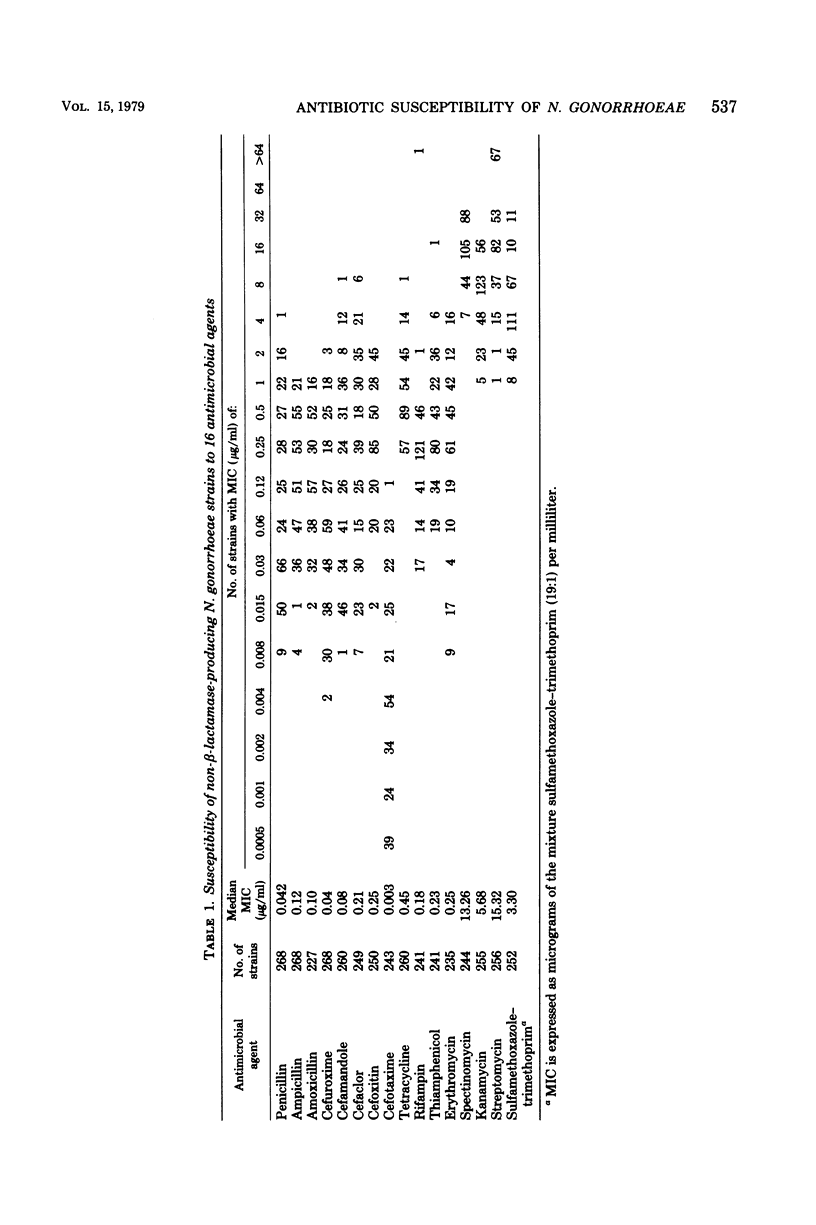
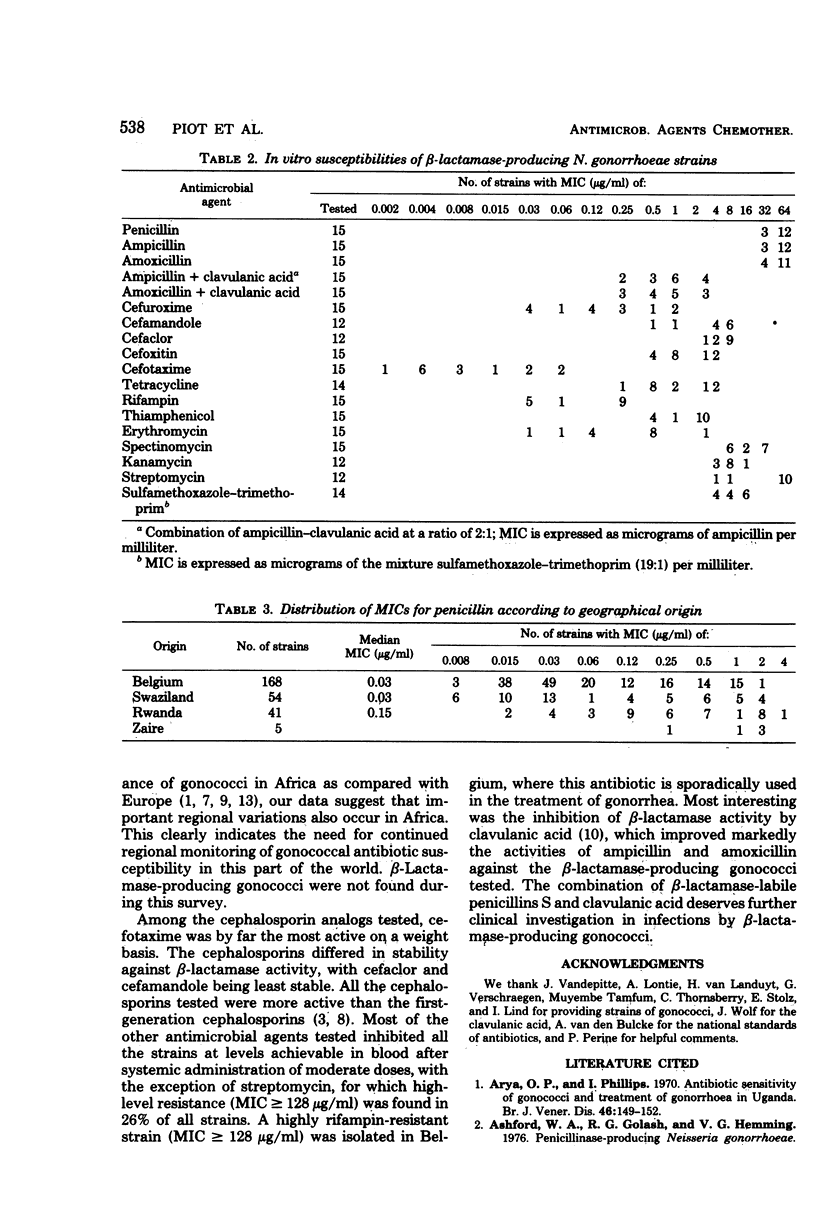
Abstract
The in vitro activities of 16 antimicrobial agents were tested by a plate dilution method against 268 unselected isolates of Neisseria gonorrhoeae from Belgium, Rwanda, Swaziland, and Zaire. Fifteen β-lactamase-producing strains isolated in Europe from various origins were also tested. There were significant regional variations in antimicrobial agent susceptibility, even among the African isolates, with the Rwandan and Zairean strains being most resistant. Benzylpenicillin and ampicillin were equally active in all but the β-lactamase-producing strains. Among the cephalosporins, cefotaxime was by far the most active, followed by cefuroxime, cefamandole, cefoxitin, and cefaclor, in that order. All strains were susceptible to spectinomycin, thiamphenicol, kanamycin, and rifampin, with the exception of one highly rifampin-resistant isolate and a moderately thiamphenicol-resistant strain. Twenty-six percent of the isolates were highly resistant to streptomycin. Six percent of the gonococci had a minimal inhibitory concentration for tetracycline greater than 2 μg/ml. Clavulanic acid inhibited the β-lactamase activity of the gonococci tested and improved markedly the activities of ampicillin and amoxicillin against β-lactamase-producing strains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya O. P., Phillips I. Antibiotic sensitivity of gonococci and treatment of gonorrhoea in Uganda. Br J Vener Dis. 1970 Apr;46(2):149–152. doi: 10.1136/sti.46.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finland M., Garner C., Wilcox C., Sabath L. D. Susceptibility of Neisseria gonorrhoeae to 66 antibacterial agents in vitro. J Am Vener Dis Assoc. 1976 Mar;2(3):33–40. [PubMed] [Google Scholar]
- Jaffe H. W., Biddle J. W., Thornsberry C., Johnson R. E., Kaufman R. E., Reynolds G. H., Wiesner P. J. National gonorrhea therapy monitoring study: in vitro antibiotic susceptibility and its correlation with treatment results. N Engl J Med. 1976 Jan 1;294(1):5–9. doi: 10.1056/NEJM197601012940102. [DOI] [PubMed] [Google Scholar]
- Meheus A., Piot P., Pattyn S., van Dyck E., van den Berghe D. Activity in vitro of ten antimicrobial agents against Neisseria gonorrhoeae. A study of the correlation between the sensitivities. Br J Vener Dis. 1976 Oct;52(5):329–332. doi: 10.1136/sti.52.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osoba A. O., Montefiore D. G., Sogbetun A. O., Alausa K. O., Anong C. N. Sensitivity pattern of Neisseria gonorrhoeae to penicillin and screening for beta-lactamase production in Ibadan, Nigeria. Br J Vener Dis. 1977 Oct;53(5):304–307. doi: 10.1136/sti.53.5.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I., King A., Warren C., Watts B. The activity of penicillin and eight cephalosporins on Neisseria gonorrhoeae. J Antimicrob Chemother. 1976 Mar;2(1):31–39. doi: 10.1093/jac/2.1.31. [DOI] [PubMed] [Google Scholar]
- Plorde J. J., Kidan T. G., Wright L. J. Penicillin sensitivity of gonococci in Ethiopia. Br J Vener Dis. 1973 Jun;49(3):260–262. doi: 10.1136/sti.49.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C., Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence M. R., Stutz D. R., Srimunta C., Duangmani C. Changing penicillin resistance of the gonococcus in Thailand. J Am Vener Dis Assoc. 1976 Sep;3(1):32–34. [PubMed] [Google Scholar]
- Stolz E., Zwart H. G., Michel M. F. Sensitivity to ampicillin, penicillin, and tetracyline of gonococci in Rotterdam. Br J Vener Dis. 1974 Jun;50(3):202–207. doi: 10.1136/sti.50.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen A. R., Van der Ham M., Heimans A. L., Kranendonk O., Maina A. N. Diminished antibiotic sensitivity of Neisseria gonorrhoeae in urban and rural areas in Kenya. Bull World Health Organ. 1971;45(6):707–717. [PMC free article] [PubMed] [Google Scholar]
- Wilson C., Rose D. L., Tramont E. C. Increased antibiotic resistance of Neisseria gonorrhoeae in Korea. Antimicrob Agents Chemother. 1976 Apr;9(4):716–718. doi: 10.1128/aac.9.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirak-Zadah T., Delavarian H., Bahavar M. A., Majidi V., Yaminifar R., Masoumi P. Penicillin-resistant strains of Neisseria gonorrhoeae in Shahre-Now. Trop Doct. 1977 Apr;7(2):57–58. doi: 10.1177/004947557700700204. [DOI] [PubMed] [Google Scholar]



