Abstract
A rich history of research documents the effects of neighborhood-level socioeconomic status (SES) conditions on health outcomes. Recent criticism of the neighborhoods and health literature, however, has stressed several conceptual and methodological challenges not adequately addressed in previous research. Critics suggest that early work on neighborhoods and health gave little thought to the spatial scale at which SES factors influence a specific health outcome. This article discusses the concept of neighborhoods and health, reviews recent criticisms of existing work, and provides a case study that exemplifies how geographic methods can address one such criticism. Using data on birth defects in North Carolina, the case study examines the relation of SES to orofacial clefts (cleft lip and cleft palate) at different spatial scales. The Brown–Forsythe test is used to select optimal neighborhood size. Results are evaluated using logistic regression models to examine the relationship between SES measures and orofacial clefts, controlling for individual-level risk factors. Results indicate modest associations between neighborhood-level measures of poverty and cleft palate but no associations with cleft lip with or without cleft palate.
Keywords: birth defects, GIS, logistic regression, neighborhoods and health, spatial scale
The medical geographic literature of research exploring differences in health outcomes among people residing in different geographic areas is extensive. Widespread documentation exists of small-area variations in morbidity, mortality, and health-related behaviors over the past 150 years (Macintyre and Ellaway 2003), and geography has often been used to pinpoint specific causes of disease. Recently, researchers have suggested that these observable differences in health between places could be due to neighborhood effects, or “the independent causal effect of a neighborhood … on a number of health and/or social outcomes” (Oakes 2004, 1938). Observable differences in health outcomes between places might be due to differences in the kinds of people who live in these places (composition) or differences in the physical or social environment (contextual; Oakes 2004). In addition, variation in health outcomes could be influenced by complex interrelationships between characteristics of people and the places in which they live. The study of neighborhood influences on health is uniquely geographic, yet much of the early research in this area was centered in major schools of public health, and research teams did not routinely include geographers. Many of the recent criticisms of neighborhoods and health research are related to the “nongeographic” lens through which public health researchers developed neighborhood studies and statistical analyses. Although tools such as geographic information systems (GIS) have been employed, analysis is often not particularly sophisticated, and huge bodies of geographic theory on the modifiable areal unit problem (MAUP) and the relevance of scale have all but been ignored.
In 2001, Ana Diez Roux, a pioneer in neighborhoods and health research, published an article on the conceptual and methodological challenges to neighborhoods and health studies (Diez Roux 2001). One specific challenge presented was how to define “neighborhoods” or relevant geographic areas. She suggested that the size and definition of the geographic area relevant to studying a specific health outcome might vary according to the processes through which the area effect is hypothesized to operate and the outcome being studied. That is to say, there is a causal pathway by which area characteristics (e.g., social or economic climate) impact health, but researchers need to understand at what scale this causal pathway operates. This requires the development and testing of hypotheses regarding the geographic area that is relevant for health outcomes.
Despite the obvious importance of scale in neighborhood and health studies, very few early studies attempted to understand the limitations of using a single geographic unit for analysis. Most health research used (and continues to use) geopolitical boundaries such as counties, census tracts, or electoral wards to examine the relationship between area-level socioeconomic and environmental variables and health outcomes. Although use of these proxies is a practical alternative because data are readily available, they are limited in that they do not necessarily correspond to the theoretically relevant geographic neighborhood. Only in the past few years have geographers actively engaged in the debate, once again revisiting geographic theories such as the MAUP and applying them to health research (Haynes et al. 2007; Spielman and Yoo 2009) and examining the effect of spatial scale on neighborhood studies (Weiss et al. 2007; Flowerdew, Manley, and Sabel 2008). In addition, advances in real-time tracking technologies have allowed researchers to define neighborhoods based on an individual’s use of and movement across space and time. Such methods challenge the assumption that neighborhood effects operate only through connections that exist among those residing in the same area and mitigate the possibility of incorrectly attributing exposure to neighborhood environments (Kwan 2009).
Overall, results from neighborhood studies tell us is that the definition of neighborhood does matter in statistical analyses of neighborhood effects. Given the importance of choosing the appropriate spatial scale for neighborhood studies, the literature is sparse and most studies choose neighborhood areas without much thought as to how or why these areas have been chosen. This article explores how geographic and statistical methods can assist in the definition, construction, and selection of neighborhoods using a case study of orofacial clefts in North Carolina.
Case Study: Orofacial Clefts in North Carolina
Orofacial clefts (OFCs) are among the most common types of birth defects in the United States. They occur when structures of the mouth fail to form properly and are divided into two distinct groups: cleft lip with or without cleft palate (CL ± P) and isolated cleft palate (CP). Nationwide, the three-year (2004–2006) estimated prevalence of CL ± P was 10.6 per 10,000 live births, and the prevalence of isolated CP was 6.4 per 10,000 live births (Parker et al. 2010). Orofacial clefts can impair the development of speech, teeth, and feeding capabilities and affected individuals require medical care from birth until adulthood. The defects therefore pose a substantial burden to individuals and their families and require significant health-related expenditures.
There is no agreement as to the effect, if any, of exposure to environmental toxicants on CP and CL ± P. What evidence we do have comes from conflicting results of studies examining presumed exposure (due to occupation or proximity) to sites that use or release specific chemical compounds (e.g., hazardous waste and toxic release sites or landfill; Dolk et al. 1998; Brender et al. 2006). Prior analysis of the data set used in this article found no association with proximity to a variety of industrial and hazardous waste sites and CP or CL ± P (Root unpublished research). Maternal smoking has also been linked to cleft lip and palate (Little, Cardy, and Munger 2004) and there is evidence of a gene–environment interaction between maternal or infant gene variations and smoking (Lammer et al. 2004). Neighborhood socioeconomic status (SES) context might also influence birth outcomes through a complex set of psychosocial, behavioral, and biological factors. Results from the few studies that have looked at the effect of both individual- and area-level characteristics on orofacial clefts are inconclusive. Some studies have found a positive association between neighborhood measures of low SES and orofacial clefts (Clark et al. 2003) and others have found no association at all (Vrijheid et al. 2000; Carmichael et al. 2003).
Given the uncertainties that exist about the etiology of orofacial clefts, examining the influence of neighborhood-level SES measures might assist in the generation of hypotheses about the underlying causal factors associated with socioeconomic risk factors. This study examines the relationship between CL ± P and isolated CP and three area-based measures of SES after adjusting for known individual-level risk factors. Three study questions guided this research: (1) To what extent are neighborhood-level SES variables related to the risk of an orofacial cleft? (2) Does this relationship differ when different spatial scales are used to define neighborhoods? (3) What do the results tell us about the intricacies of choosing a spatial scale at which to study health outcomes?
Methods
Birth Defects Data
The main source of birth defects data for this study was the North Carolina Birth Defects Monitoring Program (NCBDMP) run by the North Carolina State Center for Health Statistics (NC SCHS 2006). This study used a retrospective case-control design of North Carolina resident live births with an orofacial cleft (CL ± P or CP) between 1 January 1999 and 31 December 2004. To identify infants with an orofacial cleft, the NCBDMP database was searched using the International Classification of Diseases (ICD-9) code for orofacial clefts (codes 749.000–749.290). All live-born singleton infants with a birth certificate, without any birth defect, and born between 1 January 1999 and 31 December 2004 to North Carolina resident mothers contained in the NC Composite Linked Birth File were eligible to be controls. The data include residential address at birth, which was used to geocode cases and controls. A majority of the geocoding was completed by the NC SCHS, using geographic data technology (GDT) and parcel data from the North Carolina Department of Transportation. Records not matched by the NC SCHS were geocoded using a multistage geocoding method and different Web-based geocoding services (Lovasi et al. 2007). Using this process, 92.2 percent of cases and 93.3 percent of controls were matched.
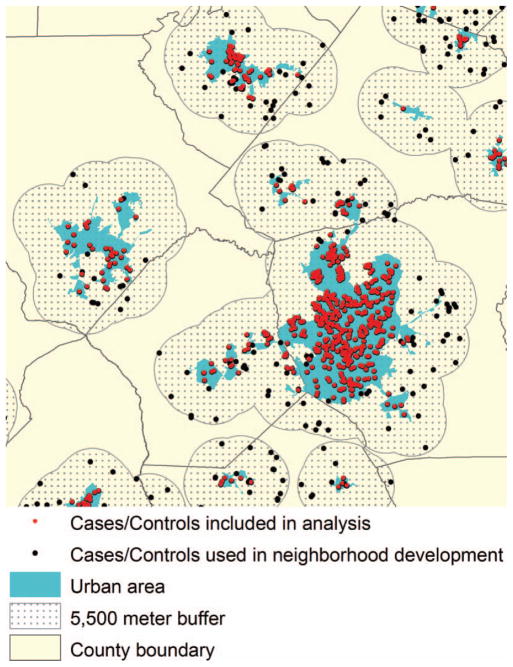
The analysis was limited to urban areas because the appropriate scale for a spatial neighborhood might differ depending on rural or urban residence. Using a GIS, each urban area was buffered at a distance of 5,500 m (the largest neighborhood size tested). Urban areas were buffered so that complete neighborhoods for individuals living near the border of an urban area could be constructed. Only cases and controls within the urban area were included in the statistical analysis, but areas contained in the buffered area were used to construct the neighborhood measures and conduct the Brown–Forsythe test (refer to Figure 1 and “Construction of Neighborhood Variables” later). The final urban sample included 319 cases of CL ± P, 206 cases of isolated CP, and 7,663 controls.
Figure 1.
Example of geographic information system used to select urban births for analysis. (Color figure available online.)
Socioeconomic Data
Socioeconomic variables for census tracts, block groups, and blocks were obtained from the U.S. Census Bureau for the 2000 Census. Following the approach of several previous studies that examine area-level effects on various birth outcomes (Krieger et al. 2003; Messer et al. 2008), three census variables were used to estimate neighborhood-level SES characteristics: percentage of the population living below 100 percent of federal poverty level, percentage of the population with less than a high school education, and percentage of the population unemployed. These measures quantify several socioeconomic domains that effect health: poverty, employment, and education. Whereas some researchers have advocated the use of indexes to measure the cumulative effects of several measures of SES (Messer et al. 2008), others have found that estimates of effects detected using a single variable measure of poverty were similar to those based on indexes (Krieger et al. 2003). The effect of a composite measure of SES similar to the Carstairs Index on OFCs was found to be statistically similar to that of the single variable measure of poverty. Therefore, this article presents single variable measures to examine separate effects of each SES domain on orofacial clefts. Each census variable was classified into quartiles based on the distribution among controls and each census tract, block group, and block given a corresponding quartile score (1 = lowest, 4 = highest).
Construction of Neighborhood Variables
Cases and controls were assigned to year 2000 census block groups and tracts. A set of “neighborhoods” was developed by creating circular buffers of various sizes around each study subject. Based on the size of the study area and the distribution of the population, the minimum size was set to a 1,000-m radius neighborhood and increased stepwise by 500 m until 5,500 m size was reached. This resulted in ten different neighborhood sizes from which to select the scale at which to conduct this analysis. The neighborhood-level SES variables were estimated by aggregating census block group data by each of the circular windows. In cases where the circular window contained only a portion of a census block group, variables were weighted by the proportion of the population from that block group that was encompassed by the window.
Selection of Neighborhood Scale
The neighborhood size used to conduct this analysis was chosen using the Brown–Forsythe (FBF) test. This method assesses whether the variance of a variable of interest (e.g., disease incidence or risk factors) is equal between two different-sized neighborhood groups. The underlying assumption of this method is that there is greater variance in disease rates among smaller neighborhoods and lower variance among larger neighborhoods. A high variance value means that the data are local or individualistic, whereas a low variance means that they capture a more global process. The optimal neighborhood ensures that the aggregate disease incidence data is somewhere in between. Use of this method is explained in detail elsewhere (Root, Meyer, and Emch 2011). The test statistic, FBF, was calculated as
where ni is the number of samples in the ith neighborhood group (e.g., the number of individual neighborhoods constructed that are the same size), N is the total number of samples for all neighborhood groups, t is the number of neighborhood groups (e.g., two different-sized neighborhood groups are compared), yij is the disease rate of the jth sample from the ith neighborhood group, ȳi is the median of disease rate from the ith neighborhood group, Dij = |yij − ȳi | is the absolute deviation of the jth observation from the ith neighborhood group median, D̄ i is the mean of Dij for neighborhood group i, and D̄ is the mean of all Dij (e.g., both neighborhood groups combined). The test assumes that the variances of two different neighborhood groups are equal under the null hypothesis. The critical value was calculated using an F distribution with (t − 1, N − t) degrees of freedom and α = 0.05 was used to test for significance.
The FBF test involves two steps. First, the variance between each neighborhood group (from 1,000 to 5,000 m) and the largest neighborhood group (5,500 m) is compared (for a total of nine separate calculations of FBF, shown in the FBF1 column of Table 1). Second, the variance between each neighborhood group (from 1,500–5,500 m) and the smallest neighborhood group (1,000 m) is compared (for a total of nine separate calculations of the FBF, shown in the FBF2 column). A significant value of FBF1 indicates that the neighborhood does not reveal the global structure of data; in essence each neighborhood is so small that it only captures disease dynamics for a small group of individuals. In contrast, a significant value in FBF2 implies that the neighborhood data are not individualistic; they are so large that local-level disease dynamics are “washed out” and undetectable. The neighborhoods between the lower and the upper limits identify a spatial scale at which local-level variation is still detectable but captures larger “neighborhood-level” disease dynamics.
Table 1.
Descriptive statistics and results for Brown–Forsythe test
| r | Population
|
Incidence rate/100,000 births
|
Brown–Forsythe
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | M | Mdn | Min | Max | M | Mdn | Variance | FBF1 | df1 | CV | FBF2 | df2 | CV | |
| 1,000 | 1 | 33 | 6.1 | 5 | 0 | 66,667 | 1003.4 | 0 | 20,571,812 | 3.99 | 9 | 1.88 | — | — | — |
| 1,500 | 1 | 64 | 11.0 | 9 | 0 | 50,000 | 1056.0 | 0 | 14,896,288 | 5.53 | 8 | 1.94 | 0.63 | 1 | 3.84 |
| 2,000 | 1 | 90 | 17.3 | 14 | 0 | 50,000 | 1081.4 | 0 | 10,762,540 | 6.59 | 7 | 2.01 | 0.84 | 2 | 3.00 |
| 2,500 | 1 | 126 | 25.0 | 20 | 0 | 33,333 | 1046.4 | 0 | 7,735,246 | 5.53 | 6 | 2.10 | 0.64 | 3 | 2.61 |
| 3,000 | 1 | 175 | 33.9 | 28 | 0 | 33,333 | 1016.9 | 0 | 5,797,722 | 4.45 | 5 | 2.21 | 0.66 | 4 | 2.37 |
| 3,500 | 1 | 221 | 44.0 | 36 | 0 | 25,000 | 989.8 | 0 | 4,489,869 | 3.36 | 4 | 2.37 | 0.93 | 5 | 2.21 |
| 4,000 | 1 | 285 | 55.2 | 45 | 0 | 20,000 | 963.9 | 0 | 3,619,704 | 2.31 | 3 | 2.61 | 1.42 | 6 | 2.10 |
| 4,500 | 1 | 345 | 67.5 | 56 | 0 | 14,286 | 941.5 | 0 | 2,935,198 | 1.48 | 2 | 3.00 | 2.09 | 7 | 2.01 |
| 5,000 | 2 | 388 | 80.7 | 67 | 0 | 12,903 | 919.3 | 0 | 2,443,491 | 0.73 | 1 | 3.84 | 2.95 | 8 | 1.94 |
| 5,500 | 2 | 427 | 94.8 | 80 | 0 | 11,111 | 899.2 | 0 | 2,081,109 | — | — | — | 3.99 | 9 | 1.88 |
Note: Bold figures in the FBF1 and FBF2 columns are the upper and lower limit of neighborhood scale, and the bold figure in the r column is the neighborhood size chosen for this analysis. r = size of the neighborhood radius in meters; df = degrees of freedom; CV1 and CV2 = critical values at 95 percent confidence level for FBF1 and FBF2.
Logistic Regression
To estimate the variation in risk of CL ± P and CP affected pregnancy associated with differences in neighborhood SES, maximum likelihood estimates of odds ratios (ORs) and 95 percent confidence intervals (CIs) were calculated from logistic regression models. For all analyses, models were estimated separately for CL ± P and CP, because initial models indicated that they are influenced by different individual-level risk factors. Individual-level risk factors included maternal age, infant sex and race or ethnicity, smoking during pregnancy, prenatal care, and Medicaid status. Regression models that included individual-level risk factors only were estimated first. Next, a set of models was estimated that included area-level SES measures defined for three different neighborhood sizes: 4,000-m radius, census tracts, and census block groups. For the models using census tracts and block groups as neighborhoods, generalized estimating equations with a logit link function were estimated to account for the block group and tract-level correlation. These models were built using independent and exchangeable within-area correlation matrices to control for the correlation. This was not necessary for models with neighborhoods defined using buffers because these neighborhoods were created for each individual case and control. All statistical analyses were conducted in R v.2.9.2 software (R Development Core Team 2011).
Results
The FBF test results for homogeneity of variance for orofacial cleft rates under various neighborhood sizes are listed in Table 1. The FBF1 test statistic shows that a neighborhood size of approximately 3,500 m is optimal and the FBF2 test statistic shows that a neighborhood size of approximately 4,500 m is optimal. Below 3,500 m, neighborhood data might only capture the characteristics of each individual, whereas above 4,500 m the neighborhoods are so large that they do not capture the influence of an individual’s proximal environment. Given these results, a neighborhood size of approximately 4,000 m was chosen for modeling. A neighborhood of approximately 4,000 m might seem large but equates to an area of approximately 5 miles across. In many U.S. urban areas, people routinely travel 5 to 10 miles to carry out many daily activities, such as shopping, taking children to school, or exercising.
Table 2 shows the prevalence of covariates among cases and controls. Risk factors were significantly different between cases and controls and between infants born with isolated CP and those born with CL ± P. Table 3 presents results from the logistic regression of individual-level variables only. Male infants showed an increased risk of CL ± P (OR = 1.61), whereas female infants showed an increased risk of isolated CP (OR = 0.66). Black infants showed a significantly decreased risk of both CL ± P (OR = 0.51) and isolated CP (OR = 0.71) compared to white infants. Finally, Medicaid status and late prenatal care (considered proxy indicators of individual-level SES) only showed an association with CL ± P.
Table 2.
Descriptive characteristics of cases and controls included in analysis
| Cleft lip ± palate
|
Cleft palate
|
|||||
|---|---|---|---|---|---|---|
| Casesa
|
Controlsb
|
p | Casesc
|
Controlsd
|
p | |
| n (%) | n (%) | n (%) | n (%) | |||
| Age | ||||||
| <25 Years | 126 (39.5) | 2,914 (38.0) | 0.596 | 63 (30.6) | 2,914 (38.0) | 0.030 |
| 25–29 Years | 81 (25.4) | 2,009 (26.2) | 0.743 | 62 (30.1) | 2,009 (26.2) | 0.212 |
| 30–34 Years | 70 (21.9) | 1,793 (23.4) | 0.547 | 56 (27.2) | 1,793 (23.4) | 0.206 |
| ≥35 Years | 42 (13.2) | 947 (12.4) | 0.668 | 25 (12.1) | 947 (12.4) | 0.924 |
| Sex | ||||||
| Female | 116 (36.5) | 3,697 (48.2) | 120 (58.3) | 3,697 (48.3) | ||
| Male | 202 (63.5) | 3,965 (51.8) | <0.0001 | 86 (41.8) | 3,965 (51.8) | 0.005 |
| Race | ||||||
| White | 202 (63.3) | 4,212 (55.0) | 0.003 | 134 (65.1) | 4,212 (55.0) | 0.004 |
| Black | 56 (17.5) | 2,129 (27.8) | <0.0001 | 45 (21.8) | 2,129 (27.8) | 0.060 |
| Hispanic | 49 (15.4) | 1,016 (13.3) | 0.279 | 24 (11.7) | 1,016 (13.3) | 0.501 |
| Other | 12 (3.8) | 306 (4.0) | 0.836 | 3 (1.5) | 306 (4.0) | 0.064 |
| Insurance | ||||||
| Private insurer | 188 (58.9) | 4,775 (62.3) | 131 (63.6) | 4,775 (62.3) | ||
| Medicaid | 131 (41.1) | 2,888 (37.7) | 0.223 | 75 (36.4) | 2,888 (37.7) | 0.708 |
| Prenatal care | ||||||
| 1st/2nd trimester | 314 (98.4) | 7,401 (96.6) | 197 (95.6) | 7,401 (96.6) | ||
| 3rd trimester | 5 (1.6) | 262 (3.4) | 0.072 | 9 (4.4) | 262 (3.4) | 0.461 |
n = 319.
n = 7,663.
n = 206.
n = 7,663.
Table 3.
Adjusted odds ratio (95 percent confidence interval) for cleft lip ± palate and cleft palate
| Cleft lip ± palate
|
Cleft palate
|
|||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | p | AOR | 95% CI | p | |
| Age | ||||||
| <25 Years | — | — | — | 0.73 | 0.51–1.05 | 0.0863 |
| 25–29 Years | — | — | — | Ref | ||
| 30–34 Years | — | — | — | 0.98 | 0.68–1.41 | 0.8994 |
| ≥35 Years | — | — | — | 0.82 | 0.51–1.32 | 0.4126 |
| Sex | ||||||
| Female | Ref | Ref | ||||
| Male | 1.61 | 1.28–2.03 | <0.0001 | 0.66 | 0.50–0.87 | 0.0038 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 0.51 | 0.37–0.69 | <0.0001 | 0.70 | 0.49–0.98 | 0.0436 |
| Hispanic | 0.95 | 0.68–1.32 | 0.7737 | 0.77 | 0.49–1.21 | 0.2560 |
| Other | 0.84 | 0.47–1.53 | 0.5738 | 0.30 | 0.10–0.95 | 0.0407 |
| Insurance | ||||||
| Private insurer | Ref | — | — | — | ||
| Medicaid | 1.36 | 1.06–1.73 | 0.0137 | — | — | — |
| Prenatal care | ||||||
| 1st/2nd trimester | Ref | — | — | — | ||
| 3rd trimester | 0.45 | 0.18–1.10 | 0.0821 | — | — | — |
Note: AOR = adjusted odds ratio; CI = confidence interval.
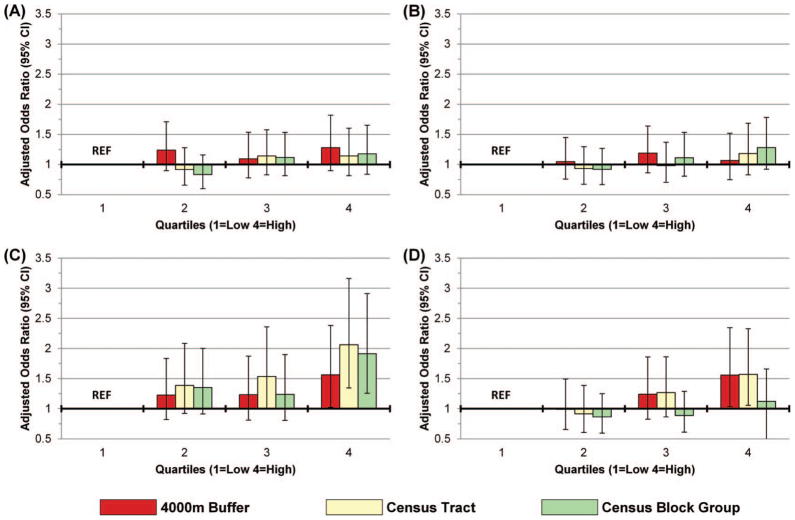
Figure 2 shows the adjusted ORs for two of the neighborhood SES measures in relation to CL ± P and CP. Only neighborhood measures of unemployment and percentage of the population below 100 percent of federal poverty level are shown because high school education showed no significant effect at any neighborhood scale. Overall, measures of neighborhood SES showed no significant association with CL ± P at any neighborhood scale. Marginal effects were present for infants born with isolated CP. Residence in a neighborhood in the highest quartile of poverty compared to the lowest quartile was associated with an increased risk for CP at all neighborhood scales. The neighborhood defined by census tracts showed the strongest association (OR = 2.06).
Figure 2.
Adjusted odds ratios (95 percent confidence interval [CI]) for neighborhood risk quartiles for (A) cleft lip ± palate (CL ± P), 100 percent of federal poverty level; (B) CL ± P, unemployment rate; (C) cleft palate (CP), 100 percent of federal poverty level; and (D) CP, unemployment rate. CL ± P adjusted for sex, race, insurance, and prenatal care; CP adjusted for age, sex, and race. (Color figure available online.)
Residence in a neighborhood in the highest quartile of unemployment compared to the lowest quartile was also associated with an increased risk for CP but only for the neighborhoods defined by the census tract and 4,000-m buffer. Women residing in a neighborhood in the highest quartile of unemployment were 1.5 times more likely to give birth to an infant with CP than women residing in the lowest quartile (4,000 m: OR = 1.56; tract: OR = 1.57). The census block group neighborhood unemployment rate showed almost no association and CIs overlapped the mean in both cases.
Discussion
This study indicates a weak association between residence in a lower SES neighborhood, as measured by poverty and unemployment, and the risk of having an infant with isolated CP, after adjusting for individual-level risk factors. The odds of a CL ± P–affected pregnancy did not appear to be affected by neighborhood-level SES, regardless of the scale at which SES was measured. Given these findings and the results of prior research, can theories about plausible links between specific socioeconomic features of the neighborhood and CP be developed? There are no data to suggest specific causal relationships, but hypotheses that merit further epidemiological research can be developed. For example, prior research on birth outcomes suggests that women who experience high levels of psychosocial stress are at greater risk for preterm and low-birth-weight births (see Hobel, Goldstein, and Barrett 2008). Although the results of this case study might be interesting by themselves, more important, they demonstrate several points about the challenge of choosing the geographic scale at which to test the effects of neighborhood-level SES on health outcomes.
At each SES quartile, the CIs for ORs measured by the 4,000-m buffer, census tract, and census block group overlapped, indicating no significant difference between ORs estimated at different scales. This suggests that too much emphasis on point estimates might be misleading, especially with cases where the point estimate at one scale (e.g., block group) is not significantly associated with the outcome, whereas the point estimate at another scale (e.g., 4,000-m buffer) is significantly associated. In this study, this was the case with fourth quartile neighborhood unemployment rates, where the buffer and tract-level OR estimates were statistically significant whereas the block group was not. The CIs for all three neighborhood scales overlapped, however, indicating that the point estimates across neighborhood scales were statistically similar. This has implications for all neighborhood studies, especially in the field of public health where so much emphasis is given to regression coefficients and ORs. In addition, it lends credibility to the ongoing debate about the usefulness of p values and significance tests in statistical inference (Cohen 1994; Stang, Poole, and Kuss 2010). In the social and medical sciences, the use of significance tests is ubiquitous, but placing too much emphasis on a p value of 0.05 might cause researchers to incorrectly reject or accept a null hypothesis. Disregarding neighborhood-level effects because CIs encompass 1.0 might impede important discoveries, especially for rare health conditions because p values and CIs are so dependent on sample size.
The neighborhood scale that is chosen for analysis is important, especially from a theoretical perspective. Researchers should use strong theory to choose a neighborhood scale that they believe represents the geographic scale at which causal pathways act between SES and the health outcome. Or, if there is uncertainty about the scale at which SES context influences a health outcome, several geographic scales could be explored and hypotheses drawn about potential causal mechanisms. In this study, for example, unemployment and poverty appear to exert some influence on CP but at different scales (poverty showed a stronger effect at smaller scales and unemployment at larger scales). It makes some sense that unemployment rate might influence CP at a higher level of geography because high unemployment tends to depress the economy in much larger areas (e.g., a whole city), whereas poverty is often localized, existing in “pockets” around a city.
Geographers have been struggling with the issue of scale for decades, discussing the MAUP in its many forms. The debate is particularly relevant to studies using GIS. The idea that aggregating data to different scales of geography might yield different results is no surprise but was given very little thought in public health, especially when neighborhoods and health studies began. Geographers have given a great deal of thought to the problems associated with scale and can contribute much to the debate of “appropriate” neighborhood scale in health studies. There are, of course, many different ways to define a neighborhood using real-time tracking, measures of distance, or even social connections or historical boundaries. The technique employed in this study (FBF test) is another method of exploring and choosing neighborhood scale. It is not, of course, the only method and might not be appropriate for choosing neighborhoods constructed using methods other than buffering, but it adds to the toolbox of methods used to understand and adapt to the MAUP. It can uncover a set of geographic scales that are relevant for assessment of health variation, allowing researchers to hypothesize why and how those scales are important. This is an important step forward in neighborhood and health research.
Finally, the skill necessary to correctly implement the GIS and the time used to construct the buffer neighborhoods might far outweigh the value added to the statistical analysis, especially because the census geography neighborhoods showed very similar statistical effects. Because the results are so similar, it begs the question of whether the extra effort is worth it. Possibly not in all health studies, but a more precise neighborhood definition could (1) validate previous findings because it reflects the geographic reality of the disease process better or (2) be more important in studies of certain health outcomes, such as infectious diseases where the mode of transmission is much better understood.
In summary, this study has identified both individual-level and neighborhood-level socioeconomic factors that contribute to the risk of isolated CP, although no neighborhood relationship was found for CL ± P. Most important, this study has shown how geographers can conceptually and methodologically contribute to neighborhoods and health studies, especially to the selection of neighborhood scale for analysis.
References
- Brender JD, Zhan FB, Suarez L, Langlois P, Moody K. Maternal residential proximity to waste sites and industrial facilities and oral clefts in offspring. Journal of Occupational and Environmental Medicine. 2006;48(6):565. doi: 10.1097/01.jom.0000214466.06076.07. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Nelson V, Shaw GM, Wasserman CR, Croen LA. Socio-economic status and risk of orofacial clefts. Paediatric and Perinatal Epidemiology. 2003;17(3):264. doi: 10.1046/j.1365-3016.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- Clark JD, Mossey PA, Sharp L, Little J. Socioeconomic status and orofacial clefts in Scotland, 1989 to 1998. Cleft Palate Craniofacial Journal. 2003;40(5):481. doi: 10.1597/1545-1569_2003_040_0481_ssaoci_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Cohen J. The Earth is round (p < .05) American Psychologist. 1994;49:997–1003. [Google Scholar]
- Diez Roux AV. Investigating neighborhood and area effects on health. American Journal of Public Health. 2001;91(11):1783. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolk H, Vrijheid M, Armstrong B, Abramsky L, Bianchi F, Garne E, Nelen V, et al. Risk of congenital anomalies near hazardous-waste landfill sites in Europe: The EUROHAZCON study. Lancet. 1998;352(9126):423. doi: 10.1016/s0140-6736(98)01352-x. [DOI] [PubMed] [Google Scholar]
- Flowerdew R, Manley D, Sabel C. Neighbourhood effects on health: Does it matter where you draw the boundaries? Social Science Medicine. 2008;66(6):1241–55. doi: 10.1016/j.socscimed.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Haynes R, Daras K, Reading R, Jones A. Modifiable neighbourhood units, zone design and residents’ perceptions. Health & Place. 2007;13(4):812–25. doi: 10.1016/j.healthplace.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clinical Obstetrics and Gynecology. 2008;51(2):333. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Soobader M-J, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (U.S.) Journal of Epidemiology and Community Health. 2003;57:186–99. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan M. From place-based to people-based exposure measures. Social Science Medicine. 2009;69(9):1311–13. doi: 10.1016/j.socscimed.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Lammer EJ, Shaw GM, Lovannisci DM, Van Waes J, Finnell RH. Maternal smoking and the risk of orofacial clefts: Susceptibility with NAT1 and NAT2 polymorphisms. Epidemiology. 2004;15(2):150–56. doi: 10.1097/01.ede.0000112214.33432.cc. [DOI] [PubMed] [Google Scholar]
- Little J, Cardy A, Munger RG. Tobacco smoking and oral clefts: A meta-analysis. Bulletin of the World Health Organization. 2004;82(3):213–18. [PMC free article] [PubMed] [Google Scholar]
- Lovasi G, Weiss J, Hoskins R, Whitsel E, Rice K, Erickson C, Psaty B. Comparing a single-stage geocoding method to a multi-stage geocoding method: How much and where do they disagree? International Journal of Health Geographics. 2007;6(1):12. doi: 10.1186/1476-072X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre S, Ellaway A. Neighborhoods and health: An overview. In: Kawachi I, Berkman LF, editors. Neighborhoods and health. Oxford, UK: Oxford University Press; 2003. pp. 20–42. [Google Scholar]
- Messer LC, Vinikoor LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, Elo I, Burke JG, O’Campo P. Socioeconomic domains and associations with preterm birth. Social Science Medicine. 2008;67(8):1247. doi: 10.1016/j.socscimed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- North Carolina State Center for Health Statistics (NC SCHS) North Carolina Birth Defects Monitoring Program. Raleigh: North Carolina State Center for Health Statistics; 2006. [last accessed 1 November 2010]. http://www.schs.state.nc.us/SCHS/bdmp. [Google Scholar]
- Oakes JM. The (mis)estimation of neighborhood effects: Causal inference for a practicable social epidemiology. Social Science Medicine. 2004;58(10):1929–52. doi: 10.1016/j.socscimed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Parker S, Mai C, Canfield M, Rickard R, Wang Y, Meyer R, Anderson P, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Research A: Clinical and Molelcular Teratology. 2010;88(12):1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing (v.9.2.9) Vienna, Austria: R Foundation for Statistical Computing; 2011. [last accessed 15 February 2012]. http://www.R-project.org. [Google Scholar]
- Root ED, Meyer R, Emch M. Socioeconomic context and gastroschisis: Exploring associations at various geographic scales. Social Science Medicine. 2011;72(4):625–33. doi: 10.1016/j.socscimed.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Spielman SE, Yoo EH. The spatial dimensions of neighborhood effects. Social Science Medicine. 2009;68(6):1098–1105. doi: 10.1016/j.socscimed.2008.12.048. [DOI] [PubMed] [Google Scholar]
- Stang A, Poole C, Kuss O. The ongoing tyranny of statistical significance testing in biomedical research. European Journal of Epidemiology. 2010;25(4):225–30. doi: 10.1007/s10654-010-9440-x. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Dolk H, Stone D, Abramsky L, Alberman E, Scott JE. Socioeconomic inequalities in risk of congenital anomaly. Archives of Disease in Childhood. 2000;82(5):349. doi: 10.1136/adc.82.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L, Ompad D, Galea S, Vlahov D. Defining neighborhood boundaries for urban health research. American Journal of Preventive Medicine. 2007;32(6 Suppl):S154–59. doi: 10.1016/j.amepre.2007.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]