Abstract
The development of pulmonary hypertension is a common accompaniment of congenital heart disease (CHD) with increased pulmonary blood flow. Our recent evidence suggests that asymmetric dimethylarginine (ADMA)-induced mitochondrial dysfunction causes endothelial nitric oxide synthase (eNOS) uncoupling secondary to a proteasome-dependent degradation of GTP cyclohydrolase I (GCH1) that results in a decrease in the NOS co-factor, tetrahydrobiopterin (BH4). Decreases in NO signaling are thought to be an early hallmark of endothelial dysfunction. As L-carnitine plays an important role in maintaining mitochondrial function in this study we examined the protective mechanisms and the therapeutic potential of L-carnitine on NO signaling in pulmonary arterial endothelial cells (PAEC) and in a lamb model of CHD and increased pulmonary blood flow (Shunt). Acetyl L-carnitine (ALC) attenuated the ADMA-mediated proteasomal degradation of GCH1. This preservation was associated with a decrease in the association of GCH1 with the Hsp70 and the C-terminus of Hsp70-interacting protein (CHIP) and a decrease in its ubiquitination. This in turn prevented the decrease in BH4 levels induced by ADMA and preserved NO signaling. Treatment of Shunt lambs with L-carnitine also reduced GCH1/CHIP interactions, attenuated the ubiquitination and degradation of GCH1, and increased BH4 levels compared to vehicle treated Shunt lambs. The increases in BH4 were associated with decreased NOS uncoupling and enhanced NO generation. Thus, we conclude that L-carnitine may have a therapeutic potential in the treatment of pulmonary hypertension in children with CHD with increased pulmonary blood flow.
Keywords: mitochondrial dysfunction, BH4, Hsp90, Hsp70, CHIP, ubiquitination
INTRODUCTION
Our recent studies have shown that mitochondrial dysfunction is an important contributing factor to the decrease in NO signaling in our lamb model of pulmonary hypertension with increased pulmonary blood flow (Shunt) [1]. Furthermore, we have also previously shown that asymmetric dimethylarginine (ADMA), an endogenous competitive inhibitor of NO synthase (NOS), induces mitochondrial dysfunction and decreases Hsp90 chaperone activity through a reduction in cellular ATP levels in cultured ovine pulmonary arterial endothelial cells (PAEC) [2]. Thus, preventing mitochondrial dysfunction may have potential for therapeutic interventions to preserve NO signaling and prevent the development of the endothelial dysfunction associated with the development of a number of cardiovascular diseases.
The mitochondrial metabolic pathways, acyl-CoA and carnitine acetyltransferase have been shown to be of critical importance for maintaining normal mitochondrial function. Previous studies implicate compromised carnitine metabolism as an important mediator in the development of mitochondrial injury [3]. Studies also show that under conditions of metabolic stress, mitochondria accumulate acyl-CoA, which is normally maintained in homeostasis with carnitine (Reviewed in [4]). Recently, we also demonstrated a disruption in carnitine homeostasis in these shunted lambs, in association with mitochondrial dysfunction and decreased eNOS/HSP90 interactions, which contributed to eNOS uncoupling and decreased NO signaling [1]. Although the mechanisms underlying the increase in eNOS uncoupling in Shunt lambs is unclear it appears to involve decreases in the NOS cofactor, tetrahydrobiopterin (BH4) [5]. Further, recent data suggest that GTP cyclohydrolase I (GCH1), the first and rate limiting enzyme in the de novo pathway of BH4 biosynthesis, is a client protein for Hsp90 [6] and we have shown that ADMA decreases Hsp90 activity in PAEC [2].
As carnitine plays an important role in maintaining mitochondrial function and has been shown to have protective effect in various pathologic conditions such as chronic renal failure [7], we hypothesized that supplementation with L-carnitine could prevent the mitochondrial dysfunction and loss of Hsp90 activity associated with increased levels of ADMA preserving BH4 levels and NO signaling. Thus in this study, we examined the effect of L-carnitine supplementation on ADMA-induced mitochondrial dysfunction in cultured PAEC and in our Shunt model. Our data demonstrate that ALC abolishes the ADMA induced decrease in GCH1 protein levels by attenuating a CHIP-dependent ubiquitination and proteasomal degradation of GCH1. This prevents the ADMA-mediated decrease in BH4 levels, which, in turn, preserved NO signaling and attenuated eNOS uncoupling. We also found that the oral administration of carnitine effectively reversed the decrease in BH4 levels in Shunt lambs and this resulted in increased NO bioavailability and decreased eNOS uncoupling. Taken together, our data indicate that L-carnitine abolishes ADMA-induced GCH1 degradation both in vitro and in vivo. Our studies suggest that L-carnitine may have therapeutic potential in the treatment of pulmonary hypertension in children with CHD with increased pulmonary blood flow.
MATERIAL AND METHODS
Surgical preparations and experimental protocol
Twelve mixed-breed Western pregnant ewes (137–141 days gestation, term = 145 days) were operated on as previously described in detail [8, 9]. Lambs received daily treatment with oral L-carnitine (n=6, 100 mg/kg/day) or its vehicle (control lambs, n=6) beginning no later than 12h after delivery. Four weeks after spontaneous delivery just prior to sacrifice the patency of the vascular graft was confirmed by inspection and changes in oxygen saturation. At the end of the protocol, all lambs were killed with a lethal injection of sodium pentobarbital followed by bilateral thoracotomy as described in the NIH Guidelines for the Care and Use of Laboratory Animals. All protocols and procedures were approved by the Committees on Animal Research at UCSF and Georgia Health Sciences University.
Cell culture and treatment
Primary cultures of ovine pulmonary arterial endothelial cells (PAEC) were isolated and cultured as described previously [10]. Before any treatments, PAEC were serum-starved for 2h, then pretreated with 100μM ALC (Sigma, St. Louis, MO) for 2h, followed by incubation with 5 μM ADMA (Sigma, St. Louis, MO) for an additional 2h. For some studies, cells were treated with the proteasome inhibitor lactacystin (20 μM, Calbiochem), vehicle (0.1% DMSO), PEG-SOD (100U/ml), or PEG-catalase (100U/ml).
Determination of mitochondrial reactive oxygen species (ROS) levels
MitoSOX™ Red (Molecular Probes), a fluorogenic dye for selective detection of ROS levels in the mitochondria of live cells was used. Briefly, cells were washed with fresh media, and then incubated in media containing MitoSOX Red (2μM), for 30 min at 37°C in dark conditions then subjected to fluorescence microscopy at an excitation of 510nm and an emission at 580nm. An Olympus IX51 microscope equipped with a CCD camera (Hamamatsu Photonics) was used for acquisition of fluorescent images. The average fluorescent intensities (to correct for differences in cell number) were quantified using ImagePro Plus version 5.0 imaging software (Media Cybernetics).
Analysis of mitochondrial membrane potential
Mitochondrial membrane potential was analyzed as previously described using the lipophilic cation 5,5′6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide [11, 12], which fluoresces red in its multimeric form in healthy mitochondria and is the active reagent in the DePsipher mitochondrial potential assay kit (Trevigen, Gaithersburg, MD). PAEC were seeded onto 24-well plates and treated in the presence or absence of ALC (100 μM, 2 h), followed ADMA exposure (5 μM, 2 h). DePsipher reagent (10 μg/ml) was then added and the samples incubated for a further 20 min. After an additional wash with Dulbecco’s PBS, the cytosolic monomer (green) form was observed and quantified by fluorescence microscopy at 530 nm.
Proteasome activity assay
Proteasome activity in PAEC was estimated using a proteasome activity assay kit (Chemicon, Billerica, MA) according to the manufacturer’s guidelines. The assay is based on detection of the fluorophore, 7-Amino-4-methylcoumarin (AMC) after cleavage from the labeled substrate, LLVY-AMC. The free AMC fluorescence was quantified using an excitation at 380nm and an emission at 460nm using a fluorescence plate reader (Thermo Fisher).
Determination of cellular ATP levels
ATP levels were estimated using a rapid, quantitative, bioluminescent determination Enzylight ATP Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s guidelines. ATP is consumed and light is emitted when firefly luciferase catalyzes the oxidation of luciferin. The amount of light emitted during the reaction is proportional to the availability of ATP. Luminescence was determined using a Fluoroscan Ascent FL plate luminometer (ThermoElectron, Corp).
Expression of a dominant negative mutant of Hsp90
The day before transfection, 1.5 × 105 cells were seeded in each well of a six-well plate and fresh DMEM containing serum and antibiotics was added. On the day of transfection, the medium was changed to one without antibiotics. The cells were transiently transfected with a dominant negative mutant of Hsp90 or the parental vector (pcDNA3) using lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. The Hsp90 dominant negative mutant exerts its affect through an inability to bind ATP [13]. The plasmid was obtained commercially (Addgene Cambridge, MA, plasmid 22480).
Hsp70 and CHIP RNA interference assays
PAEC were transfected with the appropriate small interfering RNA (siRNA) using HiPerFect transfection reagent (QIAGEN) as described previously [14]. Briefly, the day before transfection, 1.5 × 105 cells were seeded in each well of a six-well plate and fresh DMEM containing serum and antibiotics added. On the day of transfection, the medium was changed to one without antibiotics. For each well, 6μl of a 10-μM siRNA stock of Hsp70 (Santa Cruz), CHIP (Santa Cruz), or the control (a scrambled siRNA with no known homology to any human gene) was diluted into 100μl of DMEM without serum (to give a final siRNA concentration of 30 nM). To this was added 12μl of HiPerFect transfection reagent. The solution was vortexed, incubated for 10min at room temperature, and added drop-wise to the cells. As the siRNA’s utilized were designed against human mRNA sequences their ability to silence Hsp70 and CHIP was validated by Western blot 48 h after transfection (Fig. 5A & 7A, respectively).
Figure 5. Depletion of Hsp70 in pulmonary arterial endothelial cells.
PAEC were transiently transfected with a siRNA for Hsp70 or a control siRNA. Forty-eight hours after transfection, whole cell lysates (20μg) were prepared and subjected to Western blot analysis using specific antisera raised against Hsp70 (A), Hsp90 (B), and CHIP (C) with loading normalized by reprobing the membranes with an antibody specific to β-actin. A representative image is shown for each. Transfection with the Hsp70 siRNA reduces Hsp70 protein levels by ~50% (A) without altering Hsp90 (B) or CHIP (C) protein levels. Values are means ± SEM, n=6. *P < 0.05 vs. control siRNA.
Figure 7. Depletion of CHIP in pulmonary arterial endothelial cells.
PAEC were transiently transfected with a siRNA for CHIP or a control siRNA. Forty-eight hours after transfection, whole cell lysates (20μg) were prepared and subjected to Western blot analysis using specific antisera raised against CHIP (A), Hsp90 (B), and Hsp70 (C) with loading normalized by reprobing the membranes with an antibody specific to β-actin. A representative image is shown for each. Transfection with the CHIP siRNA reduces Hsp70 protein levels by ~50% (A) without altering Hsp90 (B) or Hsp70 (C) protein levels. Values are means ± SEM, n=6. *P < 0.05 vs. control siRNA.
Measurement of GCH activity
Cells were washed in PBS and lysed using 50 mM Tris-HCl buffer (pH 7.4), containing 1 mM MgCl2, 100 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM PMSF and protease inhibitors cocktail (Sigma). Aliquots (100 μl) were assayed for activity as described previously [15, 16]. Briefly, cell lysates were incubated at 37°C with 1 mM GTP and 50μg/ml bovine serum albumin, in a total volume of 200μl, for either 0- or 60-min. Reactions were terminated by rapid cooling on water-ice followed by the addition of 8 μl 5M HCl. The reaction mixtures were then oxidized with 2% KI/1% I2 for 60 min in the dark followed by the addition of 2% ascorbic acid. Samples were then incubated with 5U of alkaline phosphatase at pH 8.0 for 60 min at 37°C and the reaction stopped by acidification using 5M HCl. The neopterin content of each sample was then analyzed by HPLC (Shimadzu, Japan) using a C18 reverse-phase column eluted with aqueous 0.05% Trifluoroacetic acid (TFA) at a flow of 0.5 ml/min. Neopterin was detected by fluorescence with excitation at 350 and emission at 440 nm; authentic neopterin was used as standard. The protein content of samples was determined by the BCA assay and activity was expressed as the amount of neopterin formed (pmol/mg protein).
Quantification of tetrahydrobiopterin levels by high-performance liquid chromatography
BH4 levels were determined using the differential iodine oxidation method as we have previously published [17, 18]. Briefly, PAEC were homogenized in an extraction buffer (50 mM Tris-HCl, 1 mM EDTA, 1 mM DTT; pH 7.4) and divided into equal volumes between two centrifuge tubes containing either 1M NaOH (base) or 1M H3PO4 (acid). A solution of 1% iodine in 2% potassium iodide was added to each tube and the samples incubated in the dark at room temperature for 90 minutes. 1M H3PO4 was then added to the tubes containing NaOH. In both the acidic and basic samples the excess iodine was removed by the addition of 2% ascorbic acid and then centrifuged at 15,000g for 10 minutes to remove the precipitated protein. Each sample was then analyzed by HPLC using a Spherisorb ODS-1 column (Waters, Franklin MA) and fluorescence detection (350 nm excitation, 450 nm emission). The area under the curve obtained by oxidation in acidic conditions represents total biopterins, i.e., the sum of BH4, BH2 and free biopterin (BH4+BH2+B). In contrast, alkaline oxidation measures BH2 and free biopterin (BH2+B). Thus, BH4 levels were calculated as the difference between values obtained in acidic and alkaline conditions. A standard curve of freshly made tetrahydrobiopterin (Sigma, St. Louis, MO) was also included to allow the area under each curve to be converted into a concentration. BH4 levels were then normalized to total protein using the Bradford assay and expressed as femtomoles per microgram protein.
Western blot analysis
For the Western blot analyses, 20μg of protein prepared from total cell lysates were separated on 4–20% SDS-polyacrylamide gels, and transferred to PVDF membranes. Immunoblotting was carried out using the appropriate antibodies in Tris-base buffered saline with 0.1% Tween 20 and 5% nonfat dried milk. After washing, the membranes were probed with horseradish peroxidase-conjugated goat antiserum to rabbit or mouse. Reactive bands were visualized and quantified as mentioned above. The generation of the anti-GCH1 antibody has been previously described [14]. The anti-SOD2 antibody and the anti-UCP2 antibody were purchased from Lifespan Biosciences (Seattle, WA). Loading was normalized by reprobing the membranes with an antibody specific to β-actin.
Immunoprecipitation analysis
For the in vitro studies, PAEC were harvested in lysis buffer supplemented with protease inhibitor cocktail as we have described [1]. Cell and tissue lysates were then clarified by centrifugation at 20,000g (20 min at 4°C), the protein concentrations were determined, and 1mg of each lysate was incubated overnight at 4°C with anti-Hsp90 (BD Transduction laboratories, San Jose, CA), anti-Hsp70 (Stressgen, Ann Arbor, Michigan), or anti-CHIP (ABR Affinity BioReagents, Golden, CO) antibodies (2μg of each antibody). Immunocomplexes were adsorbed to 40μl of protein G plus protein A agarose (Calbiochem, Gibbstown, NJ) then incubated for 2h at 4°C. The immune complexes were precipitated by centrifugation, washed three times with lysis buffer, boiled in SDS sample buffer, and subjected to SDS-PAGE then transferred to PVDF membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween (TBST). A specific antiserum we have raised against GCH1 protein [5] was then added and incubated overnight at 4°C. After washing, the membranes were probed with horseradish peroxidase-conjugated goat antiserum to rabbit. Reactive bands were visualized using chemiluminescence (Super Signal West Femto; Pierce) on a Kodak 440CF image station (New Haven, CT). Bands were quantified using Kodak Image Station software, (Kodak 1D 3.6). The efficiency of each immunoprecipitation was normalized by reprobing the membranes with the appropriate immunoprecipitation antibody (Hsp90, Hsp70, or CHIP). In some cases, IP lysates were run on duplicate blots, with one blot probed for GCH1 and the other probed for CHIP to normalize the IP (Fig. 6A, Hsp70 siRNA, GCH1/CHIP interaction).
Figure 6. Depletion of Hsp70 attenuates the ADMA-mediated degradation of GCH1 in pulmonary arterial endothelial cells.

PAEC were transiently transfected with a siRNA for Hsp70 or a control siRNA. Forty-eight hours after transfection, whole cell lysates (1mg) were subjected to immunoprecipitation (IP) or ubiquitinated protein enrichment (AP) using an antibody specific to CHIP. IP samples were run on duplicate Western blot (IB), one blot was analyzed using an antibody specific for GCH1, the other blot was probed with an antibody for CHIP to normalize for the immunoprecipitation efficiency. AP samples were analyzed by Western blot (IB) analysis using an anti-GCH1 antibody. A representative image is shown for each. Depleting Hsp70 protein levels attenuated the ADMA-induced GCH1 interaction with CHIP (A) and prevented the increase in GCH-1 ubiquitination (B). Whole cell lysates (20μg) were also subjected to Western blot analysis using a specific antiserum raised against GCH1, loading was normalized by reprobing the membranes with an antibody specific to β-actin. A representative image is shown along with the densitometric analysis indicating that that reducing hsp70 protein levels preserves GCH1 protein levels in PAEC challenged with ADMA (C). Values are means ± SEM, n=6. *P < 0.05 vs. control siRNA.
Real time quantitative (q) RT-PCR
For the in vivo studies using peripheral lung tissue, qRT–PCR using SYBR green I dye for specific detection of double-stranded DNA, was employed. Briefly, total RNA was extracted using the RNeasy kit (Qiagen), and 1μg of total RNA was reverse-transcribed using QuantiTect Reverse Transcription Kit (Qiagen). Primers for GCH1 and β-actin were designed by Primer 3. The sequences were GCH1 Forward, 5′-TCT TCA CCA AGG GCT ACC AG-3′; Reverse, 5′-GGA CCT TTC CAA CAA ATG GA-3; β-actin Forward, 5′-CTC TTC CAG CCT TCC TTC CT-3′; Reverse, 5′-GGG CAG TGA TCT CTT TCT GC-3′. Real-time Quantitative PCR was conducted using an Mx4000 Multiplex Quantitative PCR System (Stratagene), using 2μl of RT product, 12.5μl of QuantiTect SYBR Green PCR Master Mix (Qiagen) and primers (400nM) in a total volume of 25μl. The following thermocycling conditions were employed: 95°C for 10 min, followed by 95°C for 30s, 55°C for 60s and 72°C 30s for 45 cycles. The threshold cycle (Ct) of a serially diluted control sample were plotted to generate a standard curve. Concentration of each sample was calculated by plotting its Ct on the standard curve and then normalized using the mRNA levels of β-actin as an internal control.
Detection of ubiquitinated GTP cyclohydrolase I
Ubiquitinated GCH1 from both PAEC and peripheral lung was enriched using affinity beads comprised of a GST-fusion protein containing a ubiquitin-associated sequence conjugated to glutathione-agarose using a ubiquitinated protein enrichment kit (Calbiochem, Gibbstown, NJ) according to the manufacturer’s guidelines. The resulting samples were then separated by SDS-PAGE then analyzed by Western blotting using our specific antiserum raised against GCH1 [17].
Exposure of pulmonary endothelial cells to shear stress
Laminar shear stress was applied using a cone-plate viscometer that accepts six-well tissue culture plates, as described previously [19, 20]. Using this apparatus we exposed PAEC to a laminar flow rate of 20 dyn/cm2 to represent physiological levels of laminar shear stress in the major human arteries, which is in the range of 5–20 dyn/cm2 with localized increases to 30–100 dyn/cm2.
Measurement of NOx levels
NO generated by PAEC was measured using an NO-sensitive electrode with a 2-mm diameter tip (ISO-NOP sensor, WPI) connected to an NO meter (ISO-NO Mark II, WPI) as described previously [21]. To measure NO levels in peripheral lung tissue, lysates were initially treated with cold ethanol to remove proteins then we utilized a Sievers 280i Nitric Oxide Analyzer (GE) to determine NOx levels as we have described. [1] Results were analyzed by measuring the area under curve of the chemiluminescence signal using the Liquid software (GE).
Measurement of superoxide levels
To detect superoxide generation in intact cells and peripheral lung tissue, EPR was carried out using the spin probe, 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine·HCl (CMH, Alexis Biochemicals, San Diego, CA) as we have described previously [22, 23]. CMH was chosen based on a prior publication in which it was shown to react more rapidly with superoxide and produce a more stable adduct than spin traps [24]. For peripheral lungs, approximately 0.1 g of tissue was sectioned and powdered from fresh-frozen biopsies of lung tissue and immediately immersed, while still frozen, in 100 μl of EPR buffer [PBS supplemented with 5 μM diethyldithiocarbamate (DETC; Sigma-Aldrich) and 25 μM desferrioxamine (Def MOS; Sigma-Aldrich)]. In addition, to determine the relative contribution of uncoupled NOS activity to superoxide production, equivalent samples were preincubated in 100 μM S-ethylisothiourea hydrobromide (ETU; Sigma), a nonspecific inhibitor of NOS isoforms. All samples were then incubated for 30 min on ice. Sample volumes were then adjusted with EPR buffer and 20 mg/ml CMH hydrochloride to achieve equal a final CMH concentration of 5 mg/ml. Samples were further incubated for 30 min on ice and then centrifuged at 14,000 g for 15 min at 4° C. For cell culture EPR, cells were plated in 6-well plates and 24h before experiment, standard medium was replaced with L-arginine and phenol red free DMEM (Athena enzyme system, Baltimore, MD). PAEC were serum-starved for 2h, followed by pretreatment with or without ALC (100μM, 2h), in the presence or absence of the isothiourea, S-ethyl-N-[4-(trifluoromethyl)phenyl]isothiourea (ETU, 100μM, 30 min, to estimated NOS-derived superoxide). Ethyl substituted isothiourea is a potent inhibitor of NOS with little isoform selectivity as previously described [25]. Cells were then treated with ADMA (5μM, 2h) followed by exposure or not to shear stress (20 dyn/cm2, 15 min). In the final hour of incubation with ADMA, 20μl of spin-trap stock solution consisting of CMH (20 μM in DPBS +25μM desferrioxamine; Calbiochem, La Jolla, CA) and 5μM diethyldithiocarbamate (Alexis Biochemicals, Lausen, Switzerland) + 2μl DMSO was added to each well. Upon completion of incubation, adherent cells were trypsinized and pelleted at 500 g. Cell pellet was washed and suspended in a final volume of 40μl DPBS (+desferrioxamine, diethyldithiocarbamate). Thirty five microliter of tissue supernatant or cell suspension was loaded into a 50-μl capillary tube and analyzed with a MiniScope MS200 ESR (Magnettech, Berlin, Germany) at a microwave power of 10 mW, modulation amplitude of 2,000 mG. The left our samples analyzed for protein content using Bradford analysis (Bio-Rad) EPR spectra were analyzed using ANALYSIS v.2.02 software (Magnettech), whereby the EPR maximum and minimum spectral amplitudes for the CM·superoxide spin-trap product waveform were quantified. NOS-derived superoxide levels were determined by subtracting the superoxide values in the presence of ETU from the superoxide values in the absence of ETU. To convert cell and tissue EPR waveforms into units of superoxide we used 1mU of xanthine oxidase to generate 1nM/min of superoxide over a 60 min period. Using this we found a linear correlation between the waveform amplitudes generated using CMH in cells and tissues and the generation of superoxide by xanthine oxidase. Using this standard curve we were able to convert waveform amplitudes into nmol of superoxide produced/min/mg protein in each reaction condition.
Mitochondrial isolation for superoxide estimation
Mitochondria were isolated from 2 × 107 cells using the Pierce Mitochondria isolation kit, as described previously [2]. Then, 2μg of isolated mitochondrial protein was adjusted with EPR buffer plus 50 μl of spin-trap stock solution consisting of CMH (20 μM in DPBS +25 μM desferrioxamine; Calbiochem, La Jolla, CA) to achieve equal protein content and CMH concentration. The samples were then incubated for 30 min on ice then pelleted. The supernatant (35μl) was then loaded into a 50-μl capillary tube and analyzed for superoxide generation as described above.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 4.01 for Windows (GraphPad Software). The mean ± SEM were calculated for all samples. Values lying >2 S.D. from the mean were not used for further analysis. Statistical significance was determined either by the unpaired t-test (for 2 groups) or ANOVA (for ≥3 groups) with Newman-Keuls post-hoc testing. A value of p<0.05 was considered significant.
RESULTS
Acetyl L-carnitine (ALC) prevents ADMA-mediated mitochondrial dysfunction and GCH1 degradation in pulmonary arterial endothelial cells
To examine the effect of ALC on mitochondrial function, we initially carried out a dose-response analysis by pre-treating PAEC with ALC (0–1mM, 2h) followed by exposure to ADMA (5μM, 2h). Our data indicate that ADMA exposure produces a significant increase in mitochondrial ROS generation (Fig. 1A), indicating an increase in oxidative stress within the mitochondria and this is dose-dependently attenuated by ALC supplementation with a maximum effect observed at 100μM ALC (Fig. 1A). The mitochondrial ROS is likely superoxide as the addition of PEG-SOD attenuates the ADMA-mediated increase in MitoSOX fluorescence (Fig. 1A). The increase in ROS induced by ADMA was further confirmed to be superoxide generation using EPR analysis on isolated mitochondria (Fig. 1B). We also found that ALC supplementation significantly attenuated the ADMA-mediated disruption of the mitochondrial membrane potential (Fig. 1C). Western blot analyses also demonstrated that ALC supplementation prevented both the ADMA-mediated decrease in SOD2 protein (Fig. 1D) and the increase in UCP2 protein levels (Fig. 1E). As the loss of SOD2 [1, 26, 27] and increase in UCP-2 [1, 28, 29] have been shown to be markers of mitochondrial dysfunction, ALC mitigates the mitochondrial dysfunction associated with ADMA exposure. We also found that ALC preserved ATP levels in PAEC exposed to ADMA (Fig. 1F). Overall these data suggest that ALC supplementation preserves mitochondrial function in ADMA-challenged PAEC.
Figure 1. Acetyl L-carnitine prevents the ADMA-mediated mitochondrial dysfunction and decreases in GTP cyclohydrolase I in pulmonary arterial endothelial cells.




PAEC were pretreated with acetyl-L-carnitine (ALC, 0–1mM) for 2h then exposed to ADMA (5μM, 2h). The effect on mitochondrial function was then determined by examining the effect on mitochondrial ROS levels, using MitoSOX red fluorescence. Representative images are shown. ADMA causes a significant increase in MitoSOX fluorescence (A) that is dose-dependently decreased in the presence of ALC (A) or when PAEC were preincubated with PEG-SOD (100U/ml, A). To confirm the MitoSOX data, mitochondrial fractions were prepared and superoxide levels were determined by EPR. ADMA increases mitochondrial superoxide levels in PAEC and ALC abolishes this increase (B). ADMA also caused a disruption of the mitochondrial membrane potential as estimated using the DePsipher compound and fluorescence microscopy (C). ALC pre-treatment preserved the mitochondrial membrane potential (C). Western blot analysis also revealed that ADMA decreased the levels of SOD2 (D) and increased the levels of UCP2 (E). Again ALC pre-treatment prevented these changes (D & E). Cumulatively, the mitochondrial dysfunction induced by ADMA led to a significant decrease in cellular ATP levels (F). The decrease in ATP levels was prevented by ALC (F). In addition protein extracts (G) and mRNA (H) were prepared from cells were treated with ALC (100μM) and ADMA and subjected to Western blot analysis and qRT-PCR respectively. A representative image is shown of the Western blot using an antibody specific for GCH1 and loading normalized by reprobing the membranes with an antibody specific to β-actin (G). ALC prevents the ADMA mediated decrease in GCH1 protein levels (G). ADMA either alone or in combination with ALC has no effect of GCH1 mRNA levels although ALC alone significantly increased GCH mRNA levels (H). Cells were incubated with ADMA for 2h then ALC was added (100μM, 0–2h post-ADMA exposure). Post-exposure with ALC preserves GCH1 protein levels (I). Further, increasing the redox scavenging potential of the cells by adding PEG-SOD (100U/ml) or PEG-catalase (100U/ml) prevents the ADMA-mediated decrease in GCH1 protein levels (J). Values are means ± SEM, n=8–10; *P<0.05 vs. untreated, † P<0.05 vs. ADMA alone: ‡ P<0.05 vs. previous dose.
Western blot analysis also demonstrated that GCH1 protein levels were reduced by ~50% by ADMA treatment (Fig. 1G) while the pretreatment of cells with ALC, significantly attenuated this ADMA-induced GCH1 protein reduction (Fig. 1H). Neither ADMA treatment nor pretreatment of PAEC with ALC, followed by ADMA-exposure altered GCH1 mRNA levels compared to untreated cells (Fig. 1H). GCH1 mRNA levels were significantly increased in cells exposed to ALC alone (Fig. 1H), even though GCH1 protein levels were not increased (Fig. 1G). To determine if there was a therapeutic window for ALC supplementation, we added ALC (100μM) after the addition of ADMA and determined the effect on GCH1 protein levels. Our data indicate that the addition of ALC post-ADMA is able to preserve GCH1 protein levels (Fig. 1I). Further, the ADMA-mediated decrease in GCH1 also appears to require increases in superoxide and hydrogen peroxide as both the addition of PEG-SOD or PEG-catalase, either alone or in combination, preserves GCH1 protein levels in ADMA-challenged PAEC (Fig. 1J).
ALC attenuates the ADMA-induced disruption of the Hsp90-GCH1 complex and the ubiquitination and proteasomal degradation of GCH1 in pulmonary arterial endothelial cells
Our recent studies have demonstrated that GCH1 is a client protein for Hsp90 while ADMA disrupts this complex in vitro and in vivo [30]. Consistent with these data we found that ADMA disrupted the complex of GCH1 with Hsp90 in PAEC (Fig. 2A) while ALC prevented the disruption of this complex (Fig. 2A). Hsp90 client proteins are usually brought into the complex with Hsp90 through a multi-protein Hsp90/Hsp70-based chaperone machinery [31]. Further, Hsp70 appears to be involved in both folding and degradation of Hsp90-client proteins [32]. ADMA exposure significantly increased the interaction of GCH1 with Hsp70 (Fig. 2B) while ALC attenuated this interaction (Fig. 2B). This ADMA-mediated decrease in Hsp90 function is likely due to the decrease in cellular ATP levels (Fig. 1E) as Hsp90 is ATP dependent. We have recently identified CHIP as the ubiquitin ligase that is responsible for the ADMA-mediated degradation of GCH1 via the ubiquitin-proteasome pathway [30] and our data indicate that ADMA increased the interaction of GCH1 with CHIP (Fig. 2C) while ALC attenuated this interaction (Fig. 2C). Our data also demonstrate that exposure of PAEC to ADMA significantly increased the ubiquitination of GCH1 (Fig. 2D) and again ALC attenuated this effect (Fig. 2D). To confirm the role of the proteasomal pathway in ADMA-mediated GCH1 degradation in PAEC, cells were pretreated with proteasomal inhibitor, lacacystin (20μM) followed by exposure to ADMA. Our data indicate that proteasome inhibition attenuates the ADMA-induced decrease in GCH1 protein levels (Fig. 2E). Our data also demonstrate that the decrease in GCH1 protein levels induced by ADMA correlates with a decrease in GCH1 activity (Fig. 2F). ALC (Fig. 2F) or lactacystin (Fig. 2F) preserved GCH1 activity. In addition, to determine if the protective effect of ALC was through an attenuation of the activity of the proteasome itself, we measured 20s proteasome activity in PAEC pretreated with ALC in the presence and absence of ADMA. Our data indicate that neither ADMA nor ALC alters proteasome activity in PAEC (Fig. 2G). Similarly, the addition of PEG-SOD or PEG-catalase, either alone or in combination, had no affect on 20s proteasome activity (Fig. 2G).
Figure 2. Acetyl L-carnitine prevents the ADMA-induced decrease in GCH1-Hsp90 interactions and GCH1 ubiquitination in pulmonary arterial endothelial cells.


PAEC were pretreated with ALC (100μM, 2h) prior to ADMA exposure (5μM, 2h). Whole cell lysates (1mg) were then subjected to immunoprecipitation (IP) using antibodies specific to Hsp90 (A), Hsp70 (B), or CHIP (C) then analyzed by Western blot (IB) analysis using a specific antiserum raised against GCH1. A representative image is shown for each Western blot. Blots were stripped and reprobed for Hsp90, Hsp70, or CHIP to normalize for the immunoprecipitation efficiency. Densitometric values were then obtained for each. ADMA significantly reduces the interaction of GCH1 with Hsp90 and significantly enhances its interaction with Hsp70 and CHIP. ALC attenuates the ADMA-induced decrease in Hsp90-GCH1 and the increase in Hsp70-GCH1 and CHIP-GCH1. In addition, cell lysates (1mg) were subjected to ubiquitinated protein enrichment (AP) followed by Western blot using specific antiserum raised against GCH1 (IB). A representative image is shown. ADMA significantly increases the ubiquitination of GCH1 and this is attenuated in the presence of ALC (D). PAEC were also incubated with ADMA for 2h in the presence or absence of the proteasome inhibitor, lactacystin (20 μM). GCH1 protein levels were determined by Western blotting with loading normalized by reprobing the membranes with an antibody specific to β-actin (E). Proteasomal inhibition prevents the decrease in GCH1 induced by ADMA (E). ALC and lactacystin also attenuate the ADMA-mediated decrease in GCH1 activity (F). ALC, ADMA, PEG-SOD, or PEG-catalase either alone or in combination had no affect on PAEC 20s proteasome activity (G). Values are mean ± SEM, n=3–6. *P<0.05 vs. untreated; † P<0.05 vs. ADMA alone.
Role of Hsp90, Hsp70 and CHIP in ADMA-induced GCH1 degradation in pulmonary arterial endothelial cells
To further dissect the cause and affect relationships between Hsp90, Hsp70 and CHIP in ADMA-mediated GCH1 degradation, we carried out a series of studies in which we either over-expressed a dominant negative Hsp90 (DNHsp90), or reduced Hsp70 or CHIP levels using specific siRNAs. Our results indicate that DN Hsp90 over-expression alone (Fig. 3A) was sufficient to decrease GCH1-Hsp90 interactions (Fig. 4A), increase the interactions of GCH1 with both Hsp70 (Fig. 4B) and CHIP (Fig. 4C), and increase the ubiquitination of GCH1 (Fig. 4D). Together these changes resulted in a decrease in GCH1 protein levels (Fig. 4E). The over-expression of DNHsp90 increased Hsp70 protein levels in PAEC as expected (Fig. 3B) but did not alter CHIP protein levels (Fig. 3C). These results confirm the key role played by Hsp90 in maintaining GCH1 protein levels in PAEC. We also found that depletion of Hsp70 (Fig. 5A) attenuated the ADMA-mediated increase in the interaction of GCH1 with CHIP (Fig. 6A) and the increase in GCH1 ubiquitination (Fig. 6B). Hsp70 depletion also prevented the ADMA-mediated decrease in GCH1 protein levels (Fig. 6C). Depletion of Hsp70 did not alter Hsp90 (Fig. 5B) or CHIP protein levels (Fig. 5C). Further, our data indicate that the depletion of CHIP (Fig. 7A) does not prevent the ADMA-mediated increase in Hsp70-GCH1 interactions (Fig. 8A). However depletion of CHIP attenuates both the increase in GCH1 ubiquitination (Fig. 8B) and the ADMA-mediated decrease in GCH1 protein levels (Fig. 8C). Depletion of CHIP did not alter Hsp90 (Fig. 7B) or Hsp70 protein levels (Fig. 7C). Collectively, these findings delineate the temporal series of events induced by ADMA that results in GCH1 ubiquitination and degradation: ADMA initially causes a reduction in Hsp90 activity that reduces its interaction with GCH1, Hsp70 is then recruited and this allows CHIP to interact with the complex. GCH1 is then ubiquitinated via CHIP and targeted for proteasomal degradation.
Figure 3. Over-expression of a dominant negative Hs90 in pulmonary arterial endothelial cells.
PAEC were transiently transfected with a DN Hsp90 or pcDNA3 (as a control). Forty-eight hours after transfection, whole cell lysates (20μg) were prepared and subjected to Western blot analysis using specific antisera raised against Hsp90 (A), Hsp70 (B), and CHIP (C) with loading normalized by reprobing the membranes with an antibody specific to β-actin. A representative image is shown for each. Transfection of DN Hsp90 increases Hsp90 protein levels by ~2-fold (A). Similarly, Hsp70 levels are also significantly increased (B). However, CHIP protein levels are unaffected (C). Values are means ± SEM, n=4–6. *P < 0.05 vs. pcDNA3 transfection.
Figure 4. Inhibition of Hsp90 alone is sufficient to stimulate GCH1 ubiquitination and degradation in pulmonary arterial endothelial cells.
PAEC were transiently transfected with a DN Hsp90 or pcDNA3 (as a control). Forty-eight hours after transfection, whole cell lysates (1mg) were subjected to immunoprecipitation (IP) using antibodies specific to Hsp90 (A), Hsp70 (B) or CHIP (C) then analyzed by Western blot (IB) analysis using an anti-GCH1 antibody. Blots were stripped and reprobed with the appropriate IP antibody to normalize for immunoprecipitation efficiency. Representative images are shown for each. The over-expression of DN Hsp90 decreases GCH1-Hsp90 interactions (A) but increases both GCH1-Hsp70 (B) and GCH1-CHIP (C) interactions. Ubiquitinated protein enrichment (AP) followed by Western blot with an anti-GCH1 antibody was also used to measure GCH1 ubquitination (IB). A representative image is shown (D). There is a significant increase in ubquitinated GCH1 (D). Whole cell lysates (20μg) were also subjected to Western blot analysis using a specific antiserum raised against GCH1 with loading normalized by reprobing the membranes with an antibody specific to β-actin. A representative image is shown along with the densitometric analysis indicating that DN Hsp90 over-expression significantly decreased GCH1 protein levels (E). Values are means ± SEM, n=4–6. *P < 0.05 vs. pcDNA3 transfection.
Figure 8. Depletion of CHIP attenuates ADMA-induced GCH1 degradation but not its interaction with Hsp70 in pulmonary arterial endothelial cells.
PAEC were transiently transfected with a siRNA for CHIP or a control siRNA. Forty-eight hours after transfection, whole cell lysates (1mg) were subjected to immunoprecipitation (IP) or ubiquitinated protein enrichment (AP) using an antibody specific to Hsp70 then analyzed by Western blot (IB) analysis using an anti-GCH1 antibody. The IP blots were stripped and reprobed for Hsp70 to normalize for the immunoprecipitation efficiency. A representative image is shown for each Western blot. Depleting CHIP protein levels did not prevent the ADMA-induced interaction of GCH1 with Hsp70 (A). However, the ADMA-mediated increase in GCH1 ubiquitination was blocked (B). Whole cell lysates (20μg) were also subjected to Western blot analysis using a specific antiserum raised against GCH1, loading was normalized by reprobing the membranes with an antibody specific to β-actin. A representative image is shown along with the densitometric analysis indicating that that reducing CHIP protein levels preserves GCH1 protein levels in PAEC challenged with ADMA (C). Values are means ± SEM, n=6. *P < 0.05 vs. control siRNA. † P<0.05 vs. CHIP siRNA without ADMA treatment.
ALC prevents the ADMA-mediated increase in NOS uncoupling in pulmonary arterial endothelial cells
Consistent with our data indicating that ADMA decreases while ALC supplementation preserves GCH1 protein levels, we also found that ADMA significantly decreased BH4 levels in PAEC (Fig. 9A) while ALC pretreatment completely attenuated this decrease (Fig. 9A). To determine the effect of these changes in BH4 levels on NO signaling, we measured shear-induced (20 dyn/cm2, 15 min) changes in NOx and NOS-derived superoxide levels in the presence or absence of ADMA and ALC. ADMA exposure increased eNOS uncoupling as determined by a significant decrease in NO generation (Fig. 9B) coupled with a significant increase in NOS-derived superoxide levels (Fig. 9C). Again ALC preserved NO signaling (Fig. 9B) and significantly attenuated ADMA-mediated increase in NOS-derived superoxide (Fig. 9C).
Figure 9. Acetyl L-carnitine attenuates the ADMA mediated decrease in tetrahydrobiopterin levels and increased eNOS uncoupling in pulmonary arterial endothelial cells.

PAEC were pretreated with ALC (100μM, 2h) prior to ADMA exposure (5μM) and the effect on cellular BH4 levels determined. The presence of ALC attenuated the ADMA-mediated and BH4 levels (A). In addition, ALC prevented the decrease in NO generation in response to shear stress (20 dyn/cm2, 15 min) mediated by ADMA (B) and decreased the ADMA-mediated increases in eNOS-derived superoxide levels (C). Values are mean ± SEM, n=6. *P < 0.05 vs. untreated cells; † P<0.05 vs. ADMA alone.
L-Carnitine supplementation prevents GCH1 proteasomal degradation in a lamb model of congenital heart disease and increased pulmonary blood flow
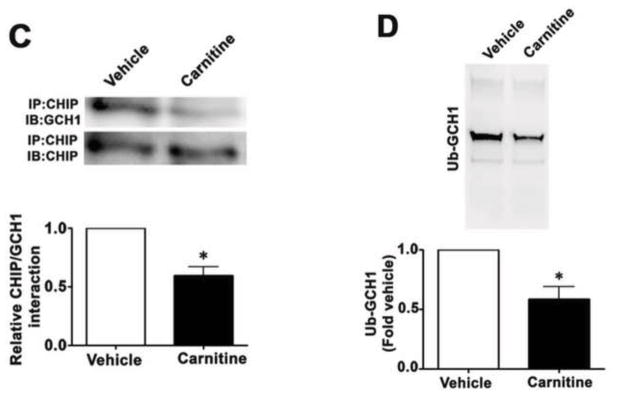
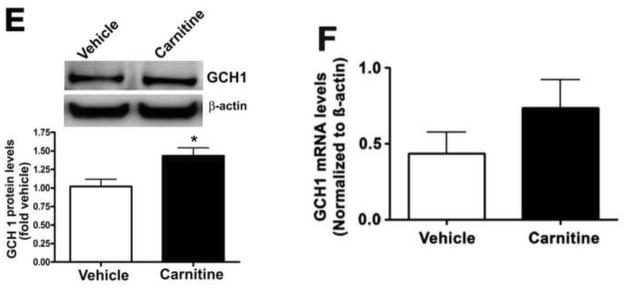
Our previous studies have demonstrated that carnitine homeostasis is disrupted [1] and BH4 levels are decreased [5] in our shunt model of increased pulmonary blood flow and this correlates with a decrease in NO signaling, decreased Hsp90 activity, and eNOS uncoupling [23]. To test the effects of L-carnitine supplementation on these events, Shunt lambs with exposed to oral L-carnitine (100mg/kg/day) or vehicle for 4-weeks. We then determined the effect on the association of GCH1 with Hsp90, Hsp70, and CHIP, as well as the levels of GCH1 ubiquitination. Our data demonstrate that the interactions of GCH1 with Hsp90 is enhanced in Shunt lambs treated with L-carnitine (Fig. 10A) while Hsp70/GCH1 (Fig. 10B) and CHIP/GCH1 (Fig. 10C) interactions are significantly decreased in Shunt lambs treated with L-carnitine. Consistent with these findings, GCH1 ubiquitination is significantly decreased in the L-carnitine treated Shunt lambs (Fig. 10D). Further, GCH1 protein levels are increased in the L-carnitine treated Shunt lambs (Fig. 10E) although GCH1 mRNA levels were unchanged (Fig. 10F) indicative of reduced GCH1 protein degradation.
Figure 10. L-carnitine supplementation preserved GCH1-Hsp90 interactions in a lamb model of increased pulmonary blood flow.

Protein extracts prepared from peripheral lung of L-carnitine (carnitine) or vehicle treated Shunt lambs were subjected to immunoprecipitation (IP) using antibodies specific to Hsp90, Hsp70, or CHIP then analyzed by Western blot (IB) analysis using a specific antiserum raised against GCH1. In addition, tissue lysates were also subjected to ubiquitinated protein enrichment (AP) followed by Western blot with an anti-GCH1 antibody (IB). A representative image is shown for each. The levels of GCH1 associated with Hsp90 (A) is significantly increased in L-carnitine treated Shunt lambs while the interaction of GCH1 with Hsp70 (B) and CHIP (C) are significantly decreased. There is also a significant decrease in ubquitinated GCH1 in carnitine treated Shunt lambs (D). Densitomeric values are mean ± SEM, n=5. *P < 0.05 compared with age-matched vehicle control Shunt lambs. GCH1 protein and mRNA were also determined by Western blot analysis or qRT-PCR respectively. The Western blot analysis was performed using an antibody raised against GCH1 with loading normalized by reprobing the membranes with an antibody specific to β-actin. A representative image is shown (E). GCH1 protein levels are significantly increased in Lcarnitine treated Shunt lambs (E). However, GCH1 mRNA levels are unchanged (F). Values expressed are mean ± SEM, n=5; *P<0.05 vs. Vehicle treated Shunt lambs.
L-Carnitine maintains pulmonary BH4 levels and preserves NO signaling in a lamb model of congenital heart disease and increased pulmonary blood flow
We next determined if the preservation of GCH1 protein levels led to a concomitant increase in BH4 levels. Our data indicate that Shunt lambs treated with L-carnitine had significantly higher lung tissue BH4 levels (Fig. 11A). Finally, we determined the effect of L-carnitine treatment on NO signaling. We identified a significant increase in NO signaling, as determined by enhanced tissue NOx levels (Fig. 11B) and this correlated with a significant decrease in NOS-derived superoxide levels (Fig. 11C) indicating that L-carnitine increases eNOS coupling.
Figure 11. L-Carnitine supplementation protects tetrahydrobiopterin levels and enhances NOS coupling in lambs with increased pulmonary blood flow.

BH4 levels in the peripheral lung of L-carnitine or vehicle treated Shunt lambs were measured by HPLC. BH4 levels are significantly increased after carnitine treatment (A). In addition, L-carnitine enhances tissue NOx levels (B) and decreases NOS-derived superoxide (C) indicative of enhanced eNOS coupling. Values expressed are mean ± SEM, n=5; *P<0.05 vs. Vehicle treated Shunt lambs.
DISCUSSION
Mitochondria are the source of superoxide anion radicals and hydrogen peroxide under physiologic and pathologic conditions. The increased production of reactive oxygen species from the mitochondria can be deleterious to the cell due to their ability to induce lipid peroxidation, protein oxidation, and DNA damage [33, 34]. Accumulating evidence suggests that mitochondrial dysfunction is associated with pulmonary hypertension [35, 36]. L-carnitine plays an important role in maintaining the balance of acyl-CoA-related functions in mitochondria and can be used pharmacologically to modulate mitochondrial dysfunction (reviewed in [4]). In this study, we found that ALC significantly attenuated the decrease in GCH1 protein levels caused by exposure to ADMA. Our data indicate that ALC preserves GCH1 protein levels by attenuating ADMA-mediated GCH1 degradation. Interestingly, we also found that ALC treatment alone significantly increased GCH1 mRNA levels although GCH1 protein levels were not significantly increased. One possible explanation is that, ALC itself may lead to some level of cellular stress [37]. Indeed i.p. injection of L-carnitine is associated with a localized inflammatory macrophage activation in the peritoneal cavity [38], and GCH1 mRNA levels can been strongly elevated in cells treated with inflammatory stimuli [39]. The fact that we did not see changes in GCH1 protein levels that mirrored the changes in mRNA may be due to the fact that our 2h study did not allow sufficient time for significant increases in GCH1 translation to occur. However, further investigations will be needed to clarify the possible regulatory mechanisms of L-carnitine on GCH1 gene and protein expression. Further, from our studies it appears that ALC had multiple affects on the cell including both ubiquitination and transcription of GCH1 as well as modifying both mitochondrial function and mitochondrion-derived superoxide. However, we speculate that the cardinal event of ALC is the preservation of mitochondrial function and that all downstream protective measures stem from this. Based on the work of Ames’ group [40–42] it is likely that ALC preserves the acetyl-CoA:Acyl-CoA balance in the mitochondria that is disrupted by ADMA. As increases in Acyl-CoA are known to decrease ATP generation the preservation of acetyl-CoA levels will preserve ATP levels. This in turn will maintain Hsp90 in an active form and preserve the Hsp90-GCH1 complex. This in turn will prevent hsp70 and CHIP recruitment and GCH1 ubiquitination and degradation.
Hsp90 has been shown to interact with a number of proteins that are required for efficient NO biosynthesis, including eNOS [43] and soluble guanylate cyclase [43]. Further, the chaperone activity of Hsp90 is ATP dependent. Our previous studies demonstrate that ADMA can have an indirect effect on eNOS coupling through its ability to cause mitochondrial dysfunction and a decrease in Hsp90 chaperone activity [2]. Our recent data have shown that GCH1 is a client protein of Hsp90 and that Hsp90-GCH1 interactions are disrupted in ADMA-exposed cells [30] and here we found that ALC administration during ADMA exposure preserved mitochondrial function and cellular ATP levels and prevented the ADMA-induced disruption of Hsp90-GCH1 complex. These findings suggest that, under the oxidative stress conditions induced by high levels of ADMA, L-carnitine can regulate Hsp90 chaperone activity by maintaining mitochondrial function and so favor the production of NO signaling molecules. Increasing evidence suggests that Hsp90-client proteins bind to Hsp70 before degradation and our recent study demonstrated that the C-terminus of Hsp70-interacting protein (CHIP) targets the proteasomal degradation of GCH1 [30]. Here, we expand on this finding by demonstrating that GCH1 is degraded via a signaling pathway that initially involves Hsp90 inhibition followed by the sequential recruitment of Hsp70 and CHIP which leads to GCH1 ubiquitination and protein degradation. ALC was able to significantly prevent these events and preserve the interaction of GCH1 with Hsp90. Recent studies from the laboratories of Zou [44] and [16, 45] and Vasquez-Vivar [46] demonstrate that ubiquitinated GCH1 levels in endothelial cells were significantly elevated under conditions of oxidative stress, which led to GCH1 degradation. Our data add to these prior studies by demonstrating that maintaining mitochondrial function through the administration of ALC significantly attenuates the GCH1 ubiquitination induced by ADMA. Further, enhancing the removal of superoxide (by the addition of PEG-SOD) or hydrogen peroxide (by the addition of PEG-catalase) also preserves GCH1 protein levels in ADMA challenged PAEC suggesting that oxidative stress is also involved in the degradation process.
Increasing evidence indicates that optimal concentrations of BH4 is of fundamental importance for the normal function of eNOS in vascular endothelial cells and endothelial BH4 bioavailability has emerged as a rationale therapeutic target in a number of vascular disease states [47]. Studies indicate that supplementation with BH4 or by increasing BH4 synthesis using adenoviral gene delivery of GCH1 can restore BH4 levels and normalize eNOS function [47]. Our results indicate that ALC preserved BH4 levels in PAEC exposed to ADMA. Furthermore, the preservation of BH4 levels was sufficient to prevent the increase in eNOS uncoupling that we have previously demonstrated in PAEC exposed to ADMA. Together, these data suggest that increasing and/or sustaining BH4 levels may be dominant over the uncoupling of eNOS associated with increases in ADMA [2]. Given the increasing number of studies that have shown an association of increases in ADMA with cardiovascular risk, enhancing BH4 levels may have therapeutic utility and L-carnitine supplementation is a potentially useful agent.
We also extended our cell culture studies to a clinically relevant lamb model that mimics a congenital heart defect (CHD) that result in increased pulmonary blood flow (Shunt). Children with these types of CHD develop early and progressive alterations in pulmonary vascular function that cause significant morbidity [8]. The mechanisms involved in this pulmonary vascular disease are not fully understood. However, our previous studies using our Shunt model have shown that there is a progressive decline in NO signaling that leads to the development of endothelial dysfunction [23]. This decline in bioavailable NO corresponds to a complex series of temporal events that involves the disruption of carnitine homeostasis and the development of mitochondrial dysfunction [1], loss of Hsp90 chaperone activity [1] and increasing in eNOS uncoupling [23] related, at least in part, to a reduction in BH4 levels [5]. Here, we show that ALC supplementation preserves Hsp90 chaperone activity and its interaction with GCH1 interaction, which suppressed the Hsp70 and CHIP-dependent GCH1 ubiquitination and degradation of GCH1 and, as a result, these alterations led to an increase in BH4 levels. Subsequently, preserved NO signaling and reduced eNOS-uncoupling (Fig. 12). As one of the most important consequences of endothelial injury is a decrease in bioavailable NO, with subsequent endothelial dysfunction, our findings that ALC preserves NO signaling could lead to a new effective therapy for children born with CHD and increased pulmonary blood flow.
Figure 12. A possible mechanism by which ADMA disrupts mitochondrial function and attenuates NO signaling in lambs with increased pulmonary blood flow.

ADMA-induces mitochondrial dysfunction reducing cellular ATP levels. As the activity of Hsp90 is ATP-dependent, GCH1/Hsp90 interactions are disrupted. This leads to increased binding of Hsp70 to GCH1 leading to the recruitment of CHIP. CHIP then ubiquitinates GCH1 targeting it for proteasomal degradation. The resulting BH4 deficiency induces eNOS uncoupling. L-carnitine supplementation short-circuits this degradation pathway by maintaining mitochondrial function.
In summary, our data show the effects of L-carnitine on ADMA-induced BH4 deficiency both in vitro and in vivo and suggest that the protective effects of L-carnitine on mitochondrial function could be an effective preventive therapy against the loss of NO signaling during the development of pulmonary hypertension. Our study provides a novel insight regarding the molecular basis for the beneficial potential of L-carnitine through its ability to preserve BH4 biosynthesis in cardiovascular disease states. Our results also reinforce the concept that preventing or attenuating the development of mitochondrial dysfunction as a therapeutic approach for the treatment of a number of cardiovascular disease states including pulmonary hypertension.
Highlights.
Acetyl L-carnitine attenuated the ADMA-mediated proteasomal degradation of GCH1.
This prevented the decrease in BH4 levels.
Treatment of Shunt lambs with L-carnitine also reduced the degradation of GCH1.
This increased BH4 levels compared to vehicle treated Shunt lambs.
The increases in BH4 enhanced NO generation in Shunt lambs.
Acknowledgments
This research was supported in part by grants, HL60190 (to SMB), HL67841 (to SMB), HL084739 (to SMB), R21HD057406 (to SMB), and HL61284 (to JRF) all from the National Institutes of Health, by a grant from the Fondation Leducq (to SMB and JRF), 09BGIA2310050 from the Southeast Affiliates of the American Heart Association (to SS), and Cardiovascular Discovery Institute Seed Awards (to SS and SK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harmon C, Oishi P, Fineman JR, Black SM. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L46–56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol. 2008;294:C1407–1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zammit VA, Ramsay RR, Bonomini M, Arduini A. Carnitine, mitochondrial function and therapy. Adv Drug Deliv Rev. 2009;61:1353–1362. doi: 10.1016/j.addr.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 6.Swick L, Kapatos G. A yeast 2-hybrid analysis of human GTP cyclohydrolase I protein interactions. J Neurochem. 2006;97:1447–1455. doi: 10.1111/j.1471-4159.2006.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calo LA, Semplicini A, Davis PA. Antioxidant and antiinflammatory effect of carvedilol in mononuclear cells of hypertensive patients. Am J Med. 2005;118:201–202. doi: 10.1016/j.amjmed.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts. A model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation. 1995;92:606–613. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- 9.Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts: a model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation. 1995;92:1–8. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- 10.Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol. 2004;286:L984–991. doi: 10.1152/ajplung.00224.2003. [DOI] [PubMed] [Google Scholar]
- 11.Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem Biophys Res Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2007;292:L165–177. doi: 10.1152/ajplung.00459.2005. [DOI] [PubMed] [Google Scholar]
- 13.Miao RQ, Fontana J, Fulton D, Lin MI, Harrison KD, Sessa WC. Dominant-negative Hsp90 reduces VEGF-stimulated nitric oxide release and migration in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:105–111. doi: 10.1161/ATVBAHA.107.155499. [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Kumar S, Tian J, Black SM. Estradiol increases guanosine 5′-triphosphate cyclohydrolase expression via the nitric oxide-mediated activation of cyclic adenosine 5′-monophosphate response element binding protein. Endocrinology. 2009;150:3742–3752. doi: 10.1210/en.2008-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milstien S, Jaffe H, Kowlessur D, Bonner TI. Purification and cloning of the GTP cyclohydrolase I feedback regulatory protein, GFRP. J Biol Chem. 1996;271:19743–19751. doi: 10.1074/jbc.271.33.19743. [DOI] [PubMed] [Google Scholar]
- 16.Kalivendi S, Hatakeyama K, Whitsett J, Konorev E, Kalyanaraman B, Vasquez-Vivar J. Changes in tetrahydrobiopterin levels in endothelial cells and adult cardiomyocytes induced by LPS and hydrogen peroxide--a role for GFRP? Free Radic Biol Med. 2005;38:481–491. doi: 10.1016/j.freeradbiomed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Sun X, Sharma S, Aggarwal S, Ravi K, Fineman JR, Black SM. GTP cyclohydrolase I expression is regulated by nitric oxide: role of cyclic AMP. Am J Physiol Lung Cell Mol Physiol. 2009;297:L309–317. doi: 10.1152/ajplung.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wainwright MS, Arteaga E, Fink R, Ravi K, Chace DH, Black SM. Tetrahydrobiopterin and nitric oxide synthase dimer levels are not changed following hypoxia-ischemia in the newborn rat. Brain Res Dev Brain Res. 2005;156:183–192. doi: 10.1016/j.devbrainres.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 20.Wedgwood S, Bekker JM, Black SM. Shear stress regulation of endothelial NOS in fetal pulmonary arterial endothelial cells involves PKC. Am J Physiol Lung Cell Mol Physiol. 2001;281:L490–498. doi: 10.1152/ajplung.2001.281.2.L490. [DOI] [PubMed] [Google Scholar]
- 21.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol. 2006;291:C555–568. doi: 10.1152/ajpcell.00509.2005. [DOI] [PubMed] [Google Scholar]
- 22.Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1444–1453. doi: 10.1152/ajplung.00175.2007. [DOI] [PubMed] [Google Scholar]
- 23.Oishi PE, Wiseman DA, Sharma S, Kumar S, Hou Y, Datar SA, Azakie A, Johengen MJ, Harmon C, Fratz S, Fineman JR, Black SM. Progressive dysfunction of nitric oxide synthase in a lamb model of chronically increased pulmonary blood flow: a role for oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2008;295:L756–766. doi: 10.1152/ajplung.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dikalov SI, Li W, Mehranpour P, Wang SS, Zafari AM. Production of extracellular superoxide by human lymphoblast cell lines: comparison of electron spin resonance techniques and cytochrome C reduction assay. Biochem Pharmacol. 2007;73:972–980. doi: 10.1016/j.bcp.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Southan GJ, Szabo C, Thiemermann C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br J Pharmacol. 1995;114:510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, Puolivali J, Scearce-Levie K, Masliah E, Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang LP, Patel M. Mitochondrial oxidative stress and increased seizure susceptibility in Sod2(−/+) mice. Free Radic Biol Med. 2004;36:542–554. doi: 10.1016/j.freeradbiomed.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 28.Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005;6:829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- 29.Hoffstedt J, Folkesson R, Wahrenberg H, Wennlund A, van Harmelen V, Arner P. A marked upregulation of uncoupling protein 2 gene expression in adipose tissue of hyperthyroid subjects. Horm Metab Res. 2000;32:475–479. doi: 10.1055/s-2007-978673. [DOI] [PubMed] [Google Scholar]
- 30.Sun X, Fratz S, Sharma S, Hou Y, Rafikov R, Kumar S, Rehmani I, Tian J, Smith A, Schreiber C, Reiser J, Naumann S, Haag S, Hess J, Catravas JD, Patterson C, Fineman JR, Black SM. CHIP-Dependent GTP Cyclohydrolase I Degradation in Lambs with Increased Pulmonary Blood Flow. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2009-0467OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 32.Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem. 1997;272:9002–9010. doi: 10.1074/jbc.272.14.9002. [DOI] [PubMed] [Google Scholar]
- 33.Tribe RM, Poston L. Oxidative stress and lipids in diabetes: a role in endothelium vasodilator dysfunction? Vasc Med. 1996;1:195–206. doi: 10.1177/1358863X9600100304. [DOI] [PubMed] [Google Scholar]
- 34.Ronson RS, Nakamura M, Vinten-Johansen J. The cardiovascular effects and implications of peroxynitrite. Cardiovasc Res. 1999;44:47–59. doi: 10.1016/s0008-6363(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 35.Daicho T, Yagi T, Abe Y, Ohara M, Marunouchi T, Takeo S, Tanonaka K. Possible involvement of mitochondrial energy-producing ability in the development of right ventricular failure in monocrotaline-induced pulmonary hypertensive rats. J Pharmacol Sci. 2009;111:33–43. doi: 10.1254/jphs.08322fp. [DOI] [PubMed] [Google Scholar]
- 36.Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol. 2009;9:287–296. doi: 10.1016/j.coph.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alves E, Binienda Z, Carvalho F, Alves CJ, Fernandes E, de Lourdes Bastos M, Tavares MA, Summavielle T. Acetyl-L-carnitine provides effective in vivo neuroprotection over 3,4-methylenedioximethamphetamine-induced mitochondrial neurotoxicity in the adolescent rat brain. Neuroscience. 2009;158:514–523. doi: 10.1016/j.neuroscience.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 38.Dionyssopoulou E, Vassiliadis S, Evangeliou A, Koumantakis EE, Athanassakis I. Constitutive or induced elevated levels of L-carnitine correlate with the cytokine and cellular profile of endometriosis. J Reprod Immunol. 2005;65:159–170. doi: 10.1016/j.jri.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Linscheid P, Schaffner A, Blau N, Schoedon G. Regulation of 6-pyruvoyltetrahydropterin synthase activity and messenger RNA abundance in human vascular endothelial cells. Circulation. 1998;98:1703–1706. doi: 10.1161/01.cir.98.17.1703. [DOI] [PubMed] [Google Scholar]
- 40.Shenk JC, Liu J, Fischbach K, Xu K, Puchowicz M, Obrenovich ME, Gasimov E, Alvarez LM, Ames BN, Lamanna JC, Aliev G. The effect of acetyl-L-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer’s disease. J Neurol Sci. 2009;283:199–206. doi: 10.1016/j.jns.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009;34:755–763. doi: 10.1007/s11064-008-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aliev G, Liu J, Shenk JC, Fischbach K, Pacheco GJ, Chen SG, Obrenovich ME, Ward WF, Richardson AG, Smith MA, Gasimov E, Perry G, Ames BN. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J Cell Mol Med. 2009;13:320–333. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang GG, Shi RZ, Jiang DJ, Chen YR, Jia C, Tang ZY, Bai YP, Xiao HB, Li YJ. Involvement of the endothelial DDAH/ADMA pathway in nitroglycerin tolerance: the role of ALDH-2. Life Sci. 2008;82:699–707. doi: 10.1016/j.lfs.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J, Wang S, Wu Y, Song P, Zou MH. Tyrosine nitration of PA700 activates the 26S proteasome to induce endothelial dysfunction in mice with angiotensin II-induced hypertension. Hypertension. 2009;54:625–632. doi: 10.1161/HYPERTENSIONAHA.109.133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitsett J, Picklo MJ, Sr, Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler Thromb Vasc Biol. 2007;27:2340–2347. doi: 10.1161/ATVBAHA.107.153742. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]