Abstract
Mutation of the Foxp3 transcription factor in Scurfy (Sf) mice results in complete absence of the CD4+Foxp3+ regulatory T cells (Tregs), severe multiorgan autoimmune syndrome, and early death at 4 wk of age. However, Sf mice simultaneously bearing the Il2−/− (Sf.Il2−/−) or Faslpr/lpr gene (Sf.Faslpr/lpr) have extended lifespan despite totally lacking Tregs, indicating a role of IL-2 and CD95 (Fas) signaling pathways in the multiorgan autoimmune syndrome beyond the Treg checkpoint. IL-2 has been implicated in regulating lymphoproliferation and CD178 (FasL) expression. However, Sf.Il2−/− mice have increased lymphoproliferation and FasL expression. Importantly, the pattern of organ-specific autoimmune response of Sf.Il2−/− mice resembled IL-2 knockout mice whereas that of Sf.Faslpr/lpr was similar to Sf mice, indicating that the distinct and weakened autoimmune manifestation in IL-2 knockout mice was not caused by the residual Tregs. Our study demonstrated a novel role of IL-2 in regulating multiorgan autoimmune inflammation beyond the Treg checkpoint and indicated that both Il2−/− and Faslpr/lpr genes prolong the lifespan of Sf mice but by different mechanisms.
Thymocyte differentiation and selection impart distinct functions into different T cell populations. In the CD4+ cell compartment, two subsets are generated: a CD25− set that responds to Ags in the host and a CD25+ set that suppresses these Ag-specific responses (reviewed in Refs. 1–3). It is now believed that autoreactive T cells with moderate affinities against self-Ags could escape negative selection and exit to the periphery (4 –7). These autoreactive T cells in the periphery are suppressed by the CD4+CD25+ regulatory T cells (Tregs)4 (1–3). In the absence of IL-2, the CD4+CD25+ Tregs in the thymus are significantly reduced but not completely absent (8, 9). The CD4+CD25+ Tregs also express the transcription factor Foxp3, and Foxp3 expression on CD4+ T cells offers a more stringent definition for the “naturally occurring” Tregs (10 –12). Thymic generation of Tregs is critically dependent on the Foxp3 gene which is linked to chromosome X (13, 14). Scurfy (Sf) mice, which bear a defective Foxp3 gene and totally lack the Tregs, invariably develop fatal multiorgan autoimmune diseases within 4 wk after birth. In newborn boys, Foxp3 mutation resulted in immune-dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome (15). Foxp3 has also been implicated in thymocyte differentiation including diversion of autoreactive clones (16–19). In addition, transfection of Foxp3 into Foxp3− T cells rendered them anergic (20). Outside the lymphoid system, Foxp3 has been shown to be a breast cancer suppressor gene through its suppressive effect on the Her-2/ErbB2 oncogene (21).
Sf mice do not develop multiorgan autoimmune inflammation until 9 days after birth (22). At 3–4 wk after birth, severe inflammation against skin and lung and moderate inflammation in the liver were observed. In contrast, IL-2 knockout (KO) mice have a significantly longer lifespan of 6 –25 wk and the most often reported organ inflammation is colitis which usually begins after weaning and progresses thereafter (23). These differences could be explained by the relatively dispensable nature of IL-2, but not Foxp3, in the generation of Tregs in the thymus. However, IL-2 is also critically important for the expansion of T cells and their effector functions, such as FasL-mediated cytotoxicity, that are involved in lymphocyte homeostasis control and tissue damage in the periphery (24 –27). Thus, lacking these regulatory mechanisms in IL-2 KO mice could also explain why they displayed significantly reduced autoimmune responses as well.
To distinguish the roles of Tregs, IL-2, and FasL in the multiorgan autoimmune syndrome that leads to early death of Sf mice, we introduced the Il2−/− or Faslpr/lpr mutant gene into Sf mice. Interestingly, these mice, despite totally lacking Tregs, still lived significantly longer than Sf mice. Introducing the Il2−/− gene into Sf mice did not reduce lymphoproliferation and FasL expression. Rather, it controlled the pattern of the multiorgan autoimmune inflammation response such that the pattern resembled that of IL-2 KO mice. Most notably, it prevented the inflammation response in the skin and greatly reduced inflammation in the lung. By contrast, the Faslpr/lpr gene did not inhibit the inflammation response associated with Sf mice. Our genetic study resolved the complex roles of IL-2 in the Treg-controlled autoimmune response. Beyond the Treg checkpoint, IL-2 was shown to regulate skin and lung inflammation in Sf mice. Inhibition of inflammation in vital organs, but not FasL expression, correlates with the ability of Il2−/− to prolong the lifespan of Sf mice. Thus, both Il2−/− and Faslpr/lpr mutation protected Sf mice from early death but by different mechanisms.
Materials and Methods
Mice
C57BL/6 (B6), B6.Il2+/−, B6.Faslpr/lpr, B6.Cg-Foxp3sf/x/J, and B6.129S7-Rag1tm/Mom/J (Rag1−/−) mice were obtained from The Jackson Laboratory. B6.Il2−/− (IL-2 KO) mice were obtained by breeding B6.Il2+/− mice as previously described (26). B6.Cg-Foxp3sf/x/J mice were bred with male B6 mice to produce Sf mice (Foxp3sf/Y). Mice (Sf.Il2−/−) carrying both Il2−/− and Foxp3sf/Y genes were generated by breeding B6.Il2+/− males with B6.Il2+/−Foxp3sf/x females. Mice (Sf.Faslpr/lpr) carrying both B6.Faslpr/lpr and Foxp3sf/Y genes were generated by breeding B6.Faslpr/lpr males with B6.Faslpr/lprFoxp3sf/x females. The presence of the Il2−/− and Foxp3sf mutation was confirmed by PCR as detailed on The Jackson Laboratory website. Mice were examined twice weekly for clinical signs of the autoimmune disease including manifestation of skin inflammation, body weight loss, and wasting, etc. Adoptive transfer experiments were conducted by injection of 20 × 106 lymph node cells into adult RAG-1 KO male recipients. Mice were examined for clinical signs of autoimmune inflammation. H&E stains of various organs and tissues were conducted at 4 wk after transfer.
Flow cytometry
Axillary, brachial, inguinal, cervical, and facial lymph nodes from sex- and age-matched B6, IL-2 KO, Sf, Sf.Il2−/−, and Sf.Faslpr/lpr mice were isolated, pooled, and single-cell suspensions were prepared in PBS. Cells (106) were suspended in 100 μl of PBS solution (containing 4 mg of BSA and 1 μg of anti-FcR mAb 2.4G2) and incubated with 0.2 μg of various fluorescent Abs for 30 min at 4°C. FITC-, PE-, or PE-Cy5-conjugated anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-CD25 (PC61), and anti-FasL (MFL-3 and Kay-10) mAb were obtained from BD Biosciences. Staining for CD4+FoxP3+ T cells was conducted using the commercial kit (eBioscience). In some experiments, lymphocytes (4 × 106 cells/2 ml/well in a 24-well plate) were activated with Con A (3 μg/ml) in a 37°C, 10% CO2 incubator for 3 days and then stained for FasL expression. At least 104 stained cells were analyzed using a FACScan equipped with CellQuest (BD Biosciences). Postacquisition analyses were conducted using FlowJo software (Tree Star).
RT-PCR
Single-cell suspensions were prepared from lymph nodes of various strains of mice as described above. T cells were purified by positive selection using magnetic beads conjugated with anti-Thy1.2 mAb (Miltenyi Biotec). The purity of the sorted population was between 96 and 98% as analyzed by flow cytometry. Total RNA was extracted from 2×106 cells with TRIzol reagent (Invitrogen Life Technologies). RT-PCR for mouse FasL and β-actin was conducted as described (28). PCR products were separated by electrophoresis through a 2% agarose gel and detected by ethidium bromide staining and photographed with a Kodak Image Station 2000R.
FasL-mediated cytotoxicity
FasL-mediated cytotoxicity was conducted as previously described using 51Cr-labeled A20 lymphoma cells as target (29). Freshly isolated lymph node cells from individual mice were used as effector cells. The cytotoxicity assay was conducted in duplicate and each assay mixture contained 2 × 104-labeled A20 cells, various numbers of effector cells, 6 mM EGTA, and 3 mM MgCl2 in a total volume of 0.2 ml. This condition has been shown to measure FasL-mediated cytotoxicity both by the specific inhibition of cytotoxicity with Fas-Ig and by the resistance to killing of Fas-negative target cells (30, 31). After incubation for 9 h at 37°C in a 10% CO2 incubator, 0.1 ml of supernatant was removed and counted with a gamma-scintillation counter (LKB). Cytotoxicity was determined and expressed as the percent-specific Cr release according to the formula: . The cpm of background release and the cpm of total release were determined by culturing target cells alone and by lysing target cells in 0.5% Nonidet P-40, respectively. Background release under this condition is between 14 and 19% of the total release.
Histology
Tissues/organs from age-matched males of various strains were fixed with 10% neutral-buffered formalin (Fisher Scientific) and sections of paraffin-embedded tissue were stained with H&E. Tissues/organs examined included colons, skin, ear, lung, and liver. We also scored inflammation levels by the extent of leukocyte infiltration in 10 randomly selected fields and categorized them as severe (4+), strong (3+), moderate (2+), mild (1+), and no inflammation (0+). The significance of differences in inflammation score was determined by the Student t test.
Results
The lifespan of Sf mice is prolonged by Il2−/− and by Faslpr/lpr mutation
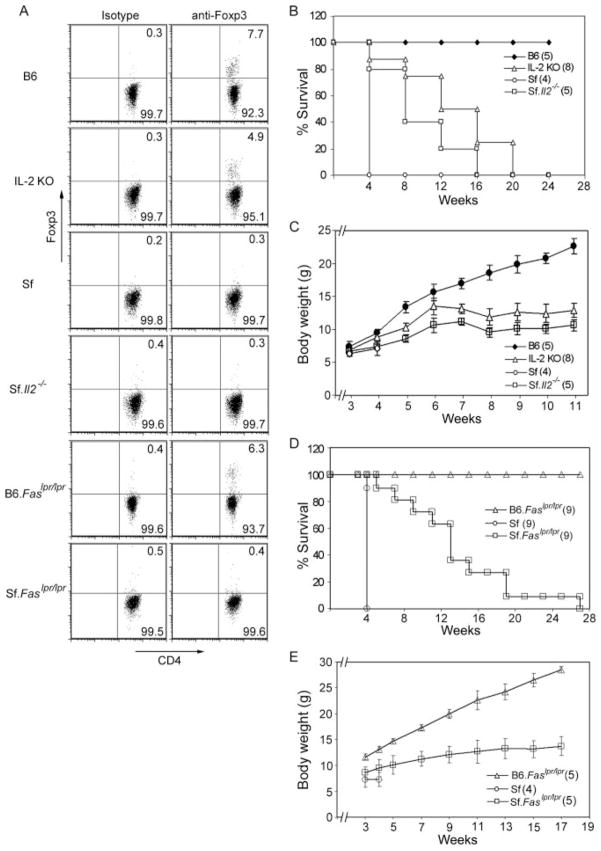
Whether Treg deficiency or FasL expression is the factor most critical to the early mortality of Sf mice was addressed by breeding experiments that introduced Il2−/− or Faslpr/lpr mutation into B6.Cg-Foxp3sf/y/J mice to generate Sf.Il2−/− and Sf.Faslpr/lpr mice, respectively. Control mice included sex- and age-matched littermates, B6.Faslpr/lpr, IL-2 KO mice, and Sf mice obtained in separate breeding. Both Sf.Il2−/− and Sf.Faslpr/lpr mice, like Sf mice, did not express the CD4+Foxp3+ Tregs (Fig. 1A). However, they all lived significantly longer than Sf mice (Fig. 1, B and D). Thus, they all survived beyond weaning which normally occurred at 4 wk after birth. Thereafter, the lifespan of individual Sf.Il2−/− and Sf.Faslpr/lpr mice varied. Interestingly, their mortality rate was comparable and approximating that of IL-2 KO mice. The prolongation of lifespan was accompanied by body weight gain in comparison with Sf mice. Although significantly heavier than Sf mice at 4 wk of age, the body weights of Sf.Il2−/− and Sf.Faslpr/lpr mice after weaning were noticeably lower than IL-2 KO mice (Fig. 1, C and E).
FIGURE 1.
Both Il2−/− and Faslpr/lpr prolonged the lifespan of Sf mice. A, CD4+Foxp3+ expression profiles among B6, IL-2 KO, B6.Faslpr/lpr, Sf, Sf.Il2−/−, and Sf.Faslpr/lpr mice. B, The mortality rate of Sf.Il2−/− mice is reduced in comparison to Sf mice. C, Body weight gain of Sf.Il2−/− mice is higher than Sf mice and approaching that of IL-2 KO mice. D, The mortality rate of Sf.Faslpr/lpr mice is reduced in comparison to Sf mice. E, Body weight gain of Sf.Faslpr/lpr mice is higher than Sf mice but significantly lower than B6.Faslpr/lpr mice. Numbers of mice examined are indicated in parentheses.
Autoimmune lymphoproliferation and mortality
Because IL-2 and Fas are important factors for T cell expansion and activation-induced T cell death (24, 25), it is possible that the prolongation of lifespan in Sf.Il2−/− and Sf.Faslpr/lpr mice was due to the two mechanisms that regulate lymphocyte homeostasis in vivo. We, therefore, compared them with age- and sex-matched controls for the total numbers of lymph node cells and the total numbers of the CD4+ T cell subset. As shown in Table I, there was a dramatic increase in the lymph node cells in the Sf mice (69 × 106) as compared with B6 control (18 ×106). A moderate but significant increase in cell number was also observed in IL-2 KO mice (33 × 106). Similar results were obtained for CD4+ T cells (Table I). The data seem to correlate the increase in lymphocyte number with the severity of autoimmune disease (more in Sf mice), the absence of Tregs (absent in Sf mice), and a normal IL-2-signaling pathway (present in Sf mice). Surprisingly, the number of total lymph node cells (or CD4+ T cells) in Sf.Il2−/− mice (153 × 106) was the highest among these strains examined. The data indicate that Il2−/− prolongs the lifespan of Sf mice not by inhibiting lymphocyte expansion caused by the absence of Tregs. The data appear consistent with the idea that inefficient expression of FasL or activation-induced cell death may be responsible for the increased lymphoproliferation in Sf.Il2−/− mice. However, such a strong lymphoproliferation over Sf mice was not observed in Sf.Faslpr/lpr mice (85 ×106) which displayed only a slightly higher lymphoproliferation over Sf mice. The data indicate that the high lymphoproliferation in Sf.Il2−/− mice is not caused by the effect of Il2−/− on Fas-based regulation of lymphocyte homeostasis.
Table I.
Comparison of lymphoproliferation among various micea
| Number of Cells (×106)
|
||
|---|---|---|
| Lymph node cells | CD4+ T cells | |
| B6 (5) | 18.3 ± 6.2 | 6.7 ± 0.4 |
| IL-2 KO (5) | 33 ± 4.4 | 13.2 ± 1.8 |
| Sf (6) | 69 ± 8.3 | 24 ± 2.9 |
| Sf.IL-2 KO (6) | 153 ± 27 | 59 ± 2.5 |
| B6.Faslpr/lpr (4) | 23 ± 3.3 | 11 ± 0.8 |
| Sf.Faslpr/lpr (4) | 85 ± 5.7 | 22 ± 2.5 |
Lymph node cells from pooled inguinal, brachial, axillary, cervical, and facial lymph nodes were obtained as described in Materials and Methods. Total lymph node cells were counted with a hemocytometer. The percentage of CD4+ T cells, determined by flow cytometry, was used to calculate the total number of lymph node CD4+ T cells.
FasL expression was not inhibited in Sf.Il2−/− mice
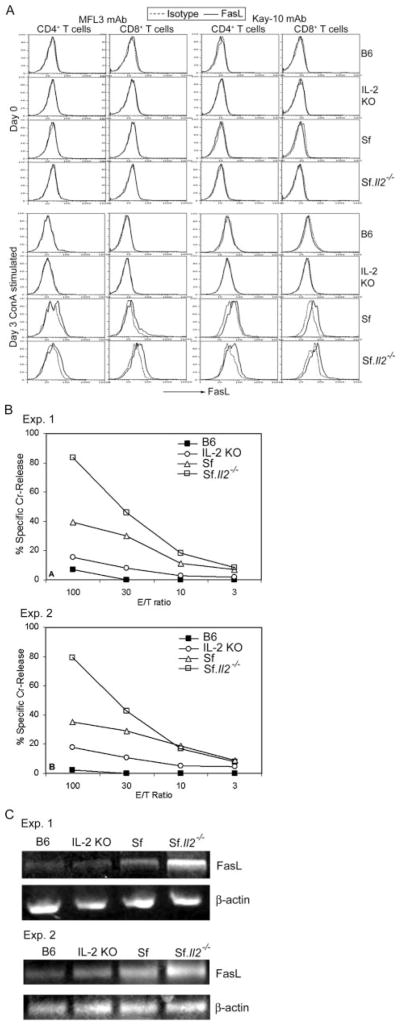
To confirm the above observation, we measured FasL expression in freshly isolated cells from the lymph nodes of B6, IL-2 KO, Sf, and Sf.Il2−/− mice using flow cytometry and cell-mediated cytotoxicity assays. Two anti-FasL mAbs were used with appropriate isotype controls. No detectable expression was observed in any samples by the staining technique. Under identical condition, T cells from Sf and Sf.Il2−/− mice that have been activated with Con A for 3 days displayed weak staining (Fig. 2A). Thus, the two mAbs were unable to stain FasL on T cells freshly isolated from mice with severe autoimmune inflammation. Cell-mediated cytotoxicity in the presence of EGTA+/Mg2+, however, readily detected FasL expression on the lymph node T cells from Sf and Sf.Il2−/− mice but not B6 mice. As shown in Fig. 2B, cells from Sf mice displayed stronger FasL-mediated cytotoxicity than cells from IL-2 KO mice that expressed marginally detectable cytotoxicity. Surprisingly, FasL-mediated cytotoxicity was strongest in Sf.Il2−/− mice. FasL-specific RT-PCR using purified T cells confirmed the results obtained with cytotoxicity assays (Fig. 2C). Thus, correlation between FasL-mediated cytotoxicity and mortality was observed between IL-2 KO and Sf mice but not between Sf and Sf.Il2−/− mice. i.e., FasL expression is not responsible for the prolongation of lifespan in Sf.Il2−/− mice.
FIGURE 2.
Comparison of FasL expression among B6, IL-2 KO, Sf, and Sf.Il2−/− mice. A, Flow cytometry was unable to detect FasL on freshly obtained lymphocytes using two mAbs. Only Con A-activated T cells displayed detectable level. FasL expression on gated CD4+ T cells and CD8+ T cells was shown. B, FasL-mediated cytotoxicity was strongly expressed by lymph node cells of Sf.Il2−/− mice, moderate for Sf mice, weakly for IL-2 KO mice but not detectable for B6 mice. C, Similar results were obtained for FasL mRNA levels among these strains as determined by RT-PCR.
Il2−/− but not Faslpr/lpr mutation regulates organ-specific autoimmune responses in Sf mice
On visual examination, Sf mice displayed autoimmune inflammation on ear, eyelid, skin, and tail whereas IL-2 KO mice did not. For the Sf.Il2−/− mice, these autoimmune diseases were either not developed or extremely mild, if any. In contrast, these autoimmune diseases were observed in Sf.Faslpr/lpr mice. These clinical observations suggest that Il2−/− and Faslpr/lpr mutation prolong the lifespan of Sf mice by different mechanisms with respect to regulation of organ-specific autoimmune inflammation, tissue damage, and organ failure.
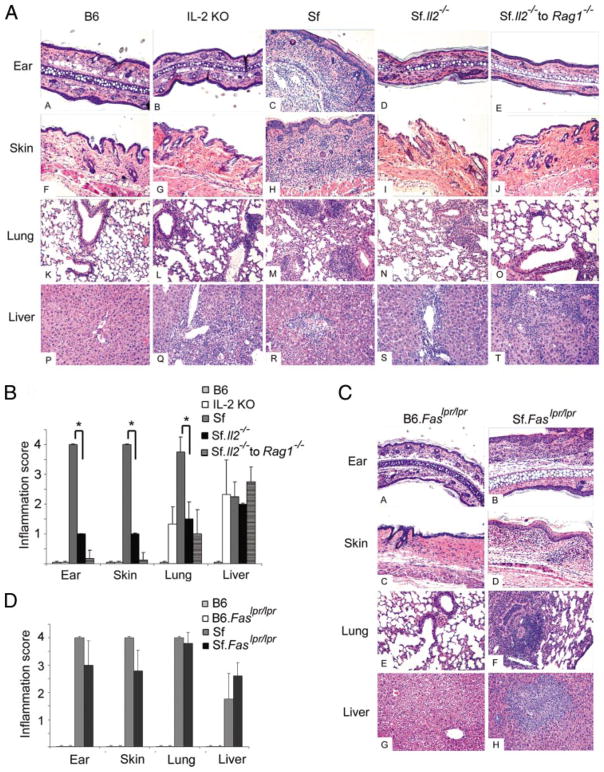
We compared the histopathology of various organs among B6, IL-2 KO, Sf, and Sf.Il2−/− mice (Fig. 3). At 4 wk old, Sf mice developed severe inflammation in the ear, lung, and skin and moderate inflammation in the liver whereas sex- and age-matched IL-2 KO mice displayed moderate inflammation in the liver and no inflammation in the ear and skin. Inflammation in the lung was nearly undetectable and occasionally observed around bronchia. In Sf.Il2−/− mice, the inflammation in the ear and skin was nearly undetectable and greatly reduced in the lung. The mild inflammation in the lung, like IL-2 KO mice, was associated with bronchia but not alveoli. In contrast, severe inflammation was observed at both places in Sf mice. Like Sf and IL-2 KO mice, perivascular infiltration of leukocytes was frequently observed in the liver of Sf.Il2−/− mice. These observations indicate that IL-2 is required for the severe autoimmune responses manifested in the lung and skin of Sf mice. Our previous study has shown that Sf mice contain dormant autoimmune T cells that can be revealed by adoptive transfer experiments (32). Therefore, lymph nodes cells from Sf.Il2−/− mice were adoptively transferred into RAG1−/− recipients and organ inflammation was determined 4 wk later (Fig. 3A). Inflammation was observed in the liver but not in the lung and skin. The result demonstrates that the inability to induce autoimmune inflammation in the skin and lung is an intrinsic property of the lymphocytes of the Sf.Il2−/− mice.
FIGURE 3.
Il2−/− but not Faslpr/lpr mutation regulates organ-specific inflammation in Sf mice. A, Ear, skin, lung, and liver of 4-wk-old B6, IL-2 KO, Sf, Sf.Il2−/− mice, and Rag1−/− mice adoptively transferred with 20 × 106 lymph node cells from Sf.Il2−/− mice were stained with H&E and examined under microscope. B, Semiquantitative measurement of inflammation in various organs demonstrated significant inhibition of inflammation in the ear, skin, and lung, but not liver, between Sf and Sf.Il2−/− mice (*, p value <0.03 by Student’s t test). C, Inflammation in ear, skin, lung, and liver was observed in Sf.Faslpr/lpr mice. Age-and sex-matched B6.Faslpr/lpr mice were used for comparison. D, Semi-quantitative determination of inflammatory response in various organs demonstrated insignificant differences between Sf and Sf.Faslpr/lpr mice.
Because Sf.Il2−/− mice lived beyond weaning and the post-weaning is an important environmental factor regulating multiorgan inflammation (32), the multiorgan autoimmune response of these mice was followed up in 8-wk-old mice. Inflammation remained undetectable in the skin and very mild in the lung. However, mild inflammation in the small intestine and severe colitis were observed (data not shown). This pattern of multiorgan autoimmune inflammation in Sf.Il2−/− mice resembled that of age-and sex-matched IL-2 KO mice (26, 33).
In contrast to Sf.Il2−/− mice, the autoimmune profile of Sf. Faslpr/lpr based on visual and histological observations was similar to that of Sf mice, i.e., severe inflammation in the ear, skin, and lung and moderate inflammation in the liver at 4 wk old (Fig. 3B). The data indicate that Faslpr/lpr mutation protected Sf mice from early mortality not by way of changing organ-specific autoimmune inflammation as IL-2 KO mice did, but by protecting tissue cell death from FasL-mediated apoptosis. Faslpr/lpr mutation also prolonged the lifespan of IL-2 KO mice (26). Collectively, the data demonstrate that the multiorgan autoimmune response in IL-2 KO and Sf mice converges on the Fas-signaling pathway and that the mortality of both IL-2 KO and Sf mice is dependent on the specific target organs and the Fas-signaling pathway.
Discussion
The major finding of this study is that blocking the IL-2-mediated signal in Sf mice greatly inhibited the autoimmune manifestation in ear, skin, and lung, improved body weight, and prolonged lifespan of the mice even though they are completely devoid of the CD4+Foxp3+ Tregs. We ruled out the role of Il2−/− in limiting T cell expansion and inhibiting FasL expression because higher lymphoproliferation and FasL expression were observed in Sf.Il2−/− mice. In addition, the autoimmune response in the ear, skin, and lung was not inhibited in Sf.Faslpr/lpr mice although their lifespan is similar to Sf.Il2−/− mice. Our animal study using genetic and de novo approaches demonstrated a heretofore unrecognized novel function of IL-2 in that it regulates the organ-specific (toward lung and skin but not liver) autoimmune responses in Sf mice.
Because the IL-2-mediated signal is important for the generation and maintenance of Tregs, the hypothesis that the IL-2-mediated signal is required mainly for self-tolerance has been proposed (34). This is based on the study that the autoimmune disease in IL-2Rβ KO mice could be completely cured when transgenic expression of IL-2Rβ was introduced in the thymocytes but not in the peripheral lymphocytes (35). What is related to the present study is that the autoimmune responses in IL-2Rβ KO mice, unlike Sf mice, occurred in the absence of IL-2- and IL-15-mediated signals. Our study showed the unexpected finding that in the absence of IL-2, severe autoimmune responses in skin are spared and the severe inflammation in lung is greatly diminished. Thus, it is possible that the autoimmune responses in IL-2Rβ KO mice was weakened and restricted to a few organs and thus easier to suppress under the condition in which a normal thymic Tregs output and an ineffective maintenance of Tregs in the periphery were established (35). In this regard, it should be emphasized that scurfy defect represents the most severe autoimmune disease known to mammals and it is significant that Il2−/− and Faslpr/lpr could inhibit disease progression and prolong the lifespan of the Sf mice.
In the type 1 diabetes mouse model, the IL-2 defect was shown to be necessary for disease development and this is attributed to the requirement of IL-2 to maintain islet-specific Tregs (36), Our results show that IL-2 regulates organ-specific autoimmune response against lung and skin not by its regulation of Tregs because IL-2 is still required for disease development even in the complete absence of Tregs. Although a normal thymic-negative selection of T cells has been demonstrated for Sf mice (22), the role of IL-2/IL-2R signaling in thymic-negative selection has not been resolved (37, 38). However, if IL-2 was required for thymic-negative selection of autoimmune T cells against self Ags (37), one would expect that Sf.Il2−/− mice should have more autoimmune T cells against ear, skin, and lung, an interpretation incompatible with our results. Moreover, because the autoimmune T cell repertoire in Treg-deficient strains is potentially very large (32), it is also difficult to envisage a mechanism of IL-2-dependent thymic selection that controls organ-specific autoimmune T cells as a whole. It remains possible that the high-affinity IL-2/IL-2R signaling is required for the expansion of certain low-affinity autoimmune T cells whose threshold of activation is high (39). The current view on multiorgan autoimmune response is that autoimmune T cells are normally present in the periphery and under the control of Tregs. Because there are Tregs in the IL-2 KO mice, albeit at a reduced level, the different display of the organ-specific autoimmune response could be attributed to the Tregs that might selectively suppress autoimmune response against certain organs. This argument is ruled out by the present study because the same pattern of multiorgan-specific autoimmune response as that of IL-2 KO mice was observed in the Sf.Il2−/− mice which completely lack Tregs.
It has been shown that mast cells, IgE, and a Th2-skewed response are strong inducing factors for skin and lung inflammation in BALB/c mice bearing mutant Foxp3 (40). In addition, histamine receptor-1 has also been implicated in lung inflammation (41). It is possible that certain organ-specific autoimmune responses are regulated by IL-2 through regulation of the expression of these factors. Another possibility is that the expression of lymphocyte trafficking receptors (CCR4, CD103, etc.) (42, 43) and their respective ligands in tissues are inhibited in Sf.Il2−/− mice. In preliminary studies, we observed a high serum IL-4 level and a high CD4+ T cell expression of CD103 in Sf mice as compared with IL-2 KO and Sf.Il2−/− mice, whereas no correlation was observed with serum IgE, IL-10, IFN-γ, TNF-α, and Th17 (Z. Zheng, R. Sharma, and S.-T. Ju, unpublished observations). Additional experiments are needed to determine the validity of the association with IL-4 and CD103. Finally, it should be noted that adoptive transfer of lymphocytes from Sf.Il2−/− mice into B6.RAG1−/− recipients failed to induce inflammation in the ear, skin, and lung (Fig. 3A) whereas transfer of CD4+ T cells of Sf mice did (32), suggesting this property, i.e., inability to induce severe inflammation in ear, skin, and lung, is intrinsic to the T cells of Sf.Il2−/− mice.
IL-2 is a critical component that facilitates activation-induced cell death by promoting FasL expression on highly activated T cells (25). Thus, FasL expression level is potentially a major regulatory mechanism for T cell homeostasis control in Treg-deficient mice in which T cell activation and reactivation occur continuously. This mechanism has often been used to explain the autoimmune lymphoproliferative response under conditions in which the IL-2-signaling pathway is impaired (24). Indeed, FasL expression is lower in IL-2 KO mice than Sf mice (Fig. 3). However, FasL expression in Sf.Il2−/− mice was higher than Sf mice. Thus, FasL expression level cannot explain the lack of inflammatory responses observed in the lung and skin of Sf.Il2−/− mice. Indeed, Faslpr/lpr mutation did not prevent inflammation in the ear, skin, and lung of Sf.Faslpr/lpr mice, yet still prolonged the lifespan. Faslpr/lpr mutation also prolonged the lifespan of IL-2 KO mice (26). These observations suggest that Fas has little influence on which target organ to attack in the multiorgan autoimmune syndrome of Sf mice. Rather, it plays an important role at the later stage of the inflammatory response involving tissue cell death as we have previously reported in IL-2 KO and IL-2Rα KO mice (26, 27). Autoimmune inflammation is also accompanied by an increase in Fas expression and serum TNF-α level and an increase in TUNEL-positive apoptotic cells in inflamed organs and (Ref. 27 and data not shown). Despite increased apoptotic mediators for tissue destruction, the lifespan of Sf.Il2−/− mice is longer than Sf mice presumably because the autoimmune inflammation in lung and skin is inhibited. Collectively, our study shows that the early mortality of Sf mice is controlled by both IL-2 and Fas, but at different stages of the autoimmune responses and by different mechanisms. Beyond the Treg checkpoint, IL-2 KO influences the mortality of Sf mice by regulating specific autoimmune inflammation in vital organs but not by preventing FasL expression and lymphoproliferation. Once initiated, inflammation induced by Il2−/− and Foxp3sf/y mutation converges on the Fas-mediated signaling pathway that leads to tissue cell death and mortality.
Acknowledgments
We thank Angela Ju for technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants DE-017579 and AR-051203 (to S.-T.J.), AR-047988 and AR-049449 (to S.M.F.), and a grant-in-aid from the Beirne B. Carter Center of Immunology (to R.S.).
Abbreviations used in this paper: Treg, regulatory T cell; Sf, Scurfy; KO, knockout.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Wing K, Fehervari Z, Sakaguchi S. Emerging possibilities in the development and function of regulatory T cells. Int Immunol. 2006;18:991–1000. doi: 10.1093/intimm/dxl044. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638– 642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81– 88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 5.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829– 840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 6.Yan J, Mamula MJ. Autoreactive T cells revealed in the normal repertoire: escape from negative selection and peripheral tolerance. J Immunol. 2002;168:3188–3194. doi: 10.4049/jimmunol.168.7.3188. [DOI] [PubMed] [Google Scholar]
- 7.Anderson AC, V, Kuchroo K. Expression of self-antigen in the thymus: a little goes a long way. J Exp Med. 2003;198:1627–1629. doi: 10.1084/jem.20031803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+CD25+CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antony PA, Paulos CM, Ahmadzadeh M, Akpinarli A, Palmer DC, Sato N, Kaiser A, Hinrichs CS, Klebanoff CA, Tagaya Y, Restifo NP. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol. 2006;176:5255–5266. doi: 10.4049/jimmunol.176.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 11.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavis MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 14.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 15.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang X, Gao JX, Jiang Q, Wen J, Seifers N, Su L, Godfrey VL, Zuo T, Zheng P, Liu Y. The Scurfy mutation of FoxP3 in the thymus stroma leads to defective thymopoiesis. J Exp Med. 2005;202:1141–1151. doi: 10.1084/jem.20050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liston A, Farr AG, Chen Z, Benoist C, Mathis D, Manley NR, Rudensky AY. Lack of Foxp3 function and expression in the thymic epithelium. J Exp Med. 2007;204:475– 480. doi: 10.1084/jem.20062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CS, Zheng Y, Liang Y, Fontenot D, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401– 410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 20.Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 2001;276:37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- 21.Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, Liu Y, Wang Y, Liu X, Chan MW, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275–1286. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Benoist C, Mathis D. How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc Natl Acad Sci USA. 2005;102:14735–14740. doi: 10.1073/pnas.0507014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 24.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615– 623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 25.Kung JT, Beller D, Ju ST. Lymphokine regulation of activation-induced apoptosis in T cells of IL-2 and IL-2Rβ knockout mice. Cell Immunol. 1998;185:158–163. doi: 10.1006/cimm.1998.1282. [DOI] [PubMed] [Google Scholar]
- 26.Xiao S, Sung SSJ, Fu SM, Ju ST. Combining Fas mutation with interleukin-2 deficiency prevents colitis and lupus. J Biol Chem. 2003;278:52730–52738. doi: 10.1074/jbc.M308707200. [DOI] [PubMed] [Google Scholar]
- 27.Sharma R, Bagavant H, Jarjour WN, Sung S-SJ, Ju S-T. The role of Fas in the immune system biology of IL-2Rα knockout mice: interplay among regulatory T cells, inflammation, hemopoiesis, and apoptosis. J Immunol. 2005;175:1965–1973. doi: 10.4049/jimmunol.175.3.1965. [DOI] [PubMed] [Google Scholar]
- 28.Ryan AE, Shanahan F, O’Connell J, Houston AM. Addressing the “Fas counterattack” controversy: blocking Fas ligand expression suppresses tumor immune evasion of colon cancer in vivo. Cancer Res. 2005;65:9817–9823. doi: 10.1158/0008-5472.CAN-05-1462. [DOI] [PubMed] [Google Scholar]
- 29.Jodo S, V, Pidiyar J, Xiao S, Furusaki A, Sharma R, Koike T, Ju ST. Cutting edge: Fas ligand (CD178) cytoplasmic tail is a positive regulator of Fas ligand-mediated cytotoxicity. J Immunol. 2005;174:4470– 4474. doi: 10.4049/jimmunol.174.8.4470. [DOI] [PubMed] [Google Scholar]
- 30.Rouvier E, Luciani MF, Golstein P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ju ST, Cui H, Panka DJ, Ettinger R, Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA. 1994;91:4185– 4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma R, Jarjour WN, Zheng L, Gaskin F, Fu SM, Ju ST. Large functional repertoire of regulatory T-cell suppressible autoimmune T cells in scurfy mice. J Autoimmun. 2007;29:10–19. doi: 10.1016/j.jaut.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma R, Zheng L, Guo X, Fu SM, Ju ST, Jarjour WN. Novel animal models for Sjögren’s syndrome: expression and transfer of salivary gland dysfunction from regulatory T cell-deficient mice. J Autoimmun. 2006;27:289–296. doi: 10.1016/j.jaut.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665– 674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 35.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice: implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 36.Liston A, Siggs OM, Goodnow CC. Tracing the action of IL-2 in tolerance to islet-specific antigen. Immunol Cell Biol. 2007;85:338–342. doi: 10.1038/sj.icb.7100049. [DOI] [PubMed] [Google Scholar]
- 37.Bassiri H, Carding SR. A requirement for IL-2/IL-2 receptor signaling in intrathymic negative selection. J Immunol. 2001;166:5945–5954. doi: 10.4049/jimmunol.166.10.5945. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki H, Hayakawa A, Bouchard D, Nakashima I, Mak TW. Normal thymic selection, superantigen induced deletion and Fas-mediated apoptosis of T cells in IL-2 receptor β chain-deficient mice. Int Immunol. 1997;9:1367–1374. doi: 10.1093/intimm/9.9.1367. [DOI] [PubMed] [Google Scholar]
- 39.Lee DS, Ahn C, Ernst B, Sprent J, Surh CD. Thymic selection by single MHC-peptide ligand: autoreactive T cells are low-affinity cells. Immunity. 1999;10:83–92. doi: 10.1016/s1074-7613(00)80009-6. [DOI] [PubMed] [Google Scholar]
- 40.Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martin MJ, Chatila TA. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 41.Bryce PJ, Mathias CB, Harrison KL, Watanabe T, Geha RS, Oettgen HC. The H1 histamine receptor regulates allergic lung responses. J Clin Invest. 2006;116:1624–1632. doi: 10.1172/JCI26150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor CCR4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Vries IJ, Langeveld-Wildschut EG, van Reijsen FC, Bihari IC, Bruijnzeel-Koomen CA, Thepen T. Nonspecific T-cell homing during inflammation in atopic dermatitis: expression of cutaneous lymphocyte-associated antigen and integrin αEβ7 on skin-infiltrating T cells. J Allergy Clin Immunol. 1997;100:694–701. doi: 10.1016/s0091-6749(97)70175-1. [DOI] [PubMed] [Google Scholar]