Abstract
Genetic variants in GPR85 (SREB2: rs56080411 and rs56039557) have been associated with risk for schizophrenia. Here, we test the hypothesis that these variants impact on brain function in normal subjects, measured with functional magnetic resonance imaging (fMRI) paradigms that target regions with greatest SREB2 expression (hippocampal formation and amygdaloid complex). During a facial emotion recognition paradigm, a significant interaction of rs56080411 genotype by sex was found in the left amygdaloid complex (male risk allele carriers showed less activation than male homozygotes for the non-risk allele, while females showed the opposite pattern). During aversive encoding of an emotional memory paradigm, we found that risk allele carriers for rs56080411 had greater activation in the right inferior frontal gyrus. Trends in the same direction were present for rs56039557 in the right occipital cortex and right fusiform gyrus. During a working memory paradigm, a significant sex-by-genotype interaction was found with male risk allele carriers of rs56080411 having inefficient activation within the left dorsolateral prefrontal cortex (DLPFC), compared with same sex non-risk carriers, while females revealed an opposite pattern, despite similar levels of performance. These data suggest that risk-associated variants in SREB2 are associated with phenotypes similar to those found in patients with schizophrenia in the DLPFC and the amygdala of males, while the pattern is opposite in females. The findings in females and during the emotional memory paradigm are consistent with modulation by SREB2 of brain circuitries implicated in mood regulation and may be relevant to neuropsychiatric conditions other than schizophrenia.
Keywords: emotion, hippocampal formation, amygdala, dorsolateral prefrontal cortex, single-nucleotide polymorphisms
INTRODUCTION
G protein-coupled receptors (GPCRs) are the largest family of membrane surface receptors, essential for signal transduction in central nervous system, and a preferred therapeutic target for psychiatric and neurological disorders (Gilchrist, 2007; Kobilka and Deupi, 2007). The SREB (Super Conserved Receptor Expressed in Brain) family—SREB1 (GPR27), SREB2 (GPR85), and SREB3 (GPR173)—is a particular subfamily of the GPCRs. They share 52–63% amino-acid identity and have lower homology to other GPCR subfamilies (Matsumoto et al, 2000). The unique characteristic of the SREB family is the high conservation of the amino-acid sequence across species: for example, SREB2 has an identical primary amino-acid sequence in humans, rats, and mice, which suggests a critical function of the protein. To date, an endogenous ligand of SREB has not been identified. Nevertheless, SREB2 has been implicated in schizophrenia both because of statistical association of gene variants with risk for illness and because transgenic mice overexpressing SREB2 display behaviors that are compatible with some models of schizophrenia (Matsumoto et al, 2008).
SREB2, located on Chromosome 7q31, is an orphan GPCR of 7.5 kB that generates nine transcripts encoding for four proteins (ECgene; http://genome.ewha.ac.kr/) through alternative splicing. Our laboratory recently demonstrated that two single-nucleotide polymorphisms (SNPs) in SREB2 were nominally associated with schizophrenia risk (Matsumoto et al, 2008). Specifically, the minor alleles of SNP rs56080411 (3′ untranslated region—of the third exon) and SNP rs56039557 (second intron) were overtransmitted to affected individuals in families (Matsumoto et al, 2008). SNP rs56039557 was also associated with risk for schizophrenia in the independent National Institute of Mental Health Genetics Initiative (NIMHGI) cohort (Wood et al, 2002). Furthermore, voxel-based morphometry—a commonly used MRI method for detecting global and regional volumetric changes in the brain—revealed an association between the same risk alleles (rs56080411 and rs56039557) and reduced hippocampal volume in schizophrenia subjects but not in normal volunteers (Matsumoto et al, 2008).
More recently, a possible role of SREB2 in autism (Trikalinos et al, 2006; Voineagu et al, 2011) and possibly attention deficit hyperactivity disorder (Anney et al, 2008), Tourette's syndrome and intellectual disability (Patel et al, 2011) has emerged. Thus, it is conceivable that genetic variation in SREB2 might contribute to a spectrum of neurodevelopmental psychiatric disorders, analogous to other genetic risk factors associated with risk for schizophrenia.
Previous work in our laboratory showed that SREB2 transcripts are expressed virtually in all neurons but most abundantly in hippocampal dentate gyrus and subventricular zone of the amygdala (Matsumoto et al, 2005). This expression pattern is consistent with the behavioral profile of the SREB2 transgenic mice that show alterations in contextual fear conditioning, ie, the behavioral response to a stressful cage environment, which is mediated by amygdala and hippocampal formation (HF) in rodents. More recently, Chen et al (2012) showed that SREB2 affects adult neurogenesis and neurogenesis-dependent learning in a transgenic mouse model. Given these data, a reasonable prediction is that the function of the amygdalar–hippocampal system would be preferentially affected in individuals carrying risk-associated alleles for rs56080411 and rs56039557 as compared with non-carriers, even in normal subjects. In this paper, we use the blood oxygenation level-dependent (BOLD) signal during fMRI as an index of brain function during the execution of tasks specifically designed to engage the amygdala and HF. Predicting the directionality of this effect is not straightforward given the lack of knowledge of SREB2's endogenous ligand and function and the effect of specific alleles on SREB2 processing in human brain. Moreover, a review of the literature linking amygdalar and hippocampal BOLD to the genetics of schizophrenia reveals conflicting information (see Supplementary Material).
In contrast to work on fMRI in hippocampus and amygdala and genetic risk for schizophrenia, previous work from our group and others has shown that excessive BOLD activation in the DLPFC for a fixed level of executive cognition is related to the genetic risk architecture of schizophrenia, constituting an intermediate biologic phenotype (Blokland et al, 2008; Callicott et al, 2003a). This phenomenon is presumed to reflect basic molecular aspects of cortical microcircuit ‘tuning' or signal to noise, and has been referred to as ‘cortical inefficiency' (Callicott et al, 2003b). Thus, cortical inefficiency might be expected to occur in carriers of the risk alleles for SREB2 as compared with non-carriers. This hypothesis also was tested with fMRI in healthy individuals performing the n-back, a working memory task that elicits dorsolateral prefrontal cortical activation (Callicott et al, 1999) and has been extensively used to characterize the intermediate phenotype of inefficient cortical information processing.
For all the tasks above, we explored sex by SREB2 genotype interactions, due to the well-recognized effects of sex on amygdala (Hamann, 2005) and hippocampal activation (Mackiewicz et al, 2006) during facial appraisal and emotional memory (Andreano and Cahill, 2009). Moreover, sex modulates the expression and possibly the frequency of psychiatric disorders, including depression and schizophrenia (Abel et al, 2010).
MATERIALS AND METHODS
Subjects
Healthy volunteers were recruited from the National Institute of Mental Health Genetic Study of Schizophrenia (NCT00001486: the Clinical Brain Disorders Branch ‘Sibling Study'), a study of neurobiological abnormalities related to genetic risk for schizophrenia (Dr Weinberger, PI). The participants included in this study had available genotypes for two SREB2 risk-associated SNPs (rs56080411 and rs56039557) and quality controlled neuroimaging data from three fMRI studies: an emotionally variable picture encoding task (PICT: 114 subjects), an emotional face matching task (FMT: 354 subjects) and a working memory task (n-back: 100 subjects). The 3 subsamples partly overlapped (45 subjects participated in all 3 studies).
Only subjects of self-described European ancestry were included in the study, to minimize the potential for population stratification artifacts. The demographic data are presented in Table 1. Subjects were screened with extensive clinical interviews and examinations, including the SCID interview for DSM-IV diagnoses. Inclusion criteria were no history of alcohol or drug abuse, no history of psychiatric (Axis I and Cluster A Axis II), neurological or medical conditions, and no current psychotropic medication. All the subjects gave written informed consent before the study, according to the guidelines of National Institute of Mental Health Institutional Review Board, who approved the study.
Table 1. Demographic and Genotypic Data for Samples Used in Imaging Analysis (PICT, FMT, and n-back).
|
FMT
|
PICT
|
n-Back
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Homozygotes for the major allele | Minor allele carriers | p | Homozygotes for the major allele | Minor allele carriers | p | Homozygotes for the major allele | Minor allele carriers | p | |
| rs56080411 (A/G) | |||||||||
| N | 249 | 105 | 77 | 37 | 64 | 29 | |||
| Sex | M/F:108/141 | M/F:54/51 | 0.16* | M/F:30/47 | M/F:24/13 | 0.016* | M/F:24/40 | M/F:15/14 | 0.258* |
| Age | 30.1±9.46 | 31.19±9.4 | 0.32 | 28.82±9.23 | 29.65±8.7 | 0.65 | 30.23±9.42 | 29.34±6.34 | 0.64 |
| IQ | 111.1±9 | 109.5±9.3 | 0.13 | 108.96±8.48 | 105.38±7.13 | 0.029 | 110.3±9.36 | 107.2±8.93 | 0.13 |
| Task accuracy | 99.6±1.3 | 99.5±1.3 | 0.70 | Ret ave: 0.89±0.15 | Ret ave: 0.91±0.09 | 0.392 | 2back: 0.86±0.15 | 2back: 0.82±0.15 | 0.302 |
| Ret neu: 0.83±0.15 | Ret neu: 0.84±0.09 | 0.62 | |||||||
| Reaction time (ms) | 1327±300 | 1358±287 | 0.37 | 1215.9±169.2 | 1253.6±163.6 | 0.263 | 2back: 452.5±208.4 | 2back: 512.3±256.4 | 0.24 |
| rs56039557 (G/C) | |||||||||
| N | 290 | 62 | 87 | 25 | 78 | 22 | |||
| Sex | M/F:134/156 | M/F:27/35 | 0.16* | M/F: 39/48 | M/F:13/12 | 0.52* | M/F:30/48 | M/F:9/13 | 1.00* |
| Age | 30.3±9.4 | 31.1±9.7 | 0.55 | 28.83±9.14 | 30.4±8.97 | 0.45 | 29.09±8.24 | 32.54±9 | 0.09 |
| IQ | 110.8±9 | 110.2±9.3 | 0.62 | 108.44±8.04 | 107.32±7.86 | 0.54 | 109.38±8.91 | 109.14±9.88 | 0.91 |
| Task accuracy | 99.6±1.2 | 99.3±1.6 | 0.03 | Ret ave: 0.89±0.12 | Ret ave: 0.9±0.19 | 0.86 | 2back: 0.86±0.15 | 2back: 0.81±0.16 | 0.204 |
| Ret neu: 0.84±0.12 | Ret neu: 0.82±0.17 | 0.483 | |||||||
| Reaction time (ms) | 1332±302 | 1361±268 | 0.48 | 1232.3±172.1 | 1220.78±151 | 0.763 | 2back: 457±218.2 | 2back: 507.4±235.1 | 0.35 |
Abbreviations: F, females; IQ, intelligence quotient; M, males; Ret ave, retrieval aversive stimuli; Ret neu, retrieval neutral stimuli.
*p-Values from χ2-test; the rest of p-values obtained with ANOVA . All values reported as mean±standard deviation.
Genotyping
Standard methods to extract DNA from white blood cells with the PUREGENE DNA purification kit (Gentra Systems; Minneapolis, MN, USA) were used. The two SNPs (rs56080411 and rs56039557) were genotyped using the Taq Man 5′-exonuclease allelic discrimination assay (Livak, 1999). Both SNPs were also checked for Hardy–Weinberg equilibrium (HWE) by using a χ2-test. The two SNPs chosen for analysis were in moderate linkage disequilibrium (r2=0.42, d'=0.87).
We also tested for significant differences in the distribution of genotypes for several other genes that have been implicated in modulating activation of the regions targeted in our fMRI analyses, including cathecol-o-methyl transferase (COMT) val158met (rs4680), brain-derived neurotrophic factor (BDNF) val66met (rs6265), and the serotonin transporter gene-linked polymorphic region (5HTTLPR) long and short promoter forms (L and S) by SREB2 genotypes to exclude chance population stratification by these other genes that are known to modulate regional neural activity during these tasks.
fMRI paradigms
FMT based on emotion identity recognition, this task, originally developed by Hariri et al (2000), has been widely used by our group and others (Hariri et al, 2002; Kienast et al, 2008) and has been shown to strongly engage the amygdala and HF. The subjects underwent two experimental conditions: emotional identity face matching and a sensorimotor task. Details of the task paradigm can be found in Supplementary Material. The subjects responded by button presses (right or left) indicating whether one of two images presented in the lower half of the screen matched to the one in the top half.
Incidental Picture Encoding Task
During the PICT fMRI paradigm the subjects visualized a set of pictures, half with negative valence and half neutral, derived from the International Affective Pictorial System—IAPS (Lang et al, 1997). This session (part of an episodic memory paradigm consisting of two runs: encoding and retrieval) was performed in a block-design manner similar to that described by Hariri et al (2003a). Briefly, four blocks of aversive and four blocks of neutral stimuli presented in counter-balanced order across subjects were displayed in each of the two runs. Further details can be found in Supplementary Material. During the stimulus presentation, the subjects were asked to decide whether the displayed image represented an indoor or outdoor scene by pressing a button. The accuracy of categorizing the scenes as ‘indoor' or ‘outdoor' and the reaction time were recorded during the scanning session.
Working Memory Task
This task has been used extensively in our group to test for genetic effects on brain function (eg, Egan et al, 2001) and was specifically designed to engage the working memory network, with emphasis on DLPFC activation. The task is a block-design paradigm that requires the subjects to recall stimuli seen on ‘N' previous screens. The version used in this study consisted of blocks of two conditions: 2 back and 0 back. During 0 back, the subjects only identified the stimulus currently presented. The stimuli were numbers (1–4) placed at fixed positions corresponding to the corners of a diamond shape and displayed for 1.8 s on a screen, in random order.
Image acquisition
The subjects were scanned with a GE Signa 3 T scanner (General Electric, Milwaukee, WI, USA) with a real-time functional imaging upgrade and equipped with a standard head coil. Echo planar imaging BOLD fMRI was acquired with 24 axial slices (4 mm thick, 1 mm gap) encompassing the whole cerebrum and the majority of the cerebellum (TR/TE=2000/30 ms, FOV=24 cm, matrix=64 × 64). The number of volumes acquired per subject was 170 during the PICT paradigm, 144 during the FMT, and 120 during the n-back task. The stimuli during the three sessions were displayed through a back-projection system and the responses were recorded through a fiber optic button-box.
fMRI data processing
fMRI data analysis was performed using statistical parametric mapping software (SPM5; http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7.2. Details of the image processing pipeline are presented in Supplementary Material. Each condition from the three fMRI studies (PICT, FMT, and ‘n-back') was modeled as a boxcar function convolved with the hemodynamic response function in SPM5 at each voxel.
The pre-processed images of each subject were entered in a first-level analysis consisting of a t-test statistics to create contrast images for task conditions relative to the control task (PICT: encoding aversive vs fixation, encoding neutral vs fixation, and encoding aversive>neutral; FMT: emotional task vs sensorimotor task; n-back: 2 back vs 0 back).
The individual contrasts were thereafter used in a between-group random-effects analysis to determine the effect of genotypes rs56080411 and rs56039557. Since the minor allele (in this case the risk-associated allele) frequency was low for both SNPs (≈0.17 for rs56080411 and ≈0.12 for rs56039557, respectively), heterozygotes and homozygotes for the risk allele were combined and compared with homozygotes for the common (non-risk) allele.
We first performed an exploratory non-hypothesis driven analysis of SREB2 effects across regions specifically activated by each of the three paradigms. For this, we explicitly masked the second-level analyses with masks corresponding to the task/condition main effect. The masks were based on the previously published studies from our laboratory and were derived from the Automated Anatomical Labeling (AAL) library implemented in WFU pick-atlas-v1.02 (Maldjian et al, 2003). Details on which AAL regions were used as masks for the main effect can be found in Supplementary Material.
Two regional prior hypotheses regarding a potential SREB2 effect on brain activation were tested with region of interest (ROI) analyses: (1) that the strongest effect would occur in amygdala and HF, ie, in regions with documented high mRNA expression during the FMT and PICT tasks; and (2) that the effect of SREB2 genotypes on risk for schizophrenia would be associated with inefficient activation in the DLPFC during the n-back task. ROIs were selected with the WFU pick-atlas-v1.02 (Maldjian et al, 2003) and comprised the HF (Hippocampus and Brodman areas 28, 35, 36), amygdala plus periamygdaloid cortex (Brodmann area 34), and DLPFC (Brodmann areas 9, 10, 45, 46). All the ROIs were dilated by 1 mm. No assumption of lateralization was made and consequently ROIs comprised both hemispheres.
The second-level analysis for all tasks was a factorial ANOVA in SPM5 with ‘genotype' and sex as ‘between-group' factors. Main effects of genotype and genotype-by-sex interactions were assessed for each SNP. All the above effects were explored by using appropriate t-contrasts. Statistical inference was based on established methods of Gaussian Random Fields Theory (Worsley et al, 1996). To correct for multiple comparisons in the imaging analyses, we considered significant all voxels surviving a threshold of p<0.05, false discovery rate (FDR) corrected (Genovese et al, 2002). This method was demonstrated to robustly control for type I (false positive) error rate in imaging genetics studies (Meyer-Lindenberg et al, 2008). In addition, we also report whether the results survived family-wise error (FWE) correction on a voxel-wise level. We also repeated the analyses in SPM8 to report whether results survived topological FDR, since this level of correction is implemented by default (Chumbley et al, 2010). Trends are reported for 0.1<FDR p-values <0.05.
RESULTS
Sample Demographics
SREB2 genotypes (rs56080411 and rs56039557) were in HWE in all the samples used for imaging analysis (Supplementary Table 1). An ANOVA indicated no significant differences in age, IQ, task performance (accuracy and reaction time) between the genotype groups (Table 1), with the following exceptions: for the PICT task, there were significantly more males among the rs56080411 risk (minor) allele carriers as compared with the homozygotes for the non-risk allele (Table 1). Also for the PICT task, risk allele carriers for rs56080411 risk (minor) allele had significantly lower IQ compared with non-risk carriers (Table 1). We tested for IQ effects and interactions with genotype in the corresponding imaging analyses. We did not find any significant main effects or interactions by genotype for IQ in the PICT task (results available upon request). Nevertheless, we added IQ as a covariate of no interest in this analysis. In the FMT task accuracy in performing the task was at ceiling, with only two possible outcomes: 96 or 100%. There was a significant difference in task performance (p=0.03) between the two genotype groups, with significantly more individuals than expected performing in the ‘low' accuracy group for rs56039557 (Table 1). Also in this case, the analysis was run using performance as a covariate.
For the n-back task, there were no significant differences in performance across genotype groups (Table 1; Supplementary Table 2). Nevertheless, age and performance were used as covariates in this analysis. There were no significant interactions of genotype by sex on the performance of any of the tasks assessed. There were no significant differences in the distribution of COMT val158met, BDNF val66met or 5HTTLPR genotypes across SREB2 genotypes.
SREB2 genotype effects on BOLD response during FMT
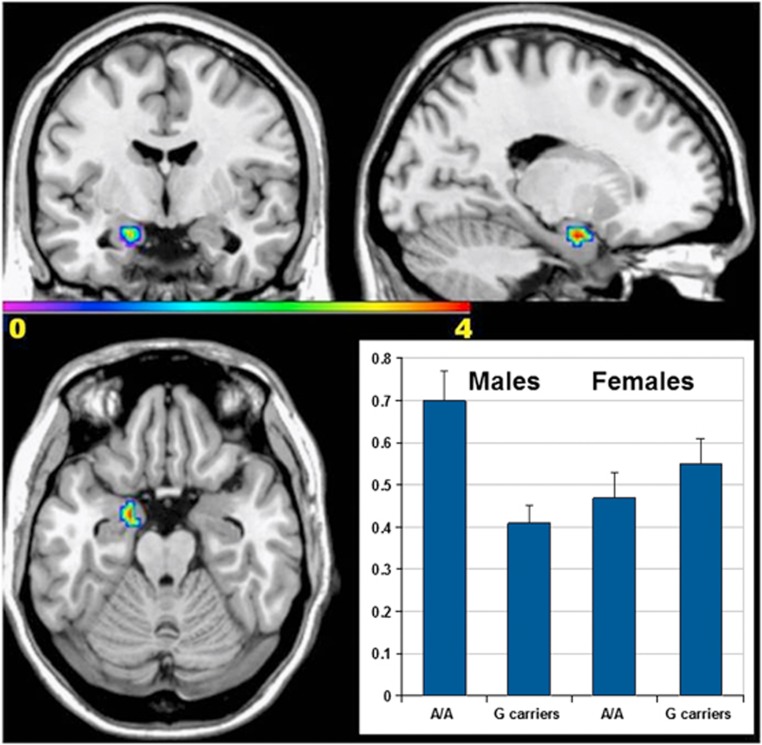
Analysis of this large sample revealed a significant interaction of genotype by sex for rs56080411 in the left amygdala-periamygdaloid cortex ROI (MNI coordinates −18 −3 −21, Z=3.54, p=0.0002, FDR and FWE corrected; Figure 1). Female carriers for the minor (risk) allele had increased activation compared with major allele homozygotes, while males had the opposite pattern. The analysis for rs56039557 did not reveal significant findings.
Figure 1.
Sex by SREB2-rs56080411 interaction effect on BOLD response during the FMT task (N=354). The three image panels are statistical parametric maps showing greater activity in left amygdaloid complex for male homozygote for the major (A, non-risk) allele vs male carriers of the risk (G) allele, while females showed the opposite effect overlaid on an MRIcro (http://www.sph.sc.edu/comd/rorden/mricro) single subject template (coronal, sagittal, and axial slices). The plot in the lower right corner shows the first eigenvariate of the β estimates for the FMT task extracted from the cluster of significance centered on MNI coordinates −18 −3 −21.
SREB2 genotype effects on BOLD response during PICT
There was a non-significant trend for the sex-by-genotype rs56080411 interaction in the left amygdaloid complex for the aversive>neutral condition (MNI coordinates −22 −11 −22, Z=2.99, p=0.001). Male homozygotes for the major allele had higher activation than male carriers of the minor (risk) allele, while females had the opposite pattern. A trend was also present for the main effect of rs56039557 in the left amygdaloid complex of the aversive>neutral contrast (MNI coordinates −19 8 −26, Z=3.05, p=0.001). Risk allele carriers had higher activation than non-risk allele homozygotes regardless of sex (Supplementary Figure 1).
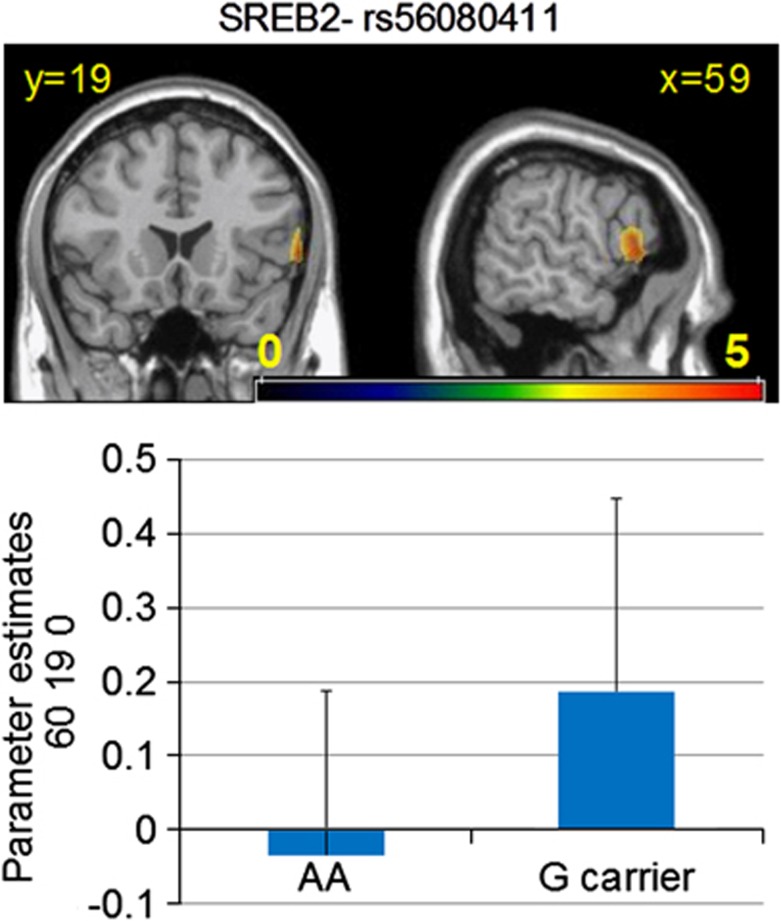
In addition, during encoding of aversive stimuli, risk allele carriers for rs56080411 had greater activation in the right inferior frontal gyrus (BA 45, MNI coordinates 60 19 0; Z-score=5.46, p=2.3 × 10−8, FWE, FDR, and topological FDR corrected; Figure 2) as compared with homozygotes for the non-risk allele. Similar but only trend level findings occurred for genotype rs56039557, where risk allele carriers showed greater activation in the right occipital cortex (BA 18, MNI coordinates 11 −79 26, Z=4.2, p=0.000013), fusiform gyrus (BA 37, MNI coordinates 41 −49 −15, Z=3.88, p=0.00005), and inferior frontal gyrus (BA 45, MNI coordinates 60 19 0, Z=3.46, p=0.00027) compared with the non-risk homozygotes (plotted in Supplementary Figure 2).
Figure 2.
SREB2-genotype effects on BOLD response during the PICT task. The top panel shows the statistical parametric maps and the bottom one the first eigenvariate of the β estimates for the aversive>fixation condition in the cluster surrounding the voxel of maximal significance (MNI coordinates 60 19 0). Risk allele carriers had higher activation compared with A homozygotes. Images were created with MRIcro (http://www.sph.sc.edu/comd/rorden/mricro). Voxel coordinates and slice locations are reported in MNI space.
SREB2 effect on BOLD response during ‘n-back task'
In this relatively large sample of normal volunteers, none of the SREB2 polymorphisms showed a genotype effect on BOLD response during 2 back vs 0 back after whole brain correction or ROI (DLPFC) analyses.
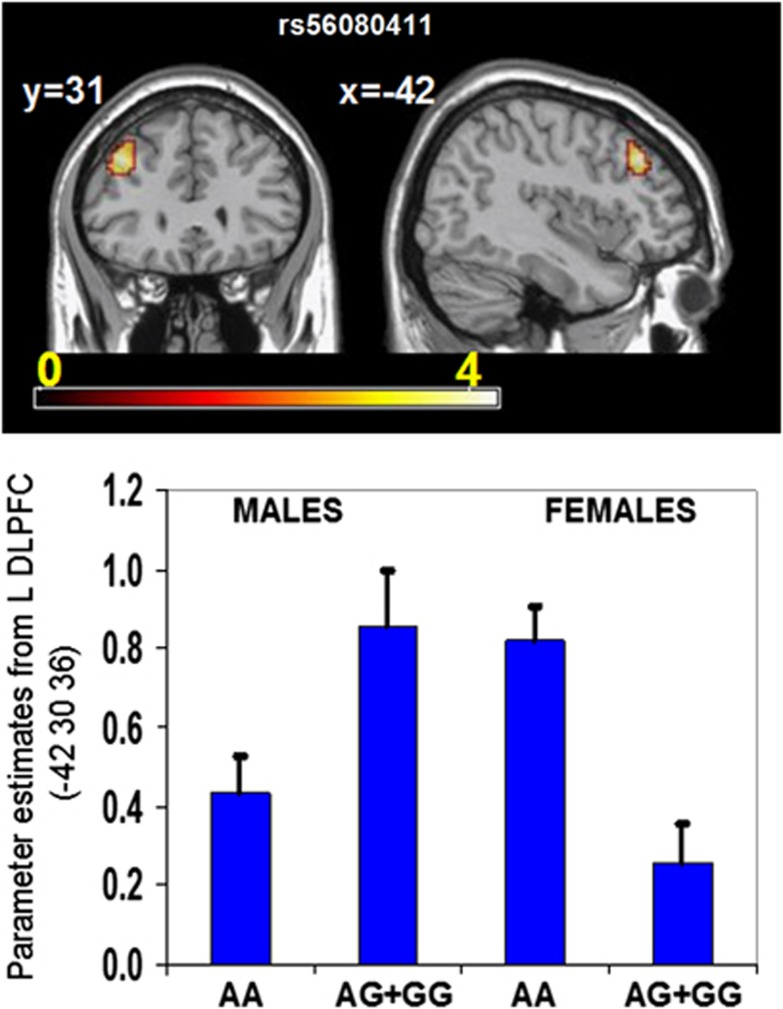
A significant genotype × sex interaction was found for rs56080411 within the left DLPFC (Talairach coordinates −42 30 36, Z-score=4.02, p=0.00003, FWE, FDR, and topological FDR corrected; Figure 3). Post hoc contrasts (Tukey HSD test) performed on the adjusted β estimates from the peak voxel above showed that male risk allele carriers had significantly higher activation compared with male non-risk carriers (p=0.014), while female risk carriers had significantly lower BOLD response compared with female non-risk allele carriers (p=0.03).
Figure 3.
Sex-by-SREB2 genotype (rs56080411) interaction in the DLPFC during the n-back task. In the L DLPFC, male carriers of the risk allele had increased BOLD activation during the n-back task as compared with non-risk homozygotes. Females displayed the opposite pattern, despite no difference in performance (% correct or reaction time) between the sex groups (Supplementary Table 2).
This result prompted us to reanalyze our clinical association data (Matsumoto et al, 2008) within subgroups of male and female participants. Because the pattern of imaging associations suggests that minor alleles have more ‘schizophrenia-like' effects (ie, prefrontal inefficiency) in normal males than in females, we predicted that the effect of minor alleles on risk for schizophrenia per se would be greater in males. A logistic regression comparing 220 male cases and 161 controls indicated that homozygotes for the minor allele of rs56080411 were significantly more likely to have schizophrenia (odds ratio (OR)=9.8, p=0.03, 95% confidence intervals (CIs)=1.25–76) than homozygotes for the major allele. The same analysis conducted in 65 female cases and 173 controls was non-significant (OR=1.54, p=0.64, 95% CI=0.26–9.1). The sex × genotype interaction showed a non-significant but provocative trend (Wald χ2=4.7, p=0.09), suggesting that the differences in effect in males and females are not simply related to sample size differences in power to detect association.
DISCUSSION
To explore the role of SREB2 on brain functioning, we used imaging genetics based on several fMRI tasks exploring hippocampal–amygdala and DLPFC function. Imaging phenotypes provide quantitative measures of the function of brain networks and are more likely to reveal genetic modulation than complex psychopathology, which implies that susceptibility genes may show measurable effects even in normal individuals within brain regions implicated in both the disease and the biology of the gene (Meyer-Lindenberg and Weinberger, 2006). Accordingly, we hypothesized that SREB2 variants will impact brain function in regions where the gene is most abundantly expressed (ie, hippocampus and amygdala; Matsumoto et al, 2005) and are particularly relevant for schizophrenia (eg, DLPFC; Weinberger et al, 2001). We tested this hypothesis in healthy volunteers by using fMRI tasks specifically designed to elicit activation in the respective regions. While our original hypothesis did not predict interactions of genotype by sex, for all tasks we found some evidence of a sex-by-genotype interaction for rs56080411. For the FMT task, the interaction between sex and genotype in the left amygdaloid complex was statistically significant (Figure 1). The directionality of the effect was consistent with the phenotype observed in patients with schizophrenia for males, ie, the risk allele was associated with reduced activation in the amygdala as compared with the homozygotes for the non-risk allele (Rasetti et al, 2009), but was the opposite in females. A similar trend for a sex-by-genotype interaction was found in the left amygdala for rs56080411 during the PICT task in the aversive>neutral contrast (Supplementary Figure 1). Whether the directionality of this effect is consistent with the schizophrenia phenotype in males is not known at the current time. Also in the n-back task, a significant interaction of rs56080411 by sex was found in the DLPFC (Figure 3), again with males carrying the risk allele showing a phenotype consistent with patients with schizophrenia (increased inefficiency) compared with homozygotes for the non-risk allele of the same sex, while females showed the opposite pattern.
The reanalysis of our clinical results, though trending toward a greater clinical risk association in males, was not conclusive, most likely due to the underrepresentation of females in our clinical sample and reduced power to detect association. However, the trend genotype-by-sex interaction raises the possibility that the sex effect is not about power alone. Nevertheless, it is worth highlighting that the reanalysis of the clinical association was based specifically on the imaging association results. In general, normal male subjects with risk-associated genotypes manifest a more ‘schizophrenia-like' pattern of prefrontal engagement during the n-back task (ie, greater inefficiency), compared with non-risk genotypes task. The opposite pattern was found in normal females. If prefrontal inefficiency is a neural mechanism of risk for schizophrenia related to SREB2 rs56080411 genotype, then it follows that the effect of this genotype on risk for clinical schizophrenia should be more apparent in males. We believe our data, in spite of the limitations of power, support this conclusion. Attempts to impute rs56080411 in other available public data sets have not been reported likely because both the SNPs reported here have not been genotyped in HapMap (http://hapmap.ncbi.nlm.nih.gov/). Further research is needed also in this area, but the hypothesis generated by the analysis of the fMRI data is that effects of SREB2 rs56080411 on amygdala reactivity to stimuli of negative valence (anxious or angry faces in the FMT and aversive scenes in the PICT) and DLPFC inefficiency and possibly on schizophrenia risk may be sex specific. It is of interest that the mouse model that supported the clinical association of these SNPs with schizophrenia was based exclusively on males (Matsumoto et al, 2008).
Evidence in favor of our original hypothesis of a main effect of genotype regardless of sex was found in the right inferior frontal gyrus during aversive encoding of the PICT task for rs56080411 (Figure 2) and at a trend level for rs56039557 in the left amygdala (Supplementary Figure 1) and in right occipital, fusiform, and inferior frontal gyrus (Supplementary Figure 2). The regions outside the HF and amygdala that were affected by genotype are well-established nodes within the circuitry underlying emotional information processing (right inferior frontal gyrus: Hariri et al, 2003b; and right fusiform gyrus-occipital cortex: Talmi et al, 2008). In all cases, emotional circuitry was more activated in the carriers of the schizophrenia risk alleles than in the non-risk homozygotes. Increased activation of the amygdala and other regions of the emotional circuitry in response to stimuli with negative valence has been observed in several patient populations with mood or anxiety disorders (eg, Beesdo et al, 2009; Nitschke et al, 2009; Sheline et al, 2001; Stein et al, 2002), but not in patients with schizophrenia, who tend to present the opposite phenotype (Aleman and Kahn, 2005). Finally, recent data from our group have shown that abnormal activation of the amygdala during the FMT is not associated with increased genetic risk for schizophrenia (Rasetti et al, 2009). Therefore, amygdala reactivity is not expected to relate to schizophrenia susceptibility genes, but could be related to the affective component of schizophrenia symptomatology, and it is possible that SREB2 regulates general characteristics of emotional processing. A recent review on candidate risk genes for schizophrenia and amygdala activation (Rasetti and Weinberger, 2011b) noted that, when an SNP–phenotype association was found, alleles associated with increased risk for affective disorders seemed to bias toward increased activation, while those associated with schizophrenia seemed to operate with reverse directionality. Our data would seem to fit in the affective disorders more than the schizophrenia pattern, although considerable genetic overlap between bipolar disorder and schizophrenia has been reported (Lichtenstein et al, 2009). We are not aware of any studies linking SREB2 to mood or anxiety disorders, with the possible exception of Palo et al (2010), but it should be kept in mind that the SNPs explored here were only very recently added to public databases and are therefore unlikely to be found on the chips commonly used for large genome-wide studies. Moreover, they are not in the Hapmap database and were therefore not imputed in prior studies. It is of interest that SREB2 is the target of several microRNAs. One of these (miR-137) has been recently associated with schizophrenia in a large genome-wide association study (Ripke et al, 2011), while another (miR-876-3p) has been found to be overexpressed in the DLPFC of patients with bipolar disorder (Miller et al, 2012). In the same report, miR-17-5p expression was found to be dysregulated both in schizophrenia and in bipolar disorder; the evidence for this miRNA binding in the SREB2 locus, however, is weaker for this miRNA (http://www.targetscan.org/; Lewis et al, 2005). Also, the recent demonstration that SREB2 is involved in hippocampal adult neurogenesis (Chen et al, 2012) may be consistent with the notion that SREB2 may not be a risk gene specific to schizophrenia alone. Moreover, there are frequent examples of genetic variation associated with multiple psychiatric phenotypes (Millar et al, 2000). Future studies in normal volunteers and clinical populations (ie, anxiety, mood disorders, and autism: see Supplementary Material) are warranted to test further the role of SREB2 in these disorders.
Although relevant for an SREB2 functional role, these results leave open the question concerning the mechanism by which SREB2 contributes to risk for schizophrenia, at least independent of sex.
The current work has the advantage of being based on a relatively large number of healthy volunteers, carefully screened for prior psychiatric disorders and of using multiple fMRI phenotypes to detect genetic effects. This approach, however, also has some limitations in that multiple testing is conducted on partly overlapping samples. The fact that the two SNPs explored are not fully independent (linkage disequilibrium r2=0.42) is also a limitation; however, repeated findings in the emotional circuitry for both SNPs in different tasks are unlikely to be due to chance alone.
In conclusion, our data show that SREB2 rs56080411 affects emotional processing in amygdala and cortical efficiency in the DLPFC in a sex-specific manner. Moreover, both SREB2 genotypes bias the responsiveness of amygdalar and right hemisphere cortical areas to aversive stimuli. These effects per se are not likely to be the mechanism of the risk association with schizophrenia, though they might relate to risk effects with other conditions, eg, autism and mood disorders. The failure to show association with DLPFC n-back-related inefficiency independent of sex also suggests that this is not the critical neural system mechanism of its genetic association with schizophrenia, at least not in females. Similar findings of schizophrenia risk-associated genes not mapping onto the simple DLPFC inefficiency phenotype have been reported (eg, ZNF804a: Esslinger et al, 2009; Rasetti et al, 2011a). It is thus conceivable that the SREB2 effect on cortical circuitry linked to risk for schizophrenia is more complex or sex dependent and will require exploration of different approaches to measuring cortical dynamics (Rasetti et al, 2011a). This is the subject of future work in our group.
Acknowledgments
This work was entirely funded by the NIMH IRP to the Weinberger Laboratory.
Mitsuyuki Matsumoto is an employee of Astellas Research Institute of America LLC, Skokie, Illinois. All other authors have no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia. Prog Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Anney RJ, Lasky-Su J, O'Dushlaine C, Kenny E, Neale BM, Mulligan A, et al. Conduct disorder and ADHD: evaluation of conduct problems as a categorical and quantitative trait in the international multicentre ADHD genetics study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1369–1378. doi: 10.1002/ajmg.b.30871. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland GA, McMahon KL, Hoffman J, Zhu G, Meredith M, Martin NG, et al. Quantifying the heritability of task-related brain activation and performance during the N-back working memory task: a twin fMRI study. Biol Psychol. 2008;79:70–79. doi: 10.1016/j.biopsycho.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003a;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003b;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Chen Q, Kogan JH, Gross AK, Zhou Y, Walton NM, Shin R, et al. 2012SREB2/GPR85, a schizophrenia risk factor, negatively regulates hippocampal adult neurogenesis and neurogenesis-dependent learning and memory Eur J Neuroscidoi: 10.1111/j.1460-9568.2012.08180.x [DOI] [PMC free article] [PubMed]
- Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. Neuroimage. 2010;49:3057–3064. doi: 10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gilchrist A. Modulating G-protein-coupled receptors: from traditional pharmacology to allosterics. Trends Pharmacol Sci. 2007;28:431–437. doi: 10.1016/j.tips.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Hamann S. Sex differences in the responses of the human amygdala. Neuroscientist. 2005;11:288–293. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003a;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003b;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Huffaker SJ, Chen J, Nicodemus KK, Sambataro F, Yang F, Mattay V, et al. A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat Med. 2009;15:509–518. doi: 10.1038/nm.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. NIMH Center for the Study of Emotion and Attention, University of Florida: Gainesville, FL; 1997. International Affective Picture System (IAPS): technical manual and affective ratings. [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc Natl Acad Sci USA. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Beltaifa S, Weickert CS, Herman MM, Hyde TM, Saunders RC, et al. A conserved mRNA expression profile of SREB2 (GPR85) in adult human, monkey, and rat forebrain. Brain Res Mol Brain Res. 2005;138:58–69. doi: 10.1016/j.molbrainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Saito T, Takasaki J, Kamohara M, Sugimoto T, Kobayashi M, et al. An evolutionarily conserved G-protein coupled receptor family, SREB, expressed in the central nervous system. Biochem Biophys Res Commun. 2000;272:576–582. doi: 10.1006/bbrc.2000.2829. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Straub RE, Marenco S, Nicodemus KK, Matsumoto S, Fujikawa A, et al. The evolutionarily conserved G protein-coupled receptor SREB2/GPR85 influences brain size, behavior, and vulnerability to schizophrenia. Proc Natl Acad Sci USA. 2008;105:6133–6138. doi: 10.1073/pnas.0710717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40:655–661. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Miller BH, Zeier Z, Xi L, Lanz TA, Deng S, Strathmann J, et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc Natl Acad Sci USA. 2012;109:3125–3130. doi: 10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palo OM, Soronen P, Silander K, Varilo T, Tuononen K, Kieseppa T, et al. Identification of susceptibility loci at 7q31 and 9p13 for bipolar disorder in an isolated population. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:723–735. doi: 10.1002/ajmg.b.31039. [DOI] [PubMed] [Google Scholar]
- Patel C, Cooper-Charles L, McMullan DJ, Walker JM, Davison V, Morton J. Translocation breakpoint at 7q31 associated with tics: further evidence for IMMP2L as a candidate gene for Tourette syndrome. Eur J Hum Genet. 2011;19:634–639. doi: 10.1038/ejhg.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti R, Mattay VS, Wiedholz LM, Kolachana BS, Hariri AR, Callicott JH, et al. Evidence that altered amygdala activity in schizophrenia is related to clinical state and not genetic risk. Am J Psychiatry. 2009;166:216–225. doi: 10.1176/appi.ajp.2008.08020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR. Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry. 2011a;68:1207–1217. doi: 10.1001/archgenpsychiatry.2011.103. [DOI] [PubMed] [Google Scholar]
- Rasetti R, Weinberger DR. Intermediate phenotypes in psychiatric disorders. Curr Opin Genet Dev. 2011b;21:340–348. doi: 10.1016/j.gde.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Talmi D, Anderson AK, Riggs L, Caplan JB, Moscovitch M. Immediate memory consequences of the effect of emotion on attention to pictures. Learn Mem. 2008;15:172–182. doi: 10.1101/lm.722908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikalinos TA, Karvouni A, Zintzaras E, Ylisaukko-oja T, Peltonen L, Jarvela I, et al. A heterogeneity-based genome search meta-analysis for autism-spectrum disorders. Mol Psychiatry. 2006;11:29–36. doi: 10.1038/sj.mp.4001750. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Wood LS, Schellin KA, Teng C.2002Single nucleotide polymorphisms diagnostic for schizophreniaWO 02086147.
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.