Abstract
Background
The serotonin (5-hydroxytryptamin; 5-HT) system has a central role in the circuitry of cognition and emotions. Multiple lines of evidence suggest that genetic variation in the serotonin transporter gene (SLC6A4; 5-HTT) is associated with schizophrenia and suicidal behavior. In this study, we wanted to elucidate whether SLC6A4 variations is involved in attempted suicide among patients with schizophrenia in a Scandinavian case–control sample.
Methods
Patients diagnosed with schizophrenia from three Scandinavian samples were assessed for presence or absence of suicide attempts, based on record reviews and interview data. Seven SLC6A4 single nucleotide polymorphisms (SNPs) were genotyped in 837 schizophrenia patients and 1,473 control individuals. Association analyses and statistical evaluations were performed with the program UNPHASED (version 3.0.9).
Results
We observed an allele association between the SNP rs16965628, located in intron one of SLC6A4, and attempted suicide (adjusted p-value 0.01), among patients with schizophrenia. No association was found to a diagnosis of schizophrenia, when patients were compared to healthy control individuals.
Conclusion
The gene SLC6A4 appears to be involved in suicidal ideation among patients with schizophrenia. Independent replication is needed before more firm conclusions can be drawn.
Keywords: Suicide ideation, Serotonin transporter gene, Association, Schizophrenia
Background
The lifetime risk of committing suicide among patients with schizophrenia is approximately 5% [1,2]. Also attempted suicide is common, with estimates ranging from 20% to 40% [3]. The heritable component of suicide attempt is partly related to psychiatric disorders but also partly independent of them [4-6].
The serotonin (5-hydroxytryptamin; 5-HT) system has a central role in the circuitry of cognition and emotions and exerts significant effects on anxiety, mood, impulsivity, sleep, ingestive behavior, reward systems, and psychosis [7]. Pharmacological agents targeting central 5-HT system has substantial effects on emotional behaviors [8-10]. Moreover, several reports imply that 5-HT may be involved in pathophysiological events associated with suicide [11-15]. In fact, postmortem examination of suicide victims shows significantly lower serotonin transporter binding in the prefrontal cortex [16].
Epidemiological and genetic studies indicate that there is a genetic component to suicidal behavior [17]. For example, a single nucleotide polymorphism (SNP) (rs1800532, A218C) in the tryptophan hydroxylase 1 gene (TPH1), which encodes a rate-limiting enzyme involved in the development of 5-HT, was previously found to be associated with suicide or suicidal attempt [18,19]. The same SNP was also investigated by us in the Scandinavian case–control material used in the present study [20]. However, no association was detected between TPH1 A218C and suicide attempt among the patients, although an association was found to schizophrenia.
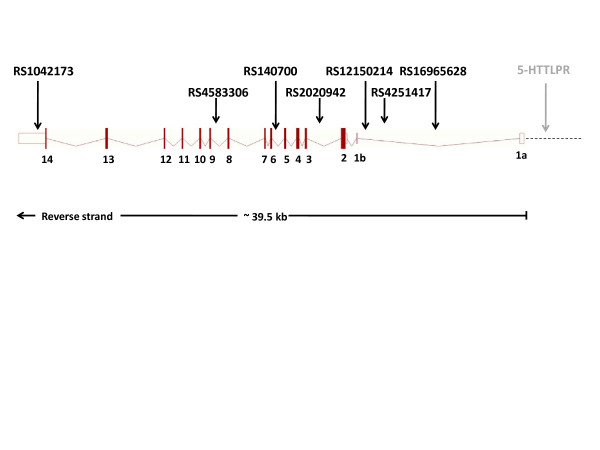
Another gene in the serotonin system, reported several times to be involved in the risk of attempting suicide, is the serotonin transporter gene (SLC6A4 or 5-HTT) [11,12,19,21]. The human SLC6A4 gene (Figure 1), located on chromosome 17q11.2, is about 39,500 base pairs long and contains 14 exons, of which exons 1a and 1b are alternatively transcribed. The gene encodes a membrane protein that plays a major role in the regulation of synaptic serotonin concentrations by recycling serotonin from the synaptic cleft.
Figure 1.
Genomic structure of the SLC6A4 gene. Exons (1a–14) are represented by filled boxes and untranslated regions are represented with open boxes. The 5’-UTR is indicated with a dashed line. The SNPs in the figure was included in present study. The 5-HTTLPR locus marked with grey color, was not included in the present association study.
Results from studies of 5-HTT knockout mouse have provided important clues regarding downstream effects of altered 5-HT function on behavior and the development of the brain, such as regulation of cell proliferation, migration and differentiation of neuronal tissue [22,23]. Interestingly, early alterations in 5-HT availability during the development of the 5-HT system might impact the distribution and density of specific 5-HT receptors, thereby altering the postsynaptic effects of 5-HT on target neurons and the reactivity of these neurons to specific stimuli [24].
In recent years, five genome-wide studies analyzing suicidal behavior in patients with bipolar disorder, alcoholism and major depression identified candidate regions on chromosome 2p11-12, 2p25 and 6q25-26 [6,25-29]. As far as we know, no genes associated with suicidal behavior have been reported in these regions yet. Neither are we aware of any genome wide studies analyzing suicidal behavior in patients with schizophrenia.
In the present study, we wanted to further explore the possible involvement of the serotonin system in suicide among patients with schizophrenia, by analyzing genetic variations in the SLC6A4 gene using a Scandinavian case–control sample. Scandinavian countries are generally considered to be well suited for genetic studies, as the populations are ethnically homogeneous and only recently have been subject to non-Caucasian immigration. Seven SNPs located in the SLC6A4 gene (Figure 1), were selected and genotyped in 837 patients suffering from schizophrenia and related disorders, of which 738 had information on suicidal behavior, and in 1,473 control individuals.
Methods
Clinical samples
The clinical samples originate from the Scandinavian Collaboration on Psychiatric Etiology (SCOPE) and were collected in Denmark (DK), Norway (NO), and Sweden (SE). Affected individuals were diagnosed according to ICD-10 (DK) or DSM-III-R/DSM-IV (NO and SE). All individuals were born in Scandinavia and of Caucasian origin, and the vast majority had two Scandinavian born parents. Detailed description of the samples was reported earlier [20,30-33]. The total sample set included 837 patients, of which 734 were diagnosed with schizophrenia, 87 with schizoaffective disorder and 16 with schizophreniform disorder, and 1473 control individuals (Table 1). Patients (N = 738) were assessed for presence or absence of suicide attempts based on record reviews (DK, SE) and interview data (NO, SE). Any previous self-harm in combination with suicidal ideation, documented or reported, was regarded as a suicide attempt. For the present analyses, subjects were divided into those who had made at least one suicide attempt and those who had not attempted suicide. The Danish Scientific Committees, the Danish Data Protection Agency, the Norwegian Scientific-Ethical Committees, the Norwegian Data Protection Agency, the Ethical Committee of the Karolinska Hospital, the Stockholm Regional Ethical Committee and the Swedish Data Inspection Board all approved the study. All participants had given informed consent prior to inclusion in the study.
Table 1.
Characteristics of Danish (DK), Norwegian (NO), and Swedish (SE) samples analyzed for association between serotonin transporter gene (SLC6A4 ) polymorphisms and schizophrenia and suicide attempt among affected (cases) and control individuals
| Country | Characteristics | Cases | Controls |
|---|---|---|---|
| DK |
N |
420 |
1004 |
| |
Gender (% women) |
42.6 |
41.5 |
| |
Age |
43.9 ± 12.3 |
43.2 ± 11.8 |
| |
Age of onset |
27.2 ± 8.9 |
|
| |
No of suicide attempters |
153 |
|
| NO |
N |
162 |
177 |
| |
Gender (% women) |
46.3 |
55.5 |
| |
Age |
37.2 ± 10.7 |
38.7 ± 10.3 |
| |
Age of onset |
27.6 ± 8.7 |
|
| |
No of suicide attempters |
42 |
|
| SE |
N |
255 |
292 |
| |
Gender (% women) |
37.2 |
37.7 |
| |
Age |
54.1 ± 15.2 |
50.3 ± 10.1 |
| |
Age of onset |
24.6 ± 6.9 |
|
| No of suicide attempters | 95 |
SNP Genotyping
Seven SNPs in the SLC6A4 gene, previously reported to be associated with schizophrenia, were analyzed. Genomic DNA was extracted from whole blood samples, and the polymorphisms were genotyped at the SNP Technology Platform in Uppsala, Sweden (http://www.genotyping.se), using the Illumina BeadStation 500GX and the 1536-plex Illumina Golden Gate assay (Illumina, Inc., San Diego, CA). The sample success rate was on average 99.7% for the genotyped SNPs and the reproducibility of the genotyping was 100% according to duplicate analysis of 2.6% of the genotypes.
Statistical analyses
Hardy–Weinberg (HW) equilibrium was tested in the control samples using Fisher’s exact test as implemented in PEDSTATS [34]. Accounting for the number of tested markers, no SNP deviated significantly from HW equilibrium. Linkage disequlibrium (D’ and R2) between SNP pairs, and haplotype block structure [35] were determined with Haploview 4.0 [36] (Additional file 1: Figure S1). The fixation index (FST) was calculated for each SNP separately, and all loci combined, in the control sample with FSTAT, grouping controls by country of origin. No evidence of population stratification was evident from the data: the fixation index for all combined loci was 0.003 (0.001-0.005) (95% bootstrap confidence interval).
Association analysis comparing allele and genotype frequencies between suicide attempters and non-attempters within the patient group and between affected individuals and controls were performed with UNPHASED (version 3.0.9) [37,38]. To account for possible population stratification and the effect of sex, we included country of origin and sex as confounding factors in the analyses. To examine whether genetic associations were homogenous, we carried out separate analyses testing if country and gender modified the association. Correction for multiple testing was done by permutation tests (n = 1000).
Results
Out of the seven markers tested, only rs16965628 located in the SLC6A4 gene, showed significant association to suicide attempt (P-value for allele association = 0.00092) and the result remained significant also after correction for multiple testing (P-value = 0.01) (Table 2). The minor allele C was underrepresented among patients with records of suicidal behavior.
Table 2.
Serotonin transporter (SLC6A4 ) single nucleotide polymorphisms (SNPs) investigated in suicidal attempters (SA) and non-suicidal (No SA) attempters with a schizophrenia diagnosis
|
SNP |
SNP localization |
Position |
|
MAF |
Base |
Genotype counts |
Allele association |
Genotype association |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
|
|
|
(SA/No SA) |
|
OR |
|
OR |
OR |
||
| (no of bases) | (1/2) | 1 1 | 1 2 | 2 2 | P-value | 2 vs 1 | P-value | 12 vs 11 | 22 vs 11 | ||||
| Rs1042173 |
3'-UTR |
28525011 |
|
0.47 |
A/C |
70/125 |
144/195 |
75/104 |
0.218 |
1.14 |
0.27 |
1.34 |
1.29 |
| Rs4583306 |
Intron 8 |
28538715 |
13704 |
0.03 |
A/G |
77/134 |
150/201 |
60/88 |
0.335 |
1.11 |
0.29 |
1.33 |
1.20 |
| Rs140700 |
Intron 5 |
28543389 |
4674 |
0.08 |
C/T |
243/348 |
43/79 |
2/0 |
0.560 |
0.89 |
0.11 |
0.80 |
2.26E9 |
| Rs2020942 |
Intron 2 |
28546914 |
3525 |
0.39 |
C/T |
108/157 |
135/201 |
44/68 |
0.777 |
0.97 |
0.94 |
0.99 |
0.93 |
| Rs12150214 |
Intron 1 |
28550888 |
3974 |
0.17 |
G/C |
213/301 |
67/117 |
10/8 |
0.735 |
0.95 |
0.23 |
0.81 |
1.72 |
| Rs4251417 |
Intron 1 |
28551858 |
970 |
0.10 |
C/T |
227/347 |
55/77 |
4/1 |
0.338 |
1.19 |
0.18 |
1.07 |
6.26 |
| Rs16965628 | Intron 1 | 28555425 | 3567 | 0.05 | G/C | 272/374 | 15/51 | 0/1 | 0.0009 (0.01) | 0.39 | 0.004 (0.02) | 0.40 | 3.92E-5 |
The table shows nominal allele and genotype association results with gender and country as covariates.
Chromosomal position, minor allele frequency (MAF), SNP-alleles (Base), genotype counts, P-values and odds ratios (OR) for allele and genotype association for the seven SNP markers are presented in the table. Global significant P-values are shown in parenthesis.
We also performed genotype association analyses. However, due to the low frequency of the minor allele of rs16965628 (0.05) only one individual was CC homozygous, and consequently the same association signal was captured with the allele and the genotype test. We did not find any evidence of heterogeneity in the association signal between the three countries, nor between male and female patients for this marker (P-value = 0.75 and 0.17, for country and gender as modifiers, respectively). In other words, all countries and both genders contributed to the observed association between rs16965628 and suicidal behavior.
For completeness, the allele and genotype frequencies of the seven polymorphisms in patients with suicidal behavior were also contrasted against the frequencies in healthy controls. The results were similar to the comparison within the patient group, and the rs16965628 C-allele was significantly lower among individuals with suicidal behavior than in the controls (p-value = 0.01 after correction for multiple testing, data not shown).
Since genetic variants in the SLC6A4 were previously found to be associated with schizophrenia in other sample sets [39-44], we analyzed the association between the seven SNPs and schizophrenia in the Scandinavian case–control sample. Two SNPs were weakly associated with the disease (Table 3), but after correction for multiple testing no association was statistically significant (P-value > 0.10).
Table 3.
Serotonin transporter (SLC6A4 ) single nucleotide polymorphisms (SNPs) investigated in patients with schizophrenia and control subjects
|
SNP |
Base |
MAF |
Genotype counts (schizophrenia/control) |
Allele association |
Genotype association |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Allele 1 |
|
Allele 2 |
|
OR |
|
|
OR |
OR |
|
| (1/2) | Homozyogotes | Heterozygotes | Homozygotes | p-value | 2 vs 1 | p-value | 12 vs 11 | 22 vs 11 | ||||
| Rs1042173 |
A/C |
0.46 |
223/410 |
402/770 |
207/291 |
0.083 |
|
1.11 |
0.022 |
|
0.93 |
1.27 |
| Rs4583306 |
A/G |
0.42 |
276/475 |
410/755 |
173/237 |
0.020 |
|
1.16 |
0.020 |
|
1.04 |
1.40 |
| Rs140700 |
C/T |
0.09 |
689/1233 |
140/221 |
2/10 |
0.435 |
|
1.09 |
0.171 |
|
1.17 |
0.39 |
| Rs2020942 |
C/T |
0.14 |
313/528 |
390/714 |
128/228 |
0.359 |
|
0.94 |
0.518 |
|
0.90 |
0.91 |
| Rs12150214 |
G/C |
0.18 |
595/982 |
219/438 |
21/46 |
0.123 |
|
0.88 |
0.282 |
|
0.86 |
0.83 |
| Rs4251417 |
C/T |
0.09 |
673/1204 |
148/257 |
9/7 |
0.214 |
|
1.14 |
0.123 |
|
1.07 |
2.78 |
| Rs16965628 | G/C | 0.06 | 753/1310 | 77/152 | 1/9 | 0.192 | 0.83 | 0.125 | 0.91 | 0.19 | ||
The table shows nominal allele and genotype association results with gender and country as covariates.
Nominal allele and genotype association results are reported with gender and country as covariates. MAF, minor allele frequencies in control individuals (allele 2 in the table).
Nominally significant; after correction for multiple testing using permutation test, all P- values > 0.10.
We analyzed linkage disequilibrium (LD) between the polymorphisms and found two blocks. All markers, except rs16965628, were located in the observed LD blocks (Additional file 1: Figure S1). Haplotype association analyzes for the two blocks, with respect to suicidal attempt and schizophrenia susceptibility, were performed with UNPHASED. The haplotype and single marker analyzes gave similar results, i.e. there were no significant haplotype association with neither phenotype (p > 0.106, data not shown).
Discussion
In the present study we found an association between the SLC6A4 rs16965628 polymorphism and attempted suicide among patients with schizophrenia (P-value = 0.00092 and global P-value = 0.01). The odds ratio was 0.39, indicating that the presence of the minor C-allele protects against suicidal behavior. The rs16965628 polymorphism is located in the first intron of the SLC6A4 gene, approximately 9 kb downstream of promoter region. The most studied polymorphisms in the SLC6A4 gene are a 44-base pair insertion–deletion in the promoter region, generating the major L (LA and LG) and S-alleles, and a 17-bp variable number of tandem repeats (VNTR) in the second intron [45]. During the years conflicting results have been reported regarding genetic variants in the SLC6A4 gene and suicidal ideation [46]. The ambiguous results from different studies possibly reflect insufficient sample sizes to obtain adequate statistical power, and heterogeneity between populations [47]. However, the two latest of three meta-analyses report association between SLC6A4 variants and suicidal behavior [11,12,21]. In the latest and largest meta-analysis, including 39 different association studies (covering all published studies up to 2006), a significant association was found between 5-HTTLPR and suicide attempts/suicide. The L-allele was underrepresented among individuals with suicidal behavior (odds ratio was 0.88), suggesting that the investigated gene variant had a protective effect against suicide [11].
The mechanism behind this association is unknown, but the 5-HTTLPR polymorphism appears to affect SLC6A4 gene expression. That is, the transcription of the SLC6A4 gene is lower in the presence of the S-allele as compared to the L or the LA-alleles [48,49]. As mentioned in the introduction, postmortem examination of suicide victims shows significantly lower serotonin transporter binding in the prefrontal cortex [16]. Thus it is possible that the protective effect of the L-allele is the result of elevated expression of the serotonin transporter protein, leading to an overall increase in serotonin transporter binding.
The relative abundance of allele transcripts of the serotonin transporter gene in human cell lines from HapMap CEPH trios, confirm the effects of 5-HTTLPR variation on the transcription of the SLC6A4 gene [50]. However, in these cell lines variation in the rs16965628 SNP was associated with considerably more transcriptional variation than the 5-HTTLPR polymorphism, and the rs16965628 C-allele was linked with increased transcription (the mean allele G/C transcript ratio was 0.47) [50]. Thus we speculate that the decreased frequency of suicidal behavior among schizophrenic patients that carried the rs16965628 C-allele, observed in this study, may have been caused by increased transcription of the SLC6A4 gene and a corresponding increase in serotonin transporter binding.
The gene SLC6A4 has frequently been implicated in psychiatric disorders [51]. Meta-analyses have reported association between 5-HTTLPR S-variant - and sometimes also the 17-bp VNTR in intron 2 polymorphism - and bipolar disorder [52-55], co-morbid bipolar disorder and tobacco use disorder [56], alcohol dependence [57,58], major depression [59,60], antidepressant-induced mania [61,62], a modulation effect of 5-HTTLPR on stress and depression [63], however highly discussed [64], and anxiety-related traits [65-67], almost all disorders with elevated rates of suicide or suicide attempts. Almost all of the above mentioned disorders show elevated rates of suicide or suicide attempts. In schizophrenia, the 5-HTTLPR and 17-bp VNTR in intron 2 polymorphisms have been associated with the disorder in individual studies and the 17-bp VNTR also in several meta-analyses [68-70]. However, the latest update in the SzGene database (http://www.szgene.org) indicates lack of overall allele association. In previous studies, 5-HTTLPR and schizophrenia was investigated by us using Swedish case–control samples, partly overlapping with the present. The results indicated that alleles within the gene were associated with age of onset [71] and disease [44]. Other researchers reported that a haplotype, including markers 5-HTTLPR and rs16965628, was associated with obsessive compulsive disorder [72]. Furthermore, rs16965628 was found to modulate task-related activation in ventral prefrontal cortex in patients with posttraumatic stress disorder [73]. Patients with obsessive compulsive disorder and posttraumatic stress disorder are known to have an elevated risk of committing suicide [74,75].
In the present study, we did not find any significant association between genetic variants in SLC6A4 and schizophrenia although an association was found to suicide attempt. This may seem contradictive but previous epidemiological studies including monozygotic and dizygotic twins showed an elevated risk of attempting suicide even after controlling for psychiatric disorders, indicating that genetic factors independent of psychiatric disorders affect the risk of suicide attempt [4,5]. Thus our results do not support that the investigated SNPs are associated with a substantial increase in the disease risk in the Scandinavian population. However, even though our sample size was large, it does not have the power to unambiguously detect weak signals, and thus a true association with an odds ratio below 1.2 cannot be ruled out [76].
Conclusions
The present results provide support for an association between a relatively uncommon SLC6A4 polymorphism and suicidal behavior among schizophrenic patients of Scandinavian origin. As the marker was not associated with the disease, the decreased risk for suicidal behavior appears to be unlinked to that of schizophrenia susceptibility. Although the estimated effect of the rs1695628 C-allele was substantial, its low frequency suggests that the potential contribution to variation in suicidal behavior in the population would be limited. Further studies in independent samples are needed to establish a link between rs1695628 and suicidal behavior in the Scandinavian and other populations.
Abbreviations
SNP: Single nucleotide polymorphism; SLC6A4 (5-HTT): Serotonin transporter gene; TPH1: Tryptophan hydroxylase 1; SCOPE: Scandinavian Collaboration on Psychiatric Etiology; DK: Denmark; NO: Norway; SE: Sweden; ICD: International Statistical Classification of Diseases and Related Health Problems; DSM: Diagnostic and Statistical Manual of Mental Disorders; DNA: Deoxyribonucleic acid; LD: Linkage Disequilibrium.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ELC wrote the draft and coordinated the preparation of the manuscript. PS participated in the study design, performed the statistical analyses and was involved in the manuscript preparation. AR and JHT contributed with data collection (DK sample). SD participated in the study design and contributed with data collection (NO sample). IM participated in the study design, clinical characterization and contributed with data collection (NO sample). OAA participated in the study design and contributed with data collection (NO sample). TW participated in the study design and contributed with data collection (DK sample). IA, HH and LT participated in the study design and contributed with data collection (SE sample). EGJ participated in the study design, clinical characterization, contributed with data collection (SE sample) and was involved in the manuscript preparation. All authors contributed to and have approved the final manuscript.
Supplementary Material
Figure S1. Linkage disequilibrium (LD) structure in the three Scandinavian control samples of seven serotonin transporter gene (SLC6A4) single nucleotide polymorphisms. Haplotype block structure (outlined), D’ (numbers, 100 not printed) and r2 (shadings) are given. The figure shows pair-wise LD among the seven SLC6A4 SNPs, as calculated by the Haploview 4.0 software. High r2 values with strong linkage disequilibrium are indicated by dark color. Gray and white colors indicate weak linkages with low r2 values. The genetic positions of the SNPs in the SLC6A4 gene are indicated in the white bar above the LD-plot.
Contributor Information
Eva Lindholm Carlström, Email: eva.lindholm@igp.uu.se.
Peter Saetre, Email: peter.saetre@ki.se.
Anders Rosengren, Email: anders.rosengren@regionh.dk.
Johan H Thygesen, Email: johan.hilge.thygesen@regionh.dk.
Srdjan Djurovic, Email: srdjan.djurovic@medisin.uio.no.
Ingrid Melle, Email: ingrid.melle@medisin.uio.no.
Ole A Andreassen, Email: o.a.andreasen@medisin.uio.no.
Thomas Werge, Email: Thomas.werge@regionh.dk.
Ingrid Agartz, Email: ingrid.agartz@medisin.uio.no.
Håkan Hall, Email: hakan.hall@pet.medkem.uu.se.
Lars Terenius, Email: lars.terenius@ki.se.
Erik G Jönsson, Email: erik.jonsson@ki.se.
Acknowledgements
We thank patients and controls for their participation and express our gratitude towards health professionals who facilitated our work. This study was financed in Denmark by grants to TW from the Lundbeck Foundation (R34-A3243); the Danish National Advanced Technology Foundation (001-2009-2); the Danish Council for Independent Research in Medical Sciences (09–065634), in Norway from the Research Council of Norway (147787, 167153), the South-Eastern Norway Health Authority (Helse Sør-Øst RHF 123/2004), Oslo University Hospital, and University of Oslo, and in Sweden from the Swedish Research Council (K2007-62X-15077-04-1, K2007-62X-15078-04-3, K2008-62P-20597-01-3. K2010-62X-15078-07-2), the regional agreement on medical training and clinical research between Stockholm County Council and the Karolinska Institutet, the Knut and Alice Wallenberg Foundation, and the HUBIN project. We thank Alexandra Tylec for technical assistance. We also thank Kristina Larsson, Per Lundmark, Tomas Axelsson and Ann-Christine Syvänen at the SNP Technology Platform for performing the genotyping. The SNP Technology Platform is supported by Uppsala University, Uppsala University Hospital and by the Knut and Alice Wallenberg Foundation.
References
- Carlborg A, Winnerbäck K, Jönsson EG, Jokinen J, Nordström P. Suicide in schizophrenia. Expert Rev Neurother. 2010;10:1153–1164. doi: 10.1586/ern.10.82. [DOI] [PubMed] [Google Scholar]
- Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24:81–90. doi: 10.1177/1359786810385490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili M, Amador XF, Girardi P, Harkavy-Friedman J, Harrow M, Kaplan K, Krausz M, Lester D, Meltzer HY, Modestin J. et al. Suicide risk in schizophrenia: learning from the past to change the future. Ann Gen Psychiatry. 2007;6:10. doi: 10.1186/1744-859X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowinski AL, Bucholz KK, Nelson EC, Fu Q, Madden PA, Reich W, Heath AC. Suicide attempts in an adolescent female twin sample. J Am Acad Child Adolesc Psychiatry. 2001;40:1300–1307. doi: 10.1097/00004583-200111000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statham DJ, Heath AC, Madden PA, Bucholz KK, Bierut L, Dinwiddie SH, Slutske WS, Dunne MP, Martin NG. Suicidal behaviour: an epidemiological and genetic study. Psychol Med. 1998;28:839–855. doi: 10.1017/S0033291798006916. [DOI] [PubMed] [Google Scholar]
- Willour VL, Seifuddin F, Mahon PB, Jancic D, Pirooznia M, Steele J, Schweizer B, Goes FS, Mondimore FM, Mackinnon DF. et al. A genome-wide association study of attempted suicide. Mol Psychiatry. 2011; : . doi: 10.1038/mp.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JA, Ansorge MS, Merker R, Weisstaub N, Zhou M. New lessons from knockout mice: The role of serotonin during development and its possible contribution to the origins of neuropsychiatric disorders. CNS Spectr. 2003;8:572–577. doi: 10.1017/s1092852900018848. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology. 1998;19:287–299. doi: 10.1016/S0893-133X(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Neumeister A. Tryptophan depletion, serotonin, and depression: where do we stand? Psychopharmacol Bull. 2003;37:99–115. [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61:765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- Li D, He L. Meta-analysis supports association between serotonin transporter (5-HTT) and suicidal behavior. Mol Psychiatry. 2007;12:47–54. doi: 10.1038/sj.mp.4001890. [DOI] [PubMed] [Google Scholar]
- Lin PY, Tsai G. Association between serotonin transporter gene promoter polymorphism and suicide: results of a meta-analysis. Biol Psychiatry. 2004;55:1023–1030. doi: 10.1016/j.biopsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- Stanley M, Virgilio J, Gershon S. Tritiated imipramine binding sites are decreased in the frontal cortex of suicides. Science. 1982;216:1337–1339. doi: 10.1126/science.7079769. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Courtet P, Jollant F, Castelnau D, Buresi C, Malafosse A. Suicidal behavior: relationship between phenotype and serotonergic genotype. Am J Med Genet C Semin Med Genet. 2005;133C:25–33. doi: 10.1002/ajmg.c.30043. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Chaste P, Malafosse A. Association between the TPH gene A218C polymorphism and suicidal behavior: a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2004;124B:87–91. doi: 10.1002/ajmg.b.20015. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Giegling I, Sato T, Hartmann AM, Moller HJ. Genetic variations in tryptophan hydroxylase in suicidal behavior: analysis and meta-analysis. Biol Psychiatry. 2003;54:465–473. doi: 10.1016/S0006-3223(02)01748-1. [DOI] [PubMed] [Google Scholar]
- Saetre P, Lundmark P, Wang A, Hansen T, Rasmussen HB, Djurovic S, Melle I, Andreassen OA, Werge T, Agartz I. et al. The tryptophan hydroxylase 1 (TPH1) gene, schizophrenia susceptibility, and suicidal behavior: a multi-centre case–control study and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2009;153B:387–396. doi: 10.1002/ajmg.b.30991. [DOI] [PubMed] [Google Scholar]
- Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. Mol Psychiatry. 2003;8:646–653. doi: 10.1038/sj.mp.4001336. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Cheng R, Juo SH, Loth JE, Nee J, Iossifov I, Blumenthal R, Sharpe L, Kanyas K, Lerer B, Lilliston B. et al. Genome-wide linkage scan in a large bipolar disorder sample from the National Institute of Mental Health genetics initiative suggests putative loci for bipolar disorder, psychosis, suicide, and panic disorder. Mol Psychiatry. 2006;11:252–260. doi: 10.1038/sj.mp.4001778. [DOI] [PubMed] [Google Scholar]
- Hesselbrock V, Dick D, Hesselbrock M, Foroud T, Schuckit M, Edenberg H, Bucholz K, Kramer J, Reich T, Goate A. et al. The search for genetic risk factors associated with suicidal behavior. Alcohol Clin Exp Res. 2004;28:70S–76S. doi: 10.1097/01.alc.0000127416.92128.b0. [DOI] [PubMed] [Google Scholar]
- Willour VL, Zandi PP, Badner JA, Steele J, Miao K, Lopez V, MacKinnon DF, Mondimore FM, Schweizer B, McInnis MG. et al. Attempted suicide in bipolar disorder pedigrees: evidence for linkage to 2p12. Biol Psychiatry. 2007;61:725–727. doi: 10.1016/j.biopsych.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Maher BS, Hughes HB, Zubenko WN, Scott Stiffler J, Marazita ML. Genome-wide linkage survey for genetic loci that affect the risk of suicide attempts in families with recurrent, early-onset, major depression. Am J Med Genet B Neuropsychiatr Genet. 2004;129:47–54. doi: 10.1002/ajmg.b.30092. [DOI] [PubMed] [Google Scholar]
- Butler AW, Breen G, Tozzi F, Craddock N, Gill M, Korszun A, Maier W, Middleton LT, Mors O, Owen MJ. et al. A genomewide linkage study on suicidality in major depressive disorder confirms evidence for linkage to 2p12. Am J Med Genet B Neuropsychiatr Genet. 2010;153:1465–1473. doi: 10.1002/ajmg.b.31127. [DOI] [PubMed] [Google Scholar]
- Hansen T, Olsen L, Lindow M, Jakobsen KD, Ullum H, Jonsson E, Andreassen OA, Djurovic S, Melle I, Agartz I. et al. Brain expressed microRNAs implicated in schizophrenia etiology. PLoS One. 2007;2:e873. doi: 10.1371/journal.pone.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson EG, Larsson K, Vares M, Hansen T, Wang AG, Djurovic S, Rönningen KS, Andreassen OA, Agartz I, Werge T. et al. Two methylenetetrahydrofolate reductase gene (MTHFR) polymorphisms, schizophrenia and bipolar disorder: an association study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:976–982. doi: 10.1002/ajmg.b.30671. [DOI] [PubMed] [Google Scholar]
- Kähler AK, Djurovic S, Kulle B, Jönsson EG, Agartz I, Hall H, Opjordsmoen S, Jakobsen KD, Hansen T, Melle I. et al. Association analysis of schizophrenia on 18 genes involved in neuronal migration: MDGA1 as a new susceptibility gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1089–1100. doi: 10.1002/ajmg.b.30726. [DOI] [PubMed] [Google Scholar]
- Saetre P, Agartz I, De Franciscis A, Lundmark P, Djurovic S, Kähler A, Andreassen OA, Jakobsen KD, Rasmussen HB, Werge T. et al. Association between a disrupted-in-schizophrenia 1 (DISC1) single nucleotide polymorphism and schizophrenia in a combined Scandinavian case–control sample. Schizophr Res. 2008;106:237–241. doi: 10.1016/j.schres.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Wigginton JE, Abecasis GR. PEDSTATS: descriptive statistics, graphics and quality assessment for gene mapping data. Bioinformatics. 2005;21:3445–3447. doi: 10.1093/bioinformatics/bti529. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M. et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25:115–121. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- Dudbridge F. Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum Hered. 2008;66:87–98. doi: 10.1159/000119108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, Zai G, Tharmalingam S, de Bartolomeis A, Wong G, Kennedy JL. Association study between the novel functional polymorphism of the serotonin transporter gene and suicidal behaviour in schizophrenia. Eur Neuropsychopharmacol. 2006;16:268–271. doi: 10.1016/j.euroneuro.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Dubertret C, Hanoun N, Ades J, Hamon M, Gorwood P. Family-based association study of the 5-HT transporter gene and schizophrenia. Int J Neuropsychopharmacol. 2005;8:87–92. doi: 10.1017/S1461145704004948. [DOI] [PubMed] [Google Scholar]
- Lin C, Tang W, Hu J, Gao L, Huang K, Xu Y, He G, Liang P, Feng G, He L, Shi Y. Haplotype analysis confirms association of the serotonin transporter (5-HTT) gene with schizophrenia in the Han Chinese population. Neurosci Lett. 2009;453:210–213. doi: 10.1016/j.neulet.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Saiz PA, Garcia-Portilla MP, Arango C, Morales B, Alvarez V, Coto E, Fernandez JM, Bascaran MT, Bousono M, Bobes J. Association study of serotonin 2A receptor (5-HT2A) and serotonin transporter (5-HTT) gene polymorphisms with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:741–745. doi: 10.1016/j.pnpbp.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Vijayan NN, Iwayama Y, Koshy LV, Natarajan C, Nair C, Allencherry PM, Yoshikawa T, Banerjee M. Evidence of association of serotonin transporter gene polymorphisms with schizophrenia in a South Indian population. J Hum Genet. 2009;54:538–542. doi: 10.1038/jhg.2009.76. [DOI] [PubMed] [Google Scholar]
- Zaboli G, Jonsson EG, Gizatullin R, De Franciscis A, Asberg M, Leopardi R. Haplotype analysis confirms association of the serotonin transporter (5-HTT) gene with schizophrenia but not with major depression. Am J Med Genet B Neuropsychiatr Genet. 2008;147:301–307. doi: 10.1002/ajmg.b.30597. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Liou YJ. Recent molecular genetic studies and methodological issues in suicide research. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:809–817. doi: 10.1016/j.pnpbp.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Arango V, Huang YY, Underwood MD, Mann JJ. Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res. 2003;37:375–386. doi: 10.1016/S0022-3956(03)00048-7. [DOI] [PubMed] [Google Scholar]
- Gonda X, Fountoulakis KN, Harro J, Pompili M, Akiskal HS, Bagdy G, Rihmer Z. The possible contributory role of the S allele of 5-HTTLPR in the emergence of suicidality. J Psychopharmacol. 2011;25:857–866. doi: 10.1177/0269881110376693. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL. et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Cleak J, Willis-Owen SA, Flint J, Shifman S. Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Mol Psychiatry. 2007;12:421–422. doi: 10.1038/sj.mp.4001952. [DOI] [PubMed] [Google Scholar]
- Levinson DF. Meta-analysis in psychiatric genetics. Curr Psychiatry Rep. 2005;7:143–151. doi: 10.1007/s11920-005-0012-9. [DOI] [PubMed] [Google Scholar]
- Furlong RA, Ho L, Walsh C, Rubinsztein JS, Jain S, Paykel ES, Easton DF, Rubinsztein DC. Analysis and meta-analysis of two serotonin transporter gene polymorphisms in bipolar and unipolar affective disorders. Am J Med Genet. 1998;81:58–63. doi: 10.1002/(SICI)1096-8628(19980207)81:1<58::AID-AJMG11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG. Meta-analysis of serotonin transporter polymorphisms and affective disorders. Psychiatr Genet. 2004;14:121–129. doi: 10.1097/00041444-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Lasky-Su JA, Faraone SV, Glatt SJ, Tsuang MT. Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:110–115. doi: 10.1002/ajmg.b.30104. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Meira-Lima I, Cordeiro Q, Michelon L, Sham P, Vallada H, Collier DA. Population-based and family-based studies on the serotonin transporter gene polymorphisms and bipolar disorder: a systematic review and meta-analysis. Mol Psychiatry. 2005;10:771–781. doi: 10.1038/sj.mp.4001663. [DOI] [PubMed] [Google Scholar]
- McEachin RC, Saccone NL, Saccone SF, Kleyman-Smith YD, Kar T, Kare RK, Ade AS, Sartor MA, Cavalcoli JD, McInnis MG. Modeling complex genetic and environmental influences on comorbid bipolar disorder with tobacco use disorder. BMC Med Genet. 2010;11:14. doi: 10.1186/1471-2350-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Hofmann SG, Asnaani A, Sawyer AT, Otto MW. The serotonin transporter gene and risk for alcohol dependence: a meta-analytic review. Drug Alcohol Depend. 2010;108:1–6. doi: 10.1016/j.drugalcdep.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Leon S, Janssens AC, Gonzalez-Zuloeta Ladd AM, Del-Favero J, Claes SJ, Oostra BA, van Duijn CM. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2008;13:772–785. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- Clarke H, Flint J, Attwood AS, Munafo MR. Association of the 5- HTTLPR genotype and unipolar depression: a meta-analysis. Psychol Med. 2010;40:1767–1778. doi: 10.1017/S0033291710000516. [DOI] [PubMed] [Google Scholar]
- Daray FM, Thommi SB, Ghaemi SN. The pharmacogenetics of antidepressant-induced mania: a systematic review and meta-analysis. Bipolar Disord. 2010;12:702–706. doi: 10.1111/j.1399-5618.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- Biernacka JM, McElroy SL, Crow S, Sharp A, Benitez J, Veldic M, Kung S, Cunningham JM, Post RM, Mrazek D, Frye MA. Pharmacogenomics of antidepressant induced mania: a review and meta-analysis of the serotonin transporter gene (5HTTLPR) association. J Affect Disord. 2012;136:e21–29. doi: 10.1016/j.jad.2011.05.038. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression A Meta-analysis. Jama-Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, Jarvelin MR, Taanila A, Flint J. 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:271–281. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JB, Sklar P. Meta-analysis reveals association between serotonin transporter gene STin2 VNTR polymorphism and schizophrenia. Mol Psychiatry. 2005;10:928–938. doi: 10.1038/sj.mp.4001690. [DOI] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: meta-analyses of 12 candidate genes. Schizophr Res. 2008;104:96–107. doi: 10.1016/j.schres.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D, Jönsson EG, Paus S, Ganguli R, Nöthen M, Nimgaonkar VL. Schizophrenia and the serotonin transporter gene. Psychiatr Genet. 1998;8:207–212. doi: 10.1097/00041444-199808040-00002. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, Murphy DL. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. 2008;17:717–723. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
- Morey RA, Hariri AR, Gold AL, Hauser MA, Munger HJ, Dolcos F, McCarthy G. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry. 2011;11:76. doi: 10.1186/1471-244X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconu G, Turecki G. Obsessive-compulsive personality disorder and suicidal behavior: evidence for a positive association in a sample of depressed patients. J Clin Psychiatry. 2009;70:1551–1556. doi: 10.4088/JCP.08m04636. [DOI] [PubMed] [Google Scholar]
- Panagioti M, Gooding P, Tarrier N. Post-traumatic stress disorder and suicidal behavior: A narrative review. Clin Psychol Rev. 2009;29:471–482. doi: 10.1016/j.cpr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Saetre P, Vares M, Andreou D, Larsson K, Timm S, Rasmussen HB, Djurovic S, Melle I, Andreassen OA. et al. DTNBP1, NRG1, DAOA, DAO and GRM3 polymorphisms and schizophrenia: an association study. Neuropsychobiology. 2009;59:142–150. doi: 10.1159/000218076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Linkage disequilibrium (LD) structure in the three Scandinavian control samples of seven serotonin transporter gene (SLC6A4) single nucleotide polymorphisms. Haplotype block structure (outlined), D’ (numbers, 100 not printed) and r2 (shadings) are given. The figure shows pair-wise LD among the seven SLC6A4 SNPs, as calculated by the Haploview 4.0 software. High r2 values with strong linkage disequilibrium are indicated by dark color. Gray and white colors indicate weak linkages with low r2 values. The genetic positions of the SNPs in the SLC6A4 gene are indicated in the white bar above the LD-plot.