Abstract
Starch is the major non-structural carbohydrate in plants. It serves as an important store of carbon that fuels plant metabolism and growth when they are unable to photosynthesise. This storage can be in leaves and other green tissues, where it is degraded during the night, or in heterotrophic tissues such as roots, seeds and tubers, where it is stored over longer time periods. Arabidopsis accumulates starch in many of its tissues, but mostly in its leaves during the day. It has proven to be a powerful genetic system for discovering how starch is synthesised and degraded, and new proteins and processes have been discovered. Such work has major significance for our starch crops, whose yield and quality could be improved by the application of this knowledge. Research into Arabidopsis starch metabolism has begun to reveal how its daily turnover is integrated into the rest of metabolism and adapted to the environmental conditions. Furthermore, Arabidopsis mutant lines deficient in starch metabolism have been employed as tools to study other biological processes ranging from sugar sensing to gravitropism and flowering time control. This review gives a detailed account of the use of Arabidopsis to study starch metabolism. It describes the major discoveries made and presents an overview of our understanding today, together with some as-yet unresolved questions.
1.INTRODUCTION
Starch is a storage carbohydrate widely synthesised in the plant kingdom and is composed of homopolymers of glucose. In Arabidopsis, as in most vascular plants, starch plays an important role in the day-to-day carbohydrate metabolism of the leaf. It is one of the primary products of photosynthesis in the chloroplast and serves to buffer the changing availability of photosynthates resulting from the diurnal cycle of light and dark. The starch accumulated during the day is degraded during the subsequent night, providing a continued supply of carbohydrate in the absence of photosynthesis. Thus, leaf starch can be seen as a short-term carbohydrate reservoir and is often termed ‘transitory starch’. The partitioning of photoassimilates into transitory starch varies from species to species. Arabidopsis partitions up to half of its assimilates into the starch pool (Zeeman and Ap Rees, 1999). This is important for normal growth in a diurnal cycle and is finely controlled to suit the growth conditions (Gibon et al., 2009). In order to understand how photoassimilates are used for plant growth and metabolism, it is essential to consider the fluxes into and out of the starch pool (Smith and Stitt, 2007; Wiese et al., 2007).
Starch also accumulates in non-photosynthetic tissues of plants, such as seeds, roots and tubers. This so-called “storage starch” is deposited in amyloplasts of heterotrophic cells serving as a medium- to long-term energy source to fuel growth processes upon germination or sprouting. In Arabidopsis, many nonphotosynthetic cell types also synthesise starch, but there are no major starch storage organs comparable to the roots of cassava or tubers of potato. Starch does accumulate transiently in developing Arabidopsis seeds, but the primary carbon store in wildtype seeds is lipid (Andriotis et al., 2010; Li-Beisson et al., 2010) and starch is almost absent at seed maturity. Nevertheless, Arabidopsis has proven to be a valuable system for studying starch metabolism. Genome studies reveal a high level of conservation in genes involved in starch metabolism between Arabidopsis and distantly related species such as cereals (Ball and Morell, 2003; Patron and Keeling, 2005), and much of what we have learned about the metabolism of transitory starch is relevant for understanding the metabolism of storage starches.
It is important to emphasise how vital storage starch is for our civilization. On average, about 50% of our daily calories are received from starch. Most is gained from the cereal crops (e.g. maize, wheat, rice) and from root or tuberous crops (e.g. cassava, potato). Additionally, a percentage of the starch produced by our crops is extracted (predicted to reach 75 million tons in 2012, International Starch Institute; http://www.starch.dk/) and used in many industrial applications (e.g. paper-, textile-, and pharmaceutical- industries). Recently, starch crops have become more important in economic terms due to their use as raw material to produce bioethanol. Given the importance of starch, it is surprising that we still do not fully understand its precise molecular structure, how it is made, and what determines the amount plants accumulate. The mapping of naturally occurring mutations that change starch content and/or properties in crops (such as maize, rice, and pea) identified the first genes coding for enzymes important in starch synthesis (e.g. Bhattacharyya et al., 1990; James et al., 1995; Gao et al., 1998). Now, such questions are also being addressed in Arabidopsis and other model systems such as Chlamydomonas reinhardtii. One of the obvious drawbacks of these model systems is that functional studies of starch properties can be difficult given the small amounts of starches they yield. Nevertheless, discoveries from these systems are extremely promising and can be readily tested in crop plants through directed breeding and biotechnological approaches. The future production of crop plants with increased starch content will help to offset the pressure on agriculture resulting from the increased use of starch crops for non-food purposes, while novel varieties could contain ‘customized starches’ specifically suited to industrial needs (Kossmann and Lloyd, 2000; Burrell, 2003; Zeeman et al., 2010; Santelia and Zeeman, 2011).
2. THE USE OF ARABIDOPSIS MUTANTS AS A TOOL TO STUDY STARCH BIOSYNTHESIS
Research into starch metabolism in Arabidopsis leaves began in the mid-1980s, with the first genetic screens for plants with altered starch levels (Caspar et al., 1985). These studies, together with subsequent functional genomics approaches, have been instrumental in confirming the pathway of starch synthesis, in elucidating the pathway of starch breakdown, and in studying the factors that determine starch structure and properties (Zeeman et al., 2007).
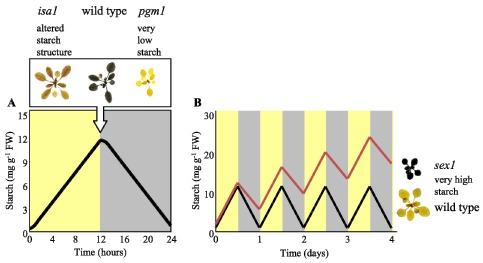
The classical approach to screen for mutations affecting starch content involved the production of randomly mutagenized populations via irradiation (e.g. X-rays or fast neutrons) or chemical mutagens (e.g. ethylmethylsulfonate). In the second generation (M2), screens for recessive, starch-related phenotypes were performed on individual plants by staining detached, ethanol-decolourised leaves with an iodine/potassium iodide solution. Iodine stains starch because the iodine molecules align within the secondary structures adopted by the glucose polymer chains (i.e. single and double helices; see Section 3 below). Although iodine staining is not quantitative, comparison with wild-type plants at specific times during the diurnal cycle allows the identification of plants with less starch or more starch than normal (Caspar et al., 1985; 1991; Figure 1A). It is also possible to use iodine staining to detect plants with altered starch structure, as this can affect the absorption spectrum of the iodine/starch complex (Zeeman et al., 1998b; Figure 1A).
Figure 1.
Identification of mutants affected in starch metabolism by iodine staining.
(A) Pattern of starch accumulation in wild-type Arabidopsis during the day (yellow) and night (grey). Inset above; images of a wild-type plant, a low-starch mutant (pgm1 - lacking plastidial phosphoglucomutase) and an altered starch structure mutant (isa1 - lacking isoamylase 1) harvested at the end of the day, decolourised in hot ethanol and iodine-stained.
(B) A scheme to illustrate the development of a starch-excess phenotype based on a decreased rate of night-time degradation. Starch synthesis during the day and degradation at night are balanced in the wild type (black line). In the starch excess line, more starch is made during the day than is broken down at night (red line). Inset right; images of a wild-type plant and a starch-excess mutant (sex1 - lacking glucan, water dikinase), harvested at the end of the night and iodine-stained, as in A.
Iodine staining at the end of the light phase, when starch content in the wild type reaches its peak, was used to isolate several Arabidopsis lines with greatly decreased starch levels (Figure 1A). These lines carry mutations in one of four genes encoding plastidial enzymes linking the Calvin cycle and starch biosynthesis (see Table 1 and Section 4.2, below). Mutants deficient in some of these enzymes have also been identified in other species such as tobacco (Hanson and McHale, 1988), pea (Harrison et al., 1998), Mesembryanthemum crystallinum (Cushman et al., 2008), Chlamydomonas reinhardtii (Ball et al., 1991), Lotus japonicus (Vriet et al., 2010), and rice (Rösti et al., 2007). The screen for starch-excess (sex) mutants differs only in that leaf starch content is visualized at the end of the dark phase, when the wild-type has metabolized almost all of its starch (Figure 1B). Mutants which still contain starch at the end of the night are assumed to have either decreased starch breakdown (Figure 1B) or increased starch synthesis rates. Numerous sex lines have been isolated and the The identification of the mutated genes has greatly improved our understanding of the process of starch breakdown (see Table 2 and Section 5, below). Several previously unknown proteins have been identified using this unbiased approach. However, the combination of classical genetics and iodine staining also has its limitations. There is functional overlap between the enzymes involved in starch metabolism and/or redundancy at the genome level, meaning that single gene mutations obtained by classical genetics will not always yield a starch phenotype. Furthermore, iodine staining is semi-quantitative at best and it is difficult to pick out mutants with subtle phenotypes using this method. The functional analysis of specific target genes through reverse genetics (i.e. using publicly-available T-DNA insertion lines, or TILLING approaches to generate knockout mutants) have complemented classical genetics. Issues of functional overlap and redundancy can be readily addressed through the generation of multiple mutant combinations.
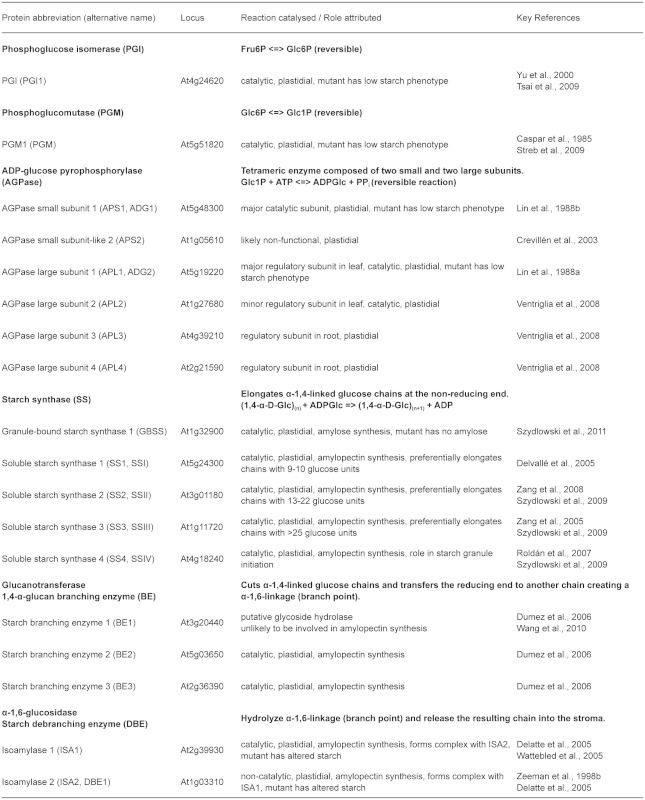
Table 1.
Arabidopsis genes coding for proteins involved in starch synthesis
A sex phenotype is not always directly associated with proteins involved in starch breakdown. Mutants lacking proteins involved in transporting starch breakdown products out of the chloroplast or metabolizing them in the cytosol also have sex phenotypes (see Section 5.8, below). This indicates that a feedback inhibition of starch breakdown occurs when mutations downstream in the pathway result in the accumulation of starch degradation intermediates. Other mutant plants can also show unexpected or pleiotropic sex phenotypes. For example, mutations affecting the triose-phosphate/phosphate translocator (TPT) cause a mild sex phenotype (Schneider et al., 2002). This results from an increased rate of starch synthesis due to the reduced export of the triose-phosphate from the chloroplast during photosynthesis. Mutant alleles of GIGANTEA (which encodes a protein involved in circadian clock function and flowering time regulation; Mizoguchi et al., 2005), also have a sex phenotype, as do plants deficient in the Snf1-related protein kinases KIN10 and KIN11 (Baena-Gonzalez et al., 2007). In these cases, it is unclear exactly how the increased starch content of the leaves is brought about. Further analyses of such mutants might be extremely helpful in evaluating the connections between the pathways of starch metabolism and the signalling pathways that regulate them.
3. THE STRUCTURE OF STARCH
Starch is a remarkable substance. It is simple in composition, yet complex in structure. Starch is almost entirely made up of the two glucose polymers: amylopectin and amylose. The glucosyl units are linked via α-1,4-glucosidic bonds to form linear chains. These chains are linked via α-1,6-glucosidic bonds - so-called branch points - which appear on average every 20–25 glucose units in amylopectin (although the lengths of individual chains can vary from 6 to more than 100 glucose units). The resulting molecule consists of 100'000 to 1'000'000 glucosyl units. In contrast, amylose is a smaller molecule (typically around 1'000 glucosyl units) and contains far fewer branch points. Amylopectin accounts for 70–90% of the granule weight of most starches, and forms a semicrystalline matrix that results in insoluble granules. Amylose is synthesised within the matrix formed by amylopectin (Denyer et al., 2001). The final granule represents a very dense and stable form of carbohydrate, presumably conferring an evolutionary and physiological advantage over the accumulation of soluble carbohydrates.
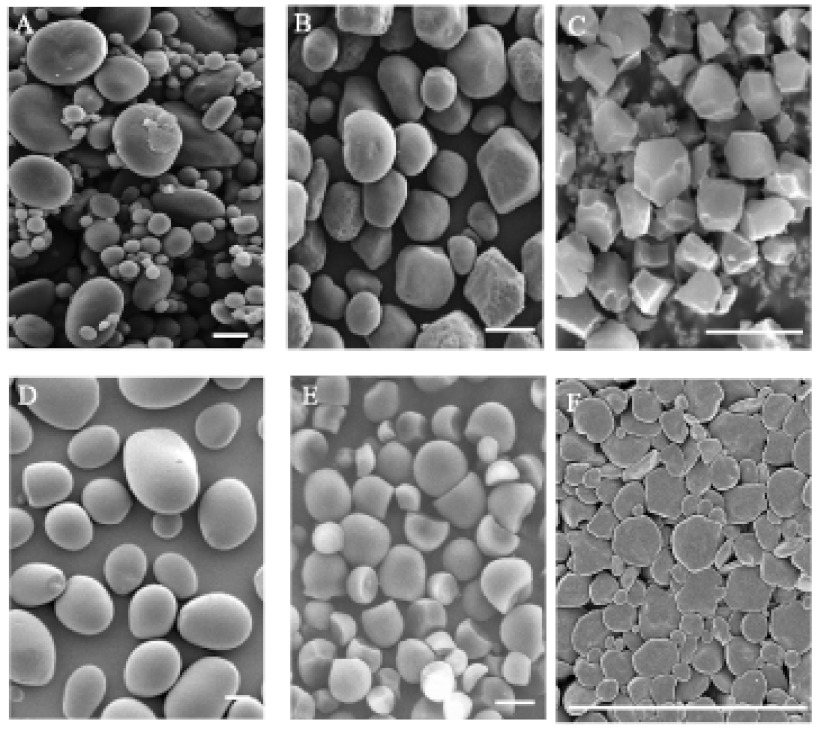
Starch granules can range in size from 1–2 µm in Arabidopsis leaves up to 100 µm in storage organs such as potato tubers. Starch granule morphology is highly diverse amongst different species (Figure 2). For example, rice has polygonal, compound granules whereas potato has smooth, round granules (Jane et al., 1994). Leaf starch granules from various species are typically lenticular or discoid, forming between the thylakoid membranes. The factors governing the differences in granule number and form have not been fully elucidated, although mutations affecting specific starch synthesising enzymes sometimes alter granule morphology. In addition to the constituent glucans, starch granules contain trace amounts of proteins, lipids and ions (the amounts of which vary depending on starch source).
Figure 2.
Scanning electron micrographs of starch granule morphology in different species.
(A) Wheat endosperm starch granules, bimodal size distribution (adapted from Li et al., 2011). B. Maize endosperm starch granules (adapted from Kubo et al., 2010). C. Rice endosperm starch granules, polygonal form (adapted from Kubo et al., 2005). D. Potato tuber starch (adapted from Bustos et al., 2004). E. Cassava root starch, cup shaped granules (adapted from Ceballos et al., 2008). F. Arabidopsis leaf starch, ellipsoid shaped granules. Scale bars = 10 µm.
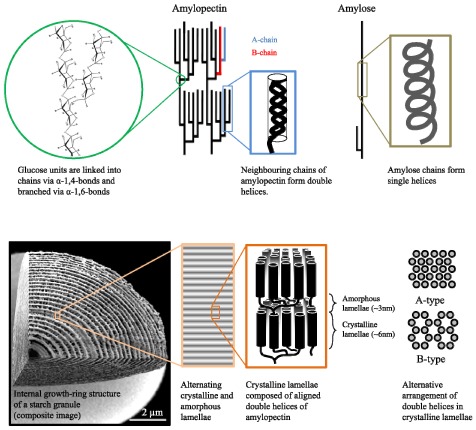
It is widely accepted that amylopectin molecules are radially oriented within starch granules. Each molecule has a single reducing end oriented towards the centre of the granule, with the non-reducing ends, where chain elongation occurs, pointing towards the surface. The chains within amylopectin molecules can be categorised into A-chains (external chains of 6 to 16 glucose units carrying no branch points), B-chains (longer chains carrying one or more branch point; Figure 3), and the C-chain (the single B-chain with a reducing end). Current models for amylopectin suggest that it has a tree-like, racemic architecture, resulting in clusters of neighbouring, unbranched chain segments, although variants on this model have been proposed (Pérez and Bertoft, 2010). The architecture of amylopectin - that is the distribution and frequency of branch points and chain lengths - enables it to adopt an insoluble form. This ability is based on the formation of double helices between neighbouring chains within clusters. The architecture further allows the double helices to align into ordered crystalline arrays or lamellae (Figure 3).
Figure 3.
The structure of the starch granule.
(Top) A racemose model of amylopectin structure. Left-handed double helices with six glucose units per turn form between A-chains or longer linear segments of B-chains. Linear amylose forms single helical structures.
(Bottom) The double helices of amylopectin arrange into ordered crystalline lamellae of two types, designated as A-type (tightly packed, typical of cereal endosperm starches), and B-type (open hexagonal pattern with a central, water-filled space, typical of tuber and leaf starches). The average external chain length is believed to influences the type of packing. Crystalline lamellae alternate with amorphous lamellae (containing the branch points) with 9-nm periodicity to form blocklets of 20–500 nm diameter (not shown; see text). These blocklets make up the growth rings that are visible with light and electron microscopy. Image adapted from Zeeman et al. (2010) with permission from Annual Reviews.
Within the crystalline lamellae, the double helices pack in a dense ‘A-type’ or a looser ‘B-type’ arrangement (Figure 3). Some starches contain a mixture of A- and B-type and are designated as ‘C-type’. The starch type seems to be determined by the underlying amylopectin structure, allowing different packing (Hizukuri, 1985). Between two crystalline lamellae is an amorphous lamella that contains most of the branch points. The alternation of crystalline and amorphous lamellae is repeated with a periodicity of 9 nm (Figure 3; Jenkins et al., 1993; Zeeman et al., 2002). There are currently two models for the macro organization of amylopectin clusters within the starch granule. Oostergetel and Vanbruggen (1993) proposed that aligned double helices form a left handed superhelix with an 18-nm diameter and a 9-nm pitch of the lamellae. This model was based on electron optical tomography and cryo-electron diffraction experiments. The second model, based on microscopic observations, proposes spherical substructures or ‘blocklets’ of 20–500 nm diameters, depending of the starch (Yamaguchi et al., 1979; Gallant et al., 1997). Higher-order structures called growth rings (as they resemble tree growth rings; Figure 3) are present in starch granules. Growth rings can be readily observed in large storage starch granules (see Pilling and Smith, 2003), and references therein). It is still unclear whether growth rings reflect periodic growth of the granule or simply a structural feature. Wild-type transitory starch granules from leaves are usually very small and thus may comprise only a single growth ring (Zeeman et al., 2002). However, growth rings can readily be seen in larger transitory starch granules such as those produced in some sex mutants. Growth rings are typically visualized by light microscopy after staining with a very dilute iodine solution, or by scanning electron microscopy after partial digestion of cracked starch granules with acid or amylolytic enzymes.
The ability of amylopectin to adopt secondary and tertiary structures distinguishes it from glycogen, the analogous storage carbohydrate in animals, fungi and bacteria. Glycogen is also an α-1,4- and α-1,6-linked glucan. However, it has more α-1,6-branch points and shorter α-1,4-linked chains. This precludes the formation of secondary structures like those seen in amylopectin (i.e. the organised arrays of double helices, see Figure 3) and thus glycogen remains water soluble. Amylopectin biosynthesis is more complex than glycogen biosynthesis and evolved early in the chloroplastida. This involved the duplication and specialisation of chain elongating and branch-forming enzymes and the recruitment of an additional enzyme to tailor their products (Deschamps et al., 2008; Ball et al., 2011).
Amylose, the second glucan in starch, accounts for 10–30% of the granule mass. Amylose is thought to adopt a single helical structure randomly orientated within the amorphous lamella. It could be viewed as ‘filling up’ the space in the amylopectin matrix (Kozlov et al., 2007). Many studies have shown that amylose is not required for starch granule formation, as mutant plants in which amylose synthesis is abolished still form starch granules (Tsai, 1974; Shure et al., 1983). However, there is much interest in amylose synthesis as amylose content has a profound impact on the functional properties of starch (e.g. during cooking of starchy foods, or when starch-based pastes are used in industrial processes; see Singh et al., 2003 and references therein).
4. STARCH BIOSYNTHESIS
4.1. Starch Biosynthesis in Leaves
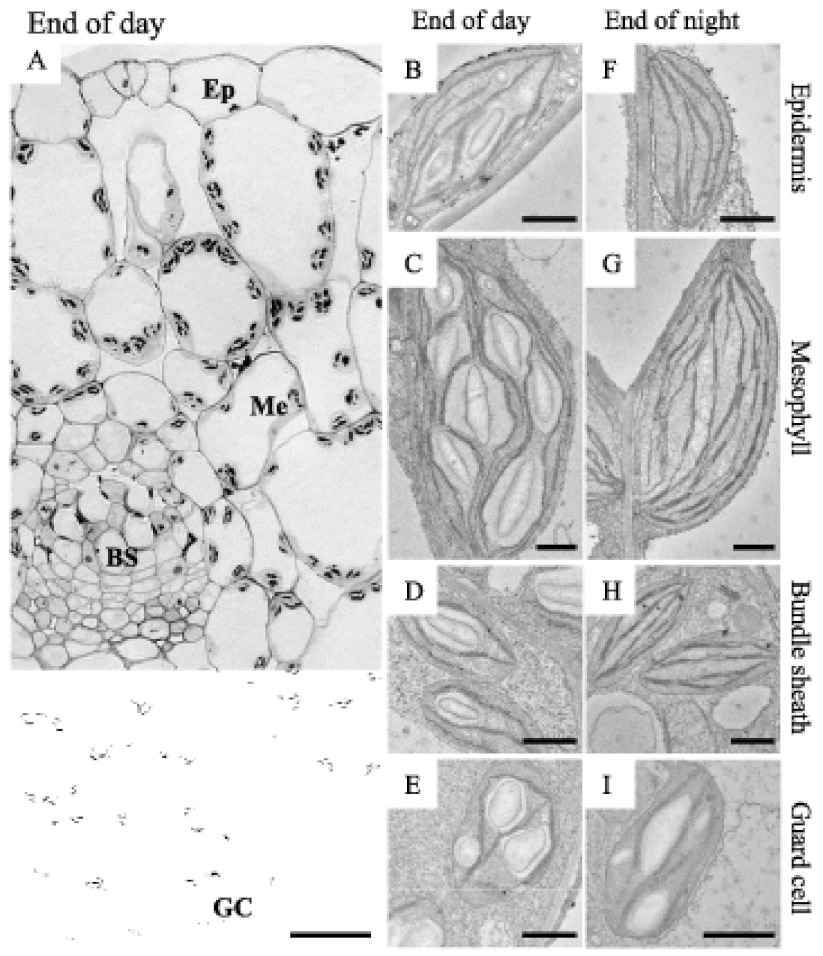
Most of the research conducted on starch metabolism in Arabidopsis focuses on leaf starch, which is made in relatively large amounts during the day in the photosynthetic palisade and spongy mesophyll cells (Figure 4). However, many other cell types, both in leaves and other organs, also synthesise starch in their plastids. Starch is synthesised in epidermal cells, in stomatal guard cells and in the bundle sheath cells (Figure 4) surrounding the vasculature (in Arabidopsis and other C3 plants the bundle sheath is present, but is not as pronounced, nor functionally specialised in the same way as in C4 plants such as maize). Starch is also made in the shoot endodermis (sometimes even referred to as the ‘starch sheath’) and in the columella cells of the root (Figure 5). In both of these locations, the starch-containing plastids serve as gravity sensing statoliths (see Section 7, below). Several floral tissues make starch, including the nectaries and the stamen filaments (Ren et al., 2007). Starch accumulates in the early stages of Arabidopsis pollen development, although mature pollen is virtually starch free (Kuang and Musgrave, 1996; Tang et al., 2009). This contrasts with other species, such as maize, where mature pollen is rich in starch reserves (Datta et al., 2002). The female gametophyte also contains starch, as do many tissues of the developing seed (both embryo and maternally derived tissues). Starch accumulation is often transitory, occurring at a specific developmental time. It is possible that starch biosynthesis serves a means to temporarily increase the local sink strength and draw in a reserve of carbohydrate, which can drive the subsequent metabolic or growth process (e.g. da Silva et al., 1997). Some tissues appear not to contain starch (e.g. root hair, mature seed, mature embryo, hypocotyl cortex). However, in sex mutants where starch degradation is compromised (see Section 5), these same tissues do contain excess starch (Vitha et al., 2007; Andriotis et al., 2010). This implies that there is either active starch turnover in these cell types or that starch is retained from an earlier point in development and cellular differentiation.
Figure 4.
Starch accumulation in leaf cell types.
(A) Light micrograph of a transverse section of a leaf harvested at the end of the light phase. The upper epidermis is on top. Epidermal cell (Ep), mesophyll cell (Me), bundle sheath cell (BS) and guard cell (GC). Scale bar = 20 µm. The leaf section was embedded for electron microscopy as described in Streb et al. (2008), and lightly stained with toluidine blue (at 20°C for 20 min). B-I. Transmission electron micrographs of cells from leaves harvested at the end of the day (B to E) and the end of the night (F to I). Scale bars = 1 µm. At the end of the night only guard cell plastids contain starch. B and F. Epidermal cell plastids. C and G. Mesophyll cell chloroplasts. D and H. Bundle sheath chloroplasts. E and I. Guard cell chloroplasts.
Figure 5.
Starch accumulation in different Arabidopsis tissues.
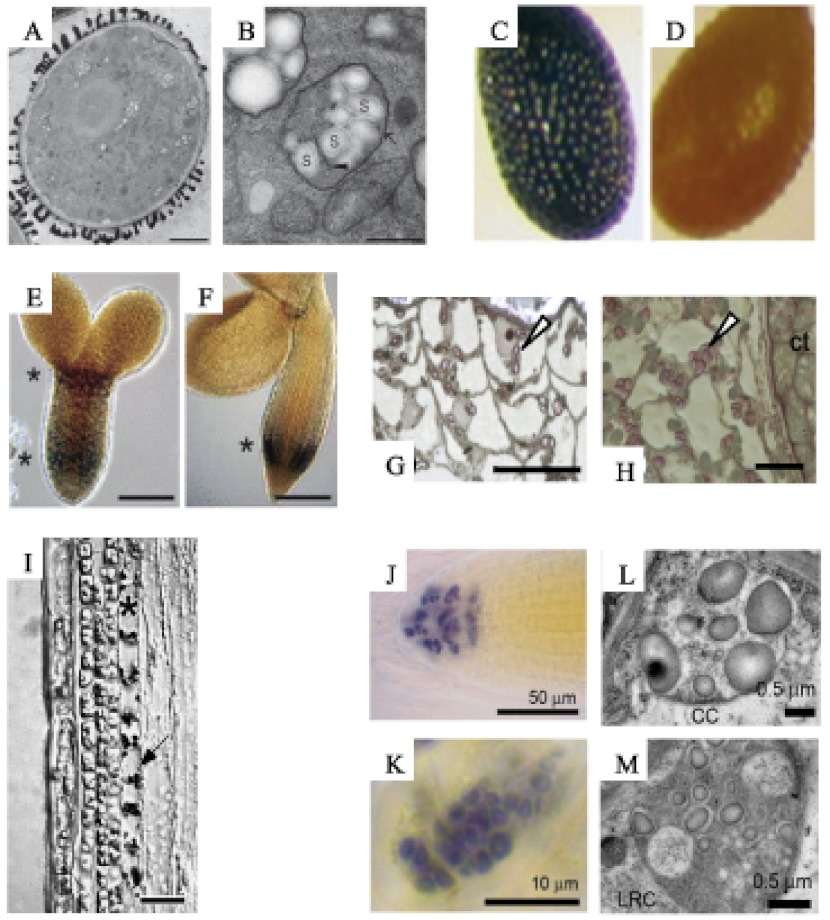
A and B. Transmission electron micrographs of a pollen grain (A) and its plastids (B). Scale bars = 5 µm (A) and 0.5 µm (B). Image adapted from Tang et al. (2009) with permission from Oxford University Press. C and D. Light micrographs of iodine-stained seeds at walking stick embryo stage (10 days after flowering, C), which contain starch and mature seed (16 days after flowering, D), which do not. Image adapted from Andriotis et al. (2010) with permission from Wiley-Blackwell Publishing. E and F. Light micrographs of iodine-stained embryos at the torpedo stage (8 days after flowering, E) and the expanded cotyledon stage (14 days after flowering, F). Two regions contain starch (marked with asterisks) above the tip of the radicle and the hypocotyl region. Scale bars = 25 µm (E) and 50 µm (F). Image adapted from Andriotis et al. (2010) with permission from Wiley-Blackwell Publishing. G and H. Light micrographs of toluidine blue- and iodine-stained endosperm at 8 and 14 days after flowering, respectively. Starch granules are indicated with arrows (adapted from Andriotis et al., 2010). Scale bars = 20 µm (G) and 10 µm (H). I. Light micrographs of a toluidine blue- and iodine-stained inflorescence stem. The endodermis is indicated by an asterisk. Arrow indicates starch-containing plastids. Image adapted from Vitha et al. (2007) with permission from Botanical Society of America. Scale bar = 25 µm. J and K. Light microscopy of iodine stained root tip (adapted from Tsai et al., 2009). Starch is visible in columella cells (J) and the root cap (K). L and M. Transmission electron micrographs of columella cell (CC) amyloplasts (L) and the root cap (LRC) cell plastids (M). Adapted from Tsai et al. (2009).
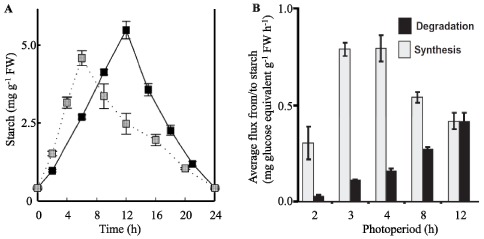
In the leaves of Arabidopsis grown in typical laboratory conditions (e.g. a 12-h photoperiod with a light intensity of 150 µmoles of photons m-2 s-1) starch accumulates during the day at a relatively constant rate, amounting to around 10 mg starch g-1 fresh weight by the end of the day (Figure 1A). During the subsequent night almost all the starch is degraded, also at a fairly constant rate (Figure 1A), to supply substrates for respiration and precursors for biosynthesis. In the past, leaf starch has been viewed as an overflow for photosynthesis - a carbohydrate pool which is built up when more sugars are available than are needed for storage in the vacuoles and for export from the leaf. While this may hold true for some species, it is probably not entirely correct for Arabidopsis. Reducing the light intensity in which Arabidopsis plants are grown reduces photosynthesis, starch accumulation and plant growth in a co-ordinated manner (Caspar et al., 1991; Schulze et al., 1991; Sun et al., 1999). This suggests that Arabidopsis partitions a fraction of its assimilates into starch even in situations of low photosynthesis. Furthermore, the amount of starch synthesised differs depending on the environmental conditions, as shown by growing Arabidopsis in different light/dark regimes (Gibon et al., 2004a; Lu et al., 2005; Smith and Stitt, 2007; Stitt et al.,2007).
Starch metabolism is important for the optimal growth of Arabidopsis in a diurnal cycle (Stitt and Zeeman, 2012). This is clearly illustrated by the severe slow-growth phenotypes of mutants unable to make starch, or unable to efficiently degrade it. The longer the night, the more compromised is the growth of such mutants. However, continuous illumination rescues this slow-growth phenotype in most cases, further illustrating that the function of transitory starch is to support night-time metabolism (Caspar et al., 1985). There is also tight relationship between starch metabolism and plant growth amongst different wild-type plants. In a study of natural Arabidopsis accessions, all of which accumulate starch, there was an inverse correlation between degree of starch accumulation and biomass accumulation (Sulpice et al., 2009). Those accessions that grew fastest invested less in starch than slowgrowing accessions, which had the highest starch levels. There are several possible explanations for this relationship: growth at night involves the utilisation of reserves for respiration as well as for biosynthetic precursors. During the day, energy and reducing power for biosynthesis may be provided via the light reactions of photosynthesis, so daytime growth may involve a lower ‘cost’ in terms of photoassimilates. Incomplete mobilisation of starch reserves would also compromise growth as it would mean that assimilated carbon, which could be used for the production of new photosynthetic biomass, languishes as an unproductive storage compound (Graf et al., 2010; Pantin et al., 2011). Conversely, premature exhaustion of carbohydrate reserves could also be detrimental for growth and it might trigger a starvation response in which valuable cellular components (e.g. lipids and proteins) are degraded to support cellular housekeeping activities and keep the plant alive (Koch, 1996; Buchanan-Wollaston et al., 2005). Thus, fine control over the metabolism of stored reserves is critical to a plant's competitiveness.
4.2. The Precursor for Starch Biosynthesis in Leaves
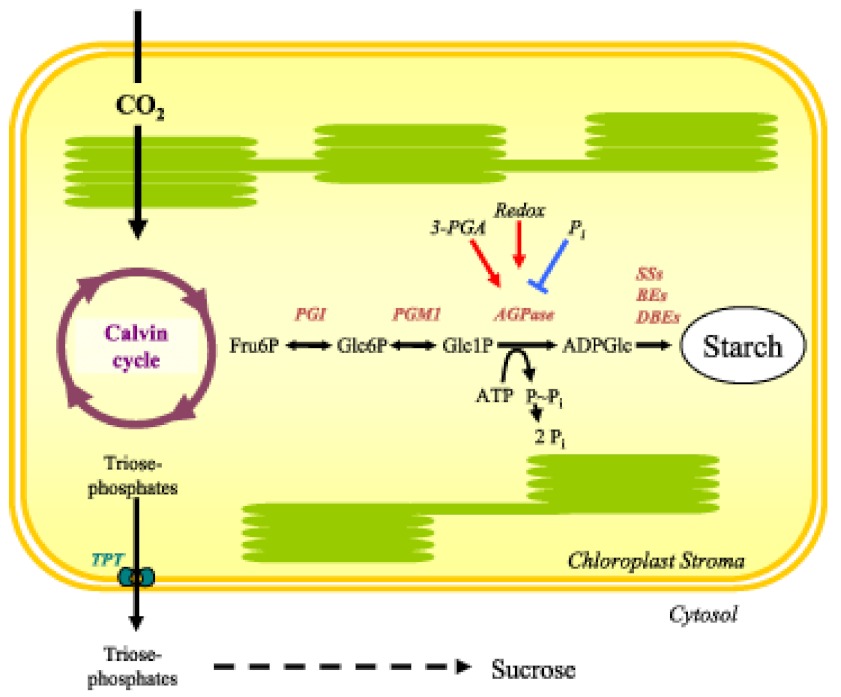
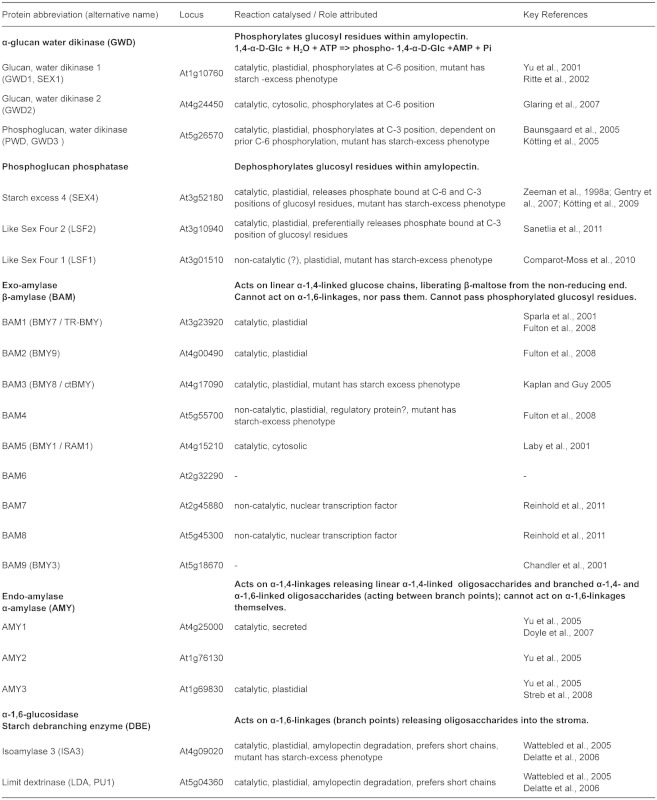
The substrate for starch synthesis in higher plants is the activated glucosyl donor, ADP-Glucose (ADPGlc). In photosynthetically active cells, the supply of ADPGlc is linked directly to the Calvin cycle via three enzymatic steps (Figure 6 and Table 1 ). Fructose-6-phosphate (Fru6P), an intermediate of the Calvin Cycle, is converted to glucose-6-phosphate (Glc6P) by plastidial phosphoglucose isomerase (PGI). Plastidial phosphoglucomutase (PGM1) converts Glc6P into glucose-1-phosphate (Glc1P). The last step is catalysed by ADP-glucose pyrophosphorylase (AGPase), which converts Glc1P and ATP into ADPGlc and inorganic pyrophosphate (PPi). All three steps are readily reversible under cellular conditions, but the last step is rendered irreversible by the immediate hydrolysis of PPi to orthophosphate (Pi) by plastidial alkaline pyrophosphatase (Weiner et al., 1987; George et al., 2010). Arabidopsis mutants in which the activities of PGI, PGM1 or AGPase are reduced or abolished have greatly reduced levels of leaf starch (Caspar et al., 1985; Lin et al., 1988b; Yu et al., 2000). Therefore, it was concluded that the whole pathway resides in the chloroplast (Figure 6).
Figure 6.
The pathway of starch biosynthesis in leaves.
Carbon assimilated via the Calvin cycle is partitioned with a fraction exported to the cytosol for sucrose synthesis and a fraction retained in the chloroplast for starch synthesis. Redox and allosteric regulation of the enzyme AGPase controls the flux of carbon into starch. Mutants deficient in plastidial PGI, PGM1 and AGPase have major reductions in starch contents, although small amounts remain, suggesting an alternative minor route (see text). Image reprinted from Zeeman et al. (2007) with permission from Portland Press.
The pathway described above is widely accepted and strongly supported by genetic and biochemical data obtained from different plant species. However, some experimental data do not entirely fit with the idea of one exclusive pathway. For example, while mutants such as pgm1 (lacking plastidial phosphoglucomutase; Caspar et al., 1985; Kofler et al., 2000) and adg1 (lacking APS1 - the small subunit of AGPase; (Lin et al., 1988b) have been generally described as ‘starchless’ or ‘starch-free’, they actually contain small amounts of starch in their chloroplasts implicating another source of precursors (Vitha et al., 2000; Streb et al., 2009; Tsai et al., 2009). Furthermore, it has been reported that ADPGlc levels are unchanged in pgm1 and adg1 mutants (Muñoz et al., 2005). These, and other observations, have renewed the debate about the pathway - or pathways - of starch synthesis in leaves and in heterotrophic organs (Muñoz et al., 2006; Streb et al., 2009). It is conceivable that ADPGlc could be synthesised by residual or alternative enzyme activities in each mutant. For example, AGPase is a heterotetramer of ‘small’ catalytic subunits and ‘large’ regulatory subunits, which are related in amino acid sequence. The adg1 mutant lacks the small subunit, but some isoforms of the large subunit have been shown to possess limited catalytic activity and may be present (although no AGPase activity has been detected in adg1; Lin et al., 1988b; Ventriglia et al., 2008). Alternatively, substrates may be imported into the plastid to support starch biosynthesis. Limited import of Glc1P into the chloroplast (Fettke et al., 2011) could explain the small amounts of starch observed in pgm1 mutants, but not adg1 mutants. It has also been suggested that ADPGlc could be synthesised by sucrose synthases (SuSy) in the cytosol and imported in the chloroplasts (Muñoz et al., 2005). SuSy is able to produce ADPGlc and fructose from sucrose and ADP in vitro (Delmer, 1972), although its normal function is considered to be the production of UDPGlc and fructose from sucrose and UDP. Quadruple mutant plants lacking SuSy in all cell types except phloem sieve elements synthesise normal amounts of leaf starch (Barratt et al., 2009). This suggests that even if SuSy does produce ADPGlc in the cytosol of mesophyll cells, it is not required for normal rates of starch synthesis.
The situation differs in the developing endosperms of cereal seeds (e.g Zea mays). In this case, ADPGlc is synthesised in the cytosol and transported into the plastid by the Brittle1 protein (BT1) in counter-exchange with ADP (Kirchberger et al., 2007). However, a cytosolic form of AGPase rather than SuSy is responsible for ADPGlc production in these tissues. This cytosolic pathway may be specific to the cereals - there is no evidence for cytosolic AGPase in Arabidopsis and the closest homologue of the BT1 in Arabidopsis (AtBT1) transports AMP, ADP and ATP but not ADPGlc (Kirchberger et al., 2008). Thus, while most data support the idea that the plastid localised pathway shown in Figure 6 is the major source of substrates for starch synthesis, it is clear that this pathway is not fully characterised and that there may be minor sources of carbohydrate derived from elsewhere.
4.3. The Regulation of Carbon Flux into Starch
The rate of starch biosynthesis in Arabidopsis leaves is finely controlled to balance the utilisation of photoassimilates with their storage for the night. Plastidial AGPase is strongly regulated through allosteric and redox control and the activity of this enzyme probably determines the flux of carbon into starch. As mentioned previously, AGPase is a heterotetramer composed of two large and two small subunits. The Arabidopsis genome encodes two small subunits, APS1 and APS2 (though APS2 is thought to be non-functional; Crevillén et al., 2003), and four large subunits (APL1, APL2, APL3, APL4, see Table 1 ). Distinct subunit compositions between APS1 and the APL isoforms confer different kinetic and regulatory properties to the heterotetramer (Crevillén et al., 2003). The AGPase small subunit is generally regarded as the catalytic subunit and the large subunit as a regulatory subunit. However, APL1 and APL2 were recently shown to be catalytically active in the presence of a mutated, non-catalytic small subunit, while APL3 and APL4 were inactive (Ventriglia et al., 2008). APS1 and APL1 are highly expressed in photosynthetic tissues and may therefore play the dominant role in leaves. This is substantiated by the fact that both mutants (adg1 and adg2, respectively) show greatly reduced AGPase activity and have decreased starch contents (Lin et al., 1988a; 1988b). The adg2 phenotype is less severe than that of adg1, because the residual APS1, possibly functioning with other APL isoforms, still affords 5% of the wild type AGPase activity (Lin et al., 1988a). APL3 and APL4 gene expression is inducible by treatment with exogenous sugars (e.g. sucrose) and both are thought to be involved in starch synthesis in heterotrophic cells (e.g. roots; Fritzius et al., 2001; Crevillén et al., 2005; Ventriglia et al., 2008).
The paradigm for AGPase regulation is that the enzyme is activated by 3-phosphoglycerate (3-PGA), an intermediate in the Calvin cycle (and an indicator of photosynthetic carbon assimilation), and inhibited by Pi (Iglesias et al., 1993). A high ratio of 3-PGA:Pi activates AGPase, promoting the synthesis of starch. This mechanism plays an important role in fine tuning the partitioning between sucrose and starch synthesis in many plants (Stitt and Quick, 1989). Specifically, when photosynthetic rate exceeds the requirements for sucrose export and storage, a buildup of phosphorylated intermediates in the cytosol feeds back to restrict export from the chloroplast. The resultant increase in 3-PGA and decrease in Pi activates starch biosynthesis, allowing photosynthesis to continue at high rates.
AGPase is also redox regulated via the reversible formation of an inter-molecular disulfide bridge between the cysteine 81 residues in the N-terminal parts of the small subunits. In its oxidised form, the enzyme is less active. When reduced, and the disulfide bridge is broken, the enzyme is activated and the affinity for its substrates increases (i.e. the Km decreases; Fu et al., 1998). In Arabidopsis leaves, redox-activation occurs during the day, presumably mediated by thioredoxins and driven by reducing power derived from photosystem I. Replacement of the endogenous small subunit with a redox-insensitive form (in which the redox-active cysteine is replaced with a serine) was performed independently by two groups. Hädrich et al. (2012) reported a degree of deregulation in starch synthesis, with slightly elevated starch levels, while Li et al. (2012) reported no change in starch levels. Interestingly, both studies revealed that the amount of the redox-insensitive protein was considerably lower than the wild type protein, suggesting instability or degradation of the mutant version. Furthermore, Hädrich et al (2012) reported increased maltose levels throughout the diurnal cycle. It is possible that the induction of concomitant starch degradation, could balance to some extent the deregulation in synthesis (Hädrich et al., 2012).
The extent of redox-activation of AGPase is also influenced by metabolites, independently of light. In this case, redox activation is probably mediated by the plastid-localized NADP-thioredoxin reductase C (NTRC; Michalska et al., 2009). In potato, high levels of glucose and sucrose increase both the AGPase activation state and starch synthesis. The signalling processes have been proposed to occur through hexokinase for glucose and through SnRK1 kinases for sucrose (Tiessen et al., 2003). Furthermore, there is evidence that trehalose-6-phosphate (Tre6P) acts as a signalling intermediate in the sucrose-dependent activation of AGPase (Kolbe et al., 2005; Lunn et al., 2006), and that levels of this metabolite might provide a direct link between sucrose and starch metabolism. However, the details of this mechanism remain to be elucidated. Overall, the convergence of regulation on AGPase strongly suggests that it is an important checkpoint determining how much starch should be made to supply the plant in the subsequent night, and how much carbohydrate the plant should use for growth during the day (Smith and Stitt, 2007). This is consistent with earlier observations that introduction of a deregulated, bacterial form of the enzyme can increase starch synthesis in plant tissues (Stark et al., 1992; Sweetlove et al., 1996).
4.4. The Biosynthesis of Amylopectin and Semi-Crystalline Starch Granules
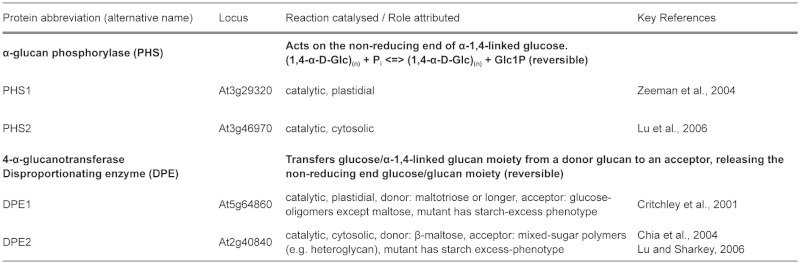
Three classes of enzymes are known to have major roles in the production of the starch granule. Most are involved in the synthesis of the amylopectin fraction. First, starch synthases (SSs) catalyse the formation of new α-1,4 glucosidic linkages by adding glucose from ADPGlc to the non-reducing end of an existing chain (Recondo and Leloir, 1961). As soon as the linear chains reach an adequate length, branching enzymes (BEs) introduce branch points by transferring a segment of 6 or more glucose residues. Interestingly, the subsequent removal of some of these branch points by debranching enzymes (DBEs) is important for starch synthesis. Additional enzymes may also contribute to the final structure of amylopectin. The roles of these different starch biosynthetic enzymes are discussed further below.
The characterized SSs can be divided into five subclasses (GBSS, SS1, SS2, SS3 and SS4) based on amino acid sequence comparisons (Ral et al., 2004; Patron and Keeling, 2005; Leterrier et al., 2008). Arabidopsis has one gene belonging to each subclass. Granule bound starch synthase (GBSS) is responsible for amylose synthesis (Tsai, 1974) and is exclusively found associated with the granule. It becomes encapsulated within the granule as amylopectin crystallises. The other four isoforms are referred to as soluble starch synthases (SSs) and are predominantly localized in the stroma. SS1, SS2, SS3 and SS4 are all involved in the elongation of amylopectin chains. Studies of mutants in both Arabidopsis and other species have led to the idea that SS1 preferentially elongates short chains (9–10 glucose units; Delvallé et al., 2005; Fujita et al., 2006), SS2 prefers intermediate chains (13–22 glucose units; Craig et al., 1998; Morell et al., 2003) and SS3 prefers long chains (more than 25 glucose units; Zhang et al., 2005; see Table 1). Thus, SS1 activity tends to create chains of a suitable length for SS2 and so on. That said, all single mutants and mutant combinations analysed thus far can synthesise starch, just with different relative proportions of chain lengths, and sometimes in lower amounts than the wild type (SS1: Delvallé et al., 2005; SS2: Zhang et al., 2008 and SS3: Zhang et al., 2005; Szydlowski et al., 2009; 2011; for an overview see Santelia and Zeeman, 2011). Therefore, the isoforms have overlapping functions and those remaining in each mutant can, in combination with branching enzymes, generate the full spectrum of chain lengths. The ss3ss4 double mutant appears to be an exception in that it is reportedly unable to initiate granules (Szydlowski et al., 2009).
Branching enzymes are glucanotransferases that generate branch points by cutting an existing α-1,4-linked chain and transferring the cut segment to another linear chain to create a new α-1,6 linkage. They act on chains with a minimum length of 12 glucose units and transfer a segment of six or more. Thus, few (if any) chains shorter than six glucose units are generated during the branching process (Takeda et al., 1993). There are two subclasses of BEs in higher plants (designated as subclasses I and II or B and A, respectively; Burton et al., 1995). Studies of cereal and potato genes suggest that subclass I BEs preferentially transfer longer chains than subclass II BEs (Takeda et al., 1993; Guan et al., 1997; Morell et al., 1997; Rydberg et al., 2001; Nakamura et al., 2010). In Arabidopsis, the situation is unusual because there are only members of subclass II (BE2 and BE3). The third gene annotated as a BE (BE1), despite being related to the BEI subclass, falls into a separate clade (Dumez et al., 2006; Wang et al., 2010; Table 1). Current evidence suggests that this gene does not encode a functional BE (Wang et al., 2010). It appears that there is no genuine subclass I BE.
There have been extensive analyses of storage starch structure in plants with reduced BE activity. Potato and maize plants lacking subclass I enzymes show only minor differences in starch structure compared to the wild type (Safford et al., 1998; Blauth et al., 2002; Satoh et al., 2003). In contrast, removal of BEII leads to an altered starch content, structure and properties in several species. For example, removal of the endosperm-specific BEIIb in maize results in amylopectin with longer chains and less branching (Stinard et al., 1993). Similar observations were made in rice (Mizuno et al., 1993). Maize plants lacking the leaf-specific isoform of BEII (BEIIa) produce transitory starch amylopectin with longer chains (Blauth et al., 2001). These findings indicate that BEII can compensate for the loss BEI, but not vice versa. This could be because BEII is required to generate the correct architecture underlying the cluster structure of amylopectin, and/or the short chains in the clusters themselves. Simultaneous repression of both BE subclasses in potato (Schwall et al., 2000) resulted in a greatly reduced production of starch which has very high apparent amylose content (or very sparsely branched amylopectin). Mutation of either subclass II BE gene in Arabidopsis results only in minor changes in starch structure suggesting functional redundancy. However, double mutant plants lacking both proteins have a striking phenotype as they are unable to make starch. Instead they accumulate high levels of maltose, probably due to the continuous degradation of newly synthesised linear glucans (Dumez et al., 2006). Dumez et al., (2006) reported that Arabidopsis plants mutated in the BE1 gene have a wild-type phenotype. However, Wang et al., (2010) have since reported that homozygous be1 null mutants have an embryo lethal phenotype. The precise function of this gene remains unclear.
Initially, it was thought that SSs and BEs activities were sufficient to synthesise amylopectin and therefore to form starch granules. However, it is now known that plants lacking a particular type of DBEs have serious defects in starch biosynthesis. Mutations abolishing this DBE result in partial or complete replacement of starch granules with a soluble glycogen-like glucan, called phytoglycogen (Figure 7). Plants have two classes of DBEs designated as isoamylases (ISA) and limit-dextrinases (LDA; also called pullulanase). Both classes can hydrolyze α-1,6 branch points but show different substrate specificities, which probably reflect the different roles they play in starch metabolism. LDA has a preference for substrates with very short branches such as β-limit dextrins (branched glucans degraded by β-amylase - an exoamylase which removes maltosyl units from external chains to within two or three glucose residues of a branch point). LDA can also act on the fungal polysaccharide pullulan (maltotriosyl units linked end-to-end by α-1,6-bonds), hence its alternative name. In contrast, ISAs cannot act on pullulan. However, the ISA class can be further divided into three subclasses, named ISA1, ISA2 and ISA3 (Hussain et al., 2003). Arabidopsis has one gene corresponding to each subclass and one gene coding for LDA (Table 1 and 2). Current evidence suggests that, while ISA1 and ISA3 genes encode active DBEs, the proteins encoded by ISA2 genes are non-catalytic due to substitutions of 6 of 8 key active-site amino acids (Macgregor, 1993; Hussain et al., 2003).
Figure 7.
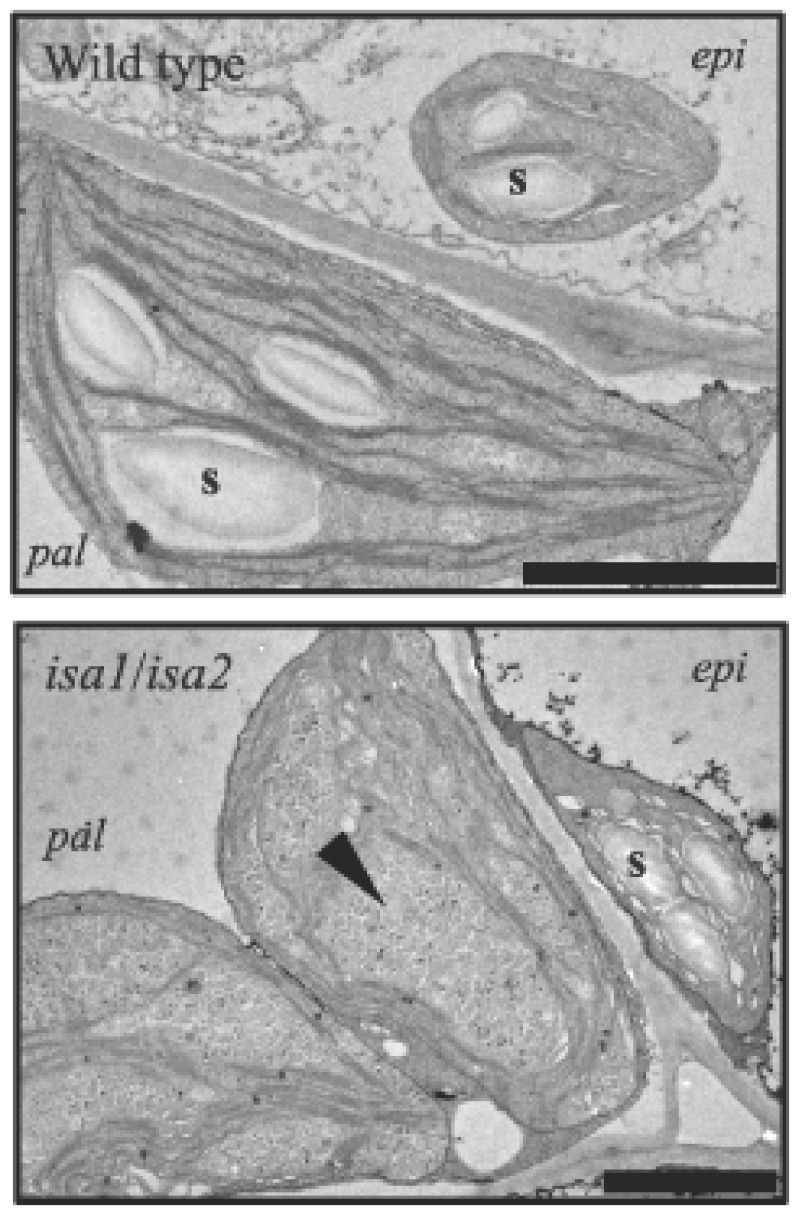
Transmission electron micrographs of phytoglycogen and starch accumulating in leaves of isa1isa2 double mutants.
Starch granules (s) are present in wild-type plastids and in the epidermal (epi) cell plastids of isa1isa2. Phytoglycogen and numerous tiny starch granules (arrowhead) are present in isa1isa2 palisade mesophyll cell (pal) chloroplasts Scale bars = 2 µm
ISA1 is primarily involved in amylopectin synthesis and, in all species studied to date, forms a multimeric enzyme with a native molecular mass between 350 kDa and 500 kDa (Ishizaki et al., 1983; Beatty et al., 1999; Fujita et al., 1999; Dauvillée et al., 2001; Delatte et al., 2005). ISA1 either forms a heteromultimer with ISA2 (as in Arabidopsis and potato; Bustos et al., 2004; Delatte et al., 2005) or a homomultimeric enzyme in addition to the heteromultimer (as in rice and maize; Utsumi and Nakamura, 2006; Kubo et al., 2010; Utsumi et al., 2011). Mutations in ISA1 in different species result in decreased insoluble, granular starch and in the production of soluble phytoglycogen, (e.g. maize: James et al., 1995; barley: Burton et al., 2002; rice: Rahman et al., 2003; Arabidopsis: Delatte et al., 2005; Wattebled et al., 2005 and Chlamydomonas: Mouille et al., 1996). Phytoglycogen is similar in composition to amylopectin, but has a higher degree of branching, more short chains, and branch points that are probably closer together. The current models for the involvement of DBEs in starch biosynthesis propose that they remove wrongly-positioned branch points that interfere with double helix formation and prevent or delay the crystallization of starch (Ball et al., 1996; Myers et al., 2000; Streb et al., 2008). Modelling studies predicted that the distance between branch points strongly influences the capacity for the alignment of double helices (O'Sullivan and Pérez, 1999). Delatte et al., (2005) suggested that the ISA1/ ISA2 complex might specifically remove branches that are too close to other branch points.
In potato and Arabidopsis, repression or mutation of the ISA2 gene results in the same phenotype as repression or mutation of ISA1 (Zeeman et al., 1998b; Hussain et al., 2003; Bustos et al., 2004; Delatte et al., 2005; Wattebled et al., 2005). It is tempting to believe that ISA2 has a regulatory function or provides substrate specificity to the multimeric enzyme. In Arabidopsis isa2 mutants, the ISA1 protein is also significantly decreased in amount. This makes it difficult to discriminate between an essential role for ISA2 in the function of the DBE multimer, or in the stability of the multimer. The presence of ISA1 homomultimers in cereals suggests that ISA2 is not always required for ISA1 stability/activity, and the loss of ISA2 in cereal endosperm does not result in a comparable phenotype to the loss of ISA1 (Kubo et al., 2010; Utsumi et al., 2011).
The extent to which debranching occurs during synthesis and the fate of the released glucans is unclear. Soluble glucans released by debranching may be degraded during the day by β-amylases (see Section 5.3, below). Maltose measurements reveal that levels are lower during the day than at night, but not zero (Chia et al., 2004; Niittylä et al., 2004). It is possible that the formation of semi-crystalline amylopectin itself limits the action of degradative enzymes and therefore prevents futile cycling of carbohydrate into and out of the starch pool. Indeed, analysis of the structure of soluble phytoglycogen in isa1 and isa2 mutants provides evidence for degradation of the outer chains (Delatte et al., 2005; Streb et al., 2008). If this is correct, it implies that the control of the pathway of starch degradation lies in the activity of the enzymes that alter the structure of amylopectin through reversible phosphorylation steps (see Section 5.1, below).
Despite playing an important role in amylopectin synthesis, it has recently been shown that DBEs are not absolutely essential for starch synthesis in Arabidopsis. In isa1 mutants, some tissues synthesise predominantly phytoglycogen (e.g. the leaf mesophyll), whereas others synthesise predominantly starch (e.g. epidermal cells, Figure 7), albeit with an altered structure (Delatte et al., 2005). Variation in the severity of the phytoglycogen-accumulation in ISA1-deficient plants is also evident between species (Mouille et al., 1996; Dauvillé;e et al., 2001; Burton et al., 2002; Posewitz et al., 2004). Subsequent removal of the two remaining DBE activities, ISA3 and LDA, in Arabidopsis abolishes starch in all cell types, seemingly supporting the idea that DBE plays a critical role (Streb et al., 2008; Wattebled et al., 2008). However, further removal of another class of glucan degrading enzyme not previously associated with starch biosynthesis (α-amylase - see Section 5.5 below) restores starch granule formation (Streb et al., 2008). This somewhat surprising result highlights the fact that glucan structure is determined by the complement of both biosynthetic and degradative enzymes and that there is interdependency in their respective activities. Streb et al., (2008) explain these observations by suggesting that the removal of branch points by ISA1 during starch synthesis promotes efficient crystallization of amylopectin, preventing the interference in starch biosynthesis by other enzymes. According to this hypothesis, α-amylase does not usually influence starch biosynthesis but, in the absence of ISA1 and other DBEs, the aberrant amylopectin can be attacked and thereby modified, further impeding granule formation.
4.5. Amylose Synthesis
Amylose, the second glucan in starch, is synthesised by GBSS. Mutant plants in several species lacking this enzyme synthesise starch containing only amylopectin (Denyer et al., 2001; Szydlowski et al., 2011). Although amylose can account for 10–30% of the starch granule weight, it is not required for granule crystallinity. GBSS has a very high affinity for the starch granule and becomes encapsulated within it (Rahman et al., 1995; Denyer et al., 2001). There it synthesises amylose using ADPGlc that diffuses into the starch granule matrix from the stroma. GBSS acts in a processive manner extending the same primer glucan molecule, which could be a malto-oligosaccharide or a side-chain of amylopectin (Denyer et al., 1996; van de Wal et al., 1998; Denyer et al., 1999; Zeeman et al., 2002). As the amylose is synthesised within the starch granule (Tatge et al., 1999; Glaring et al., 2006), it is not accessible for further modification (e.g. by branching enzymes) and remains mostly linear. The physiological role for amylose is not obvious, but the conservation of GBSS through the plant kingdom implies that amylose synthesis is important, perhaps by helping to store glucan more efficiently (i.e. more densely packed) or helping to increase stability.
4.6. Starch Granule Initiation
The mode of granule initiation is not well understood. However, starch granule number and morphology are organ and species specific and therefore it is assumed that these traits are genetically controlled (Jane et al., 1994; Crumpton-Taylor et al., 2012). In mammals and yeast, a self-glycosylating protein called glycogenin transfers glucose units from UDPGlc to a tyrosine residue to create a glucan chain, which is accessible for elongation by glycogen synthases (Lomako et al., 1988; Cheng et al., 1995). An analogous system for the initiation of starch granules is imaginable. Glycogenin-like proteins can be found in plants and reports exist where down regulation of a glycogenin homologue in Arabidopsis led to an apparently starchless phenotype (Chatterjee et al., 2005). However, this report was not detailed, and the observation needs further investigation.
Recently, it was observed that Arabidopsis plants lacking SS4 have altered patterns of starch biosynthesis and degradation and have just one large starch granule per chloroplast instead of three to five smaller ones observed in the wild type (Roldán et al., 2007). Therefore, it was hypothesized that SS4 may be involved in starch granule initiation. Furthermore, it was shown that plants lacking both SS4 and SS3 do not synthesise starch granules (Szydlowski et al., 2009) despite having 60% of the wild type activity of SS (attributable to SS1 and SS2). These observations were interpreted to mean that either SS3 or SS4 can initiate starch granules, whereas the other starch synthases cannot. Szydlowski et al., (2009) also reported that SS3 can generate glucans in vitro using only ADPGlc. This contrasts with other starch synthase isoforms which, in addition to ADPGlc as the glucosyl donor, need a glucan primer molecule as an acceptor. The capacity to initiate glucans in the absence of a primer would enable SS3 to initiate an amylopectin molecule (Szydlowski et al., 2009). Such a system has been proposed for the initiation of glycogen molecules in the bacterium Agrobacterium tumefaciens (Ugalde et al., 2003).
Altered granule size and/or numbers have also been reported in other mutants affected in starch metabolism. Plants lacking ISA1 and/or ISA2 (Zeeman et al., 1998b; Burton et al., 2002; Delatte et al., 2005; Streb et al., 2008) and some starchexcess mutants (e.g. sex4; Zeeman et al., 2002) have aberrant granules. Furthermore, it has recently been proposed that in rice endosperm, plastidial Phosphorylase also contributes to granule initiation by extending malto-oligosaccharides, which in turn serve as primers for the other starch biosynthetic enzymes (Satoh et al., 2008).
4.7. The Pathway of Starch Biosynthesis in Other Photosynthetic and Non-Photosynthetic Tissues
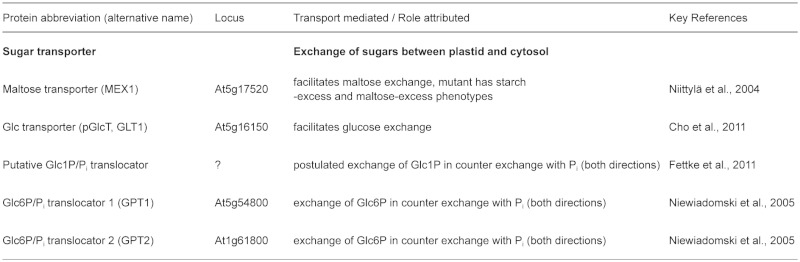
The major cell type in Arabidopsis leaves is the mesophyll, accounting for 81 % of the total cellular volume. Epidermal cells account for around 17% and vascular cells, despite representing over a third of the leaf cell number, account for only 2% of the cellular volume (Pyke et al., 1991; Figure 4A). The number of plastids/chloroplasts in each cell type and the volume they occupy varies greatly, but most if not all synthesise starch (Delatte et al., 2005; 2006; Streb et al., 2008; Tsai et al., 2009). There is evidence that the source of the precursors for starch synthesis differs between the mesophyll and the other cell types. As described above, the vast majority of precursors in the mesophyll come directly from the Calvin cycle. However, other cell types can import hexose phosphates from the cytosol to support starch synthesis in addition to, or instead of deriving them from photosynthesis. Overlach et al., (1993) reported that, unlike mesophyll cells, guard cell chloroplasts possess an active hexose-phosphate translocator (GPT) allowing uptake of Glc6P (see also Niewiadomski et al., 2005). Consistent with this, mutants lacking plastidial PGI (which interconverts Fru6P and Glc6P) are deficient in starch in the mesophyll, but are able to make starch in the guard cells (Tsai et al., 2009). Mutants deficient in plastidial PGM1 (which interconverts Glc6P and Glc1P) are deficient in starch in both cell types (Lasceve et al., 1997). These data imply that Glc6P can be efficiently transported into guard cell chloroplasts to support starch synthesis. Similar observations were made for bundle sheath cells (Tsai et al., 2009). Furthermore, Niewiadomski et al., (2005) were able to complement the low starch phenotype in mesophyll cells of the pgi mutant by constitutive expression of GPT proteins, demonstrating that these transporters are not highly expressed in the mesophyll.
It is not clear if there are other significant differences between cell types in downstream steps in the pathway of starch synthesis. It is possible that the subset of isoforms for AGPase, SSs and BEs operating in different cell types may not be the same. For example, mutants lacking APL1 (the adg2 mutant) synthesise less starch than the wild type in the leaf mesophyll but apparently have normal starch content in the root columella indicating that other large subunits (APL2, APL3 or APL4) are important for AGPase activity in these cells (Tsai et al., 2009). Furthermore, isa1 and isa2 mutants have tissue-dependent starch- and phytoglycogen-accumulating phenotypes (e.g. epidermal cells make starch whereas mesophyll cells make predominantly phytoglycogen, Figure 7). This has been attributed to possible differences in the other starch biosynthetic enzymes (Delatte et al., 2005).
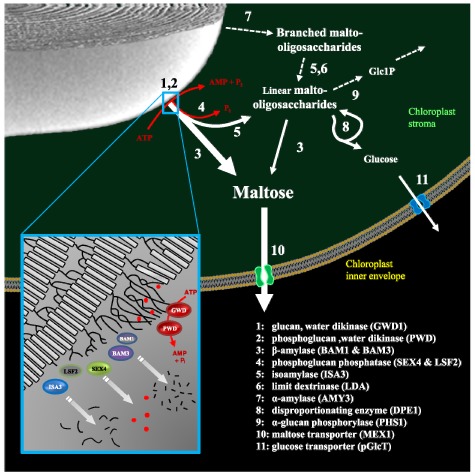
5. THE PATHWAYS OF STARCH DEGRADATION
Most studies suggest that transient starch breakdown occurs at a more-or-less constant rate throughout the night, supplying the plant with sugars. Almost all starch made during the day in Arabidopsis leaves is degraded by the end of the dark phase (Figure 1 ). The major starch breakdown products are maltose and glucose, both of which can be exported from the chloroplast to the cytosol for further metabolism (Lloyd et al., 2005; Zeeman et al., 2007; 2010). Glucose-1-phosphate can also be generated directly from starch and be metabolised in the chloroplast.
Several key enzymes involved in starch breakdown were identified by classical genetic screens, as plants impaired in this process develop sex phenotypes (see Section 2, above). This research has yielded a number of surprises. First, the pathway of starch degradation inside chloroplasts differs significantly from the recognised pathway in the endosperms of germinated cereal seeds, even though some glucan-degrading enzymes are common to both systems. Second, starch degradation in chloroplasts occurs via a network of reactions rather than a linear pathway (Figure 8; Table 2). Third, chloroplast starch degradation is facilitated by a process of reversible glucan phosphorylation, which serves to disrupt the starch granule surface, helping to initiate, and possibly control the degradation process.
Figure 8.
The pathway of starch degradation in chloroplasts.
Maltose and malto-oligosaccharides are released from the surface of the starch granule during degradation. Malto-oligosaccharides are metabolized in the stroma. Maltose and glucose are exported to the cytosol. Estimated fluxes are indicated by relative arrow size. Dashed arrows represent the minor steps in Arabidopsis (see text). Inset is a model depicting the role of phosphorylation by GWD1 and PWD in disrupting the packing of amylopectin double helices (grey boxes). This allows the release of maltose and malto-oligosaccharides (black lines) by β-amylases (BAMs) and DBE (ISA3). Phosphate (red dots) is concomitantly released by SEX4 and LSF2 to allow complete degradation. Image adapted from Zeeman et al. (2010) with permission from Annual Reviews.
5.1. Transient Glucan Phosphorylation
Evidence that glucan phosphorylation is necessary for normal starch degradation in plastids derives from initial work on potato which has been supported by subsequent studies in Arabidopsis and other species (Lorberth et al., 1998; Yu et al., 2001; Nashilevitz et al., 2009). The enzyme α-glucan water, dikinase (GWD1) phosphorylates glucosyl residues of amylopectin at the C6 position (Ritte et al., 2002). After this initial phosphorylation, a second enzyme, phosphoglucan, water dikinase (PWD, also known as GWD3) recognises the phosphorylated glucan and is able to phosphorylate a different glucosyl residue at the C3 position (Baunsgaard et al., 2005; Kötting et al., 2005). The overall frequency of phosphorylated glucosyl residues in Arabidopsis leaf starch is about 1 in 2000, and the ratio of C3:C6 phosphorylation is about 1:5 (Ritte et al., 2006; Santelia et al., 2011). This reflects the extent of phosphorylation that occurs during starch biosynthesis. When starch is being degraded, both phosphorylation and dephosphorylation are likely to proceed at considerably higher rates (Ritte et al., 2004). However, this transient phosphorylation at the granule surface is not reflected in the phosphate content of the starch.
There is good evidence that both GWD1 and PWD are needed for normal rates of starch degradation. The gwd1 mutant (also called sex1) has a very strong sex phenotype (Caspar et al., 1991; Yu et al., 2001) and starch-bound phosphate is abolished, both at C6 and C3 positions (Ritte et al., 2006; Santelia et al., 2011). In comparison, pwd mutant plants have a mild sex phenotype (Baunsgaard et al., 2005; Kötting et al., 2005), and only the C3-bound phosphate is eliminated. A third enzyme, GWD2, is also able to phosphorylate glucose units at the C6 position in vitro. However, sub-cellular localization studies identified it as extraplastidial (Glaring et al., 2007). Therefore, is unlikely to be involved in normal starch mobilization. This is consistent with the observation that gwd2 mutants do not have a sex phenotype.
Structural and modelling studies of phosphorylated glucans suggest that phosphate groups at the C6 position could locally disrupt the packing of amylopectin double helices. When phosphate groups are introduced at the C3 position, the effect on the orientation of neighbouring glucosidic bonds could destabilise the double helices themselves (Engelsen et al., 2003; Hansen et al., 2009; Blennow and Engelsen, 2010). This theory is consistent with the idea that phosphorylation solubilises the surface of the starch granule, enabling other enzymes to attack the exposed glucan chains. Direct evidence for this function comes from invitro studies. Phosphorylation by recombinant GWD1 of crystallized maltodextrins (that have structural characteristics of starch) causes the release of soluble phospho-oligosaccharides (Hejazi et al., 2008). Furthermore, GWD1 activity was shown to stimulate the degradation of intact starch granules by recombinant glucan hydrolases (Edner et al., 2007).
Until recently it was unclear what happened to the glucose residues phosphorylated by GWD1 and PWD. However, this was an important question to solve because while phosphorylation disrupts the crystalline structure of starch, it was also known from earlier studies that β-amylase activity is impeded when a phosphate group is reached (Takeda and Hizukuri, 1981). In the last few years it was shown that Arabidopsis contains two phosphoglucan phosphatases, SEX4 (for Starch Excess 4) and LSF2 (for Like Sex Four 2), which are able to dephosphorylate amylopectin (Gentry et al., 2007; Kötting et al., 2009; Santelia et al., 2011). SEX4 releases phosphate bound either to the C6 or the C3 positions (Hejazi et al., 2010), whereas LSF2 has a strong preference for phosphate bound to the C3 position (Santelia et al., 2011).
SEX4 has an important role in starch degradation. In sex4 mutant, the decreased phosphoglucan phosphatase activity results in a reduced rate of starch degradation and the accumulation of excess leaf starch (Zeeman et al., 1998a; Niittylä et al., 2006; Sokolov et al., 2006). Furthermore, soluble phospho-oligosaccharides that are not detected in the wild type accumulate in sex4 mutants (Kötting et al., 2009). These may be intermediates of starch breakdown that are normally metabolised rapidly by SEX4. Alternatively, SEX4 might normally work at the starch granule surface and phospho-oligosaccharides may only be produced in sex4 mutants because the granule surface becomes hyper-phosphorylated. Genetic analysis revealed that the phospho-oligosaccharides accumulating in sex4 mutants are liberated from the starch granule by hydrolytic enzymes that act on internal α-glucosidic bonds of amylopectin. Double mutants lacking SEX4 and either the endoamylase AMY3 or the debranching enzyme ISA3 (see Sections 5.4 and 5.5, below) have fewer phospho-oligosaccharides and higher starch levels than sex4 single mutants.
In contrast to sex4 mutants, lsf2 mutants do not exhibit a sex phenotype and nor do they accumulate phospho-oligosaccharides. Nevertheless, LSF2 is involved in starch metabolism. The lsf2 single mutant starch displays a marked increase in C3-bound phosphate, despite not having a sex phenotype. This is interesting as it demonstrates that the phosphate content of starch (an important determinant of starch properties and functionality) is determined by the balance between phosphorylation and dephosphorylation events during its biosynthesis (Santelia et al., 2011; Santelia and Zeeman, 2011). Nevertheless, the physiological role of LSF2 is probably still in starch breakdown, as when both SEX4 and LSF2 genes are mutated, the double mutant plants display a more severe sex phenotype than the sex4 single mutant.
Table 2.
Arabidopsis genes coding for proteins involved in starch breakdown and related genes.
continued.
Further evidence that a cycle of glucan phosphorylation and dephosphorylation promotes starch degradation stems from invitro experiments with isolated starch granules. When incubated together with GWD1 and hydrolytic enzymes, the presence of SEX4 enhances the release of soluble glucans from the granules (Kötting et al., 2009). Thus, it is proposed that during starch degradation, amylopectin structure is first destabilized by GWD1-and PWD-mediated phosphorylation, then simultaneously degraded by glucan hydrolytic enzymes and dephosphorylated by SEX4 and LSF2. The recent demonstration that SEX4 acts preferentially on insoluble glucans (starch) rather than soluble glucans (phospho-oligosaccharides) supports the notion that all these steps occur concurrently at the starch granule surface (Hejazi et al., 2010).
It is possible that another enzyme besides SEX4 and LSF2 is also able to dephosphorylate glucans. Arabidopsis contains an additional homolog of SEX4, called LSF1 (Like SEX Four 1). Genetic studies show that LSF1 is required for normal starch breakdown as lsf1 mutants have a sex phenotype, albeit milder than that of sex4 (Comparot-Moss et al., 2010). Interestingly, biochemical data suggest that LSF1 and SEX4 are involved in starch breakdown in different ways (Comparot-Moss et al., 2010). The lsf1 mutant does not accumulate phospho-oligosaccharides and attempts to detect phosphoglucan phosphatase activity with the recombinant LSF1 protein, or a reduction in phosphoglucan phosphatase activity in lsf1 mutant extracts, have been unsuccessful (Comparot-Moss et al., 2010). Consequently, it has been proposed that LSF1 has a regulatory rather than catalytic function.
5.2. The Degradation of the Starch Granule Surface
Upon solubilisation of the chains at the granule surface by GWD1/ PWD, hydrolytic enzymes degrade the α-1,4 and α-1,6 glucosidic bonds. Hydrolysis of linear glucans is achieved by β-amylases (BAMs) - exo-acting amylases that liberate the disaccharide maltose from the non-reducing ends. These enzymes cannot hydrolyse α-1,6 branch points, nor act close to them. Therefore, for progressive degradation of branched amylopectin molecules, DBEs are also required to hydrolyse the branch points. While BAMs and DBEs probably represent the most important starch degrading activities in Arabidopsis chloroplasts (see Sections 5.3 and 5.4, below), other glucan metabolising enzymes are also present, including the endo-acting α-amylase (which hydrolyses internal α-1,4 bonds; Section 5.5, below), α-glucan Phosphorylase (which catalyses the phosphorolysis of Glc1P from the non-reducing end of chains; Section 5.6), and disproportionating enzyme (which catalyses a range of glucanotransferase reactions; Section 5.7). The importance of each of these enzymes have been assessed using a combination of genetic and biochemical studies. Some enzymes are essential for normal rates of starch degradation and constitute a ‘major’ pathway, whereas others are non-essential (at least under laboratory conditions) and constitute a ‘minor’ pathway (Zeeman et al., 2010; Figure 8). However, there is a degree of redundancy between these pathways. Thus, the minor pathway becomes limiting when enzymes of the major pathway are missing. Furthermore, it is important to stress that the relative importance of these enzymes might differ in other Arabidopsis tissues (e.g. in parts of the developing seed; Andriotis et al., 2010) or in leaves of other species (e.g. in cereals; Dinges et al., 2003; Asatsuma et al., 2005).
5.3. The Importance Of β-Amylase
β-Amylase is a key enzyme of starch degradation. This is illustrated by the rapid increase in maltose levels in Arabidopsis leaves during the night, when starch is degraded (Chia et al., 2004; Niittylä et al., 2004; Fulton et al., 2008), and by the sex phenotypes of both potato and Arabidopsis plants lacking chloroplastic β-amylase isoforms (Scheidig et al., 2002; Kaplan and Guy, 2005; Fulton et al., 2008). In Arabidopsis, there are nine genes encoding β-amylase-like proteins (Table 2), at least four of which (BAM1 to BAM4) are targeted to the chloroplast (Lao et al., 1999; Sparla et al., 2006; Fulton et al., 2008). BAM1 and BAM3 are both active enzymes and recombinant proteins have high specific activities on glucan substrates in vitro (Fulton et al., 2008). Crude extracts of bam1 and bam3 mutants also have significant reductions in total β-amylase activity (however, see cautionary note about BAM5, below). In contrast, BAM2 has a very low specific activity and BAM4 appears to be non-catalytic due to amino acid substitutions within its active site (including one of the two catalytic glutamate residues). Crude extracts of bam2 and bam4 mutants have no reduction in total β-amylase activity.
There is partial overlap in the functions of BAM1 and BAM3. Mutants of BAM3 have a mild sex phenotype whereas mutants of BAM1 show no obvious alteration in starch metabolism compared to wild-type plants (Fulton et al., 2008). However, the bam1bam3 double mutant has a strong sex phenotype. Thus, it appears that BAM3 can compensate for the loss of BAM1 but not vice versa. So far no role for BAM2 is known. Although it is an active enzyme, no change in phenotype could be observed when BAM2 was missing either alone or in the backgrounds of other bam mutations (Fulton et al., 2008). The opposite is true for BAM4. Although the BAM4 protein has no measurable β-amylase activity, bam4 mutant plants do have a sex phenotype. It remains to be explained how this non-catalytic β-amylase-like protein influences starch breakdown. It has been speculated that it acts as a chloroplastic regulator, potentially responding to the concentration of maltose, and thereby fine-tuning the rate of starch degradation (Fulton et al., 2008). However, direct evidence for such a role is lacking.
It is conspicuous that Arabidopsis and other plants contain so many genes encoding β-amylase like proteins. Recently, it was shown that two of the m (BAM7 and BAM8) are localised to the nucleus and possess an additional protein domain that enables them to bind a specific DNA motif (Reinhold et al., 2011). These proteins act as transcriptional regulators controlling shoot growth and development, but having no direct influence over carbohydrate metabolism. It was proposed that the β-amylase-like domain may act as a metabolite sensing domain rather than catalysing the hydrolysis of glucans like true β-amylases (Reinhold et al., 2011). Little or no data are available for the functions of the remaining three BAM proteins, BAM5, BAM6 and BAM9 (Chandler et al., 2001). Localization studies showed that BAM5 is likely to be cytosolic and mainly localized in sieve elements (Monroe and Preiss, 1990; Wang et al., 1995). Therefore, it is unlikely to be directly involved in starch breakdown, consistent with the observation that bam5 mutants have normal starch levels (Laby et al., 2001). Interestingly, the expression of the BAM5 gene is highly variable, depending on growth conditions and sugar levels (Mita et al., 1997). The BAM5 protein can account for between 20% and 90% of the total β-amylase activity in crude extracts of leaves (Laby et al., 2001; Fulton et al., 2008). Furthermore, it has been previously noted that mutants affected in starch metabolism have high β-amylase activity, which in some cases has been attributed to BAM5 (Monroe and Preiss, 1990). The significance of this BAM5 regulation is unclear, but it is important to note that measurements of total β-amylase activity in crude extracts can be misleading because of it (e.g. in mutants lacking chloroplastic BAM isoforms).
5.4. The Role of Debranching Enzymes
DBE activity is required for the complete degradation of amylopectin. As described earlier in this chapter, there are four genes encoding DBE-like proteins in Arabidopsis. While two (ISA1 and ISA2) encode proteins that form a single enzyme involved in amylopectin biosynthesis (see Section 4.4, above), the other two (ISA3 and LDA) are important for starch breakdown (Wattebled et al., 2005; Delatte et al., 2006). There are differences in ISA3 and LDA in terms of the substrates they are able to attack, presumably depending on the length of the B-chain and the position and length of the A-chain attached to it (Figure 3). However, both ISA3 and LDA show high activity on β-limit dextrins (i.e. amylopectin digested with β-amylase, such that external chains are reduced to stubs of 2 or 3 glucose units), illustrating their preference for removing short branches (Wu et al., 2002; Hussain et al., 2003; Takashima et al., 2007) and consistent with roles in degradation. ISA3 seems to have a more important role in Arabidopsis than LDA, since isa3 mutants have a sex phenotype, whereas lda mutants have a wildtype phenotype. Loss of ISA3 also results in changes in the structure of the starch; isa3 amylopectin contains increased amounts of very short chains with degrees of polymerization (d.p.) 3 to 5. Double mutants lacking both ISA3 and LDA accumulate starch to a far greater extent than isa3 single mutants (Delatte et al., 2006). This shows that some starch breakdown is mediated by LDA if ISA3 is missing. Together, these results fit with a model for starch degradation in which long chains at the granule surface are degraded by β-amylases, resulting in short chains with d.p. 2 or more, which are removed by ISA3 and LDA (Figure 8).
5.5. α-Amylase
In contrast to β-amylases, α-amylases (AMY) act on internal α-1,4-bonds to release a variety of linear and branched oligosaccharides. In germinating cereal seeds, isoforms of AMY are secreted from the living aleurone cells and play a key role in the degradation of storage starch in the non-living starchy endosperm. This process fuels early seedling establishment (Fincher, 1989), as illustrated by the marked delay in seedling growth upon repression of the major secreted α-amylase expressed upon seed germination (Asatsuma et al., 2005).
In Arabidopsis, α-amylase does not have such a prominent role in starch degradation. There are three annotated α-amylases (AMY1, AMY2 and AMY3) in the Arabidopsis genome (Stanley et al., 2002). Only AMY3 has a predicted transit-peptide and cell fractionation experiments showed that it is indeed chloroplastic (Yu et al., 2005). Surprisingly, mutants lacking AMY3 alone, or all three α-amylases together, are indistinguishable from wild-type plants and have normal starch metabolism (Yu et al., 2005). It was therefore concluded that α-amylases are not part of the major pathway of starch degradation. However, subsequent studies have revealed that in situations where other enzymes are missing, AMY3 activity makes a significant impact on starch metabolism. This is particularly apparent in mutant plants lacking isoforms of DBEs. In isa3lda double mutants, starch degradation during the night is reduced compared to the wild type, but not abolished (Delatte et al., 2006). As starch is degraded, isa3lda plants accumulate the typical products of α-amylase - small soluble branched glucans (d.p. 8 to d.p. 30), which are not seen in the wild type. Furthermore, α-amylase activity and AMY3 protein amount are increased in isa3lda compared to wild-type plants (Delatte et al., 2006). Similar small branched glucans occur in quadruple mutant plants lacking all DBEs (Streb et al., 2008) although in this case, starch is almost completely replaced by the soluble phytoglycogen (see Section 4.4 above). Creation of the quintuple mutant lacking all DBEs and AMY3 abolishes the small branched glucans, showing that they are AMY3 products.
It is likely that AMY3 is the only α-amylase involved in degradation of glucans in the plastids in Arabidopsis, but little is known about the functions of AMY1 and AMY2. The AMY2 amino acid sequence does not include any obvious sub-cellular targeting information. In contrast, AMY1 possesses an amino-terminal signal peptide for targeting to the endomembrane system, and the protein is secreted rather than localized in plastids (Doyle et al., 2007). Therefore, it seems unlikely to be involved in transient starch breakdown. The AMY1 gene is induced by different stresses and by ABA and it has been hypothesized that AMY1 may play a role in disease resistance responses (e.g. degrading the starch of dead cells; Doyle et al., 2007). However, it is worth noting that different observations have been made in rice, where a secreted isoform of α-amylase most closely related to Arabidopsis AMY1 is targeted to the chloroplasts in leaves via the endomembrane system (Asatsuma et al., 2005; Kitajima et al., 2009), where it does have a function in starch metabolism. It is unclear whether this may also occur in Arabidopsis.
5.6. α-Glucan Phosphorylase
The only phosphorolytic enzyme involved in starch metabolism is the plastidial α-glucan Phosphorylase PHS1 (PHO1 in other species). The enzyme catalyses the reversible addition of the glucosyl moiety of Glc1P to the non-reducing end of an acceptor glucan, releasing orthophosphate (Pi). The reaction direction is dependent on the relative Glc1P and Pi concentrations (Rathore et al., 2009). It has been proposed that PHS1 acts predominantly in a phosphorolytic direction - i.e. contributing to starch breakdown - due to the high concentration of Pi relative to Glc1P (Wirtz et al., 1980; Kruger and ap Rees, 1983). However, genetic studies in several species and focussing on different tissues have yielded contrasting results. Thus, the precise function of plastidial Phosphorylase remains unclear. In Arabidopsis mutants lacking PHS1, there is no overall alteration in starch content (Zeeman et al., 2004), but the plants display increased sensitivity to drought stress and there is a local accumulation of starch around stress-induced lesions. In Chlamydomonas, sta4 mutants lacking PHOB (one of two plastidial Phosphorylase isoforms) have decreased starch contents under nitrogen-limiting conditions, when storage starch usually accumulates to high levels (Dauvillée et al., 2006). In this case, the authors speculated that the phenotype may be an indirect effect connected with protein-complex formation between PHOB and other starch metabolising enzymes (see Section 6.2, below). In rice, the loss of plastidial Phosphorylase (PHO1) leads to reduced endosperm starch content and shrunken seeds when grown at 20°C, though not when grown at 30°C (Satoh et al., 2008). These authors proposed that PHO1 may be involved in starch priming by elongating short chains. This view is in part derived from in-vitro studies with the heterologous PHO1 enzyme, which showed that it can elongate glucan chains even in the presence of high Pi concentrations (Hwang et al., 2010). Thus, the enzyme could generate long chains even under physiological conditions where net phosphorolytic degradation occurs (i.e. high Pi and low Glc1P). It is possible that the plastidial Phosphorylase is a versatile enzyme and that its physiological role is different between species, growing conditions and tissues. Further investigations of plastidial Phosphorylase functions are required.
An additional isoform of α-glucan Phosphorylase (PHS2) is present in the cytosol and implicated in the metabolism of cytosolic heteroglycans. These heteroglycans are proposed to form an intermediary carbohydrate pool between chloroplastic starch metabolism and cytosolic hexose-phosphates (see Section 5.9, below).
5.7. The Metabolism of Malto-oligosaccharides by Disproportionating Enzyme
The combined actions of β-amylase, α-amylase and DBEs will result in the release of maltose and longer malto-oligosaccharides into the chloroplast stroma. The malto-oligosaccharides can be further metabolised by β-amylases to yield maltose or maltotriose or by Phosphorylase to yield Glc1P and maltotetraose (maltotriose and maltotetraose are too short to act as substrates of β-amylase and Phosphorylase, respectively). Short malto-oligosaccharides are also metabolized by the glucanotransferase disproportionating enzyme (DPE1). In vitro studies showed that DPE1 can catalyse a range of reactions, transferring a part of one α-1,4-linked glucan chain to another, or to glucose. When presented with short malto-oligosaccharides, it produces glucose and a spectrum of linear oligosaccharides, the larger of which are again substrates for the other degradative enzymes. Thus, DPE1 activity, together with that of β-amylase and Phosphorylase will result in the production of maltose, glucose and Glc1P. Interestingly, maltose acts neither as an efficient donor nor acceptor for DPE1 (Lin and Preiss, 1988; Takaha et al., 1993; 1996). This exclusion of maltose means that its only fate at night is export to the cytosol (see Section 5.8, below). The importance of DPE1 in starch breakdown was confirmed by analysing dpe1 mutants (Critchley et al., 2001). The dpe1 mutant accumulates maltotriose - the by-product of β-amylolysis of malto-oligosaccharides - during starch breakdown at night. Furthermore, the mutant has a decreased rate of starch breakdown during the night resulting in a sex phenotype. This latter observation indicates that the accumulation of maltotriose may inhibit starch breakdown. Alternatively, DPE1 itself may act on the starch granule.
Interestingly, dpe1 mutants of Chlamydomonas show a lowstarch phenotype, which led to the proposal that DPE1 can contribute to starch synthesis through its capacity to transfer maltosyl residues to amylopectin in vitro (Colleoni et al., 1999; Wattebled et al., 2003). However, there is no evidence for such a role in Arabidopsis or potato (Takaha et al., 1998; Critchley et al., 2001).
5.8. Export of Starch Degradation Products to the Cytosol
The major breakdown products of starch during the night are maltose and, to a smaller extent, glucose and Glc1P. Current models for the further metabolism of these products suggest that glucose and maltose are exported to the cytosol. Glc1P can be metabolised in the chloroplast and supply chloroplast with substrates for glycolysis or the oxidative pentose phosphate pathway, either during the night or under stress or photorespiratory conditions, when starch is degraded during the day (Zeeman et al., 2004; Weise et al., 2006).
In the inner chloroplast envelope, there are distinct maltose and glucose transporters that facilitate diffusion of these sugars between the stroma and cytosol (Weber et al., 2000; Niittylä et al., 2004; Cho et al., 2011; Table 3). Mutants lacking the maltose transporter (MEX1) have a severe phenotype, as would be expected when the major export route for starch degradation products is blocked. The mex1 mutants accumulate large amounts of maltose (40-fold higher then wild-type plants) inside the chloroplast and have a sex phenotype (Niittylä et al., 2004; Lu and Sharkey, 2006). It seems likely that the elevated maltose levels lead to the feedback inhibition of starch degradation either via an as-yet uncharacterised regulatory mechanism, or by the competitive inhibition of starch degradative enzymes. In contrast, mutants lacking the glucose transporter (pGlcT) have a wild-type phenotype (Cho et al., 2011), consistent with the idea that glucose is a minor product of starch breakdown or that carbohydrate could be exported from the chloroplast in another form in pglct mutants, compensating for the glucose transport deficiency. Interestingly, when both glucose and maltose export are blocked, the plants develop a severe phenotype, with decreased chlorophyll, decreased photosynthetic rate, decreased starch content, and aberrant chloroplasts (Cho et al., 2011). This suggests that trapping starch degradation products within the chloroplast, causes a severe disruption of chloroplast function.
Table 3.
Arabidopsis genes coding for plastid inner envelope carbohydrate transporters mentioned in the text
A similar phenotype to that of the mex1pglct double mutant is seen when the mex1 and dpe1 mutations are combined (Stettler et al., 2009). The mex1dpe1 double mutant accumulates both maltose and maltotriose, and has reduced starch, chlorophyll content and photosynthesis. The similarity of the phenotypes of these double mutants is logical. On one hand, the activity of DPE1 would be a major source of glucose within the chloroplast during starch degradation (Critchley et al., 2001; Stettler et al., 2009), so loss of DPE1 should have a similar impact to the loss of pGlcT. On the other hand, the maltotriose that accumulates in the absence of DPE1 cannot cross the chloroplast envelope (Rost et al., 1996) and would thus also be trapped inside.
Ultrastructural studies of the mex1dpe1 double mutant revealed massive changes in chloroplast morphology, evidence of chloroplast lysis, and the presence of chloroplast remnants within vacuoles. These observations were used to explain the chlorotic phenotype, which was also visible (albeit to a lesser extent) in the mex1 single mutant. It was proposed that macro-autophagy may play a role in degrading non-functional chloroplasts (Stettler et al., 2009). Stettler et al., (2009) also provided genetic evidence that this severe cellular phenotype is due to the accumulation of starch degradation intermediates, as introduction of the pgm1 mutation to block starch synthesis, or mutations in GWD1 or BAMs to prevent the release of maltose from the granule, decreased the chlorosis.
It remains possible that there are other routes for the transport and/or metabolism of starch breakdown products. For example, it was recently shown that there is specific transport of Glc1P across the plastid envelope (Fettke et al., 2011) raising the possibility that Glc1P could be exported. On the other hand, Nicotiana tabacum and Physcomitrella patens both contain active plastidial hexokinases (Olsson et al., 2003; Giese et al., 2005). Thus, glucose could be metabolised in the stroma to Glc6P. The Arabidopsis hexokinase HXK3 is also located in the plastids but is expressed primarily in sink tissues (i.e. root and siliques; Karve et al., 2008). Earlier biochemical studies found that a large fraction of the hexokinase activities associated with isolated chloroplasts of spinach leaf mesophyll cells was attributable to an isoform bound to the outer envelope rather than in the stroma (Weise and Kiss, 1999). The role of stromal hexokinases in source tissues requires further investigation.
5.9. The Metabolism of Maltose in the Cytosol
Upon export to the cytosol, maltose is metabolized by a glucosyltransfer reaction catalysed by the cytosolic disproportionating enzyme DPE2 (Chia et al., 2004; Lu and Sharkey, 2004). DPE2 is specific for the β-anomeric form of maltose (Steichen et al., 2008; Dumez et al., 2006), which is the product of β-amylase (Weise et al., 2005). Arabidopsis dpe2 mutants show a sex phenotype and accumulate around 200 times as much maltose as the wild type (Chia et al., 2004; Lu and Sharkey, 2004). DPE2 transfers one glucose unit from maltose to an acceptor - probably a form of cytosolic heteroglycan - and releases the other glucose molecule (Fettke et al., 2006). The heteroglycan is composed of arabinose, galactose and, to a lesser extent mannose, fucose, xylose, rhamnose and glucose (Fettke et al., 2005). However, the polymer structure and its biosynthesis are not understood. The relatively low fraction of glucose is surprising, considering that the amount of maltose released during starch breakdown greatly exceeds the total amount of heteroglycans. This indicates that the heteroglycans do not represent a “store” of glucose per se and the glucose added to them from maltose must have a high turnover rate (Fettke et al., 2005). Hence, it was proposed that the heteroglycan serves as a short term buffer/acceptor for glucose in the cytosol (Chia et al., 2004).
The DPE2 reaction is reversible, and glucosyl moieties of the heteroglycan can be removed and added to free glucose, resulting in maltose formation (Fettke et al., 2006). Glucose can also be removed from heteroglycans by phosphorolysis via the cytosolic isoform of α-glucan Phosphorylase (PHS2), liberating Glc1P (Fettke et al., 2004). According to this metabolic scheme, equimolar amounts of glucose and Glc1P are the net products of maltose metabolism in the cytosol. However, it is worth noting that phs2 mutants have only a mild phenotype compared with dpe2 mutants. In phs2 leaves, maltose levels are elevated approximately four-fold at night, but the rate of starch degradation is normal. The heteroglycan pool has a slightly increased proportion of glucose, but again this increase is minor compared with the amount of carbon predicted to pass through the maltose pool. This suggests that, apart from PHS2, there is another route for the release of glucose transferred to cytosolic heteroglycans by DPE2 (Lu and Sharkey, 2006).
6. THE REGULATION OF STARCH METABOLISM
Establishing the key players of starch synthesis and breakdown makes it possible to address the mechanisms that regulate the pathways and determine when starch is made and how much accumulates. There is good evidence that the fluxes into and out of the starch pool in Arabidopsis leaves are regulated. This is nicely illustrated by growing plants under different light/dark cycles. In diurnal cycles with a short photoperiod, there is a relatively high rate of starch synthesis and slow rate of degradation, whereas in diurnal cycles with a long photoperiod this is reversed - starch synthesis is slow and degradation is fast (Smith and Stitt, 2007; Gibon et al., 2009; Figure 9). In both cases, the rate of degradation is essentially linear, such that the assimilated starch lasts for the duration of the night. Data consistent with this pattern was also obtained earlier with several other species suggesting that it is a common feature (Chatterton and Silvius, 1979). Several studies have also reported that there are delays in the onset of starch synthesis at the start of the day (Stitt et al., 1983; 1984; Gerhardt et al., 1987) and starch degradation at the start of the night (Gordon et al., 1980; Fondy and Geiger, 1985; Zeeman and Ap Rees, 1999), illustrating that the basis of regulation is not simply a light/dark switch. Indeed, starch degradation in the light can be induced by removing CO2 and imposing photorespiratory conditions (Weise et al., 2006). Changes in sugar levels or other metabolic intermediates may serve as the trigger for the fluxes into and out of the starch pool.
Figure 9.
Pattern of starch accumulation and degradation under different light/dark regimes.
(A) Diurnal pattern of starch content in Arabidopsis wild-type leaves. Plants grown either in 6-h light / 18-h dark (grey squares) or 12-h light /12-h dark (black squares). Image adapted from Gibon et al. (2004a) with permission from Wiley-Blackwell Publishing.
(B) The rates of starch synthesis during the light and degradation during the dark phase. In day-neutral conditions, synthesis and degradation rates are equal. In shorter photoperiods there are increased synthesis rates and decreased degradation rates (except in 2-h photoperiods, in which plants fail to grow). Graph adapted from Gibon et al. (2009) with permission from Wiley-Blackwell Publishing.
The flux of carbon into transitory starch is governed in large part by the regulation of AGPase activity (see Section 4.3, above; Geigenberger, 2011), which is effectively switched off at night through redox inactivation (Hendriks et al., 2003; Hädrich et al., 2012). It is uncertain to what extent the enzymes of starch biosynthesis themselves (i.e. SSs and BEs) are regulated. A recent survey of starch metabolic enzymes in Arabidopsis suggested that SS1, SS3 and BE2 are also redox-activated (Glaring et al., 2012). The in-vivo significance of this activation remains to be demonstrated. One study of wheat amyloplasts suggested that BE was activated through phosphorylation (Tetlow et al., 2004), but surprisingly few such data are available for the Arabidopsis enzymes (Kötting et al., 2010). One study of the Arabidopsis ss3 mutant reported increased starch contents compared to the wild type. It was proposed that SS3, in addition to its catalytic function, may play a regulatory role over other starch synthases, thereby controlling the rate of synthesis, (Zhang et al., 2005). However, this increased starch content of the ss3 mutant was not seen under all growth conditions in which it was studied (Zhang et al., 2008).
It seems likely that one or several enzymes involved in starch breakdown are inactive during the day. This seems logical as otherwise there would be a potentially futile cycle of synthesis and degradation. Pulse-chase radio-labelling studies (in which 14CO2 is supplied to photosynthesising plants for a short period and then replaced by unlabelled carbon dioxide) did not detect significant amounts of starch turnover during periods of net starch accumulation in wild-type plants (Schneider et al., 2002; Zeeman et al., 2002). If there was turnover, label incorporated into starch during the pulse would be released again during the chase, despite continued net accumulation of starch. Under certain conditions, turnover is readily detected using this method. For example, in tpt mutants where the export of those phosphates from the chloroplast is compromised, there is increased partitioning into starch and simultaneous starch degradation (Schneider et al., 2002). However, such labelling studies are relatively coarse, and would be unlikely to detect rapid turnover (such as the debranching steps integral to the maturation of amylopectin - see Section 4.4, above). A possible step for regulation is the process of reversible starch phosphorylation, as it precedes and facilitates glucan hydrolysis by solubilising the granule surface. In isa1 and isa2 mutants that accumulate soluble phytoglycogen in place of semicrystalline starch, maltose accumulates during the day (Delatte et al., 2005). This suggests that enzymes like β-amylases are active during the day but, in the wild type, may not have access to amylopectin as it rapidly assumes an insoluble form.
The potato GWD1 protein has been shown to be redox-regulated in vitro through the formation of a disulphide bridge between two cysteine residues (Mikkelsen et al., 2005). These cysteine residues are conserved in GWD1 homologues in other species, including Arabidopsis. There has been no direct demonstration that this redox-regulation is functionally significant in vivo, and in some ways it is counter-intuitive for a chloroplast-localised protein that is active at night to be redox regulated. This is because the paradigm for the reductive activation of stromal proteins is via the light-driven thioredoxin system (Buchanan and Balmer, 2005; Schürmann and Buchanan, 2008), such that the protein would be active during the day and inactive at night (as for AGPase; see above). For GWD1, there is evidence that the protein is active to an extent during the day, as newly-formed starch is phosphorylated (Blennow et al., 2000; Santelia et al., 2011). However, there is increased binding of GWD1 to starch granules and an increased rate of phosphorylation during the night, (Ritte et al., 2004), supporting a wealth of phenotypic evidence for a role of GWD1 in starch breakdown (Caspar et al., 1991; Lorberth et al., 1998; Yu et al., 2001). Thus it is necessary to consider either that there is another mechanism regulating the redox state of GWD1 at night (such as NADPH dependent thioredoxins NTRs; Michalska et al., 2009) or that redox regulation is not a major regulatory feature in vivo. Interestingly, GWD1 is not the only starch-degrading enzyme which is redox regulated. Sparla and colleagues (2006) demonstrated that one of the two active stromal β-amylases (BAM1) is also activated by reduction. This prompts the same questions as for GWD1, as BAM1 has been shown to be involved in nighttime starch degradation in the leaf mesophyll tissues (Fulton et al., 2008). However, an additional function was recently proposed for BAM1 in stomatal guard cells, where starch is believed to be degraded during the day to generate osmolites required for stomatal opening. In this case, redox-activation could be coupled to illumination (Valerio et al., 2011). Glaring et al. (2012) recently provided evidence from in-vitro studies that an additional BAM isoform is also subject to redox-activation, together with AMY3, ISA1/ISA2 and LDA.
Feedback inhibition of an enzyme-catalysed reaction by its products is another common regulatory mechanism. Although not directly demonstrated so far, product-inhibition could play a role in controlling the rate of starch breakdown. Recently, it was shown in vitro that linear glucan chains can inhibit both the phosphorylation of glucans by GWD1 (Hejazi et al., 2009) and their subsequent dephosphorylation by SEX4 (Hejazi et al., 2010). However, under normal growth conditions, longer soluble chains are hardly detectable during starch degradation making it unlikely that this is a major regulatory mechanism unless such chains occur at higher local concentrations inside the chloroplast (e.g. in close proximity to the starch granule surface). Evidence for product-inhibition also derives from mutants lacking proteins metabolising or transporting starch breakdown products. For example, blocking the export of maltose or its metabolism in the cytosol (by mutating MEX1 or DPE2 respectively) results in elevated maltose levels, but also increased starch amounts. This may result from the inhibition by maltose of one or more upstream enzymes. Furthermore, mutants lacking DPE1 also show a sex phenotype. It is plausible that maltotriose or other oligosaccharises may also feedback to inhibit starch hydrolytic activity. However, the concentrations of maltose or malto-oligosaccharides accumulating in these mutants are extremely high and probably not physiological for a wild-type plant. It is therefore unclear whether smaller changes in concentrations could serve to fine tune the starch degradation machinery.
6.1. Diurnal and Circadian Regulation of Gene Transcription
Several of the genes encoding starch metabolising enzymes show diurnal changes in expression (Harmer et al., 2000; Chandler et al., 2001; Tenorio et al., 2003; Smith et al., 2004; Lu et al., 2005). In particular, a set of about ten genes mostly involved in starch degradation have a very similar pattern of mRNA levels that peak at the end of the day, just prior to the onset of starch breakdown (Smith et al., 2004). In some cases, this expression was shown to be controlled by the circadian clock, such that when plants grown in a diurnal cycle were placed into continuous light, expression continued to oscillate with a 24-h periodicity (Harmer et al., 2000; Tenorio et al., 2003; Lu et al., 2005). In contrast, placing plants into continuous darkness abolished expression (Lu et al., 2005). The promoters of several of these genes contain a cis-regulatory ‘evening element’, through which this expression pattern is mediated (Harmer et al., 2000). Interestingly, comparable diurnal changes for some of the orthologous genes in potato have also been observed, suggesting an evolutionarily conserved mechanism of transcriptional regulation (Ferreira et al., 2010). Despite the evidence for strong diurnal/circadian regulation of transcription, the levels of the encoded proteins (when determined) generally do not fluctuate in the same way as the transcript, and instead remain relatively constant (Tenorio et al., 2003; Smith et al., 2004; Lu et al., 2005). This suggests that post transcriptional control plays an important role in regulating starch metabolism. It is possible that the diurnal cycling of transcripts is important for medium- to long-term adjustment of protein levels (Gibon et al., 2004b).
Despite the uncertainty over the significance of the circadian clock control of starch metabolic gene transcription, good evidence has been presented to show that the clock influences the fluxes into and out of leaf starch (Lu et al., 2005; Graf et al., 2010). When Arabidopsis plants grown in short days were transferred to long days, or vice versa, they were able to adjust their rate of starch degradation immediately and appropriately. If presented with an early and therefore unexpectedly long night, degradation was slower than normal. If presented with an extended day, and therefore an unexpectedly short night, the degradation rate was higher (Lu et al., 2005). It is difficult to see how the observed cycling of transcripts could explain such control. Further experiments with plants grown in abnormal diurnal cycles (17 h or 28 h rather than the normal 24 h) showed that wild-type plants were unable to adjust their starch metabolism appropriately (Graf et al., 2010). In 17-h cycles, plants always retained starch at the end of the night, whereas in 28-h cycles, plants always depleted their reserves after 24 h, before the end of the night. In the latter case, the depletion of starch was followed by the induction of starvation marker gene expression. In both abnormal cycles, growth was sub-optimal compared with plants grown in a 24-h diurnal cycle. Graf et al., (2010) also showed that plants lacking core components of the circadian clock (cca1lhy double mutants) had an altered pattern of starch degradation, with stores depleted before the end of the night, consistent with these plants having a short clock period.
6.2. Regulation by Protein Phosphorylation and Complex Formation
Further potential regulatory mechanisms include protein phosphorylation and the formation of multi-protein complexes (Kötting et al., 2010). Phosphorylation is a common mechanism to control protein function. By changing protein conformation and properties, it can change enzyme specificity and/or and interaction with other proteins. Large-scale phosphoproteomic approaches in Arabidopsis have identified a number of starch metabolic proteins as putative phosphoproteins in vivo, including plastidial PGI, PGM1, AGPase, SS3, GWD1 and GWD2, DPE2, AMY3, BAM1 and BAM3, LDA, pGlcT and MEX1 (see Kötting et al., 2010 and references therein). The list can be further extended if all plant proteomic studies are included (Kötting et al., 2010). Still the list is unlikely to be complete, as there is relatively little overlap between the phosphoproteomic studies that have been conducted to date, meaning that some phosphoproteins may have been missed. Currently, little is known about the effects of phosphorylation at the identified sites, though it seems likely that some of them are important for regulating starch metabolism.
Studies in maize and wheat endosperm showed that SS and BE isoforms occur in various combinations in protein complexes (Tetlow et al., 2004; 2008; Hennen-Bierwagen et al., 2008; 2009). Furthermore, Tetlow et al., (2004) demonstrated that wheat endosperm BEs and starch Phosphorylase assemble into a complex in a phosphorylation-dependent way. However, the protein kinases and phosphatases determining phosphorylation state, and therefore complex formation, remain to be identified. Comparable protein complexes have not yet been identified in Arabidopsis, although Sehnke et al. (2001) proposed that Arabidopsis starch metabolism could be regulated via 14-3-3 proteins, which preferentially bind phosphorylated proteins and mediate protein-protein interactions (Fu et al., 2000; Huber, 2007). The involvement of 14-3-3 proteins in starch metabolism was based on the finding that they associate with starch granules and that repression of plastid-localized isoforms changed starch properties and content in Arabidopsis leaves. An independent study of barley endosperm proteins identified a wide range of putative interaction partners of 14-3-3 proteins, several of which were involved in starch metabolism (GBSSI, SSI, SSII, BEIIa, α- and β-amylases; Alexander and Morris, 2006).
There are other intriguing proteins candidates which may be involved in protein complex formation. A recently-identified chloroplastic protein contains a carbohydrate binding module and a coiled-coil domain, but no obvious enzymatic domain (Lohmeier-Vogel et al., 2008). It was suggested that this protein might act as a scaffold, binding to starch with its CBM and interacting with other starch-related proteins via its coiled-coil domain. Another protein with a proposed regulatory function is LSF1. Although similar in sequence to the phosphoglucan phosphates SEX4 and LSF2, LSF1 appears to be inactive as a phosphoglucan phosphatase (Comparot-Moss et al., 2010). Interestingly, this protein also has a carbohydrate binding module, allowing it to bind starch granules, and a putative protein-protein interaction domain of the PDZ-type. Recently it was discovered that LSF1 forms a stable complex together with the chloroplastic β-amylase BAM1, which may serve to target the β-amylase to phosphorylated regions on the granule surface during starch degradation (Umhang and Zeeman, unpublished data). Further investigations of protein complex formation are required for understanding their importance for starch metabolism.
Although progress is being made in understanding the regulation of starch metabolism, much more work is required before we will be able to appreciate how the perception of environmental and endogenous conditions (light intensity, period and quality, temperature, biotic and abiotic stresses, sugar status, developmental stage etc.) can cause changes in allocation into and out of the starch pool. This is important because the conditions used for plant growth in the laboratory (e.g. constant light intensity, day length, temperature, nutrient and water status) do not reflect the natural environment to which the plants have adapted.
7. THE USE OF ARABIDOPSIS STARCH MUTANTS TO STUDY OTHER BIOLOGICAL PROCESSES
The central position of starch in Arabidopsis carbohydrate metabolism means that mutants affected in its metabolism can be used to study other biological processes. For example, several studies have used low starch mutants as a tool to investigate the impact of carbon starvation on plant growth and global gene expression (Gibon et al., 2004a; 2006; Bläsing et al., 2005; Wiese et al., 2007). As the mutant plants synthesise virtually no starch during the day, the buffering function is lost. Low starch plants accumulate higher levels of sugars during the day, but this only represents a small fraction of what accumulates as starch in the wild type. The sugars are rapidly depleted during the night, after which a starvation response occurs, with the induction of genes for lipid and protein degradation, and the repression of plants growth. This response is comparable to that seen when wild-type plants are kept in the dark beyond the end of a normal night (Gibon et al., 2004b; Usadel et al., 2008; Stitt et al., 2010).
It has also been recognised that mutants impaired in starch metabolism have developmental phenotypes, such as altered leaf morphology or altered flowering time. Low-starch plants such as pgm1, adg1 and pgi, or plants that are unable to degrade their starch during the night as sex1 show retarded growth and flower later than the respective wild-types, particularly in short-day conditions (Caspar et al., 1985; 1991; Lin et al., 1988b; Eimert et al., 1995; Corbesier et al., 1998; Yu et al., 2000). The late-flowering phenotypes are generally attributed to altered sucrose levels and transport of sucrose to the shoot apex. Thus they are probably indirect consequences of the defects in starch metabolism. This is consistent with experiments where applying sugars to the growth media was shown to revert the late-flowering phenotype in lowstarch pgi plants (Yu et al., 2000) or even induce early flowering, even if plants are grown in continuous darkness (Roldán et al., 1999). However, high sucrose concentrations can delay rather than promote flowering (Ohto et al., 2001) and the role of sucrose in floral initiation is still controversial. Although the pathways of floral transition are well studied at genetic level (i.e. autonomous, vernalization, photoperiodic and the gibberellins pathway, Amasino, 2010), it is unclear which of the different pathways are influenced by carbohydrate levels and the connection to starch metabolism has been mostly neglected.
Starch metabolism has also been implicated in the resistance to biotic and abiotic stresses, such as virus infection, extremes of temperature or drought stress (see Krasensky and Jonak, 2012 and references therein). Cold stress is a good example; Kaplan and Guy (2005) noted that the key intermediate of starch breakdown - maltose - increases rapidly in response to cold stress and suggested that it may serve to protect against photoinhibition (Kaplan and Guy, 2005). Plants deficient in BAM3 activity did not accumulate maltose or other soluble sugars during cold acclimation, and displayed reduced photochemical efficiency following subsequent freezing stress. A further study revealed that the failure of the gwd1 mutant (sex1) to accumulate sugars in response to a cold acclimatory period also resulted in susceptibility to subsequent freezing treatment (Yano et al., 2005). It seems likely that further investigations will reveal a central role for starch in the provision of metabolites and energy required to resist stress conditions.
Starch mutants have also been used in the study of gravitropism; the ability of the plant to detect their organ orientation relative to gravity and direct root and shoot growth accordingly (Masson et al., 2002). This is thought to occur in specialized cells (called statocytes) localized in the root cap (columella cells), and the endodermis of shoots and hypocotyls. The amyloplasts in these cells are thought to play a key role. The current view is that the amyloplasts respond rapidly to changes in the direction of gravity and sediment due to the high density of the starch they contain (around 1.5 g/cm3) compared with the surrounding cytoplasm. Although the mechanism by which the repositioning of the plastids is sensed is still debated, it was clearly demonstrated that the presence of starch promotes this response. This was illustrated by the fast realignment of root growth towards the gravity force after reorientation of wild-type plants and the very slow response of pgm1 mutants, which containing no detectable starch in the columella cells. Further use of mutants with intermediate starch revealed a good correlation between starch content and gravitropic sensitivity (Caspar et al., 1985; Kiss et al., 1996). The same mechanism seems to play a role in gravity sensing in stems (Weise and Kiss, 1999) and hypocotyls (Kiss and Edelmann, 1999), where excess starch can even cause hypersensitivity to gravity (Vitha et al., 2007)
8. UNRESOLVED QUESTIONS
There remain many gaps in our understanding of starch metabolism. For example, in the context of starch biosynthesis, the initiation and the control of granule form and size are aspects where our knowledge is sparse. Elucidating the functions of the biosynthetic enzymes at the molecular level does not provide a holistic understanding of these processes. Similarly, the molecular architecture of amylopectin is poorly defined. This is partly due to the fact that there is a complex interplay between the biosynthetic enzymes - as one enzyme acts, it produces, modifies or removes the substrate of the next, and vice versa. Added to this is the fact that each enzyme can act on a range of substrates rather than a single one. A compounding problem is that determining the actual structure of the amylopectin polymer is difficult, due to its uniform composition (i.e. just glucose units) and its branched nature. Analytical methods for parameters like chain length distributions give average measures, which can mask the structural heterogeneity that might underlie some of the features of the starch granule (see Zeeman et al., 2007).
It is likely that the remaining questions can be addressed in the future with a combination of advanced cell biology, protein analysis, carbohydrate analytical methods, and systems-level modelling. Knowledge of the precise localisation of the enzymes of starch biosynthesis within plastids and the possible proteinprotein interactions between them may give critical insight into how their activities are orchestrated. Accurate kinetics, substrate preferences, regulatory properties and three-dimensional structures of the Arabidopsis enzymes would also be of great value. This information, combined with a better understanding of amylopectin structure (in particular, the relative positioning of the branch points) should allow more refined models of amylopectin biosynthesis to be generated and tested. This type of information would complement the use of Arabidopsis as an excellent genetic system for studies of starch biosynthesis. Further goals should be to determine the generality of the models derived from Arabidopsis work in other model and non-model systems and to use the knowledge we have to improve starch crops. Furthermore, it should be possible, once the core set of protein components has been identified, to recreate starch granule biosynthesis in a heterologous system or in vitro. This should allow the biosynthetic apparatus to be manipulated and understood in much greater detail.
Some of the issues raised above for starch synthesis also apply to the pathway of starch degradation (e.g. the interdependency of the enzymes and the fact that they can work on a range of substrates). It is also a major challenge to study and understand the process that occurs at the interface between a semicrystalline substrate and the soluble phase (i.e. the granule and the plastid stroma, respectively). It is necessary to consider the association and dissociation of enzymes with the substrate surface, and their ability to modify it (e.g. through phosphorylation or hydrolysis) to allow subsequent access of other enzymes. There are some parts of the pathway of starch breakdown where far too little is known. The nature and metabolism of the proposed cytosolic heteroglycan is one such example. While we have an overview of the heteroglycan composition and its suitability as a substrate for DPE2 and PHS2, we know nothing about its structure, location in the cytosol or biosynthesis pathway.
The regulation of starch metabolism also remains a subject where more information is needed. Although some important control points have been identified (such as AGPase), together with regulatory features of the enzymes (e.g. allosteric or redox regulation), intermediate steps between the perception of endogenous and/or exogenous cues and the control of flux into and out of starch still need to be characterised. It is likely that signal transduction and response mechanisms will involve central integrators that not only control starch metabolism, but also other metabolic pathways and overall plant growth (e.g. the circadian clock, SnRK1 kinases; Baena-González et al., 2007; Graf et al., 2010). Perturbations in such core signalling processes are likely to result in the establishment of new equilibriums in plant growth and metabolism, with downstream or pleiotropic changes contributing significantly to the overall phenotype. Dissecting the components of complex responses may prove challenging.
Pleiotropic effects can influence the phenotypes of mutants affected in starch metabolism. For example, some starch-excess plants partition less carbon into starch than wild-type plants, partly compensating during the day for their inability to mobilise starch effectively at night (Zeeman and ap Rees, 1999). However, it is unclear how this change in partitioning is brought about. By offering an overview, high-throughput analytical tools (e.g. transcriptomics, proteomics, etc.) can help to reveal correlations that could potentially explain the system behaviour. When coupled with investigative molecular genetic techniques and accurate metabolic measurements, it should be possible to test these correlations and begin to understand better the control loops that integrate metabolism.
Footnotes
Citation: Sebastian Streb and Samuel C. Zeeman. (2012) Starch Metabolism in Arabidopsis. The Arabidopsis Book 9:e0160. doi:10.1199/tab.0160
elocation-id: e0160
First published on September 24, 2012: e0160. doi: 10.1199/tab.0160
REFERENCES
- Alexander R.D., Morris P.C. Aproteomic analysis of 14-3-3 binding proteins from developing barley grains. Proteomics. 2006;6:1886–1896. doi: 10.1002/pmic.200500548. [DOI] [PubMed] [Google Scholar]
- Amasino R. Seasonal and developmental timing of flowering. Plant J. 2010;61:1001–1013. doi: 10.1111/j.1365-313X.2010.04148.x. [DOI] [PubMed] [Google Scholar]
- Andriotis V.M., Pike M.J., Kular B., Rawsthorne S., Smith A.M. Starch turnover in developing oilseed embryos. New Phytol. 2010;187:791–804. doi: 10.1111/j.1469-8137.2010.03311.x. [DOI] [PubMed] [Google Scholar]
- Asatsuma S., Sawada C., Itoh K., Okito M., Kitajima A., Mitsui T. Involvement of α-amylase I-1 in starch degradation in rice chloroplasts. Plant Cell Physiol. 2005;46:858–869. doi: 10.1093/pcp/pci091. [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez E., Rolland F., Thevelein J.M., Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–942. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- Ball S., Marianne T., Dirick L., Fresnoy M., Delrue B., Decq A. A Chlamydomonas reinhardtii low-starch mutant is defective for 3-phosphoglycerate activation and orthophosphate inhibition of ADP-glucose pyrophosphorylase. Planta. 1991;185:17–26. doi: 10.1007/BF00194509. [DOI] [PubMed] [Google Scholar]
- Ball S., Guan H.P., James M., Myers A., Keeling P., Mouille G., Buléon A., Colonna P., Preiss J. From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell. 1996;86:349–352. doi: 10.1016/s0092-8674(00)80107-5. [DOI] [PubMed] [Google Scholar]
- Ball S.G., Morell M.K. From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu. Rev. Plant Biol. 2003;54:207–233. doi: 10.1146/annurev.arplant.54.031902.134927. [DOI] [PubMed] [Google Scholar]
- Ball S., Colleoni C., Cenci U., Raj J.N., Tirtiaux C. The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J. Exp. Bot. 2011;62:1775–1801. doi: 10.1093/jxb/erq411. [DOI] [PubMed] [Google Scholar]
- Barratt D.H., Derbyshire P., Findlay K., Pike M., Wellner N., Lunn J., Feil R., Simpson C., Maule A.J., Smith A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA. 2009;106:13124–13129. doi: 10.1073/pnas.0900689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunsgaard L., Lütken H., Mikkelsen R., Glaring M.A., Pham T.T., Blennow A. A novel isoform of glucan, water dikinase phosphorylates pre-phosphorylated α-glucans and is involved in starch degradation in Arabidopsis. Plant J. 2005;41:595–605. doi: 10.1111/j.1365-313X.2004.02322.x. [DOI] [PubMed] [Google Scholar]
- Beatty M.K., Rahman A., Cao H., Woodman W., Lee M., Myers A.M., James M.G. Purification and molecular genetic characterization of ZPU1, a pullulanase-type starch-debranching enzyme from maize. Plant Physiol. 1999;119:255–266. doi: 10.1104/pp.119.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya M.K., Smith A.M., Ellis T.H., Hedley C., Martin C. The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch-branching enzyme. Cell. 1990;60:115–122. doi: 10.1016/0092-8674(90)90721-p. [DOI] [PubMed] [Google Scholar]
- Bläsing O.E., Gibon Y., Günther M., Höhne M., Morcuende R., Osuna D., Thimm O., Usadel B., Scheible W.R., Stitt M. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell. 2005;17:3257–3281. doi: 10.1105/tpc.105.035261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauth S.L., Yao Y., Klucinec J.D., Shannon J.C., Thompson D.B., Guilitinan M.J. Identification of Mutator insertional mutants of starch-branching enzyme 2a in corn. Plant Physiol. 2001;125:1396–1405. doi: 10.1104/pp.125.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauth S.L., Kim K.N., Klucinec J., Shannon J.C., Thompson D., Guiltinan M. Identification of Mutator insertional mutants of starch-branching enzyme 1 (sbe1) in Zea mays L. Plant Mol. Biol. 2002;48:287–297. doi: 10.1023/a:1013335217744. [DOI] [PubMed] [Google Scholar]
- Blennow A., Bay-Smidt A.M., Olsen C.E., Moller B.L. The distribution of covalently bound phosphate in the starch granule in relation to starch crystallinity. Int. J. Biol. Macromol. 2000;27:211–218. doi: 10.1016/s0141-8130(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Blennow A., Engelsen S.B. Helix-breaking news: fighting crystalline starch energy deposits in the cell. Trends Plant Sci. 2010;15:236–240. doi: 10.1016/j.tplants.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Page T., Harrison E., Breeze E., Lim P.O., Nam H.G., Lin J.F., Wu S.H., Swidzinski J., Ishizaki K., Leaver C.J. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- Buchanan B.B., Balmer Y. Redox regulation: A broadening horizon. Annu. Rev. Plant Biol. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- Burrell M.M. Starch: the need for improved quality or quantity - an overview. J. Exp. Bot. 2003;54:451–456. doi: 10.1093/jxb/erg049. [DOI] [PubMed] [Google Scholar]
- Burton R.A., Bewley J.D., Smith A.M., Bhattacharyya M.K., Tatge H., Ring S., Bull V., Hamilton W.D., Martin C. Starch branching enzymes belonging to distinct enzyme families are differentially expressed during pea embryo development. Plant J. 1995;7:3–15. doi: 10.1046/j.1365-313x.1995.07010003.x. [DOI] [PubMed] [Google Scholar]
- Burton R.A., Jenner H., Carrangis L., Fahy B., Fincher G.B., Hylton C., Laurie D.A., Parker M., Waite D., van Wegen S., Verhoeven T., Denyer K. Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity. Plant J. 2002;31:97–112. doi: 10.1046/j.1365-313x.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- Bustos R., Fahy B., Hylton C.M., Seale R., Nebane N.M., Edwards A., Martin C., Smith A.M. Starch granule initiation is controlled by a heteromultimeric isoamylase in potato tubers. Proc. Natl. Acad. Sci. USA. 2004;101:2215–2220. doi: 10.1073/pnas.0305920101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T., Huber S.C., Somerville C. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 1985;79:11–17. doi: 10.1104/pp.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T., Lin T.P., Kakefuda G., Benbow L., Preiss J., Somerville C. Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol. 1991;95:1181–1188. doi: 10.1104/pp.95.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos H., Sanchez T., Denyer K., Tofino A.P., Rosero E.A., Dufour D., Smith A., Morante N., Pérez J.C., Fahy B. Induction and identification of a small-granule, high-amylose mutant in cassava (Manihot esculenta Crantz). J. Agric. Food Chem. 2008;56:7215–7222. doi: 10.1021/jf800603p. [DOI] [PubMed] [Google Scholar]
- Chandler J.W., Apel K., Melzer S. A novel putative β-amylase gene and ATβ-Amy from Arabidopsis thaliana are circadian regulated. Plant Sci. 2001;161:1019–1024. [Google Scholar]
- Chatterjee M., Berbezy P., Vyas D., Coates S., Barsby T. Reduced expression of a protein homologous to glycogenin leads to reduction of starch content in Arabidopsis leaves. Plant Sci. 2005;168:501–509. [Google Scholar]
- Chatterton N.J., Silvius J.E. Photosynthate partitioning into starch in soybean leaves: I. effects of photoperiod versus photosynthetic period duration. Plant Physiol. 1979;64:749–753. doi: 10.1104/pp.64.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Mu J., Farkas I., Huang D., Goebl M.G., Roach P.J. Requirement of the self-glucosylating initiator proteins Glg1p and Glg2p for glycogen accumulation in Saccharomyces cerevisiae. Mol. Cell Biol. 1995;15:6632–6640. doi: 10.1128/mcb.15.12.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia T., Thorneycroft D., Chapple A., Messerli G., Chen J., Zeeman S.C., Smith S.M., Smith A.M. A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J. 2004;37:853–863. doi: 10.1111/j.1365-313x.2003.02012.x. [DOI] [PubMed] [Google Scholar]
- Cho M.H., Lim H., Shin D.H., Jeon J.S., Bhoo S.H., Park Y.I., Hahn T.R. Role of the plastidic glucose translocator in the export of starch degradation products from the chloroplasts in Arabidopsis thaliana. New Phytol. 2011;190:101–112. doi: 10.1111/j.1469-8137.2010.03580.x. [DOI] [PubMed] [Google Scholar]
- Colleoni C., Dauvillée D., Mouille G., Buléon A., Gallant D., Bouchet B., Morell M., Samuel M., Delrue B., d'Hulst C., Bliard C., Nuzillard J.M., Ball S. Genetic and biochemical evidence for the involvement of α-1,4 glucanotransferases in amylopectin synthesis. Plant Physiol. 1999;120:993–1004. doi: 10.1104/pp.120.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comparot-Moss S., Kötting O., Stettler M., Edner C., Graf A., Weise S.E., Streb S., Lue W.L., MacLean D., Mahlow S., Ritte G., Steup M., Chen J., Zeeman S.C, Smith A.M. A putative phosphatase, LSF1, is required for normal starch turnover in Arabidopsis leaves. Plant Physiol. 2010;152:685–697. doi: 10.1104/pp.109.148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L., Lejeune P., Bernier G. The role of carbohydrates in the induction of flowering in Arabidopsis thaliana: comparison between the wild type and a starchless mutant. Planta. 1998;206:131–137. doi: 10.1007/s004250050383. [DOI] [PubMed] [Google Scholar]
- Craig J., Lloyd J.R., Tomlinson K., Barber L., Edwards A., Wang T.L., Martin C., Hedley C.L., Smith A.M. Mutations in the gene encoding starch synthase II profoundly alter amylopectin structure in pea embryos. Plant Cell. 1998;10:413–426. doi: 10.1105/tpc.10.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevillén P., Ballicora M.A., Mérida A., Preiss J., Romero J.M. The different large subunit isoforms of Arabidopsis thaliana ADPglucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme. J. Biol. Chem. 2003;278:28508–28515. doi: 10.1074/jbc.M304280200. [DOI] [PubMed] [Google Scholar]
- Crevillén P., Ventriglia T., Pinto F., Orea A., Mérida A., Romero J.M. Differential pattern of expression and sugar regulation of Arabidopsis thaliana ADP-glucose pyrophosphorylase-encoding genes. J. Biol. Chem. 2005;280:8143–8149. doi: 10.1074/jbc.M411713200. [DOI] [PubMed] [Google Scholar]
- Critchley J.H., Zeeman S.C., Takaha T., Smith A.M., Smith S.M. A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J. 2001;26:89–100. doi: 10.1046/j.1365-313x.2001.01012.x. [DOI] [PubMed] [Google Scholar]
- Crumpton-Taylor M., Grandison S., Png K.M., Bushby A.J., Smith A.M. Control of starch granule numbers in Arabidopsis chloroplasts. Plant Physiol. 2012;158:905–916. doi: 10.1104/pp.111.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva P.M.F.R., Eastmond P.J., Hill L.M., Smith A.M., Rawsthorne S. Starch metabolism in developing embryos of oilseed rape. Planta. 1997;203:480–487. [Google Scholar]
- Datta R., Chamusco K.C., Chourey P.S. Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol. 2002;130:1645–1656. doi: 10.1104/pp.006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvillée D., Colleoni C., Mouille G., Morell M.K., d'Hulst C., Wattebled F., Liénard L., Delvallé D., Ral J.P., Myers A.M., Ball S.G. Biochemical characterization of wild-type and mutant isoamylases of Chlamydomonas reinhardtii supports a function of the multimeric enzyme organization in amylopectin maturation. Plant Physiol. 2001;125:1723–1731. doi: 10.1104/pp.125.4.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvillée D., Chochois V., Steup M., Haebel S., Eckermann N., Ritte G., Ral J.P., Colleoni C., Hicks G., Wattebled F., Deschamps P., d'Hulst C., Liénard L., Cournac L., Putaux J.L., Dupeyre D., Ball S.G. Plastidial Phosphorylase is required for normal starch synthesis in Chlamydomonas reinhardtii. Plant J. 2006;48:274. doi: 10.1111/j.1365-313X.2006.02870.x. [DOI] [PubMed] [Google Scholar]
- Delatte T., Trevisan M., Parker M.L., Zeeman S.C. Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant J. 2005;41:815–830. doi: 10.1111/j.1365-313X.2005.02348.x. [DOI] [PubMed] [Google Scholar]
- Delatte T., Umhang M., Trevisan M., Eicke S., Thorneycroft D., Smith S.M., Zeeman S.C. Evidence for distinct mechanisms of starch granule breakdown in plants. J. Biol. Chem. 2006;281:12050–12059. doi: 10.1074/jbc.M513661200. [DOI] [PubMed] [Google Scholar]
- Delmer D.P. The purification and properties of sucrose synthetase from etiolated Phaseolus aureus seedlings. J. Biol. Chem. 1972;247:3822–3828. [PubMed] [Google Scholar]
- Delvallé D., Dumez S., Wattebled F., Roldán I., Planchot V., Berbezy P., Colonna P., Vyas D., Chatterjee M., Ball S., Mérida A., D'Hulst C. Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J. 2005;43:398–412. doi: 10.1111/j.1365-313X.2005.02462.x. [DOI] [PubMed] [Google Scholar]
- Denyer K., Clarke B., Hylton C., Tatge H., Smith A.M. The elongation of amylose and amylopectin chains in isolated starch granules. Plant J. 1996;10:1135–1143. [Google Scholar]
- Denyer K., Waite D., Motawia S., Moller B.L., Smith A.M. Granule-bound starch synthase I in isolated starch granules elongates malto-oligosaccharides processively. Biochem. J. 1999;340(1):183–191. [PMC free article] [PubMed] [Google Scholar]
- Denyer K., Johnson P., Zeeman S., Smith A.M. The control of amylose synthesis. J. Plant Physiol. 2001;158:479–487. [Google Scholar]
- Deschamps P., Moreau H., Worden A.Z., Dauvillée D., Ball S.G. Early gene duplication within chloroplastida and its correspondence with relocation of starch metabolism to chloroplasts. Genetics. 2008;178:2373–2387. doi: 10.1534/genetics.108.087205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges J.R., Colleoni C., James M.G., Myers A.M. Mutational analysis of the pullulanase-type debranching enzyme of maize indicates multiple functions in starch metabolism. Plant Cell. 2003;15:666–680. [Google Scholar]
- Doyle E.A., Lane A.M., Sides J.M., Mudgett M.B., Monroe J.D. An α-amylase (At4g25000) in Arabidopsis leaves is secreted and induced by biotic and abiotic stress. Plant Cell Environ. 2007;30:388–398. doi: 10.1111/j.1365-3040.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- Dumez S., Wattebled F., Dauvillée D., Delvallé D., Planchot V., Ball S.G., D'Hulst C. Mutants of Arabidopsis lacking starch branching enzyme II substitute plastidial starch synthesis by cytoplasmic maltose accumulation. Plant Cell. 2006;18:2694–2709. doi: 10.1105/tpc.105.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edner C., Li J., Albrecht T., Mahlow S., Hejazi M., Hussain H., Kaplan F., Guy C., Smith S.M., Steup M., Ritte G. Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial β-amylases. Plant Physiol. 2007;145:17–28. doi: 10.1104/pp.107.104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimert K., Wang S.M., Lue W.I., Chen J. Monogenic recessive mutations causing both late floral initiation and excess starch accumulation in Arabidopsis. Plant Cell. 1995;7:1703–1712. doi: 10.1105/tpc.7.10.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsen S.B., Madsen A.O., Blennow A., Motawia M.S., Moller B.L., Larsen S. The phosphorylation site in double helical amylopectin as investigated by a combined approach using chemical synthesis, crystallography and molecular modeling. FEBS Lett. 2003;541:137–144. doi: 10.1016/s0014-5793(03)00311-9. [DOI] [PubMed] [Google Scholar]
- Ferreira S.J., Senning M., Sonnewald S., Kessling P.M., Goldstein R., Sonnewald U. Comparative transcriptome analysis coupled to X-ray CT reveals sucrose supply and growth velocity as major determinants of potato tuber starch biosynthesis. BMC Genomics. 2010;11:93. doi: 10.1186/1471-2164-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettke J., Eckermann N., Poeste S., Pauly M., Steup M. The glycan substrate of the cytosolic (Pho 2) Phosphorylase isozyme from Pisum sativum L.: identification, linkage analysis and subcellular localization. Plant J. 2004;39:933–946. doi: 10.1111/j.1365-313X.2004.02181.x. [DOI] [PubMed] [Google Scholar]
- Fettke J., Eckermann N., Tiessen A., Geigenberger P., Steup M. Identification, subcellular localization and biochemical characterization of water-soluble heteroglycans (SHG) in leaves of Arabidopsis thaliana L.: distinct SHG reside in the cytosol and in the apoplast. Plant J. 2005;43:568–585. doi: 10.1111/j.1365-313X.2005.02475.x. [DOI] [PubMed] [Google Scholar]
- Fettke J., Chia T., Eckermann N., Smith A., Steup M. A transglucosidase necessary for starch degradation and maltose metabolism in leaves at night acts on cytosolic heteroglycans (SHG). Plant J. 2006;46:668–684. doi: 10.1111/j.1365-313X.2006.02732.x. [DOI] [PubMed] [Google Scholar]
- Fettke J., Malinova I., Albrecht T., Hejazi M., Steup M. Glucose-1-phosphate transport into protoplasts and chloroplasts from leaves of Arabidopsis. Plant Physiol. 2011;155:1723–1734. doi: 10.1104/pp.110.168716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher G.B. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989;40:305–346. [Google Scholar]
- Fondy B.R., Geiger D.R. Diurnal changes in allocation of newly fixed carbon in exporting sugar beet leaves. Plant Physiol. 1985;78:753–757. doi: 10.1104/pp.78.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzius T., Aeschbacher R., Wiemken A., Wingler A. Induction of ApL3 expression by trehalose complements the starchdeficient Arabidopsis mutant adg2-1 lacking ApL1, the large subunit of ADPglucose pyrophosphorylase. Plant Physiol. 2001;126:883–889. doi: 10.1104/pp.126.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H., Subramanian R.R., Masters S.C. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- Fu Y.B., Ballicora M.A., Leykam J.F., Preiss J. Mechanism of reductive activation of potato tuber ADPglucose pyrophosphorylase. J. Biol. Chem. 1998;273:25045–25052. doi: 10.1074/jbc.273.39.25045. [DOI] [PubMed] [Google Scholar]
- Fujita N., Kubo A., Francisco P.B., Jr, Nakakita M., Harada K., Minaka N., Nakamura Y. Purification, characterization, and cDNA structure of isoamylase from developing endosperm of rice. Planta. 1999;208:283–293. doi: 10.1007/s004250050560. [DOI] [PubMed] [Google Scholar]
- Fujita N., Yoshida M., Asakura N., Ohdan T., Miyao A., Hirochika H., Nakamura Y. Function and characterization of starch synthase I using mutants in rice. Plant Physiol. 2006;140:1070–1084. doi: 10.1104/pp.105.071845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D.C., Stettler M., Mettler T., Vaughan C.K., Li J., Francisco P., Gil M., Reinhold H., Eicke S., Messerli G., Dorken G., Halliday K., Smith A.M., Smith S.M., Zeeman S.C. β-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active β-amylases in Arabidopsis chloroplasts. Plant Cell. 2008;20:1040–1058. doi: 10.1105/tpc.107.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant D.J., Bouchet B., Baldwin P.M. Microscopy of starch: evidence of a new level of granule organization. Carbohydr. Polym. 1997;32:177–191. [Google Scholar]
- Gao M., Wanat J., Stinard P.S., James M.G., Myers A.M. Characterization of dull1, a maize gene coding for a novel starch synthase. Plant Cell. 1998;10:399–412. doi: 10.1105/tpc.10.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P. Regulation of starch biosynthesis in response to a fluctuating environment. Plant Physiol. 2011;155:1566–1577. doi: 10.1104/pp.110.170399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry M.S., Dowen R.H., 3rd, Worby C.A., Mattoo S., Ecker J.R., Dixon J.E. The phosphatase laforin crosses evolutionary boundaries and links carbohydrate metabolism to neuronal disease. J. Cell Biol. 2007;178:477–488. doi: 10.1083/jcb.200704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George G.M., van der Merwe M.J., Nunes-Nesi A., Bauer R., Fernie A.R., Kossmann J., Lloyd J.R. Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduces drought stress tolerance in Nicotiana benthamiana. Plant Physiol. 2010;154:55–66. doi: 10.1104/pp.110.157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Stitt M., Heldt H.W. Subcellular metabolite levels in spinach leaves - regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiol. 1987;83:399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y., Bläsing O.E., Palacios-Rojas N., Pankovic D., Hendriks J.H., Fisahn J., Höhne M., Günther M., Stitt M. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADPglucose pyrophosphorylase in the following light period. Plant J. 2004a;39:847–862. doi: 10.1111/j.1365-313X.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- Gibon Y., Bläsing O.E., Hannemann J., Carillo P., Höhne M., Hendriks J.H., Palacios N., Cross J., Selbig J., Stitt M. A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell. 2004b;16:3304–3325. doi: 10.1105/tpc.104.025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y., Usadel B., Bläsing O.E., Kamlage B., Höhne M., Trethewey R., Stitt M. Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol. 2006;7:R76. doi: 10.1186/gb-2006-7-8-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y., Pyl E.T., Sulpice R., Lunn J.E., Höhne M., Günther M., Stitt M. Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ. 2009;32:859–874. doi: 10.1111/j.1365-3040.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- Giese J.O., Herbers K., Hoffmann M., Klösgen R.B., Sonnewald U. Isolation and functional characterization of a novel plastidic hexokinase from Nicotiana tabacum. FEBS Lett. 2005;579:827–831. doi: 10.1016/j.febslet.2004.12.071. [DOI] [PubMed] [Google Scholar]
- Glaring M.A., Koch C.B., Blennow A. Genotype-specific spatial distribution of starch molecules in the starch granule: a combined CLSM and SEM approach. Biomacromol. 2006;7:2310–2320. doi: 10.1021/bm060216e. [DOI] [PubMed] [Google Scholar]
- Glaring M.A., Zygadlo A., Thorneycroft D., Schulz A., Smith S.M., Blennow A., Baunsgaard L. An extra-plastidial α-glucan, water dikinase from Arabidopsis phosphorylates amylopectin in vitro and is not necessary for transient starch degradation. J. Exp. Bot. 2007;58:3949–3960. doi: 10.1093/jxb/erm249. [DOI] [PubMed] [Google Scholar]
- Glaring M.A., Skryhan K., Kötting O., Zeeman S.C., Blennow A. Comprehensive survey of redox sensitive starch metabolising enzymes in Arabidopsis thaliana. Plant Physiol. Biochem. 2012;58:89–97. doi: 10.1016/j.plaphy.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Gordon A.J., Ryle G.J.A., Webb G. The relationship between sucrose and starch during dark export from leaves of uniculm barley. J. Exp. Bot. 1980;31:845–850. [Google Scholar]
- Graf A., Schlereth A., Stitt M., Smith A.M. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc. Natl. Acad. Sci. USA. 2010;107:9458–9463. doi: 10.1073/pnas.0914299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H., Li P., Imparl-Radosevich J., Preiss J., Keeling P. Comparing the properties of Escherichia coli branching enzyme and maize branching enzyme. Arch. Biochem. Biophys. 1997;342:92–98. doi: 10.1006/abbi.1997.0115. [DOI] [PubMed] [Google Scholar]
- Hädrich N., Hendriks J.H., Kötting O., Arrivault S., Feil R., Zeeman S.C., Gibon Y., Schulze W.X., Stitt M., Lunn J.E. Mutagenesis of cysteine 81 prevents dimerization of the APS1 subunit of ADPglucose pyrophosphorylase and alters diurnal starch turnover in Arabidopsis thaliana leaves. Plant J. 2012;70:231–242. doi: 10.1111/j.1365-313X.2011.04860.x. [DOI] [PubMed] [Google Scholar]
- Hansen P.I., Spraul M., Dvortsak P., Larsen F.H., Blennow A., Motawia M.S., Engelsen S.B. Starch phosphorylation - maltosidic restrains upon 3′- and 6′-phosphorylation investigated by chemical synthesis, molecular dynamics and NMR spectroscopy. Biopolymers. 2009;91:179–193. doi: 10.1002/bip.21111. [DOI] [PubMed] [Google Scholar]
- Hanson K.R., McHale N.A. A starchless mutant of Nicotiana sylvestris containing a modified plastid phosphoglucomutase. Plant Physiol. 1988;88:838–844. doi: 10.1104/pp.88.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer S.L., Hogenesch J.B., Straume M., Chang H.S., Han B., Zhu T., Wang X., Kreps J.A., Kay S.A. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 2000;290:2110–2113. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- Harrison C.J., Hedley C.L., Wang T.L. Evidence that the rug3 locus of pea (Pisum sativum L.) encodes plastidial phosphoglucomutase confirms that the imported substrate for starch synthesis in pea amyloplasts is glucose-6-phosphate. Plant J. 1998;13:753–762. [Google Scholar]
- Hejazi M., Fettke J., Haebel S., Edner C., Paris O., Frohberg C., Steup M., Ritte G. Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J. 2008;55:323–334. doi: 10.1111/j.1365-313x.2008.03513.x. [DOI] [PubMed] [Google Scholar]
- Hejazi M., Fettke J., Paris O., Steup M. The two plastidial starch-related dikinases sequentially phosphorylate glucosyl residues at the surface of both the A- and B-type allomorphs of crystallized maltodextrins but the mode of action differs. Plant Physiol. 2009;150:962–976. doi: 10.1104/pp.109.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi M., Fettke J., Kötting O., Zeeman S.C., Steup M. The Laforin-like dual-specificity phosphatase SEX4 from Arabidopsis hydrolyzes both C6- and C3-phosphate esters introduced by starchrelated dikinases and thereby affects phase transition of α-glucans. Plant Physiol. 2010;152:711–722. doi: 10.1104/pp.109.149914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks J.H., Kolbe A., Gibon Y., Stitt M., Geigenberger P. ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol. 2003;133:838–849. doi: 10.1104/pp.103.024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennen-Bierwagen T.A., Liu F., Marsh R.S., Kim S., Gan Q., Tetlow I.J., Emes M.J., James M.G., Myers A.M. Starch biosynthetic enzymes from developing maize endosperm associate in multisubunit complexes. Plant Physiol. 2008;146:1892–1908. doi: 10.1104/pp.108.116285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennen-Bierwagen T.A., Lin Q., Grimaud F., Planchot V., Keeling P.L., James M.G., Myers A.M. Proteins from multiple metabolic pathways associate with starch biosynthetic enzymes in high molecular weight complexes: a model for regulation of carbon allocation in maize amyloplasts. Plant Physiol. 2009;149:1541–1559. doi: 10.1104/pp.109.135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizukuri S. Relationship between the distribution of the chainlength of amylopectin and the crystalline-structure of starch granules. Carbohydr. Res. 1985;141:295–306. [Google Scholar]
- Huber S.C. Exploring the role of protein phosphorylation in plants: from signalling to metabolism. Biochem. Soc. Trans. 2007;35:28–32. doi: 10.1042/BST0350028. [DOI] [PubMed] [Google Scholar]
- Hussain H., Mant A., Seale R., Zeeman S., Hinchliffe E., Edwards A., Hylton C., Bornemann S., Smith A.M., Martin C., Bustos R. Three isoforms of isoamylase contribute different catalytic properties for the debranching of potato glucans. Plant Cell. 2003;15:133–149. doi: 10.1105/tpc.006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.K., Nishi A., Satoh H., Okita T.W. Rice endosperm-specific plastidial α-glucan Phosphorylase is important for synthesis of short-chain malto-oligosaccharides. Arch. Biochem. Biophys. 2010;495:82–92. doi: 10.1016/j.abb.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Iglesias A.A., Barry G.F., Meyer C., Bloksberg L., Nakata P.A., Greene T., Laughlin M.J., Okita T.W., Kishore G.M., Preiss J. Expression of the potato-tuber ADPglucose pyrophosphorylase in Escherichia coli. J. Biol. Chem. 1993;268:1081–1086. [PubMed] [Google Scholar]
- Ishizaki Y., Taniguchi H., Maruyama Y., Nakamura M. Debranching enzymes of potato tubers (Solanum tuberosum L.) .1. Purification and some properties of potato isoamylase. Agric. Biol. Chem. 1983;47:771–779. [Google Scholar]
- James M.G., Robertson D.S., Myers A.M. Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell. 1995;7:417–429. doi: 10.1105/tpc.7.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane J.L., Kasemsuwan T., Leas S., Zobel H., Robyt J.F. Anthology of starch granule morphology by scanning electron microscopy. Starch-Stärke. 1994;46:121–129. [Google Scholar]
- Jenkins J.P.J., Cameron R.E., Donald A.M. A universal feature in the structure of starch granules from different botanical sources. Starch-Stärke. 1993;45:417–420. [Google Scholar]
- Kaplan F., Guy C.L. RNA interference of Arabidopsis β-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J. 2005;44:730–743. doi: 10.1111/j.1365-313X.2005.02565.x. [DOI] [PubMed] [Google Scholar]
- Karve A., Rauh B.L., Xia X., Kandasamy M., Meagher R.B., Sheen J., Moore B.D. Expression and evolutionary features of the hexokinase gene family in Arabidopsis. Planta. 2008;228:411–425. doi: 10.1007/s00425-008-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger S., Leroch M., Huynen M.A., Wahl M., Neuhaus H.E., Tjaden J. Molecular and biochemical analysis of the plastidic ADPglucose transporter (ZmBT1) from Zea mays. J. Biol. Chem. 2007;282:22481–22491. doi: 10.1074/jbc.M702484200. [DOI] [PubMed] [Google Scholar]
- Kirchberger S., Tjaden J., Neuhaus H.E. Characterization of the Arabidopsis Brittle1 transport protein and impact of reduced activity on plant metabolism. Plant J. 2008;56:51–63. doi: 10.1111/j.1365-313X.2008.03583.x. [DOI] [PubMed] [Google Scholar]
- Kiss J.Z., Wright J.B., Caspar T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol. Plant. 1996;97:237–244. doi: 10.1034/j.1399-3054.1996.970205.x. [DOI] [PubMed] [Google Scholar]
- Kiss J.Z., Edelmann R.E. Spaceflight experiments with Arabidopsis starch-deficient mutants support a statolith-based model for graviperception. Adv. Space. Res. 1999;24:755–762. doi: 10.1016/s0273-1177(99)00409-3. [DOI] [PubMed] [Google Scholar]
- Kitajima A., Asatsuma S., Okada H., Hamada Y., Kaneko K., Nanjo Y., Kawagoe Y., Toyooka K., Matsuoka K., Takeuchi M., Nakano A., Mitsui T. The rice α-amylase glycoprotein is targeted from the Golgi apparatus through the secretory pathway to the plastids. Plant Cell. 2009;21:2844–2858. doi: 10.1105/tpc.109.068288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K.E. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Kofler H., Häusler R.E., Schulz B., Gröner F., Flügge U.I., Weber A. Molecular characterisation of a new mutant allele of the plastid phosphoglucomutase in Arabidopsis, and complementation of the mutant with the wild-type cDNA. Mol. Gen. Genet. 2000;263:978–986. doi: 10.1007/pl00008698. [DOI] [PubMed] [Google Scholar]
- Kolbe A., Tiessen A., Schluepmann H., Paul M., Ulrich S., Geigenberger P. Trehalose 6-phosphate regulates starch synthesis via post-translational redox activation of ADPglucose pyrophosphorylase. Proc. Natl. Acad. Sci. USA. 2005;102:11118–11123. doi: 10.1073/pnas.0503410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmann J., Lloyd J. Understanding and influencing starch biochemistry. Crit. Rev. Biochem. Mol. Biol. 2000;35:141–196. [PubMed] [Google Scholar]
- Kötting O., Kossmann J., Zeeman S.C., Lloyd J.R. Regulation of starch metabolism: the age of enlightenment? Curr. Opin. Plant Biol. 2010;13:321–329. doi: 10.1016/j.pbi.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Kötting O., Pusch K., Tiessen A., Geigenberger P., Steup M., Ritte G. Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiol. 2005;137:242–252. doi: 10.1104/pp.104.055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötting O., Santelia D., Edner C., Eicke S., Marthaler T., Gentry M.S., Comparot-Moss S., Chen J., Smith A.M., Steup M., Ritte G., Zeeman S.C. STARCH-EXCESS4 is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell. 2009;21:334–346. doi: 10.1105/tpc.108.064360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov S.S., Blennow A., Krivandin A.V., Yuryev V.P. Structural and thermodynamic properties of starches extracted from GBSS and GWD suppressed potato lines. Int. J. Biol. Macromol. 2007;40:449–460. doi: 10.1016/j.ijbiomac.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Krasensky J., Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012;63:1593–1608. doi: 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger N.J., ap Rees T. Properties of α-glucan Phosphorylase from pea chloroplasts. Phytochem. 1983;22:1891–1898. [Google Scholar]
- Kuang A., Musgrave M.E. Dynamics of vegetative cytoplasm during generative cell formation and pollen maturation in Arabidopsis thaliana. Protoplasma. 1996;194:81–90. doi: 10.1007/BF01273170. [DOI] [PubMed] [Google Scholar]
- Kubo A., Rahman S., Utsumi Y., Li Z., Mukai Y., Yamamoto M., Ugaki M., Harada K., Satoh H., Konik-Rose C., Morell M., Nakamura Y. Complementation of sugary-1 phenotype in rice endosperm with the wheat isoamylase1 gene supports a direct role for isoamylase1 in amylopectin biosynthesis. Plant Physiol. 2005;137:43–56. doi: 10.1104/pp.104.051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A., Colleoni C., Dinges J.R., Lin Q., Lappe R.R., Rivenbark J.G., Meyer A.J., Ball S.G., James M.G., Hennen-Bierwagen T.A., Myers A.M. Functions of heteromeric and homomeric isoamylase-type starch-debranching enzymes in developing maize endosperm. Plant Physiol. 2010;153:956–969. doi: 10.1104/pp.110.155259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laby R.J., Kim D., Gibson S.I. The ram1 mutant of Arabidopsis exhibits severely decreased β-amylase activity. Plant Physiol. 2001;127:1798–1807. [PMC free article] [PubMed] [Google Scholar]
- Lao N.T., Schoneveld O., Mould R.M., Hibberd J.M., Gray J.C., Kavanagh T.A. An Arabidopsis gene encoding a chloroplasttargeted β-amylase. Plant J. 1999;20:519–527. doi: 10.1046/j.1365-313x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- Lasceve G., Leymarie J., Vavasseur A. Alterations in lightinduced stomatal opening in a starch-deficient mutant of Arabidopsis thaliana L. deficient in chloroplast phosphoglucomutase activity. Plant Cell Environ. 1997;20:350–358. [Google Scholar]
- Leterrier M., Holappa L.D., Broglie K.E., Beckles D.M. Cloning, characterisation and comparative analysis of a starch synthase IV gene in wheat: functional and evolutionary implications. BMC Plant Biol. 2008;8:98. doi: 10.1186/1471-2229-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y., Shorrosh B., Beisson F., Andersson M.X., Arondel V., Bates P.D., Baud S., Bird D., Debono A., Durrett T.P., Franke R.B., Graham I.A., Katayama K., Kelly A.A., Larson T., Markham J.E., Miquel M., Molina I., Nishida I., Rowland O., Samuels L., Schmid K.M., Wada H., Welti R., Xu C., Zallot R., Ohlrogge J. Acyl-lipid metabolism. The Arabidopsis book / American Society of Plant Biologists. 2010;8:e0133. doi: 10.1199/tab.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.Y., Li W.H., Lee B., Laroche A., Cao L.P., Lu Z.X. Morphological characterization of triticale starch granules during endosperm development and seed germination. Can. J. Plant Sci. 2011;91:57–67. [Google Scholar]
- Li J., Almagro G., Muñoz F.J., Baroja-Fernández E., Bahaji A., Montera M., Hidalgo M., Sánchez-López A.M., Ezquer I., Sesma M.T., Pozueta-Romero J. Post-translational redox modification of ADP-glucose pyrophosphorylase in response to light is not a major determinant of fine regulation of transitory starch accumulation in Arabidopsis leaves. Plant Cell Physiol. 2012;53:433–444. doi: 10.1093/pcp/pcr193. [DOI] [PubMed] [Google Scholar]
- Lin T.P., Preiss J. Characterization of D-enzyme (4-α-glucanotransferase) in Arabidopsis leaf. Plant Physiol. 1988;86:260–265. doi: 10.1104/pp.86.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.P., Caspar T., Somerville C.R., Preiss J. A starch deficient mutant of Arabidopsis thaliana with low ADPglucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol. 1988a;88:1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.P., Caspar T., Somerville C., Preiss J. Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol. 1988b;86:1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J.R., Kossmann J., Ritte G. Leaf starch degradation comes out of the shadows. Trends Plant Sci. 2005;10:130–137. doi: 10.1016/j.tplants.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Lohmeier-Vogel E.M., Kerk D., Nimick M., Wrobel S., Vickerman L., Muench D.G., Moorhead G.B. Arabidopsis At5g39790 encodes a chloroplast-localized, carbohydrate-binding, coiled-coil domain-containing putative scaffold protein. BMC Plant Biol. 2008;8:120. doi: 10.1186/1471-2229-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomako J., Lomako W.M., Whelan W.J. A self-glucosylating protein is the primer for rabbit muscle glycogen biosynthesis. FASEB J. 1988;2:3097–3103. doi: 10.1096/fasebj.2.15.2973423. [DOI] [PubMed] [Google Scholar]
- Lorberth R., Ritte G., Willmitzer L., Kossmann J. Inhibition of a starch-granule-bound protein leads to modified starch and repression of cold sweetening. Nature Biotechnol. 1998;16:473–477. doi: 10.1038/nbt0598-473. [DOI] [PubMed] [Google Scholar]
- Lu Y., Sharkey T.D. The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta. 2004;218:466–473. doi: 10.1007/s00425-003-1127-z. [DOI] [PubMed] [Google Scholar]
- Lu Y., Gehan J.P., Sharkey T.D. Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol. 2005;138:2280–2291. doi: 10.1104/pp.105.061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Sharkey T.D. The importance of maltose in transitory starch breakdown. Plant Cell Environ. 2006;29:353–366. doi: 10.1111/j.1365-3040.2005.01480.x. [DOI] [PubMed] [Google Scholar]
- Lu Y., Steichen J.M., Yao J., Sharkey T.D. The role of cytosolic alpha-glucan Phosphorylase in maltose metabolism and the comparison of amylomaltase in Arabidopsis and Escherichia coli. Plant Physiol. 2006;142:878–889. doi: 10.1104/pp.106.086850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn J.E., Feil R., Hendriks J.H., Gibon Y., Morcuende R., Osuna D., Scheible W.R., Carillo P., Hajirezaei M.R., Stitt M. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem. J. 2006;397:139–148. doi: 10.1042/BJ20060083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor E.A. Relationships between structure and activity in the α-amylase family of starch-metabolizing enzymes. Starch-Stärke. 1993;45:232–237. [Google Scholar]
- Masson P.H., Tasaka M., Morita M.T., Guan C., Chen R., Boonsirichai K. Arabidopsis thaliana: a model for the study of root and shoot gravitropism. The Arabidopsis book / American Soc. Plant Biologists. 2002:1. doi: 10.1199/tab.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska J., Zauber H., Buchanan B.B., Cejudo F.J., Geigenberger P. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc. Natl. Acad. Sci. USA. 2009;106:9908–9913. doi: 10.1073/pnas.0903559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen R., Mutenda K.E., Mant A., Schürmann P., Blennow A. α-glucan, water dikinase (GWD): a plastidic enzyme with redox-regulated and coordinated catalytic activity and binding affinity. Proc. Natl. Acad. Sci. USA. 2005;102:1785–1790. doi: 10.1073/pnas.0406674102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S., Murano N., Akaike M., Nakamura K. Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for beta-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J. 1997;11:841–851. doi: 10.1046/j.1365-313x.1997.11040841.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T., Wright L., Fujiwara S., Cremer F., Lee K., Onouchi H., Mouradov A., Fowler S., Kamada H., Putterill J., Coupland G. Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell. 2005;17:2255–2270. doi: 10.1105/tpc.105.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Kawasaki T., Shimada H., Satoh H., Kobayashi E., Okumura S., Arai Y., Baba T. Alteration of the structural properties of starch components by the lack of an isoform of starch branching enzyme in rice seeds. J. Biol. Chem. 1993;268:19084–19091. [PubMed] [Google Scholar]
- Monroe J.D., Preiss J. Purification of a β-amylase that accumulates in Arabidopsis thaliana mutants defective in starch metabolism. Plant Physiol. 1990;94:1033–1039. doi: 10.1104/pp.94.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M.K., Blennow A., Kosar-Hashemi B., Samuel M.S. Differential expression and properties of starch branching enzyme isoforms in developing wheat endosperm. Plant Physiol. 1997;113:201–208. doi: 10.1104/pp.113.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M.K., Kosar-Hashemi B., Cmiel M., Samuel M.S., Chandler P., Rahman S., Buleon A., Batey I.L., Li Z. Barley sex6 mutants lack starch synthase IIa activity and contain a starch with novel properties. Plant J. 2003;34:173–185. doi: 10.1046/j.1365-313x.2003.01712.x. [DOI] [PubMed] [Google Scholar]
- Mouille G., Maddelein M.L., Libessart N., Talaga P., Decq A., Delrue B., Ball S. Preamylopectin processing: a mandatory step for starch biosynthesis in plants. Plant Cell. 1996;8:1353–1366. doi: 10.1105/tpc.8.8.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz F.J., Baroja-Fernández E., Moran-Zorzano M.T., Viale A.M., Etxeberria E., Alonso-Casajús N., Pozueta-Romero J. Sucrose synthase controls both intracellular ADP glucose levels and starch biosynthesis in source leaves. Plant Cell Physiol. 2005;46:1366–1376. doi: 10.1093/pcp/pci148. [DOI] [PubMed] [Google Scholar]
- Muñoz F.J., Zorzano M.T.M., Alonso-Casajús N., Baroja-Fernández E., Etxeberria E., Pozueta-Romero J. New enzymes, new pathways and an alternative view on starch biosynthesis in both photosynthetic and heterotrophic tissues of plants. Biocatal. Biotransformation. 2006;24:63–76. [Google Scholar]
- Myers A.M., Morell M.K., James M.G., Ball S.G. Recent progress toward understanding biosynthesis of the amylopectin crystal. Plant Physiol. 2000;122:989–997. doi: 10.1104/pp.122.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Utsumi Y., Sawada T., Aihara S., Utsumi C., Yoshida M., Kitamura S. Characterization of the reactions of starch branching enzymes from rice endosperm. Plant Cell Physiol. 2010;51:776–794. doi: 10.1093/pcp/pcq035. [DOI] [PubMed] [Google Scholar]
- Nashilevitz S., Melamed-Bessudo C., Aharoni A., Kossmann J., Wolf S., Levy A.A. The legwd mutant uncovers the role of starch phosphorylation in pollen development and germination in tomato. Plant J. 2009;57:1–13. doi: 10.1111/j.1365-313X.2008.03664.x. [DOI] [PubMed] [Google Scholar]
- Niewiadomski P., Knappe S., Geimer S., Fischer K., Schulz B., Unte U.S., Rosso M.G., Ache P., Flügge U.I., Schneider A. The Arabidopsis plastidic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. Plant Cell. 2005;17:760–775. doi: 10.1105/tpc.104.029124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittylä T., Messerli G., Trevisan M., Chen J., Smith A.M., Zeeman S.C. A previously unknown maltose transporter essential for starch degradation in leaves. Science. 2004;303:87–89. doi: 10.1126/science.1091811. [DOI] [PubMed] [Google Scholar]
- Niittylä T., Comparot-Moss S., Lue W.L., Messerli G., Trevisan M., Seymour M.D., Gatehouse J.A., Villadsen D., Smith S.M., Chen J., Zeeman S.C, Smith A.M. Similar protein phosphatases control starch metabolism in plants and glycogen metabolism in mammals. J. Biol. Chem. 2006;281:11815–11818. doi: 10.1074/jbc.M600519200. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A.C., Pérez S. The relationship between internal chain length of amylopectin and crystallinity in starch. Biopolymers. 1999;50:381–390. doi: 10.1002/(SICI)1097-0282(19991005)50:4<381::AID-BIP4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ohto M., Onai K., Furukawa Y., Aoki E., Araki T., Nakamura K. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol. 2001;127:252–261. doi: 10.1104/pp.127.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T., Thelander M., Ronne H. A novel type of chloroplast stromal hexokinase is the major glucose-phosphorylating enzyme in the moss Physcomitrella patens. J. Biol. Chem. 2003;278:44439–44447. doi: 10.1074/jbc.M306265200. [DOI] [PubMed] [Google Scholar]
- Oostergetel G.T., Vanbruggen E.F.J. The crystalline domains in potato starch granules are arranged in a helical fashion. Carbohydr. Polym. 1993;21:7–12. [Google Scholar]
- Overlach S., Diekmann W., Raschke K. Phosphate translocator of isolated guard-cell chloroplasts from Pisum sativum L. transports glucose 6-phosphate. Plant Physiol. 1993;101:1201–1207. doi: 10.1104/pp.101.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin F., Simonneau T., Rolland G., Dauzat M., Muller B. Control of leaf expansion: a developmental switch from metabolics to hydraulics. Plant Physiol. 2011;156:803–815. doi: 10.1104/pp.111.176289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron N.J., Keeling P.J. Common evolutionary origin of starch biosynthetic enzymes in green and red algae. J. Phycol. 2005;41:1131–1141. [Google Scholar]
- Pérez S., Bertoft E. The molecular structures of starch components and their contribution to the architecture of starch granules: a comprehensive review. Starch-Stärke. 2010;62:389–420. [Google Scholar]
- Pilling E., Smith A.M. Growth ring formation in the starch granules of potato tubers. Plant Physiol. 2003;132:365–371. doi: 10.1104/pp.102.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posewitz M.C., Smolinski S.L., Kanakagiri S., Melis A., Seibert M., Ghirardi M.L. Hydrogen photoproduction is attenuated by disruption of an isoamylase gene in Chlamydomonas reinhardtii. Plant Cell. 2004;16:2151–2163. doi: 10.1105/tpc.104.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke K.A., Marrison J.L., Leech R.M. Temporal and spatial development of the cells of the expanding 1st leaf of Arabidopsis thaliana (L.) Heynh. J. Exp. Bot. 1991;42:1407–1416. [Google Scholar]
- Rahman S., Kosarhashemi B., Samuel M.S., Hill A., Abbott D.C., Skerritt J.H., Preiss J., Appels R., Morell M.K. The major proteins of wheat endosperm starch granules. Aust. J. Plant Physiol. 1995;22:793–803. [Google Scholar]
- Rahman S., Nakamura Y., Li Z., Clarke B., Fujita N., Mukai Y., Yamamoto M., Regina A., Tan Z., Kawasaki S., Morell M. The sugary-type isoamylase gene from rice and Aegilops tauschii: characterization and comparison with maize and Arabidopsis. Genome. 2003;46:496–506. doi: 10.1139/g02-130. [DOI] [PubMed] [Google Scholar]
- Ral J.P., Derelle E., Ferraz C., Wattebled F., Farinas B., Corellou F., Buléon A., Slomianny M.C., Delvallé D., d'Hulst C., Rombauts S., Moreau H., Ball S. Starch division and partitioning. A mechanism for granule propagation and maintenance in the picophytoplanktonic green alga Ostreococcus tauri. Plant Physiol. 2004;136:3333–3340. doi: 10.1104/pp.104.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore R.S., Garg N., Garg S., Kumar A. Starch Phosphorylase: role in starch metabolism and biotechnological applications. Cht. Rev. Biotechnol. 2009;29:214–224. doi: 10.1080/07388550902926063. [DOI] [PubMed] [Google Scholar]
- Recondo E., Leloir L.F. Adenosine diphosphate glucose and starch synthesis. Biochem. Biophys. Res. Commun. 1961;6:85–88. doi: 10.1016/0006-291x(61)90389-8. [DOI] [PubMed] [Google Scholar]
- Reinhold H., Soyk S., Simková K., Hostettler C., Marafino J., Mainiero S., Vaughan C.K., Monroe J.D., Zeeman S.C. β-Amylase-like proteins function as transcription factors in Arabidopsis, controlling shoot growth and development. Plant Cell. 2011;23:1391–1403. doi: 10.1105/tpc.110.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Healy R.A., Klyne A.M., Horner H.T., James M.G., Thornburg R.W. Transient starch metabolism in ornamental tobacco floral nectaries regulates nectar composition and release. Plant Sci. 2007;173:277–290. [Google Scholar]
- Ritte G., Lloyd J.R., Eckermann N., Rottmann A., Kossmann J., Steup M. The starch-related R1 protein is an α-glucan, water dikinase. Proc. Natl. Acad. Sci. USA. 2002;99:7166–7171. doi: 10.1073/pnas.062053099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G., Scharf A., Eckermann N., Haebel S., Steup M. Phosphorylation of transitory starch is increased during degradation. Plant Physiol. 2004;135:2068–2077. doi: 10.1104/pp.104.041301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G., Heydenreich M., Mahlow S., Haebel S., Kötting O., Steup M. Phosphorylation of C6- and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett. 2006;580:4872–4876. doi: 10.1016/j.febslet.2006.07.085. [DOI] [PubMed] [Google Scholar]
- Roldán I., Wattebled F., Mercedes Lucas M., Delvallé D., Planchot V., Jimenez S., Pérez R., Ball S., D'Hulst C., Mérida A. The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J. 2007;49:492–504. doi: 10.1111/j.1365-313X.2006.02968.x. [DOI] [PubMed] [Google Scholar]
- Roldán M., Gómez-Mena C., Ruiz-García L., Salinas J., Martínez-Zapater J.M. Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsis in the dark. Plant J. 1999;20:581–590. doi: 10.1046/j.1365-313x.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- Rost S., Frank C., Beck E. The chloroplast envelope is permeable for maltose but not for maltodextrins. Biochim. Biophys. Acta. 1996;1291:221–227. doi: 10.1016/s0304-4165(96)00068-2. [DOI] [PubMed] [Google Scholar]
- Rydberg U., Andersson L., Andersson R., Aman P., Larsson H. Comparison of starch branching enzyme I and II from potato. Eur. J. Biochem. 2001;268:6140–6145. doi: 10.1046/j.0014-2956.2001.02568.x. [DOI] [PubMed] [Google Scholar]
- Safford R., Jobling S.A., Sidebottom C.M., Westcott R.J., Cooke D., Tober K.J., Strongitharm B.H., Russell A.L., Gidley M.J. Consequences of antisense RNA inhibition of starch branching enzyme activity on properties of potato starch. Carbohydr. Polym. 1998;35:155–168. [Google Scholar]
- Santelia D., Zeeman S.C. Progress in Arabidopsis starch research and potential biotechnological applications. Curr. Opin. Biotechnol. 2011;22:271–280. doi: 10.1016/j.copbio.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Santelia D., Kötting O., Seung D., Schubert M., Thalmann M., Bischof S., Meekins D.A., Lutz A., Patron N., Gentry M.S., Allain F.H., Zeeman S.C. The phosphoglucan phosphatase LIKE SEX FOUR2 dephosphorylates starch at the C3-position in Arabidopsis. Plant Cell. 2011;23:4096–4111. doi: 10.1105/tpc.111.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H., Nishi A., Yamashita K., Takemoto Y., Tanaka Y., Hosaka Y., Sakurai A., Fujita N., Nakamura Y. Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm. Plant Physiol. 2003;133:1111–1121. doi: 10.1104/pp.103.021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H., Shibahara K., Tokunaga T., Nishi A., Tasaki M., Hwang S.K., Okita T.W., Kaneko N., Fujita N., Yoshida M., Hosaka Y., Sato A., Utsumi Y., Ohdan T., Nakamura Y. Mutation of the plastidial α-glucan Phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell. 2008;20:1833–1849. doi: 10.1105/tpc.107.054007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidig A., Frohlich A., Schulze S., Lloyd J.R., Kossmann J. Downregulation of a chloroplast-targeted β-amylase leads to a starch-excess phenotype in leaves. Plant J. 2002;30:581–591. doi: 10.1046/j.1365-313x.2002.01317.x. [DOI] [PubMed] [Google Scholar]
- Schneider A., Häusler R.E., Kolukisaoglu U., Kunze R., van der Graaff E., Schwacke R., Catoni E., Desimone M., Flügge U.I. An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. Plant J. 2002;32:685–699. doi: 10.1046/j.1365-313x.2002.01460.x. [DOI] [PubMed] [Google Scholar]
- Schulze W., Stitt M., Schulze E.D., Neuhaus H.E., Fichtner K. A quantification of the significance of assimilatory starch for growth of Arabidopsis thaliana L. Heynh. Plant Physiol. 1991;95:890–895. doi: 10.1104/pp.95.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P., Buchanan B.B. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid. Redox Signal. 2008;10:1235–1274. doi: 10.1089/ars.2007.1931. [DOI] [PubMed] [Google Scholar]
- Schwall G.P., Safford R., Westcott R.J., Jeffcoat R., Tayal A., Shi Y.C., Gidley M.J., Jobling S.A. Production of very-high-amylose potato starch by inhibition of SBE A and B. Nature Biotechnol. 2000;18:551–554. doi: 10.1038/75427. [DOI] [PubMed] [Google Scholar]
- Sehnke P.C., Chung H.J., Wu K., Ferl R.J. Regulation of starch accumulation by granule-associated plant 14-3-3 proteins. Proc. Natl. Acad. Sci. USA. 2001;98:765–770. doi: 10.1073/pnas.021304198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure M., Wessler S., Fedoroff N. Molecular identification and isolation of the Waxy locus in maize. Cell. 1983;35:225–233. doi: 10.1016/0092-8674(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Singh N., Singh J., Kaur L., Sodhi N.S., Gill B.S. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003;81:219–231. [Google Scholar]
- Smith A.M., Stitt M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007;30:1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fulton D.C., Chia T., Thorneycroft D., Chapple A., Dunstan H., Hylton C., Zeeman S.C., Smith A.M. Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and post-transcriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol. 2004;136:2687–2699. doi: 10.1104/pp.104.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov L.N., Dominguez-Solis J.R., Allary A.L., Buchanan B.B., Luan S. A redox-regulated chloroplast protein phosphatase binds to starch diurnally and functions in its accumulation. Proc. Natl. Acad. Sci. USA. 2006;103:9732–9737. doi: 10.1073/pnas.0603329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparla F., Costa A., Lo Schiavo F., Pupillo P., Trost P. Redox regulation of a novel plastid-targeted β-amylase of Arabidopsis. Plant Physiol. 2006;141:840–850. doi: 10.1104/pp.106.079186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Fitzgerald A.M., Farnden K.J.F., MacRae E.A. Characterisation of putative α-amylases from apple (Malus domestica) and Arabidopsis thaliana. Biologia. 2002;57:137–148. [Google Scholar]
- Stark D.M., Timmerman K.P., Barry G.F., Preiss J., Kishore G.M. Regulation of the amount of starch in plant tissues by ADP glucose pyrophosphorylase. Science. 1992;258:287–292. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- Stettler M., Eicke S., Mettler T., Messerli G., Hörtensteiner S., Zeeman S.C. Blocking the metabolism of starch breakdown products in Arabidopsis leaves triggers chloroplast degradation. Mol. Plant. 2009;2:1233–1246. doi: 10.1093/mp/ssp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinard P.S., Robertson D.S., Schnable P.S. Genetic isolation, cloning, and analysis of a mutator-induced, dominant antimorph of the maize amylose extender1 locus. Plant Cell. 1993;5:1555–1566. doi: 10.1105/tpc.5.11.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Gerhardt R., Kurzel B., Heldt H.W. A role for fructose 2,6-bisphosphate in the regulation of sucrose synthesis in spinach leaves. Plant Physiol. 1983;72:1139–1141. doi: 10.1104/pp.72.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Kurzel B., Heldt H.W. Control of photosynthetic sucrose synthesis by fructose 2,6-bisphosphate: II. Partitioning between sucrose and starch. Plant Physiol. 1984;75:554–560. doi: 10.1104/pp.75.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Quick P. Photosynthetic carbon partitioning - its regulation and possibilities for manipulation. Physiol. Plant. 1989;77:633–641. [Google Scholar]
- Stitt M., Gibon Y., Lunn J.E., Piques M. Multilevel genomics analysis of carbon signalling during low carbon availability: coordinating the supply and utilisation of carbon in a fluctuating environment. Funct. Plant Biol. 2007;34:526–549. doi: 10.1071/FP06249. [DOI] [PubMed] [Google Scholar]
- Stitt M., Lunn J., Usadel B. Arabidopsis and primary photosynthetic metabolism - more than the icing on the cake. Plant J. 2010;61:1067–1091. doi: 10.1111/j.1365-313X.2010.04142.x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Zeeman S.C. Starch turnover: pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012;15:282–292. doi: 10.1016/j.pbi.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Streb S., Delatte T., Umhang M., Eicke S., Schorderet M., Reinhardt D., Zeeman S.C. Starch granule biosynthesis in Arabidopsis is abolished by removal of all debranching enzymes but restored by the subsequent removal of an endoamylase. Plant Cell. 2008;20:3448–3466. doi: 10.1105/tpc.108.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb S., Egli B., Eicke S., Zeeman S.C. The debate on the pathway of starch synthesis: a closer look at low-starch mutants lacking plastidial phosphoglucomutase supports the chloroplast-localized pathway. Plant Physiol. 2009;151:1769–1772. doi: 10.1104/pp.109.144931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R., Pyl E.T., Ishihara H., Trenkamp S., Steinfath M., Witucka-Wall H., Gibon Y., Usadel B., Poree F., Piques M.C., Von Korff M., Steinhauser M.C., Keurentjes J.J., Günther M., Höhne M., Selbig J., Fernie A.R., Altmann T., Stitt M. Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. USA. 2009;106:10348–10353. doi: 10.1073/pnas.0903478106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Okita T.W., Edwards G.E. Modification of carbon partitioning, photosynthetic capacity, and O2 sensitivity in Arabidopsis plants with low ADPglucose pyrophosphorylase activity. Plant Physiol. 1999;119:267–276. doi: 10.1104/pp.119.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove L., Burrell M.M., ap Rees T. Starch Metabolism in tuvers of transgenic potato (Solanum tuberosum) with increased ADPglucose pyrophosphorylase. Biochem. J. 1996;320:493–498. doi: 10.1042/bj3200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowski N., Ragel P., Raynaud S., Lucas M.M., Roldán I., Montera M., Muñoz F.J., Ovecka M., Bahaji A., Planchot V., Pozueta-Romero J., D'Hulst C., Mérida A. starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell. 2009;21:2443–2457. doi: 10.1105/tpc.109.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowski N., Ragel P., Hennen-Bierwagen T.A., Planchot V., Myers A.M., Mérida A., d'Hulst C., Wattebled F. Integrated functions among multiple starch synthases determine both amylopectin chain length and branch linkage location in Arabidopsis leaf starch. J. Exp. Bot. 2011;62:4547–4559. doi: 10.1093/jxb/err172. [DOI] [PubMed] [Google Scholar]
- Takaha T., Yanase M., Okada S., Smith S.M. Disproportionating enzyme (4-α-glucanotransferase; EC 2.4.1.25) of potato. Purification, molecular cloning, and potential role in starch metabolism. J. Biol. Chem. 1993;268:1391–1396. [PubMed] [Google Scholar]
- Takaha T., Yanase M., Takata H., Okada S., Smith S.M. Potato D-enzyme catalyzes the cyclization of amylose to produce cycloamylose, a novel cyclic glucan. J. Biol. Chem. 1996;271:2902–2908. doi: 10.1074/jbc.271.6.2902. [DOI] [PubMed] [Google Scholar]
- Takaha T., Yanase M., Takata H., Okada S., Smith S.M. Cyclic glucans produced by the intramolecular transglycosylation activity of potato D-enzyme on amylopectin. Biochem. Biophys. Res. Commun. 1998;247:493–497. doi: 10.1006/bbrc.1998.8817. [DOI] [PubMed] [Google Scholar]
- Takashima Y., Senoura T., Yoshizaki T., Hamada S., Ito H., Matsui H. Differential chain-length specificities of two isoamylasetype starch-debranching enzymes from developing seeds of kidney bean. Biosci. Biotechnol. Biochem. 2007;71:2308–2312. doi: 10.1271/bbb.70215. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Hizukuri S. Studies on starch phosphate. 5. Re-examination of the action of sweet-potato β-amylase on phosphorylated (1–4)-α-D-glucan. Carbohydr. Res. 1981;89:174–178. [Google Scholar]
- Takeda Y., Guan H.P., Preiss J. Branching of amylose by the branching isoenzymes of maize endosperm. Carbohydr. Res. 1993;240:253–263. [Google Scholar]
- Tang L.Y., Nagata N., Matsushima R., Chen Y.L., Yoshioka Y., Sakamoto W. Visualization of plastids in pollen grains: involvement of FtsZ1 in pollen plastid division. Plant Cell Physiol. 2009;50:904–908. doi: 10.1093/pcp/pcp042. [DOI] [PubMed] [Google Scholar]
- Tatge H., Marshall J., Martin C., Edwards E.A., Smith A.M. Evidence that amylose synthesis occurs within the matrix of the starch granule in potato tubers. Plant Cell Environ. 1999;22:543–550. [Google Scholar]
- Tenorio G., Orea A., Romero J.M., Mérida A. Oscillation of mRNA level and activity of granule-bound starch synthase I in Arabidopsis leaves during the day/night cycle. Plant Mol. Biol. 2003;51:949–958. doi: 10.1023/a:1023053420632. [DOI] [PubMed] [Google Scholar]
- Tetlow I.J., Wait R., Lu Z., Akkasaeng R., Bowsher C.G., Esposito S., Kosar-Hashemi B., Morell M.K., Emes M.J. Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein-protein interactions. Plant Cell. 2004;16:694–708. doi: 10.1105/tpc.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow I.J., Beisel K.G., Cameron S., Makhmoudova A., Liu F., Bresolin N.S., Wait R., Morell M.K., Emes M.J. Analysis of protein complexes in wheat amyloplasts reveals functional interactions among starch biosynthetic enzymes. Plant Physiol. 2008;146:1878–1891. doi: 10.1104/pp.108.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A., Prescha K., Branscheid A., Palacios N., McKibbin R., Halford N.G., Geigenberger P. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADPglucose pyrophosphorylase in potato tubers. Plant J. 2003;35:490–500. doi: 10.1046/j.1365-313x.2003.01823.x. [DOI] [PubMed] [Google Scholar]
- Tsai C.Y. The function of the waxy locus in starch synthesis in maize endosperm. Biochem. Genet. 1974;11:83–96. doi: 10.1007/BF00485766. [DOI] [PubMed] [Google Scholar]
- Tsai H.L., Lue W.L., Lu K.J., Hsieh M.H., Wang S.M., Chen J. Starch synthesis in Arabidopsis is achieved by spatial cotranscription of core starch metabolism genes. Plant Physiol. 2009;151:1582–1595. doi: 10.1104/pp.109.144196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde J.E., Parodi A.J., Ugalde R.A. De novo synthesis of bacterial glycogen: Agrobacterium tumefaciens glycogen synthase is involved in glucan initiation and elongation. Proc. Natl. Acad. Sci. USA. 2003;100:10659–10663. doi: 10.1073/pnas.1534787100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B., Bläsing O.E., Gibon Y., Retzlaff K., Höhne M., Günther M., Stitt M. Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol. 2008;146:1834–1861. doi: 10.1104/pp.107.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi Y., Nakamura Y. Structural and enzymatic characterization of the isoamylase1 homo-oligomer and the isoamylase1-isoamylase2 hetero-oligomer from rice endosperm. Planta. 2006;225:75–87. doi: 10.1007/s00425-006-0331-z. [DOI] [PubMed] [Google Scholar]
- Utsumi Y., Utsumi C., Sawada T., Fujita N., Nakamura Y. Functional diversity of isoamylase oligomers: the ISA1 homo-oligomer is essential for amylopectin biosynthesis in rice endosperm. Plant Physiol. 2011;156:61–77. doi: 10.1104/pp.111.173435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio C., Costa A., Marri L., Issakidis-Bourguet E., Pupillo P., Trost P., Sparla F. Thioredoxin-regulated β-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress. J. Exp. Bot. 2011;62:545–555. doi: 10.1093/jxb/erq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wal M., D'Hulst C., Vincken J.P., Buléon A., Visser R., Ball S. Amylose is synthesized in vitro by extension of and cleavage from amylopectin. J. Biol. Chem. 1998;273:22232–22240. doi: 10.1074/jbc.273.35.22232. [DOI] [PubMed] [Google Scholar]
- Ventriglia T., Kuhn M.L., Ruiz M.T., Ribeiro-Pedro M., Valverde F., Ballicora M.A., Preiss J., Romero J.M. Two Arabidopsis ADPglucose pyrophosphorylase large subunits (APL1 and APL2) are catalytic. Plant Physiol. 2008;148:65–76. doi: 10.1104/pp.108.122846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S., Zhao L.M., Sack F.D. Interaction of root gravitropism and phototropism in Arabidopsis wild-type and starchless mutants. Plant Physiol. 2000;122:453–461. doi: 10.1104/pp.122.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S., Yang M., Sack F.D., Kiss J.Z. Gravitropism in the starch excess mutant of Arabidopsis thaliana. Am. J. Bot. 2007;94:590–598. doi: 10.3732/ajb.94.4.590. [DOI] [PubMed] [Google Scholar]
- Vriet C., Welham T., Brachmann A., Pike M., Pike J., Perry J., Parniske M., Sato S., Tabata S., Smith A.M., Wang T.L. A suite of Lotus japonicus starch mutants reveals both conserved and novel features of starch metabolism. Plant Physiol. 2010;154:643–655. doi: 10.1104/pp.110.161844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Monroe J., Sjolund R.D. Identification and characterization of a phloem-specific β-amylase. Plant Physiol. 1995;109:743–750. doi: 10.1104/pp.109.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xue L., Sun J., Zuo J. The Arabidopsis BE1 gene, encoding a putative glycoside hydrolase localized in plastids, plays crucial roles during embryogenesis and carbohydrate metabolism. J. Integr. Plant Biol. 2010;52:273–288. doi: 10.1111/j.1744-7909.2010.00930.x. [DOI] [PubMed] [Google Scholar]
- Wattebled F., Ral J.P., Dauvillée D., Myers A.M., James M.G., Schlichting R., Giersch C., Ball S.G., D'Hulst C. STA11, a Chlamydomonas reinhardtii locus required for normal starch granule biogenesis, encodes disproportionating enzyme. Further evidence for a function of α-1,4 glucanotransferases during starch granule biosynthesis in green algae. Plant Physiol. 2003;132:137–145. doi: 10.1104/pp.102.016527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattebled F., Dong Y., Dumez S., Delvallé D., Planchot V., Berbezy P., Vyas D., Colonna P., Chatterjee M., Ball S., D'Hulst C. Mutants of Arabidopsis lacking a chloroplastic isoamylase accumulate phytoglycogen and an abnormal form of amylopectin. Plant Physiol. 2005;138:184–195. doi: 10.1104/pp.105.059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattebled F., Planchot V., Dong Y., Szydlowski N., Pontoire B., Devin A., Ball S., D'Hulst C. Further evidence for the mandatory nature of polysaccharide debranching for the aggregation of semicrystalline starch and for overlapping functions of debranching enzymes in Arabidopsis leaves. Plant Physiol. 2008;148:1309–1323. doi: 10.1104/pp.108.129379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Servaites J.C., Geiger D.R., Kofler H., Hille D., Gröner F., Hebbeker U., Flügge U.I. Identification, purification, and molecular cloning of a putative plastidic glucose translocator. Plant Cell. 2000;12:787–802. doi: 10.1105/tpc.12.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H., Stitt M., Heldt H.W. Subcellular compartmentation of pyrophosphate and alkaline pyrophosphatase in leaves. Biochim. Biophys. Acta. 1987;893:13–21. [Google Scholar]
- Weise S.E., Kiss J.Z. Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis. Int. J. Plant Sci. 1999;160:521–527. doi: 10.1086/314142. [DOI] [PubMed] [Google Scholar]
- Weise S.E., Kim K.S., Stewart R.P., Sharkey T.D. β-Maltose is the metabolically active anomer of maltose during transitory starch degradation. Plant Physiol. 2005;137:756–761. doi: 10.1104/pp.104.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise S.E., Schrader S.M., Kleinbeck K.R., Sharkey T.D. Carbon balance and circadian regulation of hydrolytic and phosphorolytic breakdown of transitory starch. Plant Physiol. 2006;141:879–886. doi: 10.1104/pp.106.081174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese A., Christ M.M., Virnich O., Schurr U., Walter A. Spatio-temporal leaf growth patterns of Arabidopsis thaliana and evidence for sugar control of the diel leaf growth cycle. New Phytol. 2007;174:752–761. doi: 10.1111/j.1469-8137.2007.02053.x. [DOI] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H.W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980;66:187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Colleoni C., Myers A.M., James M.G. Enzymatic properties and regulation of ZPU1, the maize pullulanase-type starch debranching enzyme. Arch. Biochem. Biophys. 2002;406:21–32. doi: 10.1016/s0003-9861(02)00412-5. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Kainuma K., French D. Electron-microscopic observations of waxy maize starch. J. Ultrastruct. Res. 1979;69:249–261. doi: 10.1016/s0022-5320(79)90114-x. [DOI] [PubMed] [Google Scholar]
- Yano R., Nakamura M., Yoneyama T., Nishida I. Starchrelated α-glucan, water dikinase is involved in the cold-induced development of freezing tolerance in Arabidopsis. Plant Physiol. 2005;138:837–846. doi: 10.1104/pp.104.056374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T.S., Lue W.L., Wang S.M., Chen J. Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiol. 2000;123:319–326. doi: 10.1104/pp.123.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T.S., Kofler H., Häusler R.E., Hille D., Flügge U.I., Zeeman S.C., Smith A.M., Kossmann J., Lloyd J., Ritte G., Steup M., Lue W.L., Chen J., Weber A. The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell. 2001;13:1907–1918. doi: 10.1105/TPC.010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T.S., Zeeman S.C., Thorneycroft D., Fulton D.C., Dunstan H., Lue W.L., Hegemann B., Tung S.Y., Umemoto T., Chapple A., Tsai D.L., Wang S.M., Smith A.M., Chen J., Smith S.M. α-Amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J. Biol. Chem. 2005;280:9773–9779. doi: 10.1074/jbc.M413638200. [DOI] [PubMed] [Google Scholar]
- Zeeman S.C., Northrop F., Smith A.M., ap Rees T. A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme. Plant J. 1998a;15:357–365. doi: 10.1046/j.1365-313x.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Zeeman S.C., Umemoto T., Lue W.L., Au-Yeung P., Martin C., Smith A.M., Chen J. A mutant of Arabidopsis lacking a chloroplastic isoamylase accumulates both starch and phytoglycogen. Plant Cell. 1998b;10:1699–1712. doi: 10.1105/tpc.10.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman S.C., Ap Rees T. Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant Cell Environ. 1999;22:1445–1453. [Google Scholar]
- Zeeman S.C., Tiessen A., Pilling E., Kato K.L., Donald A.M., Smith A.M. Starch synthesis in Arabidopsis. Granule synthesis, composition, and structure. Plant Physiol. 2002;129:516–529. doi: 10.1104/pp.003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman S.C., Thorneycroft D., Schupp N., Chapple A., Weck M., Dunstan H., Haldimann P., Bechtold N., Smith A.M., Smith S.M. Plastidial α-glucan Phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol. 2004;135:849–858. doi: 10.1104/pp.103.032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman S.C., Smith S.M., Smith A.M. The diurnal metabolism of leaf starch. Biochem. J. 2007;401:13–28. doi: 10.1042/BJ20061393. [DOI] [PubMed] [Google Scholar]
- Zeeman S.C., Kossmann J., Smith A.M. Starch: its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010;61:209–234. doi: 10.1146/annurev-arplant-042809-112301. [DOI] [PubMed] [Google Scholar]
- Zhang X., Myers A.M., James M.G. Mutations affecting starch synthase III in Arabidopsis alter leaf starch structure and increase the rate of starch synthesis. Plant Physiol. 2005;138:663–674. doi: 10.1104/pp.105.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Szydlowski N., Delvallé D., D'Hulst C., James M.G., Myers A.M. Overlapping functions of the starch synthases SSII and SSIII in amylopectin biosynthesis in Arabidopsis. BMC Plant Biol. 2008;8:96. doi: 10.1186/1471-2229-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]