Abstract
Background
Activation of ε protein kinase C (εPKC) protects hearts from ischemic injury. However, some of the mechanism(s) of εPKC mediated cardioprotection are still unclear. Identification of εPKC targets may aid to elucidate εPKC–mediated cardioprotective mechanisms. Previous studies, using a combination of εPKC transgenic mice and difference in gel electrophoresis (DIGE), identified a number of proteins involved in glucose metabolism, whose expression was modified by εPKC. These studies, were accompanied by metabolomic analysis, and suggested that increased glucose oxidation may be responsible for the cardioprotective effect of εPKC. However, whether these εPKC-mediated alterations were due to differences in protein expression or phosphorylation was not determined.
Methods and Results
Here, we used an εPKC-specific activator peptide, ψεRACK, in combination with phosphoproteomics to identify εPKC targets, and identified proteins whose phosphorylation was altered by selective activation of εPKC most of the identified proteins were mitochondrial proteins and analysis of the mitochondrial phosphoproteome, led to the identification of 55 spots, corresponding to 37 individual proteins, which were exclusively phosphorylated, in the presence of ψεRACK. The majority of the proteins identified were proteins involved in glucose and lipid metabolism, components of the respiratory chain as well as mitochondrial heat shock proteins.
Conclusion
In summary the protective effect of εPKC during ischemia involves phosphorylation of several mitochondrial proteins involved in glucose, lipid metabolism and oxidative phosphorylation. Regulation of these metabolic pathways by εPKC phosphorylation may lead to εPKC-mediated cardioprotection induced by ψεRACK.
Keywords: εPKC, ischemia, phosphorylation, mitochondria
Introduction
We previously developed and used an εPKC isoenzyme- selective activator peptide and found that εPKC activation reduces cardiac cell death induced by ischemia 1, 2. To provide insight into εPKC-mediated cytoprotective mechanisms, we used a proteomic approach combining antibodies that specifically recognize proteins phosphorylated at the PKC consensus phosphorylation site and an εPKC activator peptide 3. This approach led to the identification of mitochondrial aldehyde dehydrogenase 2 (ALDH2) as an εPKC substrate, whose phosphorylation and activation is necessary and sufficient to induce cardioprotection during an ischemic injury 3. We also demonstrated that the cytoprotective mechanism of εPKC is mediated, at least in part, by ALDH2-mediated detoxification of reactive aldehydes, such as 4-hydroxy-2-nonenal (4-HNE), that accumulate in the heart during ischemia 3-5. Studies by others, using transgenic and dominant negative εPKC mice, identified other εPKC signaling complexes, composed of proteins involved in glucose and lipid metabolism, and proteins related to transcription/ translation, suggesting that εPKC-mediated cytoprotection involves regulation of other cellular processes 6-8. A study using difference in gel eletrophoresis (DIGE) comparing hearts of mice overexpressing catalytically active and dominant negative εPKC identified alterations in the levels of proteins involved in glucose metabolism. Metabolomic studies confirmed that during ischemia/ reperfusion glucose is metabolized faster in animals expressing constitutively active εPKC 9. However, these studies did not clarify whether the differences in the identified proteins were due to differential expression or phosphorylation levels. Overexpression of εPKC lead to its mislocalization 10 and a compensatory effect observed by δPKC overexpression 9. Therefore, some of the targets identified in this study could possibly have been phosphorylated by overexpressed δPKC or mis-localized εPKC. Nevertheless, these studies suggested that the cardioprotective mechanism of εPKC is also due to the regulation of glucose and lipid metabolism.
In the present study we used an adult heart Langendorff coronary perfusion system, and treated isolated hearts with an εPKC specific activator peptide (ψεRACK) prior to ischemia, to determine phosphorylation events, following selective activation of εPKC. Proteins whose phosphorylation increased in the presence of ψεRACK were detected in 2D Gels with a phospho-specific dye. Mass spectrometry of the phosphorylated proteins demonstrated that most of the proteins identified in total heart lysates that were differentially phosphorylated upon εPKC activation were mitochondrial proteins. Isolation of mitochondria from ψεRACK treated and control hearts confirmed that εPKC activation led to an increase inphosphorylation levels of proteins involved in, the electron transport chain as well as lipid metabolism.
Materials and Methods
Ex vivo rat heart model of cardiac ischemia
Animal protocols were approved by the Stanford University Institutional Animal Care and Use Committee. Rat hearts (Wistar, 250-300g), each group consisting of three rats, were perfused via the aorta at a constant flow rate of 10 ml/min with oxygenated Krebs-Henseleit buffer (120 mM NaCl, 5.8 mM KCl, 25 mM NaHCO3, 1.2 mM NaHCO3, 1.2 mM MgSO4, 1.2 mM CaCl2, and 10 mM dextrose, pH 7.4) at 37°C. After a 20 min. equilibration period, hearts were subjected to 35 min global, no-flow ischemia. The εPKC-selective agonist ψεRACK peptide [ HDAPIGYD 11 fused to the cell permeable Tat protein transduction domain peptide, amino acids 47-57 12 (1mM) was perfused for 10 min immediately prior to ischemia onset.
Preparation of heart lysates and sub-cellular fractionation
At the end of ischemia, hearts were removed from the cannnula and immediately homogenized on ice to obtain total and mitochondrial fractions. To obtain the total lysate fraction, heart ventricles were homogenized in BufferA [7M urea, 2M tiourea, 4% CHAPS, 5mM magnesium acetate, 17μg/mL PMSF and phosphatase inhibitor cocktail diluted 1:300 (Sigma # P8340 and Sigma # P5726)]. To obtain the mitochondrial fraction, heart ventricles were homogenized in ice-cold mannitol-sucrose (MS) buffer [210 mM mannitol, 70 mM sucrose, 5 mM MOPS and 1mM EDTA containing Protease) and phosphatase Inhibitors as above]. The homogenate was centrifuged at 700g for 10 minutes (to pellet the cytoskeletal fraction), the resultant supernatant was filtered through gauze, and centrifuged at 10,000g for 10 minutes (to pellet the mitochondrial fraction). The mitochondrial pellet was washed 3x in MS buffer before the pellet was resuspended in DIGE buffer.
Two-Dimensional Gel Electrophoresis
Protein samples (300μg for analytic gels and 500 μg for preparative gels of total heart lysate and 250 μg for analytic/ preparative gels of mitochondrial fraction) were applied onto 3-10 linear immobilized pH gradient strips (13cm, GE, Healthcare, Life Science). Strips were rehydrated for 16 hours at room temperature. Isoelectric focusing (IEF) was performed on an IPGphor III apparatus (GE Healthcare Life Science) at 17 KVh. For the second dimension strips were incubated at room temperature, for 20 min in equilibration buffer [6 M urea, 2% (w/v) SDS, 50 mM Tris-HCl pH 6.8, 30% (v/v) glycerol, 0.001% (w/v) bromophenol blue] with 2% (w/v) DTT, followed by incubation with 4% (w/v) iodoacetamide in equilibrium buffer, for 20 min. The second dimension was separated using vertical SDS-PAGE. Experiments were performed in triplicates. Phospho-proteins were detected by staining with Pro-Q Diamond (Invitrogen) per manufacturer’s instructions. Gels were scanned using a Typhoon TRI scanner (Healthcare Life Science), stained with Coomassie Brilliant Blue G250 (CBB) 13 and scanned using a UTA-1100 scanner and Labscan v 5.0 software (GE Healthcare Life Science).
Image analysis was performed using Image Master Software v.5.01 (GE Healthcare Life Science). For each pair of samples analyzed, individual spot volumes of replicate gels were determined in Pro-Q Diamond stained gels (phospho-proteins), followed by normalization (individual spot volume/ volume of all spots × 100). Spots (of treated samples) that appeared or showed a change in spot volume of least 1.5 fold as compared to samples of hearts submitted to ischemia alone were excised from CBB-stained preparative gels and identified by mass spectrometry. Differences between experimental groups were evaluated by the Mann-Whitney t-test for proteomic analysis. A * p value < 0.05 was considered statistically significant.
“In-gel” protein digestion and MALDI-TOF/TOF MS
Digestion of selected spots was performed as previously described 14. Matrix-Assisted Laser Desorption ionization Time-of-Flight/Time-of-Flight Mass Spectrometry) as analysis executed aspreviously described 15. MASCOT MS/MS Ion Search (www.matrixscience.com) software was used to blast sequences against the SwissProt and NCBInr databanks. Combined MS-MS/MS searches were conducted with parent ion mass tolerance at 50 ppm, MS/MS mass tolerance of 0.2 Da, carbamidomethylation of cysteine (fixed modification) and methionine oxidation (variable modification). According to MASCOT probability analysis only hits with significant P < 0.05 were accepted Spots from total lysates were identified at the Mass Spectrometry Facility at Stanford University (mass-spec.stanford.edu).
Results
Identification of phosphoproteins
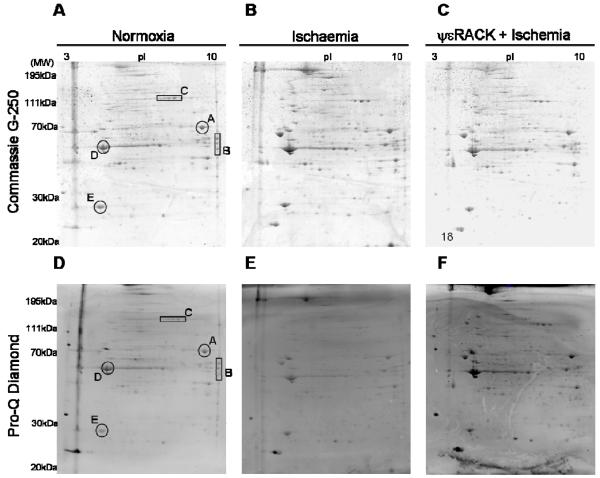
Hearts were exposed to global, no-flow ischemia (35 min) in the presence or absence of ψεRACK (1μM) applied for normoxia 10 min prior to an ischemic onset, with no wash-out, as previously described 16. Both groups had a 20 min equilibration period, after which hearts were subjected to 30 min global, no-flow ischemia. To one of the groups the εPKC-selective agonist peptide, ψεRACK, was perfused for 10 min (1μM), immediately prior to ischemia onset and kept throughout ischemia. Total lysate of 3 hearts, from 3 independent experiments, were prepared, and run individually on 2D gels. Considering phosphorylated spots that had at least a 1.5X increase, we compared phosphorylated spots from hearts of animals subjected to, ischemia and ψεRACK + ischemia. The phosphorylation of 20 spots increased only in ischemic hearts treated with ψεRACK. Of these, 18 spots were identified by mass spectrometry (Figure 1, 2 and Table 1).
Figure 1.
Detection of direct and indirect εPKC substrates in total rat heart lysates. Representative 2DE gels (n= 3 hearts of individual animals) of lysates from control hearts (A and D), hearts subjected to, ischemia alone (B and E) and Ischemia + ψεRACK (C and F) as indicated. Coommassie blue G250 stained gels (A-C) and gels stained with phospho-specific dye Pro-Q Diamond (D-F). Spots used to align gels are labeled (A and D).
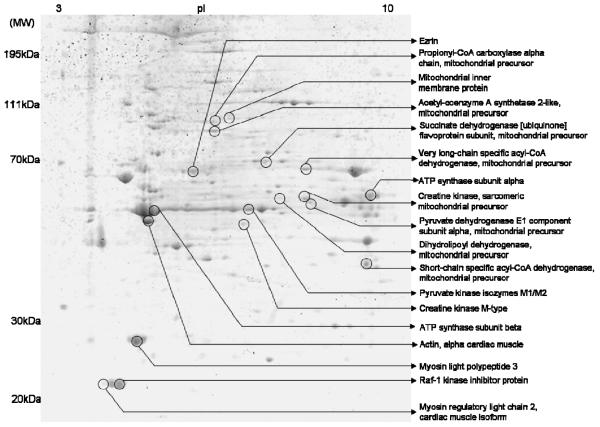
Figure 2.
Coomassie blue G250 stained gel of total heart lysate treated with ψεRACK+ ischemia indicating the spots identified by mass spectrometry whose phosphorylation significantly increased in hearts from rats treated with ψεRACK + ischemia as compared to hearts subjected to ischemia alone. For the annotation of the proteins identified see Table 1.
Table 1.
Proteins identified by mass spectrometry whose phosphorylation increased in total heart lysates of hearts subjected to ψεRACK+ ischemia relative to ischemia alone. Identified proteins indicated in figure 2 together with Uniprot accession number, number of peptides identified, Mascot score, theoretical and experimental molecular weight (M.W.) and isoeletric point, % 24 volume of ischemia where ischemia = normoxia (average of three experiments) and p-values as determined by Whitney t-test where *P<0.05 are indicated.
| Spot No. |
Protein | Accessio n No. |
Peptid e count |
Mascot prot. score |
Theorical | Experimental | % Vol | Location | P value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MW | pI | MW | pI | ||||||||
| 1 | ATP synthase subunit beta, mitochondrial precursor | P10719 | 16 | 589 | 56kDa | 5.19 | 42kDa | 5.23 | 2.3 | mitochondria | 0.02* |
| 2 | Myosin light polypeptide 3 | P16409 | 12 | 495 | 22kDa | 5.03 | 23kDa | 4.94 | 2.6 | cytosol | 0.02* |
| 3 | Succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitochondrial precursor |
Q920L2 | 29 | 693 | 71kDa | 6.75 | 65kDa | 7.32 | 2.0 | mitochondria | 0.03* |
| 4 | Creatine kinase, sarcomeric mitochondrial precursor | P09605 | 15 | 309 | 47kDa | 8.76 | 45kDa | 7.99 | 2.2 | mitochondria | 0.04* |
| 5 | Short-chain specific acyl-CoA dehydrogenase, mitochondrial precursor |
P15651 | 11 | 280 | 44kDa | 8.47 | 31kDa | 9.06 | 2.5 | mitochondria | 0,08 |
| 6 | Very long-chain specific acyl-CoA dehydrogenase, mitochondrial precursor | P45953 | 23 | 300 | 70kDa | 9.01 | 59kDa | 8.00 | 1.8 | mitochondria | 0.01* |
| 7 | Mitochondrial inner membrane protein | Q8CAQ8 | 17 | 225 | 83kDa | 6.18 | 75kDa | 6.35 | 5.1 | mitochondria | 0.02* |
| 8 | Propionyl-CoA carboxylase alpha chain, mitochondrial precursor |
P14882 | 12 | 78 | 77kDa | 6.33 | 69kDa | 6.41 | 5.1 | mitochondria | 0.02* |
| 9 | Dihydrolipoyl dehydrogenase, mitochondrial precursor |
Q6P6R2 | 13 | 207 | 54kDa | 7.96 | 48kDa | 7.33 | 4.1 | mitochondria | 0.01* |
| 10 | ATP synthase subunit alpha, mitochondrial precursor | P15999 | 20 | 694 | 59kDa | 9.22 | 45kDa | 9.14 | 3.0 | mitochondria | 0.04* |
| 11 | Creatine kinase M-type | P00564 | 14 | 568 | 43kDa | 6.58 | 39kDa | 6.87 | 4.0 | mitochondria | 0.01* |
| 12 | Pyruvate dehydrogenase E1 component subunit alpha, mitochondrial precursor |
P26284 | 13 | 266 | 43kDa | 8.49 | 44kDa | 8.06 | 3.3 | mitochondria | 0.03* |
| 13 | Actin, alpha cardiac muscle 1 | P68035 | 18 | 1030 | 41kDa | 5.23 | 40kDa | 5.14 | 6.5 | cytosol | 0.04* |
| 14 | Ezrin | P31977 | 12 | 93 | 69kDa | 5.83 | 55kDa | 5.80 | 3.5 | cytosol | 0.03* |
| 15 | Acetyl-coenzyme A synthetase 2-like, mitochondrial precursor |
Q99NB1 | 10 | 91 | 74kDa | 6.51 | 66kDa | 6.40 | 5.8 | mitochondria | 0,09 |
| 16 | Pyruvate kinase isozymes M1/M2 | P11980 | 26 | 790 | 57kDa | 6.63 | 46kDa | 7.01 | 1.7 | mitochondria | 0.01* |
| 17 | Phosphatidylethanolamine-binding protein 1 | P31044 | 5 | 326 | 20kDa | 5.48 | 19kDa | 4.65 | 6.0 | cytosol | 0.01* |
| 18 | Myosin regulatory light chain 2, ventricular/cardiac muscle isoform |
P08733 | 15 | 409 | 18kDa | 4.86 | 19kDa | 4.35 | 2.7 | cytosol | 0.01* |
Since the majority of the proteins (~70%) identified were mitochondrial proteins and since a number of previous studies demonstrated that εPKC can interact with and phosphorylate mitochondrial proteins 8, 17-20 we set out to analyze the εPKC phosphoproteome in isolated mitochondria.
Identification of phosphoproteins in mitochondrial fractions
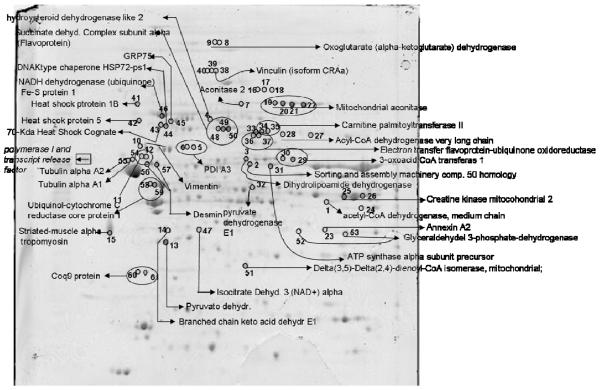
Mitochondria from, ischemia and ψεRACK+ ischemia treated hearts were isolated as described in materials and methods. In a previous study we verified the purity of our mitochondrial preparation by electron microscopy and Western blot analysis of specific mitochondrial proteins 20. Mitochondrial proteins were separated by 2-D gel electrophoresis and phosphoproteins stained with Pro-Q Diamond. Of the 183 spots that appeared or were increased in gels of mitochondria from hearts of animals treated with ψεRACK + ischemia, 62 spots were visible by Coomassie Brilliant Blue and 56 spots corresponding to 38 different proteins were identified by in-gel excision followed by mass spectrometry (Figures 3, 4 and Table 2). Twenty seven proteins were mitochondrial proteins. Nine proteins were mitochondrial inner membrane proteins and one outer membrane protein. Proteins involved in fatty acid oxidation, electron transport chain (complexes I-IV), heat shock proteins as well as structural proteins were also identified. Interestingly, protein disulfide-isomerase A3 precursor, oxoglutarate (alpha-ketoglutarate) dehydrogenase (lipoamide), tubulin alpha 1A, mitochondrial aconitase, creatine kinase, mitochondrial 2, acyl-Coenzyme A dehydrogenase very long chain, 3-oxoacid CoA transferase 1, carnitine palmitoyltransferase II, electron transfer flavoprotein-ubiquinone oxidoreductase, succinate dehydrogenase complex, subunit A, flavoprotein (Fp), glyceraldehyde 3-phosphate-dehydrogenase, desmin, ubiquinol-cytochrome c reductase core protein I and Coq9 protein had a change in more than one phospho-spot indicative of multiple phosphorylation sites.
Figure 3.
Detection of direct and indirect εPKC substrates in isolated rat heart mitochondria. Representative 2DE gels (n=3 of mitochondria isolated from individual animals) of lysates from control hearts (A and D) and hearts subjected to, Ischemia (B and E) and ψεRACK+ ischemia (C and F) as indicated. Coommassie blue G250 stained gels (A-C) and gels stained with phospho-specific dye Pro-Q Diamond (D-F). Spots used to align gels are labeled (A and D).
Figure 4.
Detection of direct and indirect εPKC substrates in isolated rat heart mitochondria. Representative 2DE gels (n=3 of mitochondria isolated from individual animals) of lysates from hearts subjected to, Ischemia and ψεRACK+ ischemia as indicated in figure 1. Coommassie blue G250 stained gels upper panels and gels stained with phospho-specific dye Pro-Q Diamond, lower panel
Table 2.
Proteins identified by mass spectrometry whose phosphorylation increased in mitochondria isolated from hearts subjected to ψεRACK+ ischemia relative to ischemia alone. Identified proteins indicated in Figure 6 are shown together with Uniprot accession number, number of peptides identified and, Mascot score, theoretical and experimental molecular weight (M.W.) and 26 isoeletric point. %volume of control (average of three experiments). * P<0.05, as determined by Whitney t-test.
| Spot No. |
Protein | Accession No. | Peptide Count |
Ion Score | Theorical | Experimental | Coverage | Vol (% Ischemia) |
||
|---|---|---|---|---|---|---|---|---|---|---|
| M.W. | pI | M.W. | pI | (%) | ||||||
| 1 | acetyl-CoA dehydrogenase, medium chain | Gi: 8392833 | 9 | 214 | 46kDa | 8.63 | 39kDa | 7.53 | 13 | appeared |
| 2 | sorting and assembly machinery component 50 homolog | gi:51948454 | 4 | 57 | 52kDa | 6.34 | 59KDa | 6.51 | 9 | appeared |
| 3 | dihydrolipoamide dehydrogenase | gi:40786469 | 5 | 102 | 54kDa | 7.96 | 61KDa | 6.43 | 9 | appeared |
| 4 | hydroxysteroid dehydrogenase like 2 [Rattus norvegicus] | gi|71043858 | 3 | 49 | 58KDa | 5.85 | 85KDa | 6.2 | 6 | appeared |
| 5 | protein disulfide-isomerase A3 precursor | gi:1352384 | 8 | 116 | 57kDa | 5.88 | 66KDa | 5.92 | 11 | appeared |
| 6 | protein disulfide-isomerase A3 precursor | gi|1352384 | 10 | 329 | 57KDa | 5.88 | 66KDa | 5.95 | 23 | appeared |
| 7 | aconitase 2 | gi|18079339 | 8 | 163 | 85KDa | 8.05 | 105KDa | 6.4 | 8 | appeared |
| 8 | oxoglutarate (alpha-ketoglutarate) dehydrogenase (lipoamide) |
gi|62945278 | 6 | 171 | 12KDa | 6.3 | 174KDa | 5.83 | 8 | appeared |
| 9 | oxoglutarate (alpha-ketoglutarate) dehydrogenase (lipoamide) | gi|62945278 | 6 | 44 | 12KDa | 6.3 | 174Kda | 5.93 | 8 | appeared |
| 10 | vimentin | gi|14389299 | 5 | 147 | 54KDa | 5.06 | 67KDa | 4.85 | 5 | appeared |
| 11 | tubulin alpha 1A | gi:38328248 | 4 | 32 | 50kDa | 4.94 | 64KDa | 5.24 | 10 | appeared |
| 12 | tubulin alpha 1A | gi:38328248 | 4 | 36 | 50kDa | 4.94 | 64KDa | 5.31 | 11 | appeared |
| 13 | pyruvate dehydrogenase (lipoamide) beta | gi|56090293 | 5 | 247 | 39KDa | 6.2 | 40KDa | 5.47 | 20 | appeared |
| 14 | branched chain keto acid dehydrogenase E1, beta polypeptide |
gi|158749538 | 4 | 267 | 43KDa | 6.41 | 42KDa | 5.48 | 13 | appeared |
| 15 | striated-muscle alpha tropomyosin | gi|207349 | 9 | 95 | 37KDa | 4.71 | 38KDa | 4.07 | 13 | appeared |
| 19 | mitochondrial aconitase | gi|10637996 | 9 | 196 | 85KDa | 7.87 | 105KDa | 7.16 | 12 | appeared |
| 20 | mitochondrial aconitase | gi|10637996 | 9 | 190 | 85KDa | 7.87 | 105KDa | 7.29 | 12 | appeared |
| 21 | mitochondrial aconitase | gi|10637996 | 8 | 229 | 85KDa | 7.87 | 104KDa | 5.23 | 12 | appeared |
| 22 | mitochondrial aconitase | gi|10637996 | 8 | 325 | 85KDa | 7.87 | 104KDa | 7.71 | 13 | appeared |
| 23 | annexin A2 | gi|9845234 | 8 | 442 | 39KDa | 7.55 | 48KDa | 7.1 | 30 | appeared |
| 24 | aldolase A | gi|202837 | 4 | 125 | 40KDa | 8.3 | 39KDa | 8.04 | 22 | appeared |
| 25 | creatine kinase, mitochondrial 2 | gi|38259206 | 6 | 326 | 47kDa | 8.64 | 46KDa | 7.57 | 21 | appeared |
| 26 | creatine kinase, mitochondrial 2 | gi|38259206 | 9 | 442 | 47kDa | 8.64 | 45KDa | 8.65 | 26 | appeared |
| 27 | acyl-Coenzyme A dehydrogenase, very long chain | gi|6978435 | 7 | 125 | 71KDa | 9.01 | 71KDa | 7.62 | 18 | appeared |
| 28 | acyl-Coenzyme A dehydrogenase, very long chain | gi|6978435 | 5 | 181 | 71KDa | 9.01 | 71KDa | 7.42 | 13 | appeared |
| 29 | 3-oxoacid CoA transferase 1 | gi|189181716 | 8 | 463 | 57kDa | 8.7 | 61KDa | 7.52 | 23 | appeared |
| 30 | 3-oxoacid CoA transferase 1 | gi|189181716 | 4 | 238 | 57kDa | 8.7 | 61KDa | 7.35 | 13 | appeared |
| 31 | ATP synthase alpha subunit precursor | gi|203055 | 8 | 327 | 59KDa | 9.22 | 59KDa | 8.25 | 20 | appeared |
| 32 | pyruvate dehydrogenase E1 alpha form 1 subunit | gi|57657 | 5 | 211 | 43KDa | 8.32 | 66KDa | 6.48 | 7 | appeared |
| 33 | carnitine palmitoyltransferase II | gi|1850592 | 8 | 257 | 74KDa | 7.02 | 74KDa | 7.1 | 7 | appeared |
| 34 | carnitine palmitoyltransferase II | gi|1850592 | 7 | 125 | 74KDa | 7.02 | 74KDa | 7.12 | 15 | appeared |
| 35 | carnitine palmitoyltransferase II | gi|1850592 | 7 | 174 | 74KDa | 7.02 | 74KDa | 7.27 | 13 | appeared |
| 36 | Electron transfer flavoprotein-ubiquinone oxidoreductase | gi|52000614 | 6 | 158 | 61KDa | 7.33 | 70KDa | 7.11 | 15 | appeared |
| 37 | Electron transfer flavoprotein-ubiquinone oxidoreductase | gi|52000614 | 6 | 321 | 61KDa | 7.33 | 70KDa | 7.18 | 14 | appeared |
| 38 | vinculin (predicted), isoform CRA_a | gi|149031250 | 5 | 41 | 123KDa | 5.54 | 146KDa | 5.84 | 5 | appeared |
| 41 | heat shock protein 1, beta (HSP90) | gi|40556608 | 7 | 268 | 83KDa | 4.97 | 105KDa | 4.65 | 9 | appeared |
| 42 | heat shock protein 5 (HSP70 ptn5) glucose regulated protein |
gi|25742763 | 9 | 296 | 72KDa | 5.07 | 84KDa | 4. 7 | 14 | appeared |
| 43 | 70-Kda Heat Shock Cognate Protein | gi|178847300 | 9 | 309 | 60KDa | 5.91 | 72KDa | 5.13 | 20 | appeared |
| 44 | DNAK-type molecular chaperone hsp72-ps1 | gi|347019 | 8 | 369 | 71KDa | 5.43 | 73KDa | 5.21 | 16 | appeared |
| 45 | grp75 | gi|1000439 | 8 | 414 | 74KDa | 5.87 | 79KDa | 5.24 | 16 | appeared |
| 46 | NADH dehydrogenase (ubiquinone) Fe-S protein 1, 75kDa | gi|53850628 | 7 | 283 | 80KDa | 5.65 | 81KDa | 5.14 | 12 | appeared |
| 47 | isocitrate dehydrogenase 3 (NAD+) alpha | gi|16758446 | 7 | 221 | 40KDA | 6.47 | 41KDa | 6.3 | 26 | appeared |
| 48 | succinate dehydrogenase complex, subunit A, flavoprotein (Fp) |
gi|18426858 | 10 | 249 | 72KDa | 6.75 | 72KDa | 6.45 | 20 | appeared |
| 49 | succinate dehydrogenase complex, subunit A, flavoprotein (Fp |
gi|18426858 | 10 | 364 | 72KDa | 6.75 | 71KDa | 6.56 | 19 | appeared |
| 50 | succinate dehydrogenase complex, subunit A, flavoprotein (Fp) |
gi|18426858 | 10 | 355 | 72KDa | 6.75 | 71KDa | 6.82 | 21 | appeared |
| 51 | Delta(3,5)-Delta(2,4)-dienoyl-CoA isomerase: Precursor | gi|6015047 | 8 | 219 | 36kDa | 8.13 | 33KDa | 6.47 | 35 | appeared |
| 52 | glyceraldehyde 3-phosphate-dehydrogenase | gi|56188 | 4 | 265 | 36KDa | 8.43 | 47KDa | 7.43 | 3 | appeared |
| 53 | glyceraldehyde 3-phosphate-dehydrogenase | gi|56188 | 3 | 74 | 36kDa | 8.43 | 47KDa | 7.65 | 17 | appeared |
| 54 | ATP synthase beta subunit | gi|1374715 | 6 | 238 | 51KDa | 4.92 | 65KDa | 4.87 | 20 | appeared |
| 55 | tubulin, beta, 2 | gi|5174735 | 6 | 110 | 50kDa | 4.79 | 66KDa | 4.75 | 11 | appeared |
| 56 | Desmin | gi|11968118 | 27 | 65 | 53kDa | 5.21 | 64KDa | 4.87 | 44 | appeared |
| 57 | Desmin | gi|11968118 | 28 | 72 | 53kDa | 5.21 | 64Kda | 5.12 | 53 | appeared |
| 58 | ubiquinol-cytochrome c reductase core protein I | gi|51948476 | 22 | 38 | 53kDa | 5.57 | 51KDa | 5.43 | 32 | appeared |
| 59 | ubiquinol-cytochrome c reductase core protein I | gi|51948476 | 7 | 385 | 53kDa | 5.57 | 52KDa | 5.59 | 21 | appeared |
| 60 | Coq9 protein | gi|51259441 | 2 | 62 | 35KDa | 5.5 | 30KDa | 4.87 | 10 | appeared |
| 61 | Coq9 protein | gi|51259441 | 8 | ND | 35KDa | 5.5 | 30KDa | 5.09 | 25 | 19,5* |
| 62 | polymerase I and transcript release factor | gi:6679567 | 3 | 46 | 44kDa | 5.43 | 64KDa | 3.31 | 7 | 19,5* |
Recently we showed that translocation of εPKC to the mitochondria is mediated by HSP90, therefore the identified substrates can be direct targets of εPKC 20. Using scansite (http://scansite.mit.edu/) we predicted PKC phosphorylation sites of the mitochondrial proteins whose phosphorylation increased upon treatment with ψεRACK. All identified mitochondrial proteins had putative PKC phosphorylation some which matched phosphorylation sites deposited in http://www.phosphosite.org/ (Table 4).
Table 4.
Predicted PKC Phosphorylation sites and validated sites of the mitochondrial proteins phosphorylated upon ischemia and ψεRACK. The phosphorylated residue is underlined.
| protein | predicted p- site |
peptide sequence1 | PKC isoenzyme |
Validated2 |
|---|---|---|---|---|
|
| ||||
| sorting and assembly machinery component 50 homolog | - | |||
|
| ||||
| T160 | LGRAEKVTFQFSYGT | PKCδ/ζ | ||
| S164 | EKVTFQFSYGTKETS | cPKC | ||
| S171 | SYGTKETSYGLSFFK | PKCε/δ | ||
| S189 | GNFEKNFSVNLYKVT | PKCζ | ||
| S203 | TGQFPWSSLRETDRG | cPKC | ||
| S216 | RGVSAEYSFPLCKTS | PKCζ | ||
| T225 | PLCKTSHTVKWEGVW | cPKCε/δ | ||
| S243 | GCLARTASFAVRKES | cPKC/ζ | ||
| S312 | NKPLVLDSVFSTSLW | PKCε | ||
| S332 | PIGDKLSSIADRFYL | PKCε | ||
|
| ||||
| dihydrolipoamide dehydrogenase | - | |||
|
| ||||
| S10 | SWSRVYCSLAKKGHF | cPKC/ζ | ||
| T165 | GKNQVTATTADGSTQ | PKCε | ||
| S170 | TATTADGSTQVIGTK | PKCδ | ||
| S208 | VSSTGALSLKKVPEK | cPKC | ||
| T279 | FKLNTKVTGATKKSD | cPKC/ζ | ||
| T282 | NTKVTGATKKSDGKI | cPKC | ||
| S502 | REANLAASFGKPINF | cPKC | ||
|
| ||||
| hydroxysteroid dehydrogenase like 2 | - | |||
|
| ||||
| T12 | TGKLAGCTVFITGAS | PKCδ | ||
| T53 | RHPKLLGTIYTAAEE | PKCδ/ζ | yes | |
| T169 | FKQHCAYTIAKYGMS | cPKC/ δ/ ζ | ||
| S237 | SIFKRPKSFTGNFII | PKCs/ δ/ ζ | ||
| S426 | TFRIVKDSLSDEVVR | PKCε | ||
| S476 | DRADVVMSMATEDFV | PKCε | ||
| T493 | FSGKLKPTMAFMSGK | cPKC/ζ/ δ/ ε | ||
|
| ||||
| protein disulfide-isomerase A3 precursor | - | |||
|
| ||||
| S239 | IKKFIQESIFGLCPH | PKCζ | ||
| T228 | AYTEKKMTSGKIKKF | PKCζ | ||
| S229 | YTEKKMTSGKIKKFI | cPKC | ||
| S239 | IKKFIQESIFGLCPH | PKCδ/ζ | ||
| S303 | KLNFAVASRKTFSHE | cPKC | ||
| T306 | FAVASRKTFSHELSD | PKCδ/ε | yes | |
| T452 | YEVKGFPTIYFSPAN | PKCε | ||
| T463 | SPANKKLTPKKYEGG | cPKC | ||
|
| ||||
| aconitase 2 | - | |||
|
| ||||
| T64 | KRLNRPLTLSEKIVY | PKCζ | ||
| T366 | HPVADVGTVAEKEGW | PKCζ | ||
| T415 | LKCKSQFTITPGSEQ | PKCδ/ε | ||
| T467 | IKKGEKNTIVTSYNR | PKCε/ ζ | ||
| T504 | TALAIAGTLKFNPET | cPKC/δ | ||
| S690 | GRAIITKSFARIHET | PKCζ | ||
| S770 | IEWFRAGSALNRMKE | PKCζ | ||
|
| ||||
|
oxoglutarate (alpha-ketoglutarate) dehydrogenase
(lipoamide) |
- | |||
|
| ||||
| T19 | RPLTASQTVKTFSQN | cPKC/e/d | ||
| S71 | AWLENPKSVHKSWDI | cPKC | ||
| S103 | PLSLSRSSLATMAHA | PKCε/χ/δ | yes | |
| T106 | LSRSSLATMAHAQSL | PKCδ | ||
| S112 | ATMAHAQSLVEAQPN | PKCδ | ||
| T190 | DKVFHLPTIIFIGGQ | PKCδ | ||
| T191 | KVFHLPIMFIGGQE | PKCδ | ||
| T262 | LARLVRSTRFEEFLQ | PKCε | ||
| S663 | AEYMAFGSLLKEGIH | PKCζ | ||
| S273 | EFLQRKWSSEKRFGL | PKCζ | ||
| S274 | FLQRKWSSEKRFGLE | cPKC/δ | ||
| S405 | TEGKKVMSILLHGDA | PKCζ | ||
| T437 | PSYTTHGTVHVVVNN | PKCδ | ||
| S861 | LIVFTPKSLLRHPEA | PKCζ | ||
|
| ||||
| aldolase A | - | |||
|
| ||||
| S39 | AADESTGSIAKRLQS | PKCδ/ζ | yes | |
| S46 | SIAKRLQSIGTENTE | PKCε | yes | |
| T227 | HHVYLEGTLLKPNMV | PKCζ | ||
| S309 | YGRALQASALKAWGG | cPKCδ | ||
| S336 | IKRALANSLACQGKY | cPKCδ | ||
|
| ||||
| acyl-Coenzyme A dehydrogenase, very long chain | - | |||
|
| ||||
| S60 | ETLSSDASTREKPAR | cPKC/ε | ||
| S72 | PARAESKSFAVGMFK | PKC8/ε | ||
| T194 | KGILLYGTKAQKEKY | PKCζ | ||
| S227 | SSGSDVASIRSSAVP | cPKCδ | ||
| S287 | TAFVVERSFGGVTHG | PKCδ | ||
| T347 | GRFGMAATLAGTMKA | PKCζ | ||
| S423 | AISKIFGSEAAWKVT | PKCζ | ||
| S517 | RRRTGIGSGLSLSGI | PKCζ | ||
|
| ||||
| 3-oxoacid CoA transferase 1 | - | |||
|
| ||||
| S16 | SGLRLCASARNSRGA | cPKC | ||
| S35 | CACYFSVSTRHHTKF | cPKC | ||
| T58 | KDIPNGATLLVGGFG | PKCδ | ||
| T140 | VELTPQGTLAERIRA | PKCζ | ||
| T163 | YTSTGYGTLVQEGGS | PKCε | ||
| S179 | IKYNKDGSVAIASKP | PKCε/ζ/δ | ||
| S253 | EEIVDIGSFAPEDIH | PKCε | ||
| S283 | EKRIERLSLRKEGEG | cPKC/ε/δ/ζ | ||
| T397 | RGGHVNLTMLGAMQV | PKCζ | ||
| T440 | SKTKVVVTMEHSAKG | cPKC/ε | ||
| T457 | HKIMEKCTLPLTGKQ | cPKCδ | ||
|
| ||||
| ATP synthase alpha subunit precursor | - | |||
|
| ||||
| T102 | ITPETFSTISVVGLI | PKCδ | ||
|
| ||||
| pyruvate dehydrogenase E1 alpha form 1 subunit | - | |||
|
| ||||
| T35 | RNFANDATFEIKKCD | PKCζ | ||
| T70 | KYYRMMQTVRRMELK | cPKC/ε | ||
| T124 | AYRAHGFTFNRGHAV | PKCδ | ||
| T139 | RAILAELTGRRGGCA | PKCδ | ||
| S152 | CAKGKGGSMHMYAKN | PKCδ/ζ | ||
| T266 | ILCVREATKFAAAYC | PKCδ | ||
| S293 | TYRYHGHSMSDPGVS | PKCε | yes | |
|
| ||||
| carnitine palmitoyltransferase II | - | |||
|
| ||||
| S15 | RAWPRCPSLVLGAPS | PKCδ | ||
| T60 | PIPKLEDTMKRYLNA | cPKC | ||
| T156 | LTRATNLTVSAVRFL | PKCδ | ||
| S320 | ETLKKVDSAVFCLCL | PKCζ | ||
| S411 | AATNSSASVETLSFN | PKCδ | ||
| S416 | SASVETLSFNLSGAL | PKCδ | ||
| T428 | GALKAGITAAKEKFD | PKCζ | ||
| T437 | AKEKFDTTVKTLSID | PKCε/δ/χ | ||
| S462 | FLKKKQLSPDAVAQL | PKCδ | ||
| T491 | ATYESCSTAAFKHGR | PKCζ | ||
| T501 | FKHGRTETIRPASIF | cPKC | ||
| S513 | SIFTKRCSEAFVRDP | PKCζ | ||
|
| ||||
| Electron transfer flavoprotein-ubiquinone oxidoreductase | - | |||
|
| ||||
| T46 | PQITTHYTIHPREKD | cPKC | ||
| T229 | KDGAPKTTFERGLEL | PKCδ | ||
| T241 | LELHAKVTIFAEGCH | PKCε/δ | ||
| S306 | DRHTYGGSFLYHLNE | PKCζ | ||
| S347 | QRWKHHPSIRPTLEG | cPKC/δ | ||
| T401 | PKIKGTHTAMKSGSL | PKCε/δ/ζ | ||
| S407 | HTAMKSGSLAAEAIF | PKCε/δ | ||
| S490 | WTLKHKGSDSEQLKP | cPKC/ε | ||
| S550 | IPVNRNLSIYDGPEQ | PKCζ | yes | |
|
| ||||
| NADH dehydrogenase (ubiquinone) Fe-S protein 1, 75kDa | - | |||
|
| ||||
| S69 | RPLTTSMSLFIIAPT | PKCε/ζ | ||
| S110 | PFILATSSLSVYSIL | PKCε | ||
| S128 | WASNSKYSLFGALRA | PKCε/δ | ||
| T139 | ALRAVAQTISYEVTM | PKCδ | ||
| S258 | YPELYSTSFMTETLL | PKCε | ||
| S276 | TFLWIRASYPRFRYD | cPKC | ||
| T297 | WKNFLPLTLAFCMWY | PKCζ | ||
|
| ||||
| isocitrate dehydrogenase 3 (NAD+) alpha | - | |||
|
| ||||
| S340 | ATIKDGKSLTKDLGG | PKCδ/ζ | ||
| T334 | IEAACFATIKDGKSL | cPKC/δ | ||
|
| ||||
|
succinate dehydrogenase complex, subunit A,
flavoprotein (Fp) |
- | |||
|
| ||||
| S28 | ATRGFHFSVGESKKA | cPKC | ||
| S36 | VGESKKASAKVSDAI | PKCδ | ||
| T118 | WRWHFYDTVKGSDWL | cPKC | ||
| S169 | QRAFGGQSLKFGKGG | cPKC/δ/ζ | ||
| S206 | RSLRYDTSYFVEYFA | PKCε/ζ | ||
| T244 | HRIRAKNTIIATGGY | cPKC/ε/δ/ζ | ||
| S462 | FGRACALSIAESCRP | cPKC/δ | ||
| S466 | CALSIAESCRPGDKV | cPKC | ||
| S484 | KANAGEESVMNLDKL | PKCδ | ||
| S497 | KLRFADGSVRTSELR | PKCε/χ/δ/ζ | ||
| S506 | RTSELRLSMQKSMQS | cPKC | ||
| S510 | LRLSMQKSMQSHAAV | PKCδ/ζ | ||
| S522 | AAVFRVGSVLQEGCE | PKCδ/ζ | yes | |
| T618 | AEHWRKHTLSYVDTK | PKCε/δ/ζ | ||
| S620 | HWRKHTLSYVDTKTG | cPKC/ζ | ||
| T630 | DTKTGKVTLDYRPVI | PKCε | ||
| T640 | YRPVIDKTLNEADCA | PKCε | ||
|
| ||||
| Delta(3,5)-Delta(2,4)-dienoyl-CoA isomerase: Precursor | - | |||
|
| ||||
| S30 | RQLYFNVSLRSLSSS | cPKC/ζ | ||
| T153 | SRYQKTFTVIEKCPK | PKCε/ζ | ||
| T225 | RSLVNELTFTARKMM | PKCδ | ||
|
| ||||
| glyceraldehyde 3-phosphate-dehydrogenase | - | |||
|
| ||||
| T57 | THGKFNGTVKAENGK | cPKC/ε | yes | |
| T185 | AITATQKTVDGPSGK | PKCδ | yes | |
| T292 | NSNSHSSTFDAGAGI | PKCε/δ | ||
|
| ||||
| ubiquinol-cytochrome c reductase core protein I | - | |||
|
| ||||
| S107 | TKSSKESSEARKGFS | PKCε/δ | ||
| T120 | FSYLVTAI IIVGVAY | PKCδ | ||
| T122 | YLVTAIIIVGVAYAA | PKCε | ||
| T180 | PLFVRHRTKKEIDQE | cPKC | ||
|
| ||||
| pyruvate dehydrogenase (lipoamide) alpha | - | |||
|
| ||||
| T35 | RNFANDATFEIKKCD | PKCζ | ||
| T70 | KYYRMMQTVRRMELK | cPKC/ε | ||
| T124 | AYRAHGFTFNRGHAV | PKCδ | ||
| T139 | RAILAELTGRRGGCA | PKCδ | ||
| S152 | CAKGKGGSMHMYAKN | PKCδ/ζ | ||
| T266 | ILCVREATKFAAAYC | PKCδ | ||
| S293 | TYRYHGHSMSDPGVS | PKCε | ||
|
| ||||
| pyruvate dehydrogenase (lipoamide) beta | - | |||
|
| ||||
| S16 | RGPLRQASGLLKRRF | PKCζ | ||
| T112 | RPICEFMTFNFSMQA | PKCζ | ||
| T235 | AKIERQGTHITVVAH | PKCζ | ||
| S282 | DIEAIEASVMKTNHL | PKCδ | ||
|
| ||||
| ATP synthase beta subunit | - | |||
|
| ||||
| S51 | RDYAAQSSAAPKAGT | PKCζ | ||
| S231 | AKAHGGYSVFAGVGE | PKCζ | ||
| T288 | RVALTGLTVAEYFRD | PKCζ | ||
| S353 | IIIIKKGSITSVQAI | PKCδ/ε/χ | ||
|
| ||||
|
Branched chain keto acid dehydrogenase E1, beta
polypeptide |
- | |||
|
| ||||
| T105 | FGGVFRCTVGLRDKY | cPKC | ||
| S177 | GDLFNCGSLTIRAPW | cPKC | ||
Predicted by Scansite (http://scansite.mit.edu).
Valic ated sites reported in phosphosite (http//www.phosphosite.org).
Discussion
Several lines of evidence suggest that selective εPKC activation reduces cardiac damage due to ischemic injury. Activation of εPKC reduces infarct size and improves functional recovery of the heart 1-3whereas εPKC inhibition or knockout negates the infarct-sparing effect of ischemic preconditioning 1, 3, 9, 21, 22. A number of mechanisms have been proposed for εPKC mediated cardioprotection, including regulation of sarcolemmal and/or mitoKATP channels 17, 23, regulation of gap-junction permeance through phosphorylation of connexin 43 24, modulation of proteasomal activity 16 or regulation of mitochondrial permeability transition pore (MPTP) opening through direct phosphorylation of MPTP components 8. We recently identified mitochondrial ALDH2 as a direct εPKC substrate whose phosphorylation and activation is essential for εPKC-mediated cardioprotection 3. The cytoprotective mechanism of ALDH2 activation by εPKC is due to the increased metabolism of reactive aldehydes, such as 4-Hydroxy-2-nonenal (4-HNE), which are produced as a by-product of ROS-induced lipid peroxidation, and accumulate, in the ischemic/ reperfused heart 25. In the present study, we used the Pro-Q Diamond phospho-specific staining method to label proteins whose phosphorylation increased by ψεRACK during ischemia. The majority (~70%) of the εPKC phosphoproteins identified in total heart homogenates treated with ψεRACK during ischemia were mitochondrial proteins. The observation that εPKC activation and cytoprotection results in phosphorylation of mitochondrial proteins and is consistent with other studies reporting that εPKC-mediated cardioprotection is mediated by phosphorylation of mitochondrial proteins 1, 3, 9, 17, 18, 22.
To provide a more extensive analysis of the εPKC mitochondrial phosphoproteome, we repeated the Pro-Q Diamond analysis on the cardiac mitochondrial-enriched subfraction. In the presence of ψεRACK we saw the appearance of 182 phosphorylated spots, suggesting that εPKC activation results in phosphorylation of a number of mitochondrial proteins. We identified novel mitochondrial εPKC phosphoproteins involved in lipid oxidation, glycolysis, electron transport chain (including proteins from complexes I-IV), ketone body metabolism, and heat shock proteins.
We found an increase in the phosphorylation of inner-mitochondrial protein components of the respiratory chain, (complexes I, II and III); NADH dehydrogenase (ubiquinone) Fe-S protein, electron transfer flavoprotein-ubiquinone oxidoreductase, succinate dehydrogenase complex, subunit A, flavoprotein (Fp) and ubiquinol-cytochrome c reductase core protein I. Our results are in agreement with a number of biochemical and functional analyses which found εPKC to interact with, and phosphorylate inner-mitochondrial proteins involved in mitochondrial respiration 7-9, 26. Further, the presence of εPKC in a highly purified inner mitochondrial membrane preparation has already been previously demonstrated 23. An increase in the activity of the electron transport chain and activation of cytochrome c oxidase subunit IV (COX) by direct εPKC phosphorylation has also been previously demonstrated 27. COX activation was suggested to be one of the cardioprotective mechanisms of εPKC, possibly due to increased electron flux through the electron transport chain, resulting in enhanced ATP generation and reduced ROS generation 22, 27, 28. An εPKC-mediated increase in cytochrome c oxidase activity was also shown to protect lens from ischemic damage 29. Selective activation of εPKC with ψεRACK increased the phosphorylation and activity of complexes I, III and IV in synaptic mitochondria, indicating that other components of the electron transport chain are also regulated by εPKC phosphorylation 30, and εPKC activation led to a decrease in mitochondrial ROS generation of neuronal mitochondria 30. In agreement with a role for εPKC in mitochondrial respiration, hearts of constitutively active εPKC transgenic mice demonstrate preserved coupling of oxidative phosphorylation, maintained mitochondrial membrane potential and decreased cytochrome c release induced by ischemic reperfusion 31. The εPKC transgenic mice used have a mutation of Ala159 to Glu in the εPKC resulting in constitutively active εPKC and increased resistance to cardiac ischemic reperfusion 8. Interestingly, in constitutively active εPKC transgenic mice, mitochondrial PKC expression is preferentially increased over cytosolic expression, suggesting that the active form of PKC results in its mitochondrial translocation 8. Taken together, these data suggest that phosphorylation of intra-mitochondrial targets is crucial for εPKC-mediated cytoprotection. In the present study we identify other components of the respiratory chain and inner mitochondrial phosphorylated proteins. However, whether there is a direct physical association between εPKC and each of the inner mitochondrial εPKC phosphoproteins identified here, and whether these are direct or indirect εPKC substrates remains to be determined. Nevertheless future studies can, be directed by the results obtained here.
We did not detect ALDH2, however this may be due to the fact that different methods of detecting protein phosphorylation have different sensitivities. Some of the εPKC targets identified can be indirect targets whose phosphorylation may be activated upon ALDH2 activation.
Using difference in gel eletrophoresis (DIGE) of cardiac mitochondria from transgenic mice expressing constitutively active or dominant negative εPKC it was found that the majority of spots unique to constitutively active εPKC corresponded to proteins involved in glucose metabolism 9. These studies were combined with metabolomic studies which detected an increase in glucose metabolites in hearts expressing constitutively active εPKC subjected to ischemia/ reperfusion 9. The authors proposed that activating glycolytic pathways during ischemia is a novel mechanism for the cardioprotective role of εPKC. In the present study we used a phospho-specific dye and ψεRACK to investigate direct protein phosphorylation events mediated by εPKC. Despite the different methods and methodology used to activate εPKC, (constitutively active transgenic vs. dynamic activation) we identified many of the same proteins, previously described in the DIGE study, including; isocitrate dehydrogenase, oxoglutarate (alpha-ketoglutarate) dehydrogenase, pyruvate dehydrogenase, succinate dehydrogenase. [6, 7, 9 and Table 4]. We also identified additional εPKC substrates involved in glycolysis, and Krebs cycle such as: aldolase A, ATP-specific succinyl-CoA synthase beta subunit, dihydrolipoamide dehydrogenase (E3), mitochondrial aconitase and aconitase 2, confirming that εPKC activation leads to phosphorylation of proteins involved in glycolysis and the Krebs cycle. Our identification of aconitase as an εPKC target suggests that regulation of the TCA cycle is mediated by εPKC. Aconitase has been previously identified as a PKCβII substrate in diabetic rats, however, aconitase phosphorylation by PKCβII impaired TCA cycle since there was an increase in reverse activity of aconitase (isocitrate to aconitase) 32. While we identified some proteins identified previously, others were not detected in the present study, such as proteins involved in the Malate/Aspartate shuttle. This could be explained by the different methodology or the sensitivity of the methods (DIGE vs ProQ Diamond) and that we only identified the more abundant phosphorylated proteins. Alternatively, some of the proteins previously detected could have their expression and not phosphorylation status altered 9. In a study identifying εPKC complexes it has been suggested that εPKC may also play a role in regulating transcription and translation processes 6. Accordingly, the phosphorylation of Coq9, a key regulator of coenzyme Q synthesis 33, was also regulated by εPKC in the present study. Further studies should be performed to determine the specific regulation of glycolytic pathways by εPKC phosphorylation and whether different isoenzymes can phosphorylate different sites.
εPKC could also have a direct or indirect role in mitochondrial protein assembly, folding, and import since we identified three mitochondrial heat shock proteins that play a role in the import and folding of proteins inside the mitochondria, and sorting and assembly machinery component 50 (SAM50), homolog of a protein involved in the assembly of outer mitochondrial membrane proteins 34.
Cardioprotective signals from G protein coupled receptors (GPCRs), activated for example by bradykinin, propagating from the plasma membrane to the mitochondria through signalosomes, vesicular multimolecular complexes derived from caveoli have been previously proposed 35. In fact εPKC was found in signalosomes and inhibition of εPKC by εV1-2 blocks signalosome stimulation of mitoKATP 35. We found two proteins that are found in caveoli, Annexin A2 and PTRF also known as Cavin 36, these proteins could be part of the signalosome probably co-purified with our mitochondrial fraction. PTRF phosphorylation has been shown to be important in caveoli formation 36.
Conclusions
A number of mechanisms have been proposed for εPKC-mediated cardioprotection by preconditioning. In the present study we identified several εPKC phosphoproteins which may be responsible for the cardioprotective effect of εPKC. The εPKC targets identified are in line with many of the previously proposed mechanisms for εPKC mediated cardioprotection. We identified components of the signalosome contributing to the idea that εPKC-mediated cardioprotection involves transduction of GPCR signaling to the mitochondria 35. We also found components of lipid and carbohydrate oxidation pathways consistent with the idea that lipid and carbohydrate metabolism is modulated by εPKC 9. Activation of the respiratory chain and increase in oxygen consumption have also been proposed to be protective mechanisms of εPKC during preconditioning, to this end we identified components of Krebs cycle, and respiratory chain, whose phosphorylation was modulated by εPKC 27, 29, 30. The exact mechanisms by which εPKC phosphorylation leads to these different cardioprotective pathways still needs to be elucidated. The data obtained in the present study can therefore direct further studies to characterize the specific role of individual mitochondrial protein phosphorylation in εPKC-mediated cardioprotection. Taken together, our data suggest that εPKC-mediated phosphorylation events in the mitochondria are important for the maintenance of metabolic activity and cardioprotection during ischemic injury.
Table 3.
Summary of the function and localization of proteins whose phosphorylation was unique or increased 1.5X (in two out of three gels, of independent samples) in mitochondria from hearts treated with ψεRACK + ischemia relative to ischemia. The biological process, mitochondrial compartment and references to previous descriptions of protein phosphorylation or expression modulated by PKCε are indicated in the table.
| Function | Protein | Localization | Reference |
|---|---|---|---|
| Fatty Acid oxidation | carnitine palmitoyltransferase II | mitochondrial inner membrane | |
| delta(3,5)-delta(2,4)-dienoyl-CoA isomerase: precursor | mitochondrial matrix | ||
| Glycolysis/ Gluconeogenesis | aldolase A | mitochondrial matrix | |
| Krebs cycle | aconitase 2 | mitochondrial matrix | |
| ATP-specific succinyl-CoA synthase beta subunit | mitochondrial matrix | ||
| isocitrate dehydrogenase 3 (NAD+) alpha | mitochondrial matrix | 6, 9 | |
| dihydrolipoamide dehydrogenase (E3) | mitochondrial matrix | ||
| mitochondrial aconitase | mitochondrial matrix | ||
| oxoglutarate (alpha-ketoglutarate) dehydrogenase (lipoamide) | mitochondrial matrix | 9 | |
| pyruvate dehydrogenase (lipoamide) beta | mitochondrial matrix | 9 | |
| pyruvate dehydrogenase E1 alpha form 1 subunit | mitochondrial matrix | 9 | |
| glyceraldehyde 3-phosphate-dehydrogenase | mitochondrial matrix | 6 | |
| Electron transport chain | electron transfer flavoprotein-ubiquinone oxidoreductase | mitochondrial inner membrane | |
| Complex I | NADH dehydrogenase (ubiquinone) Fe-S protein | mitochondrial inner membrane | |
| electron transfer flavoprotein-ubiquinone oxidoreductase | mitochondrial inner membrane | ||
| Complex II | succinate dehydrogenase complex, subunit A, flavoprotein (Fp) | mitochondrial inner membrane | 6 |
| electron transfer flavoprotein-ubiquinone oxidoreductase | mitochondrial inner membrane | ||
| Complex III | ubiquinol-cytochrome c reductase core protein I | mitochondrial inner membrane | |
| electron transfer flavoprotein-ubiquinone oxidoreductase | mitochondrial inner membrane | ||
| ATP Synthase | ATP synthase alpha subunit precursor | mitochondrial inner membrane | 6 |
| ATP synthase beta subunit | mitochondrial inner membrane | 6, 9 | |
| Ketone body metabolism | 3-oxoacid CoA transferase 1 | mitochondrial matrix | |
| branched chain keto acid dehydrogenase E1, beta polypeptide | mitochondrial matrix | ||
| vimentin | Cytosol | 6, 7 | |
| tubulin alpha 1A | Cytosol | ||
| Cytoskeletal elements | tubulin, beta, 2 | Cytosol | |
| desmin | Cytosol | 6, 7 | |
| vinculin, isoform CRA_a | Cytosol | 6, 7 | |
| heat shock protein 1, beta (HSP90) | Cytosol | ||
| Heat Shock Protein | heat shock protein 5 (HSP70 ptn5) glucose regulated protein | Mitochondria | |
| dnaK-type molecular chaperone hsp72-ps1 | Mitochondria | 6, 7 | |
| grp75 | Mitochondria | ||
| Caveoli | polymerase I and transcript release factor (PTRV) | Caveolin | |
| annexin A2 | membranes (Caveolin) | 6, 7 | |
| sorting and assembly machinery component 50 homolog | mitochondrion outer membrane |
||
| hydroxysteroid dehydrogenase like 2 [Rattus norvegicus] | mitochondrial inner membrane | ||
| Other | protein Coq9 protein | mitochondrial inner membrane | |
| protein disulfide-isomerase A3 precursor | endoplasmic reticulum | ||
| striated-muscle alpha tropomyosin | Sarcomere |
Acknowledgements
This work was supported by NIH grants AA11147 and HL52141 to D.M.-R. and in part, by an American Heart Association Western States postdoctoral fellowship to G.B.; A Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP)– Brasil grant 2005/54188-4 to D.S.; H.M.C.J. and J.C.B.F. both held a post-doctoral fellowships FAPESP 2006/52062-6 and FAPESP 2009/03143-1 respectfully.
Footnotes
Competing interests: D.M.R. is the founder of KAI Pharmaceuticals Inc, a company that aims to bring PKC regulators to the clinic. None of the research performed in her laboratory is in collaboration with or supported by the company. The other authors declare that they have no competing interests.
Authors’ contributions: G.B., H.M.C.J., A.T.D.F., J.P. and J.C.B.F. performed all experiments. D.S. and D.M-R designed the study. D.S. directed the study. D.S. and J.E.K. wrote the manuscript.
References
- 1.Chen CH, Gray MO, Mochly-Rosen D. Cardioprotection from ischemia by a brief exposure to physiological levels of ethanol: Role of epsilon protein kinase c. Proc Natl Acad Sci U S A. 1999;96:12784–12789. doi: 10.1073/pnas.96.22.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorn GW, 2nd, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase c translocation. Proc Natl Acad Sci U S A. 1999;96:12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budas GR, Disatnik MH, Chen CH, Mochly-Rosen D. Activation of aldehyde dehydrogenase 2 (aldh2) confers cardioprotection in protein kinase c epsilon (pkcepsilon) knockout mice. J Mol Cell Cardiol. 2010;48:757–764. doi: 10.1016/j.yjmcc.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budas GR, H DM, D M-R. Aldehyde dehydrogenase 2 in cardiac protection: A new therapeutic target? Trends Cardiovasc Med. 2009;19:158–164. doi: 10.1016/j.tcm.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmondson RD, Vondriska TM, Biederman KJ, Zhang J, Jones RC, Zheng Y, Allen DL, Xiu JX, Cardwell EM, Pisano MR, Ping P. Protein kinase c epsilon signaling complexes include metabolism- and transcription/translation-related proteins: Complimentary separation techniques with lc/ms/ms. Mol Cell Proteomics. 2002;1:421–433. doi: 10.1074/mcp.m100036-mcp200. [DOI] [PubMed] [Google Scholar]
- 7.Ping P, Zhang J, Pierce WM, Jr., Bolli R. Functional proteomic analysis of protein kinase c epsilon signaling complexes in the normal heart and during cardioprotection. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, P P. Protein kinase cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayr M, Liem D, Zhang J, Li X, Avliyakulov NK, Yang JI, Young G, Vondriska TM, Ladroue C, Madhu B, Griffiths JR, Gomes A, Xu Q, Ping P. Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase c epsilon and delta in regulating glucose metabolism of murine hearts. J Mol Cell Cardiol. 2009;46:268–277. doi: 10.1016/j.yjmcc.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pass JM, Gao J, Jones WK, Wead WB, Wu X, Zhang J, Baines CP, Bolli R, Zheng YT, Joshua IG, Ping P. Enhanced pkc beta ii translocation and pkc beta ii-rack1 interactions in pkc epsilon-induced heart failure: A role for rack1. Am J Physiol Heart Circ Physiol. 2001;281:H2500–2510. doi: 10.1152/ajpheart.2001.281.6.H2500. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GWn, D M-R. Opposing cardioprotective actions and parallel hypertrophic effects of delta pkc and epsilon pkc. Proc Natl Acad Sci U S A. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 13.Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: A very sensitive colloidal coomassie g-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- 14.Costa-Junior HM, Garavello NM, Duarte ML, Berti DA, Glaser T, de Andrade A, Labate CA, Ferreira AT, Perales JE, Xavier-Neto J, Krieger JE, Schechtman D. Phosphoproteomics profiling suggests a role for nuclear betaiotapkc in transcription processes of undifferentiated murine embryonic stem cells. J Proteome Res. 9:6191–6206. doi: 10.1021/pr100355k. [DOI] [PubMed] [Google Scholar]
- 15.Andrade HM, Murta SM, Chapeaurouge A, Perales J, Nirde P, Romanha AJ. Proteomic analysis of trypanosoma cruzi resistance to benznidazole. J Proteome Res. 2008;7:2357–2367. doi: 10.1021/pr700659m. [DOI] [PubMed] [Google Scholar]
- 16.Churchill EN, Ferreira JC, Brum PC, Szweda LI, D M-R. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltapkc during reperfusion. Cardiovasc Res. 2010;85:385–394. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, Shimamoto K. Opening of mitochondrial k(atp) channel occurs downstream of pkc-epsilon activation in the mechanism of preconditioning. Am J Physiol Heart Circ Physiol. 2002;283:440–447. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence KM, Kabir AM, Bellahcene M, Davidson S, Cao XB, McCormick J, Mesquita RA, Carroll CJ, Chanalaris A, Townsend PA, Hubank M, Stephanou A, Knight RA, Marber MS, Latchman DS. Cardioprotection mediated by urocortin is dependent on pkcepsilon activation. FASEB J. 2005;19:831–833. doi: 10.1096/fj.04-2506fje. [DOI] [PubMed] [Google Scholar]
- 19.Churchill EN, Disatnik MH, D M-R. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonpkc and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budas GR, Churchill EN, Disatnik MH, Sun L, D M-R. Mitochondrial import of pkcepsilon is mediated by hsp90: A role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88:83–92. doi: 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray MO, Karliner JS, D M-R. A selective epsilon-protein kinase c antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997;272:30945–30951. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- 22.Ogbi M, Johnson JA. Protein kinase cepsilon interacts with cytochrome c oxidase subunit iv and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J. 2006;393:191–199. doi: 10.1042/BJ20050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabůrek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial pkc epsilon and mitochondrial atp-sensitive k+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99:878–883. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 24.Bowling N, Huang X, Sandusky GE, Fouts RL, Mintze K, Esterman M, Allen PD, Maddi R, McCall E, Vlahos CJ. Protein kinase c-alpha and -epsilon modulate connexin-43 phosphorylation in human heart. J Mol Cell Cardiol. 2001;33:789–798. doi: 10.1006/jmcc.2000.1349. [DOI] [PubMed] [Google Scholar]
- 25.Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am J Physiol. 1999;276:H935–943. doi: 10.1152/ajpheart.1999.276.3.H935. [DOI] [PubMed] [Google Scholar]
- 26.Agnetti G, Kane LA, Guarnieri C, Caldarera CM, JE VE. Proteomic technologies in the study of kinases: Novel tools for the investigation of pkc in the heart. Pharmacol Res. 2007:55. doi: 10.1016/j.phrs.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo D, Nguyen T, Ogbi M, Tawfik H, Ma G, Yu Q, Caldwell RW, Johnson JA. Protein kinase c-epsilon coimmunoprecipitates with cytochrome oxidase subunit iv and is associated with improved cytochrome-c oxidase activity and cardioprotection. Am J Physiol Heart Circ Physiol. 2007;293:H2219–2230. doi: 10.1152/ajpheart.01306.2006. [DOI] [PubMed] [Google Scholar]
- 28.Yu Q, Nguyen T, Ogbi M, Caldwell RW, Johnson JA. Differential loss of cytochrome-c oxidase subunits in ischemia-reperfusion injury: Exacerbation of coi subunit loss by pkc-epsilon inhibition. Am J Physiol Heart Circ Physiol. 2008;294:H2637–2645. doi: 10.1152/ajpheart.91476.2007. [DOI] [PubMed] [Google Scholar]
- 29.Barnett M, Lin D, Akoyev V, Willard L, Takemoto D. Protein kinase c epsilon activates lens mitochondrial cytochrome c oxidase subunit iv during hypoxia. Exp Eye Res. 2008;86:226–234. doi: 10.1016/j.exer.2007.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A, Perez-Pinzon MA. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase c epsilon. J Neurosci. 2008;28:4172–4182. doi: 10.1523/JNEUROSCI.5471-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy J, McLeod CJ, Minners J, Essop MF, Ping P, Sack MN. Pkcepsilon activation augments cardiac mitochondrial respiratory post-anoxic reserve--a putative mechanism in pkcepsilon cardioprotection. J Mol Cell Cardiol. 2005;38:697–700. doi: 10.1016/j.yjmcc.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Lin G, Brownsey RW, MacLeod KM. Regulation of mitochondrial aconitase by phosphorylation in diabetic rat heart. Cell Mol Life Sci. 2009;66:919–932. doi: 10.1007/s00018-009-8696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh EJ, Gin P, Gulmezian M, Tran UC, Saiki R, Marbois BN, Clarke CF. Saccharomyces cerevisiae coq9 polypeptide is a subunit of the mitochondrial coenzyme q biosynthetic complex. Arch Biochem Biophys. 2007;463:19–26. doi: 10.1016/j.abb.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozjak V, Wiedemann N, Milenkovic D, Lohaus C, Meyer HE, Guiard B, Meisinger C, Pfanner N. An essential role of sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J Biol Chem. 2003;278:48520–48523. doi: 10.1074/jbc.C300442200. [DOI] [PubMed] [Google Scholar]
- 35.Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2009;46:858–866. doi: 10.1016/j.yjmcc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aboulaich N, Vainonen JP, Stralfors P, Vener AV. Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase i and transcript release factor (ptrf) at the surface of caveolae in human adipocytes. Biochem J. 2004;383:237–248. doi: 10.1042/BJ20040647. [DOI] [PMC free article] [PubMed] [Google Scholar]