Abstract
Methamphetamine (MA) is one of the most commonly abused illicit substances worldwide. Among other problems, abuse of the drug has been associated with reduced cognitive function across several domains. However, much of the literature has not attempted to differentiate cognitive difficulties caused by MA abuse from preexisting cognitive difficulties that are likely caused by other factors. Here, we address this question, evaluating evidence for a priori hypotheses pertaining to six lines of research: (a) animal studies; (b) cross-sectional human studies; (c) a twin study; (d) studies of changes in cognition with abstinence from MA; (e) studies of changes in brain structure and function with abstinence from MA; and (f) studies of the relationship between the severity of MA abuse and the extent of cognitive deficits observed. Overall the findings were mixed, with some support for a causal relationship between MA abuse and cognitive decline, and other findings suggesting that there is no relationship. The preponderance of the data, however, does support the possibility that MA abuse causes cognitive decline, of unknown duration, in at least some users of the drug. When averaged across individuals, this decline is likely to be mild in early-to-middle adulthood. However, moderator variables are likely to contribute to the presence and/or severity of cognitive decline exhibited by a given individual.
Keywords: methamphetamine, stimulant, cognition, review, cognitive, neuropsychology
INTRODUCTION
Methamphetamine (MA) abuse and dependence are major public health problems (Rawson and Condon, 2007), with amphetamines being second only to marijuana in prevalence of worldwide illicit drug use (United Nations Office on Drugs and Crime (UNODC, 2011)). Although the initiation of MA use in the United States has declined slightly since 2002 (SAMHSA, 2009), treatment admissions for MA has more than doubled between 1998 and 2007 (SAMHSA, 2009). Moreover, despite increases in treatment utilization, once dependence on MA has developed, cessation of use often proves difficult. Relapse rates following psychosocial and pharmacological treatments are high (Baker et al, 2005; Elkashef et al, 2008; Rawson et al, 2004; Shoptaw et al, 2008; Zorick et al, 2011), and for those who seek treatment, multiple treatment attempts are often the norm rather than the exception (Anglin et al, 1997; Hillhouse et al, 2007).
In addition to psychiatric and societal problems associated with MA abuse, a growing body of research has investigated whether MA abuse is associated with cognitive deficits. A meta-analysis of 17 cross-sectional studies found that humans who had abused MA exhibited significantly lower cognitive scores than control participants who did not abuse drugs (Scott et al, 2007). The effects were largest for measures of learning (d=−0.66), executive functions (d=−0.63), memory (d=−0.59), and processing speed (d=−0.52), although the majority of cognitive domains significantly differed between the groups. Cross-sectional studies, however, cannot differentiate cognitive weaknesses that may predate MA abuse from those that result from it. Notably, longitudinal studies have shown that childhood deficits in executive function can predict drug abuse in adolescence (Tarter et al, 2003, 2004), suggesting that at least some of the cognitive weaknesses noted in MA-dependent participants may be premorbid. In addition, much of the cross-sectional research available have employed flawed designs, without appropriately matching test cases and controls on potentially important variables, such as estimates of premorbid intelligence, education, and other drug and alcohol abuse. These and other limitations provoked a conclusion that the evidence for cognitive deficits in MA-dependent individuals is weak (Hart et al, 2011; however, see Payer et al (2012) for commentary).
Despite the important implications of potential MA-induced cognitive decline for public health and for the health of MA users in particular, a critical review of the evidence for a causal relationship between MA abuse and cognitive function has been lacking. This manuscript is a response to this gap in the literature. As causal experimental designs involving random assignment to chronic MA or placebo administration are ethically prohibitive in humans, the evidence provided by this review is inherently indirect and inferential. Evidence in this review is presented with respect to the following six hypotheses, with support for the hypotheses suggesting that MA does cause cognitive decline:
Animals exposed to MA will show cognitive decline, particularly when administered dosing regimens that mimic patterns of human MA abuse.
Individuals who abuse MA will have worse cognitive performance than well-matched individuals who do not abuse MA.
Twins who abuse MA will have lower cognitive performance than their twin pairs who do not abuse MA.
Individuals who abuse MA will show improvements in cognition with sustained abstinence.
MA abuse will be associated with changes in the human brain. Ideally, these brain changes will be associated with cognitive changes.
Cognitive deficits in MA-abusing individuals will be dose-related.
ANIMALS EXPOSED TO MA WILL SHOW COGNITIVE DECLINE, PARTICULARLY WHEN ADMINISTERED DOSING REGIMENS THAT MIMIC PATTERNS OF HUMAN MA ABUSE
In MA-dependent humans, MA intake typically ranges between 0.5 to 1.4 g per day (Hoffman et al, 2006; Kim et al, 2006; King et al, 2010; Simon et al, 2002), with daily intake most likely being restricted by the financial cost of MA and/or the physiological/psychological consequences of use (eg, hyperactivity, psychosis). Assuming that an average adult weighs ∼80 kg (Ogden et al, 2004), this equates to an intake of 6–17.5 mg/kg per day, although purity of the source and method of administration can affect the precise amount of MA ingested. Rodents that are allowed to self-administer infusions of MA also approach this range of consumption, with daily intake of MA exceeding 6 mg/kg after only 21 days of access (Parsegian et al, 2011; Reichel et al, 2011; Rogers et al, 2008). As MA-dependent adults typically use the drug between 1 and 5 times a day (McKetin et al, 2008; Simon et al, 2002), we estimate that most doses of MA range between 0.60 to 3.5 mg/kg per dose, although tolerant injecting users can exceed 4 mg/kg per dose, with maximum reported doses exceeding 12 mg/kg per injection (Buffum and Shulgin, 2001; Kramer et al, 1967). As rats and other animals metabolize MA more readily than humans (Caldwell et al, 1972), it is unclear whether equivalent mg/kg doses are physiologically identical between humans and animals. Nonetheless, in this review, we pay special attention to animals that have been given doses of MA that are within the range of probable human consumption (eg, <3 mg/kg per dose).
When assessed at least 1 week after MA administration, rats and mice that are administered large, binge doses of MA (⩾4 mg/kg per dose, often multiple times per day) exhibit deficits in several cognitive domains, including object recognition memory (Belcher et al, 2005; Siegel et al, 2010), odor recognition memory (O'Dell et al, 2011), spatial learning (Acevedo et al, 2007; Vorhees et al, 2009), sequential learning (Chapman et al, 2001; Daberkow et al, 2005), path integration learning (Herring et al, 2008), working memory (Mizoguchi et al, 2011), effort discounting (similar to delay discounting) (Kosheleff et al, 2012), and reversal learning (Izquierdo et al, 2010).
Similar to effects observed in humans, acute administration of moderate doses of MA (⩽2 mg/kg) to animals can improve cognitive performance, such as measures of reversal learning (Kulig and Calhoun, 1972), working memory (Shoblock et al, 2003), and the preference for larger, delayed rewards over smaller, immediate rewards (Richards et al, 1999). However, chronic, rather than acute, administration of moderate doses (⩽2 mg/kg daily) can impair cognitive performance, including measures of working memory (Lee et al, 2011; Nagai et al, 2007) and object recognition memory (Arai et al, 2009; Ito et al, 2007; Noda et al, 2010). In fact, administering mice 1 mg/kg of MA daily for 7 days produces reliable deficits in object recognition memory even when assessed at least 1 week after drug cessation (Kamei et al, 2006; Lu et al, 2010; Mizoguchi et al, 2011).
Although the previous studies document that cognitive decline can occur as a result of chronic administration of moderate doses of MA to naive animals, this pattern of drug administration does not mirror the pattern of use typical of humans, in which MA use can escalate over time in dosage and frequency (Sommers et al, 2006). For example, Segal et al (2003) have shown that an escalating dosing regimen of MA to rats (eg, starting at 0.1 mg/kg and increasing to 4 mg/kg over 14 days) can produce tolerance to subsequent binge doses of MA, such that behavioral and biological deficits produced by binge doses are attenuated. Using this paradigm, a few studies have found that deficits in object recognition memory (Belcher et al, 2008; Clark et al, 2007) and working memory (Simoes et al, 2007) caused by a binge dose in rats are largely prevented by an escalating dose beforehand. However, a recent study of vervet monkeys administered an escalating dose of MA (starting at 0.1 mg/kg and escalating to 4 mg/kg per day over a 31-day period) found that the monkeys developed selective deficits in the ability to inhibit responding to a previously rewarded stimulus (Groman et al, 2012). These deficits improved with abstinence, suggesting that recovery may occur with the cessation of MA administration.
In addition to escalating their dosing over time, humans self-administer MA, rather than being administered the drug involuntarily. To mimic this behavior, several studies have modeled escalating dosing in which MA is self-administered to rats who receive infusions after a voluntary lever press, typically using 0.02 mg/50 μl of MA per infusion. To escalate dosing over time, rats are first allowed limited access to infusions (eg, 1 h a day) for the first 5–7 days of administration, followed by more extensive access to infusions (eg, 6 h a day) for another 14–21 days. In this regimen, rats increase their administration over time, typically receiving 1 mg/kg of MA per day for the first few days, but exceeding 6 mg/kg per day by the end of the treatment. Using this paradigm, it has been shown that rats develop deficits in attentional set-shifting, when assessed 1 day after MA administration (Parsegian et al, 2011). When assessed at least 1 week after MA administration, rats exhibit deficits in object recognition memory (Reichel et al, 2011; Rogers et al, 2008) and sustained visual attention and inhibitory control (Dalley et al, 2007). Other measures of spatial reconfiguration memory and some indices of attention (eg, intradimensional shift) were not significantly affected by MA administration in these studies.
Conclusion
Chronic administration of MA doses that are likely to be within the range of human consumption produce cognitive deficits in naive animals. Although evidence suggests that the use of escalating doses of MA can prevent the cognitive deficits otherwise incurred by moderate to high doses of MA, several studies nonetheless find that self-administered and experimenter-administered escalating doses can cause cognitive deficits in both monkeys and rats (Dalley et al, 2007; Groman et al, 2012; Parsegian et al, 2011; Reichel et al, 2011; Rogers et al, 2008).
INDIVIDUALS WHO ABUSE MA WILL HAVE WORSE COGNITIVE PERFORMANCE THAN WELL-MATCHED INDIVIDUALS WHO DO NOT ABUSE MA
As literature comparing the cognitive performance of MA-dependent humans and healthy control participants is frequently plagued by poor research design, in which the groups are not comparable on extraneous variables (Hart et al, 2011), here we review only those studies that compared cognition in MA-dependent and control participants and minimally controlled for age, gender, education, and estimates of IQ, typically assessed by measures of reading/pronunciation. This review is also limited to studies that obtained a psychiatric diagnosis of MA dependence (typically with the Structured Clinical Interview for the DSM (First et al, 1996)), and attempted to exclude comorbid psychiatric and medical conditions, including current dependence on drugs other than MA. Finally, we included only studies in which abstinence from drugs at the time of cognitive testing was confirmed by urinalysis.
Seven studies that compared the cognitive performance of MA-dependent and healthy control participants met these criteria (Table 1). Of these studies, only one small study (Leland et al, 2008) did not detect significant differences in cognitive performance between the groups. The other six studies (Gonzalez et al, 2004; Henry et al, 2009, 2010; Kalechstein et al, 2003; Rendell et al, 2009; Woods et al, 2005) found that MA-dependent participants performed worse on some cognitive tests than healthy control participants, including a study of real-world functional ability (eg, measures of financial function, communication, transportation, medication management, etc.) (Henry et al, 2010). In these studies, the MA-dependent and control groups typically only differed on a subset of the tests administered. As similarly found in the meta-analysis conducted by Scott et al (2007), verbal learning and memory represented a fairly consistent area of weakness for the MA-dependent participants.
Table 1. Studies Comparing the Cognitive Performance of MA-dependent and Healthy Control Participants in which the Effect of Age, Gender, Education, and Estimated IQ was Controlled.
| Study | Sample size | Groups comparablea | Statistically controlled | Groups unmatched or unknownb | Full cog battery? | Control>MAc | No significant differencesc |
|---|---|---|---|---|---|---|---|
| Kalechstein et al (2003) | MA=27 Control=18 | Age Gender Education Estimated IQ Ethnicity Depression score | NA | Current or former other drug abuse (but not dependence) Cig. smoking | Yes | Attention/speed Learning and memory Fluency | Visuospatial Working memory Shifting/inhibition |
| Rendell et al (2009) | MA=20 Control=20 | Age Gender Education Estimated IQ English level Negative affect Sleep | NA | Current or former other drug abuse (but not dependence) Cig. smoking Ethnicity | Partial | Executive function Learning and memory Attention Prospective memory | NA |
| Henry et al (2009) | MA=12 Control=12 | Age Gender Education Estimated IQ Negative affect | NA | Current or former other drug abuse (but not dependence) Cig. smoking Ethnicity | Partial | Affect recognition Theory of mind Sentence completion Verbal learning | Phonemic fluency Delayed recall |
| Woods et al (2005) | MA=87 Control=71 | Age Gender Education Estimated IQ Ethnicity Axis I conditions | NA | Former other drug abuse/dependence Cig. smoking Hepatitis C Depression score | No | Verbal learning and recall indices | Serial clustering Recognition memory |
| Gonzalez et al (2004) | MA=53 Control=41 | Age Gender Education Estimated IQ Ethnicity Academic problems | NA | Former other drug abuse/dependence Cig. smoking | Yes | Global cognition Learning and recall (non-marijuana abusers only) | MA participants who abused marijuana did not differ from controls on any cognitive domain |
| Leland et al (2008) | MA=19 Control=19 | Age Gender Education Estimated IQ Ethnicity | NA | Current other drug dependence? Cig. smoking | No | NA | Go/No-Go indices |
| Henry et al (2010) | MA=15 Control=15 | Age Gender Education Estimated IQ Ethnicity | NA | Former other drug abuse ADHD Cig. smoking Psychotic sxs (not meeting DSM-IV diagnostic criteria) | No | Comprehension Financial skill Communication Transportation Medication management | Household skills |
| Hoffman et al (2006) | MA=41 Control=41 | Age Gender Estimated IQ | Education | Former other drug abuse/dependence? Cig. smoking Negative affect Parkinsonian sxs | Yes | Verbal memory and recall Stroop reading | Visuospatial Visual memory Attention/speed (most) Motor speed Executive function |
| Kim et al (2006) | MA=29 Control=20 | Age Gender Full scale IQ SES Social drinking | Education Cig. smoking | Ethnicity HIV status | No | Wisconsin card Sorting indices | Trailmaking test Stroop test |
| Chang et al (2005) | MA=44 Control=28 | Age Gender Education Estimated IQ | Education | Current or former other drug abuse (but not dependence) Cig. smoking Ethnicity | Yes | NA | All cognitive tests after Bonferroni correction |
| Simon et al (2010) | MA=27 Control=28 | Age Gender Estimated IQ Ethnicity ADHD score Mother's education | Education | Former other drug abuse/dependence Marijuana abuse Cig. smoking | Yes | NA | Attention/speed Working memory Learning and memory Executive function Global cognition |
| Rippeth et al (2004) | MA=47 Control=60 | Age Education Ethnicity ADHD sxs | Gender Estimated IQ Depression sxs | Former other drug abuse/dependence Cig. smoking Academic problems | Yes | Global cognition | Probably several cognitive domains, but none tested with covariates |
| Salo et al (2007) | MA=36 Control=16 | Age Gender Education Ethnicity Parental education | Education Estimated IQ | Current other drug abuse (except alcohol) Former other drug abuse/dependence Cig. smoking | No | Stroop interference | Stroop facilitation |
| Salo et al (2009) | MA=65 Control=33 | Age (subset) Gender | Education Estimated IQ | Current other drug abuse (except alcohol) Former other drug abuse/dependence Cig. smoking Ethnicity | No | Stroop interference (recently abstinent MA subjects only) | Stroop interference (long-term abstinent MA subjects only) |
| King et al (2010) | MA=54 Control=74 | Age Education Marijuana use Alcohol use | Gender Estimated IQ Cig. smoking | Current other drug abuse/dependence (but alcohol and marijuana use were comparable) Ethnicity | Yes | NA | All cognitive tests after Bonferroni correction (adolescent MA abusers) |
Abbreviations: ADHD, attention deficit hyperactivity disorder; cig., cigarette; cog, cognitive; control>MA, healthy control participants performed significantly better than MA-dependent participants; MA, methamphetamine; SES, socioeconomic status; sxs, symptoms.
The MA and control groups were considered comparable if they were not significantly different on a given characteristic.
The MA and control groups could be conceivably unmatched on numerous characteristics; we include here only those considered most salient.
Where available, we use statistics that were adjusted for multiple comparisons to determine if a particular cognitive test significantly differed between the groups.
In addition to studies that have matched the MA and control participants on key demographic variables, several studies have statistically controlled for demographic differences between the groups. Of these eight studies (see Table 1), five found that the MA-dependent participants performed worse than the control participants on a subset of the cognitive tests administered (Hoffman et al, 2006; Kim et al, 2006; Rippeth et al, 2004; Salo et al, 2007, 2009). The other three studies did not find significant differences between the groups on multiple cognitive tests (Chang et al, 2005; King et al, 2010; Simon et al, 2010), although two of these studies used Bonferroni correction for statistical significance, and this method may be overly conservative when dependent variables are interrcorrelated (Miller, 1981), as is the case with performance on most cognitive tests (Warner et al, 1987).
Conclusion
In reviewing the cognitive data from reasonably well-matched groups of MA-dependent and healthy control participants, the majority of studies have found that MA-dependent individuals have lower scores than control subjects on at least some cognitive tests, although some studies are exceptions with entirely nonsignificant differences (Chang et al, 2005; King et al, 2010; Leland et al, 2008; Simon et al, 2010). Of note, we found no well-matched studies in which the MA-dependent participants performed significantly better than control subjects on any cognitive test, which might be expected if the differences between the groups were purely an artifact of Type I statistical error. The evidence therefore suggests that at least some MA-dependent individuals do have lower cognitive function than would be expected from their demographic characteristics.
None of the studies available provided scatter plots of their cognitive data so that the overlap in performance between MA-dependent and control subjects could be observed. However, on average, the difference in performance between MA-dependent and control participants tends to be mild, as most significant differences between the groups are within (and often lower than) 1 SD of performance (based on the standard deviations for the groups reported in the studies). Assuming that the distributions of the scores are approximately normal, we suspect that it is common for the MA-dependent and control groups to exhibit a fair degree of overlap in cognitive performance.
Deficits found in MA-dependent participants in cross-sectional studies may still reflect factors that predisposed participants to drug abuse rather than resulting from the abuse, and it is impossible in cross-sectional studies to control for all potential confounds. For example, although almost all of the studies reviewed excluded current dependence on drugs other than MA (with the notable exception of nicotine), polysubstance abuse is extremely common in MA-abusing individuals. As such, when considered in isolation, the cross-sectional evidence for MA-induced cognitive decline is limited.
TWINS WHO ABUSE MA WILL HAVE LOWER COGNITIVE PERFORMANCE THAN THEIR TWIN PAIRS WHO DO NOT ABUSE MA
As genetically related individuals tend to have similar cognitive abilities (Bouchard, 1998; Winterer and Goldman, 2003) and are often raised together, twins who are discordant for MA abuse provide a valuable quasi-experimental design to control for extraneous confounds. Only one study has investigated the performance of twins who were discordant for MA abuse (Toomey et al, 2003; although Ersche et al, 2012 also compared siblings who were discordant for stimulant dependence on an inhibitory control measure, the vast majority of these participants abused cocaine, not MA). Toomey et al (2003) examined the neuropsychological performance of 50 male twin pairs from the Vietnam Era Twin Registry (31 monozygotic; 19 dizygotic), in which only one of the members had a history of heavy stimulant use. Heavy stimulant use was defined as weekly stimulant use for at least 1 year (the non-abusing twin never used stimulants weekly), but no participant endorsed use of stimulants or marijuana in the year before testing. Of those who abused stimulants, most (80%) had abused amphetamines (nine in combination with cocaine), while the rest primarily abused cocaine. The twin pairs were generally well-matched, and did not significantly differ in education, learning problems in school, history of head injury, Axis I lifetime diagnoses, alcohol use, cigarette use, marijuana dependence, or Full Scale IQ assessed by the Revised Wechsler Adult Intelligence Scale (WAIS-R; both group means=103).
The results revealed that the twins who abused stimulants had significantly worse performance than their non-abusing counterparts on two timed tests of attention (Cancellation and Trailmaking Part A) and two tests of psychomotor speed/dexterity (Finger Taping and Grooved Pegboard; p<0.05). However, the stimulant-abusing twins performed significantly better than non-abusers on one test of sustained attention (Continuous Performance Test: number correct, omissions, and sensitivity). None of the variables from the other nine tests examined significantly differed between the groups, including tests of executive function, nonverbal reasoning, reading, visual memory, or verbal memory.
Conclusion
The only relevant twin study available found that stimulant-abusing twins performed worse than non-abusing twins on a few cognitive measures, although this pattern was reversed on one test in which the stimulant-abusers performed better than the non-abusers. However, this study did not administer urinalyses to ensure that participants were drug free at the time of testing, which is important considering that acute MA can improve baseline performance (Hart et al, 2008; Mahoney et al, 2011). Further, it is unclear whether the weekly use of MA examined in this study constitutes either dependence or abuse as defined by the DSM-IV. The results here suggest that weekly stimulant use may be associated with a few cognitive weaknesses, but not global cognitive decline.
HUMANS WHO ABUSE MA WILL SHOW IMPROVEMENTS IN COGNITION WITH SUSTAINED ABSTINENCE
It is possible that any decline in cognitive function that is caused by MA abuse is permanent and irreversible. If MA abuse causes irreversible cognitive decline, the absence of a relationship between cognition and sustained abstinence from MA would have no bearing on the causal relationship between MA abuse and cognitive function. Nonetheless, improvement in the cognition of MA-abusing individuals with sustained abstinence would provide indirect evidence that MA abuse suppressed the normal state of cognitive function.
A few studies have found that MA-dependent individuals performed better on cognitive tests when tested in early abstinence and again in later abstinence (Jaffe et al, 2005; Volkow et al, 2001; Wang et al, 2004). However, these studies did not include a control group that was retested at comparable intervals, making it impossible to determine whether the improvements in test scores were an artifact of previous test exposure. Improvement associated with previous test exposure is common and can be evident for more than a year after the first testing session (Basso et al, 1999; Beglinger et al, 2005; Dikmen et al, 1999).
Three cross-sectional studies have compared the cognitive performance of MA-abusing participants who were abstinent for varying lengths of time. Using the Stroop Task, Salo et al (2009) compared the performance of MA-dependent participants who had been briefly abstinent (from 3 weeks to 6 months) to MA-dependent participants who had been abstinent for at least 1 year. The two groups of MA participants did not differ in age, gender, education, or premorbid IQ. Results showed that participants who were briefly abstinent performed significantly worse on the Stroop Task than participants who were abstinent for at least a year.
Kim et al (2006) administered three neuropsychological tests (Wisconsin Card Sorting, Stroop Test, and Trailmaking Test) to MA-dependent participants who were abstinent for a period of less than 6 months, and compared their performance to MA-dependent participants who were abstinent for more than 6 months. The groups did not differ significantly in age, gender, socioeconomic status, education, alcohol use, cigarette smoking, or duration of MA use. The participants who were abstinent for longer than 6 months performed significantly better on the Wisconsin Card Sorting Test (WCST) than those who were abstinent for less than 6 months. Stroop and Trailmaking performance did not differ significantly between the groups. Structural MRI scans were also obtained in this study, and the MA-dependent subjects with longer abstinence had larger gray-matter volumes in the right middle frontal gyrus (rMFG) than those with shorter abstinence. In addition, total and perseverative errors on the WCST were negatively correlated with gray-matter volume in the rMFG.
Simon et al (2004) administered a neuropsychological battery to treatment-seeking MA-abusing participants who were continuously abstinent during treatment for an unknown duration, and compared their performance to two other groups of MA-abusers: (1) MA-abusers who had relapsed during treatment, but were abstinent at least 4 weeks before cognitive testing; and (2) MA-abusers who had not stopped using MA during treatment and were positive for MA at each treatment session. Results revealed that the group that continued to use MA during testing performed significantly better than the relapse and abstinence groups on tests of memory. This result may be due to the acute effects of MA on performance. In addition, alternate test forms were used in this research, and it is not clear that the research groups were comparable with respect to the test forms administered.
Only two longitudinal studies have compared the neuropsychological performance of MA-dependent individuals across a period of sustained abstinence to that of healthy control subjects who were tested at comparable intervals. Simon et al (2010) administered a neuropsychological battery to MA-dependent participants at 4–9 days of abstinence and again after 1 month of sustained abstinence. A control group was tested at a comparable interval. On retesting, the MA-dependent group did not improve significantly more than the control group in any cognitive domain; they did improve 0.14 standard deviations more than the control group on the overall cognitive battery, but this difference did not approach statistical significance (p=0.33).
Iudicello et al (2010) assessed MA-dependent and healthy control subjects on a neuropsychological battery twice—once at baseline, and again approximately 1 year later. Some of the MA-dependent participants remained abstinent over that year (n=25), and some relapsed (n=58), but were clean of MA when tested. Results showed that the abstinent and non-abstinent MA groups did not change significantly more than the control group on the cognitive battery at retesting. However, post-hoc analyses revealed a trend (p=0.06) for abstinent MA-dependent participants who were impaired at baseline to improve more at retesting than relapsing MA participants and healthy controls (whether impaired at baseline or not impaired). However, some of the subgroups tested were very small (eg, n=6).
Conclusion
The evidence for cognitive improvement associated with abstinence from MA use in MA-dependent individuals is mixed. Two of three cross-sectional studies of abstinence suggest that some cognitive functions (eg, executive functioning) do improve with abstinence. However, the only longitudinal study with a strong experimental design that assessed 1 year of abstinence (Iudicello et al, 2010) found that only a certain subset of MA-dependent individuals (those impaired at baseline) improve with abstinence, whereas other MA-dependent individuals do not improve.
MA ABUSE WILL BE ASSOCIATED WITH CHANGES IN THE HUMAN BRAIN
As the structure and function of the brain influence cognitive performance (see Lezak et al (2004)), changes in the brain associated with MA abuse may imply that cognitive changes occur as well, with the best evidence provided by studies that concurrently assess both brain structure/function and cognitive performance. In animals, in addition to cognitive decline produced by chronic moderate (⩽2 mg/kg daily) or escalating doses of MA, concomitant alterations have been observed in D2-like dopamine receptor and dopamine transporter binding (DAT) (Groman et al, 2012), tissue levels of dopamine and serotonin (Lu et al, 2010), NMDA receptor binding (Lee et al, 2011), glutamate receptor (mGluR5) expression (Reichel et al, 2011), pyramidal neuron cell firing (Parsegian et al, 2011), and novelty-induced hyperphosphorylation of extracellular signal-related kinase 1/2 (Ito et al, 2007; Kamei et al, 2006; Nagai et al, 2007). In humans, cross-sectional studies likewise show differences in the brain structure and function between MA-dependent and healthy control participants (for reviews, see Berman et al (2008); Chang et al (2007); Salo and Fassbender (2012)). However, in humans, it is possible that some or all of these differences could be premorbid in nature. Thus, we focus our review here on changes in the brain that have been observed in longitudinal studies, as these changes have a higher likelihood of reflecting a consequence rather than simply a correlate of MA abuse. At present, all longitudinal studies of the brain have been conducted during abstinence from MA abuse, rather than during active use. Nonetheless, changes that occur in the brain during abstinence are likely to reflect compensatory responses to effects of MA abuse.
Using positron emission tomography (PET) and radiolabeled [11C]d-threo-methylphenidate, Volkow et al (2001) examined changes in the dopamine transporter (DAT) in the striata of five MA-dependent participants who were evaluated once after 6 or fewer months of abstinence, and again after ⩾9 months of abstinence. After protracted abstinence, DAT availability increased significantly in the caudate nucleus (+19%) and the putamen (+16%); and these increases were strongly correlated with the duration of abstinence (r=0.92). Neuropsychological tests were administered at both time points. Improvement in neuropsychological performance and increases in DAT availability showed positive trend relationships on two tests (timed gait and delayed recall, p<0.18), but none of these relationships reached significance. Although there were no controls for repeated measurement in this study, the increases in DAT availability are unlikely to be simply an artifact of repeat testing (see Meyer et al, 2002; Nurmi et al, 2000).
In another study of DAT availability in the striatum using single-proton emission computed tomography (SPECT) with Tc-99m TRODAT (Chou et al, 2007), five MA-dependent participants were assessed at baseline (while positive for MA in urinalysis) and again after 2 weeks of abstinence. A control group (n=7) was also retested after a 1-week delay period. Although the groups were not statistically compared in a within-subjects manner, striatal DAT availability increased in the MA participants from 5 to 38% (average of 20%) with abstinence, while the control participants changed from −14 to 13% with retesting. The WCST was also administered at both test sessions, and improvement on this test was correlated with the change in DAT availability. However, the control group was not used to calculate practice effects with repeated measurement, so it is unclear whether the correlation between DAT and WCST pertains to improvement in cognitive function over time, ability to benefit from previous test exposure, or both. In addition, the fact that participants were positive for MA during the first SPECT assessment significantly complicates the interpretation of DAT changes.
Wang et al (2004) measured cerebral glucose metabolism using [18F]fluorodeoxyglucose and PET in five MA-dependent participants who were tested when they were abstinent for <6 months, and again after >1 year of abstinence. Although there were no changes in absolute regional glucose metabolism, relative metabolism (regional brain values normalized to global values) significantly increased with prolonged abstinence in the thalamus (+12%), but not in the striatum or occipital cortex. When compared with neuropsychological performance also measured at both time points, the change in thalamic metabolism was positively correlated with improvement on tests of timed gait, processing speed, and delayed recall (but not immediate recall or grooved pegboard). However, no procedures were used to control for practice effects on retesting.
In another study of cerebral glucose metabolism using [18F]fluorodeoxyglucose and PET, 10 MA-dependent participants were assessed after 5–9 days of abstinence, and again after 4 weeks of sustained abstinence (Berman et al, 2007). Twelve healthy control subjects were tested at comparable times. Compared with the control subjects, the MA participants had significantly greater increases in global glucose metabolism across testing sessions, with the largest increases in the bilateral parietal lobes, orbitofrontal cortex, insula, and cingulate gyrus. The authors also administered a test of auditory vigilance during both timeframes. The MA participants tended to have slower reaction times after a month of abstinence, while the control subjects were faster at retest. However, the group by reaction time interaction was not significant (p=0.53). When reaction time was related to cerebral metabolic rate for glucose (CMRglc), reaction time slowing for MA subjects was significantly correlated with increased CMRglc in the left and right parietal lobes. In contrast, there was no relationship between reaction time and CMRglc in the control subjects.
In another study from the same laboratory, cerebral gray-matter volume of 12 MA-dependent participants was measured when they were abstinent for 4–7 days, and again after1 month of sustained abstinence (Morales et al, 2012). Twelve control subjects were also assessed at similar timeframes. Compared with the control participants, the MA participants showed significant increases in gray matter in the temporal gyrus, right angular gyrus, right insula, left precuneus, left inferior frontal gyrus, and left occipital pole (with decreased gray matter in the cerebellum). At the same statistical threshold, the control subjects did not show any significant changes in gray matter with retesting (see Figure 1). Repeat neuropsychological testing was not available for these participants.
Figure 1.
Changes in gray matter for methamphetamine (MA)-dependent individuals after 1 month of abstinence compared to control subjects re-tested after an 1 month interval. (a) Control subjects did not show any significant changes in gray matter with retesting. (b) MA-dependent participants show increases in gray matter with 1 month abstinence. Note: N=12 per group; statistical threshold: p<0.001 uncorrected, cluster extent >100 voxels.
Conclusion
Longitudinal studies of abstinence show convincing changes in the neurochemical markers (ie, DAT, glucose metabolism) and gray-matter structure of MA-dependent subjects compared with control subjects who were tested at similar intervals. Although three of four studies that included cognitive data found relationships between changes in the brain and changes in cognitive performance, it is unclear whether the changes in cognitive performance reflect practice effects from repeated testing, longitudinal changes in cognitive function, or both. However, these findings do suggest that changes in the brain during abstinence may be linked with individual differences in cognition.
COGNITIVE DEFICITS IN MA-ABUSING INDIVIDUALS WILL BE DOSE-RELATED
If MA abuse causes cognitive decline in humans, individuals who are exposed to higher amounts of MA might exhibit greater cognitive deficits than those exposed to lower amounts of MA. Although an imprecise measure of cumulative dose, several studies have obtained self-reported duration of MA use (in years, months or days) as a proxy for cumulative MA exposure. One study found that years of MA use was associated with worse performance on the Stroop Task in MA-dependent adults (Salo et al, 2009). However, the vast majority of studies correlating duration of MA use with cognitive performance in MA-abusing participants (Chang et al, 2002; Henry et al, 2010; Hoffman et al, 2006; Iudicello et al, 2011; Johanson et al, 2006; Monterosso et al, 2005; Salo et al, 2005, 2011; Simon et al, 2000; Woods et al, 2005), including a meta-analysis which included MA-abusing participants from 17 different studies (Scott et al, 2007), found nonsignificant results. In addition, in the study of twins who had used stimulants weekly (Toomey et al, 2003), better performance on a few tests of dexterity and memory was correlated with greater total days of stimulant use.
Some studies have used self-reported frequency of MA use as an estimate of MA exposure. Simon et al (2000) found that MA abusers using more frequently (both in terms of days per week and times per day) performed worse than those using less frequently on tests of memory, abstract reasoning, and executive functioning. Another study (Henry et al, 2010) found that a functional measure of financial abilities was negatively related to the number of times MA-dependent participants used per month. However, in this same study, the six other functional abilities measured were unrelated to frequency of use. Likewise, Rippeth et al (2004) found no relationship between global cognition and daily vs less than daily MA use, and Price et al (2011) found that recent frequency of MA use was associated with better performance on the Grooved Pegboard Test by male MA-dependent individuals, but worse performance by females. Similar mixed findings have been observed for measures of addiction severity, with one study finding that increased severity of amphetamine addiction was associated with worse memory and attention (McKetin and Mattick, 1998), while another study found no relationship between cognition and addiction severity (Hoffman et al, 2006).
Perhaps the best measure of exposure to MA consists of reports of the total dosage consumed, either recently or estimated across the lifespan. Two studies found that the amount of recent MA use (grams per day or per week) was associated with worse motor response inhibition (Monterosso et al, 2005) and nonverbal reasoning (King et al, 2010) in MA-dependent or -abusing individuals (King and colleagues also found a negative relationship between executive functioning and ‘lifetime METH use', but it is unclear how this was measured). In contrast, several other studies have not found a relationship between multiple cognitive measures and recent amount of MA consumed (Hoffman et al, 2006; Rippeth et al, 2004) or estimates of total lifetime quantity consumed (Chang et al, 2002; Cherner et al, 2010a; Henry et al, 2010; Iudicello et al, 2011; Woods et al, 2005).
All of the aforementioned studies evaluated relationships between parameters of MA use (often several parameters simultaneously) and cognitive function in post-hoc analyses, following up primary analyses that typically compared MA-abusing to healthy control subjects. To our knowledge, only one study has examined the relationship between parameters of MA use and cognitive function in a primary analysis. In that study (Cherner et al, 2010b), parameters of MA use for MA-dependent participants who were cognitively impaired (at least mild impairment in two or more cognitive domains based on demographically adjusted norms) were compared with those of MA-dependent participants who were not impaired on a comprehensive cognitive battery. The results revealed that the cognitively impaired and non-impaired groups did not differ on any index of MA use, including years of use, lifetime grams consumed, average grams used per year, length of abstinence, method of administration, bingeing pattern, or age of initiation.
Conclusion
The vast majority of research has not found a relationship between cognitive performance and duration of MA use. Findings from studies utilizing potentially more accurate measures of MA administration such as frequency of use or amount of recent use have been mixed, with the majority of studies not finding a relationship between cognition and estimates of cumulative lifetime dose. In addition, most of the statistically significant relationships cited previously were found in post-hoc analyses that analyzed the relationship between multiple MA use parameters and multiple cognitive tests, without consideration of Type I error rate or confounding variables. As such, the available evidence for a linear relationship between self-reported MA usage and cognitive performance is weak.
POTENTIAL MODERATORS OF THE RELATIONSHIP BETWEEN COGNITION AND MA ABUSE
Given that MA exposure does not appear to be linearly related to cognitive function in humans, but animal studies and other sources of data do suggest that MA causes cognitive decline in some individuals, authors have suggested that other factors may moderate the relationship between MA exposure and cognitive decline (Cherner et al, 2010b; Dean and London, 2010). Moderating variables may also help to explain variability in the cognitive performance of MA-dependent participants. For example, although some MA-dependent participants show significant impairment relative to demographic normative data, others perform well within expected levels (Dean and London, 2010). Likewise, animal studies show that not all animals exposed to MA decline in cognitive function, or decline to the same degree (Clark et al, 2007; Daberkow et al, 2005; Groman et al, 2012). The susceptibility to the development of cognitive decline may thus depend on moderating variables.
Age
It is well known in the neuropsychological literature that similar neurological insults do not confer the same degree of cognitive decline in all individuals. In particular, the age at which the insult occurs can significantly affect the clinical outcome. Research suggests that, with other factors controlled, older individuals generally have a worse outcome from various types of neurological injury than younger individuals (Hukkelhoven et al, 2003; Lanzino et al, 1996; Luerssen et al, 1988; Weimar et al, 2004). Such findings have supported the hypothesis that younger individuals have greater brain reserve and neural plasticity to withstand neurological injury than older adults (for review, see Satz (1993)).
Studies in drug abuse have likewise suggested that the aging brain is less able to compensate for repeated drug exposure than the younger brain (see Dowling et al (2008)). For example, research suggests that alcohol abuse may have greater neurotoxic and cognitive effects in older individuals than younger individuals, particularly after the age of 50 (Rourke and Grant, 1999; Schottenbauer et al, 2007). Likewise, in animals, MA exposure has been shown to have a greater effect on markers of neuronal damage (Teuchert-Noodt and Dawirs, 1991) and extracellular dopamine levels (Bowyer et al, 1993) in older animals than younger animals. In humans, relatively little research has examined the interaction between age and MA abuse. The meta-analysis by Scott et al (2007) found that the effect size differences in cognitive performance between MA participants and control participants increased with increasing age, but it is unclear whether this effect was due to age effects alone, or interactions between age and MA use. However, these authors also noted that most of the participants in cognitive studies of MA abuse have been relatively young, with a mean age in the 30s or younger. To the extent that deficits in cognitive function become more pronounced with increasing age, the current literature may thus underestimate the lifetime prevalence of cognitive deficits in MA-abusing individuals.
Education
Individuals with higher levels of education tend to have a better cognitive outcome in the context of a neurological injury than those with lower levels of education (Barnett et al, 2006; Elkins et al, 2006; Jones et al, 2006; Kesler et al, 2003; McDowell et al, 2007; Stern, 2002). Although brain reserve related to educational level has also been implicated as a protective factor in the development of cognitive deficits in substance use (Fein and Di Sclafani, 2004), to our knowledge, no research has examined the interaction between educational level and cognitive deficits in MA abuse.
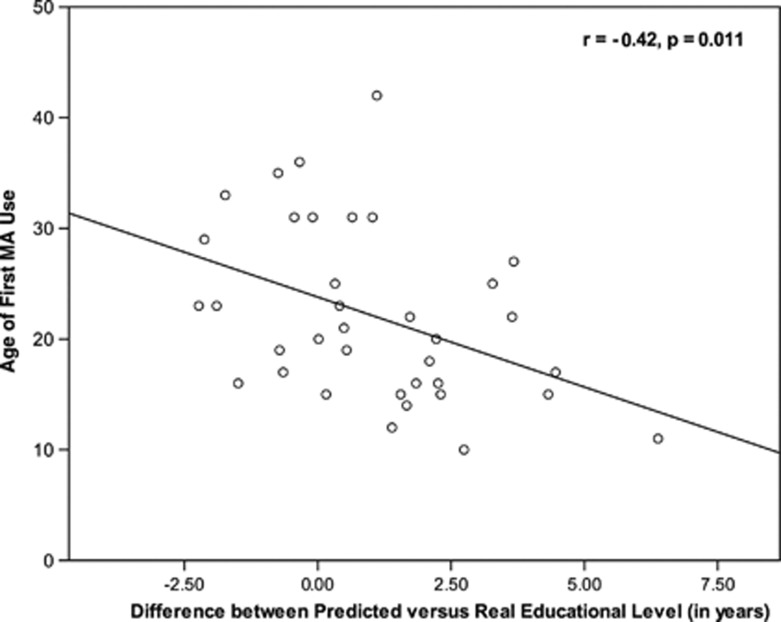
Unlike the educational level attained by adults who suffer a neurological injury later in life, MA abuse typically begins during adolescence or young adulthood, at an age in which it is common for individuals to be in school. Thus, MA abuse and exposure to schooling may not be independent phenomena. Dean et al (2012) investigated the possibility that MA usage in young adulthood interferes with the amount of education one receives. These authors hypothesized the MA use in early adulthood may interfere with educational attainment, such that some MA-dependent individuals may have the requisite cognitive abilities to go further in school, but because of MA use (and/or its psychosocial correlates), this potential is cut short. Data supported this hypothesis: not only was age of first MA use negatively related to years of education attained (r=−0.48, p<0.01), but also when the cognitive battery scores of MA-dependent participants were placed into a normative model of educational attainment, the MA participants had significantly fewer years of education than predicted by their cognitive scores. In addition, the discrepancy between actual and predicted educational levels of the MA participants was negatively correlated with the age of first MA use (r=−0.42, p=0.01; see Figure 2), indicating that those who began to use MA at an earlier age had larger discrepancy between their real and predicted educational level. Importantly, these data suggest that the years of education attained by MA-dependent individuals tends to underestimate overall cognitive function. To the extent that this phenomenon generalizes to other MA users, this suggests that studies that match MA participants and healthy control participants for educational level may actually underestimate the degree of cognitive deficit present (see Dean et al (2012) for recommendations regarding this issue).
Figure 2.
Relationship between age of first use of methamphetamine (MA) and the difference between predicted vs actual years of education. Note: N=36 MA-dependent participants. Pearson correlation. Predicted education was predicted from cognitive battery scores and demographic characteristics (age, gender, ethnicity), using a regression model developed in healthy comparison subjects (N=42). Positive difference scores indicate that predicted education was greater than actual years of education, whereas negative difference scores indicate that predicted education was less than actual years of education. Results show that larger (positive) difference scores were associated with a younger onset of MA use. Reprinted from Dean et al (2012) with permission from Elsevier.
Genetics
As genetic makeup contributes significantly to cognitive function (Bouchard, 1998; Winterer and Goldman, 2003), some of the variation in the cognitive abilities of MA-abusing individuals reflects natural variation in the human genome. However, emerging data also suggest that genetic variability may moderate the effect of MA abuse on cognitive function.
Cherner et al (2010a) hypothesized that genetic variability in the metabolism of MA influences neurotoxicity and cognitive function in MA abusers. These authors tested MA-dependent participants for common functional variants of a gene that codes for cytochrome P450, family 2, subfamily D, polypeptide 6 (CYP2D6), which catalyzes hydroxylation and demethylation of MA. MA-dependent participants with two functional alleles coding for the enzyme (extensive metabolizers of MA) had significantly worse performance than MA-dependent participants with partially functional or nonfunctional alleles (intermediate and poor metabolizers of MA, respectively) in multiple cognitive domains. As extensive metabolizers had worse cognition than the other groups, the authors theorized that the metabolic byproducts of MA may be more neurotoxic than the parent compound itself.
DISCUSSION
As chronic MA abuse cannot be manipulated experimentally in humans, the nature of the evidence regarding the causal relationship between MA abuse and cognition in humans is inherently indirect. In addition, the ability of the literature to elucidate the nature of the relationship between MA abuse and cognitive function is inevitably constrained by the quality and availability of the current state of the literature. In this respect, when evaluating the evidence related to our a priori hypotheses, we note that relatively few studies were available to draw conclusions for some of our hypotheses. Within the studies that were available, limitations were common and in part reflect natural weaknesses of quasi-experimental designs, which rely on the self-report and behavior of individuals with diverse life histories. Given these limitations, we believe that the evaluation of whether MA abuse causes cognitive decline in humans should be based on the preponderance of all the available evidence, rather than reference to one or more individual studies which do, or do not, support a particular viewpoint.
On the basis of the aforementioned perspective, we believe that the preponderance of the data suggests that MA abuse does cause mild cognitive decline in at least some individuals. We base this conclusion on the analysis of the evidence available for our six a priori hypotheses, which we summarize below:
(1) MA abuse will cause cognitive decline in animals, particularly when administered dosing regimens similar to human consumption. This hypothesis was supported. Chronic administration of doses of MA that are likely within the range of human consumption produce decline on various cognitive measures in animals. Although the use of escalating doses of MA can sometimes prevent cognitive decline otherwise observed by single moderate to high doses of MA, both experimenter and self-administered escalating dosing regimens have produced cognitive decline in rats and monkeys. As animal studies typically utilize experimental designs that can support causal inferences, evidence from these studies should likely be weighed more heavily than other forms of evidence in the overall evaluation of MA-induced cognitive decline.
(2) Individuals who abuse MA will have lower cognitive scores than well-matched individuals who do not abuse MA. This hypothesis was supported. Despite a few exceptions, the majority of studies that minimally controlled for age, gender, education, and premorbid IQ found that MA-dependent participants performed worse on at least some of the cognitive tests administered when compared with healthy participants who did not use drugs. However, because it is impossible to control fully for premorbid function and all possible confounds in cross-sectional studies, support for this hypothesis alone provides limited evidence regarding the causal relationship between MA abuse and cognitive function.
(3) Twins who abuse MA will have lower cognitive performance than their twin pairs who do not abuse MA. This hypothesis received mild support. In the only study available (Toomey et al, 2003), twins with a history of using stimulants on a weekly basis performed worse on four cognitive tests than their twin pairs who did not use stimulants (out of 14 tests), although this pattern was reversed on one test of attentional vigilance (stimulant users performed better than non-users on this test). However, it is unclear whether the weekly stimulant use endorsed in this study constitutes either dependence or abuse of stimulants based on psychiatric diagnostic criteria.
(4) Humans who abuse MA will show improvements in cognition with sustained abstinence. This hypothesis received mild support. Two of three cross-sectional studies suggested that MA-dependent participants who are abstinent for longer periods of time perform better on cognitive tests than those who are abstinent for shorter periods of time. However, the only relevant longitudinal study with a strong research design that examined 1 year of abstinence (Iudicello et al, 2010) found that only a small subset of MA-dependent individuals improve with abstinence. As a whole, the data suggest that some MA-dependent individuals do have a suppression of cognition that improves with abstinence. In addition, to the extent that decline from MA abuse is irreversible, evidence regarding this hypothesis cannot clarify whether MA abuse causes cognitive decline or not.
(5) MA abuse will be associated with changes in the human brain. This hypothesis was supported. Longitudinal studies show convincing changes in the neurochemical function (ie, DAT, glucose metabolism) and gray-matter structure of MA-dependent subjects during abstinence. These changes in the brain during abstinence are highly likely to be related to the brain alterations that occur as a direct result of MA use (eg, reflecting a compensatory reaction or recovery). Changes in the brain during abstinence have also been shown to be related to individual differences in cognitive function. Although it is unclear whether these changes in the brain during abstinence result in true cognitive improvements over time, the demonstration that the brain changes from MA abuse increases the odds that MA abuse also alters cognitive function to some degree.
(6) Cognitive deficits in MA-abusing individuals will be dose-related. Most data did not support this hypothesis. The majority of studies examining the relationship between cognitive function and duration of MA use, frequency of MA use, and/or total cumulative dose have not found significant relationships. Although some exceptions exist that have found MA usage parameters to be related to cognitive function, these findings have typically been exhibited in post-hoc analyses that examined the relationships between multiple MA use parameters and multiple cognitive tests, without consideration of Type I error rate or confounding factors.
Overall, evidence from five of our six a priori hypotheses received at least some degree of support for the notion that MA abuse causes cognitive decline. Animal studies, cross-sectional human studies, and brain studies provided fairly consistent indirect evidence for MA-induced cognitive decline. Studies of twins and cognition during abstinence provided weaker but supportive evidence, whereas studies of the dose–response relationship between MA consumption and cognition were generally not supportive. On the whole, the data support the perspective that, across individuals at the age of early-to-middle adulthood, MA abuse causes mild declines in cognitive function. These declines are likely to be observable in some, but not all, individuals who abuse MA. In addition, the extent of cognitive decline exhibited by a given individual is likely to vary depending on moderating variables such as age, educational level, and genotype.
The only hypothesis examined that received little empirical support was that relating the severity of cognitive deficits in humans to self-reported extent of MA use. It is possible that self-reported use history is too inaccurate to adequately quantify an individual's lifetime exposure to MA. In addition, given the consistent animal literature documenting the deleterious effects of binge-like dosing regimens, cumulative use may not be the best estimate of neurotoxic exposure to MA. Rather, it is possible that binge episodes differentially contribute to cognitive decline, perhaps particularly in individuals who have not yet developed considerable tolerance to the drug (Segal et al, 2003). Cherner et al (2010b) did not find a relationship between cognitive function and binge patterns in MA-dependent humans, but to our knowledge, this is the only study to have examined this issue.
In addition to challenges associated with self-reported cumulative MA use, it seems likely that MA-dependent individuals vary in their susceptibility to MA-induced cognitive decline. With other factors held constant, the literature on cognitive reserve suggests that older adults and those with less education are more susceptible to drug-induced decline than younger, more highly educated adults (Dowling et al, 2008; Satz, 1993). In addition, to the extent that drug use interferes with educational exposure, cognitive development and MA use may not be mutually exclusive phenomena (Dean et al, 2012). If cognitive development and MA use are interrelated, this may make it difficult to disentangle cognitive decline caused by MA abuse from a failure to develop particular cognitive skills (see Dean et al (2012)). Finally, if one considers that the average effect of MA abuse on cognition is relatively mild in middle adulthood (see below), it is perhaps not surprising that simple bivariate relationships between cognition and self-reported MA use would be difficult to detect in comparison to the large individual differences in cognition associated with variables such as genetic makeup (Bouchard, 1998; Winterer and Goldman, 2003), age, and educational attainment (Heaton et al, 1996). All of these factors suggest that simple linear models of MA abuse and cognition are unlikely to capture the dynamic interplay between MA abuse and a developing, adaptable organism.
As the evidence for MA-induced cognitive decline is at times ambiguous with a lack of unanimity, it may be tempting to conclude the MA abuse does not cause cognitive decline in humans. However, for this to be the case, the following conditions should be true: (a) human-like dosing of MA that causes cognitive decline in rats and monkeys does not have any cognitive effect in humans; (b) lowered cognitive scores of MA-abusing participants are entirely premorbid, despite efforts to account for premorbid function through group matching; (c) twin data on cognitive deficits in stimulant users are spurious; (d) improvements shown in cognition with abstinence are unrelated to MA use; and (e) changes in the brain likely caused by MA abuse are unrelated to cognition. Although it is possible that all of these conditions are true, we find it more reasonable to expect that MA abuse does cause at least mild cognitive decline in some people.
The notion that, on average, MA abuse causes mild rather than severe decline in cognitive function is supported by several lines of evidence (at the age of early-to-middle adulthood). First, in both animals and humans, not all studies which compared MA-exposed subjects to control subjects found cognitive deficits in the MA group (Chang et al, 2005; Clark et al, 2007; Grace et al, 2010; King et al, 2010; Leland et al, 2008; Simoes et al, 2007; Simon et al, 2010). We would suspect such null studies to be rare if MA caused severe cognitive decline. In addition, of those studies in which MA-dependent humans performed worse than control subjects, it is not uncommon for the groups to differ in one or less SD's of performance (based on data reported in the studies). According to some neuropsychological naming conventions (eg, Mitrushina et al, 2005), this would suggest that the mean performance of MA-dependent subjects falls in the low average to average range. This is consistent with studies that have referenced the cognitive performance of MA-dependent participants to published normative data (Gonzalez et al, 2004; Rippeth et al, 2004; Simon et al, 2010). Nonetheless, although MA dependence may be associated with mild cognitive decline across individuals, some susceptible individuals may exhibit considerable cognitive impairment relative to their demographically matched peers (eg, see Kalechstein et al (2003)).
As the normed cognitive performance of MA-dependent individuals frequently places outside the range of impairment according to some neuropsychological naming conventions, Hart et al (2011) concluded that the cognitive deficits of MA-dependent individuals are unlikely to be associated with functional implications. That is, because the deficits tend to be relatively mild, they are unlikely to have real-world implications. We do not share this categorical conceptualization of cognitive function. Rather, we suspect that mild deficits noted on cognitive tests likely relate to mild deficits in the cognitive construct being measured. Whether a specific cognitive deficit relates to ‘real-world' impairment is likely to be complex and dependent on a number of factors, such as the social and environmental context in which the cognitive ability is needed, and the manner in which ‘real-world' function is operationalized (eg, a mild deficit in short-term memory may affect memory for casual conversations, but have no observable relationship with household tasks or basic activities of daily living). Further, it should be noted that scores within the same normative range for those with different demographic characteristics are not necessarily equivalent in terms of the functional ability being measured. For example, low average performance for individuals with a high school degree likely relates to much lower functional ability than low average performance for individuals who graduated college (of which, MA-dependent individuals typically have lower educational attainment than their peers). For this reason, evidence suggests that scores normed to the average performance of healthy adults, without consideration of demographic characteristics (ie, ‘absolute' scores), have a tighter relationship with everyday functioning than scores that are normed to the specific demographic characteristics of the individual (Silverberg and Millis, 2009).
Certainly, functional consequences are more likely the more that cognitive scores deviate from (absolute) normative expectations, and, as such, MA-dependent individuals with mild cognitive deficits may not have difficulties in some or all measures of everyday function. However, initial studies suggest that, despite the mild (mean) deficits described, MA-dependent individuals do have lower scores on performance measures of everyday functional ability than demographically similar control subjects (Henry et al, 2010). Similarly, MA-dependent participants with mild or greater deficits across cognitive tests are more likely to be unemployed than their cognitively intact peers (Weber et al, 2012). Given the limited data available and the complexity of the relationship between cognitive test scores and everyday function, more research is needed to ascertain the impact of cognitive performance deficits on real-world function in this population (for more information, see Marcotte and Grant, (2010)).
Conclusion
Although some findings suggest that MA abuse does not cause cognitive decline in humans, the preponderance of the evidence suggests that MA abuse does cause cognitive decline in at least some individuals. When averaged across individuals, this decline appears to be mild in early-to-middle adulthood, but moderator variables likely have a role in attenuating or exacerbating the degree of decline exhibited by a given individual. The ultimate validity of our current conclusions will depend on replications and improved future research that hopefully will provide greater leverage to address the causal relationship between MA abuse and cognitive function.
Acknowledgments
This research was supported by NIH grants K23 DA927734 (ACD), DA 022539 (EDL), DA 020726 (EDL), DA 15179 (EDL), T32 DA024635 (EDL), F31 DA028812 (SMG), and endowments from the Thomas P and Katherine K Pike Chair in Addiction Studies and the Marjorie M Greene Trust.
Dr London receives funding from Phillip Morris, USA for UCLA's Adolescent Smoking Cessation Center. The remaining authors declare no conflict of interest.
References
- Acevedo SF, de Esch IJ, Raber J. Sex- and histamine-dependent long-term cognitive effects of methamphetamine exposure. Neuropsychopharmacology. 2007;32:665–672. doi: 10.1038/sj.npp.1301091. [DOI] [PubMed] [Google Scholar]
- Anglin MD, Kalechstein AD, Maglione M, Annon J, Fiorentine R. Methamphetmaine Abuse and Treatment in California: A Regional Report. University of California: Los Angeles; 1997. [Google Scholar]
- Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Kamei H, et al. GABAB receptor agonist baclofen improves methamphetamine-induced cognitive deficit in mice. Eur J Pharmacol. 2009;602:101–104. doi: 10.1016/j.ejphar.2008.10.065. [DOI] [PubMed] [Google Scholar]
- Baker A, Lee NK, Claire M, Lewin TJ, Grant T, Pohlman S, et al. Brief cognitive behavioural interventions for regular amphetamine users: a step in the right direction. Addiction. 2005;100:367–378. doi: 10.1111/j.1360-0443.2005.01002.x. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Salmond CH, Jones PB, Sahakian BJ. Cognitive reserve in neuropsychiatry. Psychol Med. 2006;36:1053–1064. doi: 10.1017/S0033291706007501. [DOI] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA, Lang JM. Practice effects on commonly used measures of executive function across twelve months. Clin Neuropsychol. 1999;13:283–292. doi: 10.1076/clin.13.3.283.1743. [DOI] [PubMed] [Google Scholar]
- Beglinger LJ, Gaydos B, Tangphao-Daniels O, Duff K, Kareken DA, Crawford J, et al. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol. 2005;20:517–529. doi: 10.1016/j.acn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O'Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O'Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- Berman S, O'Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, et al. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2007;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TJ. Genetic and environmental influences on adult intelligence and special mental abilities. Hum Biol. 1998;70:257–279. [PubMed] [Google Scholar]
- Bowyer JF, Gough B, Slikker W, Lipe GW, Newport GD, Holson RR. Effects of a cold environment or age on methamphetamine-induced dopamine release in the caudate putamen of female rats. Pharmacol Biochem Behav. 1993;44:87–98. doi: 10.1016/0091-3057(93)90284-z. [DOI] [PubMed] [Google Scholar]
- Buffum JC, Shulgin AT. Overdose of 2.3 grams of intravenous methamphetamine: case, analysis and patient perspective. J Psychoactive Drugs. 2001;33:409–412. doi: 10.1080/02791072.2001.10399926. [DOI] [PubMed] [Google Scholar]
- Caldwell J, Dring LG, Williams RT. Metabolism of [14 C]methamphetamine in man, the guinea pig, and the rat. Biochem J. 1972;129:11–22. doi: 10.1042/bj1290011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102 (Suppl 1:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, et al. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res: Neuroimaging. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Cherner M, Bousman C, Everall I, Barron D, Letendre S, Vaida F, et al. Cytochrome P450-2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: preliminary findings. J Int Neuropsychol Soc. 2010a;16:890–901. doi: 10.1017/S1355617710000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T, et al. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug Alcohol Depend. 2010b;106:154–163. doi: 10.1016/j.drugalcdep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Huang WS, Su TP, Lu RB, Wan FJ, Fu YK. Dopamine transporters and cognitive function in methamphetamine abuser after a short abstinence: a SPECT study. Eur Neuropsychopharmacol. 2007;17:46–52. doi: 10.1016/j.euroneuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Clark RE, Kuczenski R, Segal DS. Escalating dose, multiple binge methamphetamine regimen does not impair recognition memory in rats. Synapse. 2007;61:515–522. doi: 10.1002/syn.20397. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Pena Y, Bruce CC, Huszar AC, et al. Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology. 2007;32:1195–1206. doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- Dean AC, Hellemann G, Sugar CA, London ED. Educational attainment is not a good proxy for cognitive function in methamphetamine dependence. Drug Alcohol Depend. 2012;123:249–254. doi: 10.1016/j.drugalcdep.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, London ED.2010Cerebral deficits associated with impaired cognition and regulation of emotion in methamphetamine abuseIn: Kassel JD (ed)Substance Abuse and Emotion American Psychological Association: Washington, D.C.237–257. [Google Scholar]
- Dikmen SS, Heaton RK, Grant I, Temkin NR. Test-retest reliability and practice effects of expanded Halstead-Reitan Neuropsychological Test Battery. J Int Neuropsychol Soc. 1999;5:346–356. [PubMed] [Google Scholar]
- Dowling GJ, Weiss SR, Condon TP. Drugs of abuse and the aging brain. Neuropsychopharmacology. 2008;33:209–218. doi: 10.1038/sj.npp.1301412. [DOI] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Subst Abus. 2008;29:31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins JS, Longstreth WT, Manolio TA, Newman AB, Bhadelia RA, Johnston SC. Education and the cognitive decline associated with MRI-defined brain infarct. Neurology. 2006;67:435–440. doi: 10.1212/01.wnl.0000228246.89109.98. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V. Cerebral reserve capacity: implications for alcohol and drug abuse. Alcohol. 2004;32:63–67. doi: 10.1016/j.alcohol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-IP, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute: New York, NY; 1996. [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, et al. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug Alcohol Depend. 2004;76:181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Graham DL, Skelton MR, Gudelsky GA, et al. Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice. Neurotoxicol Teratol. 2010;32:346–355. doi: 10.1016/j.ntt.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, et al. Dysregulation of d2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32:5843–5852. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, et al. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2008;33:1847–1855. doi: 10.1038/sj.npp.1301578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2011;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Ryan L, Grant I, Matthews CG.1996Demographic influences on neuropsychological test performanceIn: Grant I, Adams KM (eds).Neuropsychological Assessment of Neuropsychiatric Disorders2nd edn.Oxford University Press: New York; 141–163. [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35:593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Mazur M, Rendell PG. Social-cognitive difficulties in former users of methamphetamine. Br J Clin Psychol. 2009;48 (Part 3:323–327. doi: 10.1348/000712609X435742. [DOI] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse MP, Marinelli-Casey P, Gonzales R, Ang A, Rawson RA. Predicting in-treatment performance and post-treatment outcomes in methamphetamine users. Addiction. 2007;102 (Suppl 1:84–95. doi: 10.1111/j.1360-0443.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall LF, et al. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99:666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- Ito Y, Takuma K, Mizoguchi H, Nagai T, Yamada K. A novel azaindolizinone derivative ZSET1446 (spiro[imidazo[1,2-a]pyridine-3,2-indan]-2(3H)-one) improves methamphetamine-induced impairment of recognition memory in mice by activating extracellular signal-regulated kinase 1/2. J Pharmacol Exp Ther. 2007;320:819–827. doi: 10.1124/jpet.106.114108. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Weber E, Grant I, Weinborn M, Woods SP. Misremembering future intentions in methamphetamine-dependent individuals. Clin Neuropsychol. 2011;25:269–286. doi: 10.1080/13854046.2010.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Vigil O, Scott JC, Cherner M, Heaton RK, et al. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. J Clin Exp Neuropsychol. 2010;32:704–718. doi: 10.1080/13803390903512637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O'Dell SJ, et al. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe C, Bush KR, Straits-Troster K, Meredith C, Romwall L, Rosenbaum G, et al. A comparison of methamphetamine-dependent inpatients with and without childhood attention deficit hyperactivity disorder symptomatology. J Addict Dis. 2005;24:133–152. doi: 10.1300/J069v24n03_11. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Jones RN, Yang FM, Zhang Y, Kiely DK, Marcantonio ER, Inouye SK. Does educational attainment contribute to risk for delirium? A potential role for cognitive reserve. J Gerontol A Biol Sci Med Sci. 2006;61:1307–1311. doi: 10.1093/gerona/61.12.1307. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. Neurophysiology Clin. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, et al. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol Psychiatry. 2006;59:75–84. doi: 10.1016/j.biopsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: an investigation of the cognitive reserve hypothesis. Appl Neuropsychol. 2003;10:153–162. doi: 10.1207/S15324826AN1003_04. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon SY, Kim J, et al. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- King G, Alicata D, Cloak C, Chang L. Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology (Berl) 2010;212:243–249. doi: 10.1007/s00213-010-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosheleff AR, Grimes M, O'Dell SJ, Marshall JF, Izquierdo A. Work aversion and associated changes in dopamine and serotonin transporter after methamphetamine exposure in rats. Psychopharmacology (Berl) 2012;219:411–420. doi: 10.1007/s00213-011-2367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JC, Fischman VS, Littlefield DC. Amphetamine abuse. Pattern and effects of high doses taken intravenously. JAMA. 1967;201:305–309. doi: 10.1001/jama.201.5.305. [DOI] [PubMed] [Google Scholar]
- Kulig BM, Calhoun WH. Enhancement of successive discrimination reversal learning by methamphetamine. Psychopharmacologia. 1972;27:233–240. doi: 10.1007/BF00422803. [DOI] [PubMed] [Google Scholar]
- Lanzino G, Kassell NF, Germanson TP, Kongable GL, Truskowski LL, Torner JC, et al. Age and outcome after aneurysmal subarachnoid hemorrhage: why do older patients fare worse. J Neurosurg. 1996;85:410–418. doi: 10.3171/jns.1996.85.3.0410. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim HC, Lee SY, Jang CG. Methamphetamine-sensitized mice are accompanied by memory impairment and reduction of N-methyl-d-aspartate receptor ligand binding in the prefrontal cortex and hippocampus. Neuroscience. 2011;178:101–107. doi: 10.1016/j.neuroscience.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Leland DS, Arce E, Miller DA, Paulus MP. Anterior cingulate cortex and benefit of predictive cueing on response inhibition in stimulant dependent individuals. Biol Psychiatry. 2008;63:184–190. doi: 10.1016/j.biopsych.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW.(eds) (2004Neuropsychological Assessment Oxford University Press: Oxford [Google Scholar]
- Lu P, Mamiya T, Lu L, Mouri A, Niwa M, Kim HC, et al. Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav Brain Res. 2010;207:387–393. doi: 10.1016/j.bbr.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient's age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68:409–416. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- Mahoney JJ, Jackson BJ, Kalechstein AD, De la Garza R, Newton TF. Acute, low-dose methamphetamine administration improves attention/information processing speed and working memory in methamphetamine-dependent individuals displaying poorer cognitive performance at baseline. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:459–465. doi: 10.1016/j.pnpbp.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte TD, Grant I.(eds) (2010Neuropsychology of Everyday Function Guilford Press: New York [Google Scholar]
- McDowell I, Xi G, Lindsay J, Tierney M. Mapping the connections between education and dementia. J Clin Exp Neuropsychol. 2007;29:127–141. doi: 10.1080/13803390600582420. [DOI] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users: comparison with non- drug-using controls. Drug Alcohol Depend. 1998;50:181–184. doi: 10.1016/s0376-8716(98)00022-2. [DOI] [PubMed] [Google Scholar]
- McKetin R, Ross J, Kelly E, Baker A, Lee N, Lubman DI, et al. Characteristics and harms associated with injecting versus smoking methamphetamine among methamphetamine treatment entrants. Drug Alcohol Rev. 2008;27:277–285. doi: 10.1080/09595230801919486. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl) 2002;163:102–105. doi: 10.1007/s00213-002-1166-3. [DOI] [PubMed] [Google Scholar]
- Miller RG.1981Simultaneous Statistical Inference2nd edn.Springer-Verlag Inc.: New York [Google Scholar]
- Mitrushina M, Boone B, Razani J, D'Elia LF.(eds) (2005Handbook of Normative Data for Neuropsychological Assessment Oxford University Press: Oxford [Google Scholar]
- Mizoguchi H, Ibi D, Takase F, Nagai T, Kamei H, Toth E, et al. Nicotine ameliorates impairment of working memory in methamphetamine-treated rats. Behav Brain Res. 2011;220:159–163. doi: 10.1016/j.bbr.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Morales AM, Lee B, Hellemann G, O'Neill J, London ED.2012Gray matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine Drug Alcohol Depende-pub ahead of print 22 March 2012. [DOI] [PMC free article] [PubMed]
- Nagai T, Takuma K, Dohniwa M, Ibi D, Mizoguchi H, Kamei H, et al. Repeated methamphetamine treatment impairs spatial working memory in rats: reversal by clozapine but not haloperidol. Psychopharmacology (Berl) 2007;194:21–32. doi: 10.1007/s00213-007-0820-1. [DOI] [PubMed] [Google Scholar]
- Noda Y, Mouri A, Ando Y, Waki Y, Yamada SN, Yoshimi A, et al. Galantamine ameliorates the impairment of recognition memory in mice repeatedly treated with methamphetamine: involvement of allosteric potentiation of nicotinic acetylcholine receptors and dopaminergic-ERK1/2 systems. Int J Neuropsychopharmacol. 2010;13:1343–1354. doi: 10.1017/S1461145710000222. [DOI] [PubMed] [Google Scholar]
- Nurmi E, Bergman J, Eskola O, Solin O, Hinkka SM, Sonninen P, et al. Reproducibility and effect of levodopa on dopamine transporter function measurements: a [18F]CFT PET study. J Cereb Blood Flow Metab. 2000;20:1604–1609. doi: 10.1097/00004647-200011000-00010. [DOI] [PubMed] [Google Scholar]
- O'Dell SJ, Feinberg LM, Marshall JF. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behav Brain Res. 2011;216:396–401. doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960-2002. Adv Data. 2004;347:1–17. [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Dean AC, Boileau I. What matters in measuring methamphetamine-related cognitive impairments: ‘abnormality detection' versus ‘everyday import'. Neuropsychopharmacology. 2012;37:1081–1082. doi: 10.1038/npp.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KL, DeSantis SM, Simpson AN, Tolliver BK, McRae-Clark AL, Saladin ME, et al. The impact of clinical and demographic variables on cognitive performance in methamphetamine-dependent individuals in rural South Carolina. Am J Addict. 2011;20:447–455. doi: 10.1111/j.1521-0391.2011.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Condon TP. Why do we need an addiction supplement focused on methamphetamine. Addiction. 2007;102 (Suppl 1:1–4. doi: 10.1111/j.1360-0443.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl) 2009;203:609–616. doi: 10.1007/s00213-008-1408-0. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 1999;146:432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Grant I. The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: a 2-year follow-up study. J Int Neuropsychol Soc. 1999;5:234–246. doi: 10.1017/s1355617799533067. [DOI] [PubMed] [Google Scholar]
- Salo R, Fassbender C. Structural, functional and spectroscopic MRI studies of methamphetamine addiction. Curr Top Behav Neurosci. 2012;11:321–364. doi: 10.1007/7854_2011_172. [DOI] [PubMed] [Google Scholar]
- Salo R, Gabay S, Fassbender C, Henik A. Distributed attentional deficits in chronic methamphetamine abusers: evidence from the Attentional Network Task (ANT) Brain Cogn. 2011;77:446–452. doi: 10.1016/j.bandc.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Subst Abuse Treat. 2009;37:292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Moore C, Waters C, Natsuaki Y, Galloway GP, et al. A dissociation in attentional control: evidence from methamphetamine dependence. Biol Psychiatry. 2005;57:310–313. doi: 10.1016/j.biopsych.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry. 2007;61:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2008 National Survey on Drug Use and Health: National Findings. Office of Applied Studies: Rockville, MD; 2009. [Google Scholar]
- Satz P. Brain reserve and capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295. [Google Scholar]
- Schottenbauer MA, Momenan R, Kerick M, Hommer DW. Relationships among aging, IQ, and intracranial volume in alcoholics and control subjects. Neuropsychology. 2007;21:337–345. doi: 10.1037/0894-4105.21.3.337. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O'Neil ML, Melega WP, Cho AK. Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology. 2003;28:1730–1740. doi: 10.1038/sj.npp.1300247. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maisonneuve IM, Glick SD. Differences between d-methamphetamine and d-amphetamine in rats: working memory, tolerance, and extinction. Psychopharmacology (Berl) 2003;170:150–156. doi: 10.1007/s00213-003-1522-y. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JA, Craytor MJ, Raber J. Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor levels in mice. Behav Pharmacol. 2010;21:602–614. doi: 10.1097/FBP.0b013e32833e7e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg ND, Millis SR. Impairment versus deficiency in neuropsychological assessment: implications for ecological validity. J Int Neuropsychol Soc. 2009;15:94–102. doi: 10.1017/S1355617708090139. [DOI] [PubMed] [Google Scholar]
- Simoes PF, Silva AP, Pereira FC, Marques E, Grade S, Milhazes N, et al. Methamphetamine induces alterations on hippocampal NMDA and AMPA receptor subunit levels and impairs spatial working memory. Neuroscience. 2007;150:433–441. doi: 10.1016/j.neuroscience.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dacey J, Glynn S, Rawson R, Ling W. The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat. 2004;27:59–66. doi: 10.1016/j.jsat.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71:335–344. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, et al. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Sommers I, Baskin D, Baskin-Sommers A. Methamphetamine use among young adults: health and social consequences. Addict Behav. 2006;31:1469–1476. doi: 10.1016/j.addbeh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Teuchert-Noodt G, Dawirs RR. Age-related toxicity in prefrontal cortex and caudate-putamen complex of gerbils (Meriones unguiculatus) after a single dose of methamphetamine. Neuropharmacology. 1991;30:733–743. doi: 10.1016/0028-3908(91)90181-a. [DOI] [PubMed] [Google Scholar]
- Toomey R, Lyons MJ, Eisen SA, Xian H, Chantarujikapong S, Seidman LJ, et al. A twin study of the neuropsychological consequences of stimulant abuse. Arch Gen Psychiatry. 2003;60:303–310. doi: 10.1001/archpsyc.60.3.303. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) 2011 World Drug Report (United Nations Publication, Sales No. E.11.XI.10).
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Grace CE, Schaefer TL, Graham DL, Braun AA, et al. Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. Int J Dev Neurosci. 2009;27:289–298. doi: 10.1016/j.ijdevneu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Warner MH, Ernst J, Townes BD, Peel J, Preston M. Relationships between IQ and neuropsychological measures in neuropsychiatric populations: within-laboratory and cross-cultural replications using WAIS and WAIS-R. J Clin Exp Neuropsychol. 1987;9:545–562. doi: 10.1080/01688638708410768. [DOI] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Iudicello JE, Morgan EE, Grant I, Moore DJ, et al. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125:146–153. doi: 10.1016/j.drugalcdep.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35:158–162. doi: 10.1161/01.STR.0000106761.94985.8B. [DOI] [PubMed] [Google Scholar]
- Winterer G, Goldman D. Genetics of human prefrontal function. Brain Res Brain Res Rev. 2003;43:134–163. doi: 10.1016/s0165-0173(03)00205-4. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, et al. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- Zorick T, Sugar CA, Hellemann G, Shoptaw S, London ED. Poor response to sertraline in methamphetamine dependence is associated with sustained craving for methamphetamine. Drug Alcohol Depend. 2011;118:500–503. doi: 10.1016/j.drugalcdep.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]