Abstract
Cardiac excitability and electrical activity are determined by the sum of individual ion channels, gap junctions and exchanger activities. Electrophysiological remodeling during heart disease involves changes in membrane properties of cardiomyocytes and is related to higher prevalence of arrhythmia-associated morbidity and mortality. Pharmacological and genetic manipulation of cardiac cells as well as animal models of cardiovascular diseases are used to identity changes in electrophysiological properties and the molecular mechanisms associated with the disease. Protein kinase C (PKC) and several other kinases play a pivotal role in cardiac electrophysiological remodeling. Therefore, identifying specific therapies that regulate these kinases is the main focus of current research. PKC, a family of serine/threonine kinases, has been implicated as potential signaling nodes associated with biochemical and biophysical stress in cardiovascular diseases. Thus, the role of PKC isozymes in regulating cardiac excitability has been a subject of great attention. In this review, we describe the role of PKC isozymes that are involved in cardiac excitability and discuss both genetic and pharmacological tools that were used, their attributes and limitations. Selective and effective pharmacological interventions to normalize cardiac electrical activities and correct cardiac arrhythmias will be of great clinical benefit.
INTRODUCTION
The final common pathway of electrical excitability in the heart is the generation of conducted action potentials, whereas voltage-gated ion channels and gap junctions play an important role in the action potential electrogenesis (1, 2). Cardiovascular diseases (i.e. coronary artery disease, stroke and heart failure) are associated with changes of cardiac electrical excitability due to transcriptional and post-translational modifications of ion channels and gap junction proteins (3, 4).
Conceptual and technical breakthroughs during the last decade have led to revolutionary advances in our knowledge of allosteric regulation of ion channels, whereas several studies have focused on identifying cellular nodes where signals converge and serve as multi-effector brakes to re-establish the normal electrophysiological properties inside the damaged heart. Nowadays, there is abundant evidence showing that channel phosphorylation can influence profoundly the electrical properties of cardiomyocytes and other cells (5, 6), whereas various kinases have been described as candidate mediators of the pathological cardiac electrophysiological remodeling (6–8). Among different kinases, activation of PKC isozymes has been a subject of great attention in the cardiovascular field since it is implicated in pathological settings such as heart failure (9, 10), ischemia (11, 12) and ischemic preconditioning (13–15).
We first demonstrated the relevance of PKC isozymes to cardiac excitability by showing that prolonged exposure of isolated neonatal cardiomyocytes to 4-β phorbol ester 12-myristate-13-acetate (PMA, 1–100nM, a non-selective PKC activator) enhanced the rate of contraction in a dose-dependent fashion (16). Indeed, several studies have reported the contribution of specific PKC isozymes to cardiac excitability by regulating ion channel expression (17–19) and activity (20–22), and inotropic and chronotropic effects (23). In this review, we focus on insights gained from the rational design of small molecule inhibitors and activators of selective PKC isozymes to understand their critical role in modulating cardiac excitability (Figure 1).
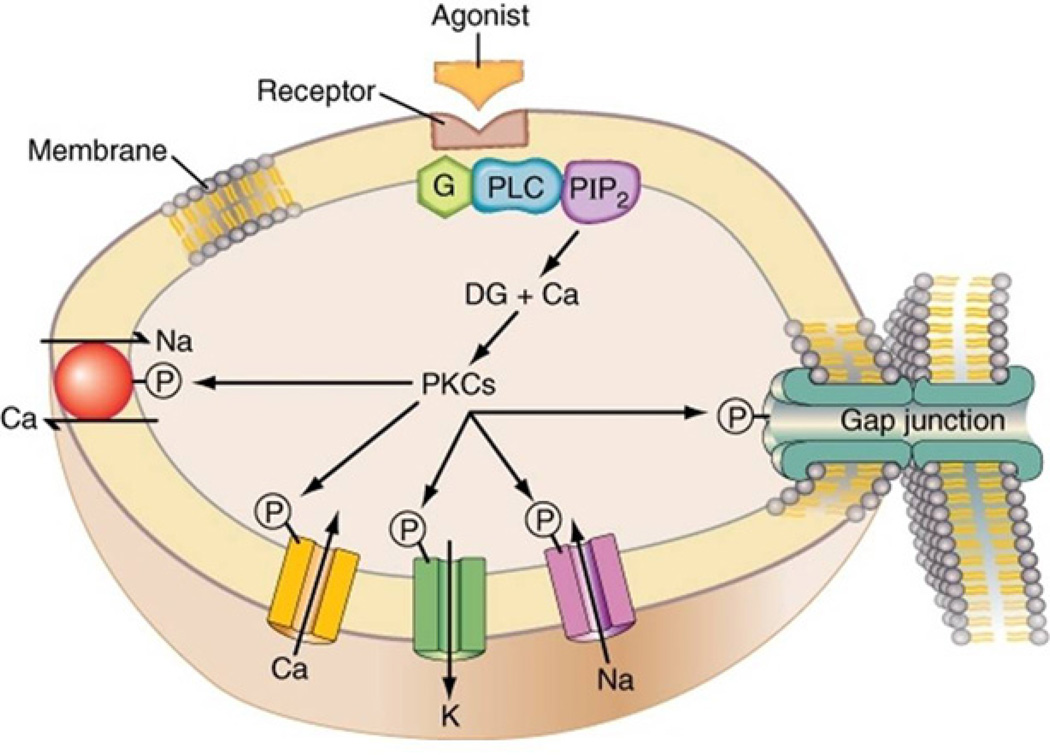
Figure 1.
Schematic representation of protein kinase C (PKC) regulation of cardiac excitability and membrane associated function through phosphorylation mechanisms. Ca: Ca channel; K: K channel; Na: Na channel; Na/Ca: Na/Ca exchanger; DG: Diacyl glycerol; G: G-protein; PLC: Phospholipase C; PIP2: Phosphoinositol di-phosphate; P: Phosphate.
PKC family
PKC is a group of ten closely related phospholipid-dependent serine-threonine protein kinases (24). These isozymes are classified based on their structure and activation requirements into three subgroups: conventional PKCs (α, βI, βII and γ), which are Ca-dependent and activated by diacylglycerol (DAG) and phosphatidylserine (PS); novel PKCs (ε, δ, η and θ), which are Ca-independent but activated by DAG and PS; and atypical PKCs (ζ and ι/λ), which are Ca and DAG-independent but activated by PS.
Of these PKC isozymes, α, βI, βII, ε, θ, λ, δ, ζ and γ PKC have been found in the heart in different animal species (25, 26), and high levels of PKC isozymes α, βI, βII, δ and ε have been reported in tsA201 and HL-1 cell lines (27). The presence of multiple PKC isozymes in the same tissue and even in the same cell type suggests that individual isozymes mediate distinct cellular functions. The effort to dissect the roles of individual PKC isozymes in cell functions has been hampered by the lack of isozyme-specific activators and inhibitors.
General PKC regulators (non-isozyme selective)
A number of small molecule drugs that regulate PKC isozymes has been generated over the last decades. Many of these compounds are competitive inhibitors of ATP binding to the catalytic site of the kinase (Table 1). However, because the catalytic site within the PKC family as well as in other protein kinases is highly conserved, generating a truly selective inhibitor of one protein kinase has proven to be a challenge (28) (Table 1). Small molecule activators of PKC isozymes are also available. Those compounds mimic the second messenger DAG and are related to the tumor promoter phorbol ester (PMA) (29), to bryostatin (30) and some other natural compounds. Some activators have higher affinity for one specific PKC subgroup over others in a dose-dependent manner (16). However, considering that these compounds are not able to distinguish the activation of PKC isozymes in the same subgroup, they are usually described as non-selective PKC activators. Furthermore, because there are multiple diacylglycerol-binding proteins (31), using them as PKC isozyme selective tools works only in a narrow concentration range and can activate proteins other than PKCs.
Table 1.
Pharmacological tools that regulate PKC isozymes
| Drug | Biological function |
Chemical characteristics |
Specificity/selectivity | Kinase binding site | Refs |
|---|---|---|---|---|---|
| PMA | PKC activator | Phorbol ester | Non-specific | DAG-binding site | (29) |
| Bryostatin | PKC activator | Macrolactones | Non-specific | DAG-binding site Some synthetic analogs are specific | (124, 125) |
| ψβRACK | PKC activator | PKC-derived peptide | cPKC-specific | Regulatory domain βPKC aa 241–246 | (126) |
| ψδRACK | PKC activator | PKC-derived peptide | δPKC | Regulatory domain δPKC aa 74–81 | (127) |
| ψθRACK | PKC activator | PKC-derived peptide | θPKC | Regulatory domain θPKC aa 73–82 | |
| ψεRACK | PKC activator | PKC-derived peptide | εPKC | Regulatory domain εPKC aa 85–92 | (120) |
| ψηRACK | PKC activator | PKC-derived peptide | ηPKC | Regulatory domain ηPKC aa 88–95 | |
| Staurosporine | PKC inhibitor | indolo-carbazole | Non-specific | Catalytic domain ATP site, affects also other kinases | (128) |
| Go6076 | PKC inhibitor | nonglycosidic indolocarbazole | cPKC, nPKC | Catalytic domain ATP site | (129, 130) |
| Bisindolmaleimide (LY333531) | PKC inhibitor | macrocyclic bis(indolyl) maleimide | βPKC | Catalytic domain ATP site, affects also other protein kinases | (131, 132) |
| GF109203X | PKC inhibitor | Bisindolylmaleimide | PKC selective | Catalytic domain ATP site, affects also other protein kinases | (133) |
| Ro31-8820 | PKC inhibitor | bis(indolyl)maleimide | PKC selective | Catalytic domain ATP site, affects also other protein kinases | (134) |
| Ro32-0423 | PKC inhibitor | bisindolylmaleimide | αPKC and βIPKC | Catalytic domain ATP site, affects also other protein kinases | (135) |
| PKC412 | PKC inhibitor | indolo-carbazole | Ser/Thr and Tyr protein kinases | Catalytic domain ATP site, affects also other protein kinases | (136) |
| UNC01 | PKC inhibitor | indolo-carbazole | cPKC>nKC | Catalytic domain ATP site, affects also other protein kinases | (137) |
| Rottlerin | PKC inhibitor | polyphenols | δPKC | Catalytic domain ATP site, inhibits also a calcium calmodulin kinases at the same IC50 | (138) |
| ISIS 3521 | PKC inhibitor | Anti-sense | αPKC | αPKC | (139) |
| ISIS9606 | PKC inhibitor | Anti-sense | αPKC | αPKC | (140) |
| βIV5-3 | PKC inhibitor | PKC-derived peptide | βIPKC | Regulatory domain βIPKC aa 646–651 | (141) |
| βIIV5-3 | PKC inhibitor | PKC-derived peptide | βIIPKC | Regulatory domain βII PKC aa 645–650 | (141) |
| γV5-3 | PKC inhibitor | PKC-derived peptide | γPKC | Regulatory domain γPKC aa 659–664 | (142) |
| δV1-1 | PKC inhibitor | PKC-derived peptide | δPKC | Regulatory domain δPKC aa 8–17 | (127) |
| εV1-2 | PKC inhibitor | PKC-derived peptide | εPKC | Regulatory domain εPKC aa 14–21 | (143) |
| θV1-1 | PKC inhibitor | PKC-derived peptide | θPKC | Regulatory domain θPKC aa 8–13 | |
| ηV1-2 | PKC inhibitor | PKC-derived peptide | ηPKC | Regulatory domain ηPKC aa 18–25 | |
| β-pseudosubstrate | PKC inhibitor | PKC-derived peptide | Pan PKC | catalytic domain, substrate site; βPKC aa 19–31 | (144) |
| ζ-pseudosubstrate | PKC inhibitor | PKC-derived peptide | aPKC subfamily | catalytic domain, substrate site; ζPKC 105–121 | (145) |
| βC2-1, 2, 4 | PKC inhibitor | PKC-derived peptide | cPKC subfamily | Regulatory domain βIIPKC aa 209–216; 186–198; 218–226 respectively | (126) |
Isozyme-selective PKC regulators
Over twenty years ago, we suggested that protein-protein interactions play a critical role in determining the selectivity of individual PKC isozymes. We identified a family of proteins, collectively termed RACKs (Receptors for Activated C Kinase) that anchor individual activated PKC isozymes near their substrates and away from others (32). We then designed peptide regulators of these protein-protein interactions as isozyme-selective inhibitors (Figure 2). The rationale used for the design of the peptides and the data used to demonstrate their selectivity were provided in over 200 publications from a variety of labs. For recent review see (33). In short, the peptides, 6–10 amino acids in length, correspond to unique sequences within the PKC isozyme-binding site on the RACK or the RACK-binding site on that PKC isozyme. Using this approach, we have identified selective inhibitors for all the PKC isozymes (see Table 1).
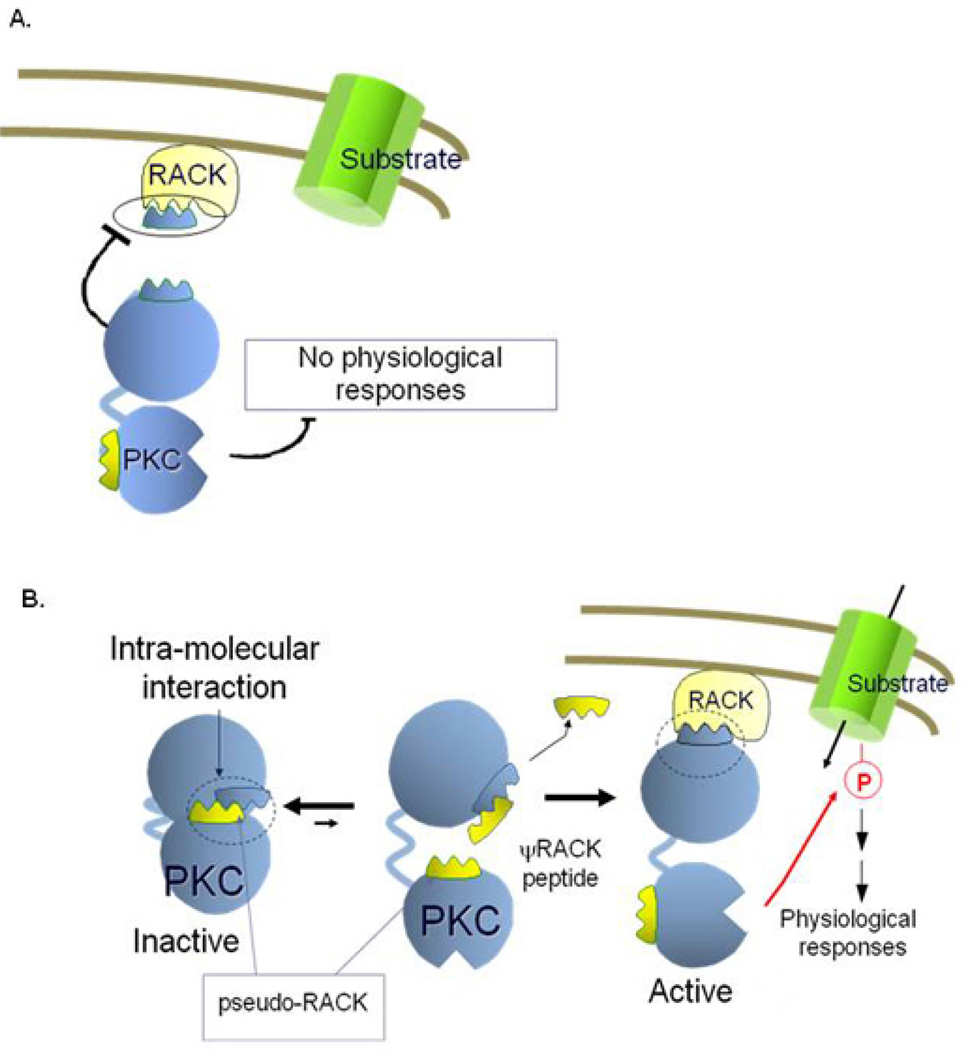
Figure 2.
Schematic representation of protein kinase C (PKC) inhibitors and activators. A: A peptide, derived from the RACK-binding site on PKC binds to the RACK and inhibits binding of the activated PKC to its RACK. Under these conditions, a substrate near the RACK (e.g., an ion channel) is not phosphorylated and therefore the physiological response mediated by this phosphorylation does not occur. B: An intra-molecular interaction between the RACK-binding site on PKC and a sequence in the enzyme that mimics the PKC-binding site on the RACK (hence pseudo-RACK), keeps the enzyme in the inactive state. A peptide corresponding to the pseudo-RACK sequence binds to the RACK-binding site on PKC and renders the enzyme active. Because the affinity of the pseudo-RACK peptide for the RACK-binding site on PKC is lower than that of the RACK, the pseudo-RACK peptide is replaced by the RACK. That results in anchoring of the active PKC near its substrate (e.g., ion channel), leading to the channel’s phosphorylation and the consequent physiological response (e.g., ion influx).
Selective 6–10 amino acid-long peptide activators of PKC isozymes were also rationally designed. These peptides are derived from intra-molecular interaction sites within each PKC isozyme (34). Each peptide activator interferes with an isozyme-specific (or sub-family-specific) inhibitory intra-molecular interaction within that PKC; each peptide is identical in sequence to one of the two intra-molecular interaction sites. By acting as a competitive inhibitor of the inhibitory intra-molecular interactions, the peptide acts as a selective activator of that isozyme. The intra-molecular interaction that we focused on is the pseudo-RACK site, the site that covers the RACK-binding site when PKC is inactive. Peptides derived from the pseudo-RACK binding site are selective activators of the corresponding PKC. Using this approach, we have identified a selective activator for all the conventional PKC isozymes, ψβRACK, and isozyme-selective activators for δ, ε, η and θPKCs (see Table 1).
Peptides that act intracellularly need to be conjugated to carrier peptides to deliver them across biological membranes. A number of such short peptide carriers were identified. We used mainly TAT47–57 (35). All the peptide regulators of PKC conjugated to such peptide carriers showed high selectivity for the corresponding isozyme. They were efficacious in vitro and in vivo at low nanomolar concentrations and were well-tolerated when acutely injected intravenously, intraperitoneally, and subcutaneously. They were also effective when delivered in a sustained fashion, using subcutaneous Alzet pumps to obtain weeks of constant delivery (3–10mg/kg/day).
PKC and Ca Channels
Ca channels consist of a central α1 subunit, which forms the ion-conducting pore, and the auxiliary subunits α2-δ, β and γ, which modulate surface expression and biophysical properties. In the sarcolemma of cardiac cells, two types of Ca channels have been identified: the high-voltage, L-type Ca channel, and the low-voltage, T-type Ca channel (2). They play an important role in normal and diseased myocytes (1).
L-type Ca channels
The influx of Ca through L-type Ca channels plays an essential role in both cardiac excitability and excitation-contraction coupling. The depolarizing current through L-type Ca channels contributes to the cardiac action potential plateau and to Ca-induced Ca release from the sarcoplasmic reticulum. Four genes encode L-type Ca channel α1 subunits in mammals: Cav1.2 (α1C), Cav1.3 (α1D), Cav1.1 (α1S) and Cav1.4 (α1F) (36). α1S and α1F expression is restricted to the skeletal muscle and the retina. α1C is assumed to be the most abundant isoform in the cardiovascular system, whereas α1D is expressed in neurons and neuroendocrine cells (1, 37). It is therefore believed that the contribution of L-type Ca currents to the physiology/pathophysiology of the heart and the therapeutic effects of Ca channel antagonists are mainly mediated through α1C Ca channels. This assumption has been challenged by recent findings that α1D knockout mice unexpectedly exhibited intrinsic sino-atrial dysfunction and predisposition to atrial fibrillation (38–40), suggesting an important functional role for the α1D Ca channel in the supraventricular tissue.
Multiple G protein-coupled receptors in the heart act through phospholipase C-dependent hydrolysis of membrane phosphoinositides to regulate many cellular proteins, including the L-type Ca channels. Early studies suggested that neurohormones (i.e. endothelin-1 and angiotensin II) modulate L-type Ca channels in a PKC isozyme-dependent manner. For example, acute endothelin 1 treatment (which activates PKC) resulted in increased, decreased or no change in basal Ca influx in isolated cardiomyocytes under different conditions (41–43).
Disparate results have also been found in studies using non-selective PKC activators. The published work from our group showed that PMA (a non-selective PKC activator) consistently inhibited Ca channels in rat ventricular cells (22, 44). However, PMA has also been reported to inhibit, have no effect, stimulate, or initially stimulate but later inhibit L-type Ca currents in cardiac myocytes (45). Non-selective PKC activators produce inconsistent responses in non-cardiac Ca channels as well. Stimulation of Ca channels by phorbol esters has been reported in Aplysia bag cell neurons (46), neuroblastomas (47), secretory RINm5f cells (48) and frog sympathetic neurons (49). Conversely, inhibition of Ca channels by phorbol esters has been reported in chick DRG cells (50), and PC-12 cells (51). Because specific PKC isozymes contribute to a wide variety of cellular responses and sometimes exert even opposite effects inside the cell, the usage of non-selective PKC activators might provide misleading information on the relative contribution of PKC isozymes to Ca channels. It seems therefore that the outcome of PKC activation is species and tissue-dependent, probably because of the isozyme type present in each cell type.
Over the last decade, the rational design of selective PKC modulators as well as the use of genetic models has contributed to better understanding the role of selective PKC isozymes on both α1C and α1D L-type Ca channels. The PKC phosphorylation sites of the cardiac α1C subunit of the L-type Ca channel have been mapped to threonines 27 and 31 of the N-terminus, in which conversion of the threonines 27 and 31 to alanine abolishes the PKC-mediated downregulation of α1C Ca channel current (ICa-L) in tsA-201 cells (52). In parallel, mutating the threonines 27 and 31 to aspartate restored the PKC sensitivity of α1C ICa-L, suggesting that change in net charge by phosphorylation of threonines 27 and 31 of the N-terminus is responsible for the α1C ICa-L inhibition (52). In order to dissect the role of specific PKC isozymes in the regulation of cardiac Ca channels, we took advantage of PKC-regulating peptides developed by our lab (24, 33). Intracellular delivery of selective εPKC activator peptide (ε V1-7) resulted in significant inhibition of ICa-L in ventricular myocytes isolated from adult rats (22). The inhibitory effect of εV1-7 was completely prevented by the εPKC peptide inhibitor (εV1-2). Because sustained εPKC activation has been associated with cardiac dysfunction and heart failure in different animal models (53–55), we tested whether chronic in vivo modulation of εPKC leads to altered ICa-L. Using transgenic mice with specific cardiac constitutive activation of εPKC (expressing the pseudo-RACK sequence of εPKC, ψεRACK), we found that sustained εPKC resulted in reduced basal ICa-L density as well as blunted the β-adrenergic activation of ICa-L (56).
Besides the negative effects of εPKC on α1C ICa-L by phosphorylating amino acid residues at N-terminal domain, Yang et al. (2005) have demonstrated in HEK293 cells that α, ε and ζ PKC isozymes phosphorylated serine 1928 at the C-terminal domain of the α1C L-type Ca channel, resulting in increased α1C L-type Ca channel activity (57). The serine 1928 phosphorylation has been previously related to up-regulation of α1C L-type Ca channel activity in a protein kinase A-dependent manner (58). Furthermore, Yang et al. (2009) have showed in Langendorff-perfused heart that both serines 1674 and 1928 were phosphorylated in response to PMA (non-selective PKC activator) (59). Using GST fusion proteins, the authors provided evidence that α, βI, βII, γ and θ PKC isozymes were able to phosphorylate the serine 1674 site in HEK293 cells. Therefore, isolated hearts from transgenic mice over-expressing αPKC displayed increased phosphorylation of serine 1674 and 1928 (59). Conversely, αPKC knockout mice presented decreased cardiac levels of serines 1674 and 1928 phosphorylation (59). Besides its effect on cardiomyocyte excitability, αPKC may affect cardiac excitation-contraction coupling through a negative regulation of sarcoplasmic reticulum Ca load, thereby affecting cardiac performance during cardiac dysfunction and heart failure (60).
Unlike the well-characterized α1C L-type Ca channels, α1D L-type Ca channels have not been highlighted in earlier studies. We have demonstrated in the tsA201 cell line, using single channel analysis, that PMA (non-selective PKC activator) treatment resulted in a decrease in open probability and increase in closed-time without any significant effect on the conductance of the α1D L-type Ca channel (61). More recently, we showed that phosphorylation of serine 81 in the N-terminal region plays a negative role in α1D Ca channel activity in tsA201 cell line (62). This response was mediated by non-selective PKC activation. The introduction of a negatively charged residue at position 81, by converting serine to aspartate, mimicked the PKC phosphorylation effect on the α1D Ca channel. The modulation of the α1D Ca channel by PKC was prevented by dialyzing cells with a 35 amino acid peptide mimicking the α1D N-terminal region comprising serine 81. In addition, we evaluated, in the tsA201 cell line, the role of specific PKC isozymes on α1D ICa-L by using different PKC isozyme-selective inhibitor peptides. We first demonstrated that βIIV5-3 (selective βIIPKC inhibitor) and εV1-2 (selective εPKC inhibitor) peptides antagonized the negative effects of PMA on α1D ICa-L (62). These results indicate that phosphorylation of serine 81 by βIIPKC and εPKC is required for down-regulation of α1D Ca channel activity. Parallel to their effects on cardiomyocyte excitability, βIIPKC and εPKC may affect cardiac excitation-contraction coupling through a negative myofilament Ca-sensitivity and cardiac hypertrophy, respectively, thereby affecting cardiac function during ventricular remodeling and heart failure (10, 54, 63).
T-type Ca channels
Transient low-voltage activated currents (ICaT) characterize T-type channels. They have been identified in the sinoatrial node, AV node, Purkinje system, vascular smooth cells, and in neurons (2, 37). The T-type Ca channel has been involved in pacemaking and in Ca signaling in secretory cells and vascular smooth muscle (37). ICaT has been implicated in triggering Ca release from the sarcoplasmic reticulum (64) and in contraction of Purkinje cells (65). There is evidence indicating that T-type Ca channels also promote cell proliferation and growth (66). In this regard, increased levels of re-expression of T-type Ca channels in certain pathologic states, such as ventricular hypertrophy, have been reported by others (67) and us (68). Only a few studies reported on the interaction of PKC with T-type Ca channels in the heart. Tseng and Boyden (69) demonstrated that PKC activation inhibited ICaT in canine cardiac cells. Similarly, Zhang et al. (2000) showed that indirect activation of PKC by arachidonic acid attenuated the recently cloned human T-type α1H current expressed in HEK 293 cells (70). At the molecular level, Cribbs et al. (1998) showed the existence of several PKC consensus motifs on the T-type Ca channel α1H subunit (71). Two sites are located on the domain I–II cytoplasmic loop and one site on the domain III–IV connector. Zheng et al. (2010) recently showed that lysophosphatidylcholine up-regulated T-type Ca channels in a PKC-dependent manner (72). However, the identity of the PKC isozyme which regulates T-type Ca channels is not yet known.
Based on the findings described above, we summarize that integration of different regulatory mechanisms controlled by specific PKC isozymes may provide sensitive modulation of Ca channel activity in response to cardiac physiopathology. In fact, it is possible that different PKC isozymes may have opposing effects on Ca channels, as previously suggested by the effect of PMA on the L-type Ca channel activity. Therefore, considering that Ca channels are the main mediators of Ca influx and are pivotal in cardiac function, further studies clarifying the physiological contribution of PKC isozyme-mediated Ca channel function need to be performed in normal and diseased hearts.
PKC and Na Channels
Voltage-gated Na channels are the primary channels responsible for the rising phase of the action potential in excitable cells. The regulation of Na channels by PKC has been studied using general PKC activators such as PMA (73) and OAG (1-oleoyl-2-acetyl-sn-glycerol) (74). In general, activation of PKC by these non-isozyme specific activators leads to a reduction in Na current in both brain and heart (75–77). Heterologously expressed rat brain (rBIIA) (73, 77) and human cardiac Na channel currents (hH1) (76) were reduced upon PKC activation. While both rBIIA and hH1 contain consensus sites for phosphorylation by PKC, most of the sites are not conserved between these two isozymes. In one study (76), elimination of conserved consensus PKC sites in the hH1 interdomain III–IV linker, which contains the putative PKC site (Ser1503) does not completely eliminate the PMA-induced Na current inhibition, implying that other phosphorylation site(s) may exist. Considering that Na channels are associated with other proteins forming a multi-protein complex (78), the remaining Na current may be due to PKC regulation of other kinases or phosphatases, and not necessarily to phosphorylation of another Na channel residue.
While these previous studies implicated PKC in the regulation of Na channels, the role and the identity of the isozyme(s) responsible for this regulation remained largely unexplored. Our laboratory has utilized rationally designed PKC modulators to gain insight into the Na channel regulation by PKC. Studies from our lab using Xenopus oocytes showed that the Na current is inhibited by PMA, a general PKC activator. In addition, the use of the peptide specific activator of εPKC, ψεRACK (Table 1), mimicked PMA effects on INa and the use of the peptide specific inhibitor of εPKC, εV1-2 (Table 1), prevented these effects, thus establishing the involvement of at least εPKC in the regulation of Na channels. Similarly, the decrease in the neuronal Nav1.8 peak current induced by PMA was prevented by a specific εPKC isozyme peptide antagonist, whereas the PMA effect on Nav1.7 was prevented by εPKC and βIIPKC peptide inhibitors (79).
Considering that βIIPKC and εPKC are key molecules involved in cardiac hypertrophy, heart failure [for recent review see (24)], and disrupted cardiac excitability (i.e. down-regulation of Ca and Na channel currents), it may be possible to consider using βIIPKC and εPKC isozyme-selective inhibitors as therapeutic tools for the treatment of chronic cardiac diseases.
PKC and K Channels
K channels are of importance in determining the shape and mostly the repolarization of the cardiac action potential (80). In cardiac myocytes, at least eleven K currents have been characterized pharmacologically or by molecular cloning. These currents include the inward rectifier K current, IKl; the transient outward K current, Ito; the ultra-rapidly activating delayed rectifier K current, IKur; and the rapidly IKr and slowly IKs activating delayed rectifier K currents (4). In general, the initial and rapid phase (phase 2) of the repolarization is caused by outward K movement (transient outward current, Ito) through rapidly activating and inactivating K channels, and the late phase (phase 3) is under the control of the delayed rectifier current (IK). As such, K currents can modulate the duration of the action potential and refractoriness of the myocardium. It is noteworthy to emphasize that there is considerable heterogeneity in the morphology of action potentials and underlying K currents from various areas of the heart and between species.
The human ether-a-go-go-related gene hERG encodes the voltage-gated K channel underlying IKr, which initiates repolarization and terminates the plateau phase of the action potential (81). Several papers have reported that either α-adrenergic or non-selective PKC stimulation by PMA reduces hERG currents in cardiomyocyte (82–85). However, this phenomenon seems to be independent of direct PKC phosphorylation of the channel, since deletion by mutagenesis of PKC-dependent phosphorylation sites in hERG does not abolish PMA-induced effects on the channel (85). The cellular mechanisms of this PKC-mediated are still not completely understood. Of interest, the non-selective PKC inhibitor, BIM-I blocks hERG currents in a PKC-independent manner (86). Thus, PKC-independent effects have to be carefully considered when using non-selective PKC inhibitors in models involving IKr currents. Other K currents such as Ito1, IKl and Kv4.2/Kv4.3 currents are also negatively regulated by non-selective PKC activation in the heart (87), whereas increased εPKC translocation (and not δPKC) indirectly attenuates K currents by suppressing the synthesis of K channel proteins in the heart (88).
IKs channel is encoded by KvLQT1 and minK genes. KvLQT1/MinK channel contributes to the phase 2 slow repolarization of cardiac action potential. PKC has been suggested as a key mediator of KvLQT1/MinK channel regulation. The inhibition of the KvLQT1/MinK channel in ventricular myocytes by angiotensin II occurs in a PKC-dependent manner (89). In contrast, PKC is implicated in KvLQT1/MinK channel activation upon beta-3 adrenergic stimulation in Xenopus oocytes (90). Considering that different PKC isozymes have opposite effects in the cell (91), future studies are required in order to better understand the contribution of specific PKC isozymes in these processes. Mouse and rat IKs currents were decreased by PKC activation (92), whereas guinea pig IKs was activated by PKC (93). These findings suggest that regulation of IKs by PKC is also species dependent. In mouse and rat minK K channel, there is a putative PKC phosphorylation site at Ser102. Position Ser102 in the minK protein has been shown to be critical in determining the effect of PKC. Work in our lab has focused on the use of rationally designed small molecules to gain insight into the modulatory actions of PKC on the delayed rectifier, IKs. The data from our lab showed that in Xenopus oocytes, activation of PKC by PMA activates the human cardiac IKs channel and that selective inhibition of βIIPKC, but not βIPKC (using βIIV5-3 and βIV5-3, respectively; Table 1) abolishes PMA-induced IKs activation. These findings support the hypothesis of specialized function of only βIIPKC (21). However, additional work is required to better understand the behavior of K channels upon βIIPKC activation during cardiac stress such as ventricular dysfunction and heart failure. In addition, the identification of the particular PKC isozymes that mediate the regulation of K currents is of importance for the understanding of the mechanism of ion channel regulation and the development of new therapeutic agents. Future studies utilizing rationally designed PKC modulators may help to answer this question.
PKC and Gap Junction
In the heart, the gap junction plays an essential role in the electrical cell-to-cell coupling and impulse propagation between cells. Gap junction proteins are encoded by the connexin multigene family, whereas connexin 43 (Cx43) is the most abundant and widespread of the connexins in the heart. Cx43 phosphorylation influences its expression, degradation and functional properties such as conductance and open-probability of channels. Thus, dysfunction of the cardiac gap junction due to Cx43 post-translational changes mainly contributes to ventricular arrhythmia. Over the last decade, PKC-mediated phosphorylation of Cx43 has been reported and associated with both increased (94, 95) and decreased conductance (7, 96). However, the contribution of specific PKC isozymes to this phenomenon remains poorly understood due to the utilization of non-selective PKC isozyme inhibitors.
Among different PKC isozymes, only αPKC and εPKC have been described to co-localize with Cx43 in the heart (3, 97, 98). Bowling et al. (2001) showed that both εPKC and αPKC co-localize with Cx43 in non-failing and failing hearts, however, only εPKC directly phosphorylates Cx43 (99). Doble et al. (2000) demonstrated that non-specific PKC activation by either fibroblast growth-factor 2 (FGF-2) or PMA increased εPKC but not αPKC co-localization with Cx43 in myocytes, resulting in decreased gap junction permeability, whereas chelerythrine treatment (a non-selective PKC inhibitor) blocked the effects of FGF-2 on Cx43 phosphorylation and permeability (7, 13). Furthermore, evidence that the εPKC isozyme is directly involved in Cx43 phosphorylation was provided by showing that over-expression of the mutated dominant-negative form of εPKC decreased myocyte Cx43 phosphorylation and permeability (7, 13). Therefore, Cx43 phosphorylation by εPKC has been associated with decreased gap junctional intercellular communication. Bao et al. (2004) proposed that phosphorylation of Ser368 by PKC produces a conformational change in the C-terminal domain that leads to a decrease in Cx43 permeability (100).
PKC and Na/Ca Exchanger
The cardiac Na/Ca exchanger (NCX) plays a pivotal role in regulating intracellular Ca homeostasis to maintain normal electrical and mechanical activities of the heart. NCX1 is particularly important to excitability because during each action potential, NCX1 rapidly extrudes the Ca which entered the myocytes and brings Ca into the myocytes during cardiac depolarization.
Ruknudin et al. (2007) have shown that NCX is a member of a macromolecular complex that includes different kinases and phosphatases (101). However, the role of protein phosphorylation in the NCX has not been well defined. Iwamoto et al. (1996, 1998) first provided evidence that the cardiac isoform of NCX can be phosphorylated by PKC in CCL39 fibroblasts, resulting in increased NCX activity (102, 103). They found that NCX1 was phosphorylated upon incubation with PMA (non-selective PKC activator). Of interest, when all the consensus sites of the PKC phosphorylation were mutated in the NCX1, the protein still showed response after PMA stimulation (102), suggesting that either PMA stimulation could result in non-specific kinase activation or that there could be nonconsensus sites for PKC on NCX1.
Zhang et al. (2001, 2009) have reported that chelerythrine (a non-selective PKC inhibitor) abolished the deleterious effect of endothelin-1 treatment on NCX1 activity in guinea-pig ventricular myocytes (8, 104). Using an animal model, Katanosaka et al. (2007) showed increased NCX phosphorylation and association of NCX1 with εPKC in the hypertrophic hearts of thoracic aortic-banded (TAB) mice (105). Indeed, transgenic mice over-expressing an NCX1 mutation in specific-PKC phosphorylation sites (NCX1-S249A/S250A/S357A) were more resistant to TAB-induced cardiac hypertrophy (105). To date, it is well-established in the literature that NCX level and activity are increased in heart failure as consequence of sarcoplasmic reticulum dysfunction and cytosolic calcium accumulation during diastole (106). Direct PKC-mediated NCX phosphorylation contributes to NCX activation. In fact, cardiac NCX1 was shown to be stimulated by acute treatment with protein kinase C (PKC) activators, such as PMA and Gq-coupled receptor agonists (103, 107). In that sense, the use of rationally designed inhibitor peptides of selective PKC isozymes might contribute to correct NCX hyper activation and improves heart failure prognosis.
Besides its direct effect on NCX activity, PKC indirectly regulates NCX function by phosphorylating phospholemman, a small transmembrane protein which belongs to a family of proteins (FXYD gene family) that bind to and regulate NCX. Many papers have reported that regulation of cardiac contractility and NCX1 activity by phospholemman is critically dependent of its phosphorylation at serines 63 and 68 by PKC in cardiac myocytes (108–110). In fact, overexpression of S68E or S63A mutations in phospholemman inhibited NCX activity. Indeed, NCX1 activity is known to be modulated by ε-adrenergic stimulation in a PKC-dependent manner (103, 111). NCX1 activity is also regulated by PKA phosphorylation at serine 68.
Phospholemman phosphorylation also regulates the membrane Na/K ATPase (Na/K pump) activity, a vital component for the maintenance of normal electrical activity. Han et al. have demonstrated that non-selective PKC activation (using PMA) increases Na/K pump Vmax (112). Of interest, PKC-associated Na/K pump activation is abrogated in phospholemman deficient mice, providing evidences that PKC regulates Na/K pump by phosphorylating phospholemman (113). Phospholemman phosphorylation at both Ser63 and Ser68 is necessary for completely relieving the phospholemman-induced Na/K pump alteration (114). Considering that NCX and the Na/K pump are found together in a functional complex in cardiac myocytes (115), the contribution of PKC-mediated phospholemman phosphorylation to this complex needs to be better exploited. Therefore, futures studies using rationally designed peptides regulators may help to clarify the contribution of PKC isozymes to direct and indirect NCX and Na/K pump regulation.
PKC and Arrhythmias
Ischemic heart disease is the leading cause of congestive heart failure and death in the Western world (116). Early reperfusion after coronary occlusion improves cardiac function and reduces infarct size, but it is invariably accompanied by an overload of intracellular Ca that mediates cellular damage and contractile dysfunction (117, 118) and fatal ventricular arrhythmias, such as ventricular fibrillation. Efforts have thus been made to minimize the adverse arrhythmic events related to myocardial ischemia-related reperfusion. While the protective role of εPKC activation in myocardial ischemia-reperfusion injury was extensively characterized regarding the alteration in hemodymamics, cellular damage, and infarction size (119), the potential protective effect of εPKC activation on the fatal ventricular arrhythmia associated with ischemia-reperfusion is just emerging.
We used optical mapping techniques to focus on the potential protective role of εPKC modulation on ischemia-reperfusion arrhythmias in two lines of transgenic mice which moderately over-express the εPKC (termed εPKC agonist mice) activator peptide (ψεRACK, Table 1) (120) resulting in a 20% increase in εPKC activity, and εPKC inhibitor peptide (εV1-2; Table 1) resulting in a 15% inhibition of εPKC activity (termed εPKC antagonist mice) in the heart only (expression of the peptides was restricted to cardiac myocytes) (121). Mice were subjected to 10 min global ischemia and 30 min reperfusion and action potentials and intracellular Ca transients were recorded simultaneously at 37 °C. In the εPKC antagonist group, in which εPKC activity was down-regulated, 10 out of 13 (76.9%) TG mice developed VT, of which six (46.2%) degenerated into sustained VF upon reperfusion (121). Interestingly, in εPKC agonist mice, in which the activity of εPKC was up-regulated, no VF was observed and only 1 out of 12 mice showed only transient VT during reperfusion. During ischemia and reperfusion, intracellular Ca transient decay was exceedingly slower in the antagonist mice compared to the agonist TG mice. The data showed that moderate in vivo activation of εPKC exerts beneficial anti-arrhythmic effects vis a vis the lethal reperfusion arrhythmias (121). Similarly, in vivo treatment of pigs with the εPKC activator, ψεRACK, early during ischemia completely blocked post-ischemic arrhythmia (122). It is interesting to note that although both εPKC inhibition (by treatment with δV1-1) and εPKC activation (by treatment with ψεRACK) resulted in similar reduction in infarct size in this transient ischemic model in pigs, only εPKC activation prevented post-ischemic arrhythmia (123). These findings have important implications for the development of PKC isozyme targeted therapeutics and subsequently for the treatment of ischemic heart disease. Further studies are required in order to identify the specific targets involved in this anti-arrhythmic PKC-mediated phenomenon.
Summary and Perspectives
In this review, we describe a continuum of responses emanating from different PKC isozymes that contribute to cardiac excitability. It is clear that PKC isozymes play critical roles in the individual ion channels, gap junction and exchangers, which ultimately interfere in the initiation and propagation of the action potential and thus cardiac excitability (Figure 1). The conflicting data on the role of individual PKC isozymes in the regulation of cardiac excitability may be due not only to the differences between species and tissue distribution, but also to the pharmacological and genetic tools used in the studies. Because PKC isozymes have unique, and sometimes, opposing functions, the use of isozyme-selective tools is critical. Using either genetic manipulation or pharmacological tools able to deliver selective agonist and antagonist peptides for the same PKC isozyme in the heart is particularly useful; it helps to determine the contribution of selective PKC isozyme(s) to cardiac excitability-related processes. Clearly, PKC isozymes are central in regulating the electrical activity of the heart. Using these isozyme-selective inhibitors and activators, we demonstrated a role for specific PKC isozymes in the regulation of Ca, Na and K channels [1–3]. It may be possible now to investigate the positive and negative contributions of specific PKC isozymes to action potential electrogenesis and excitation-contraction coupling in different pathological conditions such as ischemia, cardiac dysfunction and heart failure. Therefore, in vivo studies using different animal models will help to point out the relevance of specific PKC isozymes as therapeutic targets for the treatment of electrochemical changes induced by cardiovascular diseases.
ACKNOWLEDGEMENTS
The work in the authors’ laboratories was supported by NIH (R01-HL-077494) and the Veterans Affairs MERIT grants to MB and by an NIH (HL52141) to DM-R. JCBF holds a post-doctoral fellowship from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP 2009/03143-1). DM-R is the founder and a share holder of KAI Pharmaceuticals, Inc, a company that plans to bring PKC regulators to the clinic. However, none of the work described in this study is based on or supported by the company. JCBF and MB has no disclosure.
REFERENCES
- 1.Amin AS, Tan HL, Wilde AA. Cardiac ion channels in health and disease. Heart Rhythm. 2010;7:117–126. doi: 10.1016/j.hrthm.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann F, Lacinova L, Klugbauer N. Voltage-dependent calcium channels: from structure to function. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 3.Imanaga I. Pathological remodeling of cardiac gap junction connexin 43-With special reference to arrhythmogenesis. Pathophysiology. 2010;17:73–81. doi: 10.1016/j.pathophys.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Curr Opin Cardiol. 2010;25:29–36. doi: 10.1097/HCO.0b013e328333d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benitah JP, Alvarez JL, Gomez AM. L-type Ca(2+) current in ventricular cardiomyocytes. J Mol Cell Cardiol. 2010;48:26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doble BW, Ping P, Kardami E. The epsilon subtype of protein kinase C is required for cardiomyocyte connexin-43 phosphorylation. Circ Res. 2000;86:293–301. doi: 10.1161/01.res.86.3.293. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YH, Hancox JC. Regulation of cardiac Na+-Ca2+ exchanger activity by protein kinase phosphorylation--still a paradox? Cell Calcium. 2009;45:1–10. doi: 10.1016/j.ceca.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res. 1985;56:884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- 10.Palaniyandi SS, Sun L, Ferreira JC, Mochly-Rosen D. Protein kinase C in heart failure: a therapeutic target? Cardiovasc Res. 2009;82:229–239. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strasser RH, Braun-Dullaeus R, Walendzik H, Marquetant R. Alpha 1-receptor-independent activation of protein kinase C in acute myocardial ischemia. Mechanisms for sensitization of the adenylyl cyclase system. Circ Res. 1992;70:1304–1312. doi: 10.1161/01.res.70.6.1304. [DOI] [PubMed] [Google Scholar]
- 12.Churchill EN, Disatnik MH, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 14.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol. 1994;266(3 Pt 2):H1145–H1152. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- 15.Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc Res. 2010;85:385–394. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JA, Mochly-Rosen D. Inhibition of the spontaneous rate of contraction of neonatal cardiac myocytes by protein kinase C isozymes. A putative role for the epsilon isozyme. Circ Res. 1995;76:654–663. doi: 10.1161/01.res.76.4.654. [DOI] [PubMed] [Google Scholar]
- 17.Shubeita HE, Martinson EA, Van Bilsen M, Chien KR, Brown JH. Transcriptional activation of the cardiac myosin light chain 2 and atrial natriuretic factor genes by protein kinase C in neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A. 1992;89:1305–1309. doi: 10.1073/pnas.89.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariya K, Karns LR, Simpson PC. Expression of a constitutively activated mutant of the beta-isozyme of protein kinase C in cardiac myocytes stimulates the promoter of the beta-myosin heavy chain isogene. J Biol Chem. 1991;266:10023–10026. [PubMed] [Google Scholar]
- 19.Chien KR, Knowlton KU, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 1991;5:3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- 20.Xiao GQ, Qu Y, Sun ZQ, Mochly-Rosen D, Boutjdir M. Evidence for functional role of epsilonPKC isozyme in the regulation of cardiac Na(+) channels. Am J Physiol Cell Physiol. 2001;281:C1477–C1486. doi: 10.1152/ajpcell.2001.281.5.C1477. [DOI] [PubMed] [Google Scholar]
- 21.Xiao GQ, Mochly-Rosen D, Boutjdir M. PKC isozyme selective regulation of cloned human cardiac delayed slow rectifier K current. Biochem Biophys Res Commun. 2003;306:1019–1025. doi: 10.1016/s0006-291x(03)01095-7. [DOI] [PubMed] [Google Scholar]
- 22.Hu K, Mochly-Rosen D, Boutjdir M. Evidence for functional role of epsilonPKC isozyme in the regulation of cardiac Ca(2+) channels. Am J Physiol Heart Circ Physiol. 2000;279:H2658–H2664. doi: 10.1152/ajpheart.2000.279.6.H2658. [DOI] [PubMed] [Google Scholar]
- 23.MacLeod KT, Harding SE. Effects of phorbol ester on contraction, intracellular pH and intracellular Ca2+ in isolated mammalian ventricular myocytes. J Physiol. 1991;444:481–498. doi: 10.1113/jphysiol.1991.sp018889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira JC, Brum PC, Mochly-Rosen D. betaIIPKC and epsilonPKC isozymes as potential pharmacological targets in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.10.020. Epud ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puceat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem. 1994;269:16938–16944. [PubMed] [Google Scholar]
- 26.Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994;74:299–309. doi: 10.1161/01.res.74.2.299. [DOI] [PubMed] [Google Scholar]
- 27.Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, Negro-Vilar A, Hannun YA. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992;117:121–133. doi: 10.1083/jcb.117.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 29.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. Journal of Biological Chemistry. 1982;257:7847–7851. [PubMed] [Google Scholar]
- 30.Nelson TJ, Alkon DL. Neuroprotective versus tumorigenic protein kinase C activators. Trends Biochem Sci. 2009;34:136–145. doi: 10.1016/j.tibs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Kazanietz MG. Eyes wide shut: protein kinase C isozymes are not the only receptors for the phorbol ester tumor promoters. Mol Carcinog. 2000;28:5–11. doi: 10.1002/(sici)1098-2744(200005)28:1<5::aid-mc2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 32.Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 33.Churchill EN, Qvit N, Mochly-Rosen D. Rationally designed peptide regulators of protein kinase C. Trends Endocrinol Metab. 2009;20:25–33. doi: 10.1016/j.tem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budas GR, Koyanagi T, Churchill EN, Mochly-Rosen D. Competitive inhibitors and allosteric activators of protein kinase C isoenzymes: a personal account and progress report on transferring academic discoveries to the clinic. Biochem Soc Trans. 2007;35(Pt 5):1021–1026. doi: 10.1042/BST0351021. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Wright LR, Chen CH, Oliver SF, Wender PA, Mochly-Rosen D. Molecular transporters for peptides: delivery of a cardioprotective epsilonPKC agonist peptide into cells and intact ischemic heart using a transport system, R(7) Chem Biol. 2001;8:1123–1129. doi: 10.1016/s1074-5521(01)00076-x. [DOI] [PubMed] [Google Scholar]
- 36.Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 37.Striessnig J. Pharmacology, structure and function of cardiac L-type Ca(2+) channels. Cell Physiol Biochem. 1999;9:242–269. doi: 10.1159/000016320. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N. Functional Roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res. 2002;90:981–987. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- 39.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 40.Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bkaily G, Wang S, Bui M, Menard D. ET-1 stimulates Ca2+ currents in cardiac cells. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S293–S296. [PubMed] [Google Scholar]
- 42.He JQ, Pi Y, Walker JW, Kamp TJ. Endothelin-1 and photoreleased diacylglycerol increase L-type Ca2+ current by activation of protein kinase C in rat ventricular myocytes. J Physiol. 2000;524(Pt 3):807–820. doi: 10.1111/j.1469-7793.2000.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng TH, Chang CY, Wei J, Lin CI. Effects of endothelin 1 on calcium and sodium currents in isolated human cardiac myocytes. Can J Physiol Pharmacol. 1995;73:1774–1783. doi: 10.1139/y95-242. [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZH, Johnson JA, Chen L, El-Sherif N, Mochly-Rosen D, Boutjdir M. C2 region-derived peptides of beta-protein kinase C regulate cardiac Ca2+ channels. Circ Res. 1997;80:720–729. doi: 10.1161/01.res.80.5.720. [DOI] [PubMed] [Google Scholar]
- 45.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 46.DeRiemer SA, Strong JA, Albert KA, Greengard P, Kaczmarek LK. Enhancement of calcium current in Aplysia neurones by phorbol ester and protein kinase C. Nature. 1985;313:313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- 47.Osugi T, Imaizumi T, Mizushima A, Uchida S, Yoshida H. 1-Oleoyl-2-acetyl-glycerol and phorbol diester stimulate Ca2+ influx through Ca2+ channels in neuroblastoma × glioma hybrid NG108-15 cells. Eur J Pharmacol. 1986;126:47–51. doi: 10.1016/0014-2999(86)90736-3. [DOI] [PubMed] [Google Scholar]
- 48.Rorsman P, Arkhammar P, Berggren PO. Voltage-activated Na+ currents and their suppression by phorbol ester in clonal insulin-producing RINm5F cells. Am J Physiol. 1986;251(6 Pt 1):C912–C919. doi: 10.1152/ajpcell.1986.251.6.C912. [DOI] [PubMed] [Google Scholar]
- 49.Yang J, Tsien RW. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993;10:127–136. doi: 10.1016/0896-6273(93)90305-b. [DOI] [PubMed] [Google Scholar]
- 50.Rane SG, Dunlap K. Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci U S A. 1986;83:184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Virgilio F, Pozzan T, Wollheim CB, Vicentini LM, Meldolesi J. Tumor promoter phorbol myristate acetate inhibits Ca2+ influx through voltage-gated Ca2+ channels in two secretory cell lines, PC12 and RINm5F. J Biol Chem. 1986;261:32–35. [PubMed] [Google Scholar]
- 52.McHugh D, Sharp EM, Scheuer T, Catterall WA. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc Natl Acad Sci U S A. 2000;97:12334–12338. doi: 10.1073/pnas.210384297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inagaki K, Iwanaga Y, Sarai N, Onozawa Y, Takenaka H, Mochly-Rosen D, Kihara Y. Tissue angiotensin II during progression or ventricular hypertrophy to heart failure in hypertensive rats; differential effects on PKC epsilon and PKC beta. J Mol Cell Cardiol. 2002;34:1377–1385. doi: 10.1006/jmcc.2002.2089. [DOI] [PubMed] [Google Scholar]
- 54.Inagaki K, Koyanagi T, Berry NC, Sun L, Mochly-Rosen D. Pharmacological inhibition of epsilon-protein kinase C attenuates cardiac fibrosis and dysfunction in hypertension-induced heart failure. Hypertension. 2008;51:1565–1569. doi: 10.1161/HYPERTENSIONAHA.107.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koyanagi T, Noguchi K, Ootani A, Inagaki K, Robbins RC, Mochly-Rosen D. Pharmacological inhibition of epsilon PKC suppresses chronic inflammation in murine cardiac transplantation model. J Mol Cell Cardiol. 2007;43:517–522. doi: 10.1016/j.yjmcc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Yue Y, Qu Y, Boutjdir M. Beta- and alpha-adrenergic cross-signaling for L-type Ca current is impaired in transgenic mice with constitutive activation of epsilonPKC. Biochem Biophys Res Commun. 2004;314:749–754. doi: 10.1016/j.bbrc.2003.12.155. [DOI] [PubMed] [Google Scholar]
- 57.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, Marx SO. Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 58.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3',5'-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 59.Yang L, Doshi D, Morrow J, Katchman A, Chen X, Marx SO. Protein kinase C isoforms differentially phosphorylate Ca(v)1.2 alpha(1c) Biochemistry. 2009;48:6674–6683. doi: 10.1021/bi900322a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 61.Chahine M, Qu Y, Mancarella S, Boutjdir M. Protein kinase C activation inhibits alpha1D L-type Ca channel: a single-channel analysis. Pflugers Arch. 2008;455:913–919. doi: 10.1007/s00424-007-0342-z. [DOI] [PubMed] [Google Scholar]
- 62.Baroudi G, Qu Y, Ramadan O, Chahine M, Boutjdir M. Protein kinase C activation inhibits Cav1.3 calcium channel at NH2-terminal serine 81 phosphorylation site. Am J Physiol Heart Circ Physiol. 2006;291:H1614–H1622. doi: 10.1152/ajpheart.00095.2006. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, Grant JE, Doede CM, Sadayappan S, Robbins J, Walker JW. PKC-betaII sensitizes cardiac myofilaments to Ca2+ by phosphorylating troponin I on threonine-144. J Mol Cell Cardiol. 2006;41:823–833. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Sipido KR, Carmeliet E, Van de Werf F. T-type Ca2+ current as a trigger for Ca2+ release from the sarcoplasmic reticulum in guinea-pig ventricular myocytes. J Physiol. 1998;508(Pt 2):439–451. doi: 10.1111/j.1469-7793.1998.439bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Z, January CT. Both T- and L-type Ca2+ channels can contribute to excitation-contraction coupling in cardiac Purkinje cells. Biophys J. 1998;74:1830–1839. doi: 10.1016/S0006-3495(98)77893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Estacion M, Mordan LJ. Ca2+ influx via T-type channels modulates PDGF-induced replication of mouse fibroblasts. Am J Physiol. 1993;265(5 Pt 1):C1239–C1246. doi: 10.1152/ajpcell.1993.265.5.C1239. [DOI] [PubMed] [Google Scholar]
- 67.Nuss HB, Houser SR. T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res. 1993;73:777–782. doi: 10.1161/01.res.73.4.777. [DOI] [PubMed] [Google Scholar]
- 68.Huang B, Qin D, Deng L, Boutjdir M, Sherif N NE. Reexpression of T-type Ca2+ channel gene and current in post-infarction remodeled rat left ventricle. Cardiovasc Res. 2000;46:442–449. doi: 10.1016/s0008-6363(00)00017-1. [DOI] [PubMed] [Google Scholar]
- 69.Tseng GN, Boyden PA. Different effects of intracellular Ca and protein kinase C on cardiac T and L Ca currents. Am J Physiol. 1991;261(2 Pt 2):H364–H379. doi: 10.1152/ajpheart.1991.261.2.H364. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Cribbs LL, Satin J. Arachidonic acid modulation of alpha1H, a cloned human T-type calcium channel. Am J Physiol Heart Circ Physiol. 2000;278:H184–H193. doi: 10.1152/ajpheart.2000.278.1.H184. [DOI] [PubMed] [Google Scholar]
- 71.Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes M. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- 72.Zheng M, Wang Y, Kang L, Shimaoka T, Marni F, Ono K. Intracellular Ca(2+)- and PKC-dependent upregulation of T-type Ca(2+) channels in LPC-stimulated cardiomyocytes. J Mol Cell Cardiol. 2010;48:131–139. doi: 10.1016/j.yjmcc.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 73.Dascal N, Lotan I. Activation of protein kinase C alters voltage dependence of a Na+ channel. Neuron. 1991;6:165–175. doi: 10.1016/0896-6273(91)90131-i. [DOI] [PubMed] [Google Scholar]
- 74.Qu Y, Rogers J, Tanada T, Scheuer T, Catterall WA. Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc Natl Acad Sci U S A. 1994;91:3289–3293. doi: 10.1073/pnas.91.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M, West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993;261:1439–1442. doi: 10.1126/science.8396273. [DOI] [PubMed] [Google Scholar]
- 76.Murray KT, Hu NN, Daw JR, Shin HG, Watson MT, Mashburn AB, George AL., Jr Functional effects of protein kinase C activation on the human cardiac Na+ channel. Circ Res. 1997;80:370–376. doi: 10.1161/01.res.80.3.370. [DOI] [PubMed] [Google Scholar]
- 77.Numann R, Catterall WA, Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991;254:115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- 78.Abriel H, Kass RS. Regulation of the voltage-gated cardiac sodium channel Nav1.5 by interacting proteins. Trends Cardiovasc Med. 2005;15:35–40. doi: 10.1016/j.tcm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Vijayaragavan K, Boutjdir M, Chahine M. Modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by protein kinase A and protein kinase C. J Neurophysiol. 2004;91:1556–1569. doi: 10.1152/jn.00676.2003. [DOI] [PubMed] [Google Scholar]
- 80.Snyders DJ. Molecular Biology of K Channels. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia, PA: W.B. Saunders Company; 2000. [Google Scholar]
- 81.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barros F, Gomez-Varela D, Viloria CG, Palomero T, Giraldez T, de la Pena P. Modulation of human erg K+ channel gating by activation of a G protein-coupled receptor and protein kinase C. J Physiol. 1998;511(Pt 2):333–346. doi: 10.1111/j.1469-7793.1998.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kiehn J, Karle C, Thomas D, Yao X, Brachmann J, Kubler W. HERG potassium channel activation is shifted by phorbol esters via protein kinase A-dependent pathways. J Biol Chem. 1998;273:25285–25291. doi: 10.1074/jbc.273.39.25285. [DOI] [PubMed] [Google Scholar]
- 84.Michelotti GA, Price DT, Schwinn DA. Alpha 1-adrenergic receptor regulation: basic science and clinical implications. Pharmacol Ther. 2000;88:281–309. doi: 10.1016/s0163-7258(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 85.Thomas D, Zhang W, Wu K, Wimmer AB, Gut B, Wendt-Nordahl G, Kathofer S, Kreye VA, Katus HA, Schoels W, Kiehn J, Karle CA. Regulation of HERG potassium channel activation by protein kinase C independent of direct phosphorylation of the channel protein. Cardiovasc Res. 2003;59:14–26. doi: 10.1016/s0008-6363(03)00386-9. [DOI] [PubMed] [Google Scholar]
- 86.Thomas D, Hammerling BC, Wimmer AB, Wu K, Ficker E, Kuryshev YA, Scherer D, Kiehn J, Katus HA, Schoels W, Karle CA. Direct block of hERG potassium channels by the protein kinase C inhibitor bisindolylmaleimide I (GF109203X) Cardiovasc Res. 2004;64:467–476. doi: 10.1016/j.cardiores.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura TY, Coetzee WA, Vega-Saenz De Miera E, Artman M, Rudy B. Modulation of Kv4 channels, key components of rat ventricular transient outward K+ current by PKC. Am J Physiol. 1997;273(4 Pt 2):H1775–H1786. doi: 10.1152/ajpheart.1997.273.4.H1775. [DOI] [PubMed] [Google Scholar]
- 88.Shimoni Y, Liu XF. Role of PKC in autocrine regulation of rat ventricular K+ currents by angiotensin and endothelin. Am J Physiol Heart Circ Physiol. 2003;284:H1168–H1181. doi: 10.1152/ajpheart.00748.2002. [DOI] [PubMed] [Google Scholar]
- 89.Wang YH, Shi CX, Dong F, Sheng JW, Xu YF. Inhibition of the rapid component of the delayed rectifier potassium current in ventricular myocytes by angiotensin II via the AT1 receptor. Br J Pharmacol. 2008;154:429–439. doi: 10.1038/bjp.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kathofer S, Rockl K, Zhang W, Thomas D, Katus H, Kiehn J, Kreye V, Schoels W, Karle C. Human beta(3)-adrenoreceptors couple to KvLQT1/MinK potassium channels in Xenopus oocytes via protein kinase C phosphorylation of the KvLQT1 protein. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:119–126. doi: 10.1007/s00210-003-0772-x. [DOI] [PubMed] [Google Scholar]
- 91.Churchill EN, Mochly-Rosen D. The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem Soc Trans. 2007;35(Pt 5):1040–1042. doi: 10.1042/BST0351040. [DOI] [PubMed] [Google Scholar]
- 92.Honore E, Attali B, Romey G, Heurteaux C, Ricard P, Lesage F, Lazdunski M, Barhanin J. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. EMBO J. 1991;10:2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Varnum MD, Busch AE, Bond CT, Maylie J, Adelman JP. The min K channel underlies the cardiac potassium current IKs and mediates species-specific responses to protein kinase C. Proc Natl Acad Sci U S A. 1993;90:11528–11532. doi: 10.1073/pnas.90.24.11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moreno AP, Saez JC, Fishman GI, Spray DC. Human connexin 43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res. 1994;74:1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- 95.Kwak BR, Jongsma HJ. Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Mol Cell Biochem. 1996;157:93–99. doi: 10.1007/BF00227885. [DOI] [PubMed] [Google Scholar]
- 96.Munster PN, Weingart R. Effects of phorbol ester on gap junctions of neonatal rat heart cells. Pflugers Arch. 1993;423:181–188. doi: 10.1007/BF00374392. [DOI] [PubMed] [Google Scholar]
- 97.Weng S, Lauven M, Schaefer T, Polontchouk L, Grover R, Dhein S. Pharmacological modification of gap junction coupling by an antiarrhythmic peptide via protein kinase C activation. FASEB J. 2002;16:1114–1116. doi: 10.1096/fj.01-0918fje. [DOI] [PubMed] [Google Scholar]
- 98.Mayama T, Matsumura K, Lin H, Ogawa K, Imanaga I. Remodelling of cardiac gap junction connexin 43 and arrhythmogenesis. Exp Clin Cardiol. 2007;12:67–76. [PMC free article] [PubMed] [Google Scholar]
- 99.Bowling N, Huang X, Sandusky GE, Fouts RL, Mintze K, Esterman M, Allen PD, Maddi R, McCall E, Vlahos CJ. Protein kinase C-alpha and -epsilon modulate connexin-43 phosphorylation in human heart. J Mol Cell Cardiol. 2001;33:789–798. doi: 10.1006/jmcc.2000.1349. [DOI] [PubMed] [Google Scholar]
- 100.Bao X, Reuss L, Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J Biol Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- 101.Ruknudin AM, Wei SK, Haigney MC, Lederer WJ, Schulze DH. Phosphorylation and other conundrums of Na/Ca exchanger, NCX1. Ann N Y Acad Sci. 2007;1099:103–118. doi: 10.1196/annals.1387.036. [DOI] [PubMed] [Google Scholar]
- 102.Iwamoto T, Pan Y, Nakamura TY, Wakabayashi S, Shigekawa M. Protein kinase C-dependent regulation of Na+/Ca2+ exchanger isoforms NCX1 and NCX3 does not require their direct phosphorylation. Biochemistry. 1998;37:17230–17238. doi: 10.1021/bi981521q. [DOI] [PubMed] [Google Scholar]
- 103.Iwamoto T, Pan Y, Wakabayashi S, Imagawa T, Yamanaka HI, Shigekawa M. Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. J Biol Chem. 1996;271:13609–13615. doi: 10.1074/jbc.271.23.13609. [DOI] [PubMed] [Google Scholar]
- 104.Zhang YH, James AF, Hancox JC. Regulation by endothelin-1 of Na+-Ca2+ exchange current (I(NaCa)) from guinea-pig isolated ventricular myocytes. Cell Calcium. 2001;30:351–360. doi: 10.1054/ceca.2001.0244. [DOI] [PubMed] [Google Scholar]
- 105.Katanosaka Y, Kim B, Wakabayashi S, Matsuoka S, Shigekawa M. Phosphorylation of Na+/Ca2+ exchanger in TAB-induced cardiac hypertrophy. Ann N Y Acad Sci. 2007;1099:373–376. doi: 10.1196/annals.1387.024. [DOI] [PubMed] [Google Scholar]
- 106.Bers DM, Despa S. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J Pharmacol Sci. 2006;100:315–322. doi: 10.1254/jphs.cpj06001x. [DOI] [PubMed] [Google Scholar]
- 107.Stengl M, Mubagwa K, Carmeliet E, Flameng W. Phenylephrine-induced stimulation of Na+/Ca2+ exchange in rat ventricular myocytes. Cardiovasc Res. 1998;38:703–710. doi: 10.1016/s0008-6363(98)00039-x. [DOI] [PubMed] [Google Scholar]
- 108.Ahlers BA, Zhang XQ, Moorman JR, Rothblum LI, Carl LL, Song J, Wang J, Geddis LM, Tucker AL, Mounsey JP, Cheung JY. Identification of an endogenous inhibitor of the cardiac Na+/Ca2+ exchanger, phospholemman. J Biol Chem. 2005;280:19875–19882. doi: 10.1074/jbc.M414703200. [DOI] [PubMed] [Google Scholar]
- 109.Cheung JY, Rothblum LI, Moorman JR, Tucker AL, Song J, Ahlers BA, Carl LL, Wang J, Zhang XQ. Regulation of cardiac Na+/Ca2+ exchanger by phospholemman. Ann N Y Acad Sci. 2007;1099:119–134. doi: 10.1196/annals.1387.004. [DOI] [PubMed] [Google Scholar]
- 110.Song J, Zhang XQ, Ahlers BA, Carl LL, Wang J, Rothblum LI, Stahl RC, Mounsey JP, Tucker AL, Moorman JR, Cheung JY. Serine 68 of phospholemman is critical in modulation of contractility, [Ca2+]i transients, and Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2005;288:H2342–H2354. doi: 10.1152/ajpheart.01133.2004. [DOI] [PubMed] [Google Scholar]
- 111.Ballard C, Schaffer S. Stimulation of the Na+/Ca2+ exchanger by phenylephrine, angiotensin II and endothelin 1. J Mol Cell Cardiol. 1996;28:11–17. doi: 10.1006/jmcc.1996.0002. [DOI] [PubMed] [Google Scholar]
- 112.Han F, Bossuyt J, Despa S, Tucker AL, Bers DM. Phospholemman phosphorylation mediates the protein kinase C-dependent effects on Na+/K+ pump function in cardiac myocytes. Circ Res. 2006;99:1376–1383. doi: 10.1161/01.RES.0000251667.73461.fb. [DOI] [PubMed] [Google Scholar]
- 113.Fuller W, Shattock MJ. Phospholemman and the cardiac sodium pump: protein kinase C, take a bow. Circ Res. 2006;99:1290–1292. doi: 10.1161/01.RES.0000253090.54488.81. [DOI] [PubMed] [Google Scholar]
- 114.Han F, Bossuyt J, Martin JL, Despa S, Bers DM. Role of phospholemman phosphorylation sites in mediating kinase-dependent regulation of the Na+-K+-ATPase. Am J Physiol Cell Physiol. 2010;299:C1363–C1369. doi: 10.1152/ajpcell.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 117.Marban E, Koretsune Y, Corretti M, Chacko VP, Kusuoka H. Calcium and its role in myocardial cell injury during ischemia and reperfusion. Circulation. 1989;80(6 Suppl):IV17–IV22. [PubMed] [Google Scholar]
- 118.Zucchi R, Ronca F, Ronca-Testoni S. Modulation of sarcoplasmic reticulum function: a new strategy in cardioprotection? Pharmacol Ther. 2001;89:47–65. doi: 10.1016/s0163-7258(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 119.Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. PKC isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–599. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 120.Dorn GW, 2nd, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci U S A. 1999;96:12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yue Y, Qu Y, Boutjdir M. Protective role of protein kinase C epsilon activation in ischemia-reperfusion arrhythmia. Biochem Biophys Res Commun. 2006;349:432–438. doi: 10.1016/j.bbrc.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 122.Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by epsilon-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an epsilon-protein kinase C-activating peptide. Circulation. 2005;111:44–50. doi: 10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- 123.Inagaki K, Hahn HS, Dorn GW, 2nd, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation. 2003;108:869–875. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 124.Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. Anti-Neoplastic Agents .86. Isolation and Structure of Bryostatin-1. J Am Chem Soc. 1982;104:6846–6848. [Google Scholar]
- 125.Wender PA, DeBrabander J, Harran PG, Jimenez JM, Koehler MF, Lippa B, Park CM, Siedenbiedel C, Pettit GR. The design, computer modeling, solution structure, and biological evaluation of synthetic analogs of bryostatin 1. Proc Natl Acad Sci USA. 1998;95:6624–6629. doi: 10.1073/pnas.95.12.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ron D, Luo J, Mochly-Rosen D. C2 region-derived peptides inhibit translocation and function of beta protein kinase C in vivo. J Biol Chem. 1995;270:24180–24187. doi: 10.1074/jbc.270.41.24180. [DOI] [PubMed] [Google Scholar]
- 127.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Omura S, Iwai Y, Hirano A, Nakagawa A, Awaya J, Tsuchiya H, Takahashi Y, Masuma R. New Alkaloid Am-2282 of Streptomyces Origin Taxonomy, Fermentation, Isolation and Preliminary Characterization. J Antibiot. 1977;30:275–282. doi: 10.7164/antibiotics.30.275. [DOI] [PubMed] [Google Scholar]
- 129.Qatsha KA, Rudolph C, Marme D, Schachtele C, May WS. Go-6976, a Selective Inhibitor of Protein-Kinase-C, Is a Potent Antagonist of Human Immunodeficiency Virus-1 Induction from Latent Low-Level-Producing Reservoir Cells-Invitro. Proc Natl Acad Sci USA. 1993;90:4674–4678. doi: 10.1073/pnas.90.10.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun XG, Rotenberg SA. Overexpression of protein kinase C alpha in MCF-10A human breast cells engenders dramatic alterations in morphology, proliferation, and motility. Cell Growth Differ. 1999;10:343–352. [PubMed] [Google Scholar]
- 131.Nonaka A, Kiryu J, Tsujikawa A, Yamashiro K, Ogura Y. PKC-beta inhibitor (LY333531) attenuates leukocyte entrapment in retinal microcirculation of diabetic rats. Invest Ophthalmol Vis Sci. 1999;40:S680–S680. [PubMed] [Google Scholar]
- 132.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 133.Toullec D, Pianetti P, Coste H, Bellevergue P, Grandperret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The Bisindolylmaleimide Gf-109203x Is a Potent and Selective Inhibitor of Protein-Kinase-C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 134.Nixon JS, Bishop J, Bradshaw D, Davis PD, Hill CH, Elliott LH, Kumar H, Lawton G, Lewis EJ, Mulqueen M, Sedgwick AD, Westmacott D, Wadsworth J, Wilkinson SE. Novel, Potent and Selective Inhibitors of Protein-Kinase-C Show Oral Antiinflammatory Activity. Drugs Exp Clin Res. 1991;17:389–393. [PubMed] [Google Scholar]
- 135.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme Specificity of Bisindolylmaleimides, Selective Inhibitors of Protein-Kinase-C. Biochem J. 1993;294:335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.El Fitori J, Su Y, Buchler P, Ludwig R, Giese NA, Buchler MW, Quentmeier H, Hines OJ, Herr I, Friess H. PKC412 small-molecule tyrosine kinase inhibitor - Single-compound therapy for pancreatic cancer. Cancer. 2007;110:1457–1468. doi: 10.1002/cncr.22931. [DOI] [PubMed] [Google Scholar]
- 137.Takahashi I, Kobayashi E, Asano K, Yoshida M, Nakano H. Ucn-01, a Selective Inhibitor of Protein-Kinase-C from Streptomyces. Journal of Antibiot. 1987;40:1782–1784. doi: 10.7164/antibiotics.40.1782. [DOI] [PubMed] [Google Scholar]
- 138.Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a Novel Protein-Kinase Inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 139.Roychowdhury D, Lahn M. Antisense therapy directed to protein kinase C-alpha (Affinitak, LY900003/ISIS 3521): Potential role in breast cancer. Sem Oncol. 2003;30:30–33. doi: 10.1053/sonc.2003.37273. [DOI] [PubMed] [Google Scholar]
- 140.Levesque L, Dean NM, Sasmor H, Crooke ST. Antisense oligonucleotides targeting human protein kinase C-alpha inhibit phorbol ester-induced reduction of bradykinin-evoked calcium mobilization in A549 cells. Mol Pharmacol. 1997;51:209–216. doi: 10.1124/mol.51.2.209. [DOI] [PubMed] [Google Scholar]
- 141.Stebbins EG, Mochly-Rosen D. Binding Specificity for RACK1 Resides in the V5 Region of beta II Protein Kinase C. J Biol Chem. 2001;276:29644–29650. doi: 10.1074/jbc.M101044200. [DOI] [PubMed] [Google Scholar]
- 142.Li HF, Mochly-Rosen D, Kendig JJ. Protein kinase Cgamma mediates ethanol withdrawal hyper-responsiveness of NMDA receptor currents in spinal cord motor neurons. Br J Pharmacol. 2005;144:301–307. doi: 10.1038/sj.bjp.0706033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gray MO, Karliner JS, Mochly-Rosen D. A selective epsilon-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997;272:30945–30951. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- 144.House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 145.Braun MU, Mochly-Rosen D. Opposing effects of delta- and zeta-protein kinase C isozymes on cardiac fibroblast proliferation: use of isozyme-selective inhibitors. J Mol Cell Cardiol. 2003;35:895–903. doi: 10.1016/s0022-2828(03)00142-1. [DOI] [PubMed] [Google Scholar]