Abstract
Pyruvate kinase isoform M2 (PKM2) plays an important role in the growth and metabolic reprogramming of cancer cells in stress conditions. Here, we report that SAICAR (succinylaminoimidazolecarboxamide ribose-5′-phosphate, an intermediate of the de novo purine nucleotide synthesis pathway) specifically stimulates PKM2. Upon glucose starvation, cellular SAICAR concentration increases in an oscillatory manner and stimulates PKM2 activity in cancer cells. Changes in SAICAR levels in cancer cells alter cellular energy level, glucose uptake, and lactate production. The SAICAR-PKM2 interaction also promotes cancer cell survival in glucose-limited conditions. SAICAR accumulation is not observed in normal adult epithelial cells or lung fibroblasts regardless of glucose conditions. This allosteric regulation may explain how cancer cells coordinate different metabolic pathways to optimize their growth in the nutrient-limited conditions commonly observed in the tumor microenvironment.
An emerging hallmark of cancer cells is metabolic reprogramming, which includes elevated glucose uptake and oxygen-independent lactate fermentation known as the Warburg effect (1–3). This reprogramming is necessary for the growth and survival of tumors (4) in stress conditions and in xenografts. However, the molecular basis for this event and its role in cancer growth has remained unclear.
Several proteins, including PKM2, play important roles in the metabolic reprogramming and growth of cancer cells (4–6). PKM2 is one of four pyruvate kinases (PKs) found in mammals (7). It is highly expressed in fetal cells and cancer cells, whereas the other isoforms (PKM1, PKR, and PKL) are expressed in normal somatic tissues. Replacement of PKM2 in cancer cells with any other PK isoform drastically reduces cancer cell survival in stress conditions (hypoxia (4) or glucose-depletion (8)) and suppresses tumorigenicity (4). This suggests that subtle differences between this tumor-specific isoform and its normal cell counterparts are essential for tumor growth (4).
Biochemically, PKM2 is less active than its splice variant PKM1 due to its higher Michaelis-Menten constant (Km) for phosphoenolpyruvate [PEP, (7)]. It has been hypothesized that the PKM2 isoform allows cancer cells to divert more glycolytic intermediates to biosynthetic processes like the pentose phosphate pathway to promote cell growth (2, 9). Although this model explains many aspects of PKM2’s role in cancer cell metabolism and growth, new data suggests that this may not be the entire story. For example, knocking down PKM2 without supplying additional PK limits cell growth (4), indicating that the relationship between PK activity and cell growth is not a simple inverse correlation. Similar results were seen with inhibitors specifically targeting PKM2 (8). It has been suggested that PKM2 can balance both cell growth and energy generation in cancer, but the mechanism remains unclear (10).
We speculated that this delicate balance of cell growth and energy generation may be explained by assuming that there is an allosteric regulator of PKM2 coordinating the PK activity (and thus energy generation) in response to the cell’s metabolic demands. PKM2 is activated by the upstream glycolytic intermediate fructose-1,6-bisphosphate [FBP; (7)]. However, other PK isoforms (PKL and PKR) also activated by FBP cannot replace PKM2 for cancer cell growth in stress conditions (4, 7).
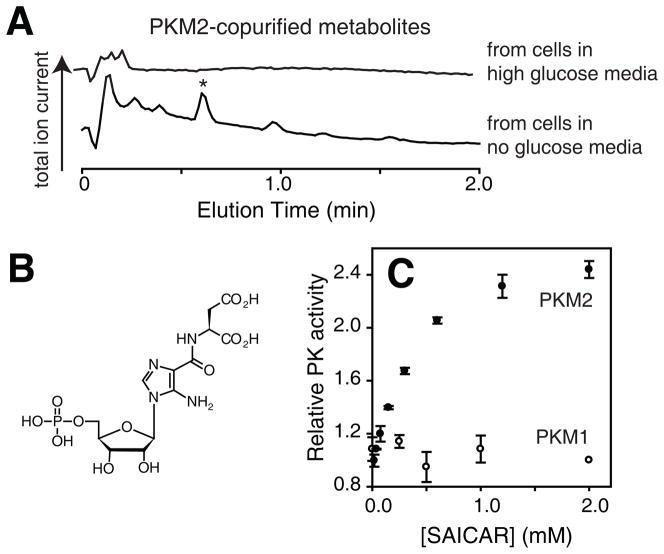
To identify other metabolites that interact with PKM2 in a glucose-dependent manner, we extracted cellular metabolites from A549 human lung cancer cells treated with high (~25 mM) or no glucose media (for 0.5 hrs). Metabolites binding to recombinant PKM2 were then identified as described in Supporting Online Materials using liquid-chromatography mass spectrometry (LC-MS, Fig. 1A). When metabolite extracts from cells grown in glucose-rich conditions were used, peaks corresponding to known PKM2-binding metabolites (FBP and pyruvate) could be identified. When metabolites from glucose-depleted cells were used, a metabolite peak (m/z in positive ion mode: 455.0817) was found (* in Fig. 1A). This compound was identified as SAICAR (succinylaminoimidazolecarboxamide ribose-5′-phosphate, Fig. 1B), a purine nucleotide biosynthesis intermediate, based on its mass (calc. exact mass, [M·H]+: 455.0815) and its fragmentation pattern (Fig. S1). There were several peaks other than SAICAR, but none were specific to PKM2 as they also co-eluted with PKM1.
Fig. 1.
Identification of SAICAR as a regulator of PKM2. (A) LC-MS total ion current chromatographs of PKM2-copurified metabolites from glucose-rich (top) and glucose-free cells (bottom). A metabolite further characterized (Fig. S1) is noted with an asterisk (*). A549 cells were incubated in fresh DMEM (10% dialyzed FBS) containing either 25 mM or no glucose for 30 min, and used for metabolite extraction as described in SOM. Extracted metabolites were mixed with recombinant human PKM2 for 30 min at 37°C, cooled on ice, and subjected to a gel-filtration chromatography (exclusion limit: 10 kDa) at 4°C. Metabolites that co-purified with PKM2 were subjected to LC-MS analysis [C18, electron-spray ionization (ESI), positive mode]. (B) Structure of SAICAR. (C) Effect of enzymatically synthesized SAICAR (Fig. S2) on PK activities of (●) PKM2 and (○) PKM1. Avg.±s.d. (n=3).
To test whether SAICAR affects PKM2’s function, we synthesized SAICAR (11) (Fig. S2) and tested its effect on PKM2 using a lactate dehydrogenase (LDH)-coupled method. SAICAR activates PKM2 with an effective concentration at the half-maximal effect (EC50) of 0.3 ± 0.1 mM (Fig. 1D). Increasing the incubation time (to 3 hours) did not alter the EC50. SAICAR did not affect other PKs tested (Fig. 1 and S3). SAICAR-binding increased the catalytic turnover number (kcat) of PKM2 by 2–3-fold while reducing the Km for PEP from ~2 mM to ~0.1 mM. SAICAR did not affect the Km for ADP. Overall, the binding of SAICAR rendered PKM2’s pyruvate kinase activity similar to that of PKM1.
When effects of other compounds related to SAICAR were tested (Fig. S4), succinyl-AMP stimulated PKM2 with an EC50 value (1.5 ± 0.4 mM) several-fold higher than that of SAICAR. None of the other tested compounds affected PKM2 activity significantly. SAICAR did not affect LDH activity (Fig. S5). The stimulation of PKM2 by SAICAR and by FBP was not additive (Fig. S6). Although this could be consistent with a competitive binding between FBP and SAICAR for an identical binding site, the study of mutant PKM2 proteins discussed later argues against this possibility.
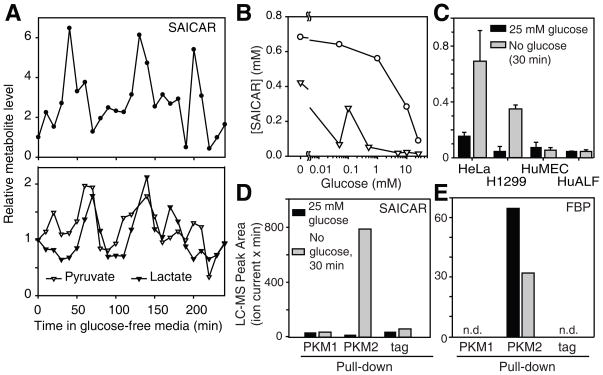
To assess the physiological significance of this interaction, we measured SAICAR concentrations in several cell lines by LC-MS. In all human cancer cells tested (HeLa cervical cancer, A549, U87 glioblastoma. and H1299 non-small cell lung cancer cells), cellular levels of SAICAR were elevated upon glucose starvation from 20–100 μM to ~ 0.3–0.7 mM (Fig. 2A-C). The concentration of SAICAR is similar to that measured in glucose-rich leukemia cells [~40μM, (12)]. The increase in cellular SAICAR level is oscillatory in HeLa (Fig. 2A) and H1299 cells (Fig S7). Pyruvate, lactate, and the ATP/ADP ratio also changed in a manner similar to the oscillation of SAICAR levels (Fig 2A and S7). SAICAR accumulation of HeLa cells was observable even after a 12-hour incubation in glucose-free media. At a fixed time point (30 min), cellular SAICAR levels in HeLa cells increased as extracellular glucose concentration decreased [Fig 2B; physiological blood glucose level in healthy patients: 4–7 mM (13)]. In NIH 3T3 mouse embryonic fibroblast cells, an increase in SAICAR level was observed only when extracellular glucose concentrations were far below the physiological range. In normal human mammary epithelial (HuMEC) and in adult lung fibroblast (HuALF) cells, SAICAR accumulation was not observed even in glucose-free media (Fig. 2C). In all cells tested, FBP levels were gradually decreased upon glucose depletion (Fig. S8). These results suggest that the cellular concentration of SAICAR increases to a level sufficient to stimulate PKM2 in glucose-deprived cancer cells. It also suggests that SAICAR accumulation in glucose-depleted conditions may be limited to highly proliferating cells, perhaps because these cells use the de novo nucleotide synthesis pathway more than normal cells. However, other proliferating normal cells expressing PKM2 may also use this regulatory mechanism.
Fig. 2.
SAICAR binds to PKM2 in glucose-depleted cancer cells. (A) Cellular concentration of SAICAR in HeLa cells in response to glucose-depletion. HeLa cells were incubated in glucose-free media, and the time course changes in SAICAR concentration were measured using LC-MS as described in SOM. Pyruvate and lactate concentration changes are also noted. (B) Effect of extracellular glucose concentration on cellular SAICAR amount. Cellular concentration of SAICAR was measured in (○) HeLa and in (▽) NIH 3T3 cells 30 min after incubating in media with various glucose concentrations. (C) Effect of cell type. HeLa, H1299, HuMEC, and HuALF were incubated in 25 mM (filled box) or in no glucose media (gray box) for 30 min and used for the analysis of SAICAR level. Avg.±s.d. (n = 3). Other cancer cell lines tested (A549 and U87) also showed glucose-depletion dependent increase of cellular SAICAR level. (D) LC-MS peak area of SAICAR co-purified with PKM1, PKM2, or StrepII tag from H1299 cells incubated for 30 min in fresh 25 mM or no glucose media. (E) Amount of FBP that co-purified with the protein of interest analyzed as in (D).
To determine if the PKM2-SAICAR interaction also occurs in cancer cells, we used an approach similar to the global metabolite-protein interaction mapping in yeast (14). HeLa cells were transfected with plasmids coding StrepII-tagged PKM2 or PKM1. These proteins were affinity purified. Metabolites co-purified with these proteins were then analyzed by LC-MS (Fig. 2D). Co-purification of SAICAR with PKM2 was observed when PKM2 was purified from glucose-depleted cells. SAICAR was not enriched with PKM1 (Fig 2E). FBP co-purified with PKM2 from glucose-rich cells as expected. These results suggest that the isozyme-specific PKM2-SAICAR interaction occurs in cells.
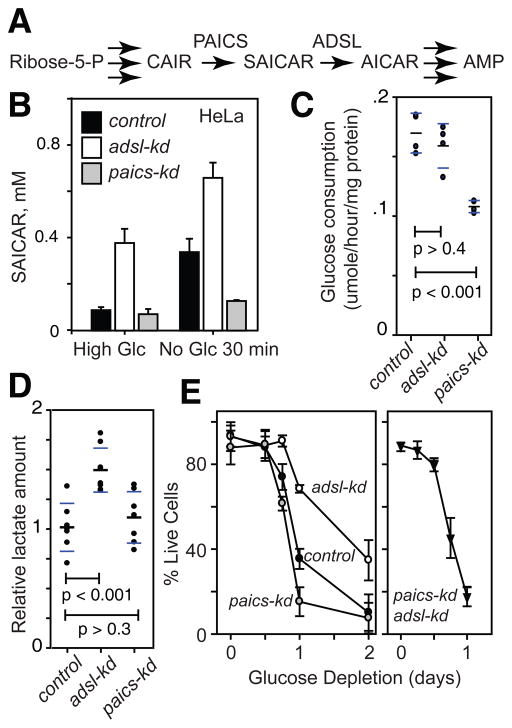
We then examined the effect of SAICAR on the pyruvate kinase activity of PKM2 in cancer cells. We prepared HeLa and H1299 cells stably transfected with shRNA vectors targeting expression of the SAICAR synthase PAICS and the SAICAR cleavage enzyme ADSL (adenylosuccinate lyase; Fig 3A). In cells where PAICS is knocked down (paics-kd), cellular SAICAR concentration was not inducible while adsl-kd cells have constitutively elevated SAICAR levels (Fig. 3B). Depletion of glucose for 30 min further increased cellular levels of SAICAR in adsl-kd cells. Prolonged (>12 hrs) glucose depletion eventually led to a decrease in cellular SAICAR levels in adsl-kd cells (Fig S9). In paics-kd adsl-kd double-knockdown cells, no increase in SAICAR levels was observed. When PK activity in cell extracts was measured, the PK activity was higher in adsl-kd cells (Fig S10). Addition of SAICAR to the cell extract elevated pyruvate kinase activity of control-kd (cells transfected with scrambled shRNA) and paics-kd cell extracts, and only slightly in adsl-kd cell extracts (Fig S10). These results support our hypothesis that elevated cellular level of SAICAR stimulates PKM2.
Fig. 3.
SAICAR level affects glucose metabolism, cancer cell survival, and proliferation. (A) A schematic diagram showing in the synthesis and metabolism of SAICAR. (B) Cellular levels of SAICAR in HeLa control-kd (black box), adsl-kd (open box), and paics-kd (gray box) before and after 30 min incubation in glucose-free media. Avg.±s.d. (n = 3). (C and D) Effect of SAICAR on (C) glucose uptake and (D) lactate fermentation. The amount of glucose and lactate in the media was measured using enzymatic assays and LC-MS analysis, respectively (normalized with total cellular protein concentration). Black and blue bars note avg. and s.d, respectively. (E) Survivals of HeLa control-kd (control; cells transfected with random shRNA vector), adsl-kd, paics-kd, and paics-kd adsl-kd cells in glucose-free media were measured using a Trypan blue exclusion method. Avg±s.d. (n=3, where >50 cells were counted to calculate % live cells in each measurement).
Next, we measured whether SAICAR affects cancer metabolism. The glucose consumption rate of paics-kd cells was reduced in comparison with control-kd and adsl-kd cells (Fig. 3C). The lactate fermentation rate of adsl-kd cells was increased in comparison with control-kd and paics-kd cells (Fig. 3D). Similar results were obtained using LC-MS analysis of cellular metabolites (Fig S11). Both adsl-kd and paics-kd cells showed increased glutamine consumption (Fig S12). These results show that commonly observed metabolic phenotypes of cancer cells are either directly or indirectly affected by SAICAR levels. In addition, in a glucose-limited condition (30 min), adsl-kd cells had more ATP than the control-kd or paics-kd cells (Fig. S13–14), suggesting that the cellular metabolism of these SAICAR-overproducing cells is adjusted in glucose-limiting conditions.
Because metabolic flux and cellular energy balance were altered by the changes in SAICAR concentration, we investigated whether SAICAR affected cancer cell survival in a glucose-dependent manner (Fig. 3E). In glucose-limited conditions, cells with higher SAICAR concentrations (adsl-kd cells or cells overexpressing PAICS, Fig S15) survived better while paics-kd cells died earlier than control-kd cells (Fig. 3E). Double knockdown of PAICS and ADSL (paics-kd adsl-kd) had an effect similar to the PAICS knock-down alone. These results indicate that SAICAR promotes cancer cell survival in glucose-limited conditions.
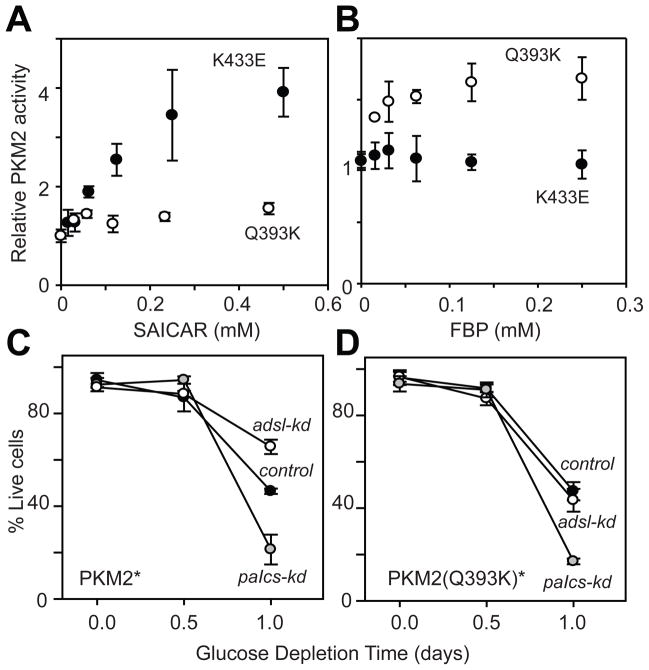
To confirm the necessity of the PKM2-SAICAR interaction for increased cell survival in glucose-limited conditions, we searched for a PKM2 mutant that is insensitive to SAICAR while retaining its other properties. Because PKM1 (only 22 amino acids different from PKM2) is insensitive to SAICAR (Fig 1), we prepared several PKM2 mutants where PKM2-specific residues were mutated to their corresponding PKM1 counterparts. The Q393K mutant was SAICAR-insensitive (Fig. 4A) and retained wild-type basal activity (Fig S16). This Q393K mutant was also stimulated by FBP (Fig. 4B). In contrast, the known phosphotyrosine– insensitive mutant, K433E [(15); K433 is also the residue contacting FBP (PDB|3ME3)], was still stimulated by SAICAR (Fig. 4A).
Fig. 4.
SAICAR-PKM2 interaction is responsible for the promotion of cancer cell survival. (A and B) Identification of a PKM2 mutant insensitive to SAICAR. Effect of (A) SAICAR and (B) FBP on pyruvate kinase activities of PKM2 Q393K (○) and PKM2 K433E (●) mutants are shown. Avg ± s.d. (n = 3). (C and D) Cell viability in glucose-depletion conditions was measured as in figure 3H in HeLa cells expressing (C) PKM2* and (D) PKM2* Q393K mutant while endogenous PKM2 expression is knocked down. Avg±s.d. (n = 3, >50 cells were counted for each measurement). Control: cells transfected with scrambled shRNA vectors.
We used the Q393K mutant to test whether the effect of cancer cell survival in adsl-kd cells is due to the PKM2-SAICAR interaction. By a previously described method (4), endogenous PKM2 was knocked down and rescued with plasmid-coded PKM2 or PKM2 Q393K (M2-rescue and Q393K rescue cells, respectively). Western blot analysis showed more than 90 % of endogenous PKM2 expression was knocked down without detectable effects on plasmid-coded PK expression. The PK activity of the Q393K-rescue cells was similar to the level observed in paics-kd cells but the activity was not stimulated by SAICAR (Fig. S16B). The PKM2-rescue cells behaved similarly to the cells with endogenous PKM2 in both glucose rich and glucose-limited conditions (Fig. 4C). The expression level of PKM2 was similar in untransfected and in Q393K-rescue cells. Q393K-rescue cells behaved nearly identical to cells with endogenous PKM2 in glucose-rich conditions. However, knockdown of ADSL in Q393K-rescue cells no longer promoted cell survival in glucose-limited conditions (Fig. 4D), indicating that the effect of SAICAR on cell survival in this condition is due to the SAICAR-PKM2 interaction.
Based on these results, we conclude that at least some aspects of PKM2’s role in tumor biology can be explained by SAICAR-mediated allosteric stimulation. This feature of PKM2 may allow cells to adjust their energy generation in response to nutritional and metabolic demands. When sufficient levels of glucose are provided, diverting glycolytic intermediates to biosynthetic processes like the pentose phosphate pathway can promote cell growth. However, in energy-depleting conditions such as nutrient-limitation, continued diversion of glycolytic intermediates to biosynthetic processes can hamper cells by depleting cellular energy below the minimal level required. The SAICAR-PKM2 interaction described here can be a molecular mechanism for this delicate balance. Ribose-5-phosphate, the starting material of nucleotide biosynthesis, is produced from the pentose phosphate pathway, which diverts glycolytic intermediates away from energy production (16). Connecting PKM2’s PK activity with an intermediate of the de novo purine nucleotide biosynthesis pathway could give cells fine-tuned control of their metabolism in demanding conditions. SAICAR is produced from L-aspartate, a byproduct of glutaminolysis, which is known to be important in cancer cell metabolism (17), and the cleavage of SAICAR yields fumarate, which contributes to the citric acid cycle in mitochondria (18). SAICAR is therefore positioned to convey cellular metabolic demands to PKM2.
Acknowledgments
We thank T. Coupet, D. Meyer, and E. Pryce for technical assistance; R. Coleman, Y. Wang, C. Arrowsmith, and members of our department for sharing their equipment and materials; and A. Le, E. O’Shea, and members of our department for comments on the manuscript. Supported by Johns Hopkins University Krieger School of Arts & Sciences startup fund to Y.S.L.
Footnotes
The authors declare no conflict of interest.
References and Notes
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 4.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 5.Le A, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107(5):2037. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf A, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43(7):969. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Spoden GA, et al. Pyruvate kinase isoenzyme M2 is a glycolytic sensor differentially regulating cell proliferation, cell size and apoptotic cell death dependent on glucose supply. Exp Cell Res. 2009;315(16):2765. doi: 10.1016/j.yexcr.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macintyre AN, Rathmell JC. PKM2 and the Tricky Balance of Growth and Energy in Cancer. Mol Cell. 2011;42(6):713. doi: 10.1016/j.molcel.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee P, Colman RF. Expression, purification, and characterization of stable, recombinant human adenylosuccinate lyase. Protein Expr Purif. 2007;51(2):227. doi: 10.1016/j.pep.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Sant ME, Lyons SD, Phillips L, Christopherson RI. Antifolates induce inhibition of amido phosphoribosyltransferase in leukemia cells. J Biol Chem. 1992;267(16):11038. [PubMed] [Google Scholar]
- 13.Standards of medical care in diabetes--2006. Diabetes Care. 2006;29(Suppl 1):S4. [PubMed] [Google Scholar]
- 14.Li X, Gianoulis TA, Yip KY, Gerstein M, Snyder M. Extensive in vivo metabolite-protein interactions revealed by large-scale systematic analyses. Cell. 2010;143(4):639. doi: 10.1016/j.cell.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452(7184):181. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 16.Tong X, Zhao F, Thompson CB. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev. 2009;19(1):32. doi: 10.1016/j.gde.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dang CV. Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell Cycle. 2010;9(19):3884. doi: 10.4161/cc.9.19.13302. [DOI] [PubMed] [Google Scholar]
- 18.Raimundo N, Baysal BE, Shadel GS. Revisiting the TCA cycle: signaling to tumor formation. Trends Mol Med. 2011;17(11):641. doi: 10.1016/j.molmed.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannone RJ, et al. Dual-tagging system for the affinity purification of mammalian protein complexes. Biotechniques. 2007;43(3):296. doi: 10.2144/000112550. [DOI] [PubMed] [Google Scholar]
- 20.Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967;6(7):1948. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz MA, Burant CF, Kennedy RT. Reducing time and increasing sensitivity in sample preparation for adherent mammalian cell metabolomics. Anal Chem. 2011;83(9):3406. doi: 10.1021/ac103313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flaks JG, Erwin MJ, Buchanan JM. Biosynthesis of the purines. XVI. The synthesis of adenosine 5′-phosphate and 5-amino-4-imidazolecarboxamide ribotide by a nucleotide pyrophosphorylase. J Biol Chem. 1957;228(1):201. [PubMed] [Google Scholar]
- 23.Lukens LN, Buchanan JM. Biosynthesis of the purines. XXIII. The enzymatic synthesis of N-(5-amino-1-ribosyl-4-imidazolylcarbonyl)-L-aspartic acid 5′-phosphate. J Biol Chem. 1959;234(7):1791. [PubMed] [Google Scholar]
- 24.Janet OHL, Passonneau V. Enzymatic analysis: A practical guide. Humana Press; 1993. [Google Scholar]