Abstract
Rationale
Adolescent binge drinking is concerning, as important neurodevelopments occur during this stage. Previous research suggests that binge drinking may disrupt typical brain development, and females may be particularly vulnerable.
Objectives
We used magnetic resonance imaging (MRI) to examine cortical thickness in adolescent females and males with and without histories of binge drinking.
Methods
Participants (N=59) were 16–19-year-old adolescents recruited from local schools. Recent binge drinkers (n=29, 48% female) were matched to non-drinkers (n=30, 50% female) on age, gender, pubertal development, and familial alcoholism. Participants completed a neuropsychological battery and MRI session. Cortical surfaces were reconstructed with FreeSurfer.
Results
Binge × gender interactions (p<.05) were seen for cortical thickness in four left frontal regions: frontal pole, pars orbitalis, medial orbital frontal, and rostral anterior cingulate. For all interactions, female bingers had thicker cortices than female controls, while male bingers had thinner cortices than male controls. Thicker left frontal cortices corresponded with poorer visuospatial, inhibition, and attention performances for female bingers (r=−0.69 to 0.50, p<0.05) and worse attention for male bingers (r=−0.69, p=0.005).
Conclusions
Adolescent females with recent binge drinking showed ~8% thicker cortices in left frontal regions than demographically similar female non-drinkers, which was linked to worse visuospatial, inhibition, and attention performances. In contrast, adolescent binge-drinking males showed ~7% thinner cortices in these areas than non-drinking males. These cross-sectional data suggest either different gray matter risk factors for males as for females toward developing heavy drinking, or differential adverse sequelae.
Keywords: Adolescence, Alcohol, Binge, Gender, FreeSurfer, Cortical thickness, Neurodevelopment, Alcohol use disorder
Introduction
Adolescence is associated with marked increases in binge drinking, defined as ≥4 drinks per occasion for females, or ≥5 drinks per occasion for males (Wechsler and Isaac 1992). Alarmingly, 23% of twelfth graders report engaging in a binge-drinking episode in the past 2 weeks (Johnston et al. 2011). Acute excessive alcohol consumption is of great public health concern as it increases an adolescent's likelihood of engaging in risky behaviors like drunk driving, riding with impaired drivers, violence, unsafe sex, and other substance use (Miller et al. 2007). While males have historically exhibited higher rates of binge drinking than females, recent surveys have demonstrated a narrowing of this gap, with 31% of males and 20% of females engaging in recent binge drinking during their senior year of high school (Johnston et al. 2009). Female binge drinkers may be more vulnerable to psychopathology (SAMHSA 2008) as well as neurocognitive decrements (Hommer et al. 1996, 2001; Jacobson 1986; Mann et al. 1992; Medina et al. 2008; Schweinsburg et al. 2003; Squeglia et al. 2009b, 2011), highlighting the importance of characterizing gender differences in alcohol-use-linked features among adolescents.
Repeated binge drinking may present potential deleterious effects on adolescent brain development (Brown et al. 2008; Squeglia et al. 2009a). Dynamic and critical brain changes occur between roughly ages 12–20 years (Bava et al. 2010; Silk and Wood 2011), including thinning of cortices (i.e., decreased gray matter volume) and myelination (i.e., increases in white matter volumes), which correspond to more efficient and specialized information processing (Giedd 2004; Sowell et al. 2004; Spear 2009). Cortical thinning during late childhood and adolescence is generally considered to be related to pruning of excess neurons and begins primarily in dorsal parietal cortices, continuing anteriorly to the prefrontal cortex, then posteri-orly to the parietal, occipital, and, finally, temporal cortices (Gogtay et al. 2004), with decreases in dorsal prefrontal cortical volume by late adolescence (Sowell et al. 2001). Importantly, sexually dimorphic trajectories in neuromatu-ration have been identified, with development of white and gray matter occurring 1–2 years earlier in girls (Giedd 2004; Lenroot and Giedd 2006). The effect of binge drinking on this process of cortical thinning during adolescence has not yet been explored.
Deficits related to binge drinking during adolescence and adulthood have been observed in both females and males, but females may be particularly vulnerable to its neurocognitive effects (Caldwell et al. 2005; Hommer et al. 1996, 2001; Jacobson 1986; Mann et al. 1992; Medina et al. 2008; Schweinsburg et al. 2003; Squeglia et al. 2009b, 2011). In a recent functional magnetic resonance imaging study of 40 adolescent binge drinkers and 55 demographically similar controls, heavy binge drinking in the past 3 months was associated with gender-specific differences in frontal, temporal, and cerebellar brain activation during a spatial working memory task (Squeglia et al. 2011). Specifically, female binge drinkers showed less brain activation compared with female controls in eight areas, while male binge drinkers showed greater activation than male controls to the task in four areas, despite equivalent task performance. For female bingers, less brain activation was associated with poorer performance on more sensitive out-of-scanner neuropsychological tests of attention and working memory, while, for male bingers, greater activation was linked to better spatial performance (Squeglia et al. 2011). These findings support previous reports of altered brain activation patterns associated with heavy drinking in females, while male binge drinkers demonstrated similar response as controls, despite equivalent task performance (Caldwell et al. 2005). Similarly, longitudinal work has shown that female teens who initiated heavy drinking declined on tests of spatial functioning in contrast to their pre-drinking baseline, whereas girls who did not initiate heavy drinking improved on such tasks (Squeglia et al. 2009b), and male heavy drinkers did not exhibit the same degree of neurocognitive deficits when compared with male controls.
Gender-moderated differences have also been found in adolescent morphometric studies, where females diagnosed with alcohol use disorders exhibited smaller prefrontal volumes than non-using females, while males with alcohol use disorders had larger prefrontal volumes than male controls (Medina et al. 2008). These differences were apparent despite males and females having similar drinking histories.
Currently, the only study of cortical thickness in adolescent substance users compared 18 heavy marijuana users to 18 healthy controls and found that marijuana use was associated with both increased and decreased cortical thickness in frontal, parietal, temporal, and insular regions (Lopez-Larson et al. 2011). Participants did not meet criteria for an alcohol use disorder, but other information on their alcohol use was not indicated. Understanding the effect of binge drinking, in particular, on cortical maturation is important due to its widespread occurrence.
This study used magnetic resonance imaging (MRI) data to examine cortical thickness in adolescent males and females with and without histories of binge drinking. Given the relative immaturity in frontal systems during adolescence and the vulnerability of the frontal lobe to alcohol-related insults, we hypothesized that teen binge drinkers would show suggestions of impinged cortical pruning. Specifically, we hypothesized that binge drinkers would have thicker cortices in frontal regions. We also hypothesized that thicker frontal cortices would be more pronounced for females, as previous research suggests females are more susceptible to alcohol-related neuro-cognitive deficits and that this would be linked to cognitive inefficiency.
Methods and materials
Participants
Participants (N=59) were 16–19-year-old adolescent males (n=30) and females (n=29) recruited from San Diego area public schools as part of neuroimaging studies on adolescent substance use (Bava et al. 2009; McQueeny et al. 2009; Squeglia et al. 2009b; Tapert et al. 2007). Males with ≥5 and females with ≥4 drinks on more than one occasion in the past 3 months were categorized as binge drinkers (n=29) (McQueeny et al. 2009; NIAAA 2004; Wechsler et al. 1994) and were matched to non-using adolescents (n=30; <3 drinks total in the past 3 months; no lifetime binge drinking episode) on age, gender, pubertal development, and family history of alcohol use disorder (see Table 1). Consent and assent (for participants under age 18) were obtained, and participants were screened for eligibility via youth and parent interviews.
Table 1.
Demographic and substance use characteristics of participants
| Binge drinkers (n=29) |
Controls (n=30) |
||||
|---|---|---|---|---|---|
| Females | Males | Females | Males | ||
| M (SD) (n=14) | M (SD) (n=15) | M (SD) (n=15) | M (SD) (n = 15) | ||
| Demographics | Agebe (range, 16 to 19 years) | 17.81 (1.01) | 18.59 (0.56) | 18.02 (1.08) | 17.89 (1.15) |
| Race (% Caucasian) | 71% | 87% | 80% | 67% | |
| Family history of alcoholism density (range, 0–2) | 0.39 (0.62) | 0.35 (0.56) | 0.18 (0.35) | 0.32 (0.58) | |
| Hollingshead Index of Social Position score | 28.21 (15.08) | 26.33 (19.08) | 24.73 (12.50) | 29.33 (15.06) | |
| Body mass Indexe | 20.86 (2.82) | 24.25 (2.61) | 22.69 (4.99) | 22.13 (4.19) | |
| Pubertal Development Scale total | 19.00 (8.2) | 18.33 (1.40) | 18.93 (1.07) | 17.80 (1.86) | |
| Years of education completedbe | 11.29 (0.91) | 12.13 (6.40) | 11.53 (1.25) | 11.27 (0.96) | |
| Substance use | Peak drinks on an occasion, past 3 monthsabcde | 6.43 (2.77) | 12.10 (3.81) | 0.00 (0.00) | 0.07 (0.26) |
| Estimated peak BAC, past 3 monthsabc | 0.26 (0.10) | 0.28 (0.10) | 0.00 (0.00) | 0.00 (0.00) | |
| Lifetime alcohol use occasionsabc | 102.29 (78.94) | 66.66 (43.82) | 0.07 (0.26) | 0.13 (0.35) | |
| Average no. drinks per drinking day, past monthabc | 2.36 (2.68) | 4.67 (3.37) | 0.00 (0.00) | 0.07 (0.26) | |
| Days since last alcohol use | 24.85 (22.57) | 17.13 (13.80) | n/a | n/a | |
| Tobacco cigarettes per day | 0.07 (0.27) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | |
| Lifetime marijuana use occasionsbc | 16.93 (33.54) | 3.67 (4.13) | 0.00 (0.00) | 0.07 (0.28) | |
| Marijuana use days per month, past 3 monthsabc | 0.50 (0.90) | 0.38 (0.65) | 0.00 (0.00) | 0.00 (0.00) | |
| Lifetime other drug use occasionscde | 5.71 (8.15) | 0.67 (1.29) | 0.00 (0.00) | 0.00 (0.00) | |
| NP measures | Complex Figure copy accuracy | 29.21 (3.10) | 28.86 (3.38) | 30.59 (2.76) | 28.33 (3.07) |
| Complex Figure delay accuracy | 21.17 (5.40) | 20.50 (5.73) | 20.22 (5.69) | 19.23 (4.79) | |
| WAIS-III Digits forward | 10.53 (2.00) | 11.07 (2.66) | 10.83 (1.83) | 10.83 (1.64) | |
| WAIS-III Digits backward | 6.80 (1.52) | 8.00 (3.15) | 7.25 (2.15) | 7.23 (1.99) | |
| D-KEFS Color Word Interference Cond 3 time (s) | 46.00 (8.09) | 44.09 (11.03) | 43.38 (10.62) | 45.38 (7.83) | |
| D-KEFS Towers Total Achievement Score | 19.20 (3.99) | 18.04 (3.70) | 19.41 (2.80) | 18.19 (3.18) | |
| WRAT-3 Reading standard score | 107.43 (5.77) | 112.00 (8.20) | 108.14 (7.40) | 107.54 (13.80) | |
| Cortical thickness | Left frontal pole (mm)ae | 3.24 (0.19) | 2.99 (0.26) | 3.00 (0.23) | 3.12 (0.34) |
| Left pars orbitalis (mm)be | 2.86 (0.13) | 2.72 (0.17) | 2.76 (0.17) | 2.84 (0.19) | |
| Left medial orbital frontal gyrus (mm)b | 2.60 (0.12) | 2.53 (0.15) | 2.55 (0.15) | 2.64 (0.15) | |
| Left rostral anterior cingulate (mm)bde | 3.14 (0.24) | 2.83 (0.27) | 3.10 (0.18) | 3.03 (0.21) | |
All NP scores are raw values, unless otherwise noted. For the full sample, ethnicity was 12% Latino; race was 76% Caucasian, 16% multiracial, 3% African-American, 3% Asian, and 2% Native American
BAC blood alcohol concentration; D-KEFS Delis–Kaplan Executive Function System, NP neuropsychological; WAIS-III Wechsler Adult Intelligence Scale, 3rd edition, WASI Wechsler Abbreviated Scale of Intelligence, WRAT-3 Wide Range Achievement Test, 3rd edition
Female binge drinkers ≠ female controls, p<.05
Male binge drinkers ≠ male controls, p<.05
Binge drinkers ≠ controls, p<.05
Female ≠ male, p<.05
Female binge drinkers ≠ male binge drinkers, p<.05
Exclusionary criteria were relatively rigid and designed to reduce potential influences other than binge drinking on brain integrity measures: parental history of psychotic, bipolar, or antisocial personality disorder; prenatal exposure to alcohol (>2 drinks on an occasion or >4 drinks in a week) or any illicit drugs; premature birth (<36 weeks gestation); history of any neurological (e.g., migraine, traumatic brain injury with loss of consciousness >2 min) or serious medical problem; left-handedness; lifetime use of psychotropic medications; current or past probable DSM-IV Axis I diagnosis (APA 1994) other than conduct disorder, oppositional defiant disorder, simple phobia, or alcohol use disorder (AUD), assessed by the Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al. 2001) administered to youth and parent; marijuana use >3×/month in the past 3 months; >25 lifetime total uses of other illicit substances including prescription medication misuse; substance use in the 72 h before scanning (confirmed with breathalyzer and urine toxicology); no parent or guardian available to provide corroborating information; and MRI contraindications (e.g., braces). Approximately 12% of respondents to flyer distributions at local schools met eligibility criteria. The study protocol was executed in accordance with the University of California, San Diego Human Research Protections Program.
Participants were well-matched between binge drinking groups and genders (see Table 1), with the exception that binge drinking males were about 8 months older than female binge drinkers and male controls, and had larger body mass indices than male controls (p<.05).
Measures
Substance use
The Customary Drinking and Drug Use Record (Brown et al. 1998) obtained self-reported quantity and frequency of lifetime, past year, and past 3 month alcohol, tobacco, marijuana, and other drug use. Estimates of blood alcohol concentration were calculated using peak number of drinks, duration of consumption, gender, and body mass index (Fitzgerald 1995; Widmark 1922). The Timeline Followback (Sobell and Sobell 1992) assessed substance use quantity and frequency for the 30 days prior to scanning, with temporal cues to aid recall. Breathalyzer, urine toxicology, and corroborating information from a parent and one other biological relative were collected to increase accuracy of self-report information (Jones and Sigall 1971).
Neuropsychological measures
A neuropsychological battery was completed within 1 week of scanning to assess cognitive domains previously associated with alcohol-related deficits. Measures of executive functioning, attention, and planning/spatial skills hypothesized to be correlated with frontal cortical thickness were: Delis–Kaplan Executive Function System (D-KEFS) Color-Word Interference (condition 3 time to complete) and Towers tests (total achievement score; Delis et al. 2001), Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Span (Wechsler 1997), and Complex Figure copy and 30-min accuracy and delay (Loring and Meador 2003; Meador et al. 1993; Rey and Osterrieth 1993; Taylor 1969). Wide Range Achievement Test-3 Reading scores (Wilkinson 1993) were obtained as a measure of premorbid functioning and intellectual capacity. Complete neuropsychological data were available for 11 female bingers and 13 male bingers.
Family background
The Family History Assessment Module (Rice et al. 1995), administered to youth and parents, ascertained familial density of substance use disorders by adding 0.5 for each biological parent and 0.25 per biological grandparent (Zucker et al. 1994), endorsed by youth or parent as having an AUD or other substance use disorder. Socioeconomic background information (i.e., educational attainment, occupation, and salary of each parent) was obtained from parents and converted to a Hollingshead Index of Social Position score (Hollingshead 1965).
Development
Pubertal Development Scale total scores (Petersen et al. 1988) were obtained to assess pubertal staging, which was calculated separately by gender.
Psychopathology and mood
Parents of participants aged 16 to 18 years completed the Child Behavior Checklist (Achenbach and Rescorla 2001), and youths aged 18 to 19 years living independently from parents completed the Adult Self Report (Achenbach and Rescorla 2001) to obtain age- and gender-normed continuous measures of internalizing and externalizing psychopathological syndromes. The Beck Depression Inventory-II (Beck et al. 1996) and Spielberger State Anxiety Inventory (Spielberger et al. 1970) assessed mood state at the time of scanning. No participant scored in the clinical range on any psychopathological or mood measure.
Procedures
Imaging
High-resolution anatomical images were collected at the UCSD Keck fMRI Center from a 3-Tesla CXK4 short bore Excite-2 MR system (General Electric, Milwaukee, WI) with an eight-channel phase-array head coil. Participants were placed comfortably on the scanner table, and the head was stabilized within the head coil using foam cushions (NoMoCo Pillow, La Jolla, CA). Scan sessions involved a 10-s scout scan to assure good head placement and slice selection covering the whole brain, followed by a sagittally acquired high-resolution 3D T1-weighted anatomical MRI that lasted 7 min and 26 s (FOV 24 cm, 256×256×192 matrix, 0.94×0.94×1 mm voxels, 176 slices, TR=20 ms, TE=4.8 ms; flip angle 12°).
Data analysis
Cortical thickness measurement
Cortical surface reconstruction and cortical thickness estimation were performed using FreeSurfer (version 5.0, http://surfer.nmr.mgh.harvard.edu). The FreeSurfer program utilizes a series of automated imaging algorithms to produce measures of cortical thickness (Dale et al. 1999; Fischl and Dale 2000; Fischl et al. 1999, 2004). The process first involves intensity normalization, Talairach transformation, skull-stripping, and labeling of the subcortical white matter. The white matter border is then tessellated by placing two triangles at each face (square) that separates white matter voxels from other voxels (i.e., gray matter). This initial coarse tessellation is then smoothed via surface deformation algorithm that is guided by local MRI intensity gradients to optimally place smooth gray/white and, by deforming outward, gray/cerebrospinal fluid (CSF) borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al. 1999; Dale and Sereno 1993; Fischl and Dale 2000). The smoothing produced from the spatial intensity gradients across tissue classes frees the surfaces from being reliant on the absolute signal intensity. Thus, this surface rendering process yields data that are not constrained to the voxel resolution of the original images, allowing for the quantification of submillimeter group differences (Fischl and Dale 2000). Cortical thickness was calculated as the closest distance from the gray/white matter boundary to the gray matter/CSF boundary at each vertex on the cortical surface (Fischl and Dale 2000). The validity of the cortical thickness measurement procedures has been verified using manual measurements (Kuperberg et al. 2003; Salat et al. 2004) and histological analysis (Rosas et al. 2002).
One rater (LMS), blind to participant characteristics, followed the reconstruction procedures (http://surfer.nmr.mgh.harvard.edu/fswiki/RecommendedReconstruction) to identify and correct any errors made during the cortical reconstruction. This involved verification of the Talairach transformation and of the automated skull stripping, as well as a coronal plane slice-by-slice inspection of the gray/white and gray/CSF surfaces. Modifications to the surfaces were made as necessary to correct for tissue misclassifications (e.g., residual dura matter classified as cortex). Following inspection, an automated parcellation procedure divided each hemisphere into 32 independent cortical regions, including 13 frontal lobe regions, based on gyral and sulcal features (Desikan et al. 2006; Fischl et al. 2004). Cortical thickness estimates of each frontal region were extracted for subsequent statistical analysis.
Statistical analyses
Cortical thickness values, averaged across each parcellation region, were imported from FreeSurfer to SPSS for each participant. In SPSS, analyses of covariance (ANCOVA) examined main effects of binge drinking status, gender, and binge × gender interactions (p<.05, per region of interest) on cortical thickness values (i.e., 13 left and right frontal regions), controlling for demographic factors that varied between groups (i.e., age and body mass index) as well as intracranial volume. While male binge drinkers reported greater recent drinking quantities (i.e., approximately twice as many peak drinks per binge drinking occasion and twice as many average number of drinks per drinking day) than female binge drinkers, female binge drinkers reported more other substance use than male binge drinkers (see Table 1). Therefore, lifetime drinking, marijuana, and other drug use days were controlled for in follow-up analyses, in addition to previously mentioned demographic factors. Post hoc ANCOVAs were used for pairwise comparisons (p<.05). Exploratory follow-up analyses correlated cortical thickness and neuropsychological test scores using SPSS, with Type I error correction set at p<.05 per region of interest.
Results
Main effects
There was no main effect of binge status or gender on cortical thickness.
Binge × gender interactions in regions of interest
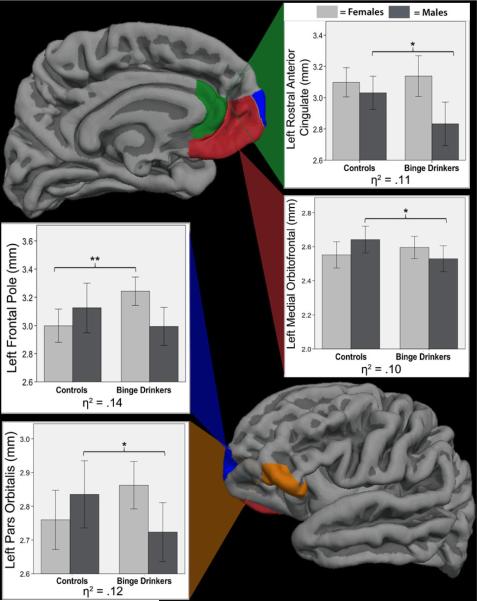
Significant binge × gender interactions were found in four left frontal regions: left frontal pole [F(1,52)=4.24, p=0.02, η2=0.14], left pars orbitalis [F(1,52)=3.58, p=0.03, η2=0.12], left medial orbital frontal gyrus [F(1,52)=2.72, p=0.05, η2=0.10], and left rostral anterior cingulate [F(1,52)=3.21, p=0.05, η2=0.11], above and beyond effects attributable to age, body mass index, or intracranial volume (see Fig. 1 and Table 1). While findings would not have survived strict Bonferroni corrections, the effect sizes were medium. Generally, each interaction showed the same pattern: female binge drinkers had thicker cortices than female controls, while male binge drinkers had thinner cortices than male controls (see Fig. 1). Post hoc ANCOVAs, controlling for age, body mass index, and intracranial volume, were run on males and females separately in the four regions with significant binge × gender interactions. Male binge drinkers had significantly thinner cortices than male controls in three regions: left pars orbitalis, left medial orbital frontal, and left rostral anterior cingulate (p<0.05; 4–7% thinner). Female binge drinkers had significantly thicker cortices than female controls in the frontal pole (p=0.005; 8% thicker).
Fig. 1.
Left hemisphere frontal lobe parcellation regions with significant binge × gender interactions (N=59; female bingers=14, female controls=15, male bingers=15, male controls=15). For all interactions, female bingers had thicker cortices compared with female controls, and male bingers had thinner cortices than male controls. Bar graphs represent raw data. *p<.05, **p<.005
A whole-brain analysis examined if additional regions (i.e., other than frontal regions of interest) exhibited significant binge × gender interactions (p<.05); no additional interactions were observed. To ensure that lifetime substance use variables that differed between genders did not account for these findings, lifetime alcohol, marijuana, and other drug use days (see Table 1) were controlled for in addition to demographic factors and intracranial volume; results remained unchanged.
Behavioral correlates
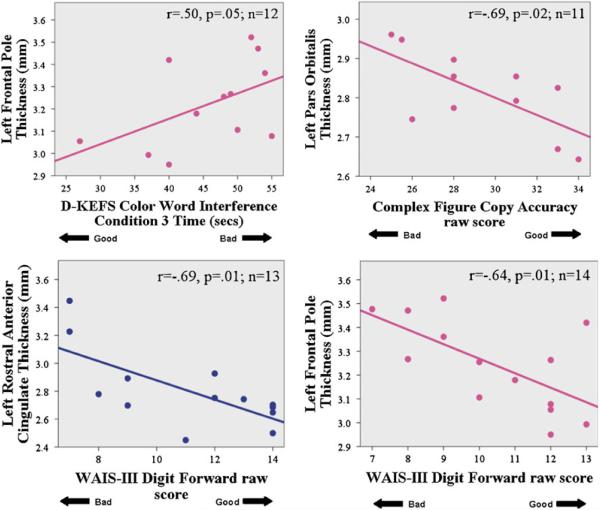
For binge drinkers, two-tailed Pearson correlations examined relationships between cortical thickness in the four areas with significant binge × gender interactions and neuropsychological tests of spatial functioning, attention, and executive functioning (see Table 1 for specific tests), to determine if cortical thickness was associated with behavioral deficits. For female binge drinkers (n=14), thicker cortices corresponded with worse performance on tests of visuospatial construction, inhibition, and attention. Specifically, thicker left pars orbitalis areas correlated with worse visuospatial construction accuracy scores (Rey–Osterrieth Complex Figure copy, r=−0.69, p=0.02), and the thicker left frontal poles significantly correlated with worse inhibition (slower D-KEFS Color Word Interference Condition 3 time, r=0.50, p=0.05) and worse attention (WAIS-III Digit Forward raw scores, r=−0.64, p=0.01). For male bingers (n=15), thicker rostral anterior cingulate correlated with worse attention (WAIS-III Digit Forward raw scores, r=−0.69, p=0.01; see Fig. 2). Cortical thickness was not correlated with marijuana or other substance use.
Fig. 2.
Correlations between neuropsychological measures and cortical thickness in female (magenta) and male (blue) binge drinkers
Discussion
This is the first study we are aware of that used cortical thickness indices to examine the effect of alcohol use on adolescent brain development. This study utilized high-resolution MRI datasets to examine cortical thickness in a sample of 59 well-matched healthy adolescent males and females with and without histories of binge drinking (i.e., ≥5 drinks for males and ≥4 drinks for females on at least one occasion in the past 3 months). As hypothesized, significant binge × gender interactions on cortical thickness were found in four left frontal regions: frontal pole, pars orbitalis, medial orbital frontal gyrus, and rostral anterior cingulate. For each interaction, female binge drinkers had thicker cortices than female controls, while male binge drinkers had thinner cortices than male controls. Thicker frontal cortices were associated with poorer neuropsychological functioning for female binge drinkers. The effect of binge drinking on cortical thickness held after controlling for substance use history, suggesting that binge drinking in particular is associated with greater alterations in neuroarchitecture than more frequent drinking at lesser quantities. These findings are compelling because these adolescents are relatively high-functioning, involved in fairly normative levels of drinking that do not reach alcohol dependence criteria, have no current or past psychological or neurological disorders, and have minimal, if any, current other substance use.
Female binge drinkers in the current study demonstrated thicker cortices, which in turn corresponded to poorer neuropsychological performance. Previous evidence of cortical thinning throughout adolescence suggests that gray matter reductions are the result of synaptic refinement that subserve efficient neural processing (Giedd 2004; Sowell et al. 2004; Spear 2009). Our finding that female drinkers have thicker cortices than male drinkers is particularly notable, considering female cortices begin thinning 1–2 years earlier than males on average (Giedd 2004; Lenroot and Giedd 2006) and could suggest an even greater deleterious effect of drinking on female brain maturation than statistical comparisons indicate. N-methyl d-aspartate (NMDA) receptor functioning is crucial for strengthening synapses and contributing to the loss of less important connections throughout development (Stoneham et al. 2010). Thus, it is possible that repeated alcohol exposure during adolescence may interfere with normal NMDA-mediated synaptic pruning. In particular, differences in cortical thickness were observed in regions that continue to thin throughout adolescence, such as lateral prefrontal cortex (Giedd 2004; Lenroot and Giedd 2006), but not in regions that have already matured. This supports the notion that thicker cortices represent altered pruning and could provide a biological basis for previous findings attributing adolescent substance use to worse neuropsychological performance (Squeglia et al. 2011; Tapert and Brown 1999; Tapert et al. 2002). A typical cubic millimeter of gray matter in an adult contains 35 to 70 million neurons and almost twice as many glial cells (Lenroot and Giedd 2006; Pakkenberg and Gundersen 1997), as well as over 500 billion synapses (Scheff et al. 2001), so even slight differences in cortical thickness could be associated with significant divergence from typical synaptic pruning/gray matter loss across adolescent development. Contrary to hypotheses, male binge drinkers showed thinner (i.e., more mature) cortices than male controls, suggesting binge drinking was not as disruptive to cortical pruning in male adolescents. Male binge drinkers were approximately 8 months older than male controls, so it is expected that they would have thinner cortices; while we controlled for age, this may not have fully accounted for age or pubertal influences.
Since this study is cross-sectional, it is possible that females who develop heavy drinking patterns are more likely to have neural abnormalities that predate substance involvement. Therefore, thicker frontal cortices could be a risk factor for initiating heavy substance use (e.g., less efficient processing of information and problem solving abilities, decreased ability to weigh risks vs. benefits), rather than a consequence of binge drinking. Longitudinal studies are needed to disentangle premorbid differences from alcohol-induced changes in cortical morphometry and establish temporal ordering of these features and behaviors.
In the current study, thicker cortices in adolescents were associated with worse behavioral performance. This was particularly the case for females, which corresponds with our previous findings that less brain activation in female bingers was associated with worse sustained attention and working memory (Squeglia et al. 2011). Although overall, binge drinking males had thinner cortices than male controls, those binge drinking males with thicker cortices were more likely to have worse attention scores. This corresponds to our overall hypothesis that thicker cortices were associated with less neurodevelopment. These neuro-behavioral findings may suggest greater disruptions in brain–behavior relationships among females and a heightened vulnerability to subtle yet deleterious effects of alcohol use on neuromaturation, but this notion requires confirmation in longitudinal studies. Previous evidence has similarly suggested greater susceptibility to alcohol-related brain changes among women as compared with men (Caldwell et al. 2005; Hommer et al. 1996, 2001; Jacobson 1986; Mann et al. 1992; Medina et al. 2008; Schweinsburg et al. 2003; Squeglia et al. 2009b, 2011).
These results also parallel past morphometric (Medina et al. 2008) and recent functional MRI findings (Squeglia et al. 2011) that showed analogous alcohol use × gender interactions in frontal regions for adolescents who engage in heavy drinking. The observed gender-specific patterns may relate to divergent neuromaturation trajectories, hormonal vacillations, and differences in alcohol metabolism between female and male adolescents. Hormonal fluctuations stimulated by alcohol use (Emanuele et al. 2001; Kim et al. 2003) or differences in female metabolism, body fat ratios, and lower body weight (Frezza et al. 1990; Wechsler et al. 1995) could also explain the observed gender-specific differences.
Limitations of this study include the cross-sectional design, as differences in cortical thickness could precede or follow binge drinking. While marijuana and other drug use were statistically controlled for in analyses, it is possible these variables continue to confound the findings. Illicit drug use was relatively limited (on average, 16 lifetime marijuana use days for females, 4 for males; 6 lifetime other substance use days for females, 1 for males), and no participant met criteria for a marijuana or other substance use disorder. Therefore, it is possible but unlikely that co-occurring limited drug use influenced outcomes. Longitudinal research, already in progress, will help determine temporal sequence of alcohol use and brain development abnormalities. Additionally, the sample is comprised of healthy, high-functioning adolescents, so findings may not generalize to clinical or lower-functioning samples. However, these findings have important clinical and public health implications, particularly given the participants' limited, sub-diagnostic alcohol use, limited other substance use, and absence of psychopathology. The normative practice of binge drinking during this crucial maturational period could have a negative impact on long-term academic, occupational, and social functioning extending into adulthood.
In conclusion, binge drinking during adolescence is associated with gender-specific differences in typical frontal cortical thickness. This raises the possibility that females may be more susceptible to the adverse neurodevelopmental effects of heavy alcohol use during adolescence. Greater cortical thinning is related to more proficient performance for both female and male adolescents. This is the only study we are aware of that has examined cortical thickness in heavy-drinking adolescents and one of only two to examine cortical thickness in adolescent substance users (Lopez-Larson et al. 2011). Future longitudinal research should examine the significance of cortical thickness in relation to alcohol consumption, as heavy binge drinking during this time may impact behaviors such as academic performance, driving safety, and neuropsychological performance.
Acknowledgments
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (R01 AA13419, PI: Tapert; F31 AA018940, PI: Squeglia, F32 AA018597; R21 AA019748, PI: Pulido) and the National Institute on Drug Abuse (R01 DA021182, P20 DA024194, P20 DA027834).
The authors thank Veronique Boucquey, Norma Castro, Sonja Eberson, Diane Goldenberg, Joanna Jacobus, Anthony Scarlett, Rachel Thayer, Dr. Sunita Bava, Dr. Sandra Brown, Dr. Karen Hanson, Dr. Omar Mahmood, Dr. M.J. Meloy, and the participating families and schools.
Footnotes
Conflicts of interest and financial disclosures The authors report no conflicts of interest or financial disclosures.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington: 2001. [Google Scholar]
- APA . Diagnostic and statistical manual of mental disorders. 4th edn. vol DSM-IV. American Psychiatric Association; Washington: 1994. [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res: Neuroimaging. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-2. Psychological Corporation; San Antonio: 1996. [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Supplement 4):S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan executive function system: examiner's manual. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Steiner J, Kirsteins L, Emanuele MA. Effect of chronic ethanol exposure on female rat reproductive cyclicity and hormone secretion. Alcohol Clin Exp Res. 2001;25:1025–1029. [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF. Intoxication test evidence. 2nd edn. Clark Boardman Callaghan; Deerfield: 1995. [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004:1021. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TFr, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. Yale University; New Haven: 1965. [Google Scholar]
- Hommer DW, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, Eckardt M. Decreased corpus callosum size among alcoholic women. Arch Neurol. 1996;53:359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Hommer DW, Momenan R, Kaiser E, Rawlings RR. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Jacobson R. The contributions of sex and drinking history to the CT brain scan changes in alcoholics. Psychol Med. 1986;16:547–549. doi: 10.1017/s003329170001031x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings. National Institute on Drug Abuse; Bethesda: 2009. 2008 (NIH Publication No. 09-7401) [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings. National Institute on Drug Abuse; Bethesda: 2011. [Google Scholar]
- Jones E, Sigall H. The bogus pipeline: a new paradigm for measuring affect and attitude. Psychol Bull. 1971;76:349–364. [Google Scholar]
- Kim JH, Kim HJ, Noh HS, Roh GS, Kang SS, Cho GJ, Park SK, Lee BJ, Choi WS. Suppression by ethanol of male reproductive activity. Brain Res. 2003;989:91–98. doi: 10.1016/s0006-8993(03)03372-9. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring DW, Meador KJ. The Medical College of Georgia (MCG) complex figures: four forms for follow-up. In: Knight J, Kaplan E, editors. Rey–Osterrieth handbook. Psychological Assessment Resources; Odessa: (2003. [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Mann K, Batra A, Gunthner A, Schroth G. Do women develop alcoholic brain damage more readily than men? Alcohol Clin Exp Res. 1992;16:1052–1056. doi: 10.1111/j.1530-0277.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Moore EE, Nichols ME, Abney OL, Taylor HS, Zamrini EY, Loring DW. The role of cholinergic systems in visuospatial processing and memory. J Clin Exp Neuropsychol. 1993;15:832–842. doi: 10.1080/01688639308402599. [DOI] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- NIAAA . NIAAA Council approves definition of binge drinking. vol 3. NIAAA Newsletter; Bethesda: 2004. [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolescence. 1988:17. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Rey A, Osterrieth PA. Corwin J, Bylsma FW, translators. Translations of excerpts from Andre Rey's “Psychological examination of traumatic encephalopathy” and P.A. Osterrieth's “The complex figure copy test”. Clin Neuropsychol. 1993;7:3–21. [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JIJ, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2007 National Survey on Drug Use and Health. National Findings; Rockville: 2008. Substance Abuse and Mental Health Services Administration, Office of Applied Studies (2008) (NSDUH Series H-34). DHHS Publication No. SMA 08-4343. [Google Scholar]
- Scheff SW, Price DA, Sparks DL. Quantitative assessment of possible age-related change in synaptic numbers in the human frontal cortex. Neurobiol Aging. 2001;22:355–365. doi: 10.1016/s0197-4580(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I. Effects of alcoholism and gender on brain metabolism. Am J Psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Wood AG. Lessons about neurodevelopment from anatomical magnetic resonance imaging. J Dev Behav Pediatr. 2011;32:158–168. doi: 10.1097/DBP.0b013e318206d58f. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: psychosocial and biological methods. Humana; Totowa: 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Thompson PM, Tessner K, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. 1st edn. W. W. Norton & Co Inc; New York: 2009. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto: 1970. [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. J Clin EEG Neurosci. 2009a;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009b;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Dager Schweinsburg A, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcoholism: Clin Experiment Res. 2011 doi: 10.1111/j.1530-0277.2011.01527.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneham ET, Sanders EM, Sanyal M, Dumas TC. Rules of engagement: factors that regulate activity-dependent synaptic plasticity during neural network development. Biol Bull. 2010;219:81–99. doi: 10.1086/BBLv219n2p81. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. J Int Neuropsychol Soc. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–184. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LB. Localisation of cerebral lesions by psychological testing. Clin Neurosurg. 1969;16:269–287. doi: 10.1093/neurosurgery/16.cn_suppl_1.269. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd edn. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Wechsler H, Isaac N. `Binge' drinkers at Massachusetts colleges. Prevalence, drinking style, time trends, and associated problems. J Am Med Assoc. 1992;267:2929–2931. doi: 10.1001/jama.267.21.2929. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S. Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. J Am Med Assoc. 1994;272:1672–1677. [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Davenport A, Rimm EB. A gender-specific measure of binge drinking among college students. Am J Public Health. 1995;85:982–985. doi: 10.2105/ajph.85.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmark E. A micromethod for the estimation of alcohol in blood. Biochemistry. 1922;131:473–484. [Google Scholar]
- Wilkinson GS. WRAT-3: Wide Range Achievement Test administration manual. 3rd edn. Western Psychological Services; Wilmington: 1993. [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms: I. Biopyschosocial variation among pathways into symptomatic difficulty. In: Babor TF, Hesselbrock V, Meyer RE, Shoemaker W, editors. Types of alcoholics: evidence from clinical, experimental and genetic research. The New York Academy of Sciences; New York: 1994. pp. 134–146. [DOI] [PubMed] [Google Scholar]