JM, Buring JE, Lee IM. Association between modifiable lifestyle factors and residual lifetime risk of diabetes. Nutr Metab Cardiovasc Dis. 2011 doi: 10.1016/j.numecd.2011.08.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon KJ, Lee O, Kim HK, Han SN. Comparison of the dietary intake and clinical characteristics of obese and normal weight adults. Nutr Res Pract. 2011;5(4):329–36. doi: 10.4162/nrp.2011.5.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Temelkova-Kurktschiev T, Stefanov T. Lifestyle and genetics in obesity and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2012;120(1):1–6. doi: 10.1055/s-0031-1285832. [DOI] [PubMed] [Google Scholar]
- 30.Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54(12):3003–6. doi: 10.1007/s00125-011-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


