Abstract
Lipid droplets (LDs) and peroxisomes are central players in cellular lipid homeostasis: some of their main functions are to control the metabolic flux and availability of fatty acids (LDs and peroxisomes) as well as of sterols (LDs). Both fatty acids and sterols serve multiple functions in the cell—as membrane stabilizers affecting membrane fluidity, as crucial structural elements of membrane-forming phospholipids and sphingolipids, as protein modifiers and signaling molecules, and last but not least, as a rich carbon and energy source. In addition, peroxisomes harbor enzymes of the malic acid shunt, which is indispensable to regenerate oxaloacetate for gluconeogenesis, thus allowing yeast cells to generate sugars from fatty acids or nonfermentable carbon sources. Therefore, failure of LD and peroxisome biogenesis and function are likely to lead to deregulated lipid fluxes and disrupted energy homeostasis with detrimental consequences for the cell. These pathological consequences of LD and peroxisome failure have indeed sparked great biomedical interest in understanding the biogenesis of these organelles, their functional roles in lipid homeostasis, interaction with cellular metabolism and other organelles, as well as their regulation, turnover, and inheritance. These questions are particularly burning in view of the pandemic development of lipid-associated disorders worldwide.
WORK for the past five decades on the yeast Saccharomyces cerevisiae has contributed fundamental insight into peroxisome biogenesis and function that is also relevant for mammalian cells. While LD research in yeast is still in its infancy and looks back to a much shorter history—the previous edition of YeastBook did not even mention LDs as an “organelle”—combined biochemical, cell biological, lipidomic, and proteomic studies in recent years have already contributed significant insight into LD biogenesis and function.
Lipid Droplets
LDs, also termed “lipid particles,” “lipid bodies,” or “oil bodies,” are ubiquitous subcellular structures that have only in recent years been recognized as metabolically highly dynamic organelles (Daum et al. 2007a; Fujimoto et al. 2008; Goodman 2008, 2009; Guo et al. 2009; Krahmer et al. 2009; Murphy et al. 2009; Olofsson et al. 2009; Walther and Farese 2009, 2012; Athenstaedt and Daum 2011). In the past, LDs were primarily considered as rather inert storage depots for the ‘”neutral lipids,” triacylglycerols (TAG) and steryl esters (SE). However, the increased biomedical interest in understanding neutral lipid homeostasis, fueled by the pandemic increase in lipid-associated disorders, has moved LDs into the spotlight of biomedical research (Farese and Walther 2009; Walther and Farese 2012). Given the significant homology of lipid biosynthetic processes to mammalian cells, yeast LD research has gained a great momentum to address the fundamental mechanisms of LD assembly and the regulation of neutral lipid homeostasis (Athenstaedt and Daum 2006, 2011; Czabany et al. 2007; Daum et al. 2007a,b; Rajakumari et al. 2008; Kohlwein 2010a,b).
Among subcellular organelles, LDs are unique in their structure, as they appear to harbor only a monolayer of phospholipids that surrounds the hydrophobic core consisting of TAG and SE. A second feature standing out is that LDs, like peroxisomes, are organelles that are not essential under standard nutritional conditions, i.e., in the presence of carbon sources other than fatty acids (FA). Unlike other organelles, LD biogenesis and degradation need to be discussed in the context of the synthesis and turnover of their major components, namely neutral lipids: their biogenesis is driven by the availability of precursors for the synthesis of their core compounds, TAG and SE, and cells are devoid of LDs in the absence of the cellular capacity to synthesize these lipids (Garbarino et al. 2009; Petschnigg et al. 2009). On the other hand, TAG synthesis—and concomitant formation of LDs—is essential for cell survival in the presence of excess FAs (Garbarino et al. 2009; Petschnigg et al. 2009; Fakas et al. 2011b). The LD surface is decorated with numerous proteins that are, in part, also present in the endoplasmic reticulum (ER) membrane, raising the question as to the specific signals that target proteins to the LD surface. The highly dynamic nature of LDs in growing cells reflects the importance of neutral lipids in various stages of cell growth and in response to the nutritional status of the cell; the metabolic role of LDs is highlighted by the recent discoveries that TAG-derived metabolites are required for efficient cell cycle progression (Kurat et al. 2009) and that TAG play an essential role in counteracting FA-induced lipotoxicity (Garbarino et al. 2009; Petschnigg et al. 2009; Fakas et al. 2011b).
Experimental approaches to studying LD biology
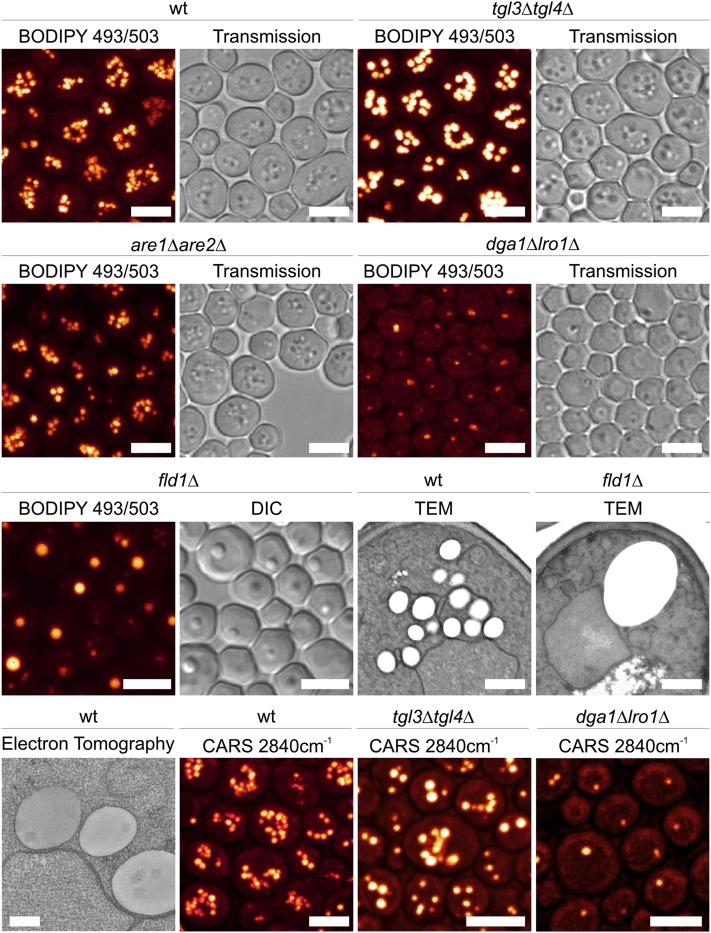
In vivo, LDs are readily detectable by transmission light microscopy (differential interference contrast (DIC; Nomarski optics) due to their high refractive index (Figure 1). Numerous cell-permeable hydrophobic fluorescence dyes that label LDs with high specificity, including Nile Red, LD540, and BODIPY dyes, exist (Szymanski et al. 2007; Fei et al. 2008; Wolinski and Kohlwein 2008; Spandl et al. 2009; Wolinski et al. 2011, 2012). It should be noted, however, that these dyes are potential substrates of the pleiotropic drug resistance pumps (Ivnitski-Steele et al. 2009), and staining efficiency may strongly depend on the activity of these pumps in the respective strain backgrounds. Thus, staining of LDs in growing cultures that contain both young and aged cells may appear quite heterogeneous; fixation of cells with formaldehyde or elimination of Pdr pumps strongly increases labeling efficiency (Wolinski and Kohlwein 2008; Wolinski et al. 2009a, 2012). Given the specificity and ease of labeling of both living and fixed cells, several microscopy- or photometry-based screens of yeast mutant collections have been performed to identify mutants with altered LD morphology and content (Szymanski et al. 2007; Fei et al. 2008; Bozaquel-Morais et al. 2010; Adeyo et al. 2011; Fei and Yang 2012). In addition, green fluorescent protein-tagged reporter constructs of LD-associated proteins provide an additional tool for studying LD dynamics and inheritance (Kurat et al. 2006; Jacquier et al. 2011; Wolinski et al. 2012). It should be noted that the number and size of LDs vary greatly between various yeast wild-type strains, and it is currently unclear which genetic traits are responsible for this heterogeneity. Microscopy-based screens of the GFP-labeled protein collection (Huh et al. 2003) have also led to the identification of numerous novel LD-associated proteins (Natter et al. 2005; see below). In addition to the use of hydrophobic fluorescent dyes, recent advances in spectroscopic imaging techniques such as coherent anti-Stokes Raman scattering (CARS) microscopy allow the label-free imaging of yeast LDs (brackmann et al. 2009; Kohlwein 2010b; Wolinski et al. 2012). This technology is based on the C-H molecular vibrations in the FA acyl chains that are packed in high density as TAG in the LD, and thus independent of exogenously supplied fluorescent dyes or endogenously expressed fluorescent protein reporter constructs (see also Figure 1).
Figure 1 .
Morphological characteristics of yeast lipid droplets. (Rows 1–3, left panels) Fluorescence images of LDs that are labeled with BODIPY 493/503 (Wolinski and Kohlwein 2008; Wolinski et al. 2009a, 2012). (Right panels) Corresponding transmission images. All strains, except the fld1Δ mutant, were cultivated for 72 h in YPD complete medium; fld1Δ mutants were grown in synthetic complete (minimal) medium with 2mg/liter inositol for 12 h. Images were obtained by confocal laser scanning microscopy and represent projections of 8–12 optical sections. wt, wild type; tgl3Δ tgl4Δ, mutant lacking the major TAG lipases; are1Δ are2Δ, mutant lacking the steryl ester synthases and thus harboring LDs that contain TAG only; dga1Δ lro1Δ, mutant lacking acyl-CoA and phospholipid-dependent diacylglycerol (DAG) acyltransferases and thus harboring LDs that contain SE only; fld1Δ mutant, lacking the yeast ortholog of seipin. TEM: transmission electron microscopy images of wild type (wt) and the fld1Δ mutant. (Row 4) Electron tomography (ET) of LDs in wild type, showing close association of LDs with the ER membrane. CARS: coherent anti-Stokes Raman scattering microscopy of LDs in wild type, tgl3Δ tgl4Δ mutant, and dga1Δ lro1Δ mutant. CARS is a label-free imaging technique that generates contrast by imaging molecular vibrations at 2840 cm−1. Scale bar: 500 nm in the TEM images, 200 nm in the ET image, and 5 μm in the fluorescence/transmission images. See text for details. Images courtesy of H. Wolinski (fluorescence and CARS microscopy) and D. Kolb (electron microscopy and tomography).
Higher resolution images of LDs are obtained by electron microscopy and electron tomography (Binns et al. 2006; Perktold et al. 2007; Czabany et al. 2008; Jacquier et al. 2011; Wolinski et al. 2011) (Figure 1), which also demonstrate their close physical interactions with other intracellular organelles, in particular the ER, mitochondria, and peroxisomes (Binns et al. 2006; Pu et al. 2011). Biophysical studies on isolated LDs have been performed using X-ray small-angle scattering analyses, dynamic light scattering, and differential scanning calorimetry to unveil LD size distribution and structural organization, depending on lipid composition (Czabany et al. 2008; Spanova et al. 2012; see below).
LD can be purified from cellular extracts by ultracentrifugation/flotation (Leber et al. 1994; Athenstaedt et al. 1999; Connerth et al. 2009). Since LDs are in close contact with other intracellular organelles, a clean LD preparation requires careful cell lysis (e.g., enzymatic digestion of the cell wall with Zymolyase), followed by differential centrifugation, to obtain a layer of LDs—together with vacuolar membranes—floating on top of the centrifuge tube. Attached vacuolar membranes are separated by an additional centrifugation step that requires a pH/buffer change (Athenstaedt et al. 1999; Connerth et al. 2009; Grillitsch et al. 2011). This protocol restricts LD preparations to cells in late log/early stationary phase that are susceptible to Zymolyase lysis of the cell wall.
Lipid droplet structure
LDs isolated from yeast are rather homogeneous in size, ranging from typically 300 nm (in late log phase) to 1 μm (in stationary phase) in diameter. In the late log/early stationary phase of growth, the majority of LDs fall into a rather narrow 350- to 450-nm size range, largely independent of their lipid composition (Czabany et al. 2008). As discussed below, LDs are subject to high metabolic turnover and may be almost completely degraded during the early log phase of growth (Kurat et al. 2006). Wild-type LDs containing about equal amounts of TAG and SE are typically spherical structures, in which the core of neutral lipids is surrounded by a monolayer of phospholipids, which, according to the current biogenesis models, is derived from the ER membrane (Mechanisms of LD biogenesis and inheritance). X-ray small-angle scattering experiments have unveiled some level of supramolecular organization of LDs, indicating that SE form a shell surrounding the rather fluid disordered TAG core (Czabany et al. 2008). A hem1 mutant that is defective in sterol synthesis accumulates the sterol intermediate squalene in LDs, which is found in subcellular membranes as well as in LD and leads to a disordering of the shell structure (Spanova et al. 2012).
Lipid composition of purified LDs
The major lipid components of LDs are the neutral lipids, TAG and SE (Zinser et al. 1991; Leber et al. 1994; Connerth et al. 2009). In mammalian cells, LD composition may vary, depending on cell type, and contain mostly TAG (as in adipocytes) or TAG and cholesteryl esters, retinylesters, and free cholesterol (as in liver). It should be emphasized that the designation “neutral lipid’ of these compounds refers to their uncharged and highly hydrophobic structure, but not to their (active) involvement in cellular metabolism. TAG and SE are present in about equal amounts in LDs (Daum et al. 2007b; Czabany et al. 2008; Rajakumari et al. 2008; Connerth et al. 2009; Grillitsch et al. 2011). The TAG molecular species distribution reflects the cellular content of long-chain FAs, namely predominantly C16 and C18 saturated and mono-unsaturated FAs, giving rise to the most prominent 48:2, 50:2, 50:3, 52:2, and 52:3 TAG molecular species1 in wild-type cells grown on glucose (Connerth et al. 2009; Grillitsch et al. 2011). Somewhat different TAG profiles were obtained for cells grown on raffinose (Ejsing et al. 2009). This finding also reflects the dynamic nature of LDs [and the entire yeast lipidome for that matter (Klose et al. 2012)] that respond quickly to growth rate and carbon source. Accordingly, growth of yeast in the presence of oleic acid as the sole carbon source results in TAG species predominantly composed of TAG 54:3 (Grillitsch et al. 2011). The SE fraction is mainly composed of ergosterol esterified with oleic acid (C18:1) and palmitoleic acid (Czabany et al. 2008), but sterol intermediates, such as zymosterol, episterol, and fecosterol are also found esterified in the SE fraction (Zweytick et al. 2000b; Czabany et al. 2008).
The phospholipid monolayer of LDs is enriched in the anionic phospholipid, phosphatidylinositol, compared to total cellular phospholipids (Schneiter et al. 1999; Connerth et al. 2009; Grillitsch et al. 2011); notably, the molecular species distribution of LD phospholipids is quite distinct from that of the ER membrane, from which it is presumably derived (Connerth et al. 2009; Grillitsch et al. 2011), and appears to be enriched in double-unsaturated species (Schneiter et al. 1999). Notably, phosphatidylinositol molecular species with medium-chain fatty acids (C12 and C14), which are quite prominent in subcellular membranes (Ejsing et al. 2009; Klose et al. 2012), are excluded from the LD phospholipid monolayer (Schneiter et al. 1999).
Protein composition of LDs
Although LDs are present in almost all cell types, ranging from bacteria to mammals, their protein composition is rather divergent (Murphy 2001; Yang et al. 2012). The proteome of highly purified LDs from yeast is composed of a characteristic set of proteins, but the overall protein content is rather low (Table 1). Notably, most of the LD-resident enzymes identified so far play a role in lipid metabolism, emphasizing the active role of this organelle in cellular metabolism (Athenstaedt and Daum 2006; Czabany et al. 2007; Daum et al. 2007a,b; Rajakumari et al. 2008; Kohlwein 2010b; Grillitsch et al. 2011). Notably, the set of LD-associated proteins may substantially change during cellular growth, in particular if FAs such as oleic acid are supplied, to induce formation of LDs and peroxisomes (Grillitsch et al. 2011). Also, size and phospholipid composition that are dependent on growth conditions and media composition (e.g., presence or absence of the phospholipid precursor inositol) may influence the LD proteome (Fei et al. 2011c). Numerous LD-associated proteins display a dual localization also to the ER (Table 1; see below), and their relative distribution to both organelles may change during various stages of growth. Since LDs closely interact with other subcellular organelles, some of the identified proteins may actually be contaminants during preparation. On the other hand, a transient association of non-LD-resident proteins may also be of physiological significance (see Physiological role of LDs): evidence suggests that association of proteins with LDs, at least in mammalian cells, may serve a protective or regulatory role (Hodges and Wu 2010).
Table 1 . Neutral lipid metabolism enzymes and LD-associated proteins.
| Gene | Enzyme | Molecular mass (kDa) | Isoelectric point | Molecules per cella | Locationb | Transmembrane domains | Phosphorylation sitesc | |
|---|---|---|---|---|---|---|---|---|
| Neutral lipid synthesis enzymes | ||||||||
| SCT1 (GAT2) | Glycerol-3-P/dihydroxyacetone-P acyltransferase | 85.7 | 7.27 | 1,050 | ER | 4 | Few | |
| GPT2 (GAT1) | Glycerol-3-P/dihydroxyacetone-P acyltransferase | 83.6 | 10.3 | 3,100 | ER, lipid droplets | 4 | Several | |
| AYR1 | Acyl DHAP reductase | 32.8 | 9.92 | 3,670 | ER, lipid droplets | None | None | |
| SLC1 | LysoPA/Acylglycerol-3-P acyltransferase | 33.8 | 10.41 | NDd | ER, lipid droplets | 1 | None | |
| ALE1 (SLC4, LPT1, LCA1) | LysoPA/Acylglycerol-3-P acyltransferase | 72.2 | 10.3 | ND | ER | 7 | Several | |
| PHM8 | LysoPA phosphatase | 37.7 | 5.14 | 195 | Cytoplasm, nucleus | None | None | |
| LOA1 (VPS66) | LysoPA acyltransferase | 33.8 | 10.5 | 6,630 | ER, lipid droplets | 1 | None | |
| PAH1 (SMP2) | PA phosphatase | 95 | 4.68 | 3,910 | Cytoplasm, ER | None | Several | |
| DGK1 (HSD1) | DAG kinase | 32.8 | 9.48 | 784 | ER | 4 | Few | |
| DGA1 | Acyl-CoA diacylglycerol acyltransferase | 47.7 | 10.39 | 907 | ER, lipid droplets | 1 | Few | |
| LRO1 | Phospholipid diacylglycerol acyltransferase | 75.3 | 6.67 | ND | ER | 1 | Few | |
| ARE1 (SAT2) | Acyl-CoA sterol acyltransferase | 71.6 | 8.27 | ND | ER | 9 | Several | |
| ARE2 (SAT1) | Acyl-CoA sterol acyltransferase | 74.0 | 7.71 | 279 | ER | 9 | Several | |
| Neutral lipid turnover enzymes | ||||||||
| LDH1 | Triacylglycerol lipase, hydrolase | 43.3 | 6.51 | ND | Lipid droplets | None | None | |
| TGL1 (YKL5) | Triacylglycerol lipase, sterylester hydrolase | 63.0 | 6.83 | 1,470 | ER, lipid droplets | 1 | Several | |
| TGL2 | Acylglycerol lipase | 37.5 | 8.41 | ND | Mitochondria | None | None | |
| TGL3 | Triacylglycerol lipase, lysoPA acyltransferase | 73.6 | 8.50 | 3,210 | Lipid droplets | 1 | Few | |
| TGL4 (STC1) | Triacylglycerol lipase, Ca++ dependent phospholipase A2, lysoPA acyltransferase | 102.7 | 8.05 | 195 | Lipid droplets | None | Several | |
| TGL5 (STC2) | Triacylglycerol lipase, lysoPA acyltransferase | 84.7 | 9.84 | 358 | Lipid droplets | 1 | Several | |
| YEH1 | Sterylester hydrolase | 66.5 | 6.33 | 7,770 | Lipid droplets | 1-2 | None | |
| YEH2 | Sterylester hydrolase | 62.4 | 8.91 | 1,630 | Plasma membrane | 1 | Few | |
| YJU3 | Monoacylglycerol lipase | 35.6 | 8.5 | 2,140 | Lipid droplets, ER | None | None | |
| Lipid droplet-associated proteinsd | ||||||||
| ATF1 | Alcohol-O-acetyltransferase | 61.0 | 6.94 | 1,990 | Lipid droplets | None | None | |
| AYR1 | Acyl DHAP reductase | 32.8 | 9.92 | 3,670 | ER, lipid droplets | None | None | |
| BSC2 | Unknown function | 26.6 | 5.10 | 922 | Lipid droplets | 1 | None | |
| COY1 | Unknown function, similarity to mammalian CASP | 77.5 | 5.93 | 2,650 | Lipid droplets, Golgi | 1 | Several | |
| CPR5 (CYP5) | Peptidyl-prolyl cis-trans isomerase (cyclophilin) | 25.3 | 5.40 | ND | Lipid droplets, ER | 1 | 1 | |
| CSR1 (SFH2) | Phosphatidylinositol transfer protein | 47.5 | 6.61 | 9,600 | Lipid droplets, cytoplasm | None | 1 | |
| CST26 (PSI1) | Stearoyl-CoA acyltransferase, phosphatidylinositol-specific | 45.5 | 10.15 | 2,010 | Lipid droplets, ER | Four | None | |
| EHT1 | Acyl-coenzymeA ethanol O-acyltransferase | 51.3 | 7.83 | 2,550 | Lipid droplets, ER | None | None | |
| ENV9 | Unknown function, similarity to oxidoreductases | 37.5 | 8.35 | 967 | Lipid droplets | 1 | None | |
| ERG1 | Squalene epoxidase | 55.1 | 6.45 | 65,400 | Lipid droplets, ER | 2 | None | |
| ERG27 | 3-Keto sterol reductase | 39.7 | 8.90 | ND | Lipid droplets | None | 1 | |
| ERG6 (ISE1, LIS1, SED6, VID1) | Δ24-Sterol C-methyltransferase | 43.3 | 5.60 | 53,800 | Lipid droplets, ER | None | Few | |
| ERG7 | Lanosterol synthase | 83.5 | 6.59 | 2,190 | Lipid droplets, ER | None | None | |
| FAA1 | Fatty acyl-CoA synthetase | 77.8 | 7.58 | 7,470 | Lipid droplets, ER | None | None | |
| FAA4 | Fatty acyl-CoA synthetase | 77.2 | 6.52 | 31,200 | Lipid droplets, ER | None | None | |
| FAT1 | Fatty acid transporter and fatty acyl-CoA synthetase | 77.1 | 8.47 | 16,900 | Lipid droplets, ER | 3 | None | |
| GTT1 | Glutathione S-transferase | 26.8 | 6.65 | ND | Lipid droplets, ER | None | None | |
| HFD1 | Hexadecenal dehydrogenase | 60.0 | 6.73 | 2,930 | Lipid droplets, mitochondria | 1 | 1 | |
| KES1 (LPI3, OSH4, BSR3) | Member of the oxysterol binding protein family | 49.5 | 5.92 | 32,200 | Lipid droplets, cytoplasm | None | None | |
| LDB16 | Unknown function | 29.0 | 8.07 | 149 | Lipid droplets | 2 | 1 | |
| LOA1 (VPS66) | LysoPA acyltransferase | 33.8 | 10.5 | 6,630 | Lipid droplets, ER | 1 | None | |
| NUS1 | Putative prenyltransferase | 42.6 | 6.94 | ND | Lipid droplets, ERe | 1 | None | |
| OSW5 | Unknown function | 16.4 | 9.94 | 922 | Lipid droplets | 2 | 1 | |
| PDI1 (MFP1, TRG1) | Protein disulfide isomerase | 58.2 | 4.22 | ND | Lipid droplets, ER (lumen) | None | None | |
| PDR16 (SFH3) | Phosphatidylinositol transfer protein | 40.7 | 8.16 | 15,400 | Lipid droplets, cytoplasm | None | 2 | |
| PET10 | Unknown function | 31.2 | 8.85 | 2,160 | Lipid droplets | None | None | |
| RRT8 | Unknown function | 39.6 | 10.66 | ND | Lipid droplets | 5 | None | |
| SLC1 | LysoPA/Acylglycerol-3-P acyltransferase | 33.8 | 10.41 | ND | Lipid droplets, ER | 1 | None | |
| SNA2 | Unknown function | 9.2 | 6.50 | 20,400 | Lipid droplets | 2 | 1 | |
| SNX41 | Sorting nexin | 70.7 | 7.02 | 1,800 | Lipid droplets, cytoplasm | None | None | |
| SRT1 | Cis-prenyl transferase | 40.2 | 10.30 | ND | Lipid droplets | 1 | None | |
| SSO1 | t-SNARE | 33.1 | 5.06 | 450 | Lipid droplets, vesicles | 1 | 1 | |
| TDH1 (GLD3) | Glyceraldehyde-3-phosphate dehydrogenase, isozyme 1 | 35.8 | 8.59 | 120,000 | Lipid droplets, cytoplasm | None | Several | |
| TDH2 (GLD2) | Glyceraldehyde-3-phosphate dehydrogenase, isozyme 2 | 35.8 | 6.96 | 121,000 | Lipid droplets, cytoplasm | None | Several | |
| TDH3 (GLD1, HSP35, HSP36, SSS2) | Glyceraldehyde-3-phosphate dehydrogenase, isozyme 3 | 35.7 | 6.96 | 169,000 | Lipid droplets, nucleus, cytoplasm | None | Several | |
| TGL1 (YKL5) | Triacylglycerol lipase, sterylester hydrolase | 63.0 | 6.83 | 1,470 | Lipid droplets, ER | 1 | Several | |
| TGL3 | Triacylglycerol lipase, lysoPA acyltransferase | 73.6 | 8.50 | 3,210 | Lipid droplets | 1 | Few | |
| TGL4 | Triacylglycerol lipase, Ca++ dependent phospholipase A2, lysoPA acyltransferase | 102.7 | 8.05 | 195 | Lipid droplets | None | Several | |
| TGL5 | Triacylglycerol lipase, lysoPA acyltransferase | 84.7 | 9.84 | 358 | Lipid droplets | 1 | Several | |
| UBX2 (SEL1) | Bridging factor involved in ER-associated protein degradation (ERAD) | 66.8 | 5.22 | 12,600 | Lipid droplets, ER | None | None | |
| USE1 (SLT1) | SNARE | 28.1 | 5.01 | 973 | Lipid droplets, ER | 1 | None | |
| YEH1 | Sterylester hydrolase | 66.5 | 6.33 | 7,770 | Lipid droplets | 1-2 | None | |
| YIM1 | Unknown function | 41.6 | 7.98 | 6,540 | Lipid droplets, ER | None | None | |
| YJU3 | Monoacylglycerol lipase | 35.6 | 8.5 | 2,140 | Lipid droplets, ER | None | None | |
| YPT7 (AST4, VAM4) | Rab family GTPase | 23.0 | 4.62 | 5,530 | Lipid droplets, cytoplasm | None | None | |
| YOR059c | Putative lipase | 51.1 | 9.81 | 1,210 | Lipid droplets | 1 | None | |
Much of the information in this table may be found in the Saccharomyces Genome Database. ND, not determined.
Habeler et al. (2002); Grillitsch et al. (2011); Kumar et al. (2002); Huh et al. (2003); Natter et al. (2005); Athenstaedt et al. (1999).
Based on PhosphoGrid (http://www.phosphogrid.org) and PhosphoPed (http://www.phosphopep.org) Databases.
Notably, yeast LDs do not contain proteins related to the perilipin family of proteins in mammals (Brasaemle 2007) or oleosins in plants (Chapman et al. 2012). Perilipins are prominent LD surface proteins that regulate the access of enzymes to the LD surface during lipogenesis or lipolysis (Brasaemle 2007). Oleosins and related proteins are characteristically shaped proteins that reside on the surface of oil droplets in plant seeds and nonseed tissues and play a role in stress response, hormone signaling, and plant growth and development (Chapman et al. 2012). Both types of surface proteins are believed to play important roles in LD biogenesis and structure and lipid mobilization; thus, the question remains how the size of LDs and processes acting on TAG and SE substrates are regulated in yeast in the absence of such LD coat proteins.
Biosynthesis of triacylglycerol and steryl esters
Formation of LD is driven by the synthesis of TAG and SE; in the absence of the biosynthetic capacity to form these lipids, no LD are present and LD-resident proteins may mis-localize to the ER or other intracellular structures and the cytosol (Athenstaedt and Daum 2006, 2011; Daum et al. 2007a,b; Rajakumari et al. 2008; Garbarino et al. 2009; Petschnigg et al. 2009; Jacquier et al. 2011). The enzymes involved in TAG and SE metabolism are listed in Table 1 (see also Henry et al. 2012).
The major substrates for the synthesis of TAG and SE are activated FAs and glycerol-3-phosphate or dihydroxyacetone phosphate (DHAP) and sterols, respectively. The first and rate-limiting step in FA synthesis is catalyzed by acetyl-CoA carboxylase, encoded by ACC1 (Roggenkamp et al. 1980; Al-Feel et al. 1992; Hasslacher et al. 1993; Tehlivets et al. 2007; Henry et al. 2012) (Figure 2). Acc1 converts acetyl-CoA to malonyl-CoA in an ATP, biotin, and CO2-dependent reaction. Malonyl-CoA is used by FA synthase, which consists of a hexameric α6β6 complex of two subunits encoded by FAS2 (α-subunit) and FAS1 (β-subunit) for the step-wise elongation of the growing acyl chain (Tehlivets et al. 2007). In contrast to mammalian FA synthase that releases free FAs, the yeast FAS complex generates acyl-CoAs that may be directly channeled into phosphatidic acid (PA), TAG and SE synthesis (Tehlivets et al. 2007; Henry et al. 2012). FA de novo synthesis is a major consumer of acetyl-CoA and NADPH, similar to sterol synthesis. Free FAs that are derived from exogenous supply or from endogenous lipid degradation need to be activated by one of five acyl-CoA synthetases, encoded by FAA1, FAA2, FAA3, FAA4, and FAT1 genes, which differ in their substrate specificities (Black and Dirusso 2007). Faa2 is required for the activation of FAs that are directed toward β-oxidation (see below). Faa1, Faa4, and Fat1 activate exogenously supplied FAs and free FAs that derive from phospholipid, TAG, and SE breakdown. In the absence of these acyl-CoA synthetases, yeast secretes lipolysis-derived FAs (Scharnewski et al. 2008), and growth and membrane lipid composition depend solely on the FAs that are generated by de novo synthesis, FA desaturation, and elongation (Tehlivets et al. 2007).
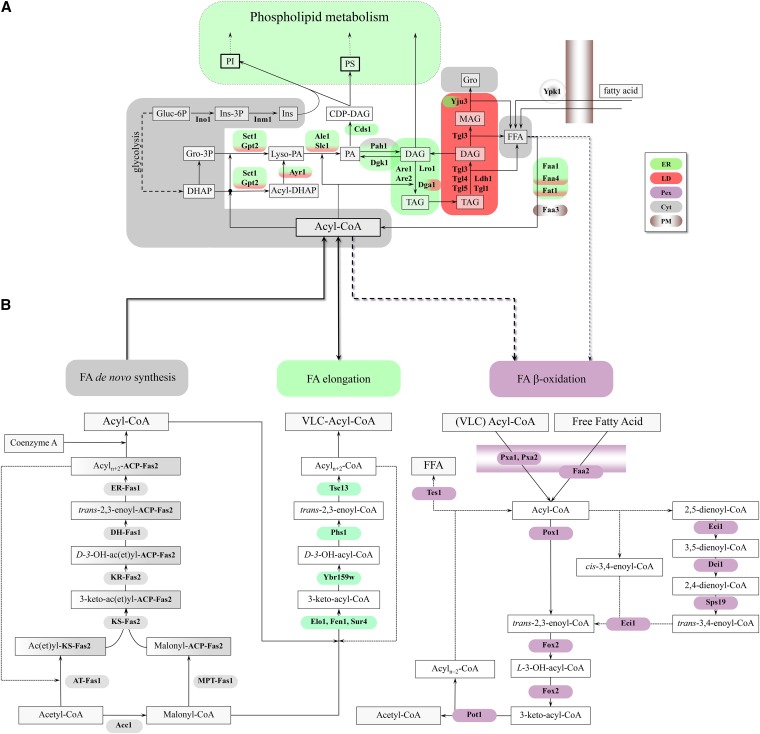
Figure 2 .
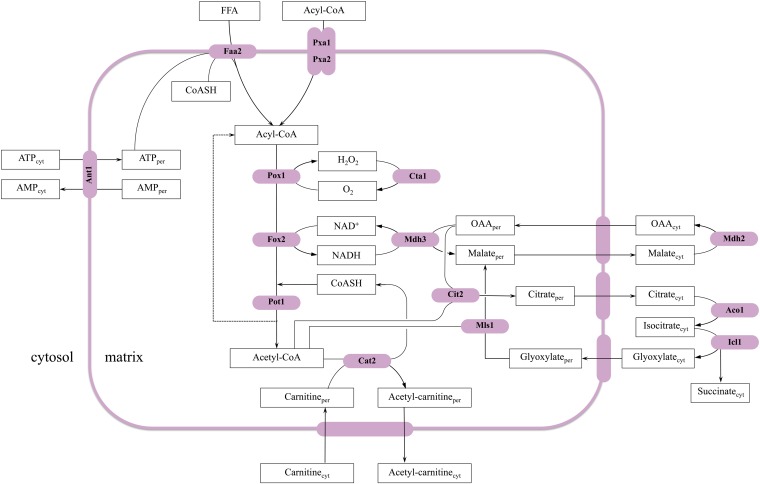
(A) Metabolic pathways of TAG synthesis and degradation and their subcellular localization (adapted from Kohlwein 2010b and Henry et al. 2012). Phospholipids and TAG share DAG and PA as common precursors. In the de novo synthesis of phospholipids, PA serves as the immediate precursor of CDP-DAG, precursor to PI, PGP, and PS. PA is dephosphorylated to DAG, which serves as the precursor of PE and PC in the Kennedy pathway. DAG also serves as the precursor for TAG and can be phosphorylated, regenerating PA. The names of the enzymes that are discussed in detail in the text are shown adjacent to the arrows of the metabolic conversions in which they are involved, and the gene–enzyme relationships are listed in Table 1. Lipids and intermediates are boxed, with the most abundant lipid classes boxed by bold lines. Enzyme names are indicated in boldface type. TAG, triacylglycerols; PI, phosphatidylinositol; PA, phosphatidic acid; CDP-DAG, CDP-diacylglycerol; DAG, diacylglycerol; MAG, monoacylglycerol; Gro, glycerol; Gluc-6P, glucose-6 phosphate; DHAP, dihydroxyacetone phosphate, PS, phosphatidylserine; FFA, free fatty acids; Ins, inositol. Nucl, nucleus; ER, endoplasmic reticulum; Mito, mitochondria; LD, lipid droplets; G/E/V, Golgi, endosomes, vacuole; Pex, peroxisomes; Cyt, cytoplasma; PM, plasma membrane. See text for details. (B) Metabolic pathways of fatty acid metabolism. FA de novo synthesis and elongation: FA (type I) de novo synthesis requires the synthesis of malonyl-CoA by the acetyl-CoA carboxylase Acc1. This cytosolic trifunctional enzyme harbors a covalently bound biotin, an N-terminal biotin carboxylase domain, and a C-terminal transcarboxylase domain (Tehlivets et al. 2007). Malonyl-CoA is used by the cytosolic FA synthase complex, consisting of Fas1 (β-subunit) and Fas2 (α-subunit), which are organized in a hexameric α6β6 complex. Fas1 harbors acetyl transferase (AT), enoyl reductase (ER), dehydratase (DH), and malonyl-palmitoyl transferase (MPT) activities; Fas2 contains the acyl carrier protein (ACP), 3-ketoreductase (KR), 3-ketosynthase (KS), and phosphopantheine transferase activities. The product of FA synthesis in yeast is acyl-CoA, typically C14–C16 carbon atoms in length (Tehlivets et al. 2007). Activated FAs may be elongated to VLCFAs by the activity of Elo1, Fen1/Elo2, and Sur4/Elo3 (condensing enzymes); Ybr159w (reductase); Phs1 (dehydratase); and Tsc13 (enoyl-CoA reductase). Yeast also expresses a set of bacterial type II enzymes (as individual polypeptides) that perform the same reactions in mitochondria, but are encoded by nuclear genes (Tehlivets et al. 2007). Mitochondrial FA synthesis presumably generates FA only up to C8, which is a precursor for lipoic acid synthesis. FAs are degraded by β-oxidation. β-oxidation in yeast occurs exclusively in peroxisomes. Medium chain fatty acids enter peroxisomes as free fatty acids (FFA) and are activated by a peroxisomal acyl-CoA synthetase, Faa2. ATP that is required for this activation step is imported into the organelle via Ant1. Long chain fatty acids, such as oleate, are activated outside the organelle by Fat1, Faa1, or Faa4 and taken up as CoA esters (acyl-CoA) via a peroxisomal ABC transporter that consists of the heterodimer Pxa1/Pxa2. Inside peroxisomes, CoA esters undergo dehydrogenation by Pox1, hydratation/dehydrogenation by Fox2, and ultimately thiolytical cleavage by Pot1, leading to acetyl-CoA and an acyl-chain shortened by two carbon atoms. Hydrogen peroxide produced by Pox1 is degraded by peroxisomal catalase T, Cta1. NADH is exported to the cytosol via a malate shuttle that involves peroxisomal (Mdh3) and cytosolic (Mdh2) malate dehydrogenases. The transporter for malate and oxaloacetate has not been identified yet. Acetyl-CoA is transported to the cytosol via carnitine-dependent acetyl-CoA transport (involving Cat2) or via the glyoxylate cycle (see Figure 5). Unsaturated FAs, such as oleic acid with the double bond between C9 and C10, can be fully oxidized only in the presence of auxiliary enzymes, but the precise mechanism is controversial. Eci1 is a Δ3,Δ2-enoyl-CoA isomerase in the so-called isomerase-dependent major pathway, which catalyzes the positional and stereochemical isomerization of cis-3-enoyl-CoA to trans-2-enoyl-CoA; this reaction is required after oleic acid (as coenzyme A derivative) has been shortened by three rounds of β-oxidation, since only trans-2-enoyl-CoA is a β-oxidation substrate. Eci1 also isomerizes a fraction of 2-trans, 5-cis-dienoyl-CoA to 3,5-dienoyl-CoA, which has two conjugated double bonds in trans (3) and cis (5) configuration. This compound is presumably degraded by the minor pathway that involves Dci1, Sps19, and Eci1. Alternatively, 3,5-dienoyl-CoA is hydrolyzed by Tes1 thioesterase-dependent pathway) to the free FA and coenzyme A.
The central intermediate in glycerolipid metabolism from which TAG and phospholipids are derived is PA (Athenstaedt and Daum 1997, 1999; Kohlwein 2010b) (Figure 2A). PA is synthesized by a two-step acylation reaction: first, glycerol-3-phosphate is acylated by Sct1 and Gpt2 acyltransferases to sn1-acylglycerol-3-phosphate (also termed lyso-PA) (Zheng and Zou 2001; Zaremberg and McMaster 2002). Alternatively, Sct1 and Gpt2 may also acylate dihydroxyacetone phosphate to 1-acyl-DHAP, which is subsequently reduced by the Ayr1 reductase to sn1-acylglycerol-3-phosphate (Athenstaedt and Daum 2000). The acyltransferases and Ayr are predominantly localized to the ER membrane, but, notably, Ayr and Gpt2 also partially localize to the LD (Athenstaedt et al. 1999; Athenstaedt and Daum 2000; Marr et al. 2012), indicating that at least the first steps in PA synthesis are also LD resident. Gpt2 and Sct1 acyltransferases exhibit different substrate specificities, giving rise to different populations of phospholipids and TAG molecular species (Zaremberg and McMaster 2002; Marr et al. 2012).
Sct1 and Gpt2 generate lyso-PA, which is further acylated by the ER-resident Slc1 and Ale1 acyltransferases to sn1,2-diacylgycerol-3-phosphate (PA) (Benghezal et al. 2007; Chen et al. 2007; Jain et al. 2007; Riekhof et al. 2007; Henry et al. 2012). Slc1 and Ale1 are members of the MBOAT, the membrane-bound O-acyltransferase family of proteins and also involved in the Lands cycle of phospholipid acyl-chain remodeling (Hofmann 2000; Benghezal et al. 2007; Chen et al. 2007; Jain et al. 2007; Riekhof et al. 2007; Pagac et al. 2011). PA is the central glycerolipid intermediate that is utilized both for TAG and for phospholipid synthesis (for details see Henry et al. 2012). In addition to its role as glycerolipid precursor, PA also plays an important role in regulating cellular lipid metabolism (Henry et al. 2012), and its dephosphorylation to diacylglycerol (DAG) is a key step in driving LD formation (Adeyo et al. 2011; Fei et al. 2011c).
The gatekeeper and major regulator of TAG synthesis—and therefore of LD formation–is the Mg++-dependent PA phosphohydrolase, Pah1/Smp2 (Carman and Han 2006, 2011; Han et al. 2006, 2007; O’Hara et al. 2006; Pascual and Carman 2012): in the absence of this enzyme in pah1 mutants, TAG synthesis is reduced by at least 70%, which also results in a drastically reduced LD formation (Adeyo et al. 2011; Fei et al. 2011c) (Figure 1). Mammals express the Pah1/Smp2 ortholog, lipin (encoded by LPIN1-3 genes), mutations of which may cause lipodystrophy in the mouse (Garg 2004; Csaki and Reue 2010). Two additional enzymes, diacylglycerolpyrophosphate phosphatase, encoded by DPP1 and LPP1, may also be involved in DAG formation; however, they serve a regulatory function and their quantitative contribution to TAG formation is unlikely (Henry et al. 2012).
Diacylglycerol that is formed by dephosphorylation of PA is converted either by the acyl-CoA-dependent acyltransferase Dga1 [ortholog of mammalian DGAT (Oelkers et al. 2002; Sandager et al. 2002; Sorger and Daum 2002, 2003)], or the phospholipid-dependent acyltransferase Lro1 [ortholog of mammalian lecithin-cholesterol acyltransferase LCAT (Oelkers et al. 2000)] to TAG. Lro1 localizes to the ER whereas Dga1 localizes both to the ER and LDs (Natter et al. 2005; Choudhary et al. 2011; Jacquier et al. 2011). The primary acyl donors of the Lro1-catalyzed reaction are phosphatidylethanolamine and phosphatidylcholine; thus, this reaction not only contributes to the synthesis of TAG but also serves to remodel the acyl chain composition of these phospholipids (Kohlwein 2010b; Horvath et al. 2011). Minor contribution to TAG synthesis from DAG stems from the activity of the sterol acyltransferases Are1 and Are2 (Yang et al. 1996) (see below).
The second major neutral lipid components of LD are the SEs. Sterols are synthesized in the ER membrane, which also harbors the acyl-CoA-dependent acyltransferases Are1 and Are2 that are required for SE synthesis (Yang et al. 1996; Yu et al. 1996; Zweytick et al. 2000b) (Table 1). Are1 and Are2 share 49% sequence identity with each other, and some 24% identity with mammalian acyl-CoA:cholesterol acyltransferases (ACAT; hence their names ACAT-related enzymes, or Are). Like Slc1 and Ale1, Are1 and Are2 are members of the MBOAT family of membrane-bound O-acyltransferases (Pagac et al. 2011). Notably, both enzymes acylate not only ergosterol, but also intermediates in the ergosterol biosynthetic pathway: whereas the major SE synthase Are2 prefers ergosterol as the substrate, Are1 has a preference for the sterol precursor, lanosterol, giving rise to distinct SE compositions in mutants lacking either one of the enzymes (Zweytick et al. 2000b; Czabany et al. 2007, 2008). Are1 was also found to contribute most to SE synthesis under anaerobic conditions (Hronska et al. 2004).
The localization of the four acyltransferases involved in TAG and SE formation to the ER membrane poses an interesting puzzle as to the transfer mechanism of their products, TAG or SE, to the LD. The close association between the ER and LDs (Figure 1) may be instrumental in supporting this exchange, but the proteins required for this process are presently unknown. Notably, since also intermediates of the ergosterol biosynthesis are stored as SEs in LD, their mobilization and further processing to “mature” ergosterol requires their reshuffling to the ER-resident sterol biosynthetic enzymes (Espenshade and Hughes 2007). The mechanism underlying this transfer and its regulation are unknown.
Turnover of lipid droplets
A systematic microscopic analysis in growing cells has shown that LDs are readily degraded and their content mobilized by up to 80% within 4–6 hr after transfer of stationary-phase cells into fresh, glucose-containing media (Kurat et al. 2006); the LDs are subsequently replenished until cells reach stationary phase. The neutral lipid content of LD is degraded by the activity of TAG lipases and SE hydrolases. Tgl3, Tgl4, and Tgl5 are members of the conserved patatin-domain-containing family of hydrolases (Athenstaedt and Daum 2003, 2005, 2006; Czabany et al. 2007; Daum et al. 2007a,b) that are characterized by a serine active residue embedded in a G-x-S-x-G motif in a patatin domain (Kienesberger et al. 2009); however, in contrast to typical lipases that harbor a Ser-Asp-His catalytic triad (see below), these enzymes harbor only a catalytic dyad, composed of a serine and an aspartic acid residue. Yeast Tgl4 is the functional ortholog of the mammalian adipose triglyceride lipase, ATGL (Zimmermann et al. 2004; Kurat et al. 2006), which is the major TAG-hydrolyzing enzyme in adipose tissue and in other cell types (Lass et al. 2011; Zechner et al. 2012). ATGL deficiency in humans is associated with neutral lipid storage disease with myopathy, NLSDM (Schweiger et al. 2009; Zechner et al. 2012). Tgl3 and Tgl4 are the major TAG lipases in yeast, and deletion of these genes leads to markedly increased LD size and number (Figure 1); Tgl5 only marginally contributes to TAG hydrolysis under standard growth conditions. TAG content is increased by ∼15% in the tgl3 mutant, whereas overexpression reduces TAG content <10% (Athenstaedt and Daum 2003). However, since TAG content is strongly dependent on growth conditions, somewhat different values were obtained in another study from the same lab, showing that TAG levels in the tgl3 mutant increased to 4.11 μg/mg dry cells compared to 1.72 μg TAG/mg dry cells of the wild-type strain (BY4741). Tgl4-deficient cells contained 2.97 μg TAG/mg cell dry weight. Notably, whereas Tgl5-deficient cells had TAG levels identical to wild type, TAG levels were even further increased in tgl3tgl5 double mutants to 5.38 μg/mg dry weight (Athenstaedt and Daum 2005).2 Not only the quantitative contribution to TAG lipolysis between the yeast lipases differs, but also the lipase substrate specificities differ: Tgl3 preferentially hydrolyzes TAG species containing C14, C16, C20, and C26 saturated acyl chains (Athenstaedt and Daum 2003). Similarly, Tgl4 prefers TAG species with C14 and C16 acyl chains. Notably, cells lacking the Tgl5 lipase showed markedly increased levels of C26 acyl chain-containing TAG molecular species, indicating a substrate preference of this lipase for very long chain FAs (Athenstaedt and Daum 2005). In addition to being an efficient TAG lipase, Tgl3 also harbors substantial DAG lipase activity; thus, overexpression of Tgl4 in a tgl3 mutant background leads to increased accumulation of DAG, which is also accompanied by a slight growth defect (Kurat et al. 2006). Tgl4, in addition to being a major TAG lipase, also displays steryl ester hydrolase and phospholipase A2 activities in vitro. Furthermore, this enzyme also catalyzes acyl-CoA dependent re-acylation of lyso-PA to PA (Rajakumari and Daum 2010b). The efficacy of this reaction in contributing to the synthesis of PA in vivo is not clear and apparently not sufficient to support growth of an slc1ale1 double mutant, lacking the two major yeast lyso-PA acyltransferases (see above). Similarly, Tgl3 and Tgl5 lipases also harbor lyso-PA and lyso-phosphatidylethanolamine acyltransferase activities in vitro (Rajakumari and Daum 2010a). Thus, Tgl3, Tgl4, and Tgl5 lipases not only catalyze TAG breakdown to various degrees, but may also be involved in establishing specific acyl-chain compositions to phospholipids.
Despite catalyzing the majority of TAG breakdown, deletion of all three lipases does not result in a significant growth phenotype in logarithmically growing cells (Athenstaedt and Daum 2005; Kurat et al. 2006); however, lipase mutants are sensitive to the FA synthesis inhibitor cerulenin, consistent with the role of lipolysis-derived metabolites (FAs, DAG) for the synthesis of membrane phospholipids. Notably, initiation of the cell division cycle upon transfer of stationary phase/quiescent cells into fresh growth media is delayed in mutants lacking Tgl3 and Tgl4 lipases (Kurat et al. 2009): in these mutants, G1/S transition is extended by some 30 min, indicating that lipolysis-derived metabolites are required for efficient cell cycle progression (see below). Evidence suggests that lack of lipolysis affects the formation of sphingolipids (Rajakumari et al. 2010), which play multiple regulatory and structural roles (Dickson 2010). Also, lipase-deficient mutants are defective in phosphatidylinositol (PI) synthesis: addition of inositol to wild-type cells that were grown in the absence of this lipid precursor results in a rapid burst in PI synthesis, which is significantly attenuated in tgl3tgl4tgl5 lipase mutants. Additional inhibition of de novo FA synthesis by cerulenin abolishes the burst in PI synthesis after inositol addition, indicating that both de novo-synthesized FAs and metabolites derived from TAG breakdown are required to support PI synthesis (Gaspar et al. 2011). PI is also a precursor for the synthesis of complex sphingolipids, which may be the underlying reason for attenuated sphingolipid synthesis in lipase-deficient cells (Rajakumari et al. 2010).
Homozygous diploid tgl3/tgl3 and tgl5/tgl5 mutants are unable to sporulate, indicating that Tgl3 and Tgl5 provide essential activities that are required for the generation of functional spores (Rajakumari and Daum 2010a). Indeed, it was shown that the Tgl3 acyltransferase activity, rather than the lipase activity, is required for sporulation (Rajakumari and Daum 2010a). However, the specific step in the sporulation program that requires this activity is not known.
The role of the TAG lipases in sustaining viability during stationary phase, in the absence of other carbon sources, is not known. Notably, mutants defective in the DAG kinase Dgk1 also display a delay in growth resumption after transfer of stationary cells into fresh growth medium (Fakas et al. 2011a), similar to tgl3tgl4 mutants, and TAG degradation is defective, even in the presence of cerulenin. Choline supplementation partially suppresses this defect; it was suggested that lipotoxic lipolysis-derived DAG might accumulate under these conditions, which is drained into the synthesis phospholipids via the cytidine diphosphate (CDP)–choline (Kennedy) pathway if choline is present (Fakas et al. 2011a). Dgk1 is localized to the ER, and the transfer of its substrate DAG from the LDs may be facilitated by the close physical interaction between both organelles (Szymanski et al. 2007).
Common to Tgl3, Tgl4, and Tgl5 TAG lipases is their exclusive localization to LDs, which is in contrast to other LD proteins—mostly enzymes involved in anabolic processes—that are additionally associated with the ER membrane (Athenstaedt and Daum 2006; Kurat et al. 2006; Daum et al. 2007a; Rajakumari et al. 2008; Kohlwein 2010b). Localization of Tgl3 to LDs may be regulated by the yeast seipin ortholog Fld1 (Wolinski et al. 2011).
In addition to the major TAG lipases Tgl3 and Tgl4, which catalyze the majority of TAG and also DAG breakdown, yeast also expresses a monoacylglycerol (MAG) lipase to complete the “lipolytic cascade” analogous to mammalian cells (Zechner et al. 2012). Yeast MAG lipase is encoded by the YJU3 gene (Heier et al. 2010) and localizes to both ER and LDs. Deletion of the YJU3 gene results in accumulation of MAG, but does not lead to a detectable phenotype under numerous experimental conditions. This is surprising since the specific activity of the Yju3 protein is several orders of magnitude higher than that of the TAG lipases (Heier et al. 2010).
The TGL1-, YEH1-, and YEH2-encoded steryl ester hydrolases are involved in SE degradation. These enzymes are related to mammalian acid lipases, and, as “prototypic” hydrolases, they harbor a serine-active site embedded in a G-x-S-x-G motif, a catalytic triad consisting of Ser-Asp-His residues, and an α/β-hydrolase fold (Jandrositz et al. 2005; Koffel et al. 2005; Mullner et al. 2005; Koffel and Schneiter 2006; Wagner et al. 2009). Whereas Tgl1 and Yeh1 localize predominantly to LDs, Yeh2 is enriched in the plasma membrane (Koffel et al. 2005; Mullner et al. 2005; Wagner et al. 2009), consistent with previous findings derived from cell fractionation experiments that showed significant SE hydrolase activity in the plasma membrane (Zinser et al. 1993; Leber et al. 1995). In addition to its activity as an SE hydrolase, Tgl1, which shares similarities to mammalian lysosomal acid lipases, also degrades TAG in vitro. This activity, however, does not appear to significantly contribute to TAG turnover in vivo under standard growth conditions (Jandrositz et al. 2005) and does not affect LD abundance and structure.
Regulation of neutral lipid synthesis
Very little is known about the specific regulation of enzymes involved in TAG and SE synthesis and, therefore, in LD biogenesis. Formation of LDs is clearly driven by the availability of lipid precursors, sterols, and FAs and therefore is dependent on the regulatory processes that control the biosynthesis of their lipid constituents. Notably, none of these processes has been specifically investigated in the context of LD formation. Microscopic analysis of LD in growing cells indicated that their degradation and new synthesis may be processes that at least partially overlap (Kurat et al. 2006). Similarly, dynamic flux balance analysis also unveiled that degradation and de novo formation of LD may occur in parallel to maintain FA and lipid homeostasis (Zanghellini et al. 2008). This appears to be a conserved mechanism that also occurs in mammalian cells: FAs taken up from the blood stream into cells may first be incorporated into TAG prior to their release by lipolysis. Failure to degrade TAG in homozygous lipase-deficient ATGL−/− mouse mutants leads to a lack of PPAR-agonist release and impaired mitochondrial function (Haemmerle et al. 2011; Zechner et al. 2012).
A major determinant of TAG synthesis is the availability of FAs and glycolysis-derived glycerol-3-phosphate or DHAP. Exogenously supplied FAs are preferentially stored as TAG, but are also incorporated into membrane phospholipids upon FA supplementation (Grillitsch et al. 2011). Very little is known about the regulation of acyl-CoA synthetase activities that are required to activate free FAs (Black and Dirusso 2007), and it can only be speculated that glycolysis derived glycerol-3-phosphate or DHAP are the limiting compounds that determine cellular TAG levels in the presence of a surplus of exogenous FAs. TAG levels are increased about fivefold when cells are grown in the presence of oleic acid as the sole carbon source (Grillitsch et al. 2011). Endogenous FA synthesis is under transcriptional and post-translational control at the level of Acc1 and the FA synthase complex (Tehlivets et al. 2007; Kohlwein 2010b; Henry et al. 2012). Acc1 is phosphorylated and inactivated by Snf1 kinase, the ortholog of mammalian AMP-activated protein kinase (Woods et al. 1994), under conditions of scarce energy. Thus, Snf1 is an important regulator of TAG homeostasis by regulating the activity of Acc1 and, thus, FA de novo synthesis and TAG accumulation (Tehlivets et al. 2007). Recent evidence suggests that TOR and Snf1/AMPK pathways are connected to the control of TAG formation through the Sit4-Sap190 protein phosphatase complex that may control the activity of Acc1 and/or Snf1 (Bozaquel-Morais et al. 2010). FA desaturation is regulated by the membrane-bound transcription factors Spt23 and Mga2, which are processed in an Rsp5 ubiquitin ligase-dependent reaction (Hoppe et al. 2000; Rape et al. 2001); soluble Spt23 and Mga2 fragments translocate into the nucleus to regulate the expression of the OLE1 gene encoding the single FA desaturase in yeast (Stukey et al. 1989, 1990; Hoppe et al. 2000; Chellappa et al. 2001; Rape et al. 2001; Martin et al. 2002, 2007; Tehlivets et al. 2007; Henry et al. 2012). Notably, overexpression of constitutively active Mga2 or Spt23 fragments stimulates TAG synthesis and leads to altered LD morphology, indicating a regulatory link between Rsp5, Spt23, and Mga2 function and lipid homeostasis (Kaliszewski and Żołądek 2008).
The initial steps in glycerolipid synthesis require the activity of Sct1 and Gpt2 acyltransferases (Figure 2). Sct1 localizes to the ER membrane (Bratschi et al. 2009), and the SCT1 gene was originally identified as a suppressor of a choline transport mutant, indicating a functional relationship to phosphatidylcholine synthesis (Matsushita and Nikawa 1995). Indeed, establishment of the acyl-chain composition in phosphatidylcholine requires Sct1 (Boumann et al. 2003). Sct1 activity, which is regulated by phosphorylation by an as-yet-unknown kinase, competes with the OLE1-encoded FA desaturase for their common substrate, palmitoyl-CoA. Thus, overexpression of Sct1 leads to increased phosphatidylinositol and TAG levels at the expense of phosphatidylethanolamine and a general shift in FA profiles toward more saturated species (De Smet et al. 2012). Notably, deletion of the SCT1 gene has a significant impact on the turnover of phosphatidylcholine that is generated through the CDP-choline (“Kennedy”) pathway (Zaremberg and Mcmaster 2002), and further evidence suggests that this phospholipid, next to TAG, functions as a reservoir for FAs, in particular for C16:0 (De Smet et al. 2012). This is also consistent with the observation that cells become more sensitive to C16:0 supplementation when both TAG synthesis and the phospholipid methylation pathway are blocked (Garbarino et al. 2009).
Deletion of the second acyltransferase encoded by the GPT2 gene has the opposite effect on phosphatidylcholine turnover than a deletion of SCT1, namely a highly stimulated turnover of this phospholipid synthesized via the CDP-choline pathway (Zaremberg and McMaster 2002). In contrast to wild-type cells, mutants defective in Gpt2 acyltransferase are sensitive to oleate supplementation and fail to synthesize TAG and induce LD formation (Marr et al. 2012). Oleate may indeed regulate Gpt2 abundance and its activity by phosphorylation; furthermore, Gpt2-containing crescent ER structures that are observed in close vicinity to LDs in the presence of oleate indicate a regulatory crosstalk between LD formation and activity of the initial steps of glycerolipid synthesis (Marr et al. 2012).
The redundant lyso-PA acyltransferases encoded by SLC1 and ALE1 both contribute to the typical FA spectrum in cellular glycerolipids, whereby Ale1 may have a somewhat higher preference for C16:1-CoA than Slc1 (Benghezal et al. 2007). Although Slc1 harbors the majority of cellular sn1-acylglycerol-3-phosphate acyltransferase activity, deletion of either SLC1 or ALE1 genes does not significantly affect total cellular glycerolipid content (Benghezal et al. 2007). This is surprising since these enzymes together execute an essential reaction, indicated by the synthetic lethal phenotype of slc1ale1 double mutants, which also suggests the absence of significant additional lyso-PA acyltransferase activities in yeast (Jain et al. 2007).
The dephosphorylation of PA to DAG is considered the rate-limiting step in TAG formation, and mutants lacking the PA phosphatase Pah1 are characterized by drastically reduced TAG levels (Carman and Han 2006, 2009; Han et al. 2006; Fakas et al. 2011b; Henry et al. 2012; Pascual and Carman 2012). Pah1 is under multiple levels of regulation by phosphorylation, which controls its localization to the cytosol (phosphorylated) or its association with the ER membrane (dephosphorylated) (Carman and Han 2009; Karanasios et al. 2010; Choi et al. 2011). Dephosphorylation of Pah1 by the Nem1-Spo7 phosphatase complex favors its association with the ER membrane and facilitates generation of the TAG precursor DAG (Siniossoglou et al. 1998; Santos-Rosa et al. 2005).
Notably, Pah1 is phosphorylated by the cyclin-dependent protein kinases Cdc28/Cdk1 and Pho85 (Karanasios et al. 2010; Choi et al. 2011), indicating that its membrane association and activity are regulated in a cell cycle-dependent manner. Since TAG degradation also is regulated in a cell cycle-dependent manner (Kurat et al. 2009) (see below), the picture emerges that TAG synthesis and degradation may indeed oscillate during the cell cycle (Kurat et al. 2009; Kohlwein 2010b).
The activity of Pah1 is counteracted by the CTP-dependent diacylglycerol kinase Dgk1 and may thus contribute to the regulation of TAG homeostasis. Overexpression of the DGK1 gene results in proliferation of ER membranes, consistent with an overproduction of PA that is preferentially channeled into phospholipid synthesis (Han et al. 2008a,b). Whereas the impact of DGK1 overexpression on cellular TAG levels is unclear, deletion of this gene hardly has any affect on cellular TAG content in growing cells. However, Dgk1 activity is important during periods of growth resumption, i.e., after transfer of stationary-phase cells to fresh media, presumably to convert lipolysis-derived DAG to PA and subsequently to phospholipids. Absence of Dgk1 activity leads to the accumulation of DAG with potentially detrimental effects on the cells, which can be attenuated by utilizing DAG for the CDP-choline pathway in the presence of choline (Fakas et al. 2011a).
The specific regulatory mechanisms that control the activity of Dga1, Lro1, Are1, and Are2 acyltransferases are unknown (Yang et al. 1996; Oelkers et al. 2000, 2002; Zweytick et al. 2000b; Sorger and Daum 2002). Mutant analysis indicates that Dga1 contributes more significantly to TAG synthesis in the stationary phase, whereas Lro1 apparently is more active during logarithmic growth (Oelkers et al. 2002). Notably, supplementation of wild-type cells with oleic acid, which stimulates TAG synthesis, simultaneously reduces cellular SE levels (Connerth et al. 2010). This observation indicates a regulatory crosstalk between TAG synthesis and SE synthesis, the molecular basis of which, however, has not been uncovered yet. These findings also raise the question of whether distinct types of yeast LDs that harbor either SE or TAG may exist (see below). Clearly, biophysical properties differ between SE- or TAG-only LDs, despite similar size distribution (Czabany et al. 2008). For example, tri-oleoyl glycerol and tri-palmitoleoyl glycerol, which are the major TAG species, have a melting point below –4°, whereas cholesteryl oleate (related to the yeast ergosteryl oleate) has a melting point above +40° (PubChem Substance database).
Acylation of sterols may regulate the flux through the ergosterol biosynthetic pathway by sequestering and storing intermediate products as SEs in the LD. Thus, acylation may prevent buildup of potentially harmful sterol intermediates. Sterol synthesis is under a tight feedback regulatory loop that controls the expression of HMG-CoA reductase, the key enzyme of sterol synthesis both in yeast and in mammals (Espenshade and Hughes 2007; Burg and Espenshade 2011; Raychaudhuri et al. 2012). Mutants lacking Dga1, Lro1, Are1, and Are2 acyltransferases altogether display a defect in sterol synthesis, which is due to the reduced amount of squalene epoxidase, Erg1 (Sorger et al. 2004). This reduction in Erg1 abundance is not due to attenuated expression but rather is a result of decreased protein stability in the dga1lro1are1are2 quadruple mutant. Erg1 typically localizes both to the ER and LDs (Leber et al. 1998), which, however, are absent in the quadruple mutant. Thus, the decreased Erg1 stability indicates a tight regulation of the amount of ER-resident Erg1 protein. It furthermore suggests that localization of Erg1 to the LD provides a mechanism to store (catalytically inactive) enzyme that is not subject to this regulation (Leber et al. 1998), but may be relocalized to the ER upon metabolic requirements. The mechanisms that govern sterol lipid exchange between the ER and LDs, and the regulation of these processes, remain obscure.
TAG accumulation is also influenced by the cellular capacity to synthesize phospholipids: attenuated phosphatidylcholine synthesis, i.e., in mutants lacking the CHO2- and OPI3-encoded phospholipid methyltransferases or defective in S-adenosylhomocysteine hydrolase (Sah1), which affects the methylation activity, leads to an increased synthesis of TAG and LD proliferation (malanovic et al. 2008). The reduced flow of FAs into phosphatidylcholine synthesis presumably leads to the accumulation of PA, which is preferentially channeled into the synthesis of TAG. However, these observations also support the notion that PC synthesis, in addition to TAG, may also provide some (limited) buffering capacity for accommodating excess FA.
Regulation of neutral lipid degradation
Lipolysis is most active during growth resumption of stationary-phase cells that are transferred into fresh, glucose-containing medium (Kurat et al. 2006; Zanghellini et al. 2008). TAG is degraded by Tgl3 and Tgl4 lipases that both reside on the lipid droplet (Czabany et al. 2007; Daum et al. 2007a; Rajakumari et al. 2008; Kohlwein 2010b; Henry et al. 2012). Neither protein abundance nor localization appear to change during the phase of lipolysis. Tgl5 and Tgl1, which are also TAG lipases in vitro, do not appear to contribute significantly to TAG degradation. Indeed, Tgl1 is more active as a SE hydrolase (Koffel et al. 2005). Tgl4 is phosphorylated and activated by the cyclin-dependent kinase Cdk1/Cdc28 (Kurat et al. 2009) at the G1/S transition of the cell cycle, suggesting that lipolysis-derived products (i.e., FAs or DAG) are required to drive cell cycle progression. The specific checkpoint-monitoring availability of lipolysis products is unknown. Similarly, Tgl5 may also be a substrate of Cdk1/Cdc28 (Ubersax et al. 2003), and Tgl3 lipase is a potential target of the second, nonessential cyclin-dependent kinase Pho85, according to large-scale studies (Ptacek et al. 2005). Whether Tgl3 and Tgl5 activities are indeed regulated during the cell cycle is not known.
The observation that lipolysis in yeast is linked to cell cycle progression is unexpected; indeed, Tgl4 and Pah1 are among the very few direct enzymatic targets of the cyclin-dependent kinase Cdk1/Cdc28. Since both de novo TAG synthesis, driven by the activity of PA phosphatase Pah1, and lipolysis are regulated in a cell cycle-dependent manner, it becomes obvious that maintenance of lipid homeostasis during the cell cycle is critical (Kurat et al. 2009; Kohlwein 2010b). Neither the TAG degradation products nor the checkpoint regulator that senses their availability are known. Notably, Tgl4 phosphorylation—and activation—occurs at the G1/S transition of the cell cycle, at bud emergence, whereas Pah1 phosphorylation—and inactivation—occurs at the G2/M transition. This leaves both enzymes active during a large part of the cell cycle, consistent with a model that lipogenesis and lipolysis may occur in parallel to sustain cellular lipid homeostasis (Zanghellini et al. 2008; Kohlwein 2010b).
The mechanisms of the regulation of steryl ester hydrolysis by Yeh1, Yeh2, and Tgl1 are currently unknown. Since LDs that are composed of about equal amounts of TAG and SEs are mobilized by 80% during the initial phase (∼6 hr) of growth resumption (Kurat et al. 2006), one can assume the highest activity of these enzymes during this period of growth. The LD-resident enzymes Yeh1 and Tgl1, but not the plasma membrane-resident enzyme Yeh2, harbor potential cAMP-dependent protein kinase A phosphorylation sites, which may be responsible for stimulation of activity (Koffel et al. 2005). Also, Yeh1 is the major SE hydrolase in hem1-deficient mutant cells that lack de novo sterol synthesis and require ergosterol supplementation (Koffel and Schneiter 2006).
Mechanisms of LD biogenesis and inheritance
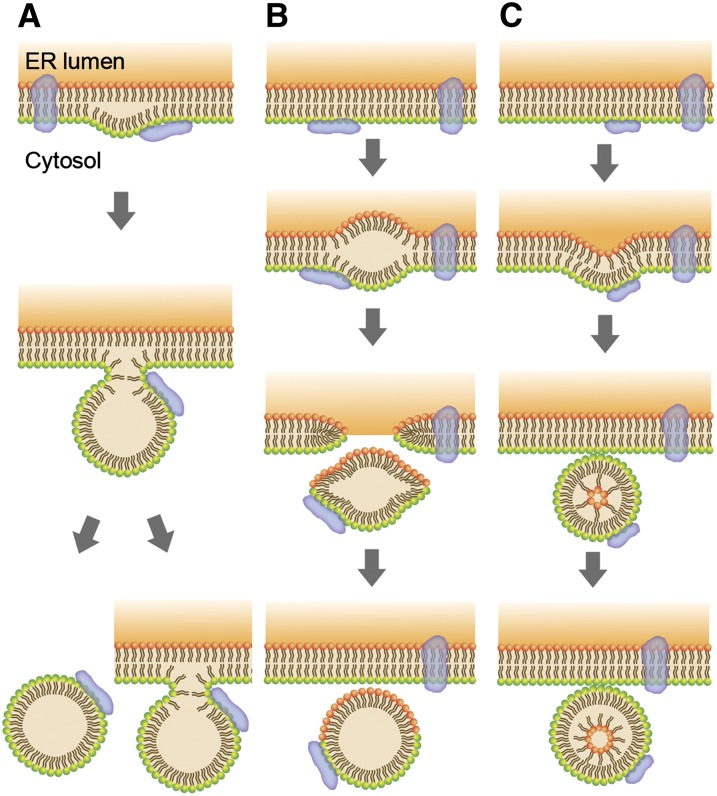
No clear picture currently exists of how LDs are actually assembled, neither in yeast nor in other cell types. Current models of LD formation are summarized in Figure 3 (Zweytick et al. 2000a; Mullner and Daum 2004; Czabany et al. 2007; Daum et al. 2007a; Jacquier et al. 2011) (see below). The “lensing” model (Figure 3A) and the “bicelle” model (Figure 3B) share the idea that TAG accumulates between the leaflets of the ER membrane; after reaching a critical size, LD may bud off toward the cytosol (lensing model) or are excised from the ER, leaving behind a gap in the membrane, which, however, may be quickly filled up again. In the former model, the monolayer surrounding the LD is solely derived from the cytosolic leaflet of the ER membrane, whereas in the bicelle model both ER membrane leaflets contribute to the LD surface monolayer. The “vesicle budding” model (Figure 3C) suggests the formation of TAG-filled secretory vesicles that undergo remodeling of the ER-derived phospholipid bilayer to yield the observed phospholipid monolayer covering the LDs. Common to these models is a tight functional interaction between emerging LDs and the endoplasmic reticulum from which they presumably derive. Indeed, LDs may be in continuous ER contact throughout their life cycle (Wolinski et al. 2011). Recently, first attempts have been made to understand LD formation from a theoretical point of view, based on biophysical models (Zanghellini et al. 2010a,b). According to these models, LDs bud off the ER membrane in a process that is driven by lipid de-mixing in the membrane, when a critical size of some 12 nm is reached. Since this size is more than an order of magnitude below the observed LD size in vivo, the authors concluded that LD formation is a two-step process in which initial LD formation is followed by fusion events, giving rise to native “ripe” LDs (Zanghellini et al. 2010a,b).
Figure 3 .
Models of lipid droplet biogenesis (adapted from Guo et al. (2009). (A) According to the “lensing model,” neutral lipids are deposited between the leaflets of the ER membrane: after reaching a critical size, the neutral lipid core bulges out and the LD is formed; the LD surface monolayer is derived solely from the cytosolic leaflet of the ER membrane. Subsequently, the LD may completely separate from the ER membrane, or remain attached, with the surface layer forming a continuum with the ER. (B) Bicelle formation: LD formation similar to model in A, but the LD is excised from the ER membrane, and both ER membrane leaflets contribute to the LD surface monolayer. (C) Vesicle formation. Inclusion of the neutral lipid core in the membrane vesicle requires rearrangement of the inner leaflet of the bilayer. These models explain the origin of the phospholipid membrane, which stems either from the cytoplasmic leaflet or from both leaflets of the ER membrane, respectively. Unclear is what limits the expansion of the neutral lipid core between the leaflets, what determines the orientation of LD extrusion toward the cytosol, and how the integrity of the ER membrane is maintained. Notably, none of the intermediate stages representing neutral lipid deposits between the ER membrane leaflets, nascent lipid droplets in the ER, or lipid-filled vesicular structures have been experimentally observed in wild-type cells.
Contribution of acyl transferases to LD biogenesis
In the absence of both DAG acyltransferases, Dga1 and Lro1, LDs are solely composed of SE. Notably, despite the fact that SE make ∼50% of the total neutral lipid content in wild-type cells, LD numbers are drastically reduced in dga1lro1 double mutants to one or two LDs (Oelkers et al. 2002; Sorger and Daum 2002; Athenstaedt and Daum 2006; Czabany et al. 2007; Daum et al. 2007b; Rajakumari et al. 2008; Walther and Farese 2009; Kohlwein 2010b) (Figure 1). In contrast, simultaneous deletion of Are1 and Are2 sterol acyltransferases has only a marginal effect on LD content. No LDs are present in mutants lacking Dga1 Lro1 Are1 and Are2 acyltransferases (Oelkers et al. 2002; Sandager et al. 2002; Garbarino et al. 2009; Petschnigg et al. 2009; Kohlwein 2010b). Thus, LD formation is clearly correlated with the activity of these acyltransferases. This has also led to the establishment of a test system to study LD biogenesis by expressing the major DAG acyltransferases, Dga1 or Lro1, under control of the galactose-inducible GAL1p promoter, in cells lacking other acyltransferases (Jacquier et al. 2011). In this system, LDs are absent from cells grown on glucose, but LD formation is induced upon shift of cells to galactose medium; LD formation could be observed within 2 hr of induction of the acyltransferases. Formation of new LDs occurs close to the nuclear ER, consistent with the current biogenesis model that LD may derive from the ER. Furthermore, LD proteins that relocalize to the ER in the absence of LDs translocate to the newly formed, nascent LD; this protein relocalization is independent of de novo protein synthesis or energy (Jacquier et al. 2011). Fluorescence recovery after photobleaching and fluorescence loss in photobleaching experiments suggest that ER and LD membrane may indeed form a continuum that allows the free diffusion of LD-resident proteins from the ER to the growing LD, and back to the ER, upon stimulation of TAG breakdown (Jacquier et al. 2011).
The topology of acyltransferases involved in TAG formation may provide some clues as to the origin of the LD core lipids and thus the mechanism of LD formation (Choudhary et al. 2011; Jacquier et al. 2011; Pagac et al. 2011). Dga1 harbors a stretch of hydrophobic amino acids compatible with two membrane-spanning domains; since the enzyme is active both in the ER and on LDs, which contain only a phospholipid monolayer, any potential rearrangement of the enzyme does not appear to affect its activity (Jacquier et al. 2011). In contrast, Lro1, the phospholipid-dependent acyltransferase, which is exclusively localized to the ER, harbors only one membrane-spanning domain. Its presumed active site residing in the lumen of the ER suggests that TAG may indeed be formed in the lumen of the ER, rather than between ER membrane leaflets (Choudhary et al. 2011). Similarly, evidence suggests that in the MBOAT enzymes Are1 and Are2, the conserved histidine residue involved in catalysis is also exposed to the luminal side of the ER (Pagac et al. 2011).
Targeting of proteins to LDs
Unlike other proteins targeted to organelles, LD-associated proteins apparently do not harbor targeting consensus sequences as determined by primary structure comparison of LD-associated proteins. However, a common feature appears to be the presence of hydrophobic domains, although exceptions exist (Leber et al. 1998; Mullner et al. 2004; Grillitsch et al. 2011). As shown in Table 1, several of the LD-associated proteins contain even one or two (predicted) transmembrane domains, which appear to be incompatible with the generally accepted view that the LD surface is covered by a phospholipid monolayer. Thus it is unclear how these extended stretches of hydrophobic amino acids are accommodated in the LD surface layer. Also, numerous LD proteins lack hydrophobic stretches indicative of membrane-anchoring sequences altogether (Table 1), suggesting that their interaction with LDs may be indirect and through the interaction with LD-anchored proteins.
Notably, numerous LD-associated proteins are dually localized also to the ER membrane (Table 1), including the enzymes involved in sterol synthesis Erg1, Erg6, and Erg7 (Mullner et al. 2004). The physiological relevance of this dual localization is unclear, since the other enzymes of ergosterol biosynthesis are ER-resident; However, as shown for Erg1, localization to the LD may serve a regulatory function—to provide a pool of enzyme that is inactive on the LD, but which may readily relocalize to the ER upon demand (Sorger et al. 2004). Truncated versions of the Erg1 lacking a single C-terminal hydrophobic stretch of 55 amino acids lost their affinity to the LDs and relocalized prodominantely to the ER. Deletion of 87–139 C-terminal amino acids of the Erg7 protein also led to significant retention of protein in the ER and reduced association to LDs. A C-terminal deletion of 26 hydrophobic amino acids in Erg6 did not significantly alter its localization, whereas a deletion of 66 C-terminal amino acids abolished LD association and led to full translocation of the truncated Erg6 protein to the ER (Mullner et al. 2004). It should be noted, however, that the relative distribution of proteins to the ER and LDs might depend on protein abundance, which somewhat limits the use of episomal overexpression clones for LD localization studies. Apparently, hydrophobic stretches are required for LD association, but the factors that discriminate relative distribution to LDs and the ER are currently unknown. Notably, heterologous LD proteins expressed in yeast also localize faithfully to LDs, such as mammalian adipose triglyceride lipase, ATGL (Kurat et al. 2006), or methyltransferase like 7B (AAMB) (Zehmer et al. 2008), despite the absence in yeast of perilipins that play an important role in regulating the access of proteins to the LDs in mammals (Brasaemle 2007).
Lipid droplet morphology and inheritance
Notably, in a given population of cells, LD size distribution is quite homogeneous, and it is currently unclear which factors regulate LD size independently of neutral lipid composition (Czabany et al. 2008). Yeast expresses the protein Fld1 that is distantly homologous to mammalian seipin, implicated in the serious inheritable Berardinelli–Seip congenital lipodystrophy type 2 that results from defects in the BSCL2 gene (Szymanski et al. 2007; Fei et al. 2008, 2011a,b). Mutations in the FLD1 gene lead to “supersized” LDs under inositol-limiting conditions, indicating that Fld1 may play a role in LD biogenesis and organization. Indeed, morphological analysis in growing yeast cells indicates that Fld1 plays a role in LD subcellular distribution and inheritance (Wolinski et al. 2011). Furthermore, access of the TAG lipase Tgl3 to LDs seems to be impaired in fld1 mutants. Its role in LD formation is derived from observations that Fld1 may form homo-oligomers and localizes at the interface between the ER membrane and LDs (Szymanski et al. 2007; Binns et al. 2010). Thus, although not itself an LD-resident protein, Fld1 is a potential regulator of LD assembly.
Physiological role of LDs
LDs function as the storage depot for TAG and SE. Thus, processes that depend on TAG and SE formation, or metabolites derived from TAG or SE, are affected by the cell’s capacity to generate LDs. LDs lacking SE are more sensitive to sterol synthesis inhibitors, such as terbinafine (Zweytick et al. 2000b), which is in line with the function of SE as storage molecules. In the presence of sterol synthesis inhibitors, SE are degraded and sterols incorporated into membranes until the SE content of the cell is exhausted and growth ceases (Zweytick et al. 2000b). Similarly, inhibition of FA de novo synthesis by cerulenin results in rapid mobilization of TAG and the utilization of released FAs or DAG for membrane lipid synthesis (Kurat et al. 2006; Fakas et al. 2011a).
A second major function of TAG (and LDs) is to serve as a buffer to “neutralize” excess FA. Cells lacking the capacity to synthesize TAG, i.e., dga1lro1are1are2 quadruple mutants, are highly sensitive to supplementation with unsaturated FAs: in the absence of TAG formation, oleic acid is preferentially incorporated into phospholipids, which leads to massive membrane proliferation and rapid loss of viability (Kohlwein and Petschnigg 2007; Garbarino et al. 2009; Petschnigg et al. 2009). This also reflects the sensitivity of mammalian cells to FA overload (Listenberger et al. 2003; Schaffer 2003). Notably, quadruple mutants exposed to oleic acid appear to “adapt” to this challenge and recover after an extended lag period (Connerth et al. 2010). This adaptation, however, seems to be a stable trait and suggests the appearance of suppressor mutations that allow cells devoid in TAG synthesis to sustain oleic acid challenge. Indeed, mutations in mitochondrial DNA confer resistance of the quadruple mutant to oleic acid-induced cell death (Rockenfeller et al. 2010). Similarly to the quadruple mutant that lacks TAG altogether, pah1 mutants lacking PA phosphatase and containing drastically reduced levels of TAG are also highly sensitive to unsaturated FA supplementation (Fakas et al. 2011b). Thus, the picture emerges that FA overload leads to a critical imbalance in cellular phospholipid composition in the absence of TAG synthesis (Kohlwein and Petschnigg 2007; Garbarino et al. 2009; Kohlwein 2010a).
As mentioned above, attenuation of phosphatidylcholine synthesis in cho2opi3 mutants or in mutants defective in S-adenosylhomocysteine hydrolase, which regulates the cellular methylation potential (Malanovic et al. 2008; Tehlivets 2011), leads to an increased flux of FAs into TAG and subsequent LD accumulation. Notably, levels of phosphatidylcholine can be substantially reduced in yeast cells without leading to a significant growth phenotype (Henry et al. 2012), indicating that this phospholipid may also serve, at least in part, as a buffer for FAs. Similarly, a block of the early secretory pathway that can also be considered as a process to regulate the metabolic flux of phospholipids out of the ER leads to elevated TAG levels (Gaspar et al. 2008). These observations support the notion that phospholipid and TAG metabolism are metabolically tightly interconnected and that inactivation of either biosynthetic branch forces the channeling of FAs into the other, with potentially detrimental consequences for the cell. Furthermore, TAG and LD formation appear to play a crucial role in modulating ER stress that is induced by altered phospholipid composition or turnover (Hapala et al. 2011).
In addition to serving as an overflow reservoir for excess FAs, TAG—and LD altogether—may also serve as an overflow storage compartment for proteins. For example, Erg1 is a prominent protein residing on LDs, but inactive in the absence of the ER-resident reductase (Leber et al. 1998). Thus, localization of Erg1 to LD may serve as a reservoir to control the catalytic capacity of the ER-resident sterol biosynthetic pathway. Absence of LDs leads to relocalization of Erg1 to the ER membrane and its partial degradation to regulate the concentration of Erg1 in the ER (Sorger et al. 2004).
Notably, induction of LDs was observed in cells expressing mammalian α-synuclein (Outeiro and Lindquist 2003). α-Synuclein is implicated in neurogenerative diseases, such as Parkinson’s and Alzheimer’s, and its expression in yeast is toxic and leads to impaired vesicular trafficking and inhibits phospholipase D expression (Outeiro and Lindquist 2003). Notably, yeast quadruple mutants lacking Dga1 Lro1 Are1 and Are2 acyltransferases and, therefore, LDs altogether (see above) are more resistant to α-synuclein expression (Sere et al. 2010). In the quadruple mutant, the basal levels of reactive oxygen species (Sere et al. 2010) as well as unfolded protein response (Petschnigg et al. 2009) are elevated in the absence of neutral lipid synthesis; thus it was suggested that upregulated oxidative defense mechanisms may protect LD-deficient cells from α-synuclein toxicity. Notably, the sterol precursor, squalene, may play an important role in oxidative stress defense (Sere et al. 2010). Squalene accumulates in LDs, but also in subcellular membranes, when LDs are absent (Spanova et al. 2010, 2012).
Physiological interaction of LDs with other organelles
It is currently unclear whether and how TAG homeostasis and peroxisome (PEX) function (see Peroxisomes) are coupled in yeast, despite their apparent close physical interaction in vivo (Binns et al. 2006). A physiological interaction between these organelles may be restricted to FA β-oxidation, which, in S. cerevisiae, occurs exclusively in peroxisomes and is absent from mitochondria, which are the major site of β-oxidation in mammalian cells. Tgl3 and Tgl4 lipases are not required for induction of peroxisome formation after glucose depletion in the absence of exogenous FA supplementation (Petschnigg et al. 2009). By using bi-molecular fluorescence complementation, Pu et al. (2011) identified several interactions of LD-resident proteins with peroxisomal and mitochondrial proteins, indicating their direct physical interaction. This analysis technique is based on the reconstitution of a fluorescent protein (Venus) from two nonfluorescent fragments that is driven by the interaction of two proteins fused to these fragments. Most significant interactions were observed for the LD-resident Erg6 and Pet10 with other LD proteins, but also with mitochondrial and peroxisomal proteins (Pu et al. 2011). According to this analysis, Tgl3 lipase interacts with the Ayr1 protein that catalyzes the reduction of 1-acyl-DHAP to lyso-PA (Figure 2), thus indicating a feedback loop between lipolysis and de novo glycerolipid synthesis. Furthermore, the interaction of the TAG lipase Tgl3 with the peroxisomal protein Pex11 is consistent with the concept of metabolic channeling of TAG-derived FAs to peroxisome biogenesis and β-oxidation.
Yeast as a model to investigate and understand lipid-associated disorders
The pandemic development of obesity and related disorders, such as cardiovascular disease and type 2 diabetes, has led to significant efforts to study the molecular basis of lipid-associated disorders in various experimental model systems, including mice, worms, flies, and yeast. The similarities in the metabolic pathways involved in TAG and SE metabolism between yeast and “larger” cells make yeast an attractive experimental system to study lipid function and malfunction at the molecular and cellular levels (Kohlwein and Petschnigg 2007; Kohlwein 2010a,b; Zechner et al. 2012). Although lipid-associated disorders typically affect multiple organs and cell types, fundamental insights into key factors of mammalian TAG synthesis have been generated in yeast, e.g., by the discovery of lipin function as a phosphatidic acid hydrolase. Lipin is a protein known for a long time to be implicated in lipodystrophies in mouse model systems (Peterfy et al. 2001; Carman and Han 2006; Reue and Zhang 2008; Csaki and Reue 2010; Han and Carman 2010), yet its enzymatic function was identified by studies in yeast (Han et al. 2006, 2007; O’Hara et al. 2006; Carman and Wu 2007).
Sah1, a key enzyme in methylation metabolism, is one of the most highly conserved proteins, sharing some 60% sequence identity between yeast and the mammalian enzymes (Tehlivets et al. 2004; Malanovic et al. 2008; Tehlivets 2011). Notably, the SAH1 gene in yeast was found to be transcriptionally coregulated with phospholipid biosynthetic genes, indicating a functional link between methylation and lipid metabolism. Indeed, phospholipid methylation is a major consumer of S-adenosylmethionine (AdoMet), leading to the accumulation of S-adenosylhomocysteine (AdoHcy) as a by-product, which also acts as a potent product inhibitor. Sah1 is responsible for the degradation of AdoHcy to adenosine and homocysteine, thus regulating the cellular methylation potential (Tehlivets 2011); however, this reaction is reversible, and an accumulation of homocysteine may, in fact, drive the formation of the methylation inhibitor, AdoHcy. This puts the risk factor for atherosclerosis, homocysteine, into a new perspective, as a regulator of cellular methylation by its Sah1-dependent conversion to AdoHcy (Tehlivets 2011). Since Sah1 is an essential enzyme, studies in mouse model systems are very limited due to embryonic lethality of the ko mutation. On the other hand, mammalian Sah1 complements a yeast sah1 deletion, providing an attractive experimental system for studies on structure–function relationships (Malanovic et al. 2008; Tehlivets 2011).
A high level of functional of structural conservation was also observed for the “lipolytic cascade,” a sequence of enzymatic steps that results in complete TAG degradation via DAG and MAG to glycerol and free FAs (Kurat et al. 2006; Zechner et al. 2012). These reactions are governed in yeast by the Tgl3 and Tgl4 TAG lipases, of which Tgl3 also harbors DAG lipase activity (Kurat et al. 2006); the final step of MAG hydrolysis is catalyzed by Yju3 in yeast (Heier et al. 2010). In mammals, ATGL is responsible for TAG breakdown to DAG, which is further subject to degradation by hormone-sensitive lipase and monoacylglycerol lipase. Notably, Tgl3, Tgl4, and ATGL are members of the patatin-domain-containing family of enzymes (Kienesberger et al. 2009), and Tgl4 deficiency in yeast can be functionally complemented by mouse or human ATGL, which also correctly localize to the LD, despite the absence of perilipins in yeast that regulate access of ATGL to LDs in mammalian cells (Kurat et al. 2006). Lypolysis clearly not only provides FAs for β-oxidation and energy production but also generates TAG-derived signaling molecules important for mitochondrial function in mammals (Zechner et al. 2012) or cell cycle progression in yeast (Kurat et al. 2009).
Many of the enzymes involved in TAG synthesis have orthologs in mammals and are now extensively studied with respect to their topology in the ER membrane and with respect to their contribution to LD formation (Choudhary et al. 2011; Jacquier et al. 2011; Pagac et al. 2011). A class of proteins implicated in TAG storage and LD biogenesis are the mammalian FIT proteins [fat storage-inducing transmembrane proteins (Kadereit et al. 2008; Gross et al. 2010, 2011; Moir et al. 2012)], of which two orthologs exist in yeast, encoded by SCS3 and YFT2. Yeast mutants lacking Scs3 are inositol auxotrophs, indicating a functional link to the transcriptional regulation of phospholipid synthesis (Henry et al. 2012); however, their specific roles in TAG metabolism and LD formation in yeast are unknown. Large-scale interaction studies indicate that both SCS3 and YFT2 have shared and unique functions and may be required for ER membrane biosynthesis in response to perturbations in lipid metabolism and ER stress (Moir et al. 2012). In two independent imaging-based screens, Fei et al. (2008, 2011b) and Szymanski et al. (2007) identified yeast mutants with aberrant LD morphology (“supersized LDs”) that are defective in the FLD1 gene. FLD1 is an ortholog of the mammalian BSCL2 gene encoding seipin that is associated with severe inherited Berardinelli–Seip congenital lipodystrophy type 2 (Agarwal and Garg 2003; Agarwal et al. 2004; Garg and Agarwal 2009). Notably, the supersized LD phenotype in yeast fld1 mutants can be complemented by mammalian wild-type BSCL2, supporting the high level of functional conservation in mammals and yeast (Fei et al. 2008, 2011b).
In addition to the lipogenic and lipolytic pathways, the major regulatory and signaling processes, such as TOR and AMPK/Snf1, are also conserved in yeast (Zaman et al. 2008; De Virgilio 2012) and apparently are connected to lipid homeostasis (Bozaquel-Morais et al. 2010); however, this “lipid connection” clearly needs further exploration. Notably, many yeast wild-type strains significantly differ in their LD content, but the genetic basis for these diverse lipid phenotypes is not known. This observation clearly reflects the polygenic nature of lipid and energy metabolism in mammals and requires QTL analysis to obtain further insight into the specific contributions of genes and pathways to cellular lipid homeostasis.
Peroxisomes
Microbodies were first described on the basis of their simple morphology in mouse renal tubule cells by Rhodin (1954). Later, biochemical functions could be attributed to these organelles, and they were functionally subdivided into peroxisomes (containing at least one hydrogen peroxide-producing oxidase and catalase), glyoxysomes (containing enzymes of the glyoxylate cycle) (Tolbert and Essner 1981), glycosomes (harboring glycolytic enzymes, observed only in trypanosomes) (Opperdoes and Borst 1977), Woronin bodies that are involved in plugging of septal pores in filamentous ascomycetes (Dhavale and Jedd 2007), and hydrogenosomes (producing hydrogen, observed only in anaerobic fungi) (Martin and Muller 1998). Of these, peroxisomes were first characterized as organelles implicated in hydrogen peroxide metabolism. However, today various other peroxisomal functions are known (for reviews see Nyathi and Baker 2006; van der Klei and Veenhuis 2006b; Wanders and Waterham 2006), including biosynthetic [e.g., in secondary metabolite biosynthesis (Bartoszewska et al. 2011)] and non-metabolic ones [e.g., in the innate immune response (Lazarow 2011)].
In yeast, the morphology of microbodies was described for the first time in S. cerevisiae by Avers and Federman (1968). However, it took almost 20 years before collaborative efforts of the groups of Veenhuis and Kunau demonstrated that peroxisomes play a crucial role in oleate metabolism in S. cerevisiae and that, consequently, these organelles are massively induced during growth of yeast on oleate as the sole carbon and energy source (Veenhuis et al. 1987). These findings opened a new era in peroxisome research, which contributed to the identification of the first PEX genes (Erdmann et al. 1989, 1991) involved in peroxisome development. This information subsequently allowed unraveling of the principles of human peroxisome biogenesis disorders because of the strong conservation of the molecular mechanisms of peroxisome development between lower and higher eukaryotes.
Peroxisome composition
Peroxisomes consist of a single membrane encompassing a protein-rich matrix. A typical feature of peroxisomal membranes is their low protein content, which is supported by their very smooth fracture faces in freeze-etch replicas (Figure 4A). The peroxisomal matrix is generally considered to represent the site of the highest protein concentration in eukaryotic cells. Indeed, peroxisomes are the highest density organelles after density gradient centrifugation of post-nuclear cell homogenates. The high protein concentration in these organelles often results in the formation of electron dense inclusions or protein crystalloids in the peroxisomal matrix, e.g., in peroxisomes of plants or methylotrophic yeasts. However, in wild-type S. cerevisiae this is never observed (Figure 4D): in this organism, peroxisome proliferation is induced by oleate and strongly repressed by glucose (Figure 4, C and D). Repression of peroxisome proliferation is especially evident in certain S. cerevisiae strains such as G910 (Veenhuis et al. 1987). These cells contain only one or very few peroxisomes during exponential growth on glucose (Figure 4C), in contrast to oleate-grown cells in which the abundance and size of peroxisomes is strongly increased (Figure 4D). However, in various currently used strains, peroxisome numbers in glucose-grown cells are only slightly lower compared to cells grown on oleate (Figure 4, E and F; S. cerevisiae BY4742).
Figure 4 .
Morphological characteristics of yeast peroxisomes. (A) Freeze-etch replica of oleic acid-grown S. cerevisiae cells showing the fraction faces of the different organelles. Peroxisomes contain very smooth fracture faces, indicative of a low abundance of integral membrane proteins. (B) Thin section of a cell grown on oleic acid cytochemically stained for catalase activity, using diamino-benzidine and hydrogen peroxide. In these cells, numerous stained, electron-dense peroxisomes are present. Staining of the mitochondrial cristae is due to cytochrome c peroxidase activity. Ultrathin sections of (C) glucose-grown and (D) oleic acid-grown and KMnO4-fixed S. cerevisiae G910 cells (Veenhuis et al. 1987). The cell grown on glucose displays only a few very small peroxisomes (arrows), whereas strong peroxisome proliferation is evident in the oleic acid-grown cell. (E and F) Fluorescence microscopy of (E) glucose-grown and (F) oleic acid-grown cells expressing GFP-SKL to label peroxisomes in wild-type strain BY4742. Notably, in this strain background, the difference in peroxisome number on glucose and oleic acid media is far less pronounced compared to strain G910. N, nucleus; M, mitochondrion; P, peroxisome; V, vacuole; LD, lipid droplet. Scale bar: 200 nm in A, 1 μm in B–D, and 3 μm in E and F.
The peroxisomal matrix almost exclusively contains enzymes, which generally harbor cofactors and are mostly oligomeric. Peroxisomal membrane-bound enzymes are rare. This may explain the relatively low membrane surface/volume ratio and the very low protein/phospholipid ratio of peroxisomal membranes. All characterized peroxisomal membrane proteins (PMPs) are involved in peroxisome biogenesis and dynamics or in solute transport processes; protein modifications, such as glycosylation or phosphorylation, have been reported for only a few PMPs and are absent from matrix proteins.
The Saccharomyces cerevisiae Genome Database currently contains 66 proteins that have been demonstrated to reside at peroxisomes (listed in Table 2). Of these, 24 encode enzymes, whereas only 3 represent membrane transporters for solutes. The remaining proteins are peroxins or proteins involved in various other peroxisomal processes, such as fission or inheritance (Table 2).
Table 2 . Peroxisomal proteins.
| Gene | Required for growth on oleate | Expression induced by oleate | Enzyme/activity | Molecular mass (kDa) | Isoelectric point | Molecules per cella | Localizationb | Function |
|---|---|---|---|---|---|---|---|---|
| β-Oxidation enzymes | ||||||||
| PCS60 (FAT2) | No | Yes | Medium chain fatty acyl-CoA synthetase | 60.5 | 9.98 | 8,770 | Peripheral peroxisomal membrane and matrix | Activates fatty acids with a preference for medium chain lengths, C9–C13 |
| FAT1 | No | Very long chain fatty acyl-CoA synthetase and long chain fatty acid transporter | 77.1 | 8.47 | 16,900 | Lipid droplet, ER, peroxisome Three predicted TM | Activates fatty acids with a preference for very long chain lengths, C20–C26 | |
| POX1 | Yes | Yes | Acyl-CoA oxidase | 84.0 | 8.73 | ND | Peroxisomal matrix | Oxidation of acyl-CoA |
| CTA1 | No | Yes | Catalase | 58.6 | 7.46 | 623 | Peroxisomal matrix | Degrades hydrogen peroxide produced by Pox1 |
| FOX2 (POX2) | Yes | Yes | Multifunctional enzyme; 3-hydroxyacyl-CoA dehydrogenase and enoyl-CoA hydratase | 98.7 | 9.75 | ND | Peroxisomal matrix | |
| POT1 (FOX3, POX3) | Yes | Yes | 3-Ketoacyl-CoA thiolase | 44.7 | 7.56 | ND | Peroxisomal matrix | Cleaves 3-ketoacyl-CoA into acyl-CoA and acetyl-CoAs |
| DCI1 (ECI2) | Δ(3,5)-Δ(2,4)-dienoyl-CoA isomerase (putative) | 30.1 | 8.83 | ND | Peroxisomal matrix | Auxiliary enzyme of fatty acid β-oxidation; role in β-oxidation debated | ||
| SPS19 (SPX19) | Yes | Yes | 2,4-Dienoyl-CoA reductase | 31.1 | 9.67 | ND | Peroxisomal matrix | Auxiliary enzyme of fatty acid β-oxidation |
| ECI1 | Yes | Yes | Δ3, Δ2-enoyl-CoA isomerase | 31.7 | 8.21 | ND | Peroxisomal matrix | Auxiliary enzyme of fatty acid β-oxidation |
| TES1 (PTE1) | Yes | Yes | Acyl-CoA thioesterase | 40.3 | 9.58 | ND | Peroxisomal matrix | Auxiliary enzyme of fatty acid β-oxidation |
| MDH3 | Yes | Yes | Malate dehydrogenase | 37.3 | 10.00 | 3,300 | Peroxisomal matrix | Required for the malate-oxaloacetete shuttle, to exchange peroxisomal NADH for cytosolic NAD+, part of the glyoxylate cycle |
| IDP3 | Yes | Yes | NADP+ dependent isocitrate dehydrogenase | 47.91 | 10.02 | ND | Peroxisomal matrix | Required for the 2-ketoglutarate/isocitrate shuttle, exchanging peroxisomal NADP+ for cytosolic NADPH |
| CAT2 | No | No | Carnitine acetyl-CoA transferase | 77.2 | 8.34 | 470 | Peroxisome, mitochondria | Transfers activated acetyl groups to carnitine to form acetylcarnitine which can be shuttled across membranes |
| Glyoxylate cycle | ||||||||
| CIT2 | No | Citrate synthase | 51.4 | 6.34 | 2,310 | Peroxisomal matrix | Condensation of acetyl CoA and oxaloacetate to form citrate | |
| MDH3 | Yes | Yes | Malate dehydrogenase | 37.3 | 10.00 | 3,300 | Peroxisomal matrix | Required for the malate–oxaloacetete shuttle, to exchange peroxisomal NADH for cytosolic NAD+ |
| MLS1 | Yes | Yes | Malate synthase | 62.8 | 7.18 | ND | Peroxisomal matrix | Required for utilization of nonfermentable carbon sources |
| Other peroxisome-associated enzyme activities | ||||||||
| GPD1 (DAR1, HOR1, OSG1, OSR5) | NAD-dependent glycerol-3-phosphate dehydrogenase | 42.9 | 5.26 | 807 | Peroxisome, cytosol, nucleus | Key enzyme of glycerol synthesis, essential for growth under osmotic stress | ||
| PNC1 | Nicotinamidase | 25.0 | 6.23 | 7,720 | Peroxisome, cytosol | Converts nicotinamide to nicotinic acid as part of the NAD(+) salvage pathway | ||
| NPY1 | NADH diphosphatase | 43.5 | 6.26 | 846 | Peroxisome, cytosol | Hydrolyzes the pyrophosphate linkage in NADH and related nucleotides | ||
| STR3 | Cystathionine β-lyase | 51.8 | 7.96 | ND | Peroxisome | Converts cystathionine into homocysteine | ||
| GTO1 | ω-Class glutathione transferase | 41.3 | 9.53 | Peroxisome | Induced under oxidative stress | |||
| AAT2 (ASP5) | Yes | Aspartate aminotransferase | 46.1 | 8.50 | 7,700 | Cytosol, peroxisome | Involved in nitrogen metabolism | |
| PCD1 | Nudix pyrophosphatase with specificity for coenzyme A and CoA derivatives | 39.8 | 6.59 | 238 | Peroxisome | May function to remove potentially toxic oxidized CoA disulfide from peroxisomes | ||
| LPX1 | Yes | Triacylglycerol lipase | 43.7 | 8.16 | 2,350 | Peroxisomal matrix | ||
| Peroxisomal transporters | ||||||||
| PXA1 (LPI1, PAL1, PAT2, SSH2) | Subunit of a heterodimeric ATP-binding cassette transporter complex | 100.0 | 10.34 | ND | Peroxisomal membrane | Import of long-chain fatty acids into peroxisomes | ||
| PXA2 (PAT1) | Subunit of a heterodimeric ATP-binding cassette transporter complex | 97.1 | 9.47 | ND | Peroxisomal membrane | Import of long-chain fatty acids into peroxisomes | ||
| ANT1 (YPR128C) | Adenine nucleotide transporter | 36.4 | 10.6 | 2,250 | Peroxisomal membrane | Involved in β-oxidation of medium-chain fatty acids | ||
| Peroxins | ||||||||
| PEX1 (PAS1) | AAA ATPase | 117.3 | 6.93 | 2,100 | Peroxisomal membrane | Involved in recycling of Pex5, forms heterodimer with Pex6 | ||
| PEX2 (CRT1, PAS5) | E3 ubiquitin ligase | 30.8 | 9.02 | 339 | Peroxisomal membrane | RING finger protein, forms complex with Pex10 and Pex12. Involved in matrix protein import | ||
| PEX3 (PAS3) | 50.7 | 6.29 | 1,400 | Peroxisomal membrane | Required for proper localization of PMPs | |||
| PEX4 (PAS2, UBC10) | Ubiquitin-conjugating enzyme | 21.1 | 5.36 | ND | Peroxisomal membrane | Involved in matrix protein import | ||
| PEX5 (PAS10) | Soluble PTS1 receptor | 69.3 | 4.79 | 2,070 | Cytosol and peroxisomal matrix | Required for import of PTS1-containing peroxisomal proteins, contains TPR domains | ||
| PEX6 (PAS8) | AAA ATPase | 115.6 | 5.44 | 1,630 | Peroxisomal membrane | Involved in recycling of Pex5, forms heterodimer with Pex1 | ||
| PEX7 (PAS7, PEB1) | Soluble PTS2 receptor | 42.3 | 8.34 | 589 | Cytosol and peroxisomal matrix | Requires Pex18 and Pex21 for association to the receptor docking site, contains WD40 repeat | ||
| PEX8 (PAS6) | Intraperoxisomal organizer of the peroxisomal import machinery | 68.2 | 7.62 | 538 | Peroxisomal matrix and luminal membrane face | Pex5-cargo dissociation | ||
| PEX10 (PAS4) | E3 ubiquitin ligase | 39.1 | 9.88 | ND | Peroxisomal membrane | RING finger protein involved in Ubc4-dependent Pex5 ubiquitination. Forms complex with Pex2 and Pex12 | ||
| PEX11 (PMP24, PMP27) | 26.9 | 10.65 | 1,630 | Peroxisomal membrane | Involved in peroxisome fission, required for medium-chain fatty acid oxidation | |||
| PEX12 (PAS11) | E3 ubiquitin ligase | 46.0 | 9.86 | 907 | RING finger protein, forms complex with Pex2 and Pex10 | |||
| PEX13 (PAS20) | Component of docking complex for Pex5 and Pex7 | 42.7 | 9.83 | 7,900 | Peroxisomal membrane | Forms complex with Pex14 and Pex17 | ||
| PEX14 | Central component of the receptor docking complex | 38.4 | 4.61 | 2,570 | Peroxisomal membrane | Interacts with Pex13 | ||
| PEX15 (PAS21) | 43.7 | 8.42 | 1,070 | Peroxisomal membrane | Recruits Pex6 to the peroxisomal membrane, tail anchored PMP | |||
| PEX17 (PAS9) | Component of docking complex for Pex5 and Pex7 | 23.2 | 10.24 | 656 | Peroxisomal membrane | Forms complex with Pex13 and Pex14 | ||
| PEX18 | Required for PTS2 import | 32.0 | 4.78 | ND | Interacts with Pex7; partially redundant with Pex21 | |||
| PEX19 (PAS12) | Chaperone and import receptor for newly synthesized PMPs | 38.7 | 4.08 | 5,350 | Cytosol, peroxisome; farnesylated | Interacts with PMPs, involved in PMP sorting. Also interacts with Myo2 and contributes to peroxisome partitioning | ||
| PEX21 | Required for PTS2 protein import | 33.0 | 6.67 | ND | Cytosol | Interacts with Pex7, partially redundant with Pex18 | ||
| PEX22 (YAF5) | Required for import of peroxisomal proteins | 19.9 | 8.33 | 259 | Peroxisomal membrane | Recruits Pex4 to the peroxisomal membrane | ||
| PEX25 | Involved in the regulation of peroxisome size and maintenance, required for re-introduction of peroxisomes in peroxisome deficient cells | 44.9 | 9.77 | 2,420 | Peripheral peroxisomal membrane | Recruits GTPase Rho1 to peroxisomes, interacts with homologous protein Pex27 | ||
| PEX27 | Involved in the regulation of peroxisome size and number | 44.1 | 10.49 | 382 | Peripheral peroxisomal membrane | Interacts with homologous protein Pex25 | ||
| PEX28 | Involved in the regulation of peroxisome size, number and distribution | 66.1 | 7.09 | ND | Peroxisomal membrane | May act upstream of Pex30, Pex31 and Pex32 | ||
| PEX29 | Involved in the regulation of peroxisome size, number and distribution | 63.5 | 6.8 | 5,040 | Peroxisomal membrane | May act upstream of Pex30, Pex31 and Pex32 | ||
| PEX30 | Involved in the regulation of peroxisome number | 59.5 | 5.59 | 4,570 | Peroxisomal membrane | Negative regulator, partially functionally redundant with Pex31 and Pex32 | ||
| PEX31 | Involved in the regulation of peroxisome number | 52.9 | 10.15 | 238 | Peroxisomal membrane | Negative regulator, partially functionally redundant with Pex30 and Pex32 | ||
| PEX32 | Involved in the regulation of peroxisome number | 48.6 | 9.14 | ND | Peroxisomal membrane | Negative regulator, partially functionally redundant with Pex30 and Pex31 | ||
| PEX34 | Involved in the regulation of peroxisome number | 16.6 | 10.30 | ND | Peroxisomal membrane | |||
| Peroxisome fission and inheritance | ||||||||
| DYN2 (SLC1) | Light chain dynein | 10.4 | 9.03 | 1,310 | Cytosol | Microtubule motor protein | ||
| SEC20 | v-SNARE | 43.9 | 5.92 | 4,910 | Golgi, ER | Involved in retrograde transport from the Golgi to the ER; interacts with the Dsl1 complex through Tip20 | ||
| SEC39 (DSL3) | Component of the Dsl1p-tethering complex | 82.4 | 4.65 | 1,840 | ER, nuclear envelope | Proposed to be involved in protein secretion | ||
| DSL1 (RNS1) | Component of the ER target site that interacts with coatomer | 88.1 | 4.69 | 8,970 | Peripheral ER, Golgi membrane | Forms a complex with Sec39 and Tip20 that interacts with ER SNAREs Sec20 and Use1 | ||
| FIS1 (MDV2) | Required for peroxisome fission | 17.7 | 9.87 | 2,410 | Peroxisomal membrane, mitochondria | Tail-anchored protein; recruits Dnm1 via Mdv1/Caf4; also involved in mitochondrial fission | ||
| DNM1 | GTPase, dynamin-like protein involved in peroxisome fission | 85.0 | 5.25 | 9,620 | Also involved in mitochondrial fission | |||
| VPS1 (GRD1, LAM1, SPO15, VPL1, VPT26) | GTPase, dynamin-like protein involved in peroxisome fission | 78.7 | 8.15 | 5,960 | Also involved in vacuolar protein sorting | |||
| VPS34 (END12, PEP15, VPL7, VPT29, STT8, VPS7) | Phosphatidylinositol 3-kinase | 100.9 | 7.79 | 1,080 | Forms complex with Vps15 | |||
| INP1 | Involved in retention of peroxisomes in mother cells | 47.3 | 8.34 | 639 | Peroxisomal membrane | Recruited to the peroxisome by binding to Pex3 | ||
| INP2 | Myo2 receptor, involved in peroxisome inheritance | 81.5 | 9.41 | 736 | Peroxisomal membrane | |||
| RHO1 | GTP-binding protein of the Rho subfamily of Ras-like proteins; involved in actin assembly at the peroxisome | 23.2 | 6.07 | ND | Involved in de novo peroxisome formation, recruited to peroxisomes by Pex25 | |||
Much of the information in the table may be found in the Saccharomyces Genome Database. ND, not determined.
The lipid composition of oleate-grown S. cerevisiae peroxisomes has been determined by Zinser et al. (1991). The peroxisomal membrane contains the major cellular phospholipids—phosphatidylcholine (48.2%), phosphatidylethanolamine (22.9%), and phosphatidylinositol (15.8%)—but also has a remarkably high cardiolipin content (7%). The relative abundance of cardiolipin is noteworthy since this lipid is synthesized in mitochondria (Henry et al. 2012). The other lipids are derived from the ER; however, the mechanisms by which these lipids reach the peroxisomes are not yet firmly established; some evidence suggests that this process involves vesicular transport both from the ER and from mitochondria (Braschi et al. 2010).
Peroxisome metabolic functions
By definition, peroxisomes contain at least one hydrogen peroxide-producing oxidase together with catalase, which decomposes the hydrogen peroxide by-product of the oxidation reaction. S. cerevisiae contains only one oxidase, namely the flavo-enzyme Pox1 (acyl-CoA oxidase), an enzyme of the β-oxidation pathway. Unlike most other species, S. cerevisiae contains a second, cytosolic catalase T, Ctt1, in addition to the peroxisomal catalase A isoenzyme, Cta1 (Skoneczny et al. 1988).
The two best-characterized peroxisomal metabolic pathways in S. cerevisiae are the β-oxidation of fatty acids (Poirier et al. 2006) (Figure 2B, Figure 5) and the glyoxylate cycle (Kunze et al. 2006) (Figure 5). In most other yeast species (e.g. Candida tropicalis, Hansenula polymorpha, Pichia pastoris), several other important peroxisome-bound pathways occur, such as the metabolism of alkanes and methanol and the oxidation of various organic nitrogen sources such as primary amines, purines, and D-amino acids (for a review see van der Klei and Veenhuis 2006b).
Figure 5 .
Compartmentalization of the β-oxidation pathway and the glyoxylate cycle. The enzymes of the β-oxidation (see Figure 2B) as well as key enzymes of the glyoxylate cycle are localized to peroxisomes in S. cerevisiae. In addition to the β-oxidation enzymes, three peroxisomal membrane-associated proteins are required for fatty acid oxidation in peroxisomes, namely the ABC transporter Pxa1/Pxa2 for the import of long-chain acyl-CoA, Faa2 for the activation of medium chain FAs, and the transporter Ant1 for import of ATP. The glyoxylate cycle converts two acetyl-CoA molecules into succinate and contributes to the export of acetyl-CoA that is produced in the β-oxidation cycle. Glyoxylate and the first acetyl-CoA molecule are condensed by malate synthase (Mls) to malate; malate dehydrogenase (Mdh) converts malate to oxaloacetate (OAA); and isocitrate synthase (Cit) condenses OAA and a second acetyl-CoA molecule to form citrate. Aconitase (Aco) catalyzes the isomerization of citrate into isocitrate, which is cleaved by isocitrate lyase (Icl) into succinate and glyoxylate. Glyoxylate can be used for the next round of the glyoxylate cycle, whereas succinate is used to replenish the citric acid cycle or to function as a precursor for amino acid or carbohydrate biosynthesis. In S. cerevisiae, citrate synthase, Cit2, and malate synthase Mls1 are peroxisomal enzymes, whereas Icl1 is cytosolic. This is in contrast to plants, filamentous fungi, and other yeast species, in which Icl is peroxisomal as well. Acetyl-CoA can also be exported via the carnitine shuttle, which involves peroxisomal Cat2. The malate shuttle is responsible for NADH export. The predicted small molecule transporters involved in both shuttles or in the glyoxylate cycle have not been identified yet.
β-Oxidation:
S. cerevisiae can utilize a range of saturated and unsaturated fatty acids of different chain lengths, which, in the absence of glucose, are oxidized by peroxisomal β-oxidation. β-Oxidation involves four steps, namely CoA activation, oxidation, hydratation/dehydrogenation, and thiolytical cleavage to generate acetyl-CoA and an acyl chain that is shortened by two carbon atoms (Figure 2B, Figure 5). Activation of medium chain fatty acids occurs in the organelle matrix via acyl-CoA synthetase, Faa2 (Figure 5). This process requires ATP, which is imported into the organelle by the adenine nucleotide transporter Ant1 (Table 2) (Palmieri et al. 2001). It has been suggested that Faa2 produces AMP and pyrophosphate; hence, most likely Ant1 exchanges AMP for ATP across the peroxisomal membrane. Long-chain fatty acids are activated outside the organelle by Fat1 and taken up as CoA esters via the heterodimeric ABC transporter consisting of Pxa1 and Pxa2 (Hettema et al. 1996) (Figure 5). A portion of the cellular Fat1 activity is associated with peroxisomes (Watkins et al. 1998). The acetyl-CoA product of the β-oxidation is transported to mitochondria for further oxidation by the citric acid cycle. Export from peroxisomes occurs via two different pathways, namely via carnitine-dependent acetyl-CoA transport that involves Cat2 or via the glyoxylate cycle (Figure 5). NADH is transferred to the cytosol via the malate shuttle (Figure 5) (for an excellent review see van Roermund et al. 2003).
For β-oxidation of unsaturated fatty acids with trans and cis double bonds at odd-numbered positions or cis double bonds at even positions, auxiliary peroxisomal enzymes are required, i.e., Eci1, Dci1, Tes1, and Sps19 (van Roermund et al. 2003) (Table 2, Figure 2). For example, oleic acid metabolism requires the auxiliary enzyme Eci1, which catalyzes the isomerization of the cis double bond of oleate after shortening of the oleoyl-CoA chain by three rounds of β-oxidation (Kunau et al. 1995). Eci1 also isomerizes a fraction of 2-trans, 5-cis-tetradecadienoyl-CoA, an intermediate of oleic acid β-oxidation, to 3,5-cis-tetradecadienoyl-CoA, which has two conjugated double bonds that prevent further β-oxidation. Gurvitz et al. (1999) proposed that 3,5-cis-tetradecadienoyl-CoA is oxidized by the reductase-dependent pathway and involves the dienoyl isomerase Dci1. However, recent data also indicate that the thioesterase-dependent pathway, which involves the acyl-CoA thioesterase Tes1, is operative in S. cerevisiae (Ntamack et al. 2009).
The β-oxidation of unsaturated fatty acids with a double bond at an even position requires the function of the NADPH−dependent 2,4-dienoyl-CoA reductase Sps19. The NADP+ generated by this reaction is reduced by the peroxisomal isocitrate dehydrogenase Idp3, and, for the regeneration of NADPH, an isocitrate/2-oxoglutarate shuttle exists (reviewed in van Roermund et al. 2003).
The role of Pcs60/Fat2, which is associated with the inside of the peroxisomal membrane, is still unclear. Pcs60/Fat2 belongs to the family of proteins that act via an ATP-dependent covalent binding of AMP to their substratesand shows high similarity to Escherichia coli long-chain acyl-CoA synthetases (Blobel and Erdmann 1996). However, Pcs60/Fat2 is not required for growth of S. cerevisiae on oleate. Interestingly, this protein binds mRNAs encoding proteins involved in triglyceride metabolism (Tsvetanova et al. 2010).
Glyoxylate cycle:
The glyoxylate cycle allows cells to convert two acetyl-CoA molecules into succinate, which can be used to replenish the citric acid cycle or to function as precursors for amino acid or carbohydrate biosynthesis (Figure 5). In yeast, this cycle is essential for growth on oleate or C2 substrates such as ethanol or acetate. In S. cerevisiae, three glyoxylate cycle enzymes are cytosolic, namely the malate dehydrogenase Mdh2, the aconitase Aco1, and the isocitrate lyase Icl1, whereas two are peroxisomal: namely citrate synthase Cit2 and malate synthase Mls1 (McCammon et al. 1990; Taylor et al. 1996) (Figure 5). Remarkably, in most other yeast species, in plants, and in filamentous fungi, isocitrate lyase also is a peroxisomal enzyme (for a review see Kunze et al. 2006). The presence of Icl1 in the cytosol of S. cerevisiae is surprising as it catalyzes the production of the reactive compound glyoxylate. Thus, the compartmentalization of the enzymes of the glyoxylate cycle predicts the presence of solute transporters for glyoxylate cycle intermediates in the peroxisomal membrane (Figure 5). It is, however, also possible that no specific transport proteins are required since yeast peroxisomal membranes contain pore-forming proteins that allow passage of small molecules with a molecular mass up to 400 Da, which is sufficient to allow passage of these intermediates (Antonenkov et al. 2009). However, the genes encoding these pore-forming proteins have not yet been identified.
Other peroxisomal enzymes:
Except for the key enzymes involved in fatty acid utilization and the glyoxylate cycle, additional enzymes are (perhaps only transiently) associated with peroxisomes in S. cerevisiae. However, for many of these proteins the physiological relevance is unclear (Table 2). Moreover, in some cases their peroxisomal localization has not yet been firmly established. Some examples are detailed below. Lpx1 is a peroxisomal protein that has acyl hydrolase and phospholipase A activity in vitro. Deletion of LPX1 results in peroxisomes with aberrant morphology that is characterized by intraperoxisomal vesicles or invaginations, which may point to a role of vesicle fusions in peroxisome development (Thoms et al. 2008). Several peroxisomal matrix proteins have been implicated to play a role in (oxidative) stress response or aging. Glycerol-3-phosphate dehydrogenase 1 (Gpd1) is a key enzyme in glycerol biosynthesis and essential for S. cerevisiae to cope with osmotic stress (Albertyn et al. 1994); its expression is regulated by the high-osmolarity glycerol response pathway. Fluorescence microscopy (Huh et al. 2003) and proteomics analysis of peroxisomal fractions (Marelli et al. 2004) indicated a (partial) peroxisomal localization of Gpd1. This was recently confirmed by Jung et al. (2010) who showed that Gpd1 is targeted to peroxisomes via the PTS2 pathway (see below). However, under stress conditions, Gpd1 is localized to the cytosol and the nucleus, a process that is regulated by phosphorylation of a residue adjacent to the PTS2 in Gpd1. The physiological relevance of the different subcellular locations remains to be established.
Interestingly, Pnc1, whose import into peroxisomes also depends on the PTS2 pathway, shows a similar shift in localization in response to stress (Anderson et al. 2003). Moreover, the expression of GPD1 and PNC1 is strongly correlated (Jung et al. 2010). Pnc1 converts nicotinamide to nicotinic acid as part of the NAD+ salvage pathway. Nicotinamide strongly inhibits Sir2, a protein important for life-span extension by calorie restriction (Kaeberlein et al. 1999), and, like Sir2, Pnc1 also functions in life-span extension (Anderson et al. 2003). Most likely, Pnc1 regulates longevity by reducing nicotinamide levels, which activates Sir2 (Gallo et al. 2004). Whether the localization of Pnc1 to peroxisomes is important for its function in life-span extension is not yet known.
GTO1 encodes a PTS1 containing ω-class peroxisomal glutathione transferase (Barreto et al. 2006), whose expression is induced by oxidative stress. The role of Gto1 may be related to the redox regulation of cystathionine β-lyase, Str3, another putative peroxisomal protein (Schafer et al. 2001; Yi et al. 2002). Str3 is involved in transulfuration of cysteine to homocysteine (Table 2).
Finally, two phosphatases, Npy1 and Pcd1, were found to be localized to peroxisomes. Npy1 is a PTS1-containing diphosphatase, which utilizes NADH as its preferred substrate. This enzyme may function in the regulation of nicotinamide coenzyme concentrations or in the elimination of oxidized nucleotide derivatives (Abdelraheim et al. 2001). Pcd1 is a PTS2-containing diphosphatase, which is active toward coenzyme A and its derivatives (Cartwright et al. 2000). A proposed role for this enzyme is the removal of potentially toxic oxidized CoA disulfide in peroxisomes (Cartwright et al. 2000).
Methods to identify proteins involved in peroxisome biology
Identification of peroxisomal enzymes:
Peroxisome-borne proteins were first identified by biochemical analysis of fractions enriched in peroxisomes obtained by subcellular fractionation. This approach became feasible because of the pioneering work of the Nobel Laureate Christian de Duve, who was the first to isolate peroxisomes from rat liver (De Duve 1965). However, it took a decade before the first report appeared on the isolation of S. cerevisiae peroxisomes from derepressed cells (Parish 1975), and controversies regarding the yeast peroxisomal protein content remained over several years. Major improvements in the preparation techniques were achieved after the finding that peroxisome proliferation in S. cerevisiae can be induced by oleate (Veenhuis et al. 1987). Together with the optimization of cell fractionation protocols for S. cerevisiae, McCammon et al. (1990) were able to convincingly show the localization of enzymes of the glyoxylate and β-oxidation in highly purified peroxisomal fractions of oleate-grown yeast. Today, these procedures are classic in peroxisome research and still in use (among others) to determine the organelle proteome and to characterize defects in peroxisome assembly in mutant strains (for detailed protocols see Distel and Kragt 2006).
Discovery of peroxins by genetic approaches:
Growth of S. cerevisiae on oleate requires intact peroxisomes; hence peroxisome-deficient mutants were readily selected from collections of oleate utilization-deficient mutants (Erdmann et al. 1989). The corresponding PEX genes were cloned by functional complementation upon transformation of the selected mutant with a genomic library. In this way, the first PEX gene was identified in baker’s yeast (Erdmann et al. 1991). Subsequently, similar approaches were used for other yeast species, including Yarrowia lipolytica and Pichia pastoris, two yeast species that are also capable of growing on oleate, as well as Hansenula polymorpha, which, like P. pastoris, can grow on methanol as the sole source of carbon and energy (van der Klei et al. 1991; Liu et al. 1992; Nuttley et al. 1994). In these latter two species, H. polymorpha and P. pastoris, peroxisomes are essential for growth on methanol, a property used for the identification of PEX genes. The identification of novel PEX genes in the other yeast species facilitated the work in S. cerevisiae because homologous genes were readily cloned on the basis of sequence homology, especially after completion of the sequencing of the entire S. cerevisiae genome (Goffeau et al. 1996). This was particularly advantageous because of the functional redundancy of some S. cerevisiae PEX genes and the lack of clear phenotypes of certain pex mutants.
Most of the S. cerevisiae PEX genes identified by the above approaches appear to be involved in matrix protein import—namely PEX1, -2, -4, -5, -6, -7, -8, -10, -12, -13, -14, -15, -17, -18, -21, -22 (Table 2)—whereas two genes, PEX3 and PEX19, are proposed to be essential for the formation of functional peroxisomal membranes. In the first group of mutants, remnant peroxisomal membrane structures (also termed “ghosts”) are still present, indicating that the formation of peroxisomal membranes is independent of the matrix protein import process. In pex3 and pex19 mutants, however, peroxisome membrane structures are not detectable. In both groups of mutants, most of the peroxisomal matrix proteins are mislocalized to the cytosol, where they are relatively stable. PMPs are also stable in mutants of the first group and localize to the peroxisomal ghosts. In pex3 or pex19 mutants, however, PMPs are mislocalized to the cytosol or the ER, or are rapidly degraded (Hettema et al. 2000; Otzen et al. 2004).
Most of the recently identified PEX genes (Table 2) have been identified by alternative approaches (see section below). In general, deletion of these genes results in alterations of peroxisome numbers and/or size and is not accompanied by mislocalization of peroxisomal protein or major defects in growth on oleate media.
As shown in Table 2, not all PEX genes that have been identified so far are also present in S. cerevisiae. PEX9 was solely identified in the yeast Y. lipolytica, but later studies revealed that it was incorrectly annotated and in fact encodes PEX26 (Kiel et al. 2006). PEX16 occurs only in higher eukaryotes and in filamentous fungi, but is absent from most yeast species, with the exception of Y. lipolytica (Kiel et al. 2006). PEX20 encodes a peroxin in filamentous fungi, which is the functional ortholog of S. cerevisiae Pex18 and the partially redundant protein Pex21, coreceptors in the PTS2 matrix protein import pathway (for details see below). Similarly, Pex26 occurs only in higher eukaryotes and filamentous fungi, but can be regarded as the functional homolog of S. cerevisiae Pex15, which is a PMP that recruits the two ATPases and members of the AAA-protein family, Pex1 and Pex6, to the peroxisomal membrane.
Pex33 was identified in the filamentous fungus Neurospora crassa and shows homology to a short N-terminal domain of Pex14 (Managadze et al. 2010). A similar protein was identified in the fungus Penicillium chrysogenum, where it was designated Pex14/17 (Opalinski et al. 2010).
Y. lipolytica Pex23 has three homologs in S. cerevisiae that are designated Pex30, Pex31, and Pex32 in this organism; Y. lipolytica Pex24 is homologous to S. cerevisiae Pex28 and Pex29 (Table 2).
Identification of peroxisomal proteins by organelle proteomics:
PEX11 is the first peroxin that has not been cloned by classic genetic approaches. Instead, it was identified by sequencing of a protein present in purified peroxisomal membranes followed by reversed genetics (Erdmann and Blobel 1995; Marshall et al. 1995). The same approach resulted in the identification of other novel PMPs, such as Psc60, Pex13, and Ant1. The first mass spectrometry analyses of purified peroxisomal membrane fractions were reported in 2001 (Schafer et al. 2001) and in 2002 (Yi et al. 2002) and resulted in the identification of several novel candidate peroxisomal proteins. An extensive analysis of the proteome of isolated S. cerevisiae peroxisomes was reported by Marelli et al. (2004). In this study, classic subcellular fractionation procedures were combined with immuno-isolation to obtain fractions enriched in peroxisomes from oleate-grown cells. This quantitative proteomics study resulted in a list of 71 candidate proteins that had a high likelihood of being peroxisomal because 28 of them were already annotated as being peroxisomal. Interestingly, the list contained several candidate proteins that were previously localized to other cell compartments, such as the LD protein Faa1 and six proteins linked to the secretory pathway, namely Dpm1, Ybr159w, Yor086c, Ygr266w, Rho1, and Cdc42. Biochemical and microscopic studies of a selection of eight of the candidate proteins confirmed that most of these proteins were, at least transiently, located to peroxisomes such as Rho1, Emp24, Faa1, and Erg6 (Marelli et al. 2004).
Rho1 is a small GTPase typically localizing to the plasma membranes and endomembranes in glucose-grown cells. However, Rho1 also localizes to the peroxisomal surface where it is recruited by the PMP Pex25 (Marelli et al. 2004); it is suggested that it regulates the assembly state of actin and thereby controls peroxisome membrane dynamics. Recent data in H. polymorpha revealed that in that yeast species a significant portion of Rho1 also colocalizes with peroxisomes (Saraya et al. 2011); Rho1 as well as Pex25 are essential for the generation of peroxisomes in H. polymorpha pex3 cells upon transformation and expression of PEX3 (for details see below).
The role of S. cerevisiae Emp24 in peroxisome biology has not yet been analyzed in greater detail. Emp24 is a member of the family of p24 proteins, which are membrane proteins that are abundantly present in the membranes of the early secretory pathway (for a review see Strating et al. 2009). Studies in H. polymorpha showed that a (minor) portion of H. polymorpha Emp24 colocalizes to peroxisomes, where it is important for peroxisome fission by an as-yet-unknown mechanism (Kurbatova et al. 2009).
Erg6 and Faa1 are typically localized to the ER and LDs (see above; Table 1), but were also found to colocalize with peroxisomes, which may reflect the close physical and physiological interaction between these organelles. Alternatively, however, the peroxisomal fractions analyzed may have been contaminated with LDs, which are also induced in the presence of oleate (Binns et al. 2006; Pu et al. 2011). A large number of candidate peroxisomal proteins identified in the study by Marelli et al. (2004) have not yet been further analyzed, but are of high interest for future peroxisome research.
In silico prediction of peroxisomal proteins:
Comparative genomics approaches have been used to identify putative peroxisomal matrix proteins based on consensus sequences of the peroxisomal targeting signals (PTSs) PTS1 and PTS2. The first report on in silico prediction of S. cerevisiae peroxisomal matrix proteins was by Kal et al. (2000). Emanuelsson et al. (2003) designed an improved predictor (PeroxiP, http:www.bioinfo.se/PeroxiP/) in which the sequence of the nine amino acids that immediately precede the PTS1 consensus sequence also was included as a criterion. Another predictor specific for PTS1 proteins has been generated by Neuberger et al. (2003a,b; mendel.imp.ac.at/mendeljsp/ sat/pts1/PTS1predictor.jsp). Although very successful for metazoans and plants, these methods have not been very effective in identifying novel peroxisomal yeast proteins.
The limited success of using peroxisomal targeting sequences for the prediction of yeast peroxisomal proteins may be due to the fact that several peroxisomal matrix proteins exist that lack a consensus PTS, e.g., Pox1 and Cat2 (van der Klei and Veenhuis 2006a). On the other hand, proteins that do have a PTS based on computer predictions may not be peroxisomal because these signals are masked by protein conformation or altered/removed by post-translational processes. Moreover, it was recently established that in certain fungi some glycolytic enzymes obtain a PTS only upon ribosomal readthrough or by differential splicing, resulting in partial peroxisomal localization of these predominantly cytosolic enzymes (Freitag et al. 2012). In addition, in silico prediction is not yet possible for PMPs because no specific signature is currently known for these proteins.
Transcriptome analysis:
Another approach that has been used to identify peroxisomal proteins is the analysis of transcripts that are induced upon stimulation of peroxisome proliferation. Serial Analysis of Gene Expression analysis (Kal et al. 1999) revealed that predominantly genes encoding peroxisomal enzymes of the β-oxidation pathway and other proteins participating in oleate metabolism were upregulated upon shifting S. cerevisiae from glucose to oleate media. This study resulted in the first identification of the acyl-CoA thioesterase Tes1. The rather surprising outcome of this study was that, with the exception of PEX11, the messenger RNA levels of PEX genes remained unaltered after the shift from glucose to oleate medium (Kal et al. 1999).
Smith et al. (2002) were the first to perform microarray analysis to identify novel oleate-inducible peroxisomal proteins. In this study, candidate genes were identified by pattern matching of profiles of genes known to be involved in peroxisome biogenesis or function by using three complementary clustering algorithms. Thus, in addition to highly induced genes, genes that have lower levels of induction but similar induction patterns were identified. The screen resulted in a list of 225 candidate genes of which 2 were further analyzed to validate the approach. Indeed, both genes, LPX1 and PEX25 (Table 2), encoded novel peroxisomal proteins. Microarray studies using the methylotrophic yeast H. polymorpha also showed that predominantly the peroxisomal enzymes of methanol metabolism are strongly induced upon a shift of cells from glucose to methanol medium (van Zutphen et al. 2010). Again, PEX genes were not or were only slightly induced under these conditions; the highest increase in gene expression (four- to fivefold) was observed for PEX11 and PEX32 upon induction of peroxisome proliferation.
Microscopic analysis of peroxisomes:
A direct way to identify peroxisomal proteins is by fluorescence microscopy. Today several genome-wide studies to localize the entire S. cerevisiae proteome have been reported. The first study was performed by Kumar et al. (2002) who used indirect, high-throughput immunofluorescence. These authors epitope-tagged 2085 S. cerevisiae ORFs and also randomly tagged genes by transposon mutagenesis. However, novel peroxisomal proteins were not identified by this approach.
In 2003, Huh et al. performed fluorescence microscopy of glucose-grown cells of an S. cerevisiae collection of strains that express C-terminal GFP fusion proteins. This study resulted in 21 proteins with a putative peroxisomal localization. This rather low number may be related to the fact that the proteins were C-terminally tagged, which masks the function of the C-terminal PTS1 sequences. Moreover, cells were grown on glucose in this study, and thus peroxisome proliferation was not strongly induced (Huh et al. 2003). This study, however, resulted in the detection of novel peroxisomal proteins and showed the association of Gpd1 with peroxisomes (Table 1). Natter and colleagues (Kals et al. 2005; Natter et al. 2005) carried out a similar study with plasmid-encoded C-terminal GFP fusion proteins. This study by Natter et al. was limited to 493 proteins, which were selected on the basis of their potential participation in lipid metabolism and membrane assembly; the localization of 16 known yeast peroxisomal proteins was also confirmed by that study.
Wolinski et al. (2009b) used GFP fused to a PTS1 under the control of the ADH1 promoter to analyze all 4740 viable yeast deletion strains by confocal imaging and automated quantitative analysis for peroxisome deficiency or morphological alterations. In addition to all previously known pex mutants defective in PTS1 protein import, mdh2 and afg1 deletion mutants showed a significant amount of cytosolic GFP-PTS1 as well. MDH2 encodes a cytosolic malate dehydrogenase, whose function is important in peroxisomal β-oxidation (Figure 5). AFG1 encodes a mitochondrial member of the AAA family of proteins. Analysis of the localization of additional peroxisomal membrane and matrix markers in mdh2 and afg1 cells indicated that both strains are defective in PTS1 and PTS2 matrix protein import, but not in the formation of peroxisomal membranes. How these proteins specifically function in matrix protein import is not yet known.
Saleem et al. (2008) analyzed a collection of 249 mutant strains lacking nonessential kinases, phosphatases and cyclins for alterations in peroxisome biology. In this study, GFP was fused to the C terminus of the PTS2 protein Pot1. The POT1 promoter contains an oleate-responsive element, which is typical for oleate-induced peroxisomal β-oxidation enzymes (for a review see Gurvitz and Rottensteiner 2006). Cells were analyzed using a combination of confocal laser scanning microscopy to monitor peroxisome abundance and morphology and fluorescence activated cell sorting (FACS) to measure Pot1-GFP fluorescence levels. Different classes of regulatory proteins were found to regulate POT1 expression and peroxisome number and size; interestingly, the nonessential cyclin-dependent kinase Pho85 was shown to be involved in both. Prominent effectors in peroxisome biogenesis identified in that study include actin-regulating proteins, which function through the action of Rho regulators (e.g., Rho1), as well as proteins involved in phosphatidylinositol metabolism (e.g., Vps34). The latter group of proteins may mediate so far unknown membrane fusion processes in peroxisome biogenesis (Saleem et al. 2008). In a recent update to this study, Saleem et al. (2010) analyzed some 4000 S. cerevisiae deletion strains expressing chromosomally integrated Pox1-GFP. In this screen, most of the known PEX genes required for PTS2 protein import were identified. In addition, deletion of CBS1 resulted in mislocalization of Pot1-GFP. This was a very unexpected finding because Cbs1 is a mitochondrial translational activator. Interestingly, Pot1-GFP import into peroxisomes partially recovered when cbs1 cells were maintained for several days on solid growth media. In addition, the screen revealed three novel proteins involved in peroxisome inheritance: Vps52, a regulator of actin; Pir3, involved in cell-wall organization; and Ykr015c, a protein of unknown function. Several additional mutants that displayed alterations in peroxisome size and/or number were identified (see the Yeast Peroxisome Cellular Imaging Resource at http://pbeid.systemsbiology.net). These include the known genes involved in the regulation of peroxisome size, PEX11, VPS1, and DNM1, as well as two novel genes, MNN11, encoding a subunit of a Golgi-resident mannosyltransferase, and HSL7, encoding the bud-neck-localized protein arginine N-methyltransferase. The role of these proteins in regulating peroxisome proliferation still needs to be established.
Many of the proteins identified in the various imaging-based screens do not localize to peroxisomes or are rather known to be involved in other cellular processes. Deletion of genes encoding mitochondrial proteins leads to a reduction in peroxisome numbers, e.g., Cox6, Cox9, and Cox10, which are involved in mitochondrial respiration, further stressing the functional and metabolic links between peroxisomes and mitochondria. Indeed, defects in mitochondrial function have previously been indicated to stimulate peroxisome proliferation—the so-called retrograde response. Also, deletion of two genes encoding vacuolar proteins results in reduced numbers of peroxisomes, i.e., the vacuolar t-SNARE Vam3 or the subunit of the vacuolar ATPase Vph1. Similarly, deletion of the VAM3 and VAM7 genes encoding two vacuolar t-SNARES in H. polymorpha also results in unusual peroxisomal structures with multiple membrane-enclosed compartments (Stevens et al. 2005).
Surprisingly, given the important proposed role for the ER in peroxisome biogenesis (see below), no ER proteins were identified in the screen of Saleem et al. (2010) that were important for peroxisome biogenesis, inheritance, or regulation of organelle number. Only deletion of FEN1, a gene required for fatty acid elongation (Henry et al. 2012), showed an effect on peroxisome size and resulted in enlarged organelles (Saleem et al. 2010).
Peroxisome Biogenesis
Peroxisomal matrix protein import
Targeting signals and their receptors:
The peroxisomal matrix protein import machinery has unique properties and differs fundamentally from import systems of other eukaryotic organelles in that it can translocate folded proteins and even protein complexes across the membrane. Peroxisomal matrix proteins are nuclear encoded, synthesized in the cytosol, and subsequently post-translationally imported into the organelle. These proteins harbor PTSs that are recognized by the soluble receptor proteins Pex5p (PTS1) and Pex7p (PTS2), respectively. It is generally assumed that PTS1 signals are not cleaved upon import, whereas a few PTS2 sequences were indeed shown to be processed upon import in higher eukaryotes.
PTS1 and its receptor Pex5:
The first PTS1 that has been described is the C-terminal tripeptide SKL in firefly luciferase (Gould et al. 1987). This PTS1 tripeptide, as well as the PTS1 peroxisomal import machinery, is highly conserved in yeast, plants, insects, and mammals (Gould et al. 1990). Detailed sequence analysis and mutagenesis studies revealed that the PTS1 tripeptide invariably consists of a small neutral amino acid at the first position, followed by an amino acid residue capable of hydrogen bonding at the penultimate position and a hydrophobic residue at the extreme C terminus. Also, the tripeptide must be present at the extreme C terminus of the protein. The most commonly used consensus of the PTS1 tripeptide is [(S/A/C)(K/R/H)(L/M)]. Studies by Lametschwandtner et al. (1998) have suggested a broader degeneracy in yeast and human PTS1 sequences. Also, not all PTS1 peptides that bind human Pex5 can bind yeast Pex5 as well, demonstrating some species specificity.
Detailed studies on the interaction of the PTS1-binding domain of Pex5 with various proteins/peptides indicate that residues upstream of the PTS1 tripeptide also are important for cargo recognition. Therefore, the PTS1 is now defined as a C-terminal 12-amino-acid sequence, which consists of the C-terminal tripeptide that interacts with the PTS1-binding site in Pex5, a tetrapeptide immediately upstream of this tripeptide, which may interact with the surface of Pex5, and a flexible hinge region of five residues (reviewed by Brocard and Hartig 2006).
The PTS1 receptor Pex5 consists of a relatively poorly conserved N-terminal domain and a C-terminal domain, which contains six tetratricopeptide repeats (TPR). A typical feature of the N terminus of Pex5 is the presence of several WxxxF/Y motifs. In human Pex5, these motifs are required for binding of the receptor to Pex13 and Pex14, but in yeast the WxxxF/Y motif is important only for Pex13 binding (Williams et al. 2005). The N terminus of Pex5 also is the region where the protein is modified by mono- or multiubiquitination, which is important for receptor recycling (see below).
The Pex5 TPR domain is the actual PTS1-binding site (Brocard et al. 1994). According to a crystal structure of the TPR domain of human Pex5, the PTS1 is locked in a groove allowing various interactions with two sets of three TPR motifs linked together by a helical hinge (Gatto et al. 2000). Structural studies by Stanley et al. (2006) using the human Pex5 TPR domain and a full-length peroxisomal matrix protein, the sterol carrier protein 2 (SCP2), indicated that the cargo is bound to the receptor by two separate binding sites: a C-terminal PTS1 motif and a topologically separated secondary site. These studies also revealed major conformational changes of the receptor that occur upon cargo loading. Unfortunately, so far no structural data on a full-length Pex5 protein have been reported. For further details on the structural properties of Pex5, the reader is referred to the excellent review by Stanley et al. (2007).
As discussed above for the SCP2 protein, the PTS1-binding site in the C-terminal TPR domain of the Pex5 receptor is not the only site of interaction with cargo. Some matrix proteins without (Klein et al. 2002) or with a redundant PTS1 (Gunkel et al. 2004) also bind to the N terminus of Pex5, such as Pox1 and Cat2 in S. cerevisiae (Schafer et al. 2004) and alcohol oxidase in H. polymorpha (Gunkel et al. 2004). Mutational analysis of the S. cerevisiae Pex5 N terminus indicates that the domains that are required for Cat2 and Pox1 binding are overlapping but not identical. Hence, most likely multiple residues in the N terminus of Pex5 are involved in the recognition of non-PTS1 proteins.
The occurrence of different matrix protein-binding sites in Pex5 variants was nicely illustrated by studies of Ozimek et al. (2006), who showed that S. cerevisiae Pex5 (via its TPR domain) and H. polymorpha Pex5 (via its N-terminal domain) recognize different, independent binding sites in the same peroxisomal matrix protein. Also, S. cerevisiae peroxisomal catalase A has a redundant PTS1, which is the very unusual hexapeptide SSNSKF. Catalase A has a second internal PTS, but it is unknown to which region (N or C terminus) of Pex5 this sequence actually binds (Kragler et al. 1993).
PTS2 and Pex7 with its coreceptors:
PTS2 was first identified in rat liver thiolase, where it is present as an N-terminal presequence that is processed upon import. Only a few proteins have a PTS2 for which the consensus sequence is (R/K)(L/V/I)X5(Q/H)(L/A/I). S. cerevisiae expresses only three peroxisomal proteins with a firmly established PTS2, namely Pot1, Pcd1, and Gpd1.
The PTS2 is recognized by the soluble receptor Pex7, which is characterized by the presence of six WD repeats. On the basis of its sequence, the receptor is predicted to fold as a seven-bladed β-propeller domain, in which each “blade” comprises a so-called WD repeat (Li and Roberts 2001; Stanley et al. 2007); however, no Pex7 crystal structures have been resolved yet.
Pex7 requires additional proteins that function as coreceptors. In S. cerevisiae, these are the partially redundant, homologous proteins Pex18 and Pex21 (for a review see Schliebs and Kunau 2006). In other yeast species and filamentous fungi, this function is fulfilled by Pex20 (Einwachter et al. 2001). The coreceptors share a characteristic N-terminal region that contains WxxxF/Y sequence motifs, such as the N terminus of Pex5 and a Pex7-binding region. Also, like the Pex5 N terminus, these proteins are ubiquitinated, a process that is required for recycling of the proteins (Leon and Subramani 2007). Additional functions have also been attributed to the coreceptor proteins, e.g., in oligomerization of the cargo protein (Titorenko et al. 1998) or in PTS2 binding (Otzen et al. 2005).
Piggy-back import:
Some peroxisomal proteins without a recognizable PTS still can be imported into peroxisomes in complex with a PTS-containing protein. Recently, the first example of piggy-back import was reported for the mammalian peroxisomal protein, superoxide dismutase (Islinger et al. 2009). The examples that have been reported in yeast (Glover et al. 1994; McNew and Goodman 1994) all comprise artificial co-expression of oligomeric proteins with and without PTS sequences, and it is currently unclear whether piggy-back import indeed occurs for endogenous yeast proteins in vivo.
Receptor docking site:
The receptor-cargo complex docks at the outer surface of the peroxisomal membrane to a proteinaceous receptor docking site that consists of the three peroxisomal membrane proteins Pex13, Pex14, and Pex17. Pex17, which interacts with Pex14, occurs only in S.cerevisiae, and its function is still enigmatic. Pex13 and Pex14 are highly conserved, and they interact with each other as well as with both Pex5 and Pex7 import receptors (Figure 6A). Pex13 is an integral membrane protein that contains two transmembrane regions and a Src homology 3 (SH3) domain at the extreme C terminus, which is exposed to the cytosol. The extreme N terminus of Pex13 binds Pex7 (Stein et al. 2002), whereas the SH3 domain has distinct binding sites for Pex5 and Pex14 (Pires et al. 2003). In Pex14, a PxxP domain, as well as other conserved residues, is involved in the interaction with the Pex13 SH3 domain (Girzalsky et al. 1999; Bottger et al. 2000). Pex14 also binds to a region in between the two transmembrane domains of Pex13 (Schell-Steven et al. 2005). In addition, indirect interactions may occur between both peroxins, which could explain why disruption of individual interaction sites often does not significantly affect protein import in vivo (Williams and Distel 2006).
Figure 6 .
Peroxisomal protein import. (A) Hypothetical model of PTS1 protein import. First, cytosolic Pex5 binds a newly synthesized PTS1-containing cargo protein (“cargo”). The PTS1 binds to the C-terminal TPR domain of Pex5. Next, Pex5 docks to the receptor-docking complex at the peroxisomal membrane, which is composed of Pex13, Pex14, and Pex17. Docking involves the N-terminal domain of Pex5, indicated as a spiral. Subsequently, the Pex5-cargo complex is imported into the organellar matrix. Pex5 most likely forms a transient pore in the peroxisomal membrane. The Pex5-cargo complex than dissociates in a process that involves Pex8, a peripheral membrane protein in the peroxisomal matrix. Finally, Pex5 is recycled back to the cytosol, a process that enables it to bind the next PTS1 cargo protein. Recycling involves mono-ubiquitination of Pex5 by the UBC protein Pex4, which is recruited to the peroxisomal membrane by Pex22. The three RING finger proteins Pex2, Pex10, and Pex12 are proposed to serve as E3 ligases. The ubiquitinated Pex5 is pulled out of the membrane by the AAA proteins Pex1 and Pex6, which are associated with the peroxisomal membrane via Pex15. When Pex5 recycling fails, it becomes polyubiquitinated by Ubc4/5 and degraded by the proteasome. (B) Hypothetical model of PTS2 protein import. Dimeric Pot1 is shown as an example of a typical PTS2 protein. Dimeric Pot1 first binds to the PTS2 receptor Pex7. Subsequently, the coreceptor Pex18 (and possibly also Pex21) binds to the receptor/cargo complex. Pex7 associates with Pex13 of the docking complex. After import of the Pot1 cargo into peroxisomes by an as-yet-unknown mechanism Pex7 recycles back to the cytosol. Pex18, however, first forms a complex with Pex14. Whether and how Pex18 recycles for another round of import is unknown.
Although Pex13 can directly interact with Pex5, Pex14 is most likely the initial docking protein in the peroxisomal membrane in the course of PTS1 protein import (Urquhart et al. 2000); Pex13 may have a similar function in docking of Pex7. Recently, Grunau et al. (2009) showed that the PTS2 protein Pot1 first binds Pex7 in a process that is independent of the coreceptors Pex18 and Pex21. Next, a cytosolic Pot1-Pex7-Pex18 complex that docks to the peroxisomal membrane via the interaction between Pex7 and Pex13 is formed. It was proposed that Pex7 and the cargo dissociate during or after assembly of a large complex with Pex14 and Pex13 (for details see Figure 6B).
The first PEX14 gene was cloned in H. polymorpha by functional complementation of a mutant defective in growth on methanol (Komori et al. 1997). The S. cerevisiae homolog was cloned by the Kunau group, based on the sequence homology with H. polymorpha PEX14, and was shown to represent the Pex5 docking site (Albertini et al. 1997). At the same time, Brocard et al. (1997) isolated the yeast homolog by a two-hybrid screen using Pex5 as a bait. Pex14 is characterized by a coiled-coil region in the middle part of the protein that is involved in homo-dimerization; however, its topology in the membrane is still debated (Azevedo and Schliebs 2006).
The conserved extreme N-terminal region adopts an α-helical structure (de Vries et al. 2007). The N terminus of S. cerevisiae Pex14 has been implicated in Pex5 binding, whereas its C terminus contains overlapping Pex5- and Pex7-binding sites (Niederhoff et al. 2005; Williams et al. 2005). In H. polymorpha, however, no receptor binding sites have been identified in the Pex14 C terminus (Bellu et al. 2001); instead, in H. polymorpha the Pex14 N terminus most likely binds both Pex5 and Pex7, as it is involved in both PTS1 and PTS2 protein import (de Vries et al. 2007). Structural studies using purified proteins showed that the N terminus of human Pex14 binds Pex19 in addition to Pex5 (Neufeld et al. 2009). Interestingly, an F/YFxxxF motif in the N terminus of Pex19 associates with the same site in Pex14 as the Pex5 WxxxF/Y motif. Although the key role of Pex19 is in sorting of PMPs (see below), in this case it possibly plays a role in the assembly of the docking complex (Fransen et al. 2004).
Although it is generally accepted that Pex14 is the initial Pex5 receptor in the peroxisomal membrane, the molecular mechanisms involved in its function are still unknown. Moreover, H. polymorpha Pex14 has additional functions, for example, in peroxisome degradation by autophagy (Bellu et al. 2001; Zutphen et al. 2008). Interestingly, data in H. polymorpha also indicate that PTS1 protein import can proceed in a PEX14 deletion strain upon overexpression of Pex5 (Salomons et al. 2000). This suggests that Pex14 is not essential but rather important for the efficacy of matrix import and that Pex5 also takes part in the protein translocation process.
RING finger complex:
The “really interesting new gene” (RING) complex involved in peroxisome biogenesis and function consists of three proteins—Pex2, Pex10, and Pex12—that interact with each other. They contain RING domains at their cytosolic C termini, which bind zinc ions through characteristic conserved cysteine and histidine residues. As these proteins are integral membrane proteins, it was proposed that they form the actual translocation pore through the membrane. Recent findings, however, indicate that these proteins have E3 ubiquitin ligase activity and function together with the ubiquitin-conjugating E2 enzymes Pex4 or Ubc4. Indeed, Pex4 plays an important role in matrix protein import (Wiebel and Kunau 1992), whereas Ubc4 is not required for this process. Instead, Ubc4 is responsible for a quality control mechanism that results in degradation of nonfunctional Pex5 molecules by polyubiquitination and their subsequent degradation by the proteasome (Figure 6A) (Kiel et al. 2005b).
Williams et al. (2008) were the first to show that the RING domain of Pex10 exhibits E3 ligase activity and acts as E3 ligase for Ubc4-dependent polyubiquitination of Pex5. Platta et al. (2009) subsequently demonstrated that all three RING finger peroxins exhibit ubiquitin-ligase activity. These authors, however, reported that Pex2, but not Pex10, is essential for Ubc4-dependent polyubiquitination, whereas Pex12 catalyzes Pex4-dependent monoubiquitination of Pex5. Monoubiquitination is important for recycling of functional Pex5 molecules to the cytosol to mediate another round of PTS1 protein import (Figure 6A). pex10 mutants show a severe defect in matrix protein import, which could be due to the stabilizing function of Pex10 on the other RING finger proteins. However, as Pex10 also has E3 ligase activity, the important current question concerns what the substrate of Pex10 actually is.
Translocation pore:
The general view on the peroxisomal translocon is that it is composed of two subcomplexes, the receptor docking complex and the RING finger complex, which are organized into the so-called importomer by Pex8 (Agne et al. 2003). Studies in H. polymorpha indicated that Pex8 is also required for cargo release from Pex5 (Wang et al. 2003), indicating a dual role for Pex8. A recent proteomics study confirmed the presence of proteins of the docking complex and the RING finger complex, together with Pex8, in the core complex of the peroxisomal translocon. In addition, Pex11 and Dyn2, the microtubule motor protein, also were observed to be closely associated with the peroxisomal core translocon (Oeljeklaus et al. 2012).
In Pichia pastoris Hazra et al. (2002) proposed that Pex3, rather than Pex8, is the protein that sequesters both subcomplexes into the translocon. However, this protein was identified as a component of neither the 9 core nor the 12 transient components of the importomer in S. cerevisiae (Oeljeklaus et al. 2012). Rather, Pex3 is a peroxin that is implicated in peroxisomal membrane biogenesis (see below).
The actual mode of matrix protein import is still elusive. Recently, the group of Erdmann (Meinecke et al. 2010) provided evidence for the concept of a transient pore, composed of Pex5 and Pex14 oligomers. This transiently gated ion-conducting channel may form pores of variable sizes up to 9 nm, which are induced by the interaction with the receptor cargo complex. This model also explains that the peroxisomal translocon pore may accommodate 9-nm gold particles (Walton et al. 1995). The occurrence of Pex5 oligomers would be in line with electron microscopy data obtained from isolated H. polymorpha Pex5 (Moscicka et al. 2007). On the other hand, by using small-angle X-ray scattering, Shiozawa et al. (2009) suggested that human Pex5 is rather monomeric in solution. Nevertheless, the option of a transient pore consisting of Pex14 and Pex5 oligomers to mediate matrix protein import is quite attractive. As it stands now, however, this model still leaves many questions open, i.e., how the import process is regulated and how the translocon is able to accommodate large receptor-bound oligomeric matrix proteins.
Receptor recycling and the role of ubiquitin:
The final step in PTS1 matrix protein import is the recycling of Pex5 to the cytosol. The first indication that Pex5 is indeed a cycling receptor came from the observation that in wild-type H. polymorpha Pex5 shows a dual localization in the cytosol and the peroxisomal matrix (van der Klei et al. 1995). Subsequent studies in the same organism indicated that the absence of Pex4 resulted in a specific block in PTS1-protein import that could be suppressed by overexpression of PEX5. Whereas in wild-type cells overproduced Pex5 was localized mainly to the cytosol, it accumulated in pex4 cells at the inner surface of the peroxisomal membrane. This observation led to the proposal that Pex4, and hence ubiquitination, is involved in receptor recycling (van der Klei et al. 1998). Erdmann’s group has significantly contributed to unraveling the principles of the Pex5 recycling process (for a review see Schliebs et al. 2010). In the normal receptor recycling process, Pex5 is mono-ubiquinated at a conserved cysteine by Pex4 and Pex12. Poly-ubiquitination of Pex5 occurs on a lysine residue (Kiel et al. 2005a). Release of the receptor from the membrane is an ATP-dependent process that is facilitated by the two AAA ATPases, Pex1 and Pex6, which are recruited to the membrane by Pex15. Debelyy et al. (2011) recently identified a novel component, Ubp15, which is capable of removing ubiquitin from Pex5. Although the model of a cycling Pex5 receptor is today generally accepted, it is still debated whether in yeast Pex5 associates to the outer surface of the membrane, inserts into the membrane, or even transiently enters the peroxisomal membrane, as suggested by the data obtained in H. polymorpha. The current knowledge of protein import and receptor recycling is summarized in Figure 6A.
On this point only the PTS1 peroxisomal protein import pathway has been studied in greater detail, but evidence suggests that the PTS2 receptor Pex7 may also cycle and enter the matrix, based on the observed localization of this protein in the peroxisomal matrix (for a review see Lazarow 2006) (Figure 6B).
Sorting and insertion of PMPs
Like matrix proteins, PMPs are synthesized in the cytosol. However, whether they immediately insert into the peroxisomal membrane or traffic first to the ER is currently still strongly debated. PMP-targeting signals (mPTSs) may involve different regions of the PMPs and are not characterized by a simple consensus sequence. Two classes of PMPs have been defined: class I PMPs contain an mPTS that is recognized by the soluble farnesylated protein Pex19. Class II PMPs, such as Pex3, Pex16, and Pex22, contain membrane-targeting information that is not recognized by Pex19 (Jones et al. 2004). The prevailing model for class I PMPs sorting suggests that cytosolic Pex19 serves as a cycling mPTS receptor, which docks to Pex3 on the peroxisomal surface, after which the cargo PMP is inserted into the membrane via a still-unknown pathway (reviewed in Schliebs and Kunau 2004). In line with this model is the observation that Pex19 binds mPTSs that are required for targeting (Jones et al. 2004). Recently, the crystal structure of the C-terminal part of human Pex19 was solved, which revealed that mPTSs bind to a globular α-helical domain in the C terminus of the protein (Schueller et al. 2010). However, there are conflicting data on the exact role of Pex19 in the biogenesis of the peroxisomal membrane. For example, in H. polymorpha and S. cerevisiae, pex19 mutant cells that overproduce Pex3 peroxisomal membrane structures that contain PMPs exist, suggesting that Pex19 is not essential for the insertion of PMPs into peroxisomal membranes (Otzen et al. 2004). Similarly, in Y. lipolytica cells lacking Pex19 structures are present that resemble normal peroxisomes even without Pex3 overproduction (Lambkin and Rachubinski 2001). On the basis of these and other observations it has been suggested that Pex19 is required mainly to assemble PMP complexes at the peroxisomal membrane and, hence, may function as a chaperone instead of being an mPTS receptor protein.
Data by van der Zand et al. (2010) suggest, on the basis of live-cell imaging experiments, that in S. cerevisiae all PMPs travel via the ER to peroxisomes. The authors propose novel functions for Pex3 and Pex19 in the budding of peroxisomal vesicles from the ER. According to these studies, insertion of PMPs into the ER depends on the Sec61 translocon, except for the tail-anchored protein Pex15, which requires Get3. Notably, in previous studies by South et al. (2001) no effect of the inactivation of Sec61 on peroxisome formation could be demonstrated.
The process of budding of PMP-containing vesicles from the ER proposed by van der Zand et al. (2010) is supported by data generated by Lam et al. (2010), who used a cell-free in vitro vesicle-budding assay. Using this assay, S. cerevisiae Pex15 and Pex3 were shown to be packaged into small vesicles that derived from the ER in a process that was dependent on Pex19. These vesicles were formed by a novel budding mechanism and independently of the COPII machinery that is responsible for secretory cargo packaging at the ER. Possibly, these vesicles fuse homotypically to create a new peroxisome, the so-called de novo peroxisome formation process (see below), or they fuse with preexisting peroxisomes as a mode to transfer PMPs and lipids from the ER to these organelles. In a similar study using permeabilized P. pastoris cells, Pex3 and Pex11 were shown to be copackaged into ER-derived vesicles in a Pex19-dependent process (Agrawal et al. 2011). Interestingly, this study revealed that the vesicles were also formed in the absence of Pex3, suggesting that this peroxin is not essential for vesicle formation. This finding also implies that Pex19 can dock to the ER membrane without its presumed membrane docking protein Pex3. Notably, this observation is in line with earlier data, which indicate that, in P. pastoris pex3 mutant cells, small structures that contain PMPs are present (Hazra et al. 2002).
Although the above studies indicate that some PMPs initially may insert into the ER membrane, data have been reported that indicate that targeting of several PMPs (including Pex3) to the ER is rather inefficient, especially when endogenous promoters are used to drive gene expression of the reporter constructs. For example, a fusion protein of full-length Pex3 and GFP, expressed under control of the PEX3 promoter in H. polymorpha pex19 cells, localizes to the cytosol (Otzen et al. 2004). Similarly, tagged Pex11 produced under control of the endogenous promoter is localized to the cytosol in S. cerevisiae pex3 and pex19 mutant strains (Hettema et al. 2000). Also, in H. polymorpha pex11 pex25 double-mutant cells, which are fully devoid of peroxisomal membrane structures, Pex3-GPF produced under control of the endogenous PEX3 promoter is cytosolic (Saraya et al. 2011).
Although in most studies PMPs were shown to colocalize with ER markers even when expressed at wild-type levels, the kinetics of their production (e.g., pulsed induction of GFP-tagged proteins using strong inducible promoters) may be quite different from the wild-type situation. Hence, it cannot be excluded that deregulated expression contributes to enhanced targeting of these proteins to the ER. The apparently contradictory data on PMP sorting may also depend on the model and reporter proteins used. This may be concluded from data of the Fujiki laboratory (Matsuzaki and Fujiki 2008), who demonstrated that in mammals Pex3 is directly sorted to peroxisomes. For this sorting, Pex19 serves as a chaperone for full-length Pex3 to form a soluble complex in the cytosol, which docks to Pex16 at the peroxisomal membrane.
A number of PMPs in various cell types show a dual localization to the ER and peroxisomes under steady-state conditions, for example, plant Pex16 (Karnik and Trelease 2005), P. pastoris Pex30 and Pex31 (Yan et al. 2008), and S. cerevisiae Pex11. In the case of S. cerevisiae Pex11, the localization varies with the phosphorylation state of the protein (Knoblach and Rachubinski 2010). Whether these ER-localized proteins are on their way to peroxisomes is, however, not firmly established. The key questions to date are whether in wild-type cells all or a subset of the PMPs travel via the ER to peroxisomes under normal physiological conditions and whether the vesicles formed in the in vitro assays represent transport vesicles or preperoxisomes of a de novo synthesis pathway. The current availability of in vitro assays will significantly help in further understanding these important questions in PMP sorting.
Formation of peroxisomes from the ER
Several observations based on electron microscopy have suggested that new peroxisomes can be formed from the ER. Membrane connections appear to exist between peroxisomes and the ER in mouse dendritic cells, allowing new peroxisomes to pinch off from specialized regions of the ER (Geuze et al. 2003). Also, evidence obtained in yeast pex3 and pex19 mutants that lack peroxisomal membrane structures suggests that peroxisomes can be formed from the ER upon reintroduction of the corresponding PEX3 or PEX19 wild-type genes (Hoepfner et al. 2005; Kragt et al. 2005; Tam et al. 2005; Haan et al. 2006). In vivo pulse-chase experiments using Pex3-GFP in S. cerevisiae pex3 cells (Hoepfner et al. 2005) revealed that Pex3-GFP is first targeted to the ER, but later present in new, small peroxisomes. Very similar observations have been made in other laboratories using S. cerevisiae and in H. polymorpha (for a review see Tabak et al. 2008).
An alternative explanation for the ER-localized Pex3 in these experiments, however, may be that peroxisomes are formed by an alternative, relatively slow process, thus promoting excess Pex3 to sort to the ER, when insufficient peroxisomal membrane is present. Likely, Pex3 and other PMPs may contain ER-targeting signals, but these are not yet resolved. Therefore, during de novo synthesis, these proteins, including Pex3, may initially sort to the ER but later on be targeted directly from the cytosol to the newly formed organelle without a requirement for translocation via the ER. In line with this suggestion is the general observation that these PMPs are never observed at the ER in wild-type cells grown under normal conditions. However, this could also be explained by an extremely short residence time of these proteins at the ER.
A role for the ER in peroxisome biogenesis is also indicated by observations of Perry et al. (2009), who showed that Pex3-GFP localizes to tubular-vesicular structures in cells suppressed for Sec20, Sec39, and Dsl1, which form a complex in the ER. Also, deletion of ARF1 and ARF3 in S. cerevisiae affects peroxisome proliferation (Anthonio et al. 2009). However, details on the exact function of these proteins in peroxisome biogenesis are still lacking.
Several attempts have been made to identify genes that are essential for the de novo peroxisome formation process. In H. polymorpha, several gene deletions have been tested for their effect on the reintroduction of peroxisomes in pex3 mutant cells upon induction of Pex3-GFP synthesis. These studies revealed that Dnm1, Vps1, Emp24, Pex11, and Pex11C are not required (Nagotu et al. 2008b; Kurbatova et al. 2009; Saraya et al. 2011). However, Saraya et al. (2011) showed that Pex25 as well as the GTPase Rho1 are necessary to allow the formation of new peroxisomes in pex3 cells. Huber et al. (2012) showed that in S. cerevisiae Pex25 also is essential for reintroduction of peroxisomes in Pex3-deficient cells. Since H. polymorpha pex25 and S. cerevisiae pex25 cells contain normal peroxisomes, the above process apparently is not essential in wild-type cells to maintain peroxisomes.
Recent findings by van der Zand et al. (2012) suggest that in S. cerevisiae the process of peroxisome formation from the ER involves at least two biochemically distinct types of preperoxisomal vesicles that are initially formed from the ER, which each carry half a peroxisomal translocon complex and hence are unable to import peroxisomal matrix proteins. Upon their fusion, mediated by the AAA proteins Pex1 and Pex6, a functional translocon is formed allowing uptake of peroxisomal matrix proteins from the cytosol. This model suggests a novel role for Pex1 and Pex6 in vesicle fusion, in addition to their established function in Pex5 recycling. Notably, earlier data obtained in the yeast Y. lipolytica also suggested the formation of mature peroxisomes upon fusion of different subtypes of small preperoxisomal vesicles (Titorenko et al. 2000). Fusion of various types of vesicles was mediated by Pex1 and Pex6; however, the origin of the vesicles remains unknown. All vesicles were matrix protein import competent, although the vesicle subtypes contained different combinations of peroxisomal proteins.
In summary, peroxisome formation can occur de novo in cells lacking pre-existing peroxisomes. However, the significance of de novo peroxisome formation in cells that already have peroxisomes is still debated.
Peroxisome fission
Pex11 and the Fis1/Dnm1 fission machinery:
For decades, peroxisomes have been considered to be autonomous organelles that multiply by growth and division (Lazarow and Fujiki 1985). Various proteins are known to function in peroxisome fission. The first step in fission is organelle elongation, a process that is mediated by Pex11 (Opalinski et al. 2011). In all studies performed in various species so far artificial modulation of Pex11 levels has resulted in variations in peroxisome size and abundance; deletion of the PEX11 gene invariably leads to fewer enlarged organelles, whereas PEX11 overexpression leads to an increased number of smaller and often tubulated peroxisomes. Studies in H. polymorpha (Opalinski et al. 2011) revealed that the insertion of the N-terminal amphipathic α-helix of Pex11 into the membrane causes the initial membrane curvature, which initiates organelle elongation. The activation of Pex11 in organelle fission may be regulated by phosphorylation/dephosphorylation (Saleem et al. 2008; Knoblach and Rachubinski 2010). Mutant studies indicate that strains producing constitutively dephosphorylated Pex11 show a phenotype similar to pex11 cells, whereas strains expressing a phosphomimetic Pex11 mutant allele show enhanced peroxisome proliferation, similar to cells overexpressing PEX11.
Recent observations indicate that in the yeasts S. cerevisiae and H. polymorpha most of the organelles are formed by fission of existing peroxisomes by the activity of the dynamin-related proteins (DRP) Dnm1 and Vps1 (Hoepfner et al. 2001; Motley and Hettema 2007; Nagotu et al. 2008b). This is, among other observations, suggested by the finding that, in cells of a dnm1vps1 double-deletion strain, peroxisome fission is completely blocked, resulting in the presence of a single enlarged peroxisome per cell, even after prolonged cultivation under peroxisome-inducing conditions. In these cells, peroxisome formation from the ER is not affected, but generation of additional organelles was never observed (Motley and Hettema 2007; Nagotu et al. 2008b).
DRPs are large GTPases that are involved in multiple membrane fission and fusion events. Vps1 was initially found to be involved in vacuolar protein sorting, whereas Dnm1 was first identified as a protein required for mitochondrial fission. The peroxisomal fission machinery is therefore not unique for this organelle, but shares components with other membrane fission/fusion processes. Dnm1 is essential for peroxisome fission under conditions of peroxisome induction by oleate, whereas Vps1 functions under glucose-repressing conditions (Hoepfner et al. 2001; Kuravi et al. 2006). In S. cerevisiae, Dnm1 is recruited to peroxisomes by Mdv1 or its paralog Caf4, which are both associated with the peroxisomal membrane via the tail-anchored protein Fis1 (Motley and Hettema 2007; Motley et al. 2008). Mdv1 and Caf4 are WD repeat proteins, which are absent in higher eukaryotes. How Vps1 is recruited to peroxisomes is not yet known. Notably, in H. polymorpha, Vps1 does not play a role in peroxisome fission, and, thus, this yeast species seems to be more similar to higher eukaryotes in this respect, in which a single DRP is involved in mitochondrial and peroxisome fission, as well as in chloroplast fission, in plants.
It is an intriguing question how the Fis1/Drp fission machinery is properly distributed over the individual organelles. In higher eukaryotes, Pex11 has been implicated in the recruitment of Fis1 to peroxisomes (Kobayashi et al. 2007; Lingard et al. 2008); in mammals, a role of Pex19 in Fis1 targeting was established (Delille and Schrader 2008), but these processes have not yet been confirmed in yeast. Fluorescence microscopy studies in H. polymorpha revealed that GFP-tagged Dnm1 is not evenly distributed in the cell, but rather present in multiple spots to which Mdv1 colocalizes. These spots dynamically associate and disassociate from mitochondria and peroxisomes, demonstrating that the same protein molecules may be involved in the fission of either one of these organelles (Nagotu et al. 2008a).
Peroxisome fission in H. polymorpha is fully blocked in dnm1 cells. Growing H. polymorpha dnm1 mutant cells contain a single enlarged peroxisome, which forms a long extension that protrudes into the developing bud. These extensions are not observed in dnm1 pex11 cells, which is in agreement with the notion that Pex11 plays a role in peroxisome elongation (Nagotu et al. 2008b). Remarkably, Pex11 protein concentrates at the base of these peroxisome extensions, indicating that during fission of the organelles Pex11 segregates into Pex11-enriched patches at the membrane. Recent findings show that other peroxins including Pex10, Pex14, but also the Pex11-family protein Pex25, do not segregate to the same patches; instead, these proteins move to the developing new organelles or extensions in dnm1 cells (Cepinska et al. 2011).
Role of the other Pex11 protein family members:
Most organisms contain at least three Pex11 protein family members (see Table 2). The human genome encodes three family members, namely Pex11α, Pex11β, and Pex11γ, all of which have high similarity to S. cerevisiae Pex11. S. cerevisiae expresses, in addition to Pex11, the weakly homologous Pex25 protein and its partially redundant paralogue, Pex27 (Rottensteiner et al. 2003), which have also been implicated in peroxisome proliferation. Quantitative analysis of electron microscopy images revealed that peroxisomes are enlarged in pex11, pex25, or pex27 mutant cells (Tam et al. 2003). The molecular function of Pex27 is still unclear, whereas Pex25 plays a role in recruiting Rho1 to the peroxisomal membrane (Marelli et al. 2004). In H. polymorpha, Pex25, but not Pex11 or Pex11c, has been shown to be important for the formation of peroxisomes from the ER (Saraya et al. 2011). Large-scale protein interaction studies by two-hybrid analysis (Yu et al. 2008), together with information from a global analysis of protein localization (Huh et al. 2003), led to the identification of a novel peroxisomal interaction partner of Pex11, Pex25, and Pex27, named Pex34; Pex34 was proposed to act as a positive effector of peroxisome division (Tower et al. 2011).
Pex23 protein family:
Based on sequence homology, Kiel et al. (2006) defined the Pex23 protein family, which contains the membrane proteins Pex23, Pex24, Pex28, Pex29, Pex30, Pex31, and Pex32. This family of peroxisomal proteins can be divided into two groups of proteins with weak similarity (see Table 2; Kiel et al. 2006): The first group consists of Y. lipolytica Pex23 (Brown et al. 2000) and the related S. cerevisiae proteins Pex30, Pex31, and Pex32 (Vizeacoumar et al. 2004) and P. pastoris Pex30 and Pex31 (Yan et al. 2008). These proteins contain a DysF motif with an unknown function that was first observed in human dysferlin. The second group contains Y. lipolytica Pex24 (Tam and Rachubinski 2002) and S. cerevisiae Pex28 and Pex29 (Vizeacoumar et al. 2003).
Y. lipolytica Pex23 is an integral peroxisomal membrane protein. S. cerevisiae Pex30, Pex31, and Pex32 are localized to peroxisomes; however, a significant portion of Pex30 is present in lighter fractions in sucrose gradients. These fractions may represent the ER, as P. pastoris Pex30 and Pex31 show a dual localization to the ER and peroxisomes. In Y. lipolytica pex23 mutant cells, the majority of the peroxisomal matrix proteins is mislocalized to the cytosol, but the cells still contain small vesicular structures that contain PTS1 and PTS2 proteins. The phenotypes of S. cerevisiae pex30, pex31, and pex32 cells are quite different because they contain peroxisomes in which matrix proteins are normally imported. In pex30 mutant cells, the number of peroxisomes is increased, and pex31 and pex32 cells have enlarged peroxisomes. It was proposed that, in S. cerevisiae, Pex30 is a negative regulator of peroxisome number, whereas Pex31 and Pex32 are negative regulators of peroxisome size (Vizeacoumar et al. 2004). Notably, in P. pastoris, deletion of PEX30 or PEX31 results in the opposite effect, namely a reduction in peroxisome number (Yan et al. 2008).
Y. lipolytica PEX24 encodes for a peroxisomal membrane protein, and PEX24-deficient mutants lack morphologically recognizable peroxisomes, but instead contain unusual extended membrane structures (Tam and Rachubinski 2002). Immunofluorescence microscopy suggested a cytosolic localization for PMPs and matrix proteins in these mutants; however, using biochemical approaches, membrane fractions could be detected that contained minor amounts of matrix marker proteins. Hence, pex24 mutant cells apparently fail to assemble functional peroxisomes, but still contain membrane structures that exhibit some peroxisomal characteristics.
In S. cerevisiae cells in which either one or both PEX28 and/or PEX29 are deleted, peroxisome assembly is not affected. These mutant cells contain organelles that have a lower density, are smaller, are more abundant, and tend to cluster (Vizeacoumar et al. 2003). Two-hybrid studies revealed that Pex28 and Pex29 interact with Pex30, Pex31, and Pex32. Systematic deletion of genes demonstrated that PEX28 and PEX29 function upstream of Pex30, Pex31, and Pex32 and function together with these proteins in the regulation of peroxisome proliferation (Vizeacoumar et al. 2004).
In summary, the Y. lipolytica members of the Pex23 family seem to play a key role in peroxisome assembly, whereas the S. cerevisiae and P. pastoris members are important in the regulation of peroxisome proliferation. Detailed knowledge about the function of these proteins is required to understand the major species-specific differences in the phenotypes of the respective deletion strains.
Peroxisome inheritance
During budding of yeast cells, one or a few organelles are actively transported to the developing bud, a process that is mediated by the class V myosin motor protein Myo2 and actin filaments (Hoepfner et al. 2001). The remaining peroxisomes are retained in the mother cell. Inp1 has been identified as the peroxisome-specific retention factor, connecting peroxisomes to an as-yet-unknown anchoring structure in the mother cell. The Hettema laboratory demonstrated that Pex3 recruits Inp1 to the peroxisomal membrane (Munck et al. 2009). Importantly, the Inp1-binding region in the Pex3 protein is distinct from the regions involved in membrane formation. Unexpectedly, in the absence of Pex11, peroxisome retention is also defective in H. polymorpha, despite the fact that Inp1 is properly localized to peroxisomes (Krikken et al. 2009). Hence, Pex11 appears to have a second function in organelle retention, in addition to its role in peroxisome fission.
Inp2 is a PMP that acts as the peroxisomal receptor for Myo2 and attaches the globular tail of Myo2 to the peroxisome, thus allowing transport of the organelle to the bud. The region of Myo2 involved in Inp2 binding was identified using mutant variants of Myo2. These studies also showed that Inp2 is a phosphoprotein whose level of phosphorylation is coupled to the cell cycle (Fagarasanu et al. 2006; Fagarasanu et al. 2009). Recently, Otzen et al. (2012) provided evidence that Pex19 also plays a role in peroxisome inheritance by associating peroxisomes to Myo2. Interestingly, mutations that affect the interaction between Myo2 and Pex19 do not abolish the Inp2–Myo2 interaction.
Chang et al. (2009) suggested that Inp2 is unique for S. cerevisiae and related species because in Y. lipolytica Pex3 and its paralog Pex3B function as peroxisome-specific receptors of Myo2. However, Inp2 is also present in other yeast species. The finding that H. polymorpha Inp2 interacts with Myo2 points to a conserved function of Inp2 as a binding protein for Myo2 (Saraya et al. 2010). Remarkably, in H. polymorpha, Myo2–Inp2 binding was dependent on Pex19. This is consistent with the view that Pex19 may have a stabilizing role in the interaction between Inp2 and Myo2 and is also in line with the previously observed defect in peroxisome inheritance in H. polymorpha pex19 cells (Otzen et al. 2006).
Peroxisome degradation
The actual peroxisome population per cell is largely prescribed by physiological needs and determined by the machineries of organelle proliferation, inheritance, and degradation. Peroxisome inactivation can be achieved by degradation of (part of) their constituents or by turnover of the whole organelle by autophagy (Zwart et al. 1979). Organelle degradation by autophagy can serve three main cellular functions, namely nonselective degradation, e.g., under nutrient depletion conditions to recycle cellular material; selective degradation of redundant organelles; and constitutive degradation to remove exhausted organelles as a mode to continuously rejuvenate the organelle population (Aksam et al. 2007). Selective peroxisome degradation is also designated as “pexophagy” (Klionsky et al. 2007). Two distinct mechanisms of pexophagy, termed “macropexophagy” and “micropexophagy,” which can be morphologically distinguished, may occur. During macropexophagy, individual organelles are sequestered by a double-membrane structure, the autophagosome, which fuses with the vacuole and releases the organelle into the lytic environment of the vacuole. During micropexophagy an organelle or a cluster of organelles is engulfed by vacuolar extensions, followed by incorporation of the organelle into the vacuole.
Most studies of the molecular mechanisms involved in pexophagy have been performed with methylotrophic yeast species such as H. polymorpha or P. pastoris. When these organisms are grown on methanol, peroxisomes are massively induced. However, upon transfer of methanol-grown cells to glucose media, these organelles become redundant for growth and are therefore rapidly degraded by pexophagy. Pexophagy involves a core set of ATG genes that also plays a role in other autophagy processes (Meijer et al. 2007). In addition, a few genes that are specifically involved in pexophagy are known (for recent reviews see Sakai et al. 2006 and Manjithaya et al. 2010). Although homologous genes exist in S. cerevisiae, their specific roles in pexophagy in this organism have not been elucidated yet.
Peroxisome function and biology in yeast as a paradigm for metabolic disorders in humans
Yeast is a very useful model (“reference organism”) to understand basic processes of peroxisome biology in humans. As in yeast, human peroxisomes harbor enzymes of the β-oxidation pathway. However, in addition, they are involved in other processes that do not occur in S. cerevisiae, such as ether phospholipid biosynthesis, fatty acid α-oxidation, and the oxidation of D-amino acids and of polyamines (for reviews see Wanders and Waterham 2006 and Wanders et al. 2010). Also, unlike in yeast, mammalian peroxisomal β-oxidation is restricted to very long chain fatty acids (VLCFAs); short- and medium-chain FAs are oxidized by mitochondrial enzymes in mammals, whereas mitochondrial β-oxidation does not exist in S. cerevisiae, and short-, medium-, and long-chain fatty acids are solely degraded in peroxisomes in that organism. Since the metabolic pathways in yeast and human peroxisomes are quite distinct, yeast research has only poorly contributed to the understanding of the metabolism of human peroxisomes. In marked contrast, however, studies of yeast peroxisome biogenesis have been instrumental in identifying human counterparts of yeast peroxins and in understanding the molecular basis of peroxisome biogenesis disorders (PBDs). In PBD patients, normal peroxisomal structures are absent, causing dramatic defects in peroxisomal metabolism. Among other deficiencies, PBDs lead to the accumulation of very long chain fatty acids or defective plasmalogen synthesis, and PBD patients develop liver diseases, variable neurodevelopmental delay, retinopathy, and perceptive deafness with onset in the first months of life. The most severe PBD is Zellweger syndrome, and patients suffering from this disease typically die before one year of age (Steinberg et al. 2006).
In 1973 Goldfischer et al. (1973) described that cells of Zellweger syndrome patients were fully devoid of peroxisomal structures. However, it was not until 1992 that the first gene associated with a peroxisome biogenesis disorder was identified by complementation of fibroblasts isolated from a patient with Zellweger syndrome (Shimozawa et al. 1992). In later studies, the cloning and sequencing of yeast PEX genes has greatly facilitated the identification of the corresponding human genes by homology probing and searches in human genome databases. The genes that have been shown to be defective in PBDs are so far limited to those that play a role in matrix protein import and in the formation of the peroxisomal membrane, i.e., Pex3 and Pex19. In addition, Pex16 is required for membrane biogenesis in humans. However, several of the other PEX genes appear to be yeast specific and do not have a clearly defined structural ortholog in humans (for a review see Steinberg et al. 2006).
Most of the research on yeast peroxisomes is performed using S. cerevisiae due to the extensive set of experimental tools that was first available for this species; however, other yeast species have specific advantages for peroxisome research, as their peroxisomes are more similar to their human counterparts in certain aspects. This has become apparent from the presence of orthologs of typical human peroxisomal enzymes in several yeast species, such as D-amino acid oxidases and polyamine oxidases, which are absent from S. cerevisiae. As outlined above, this is also true for peroxisomal processes, such as organelle fission. All eukaryotes, with the exception of S. cerevisiae, contain a peroxisomal Lon protease (Aksam et al. 2007), and studies in H. polymorpha and Penicillium chysogenum revealed that this protease is very important for quality control processes in the organelle (Aksam et al. 2007; Bartoszewska et al. 2012). Hence, studies on peroxisome biology in other fungi may have specific advantages over S. cerevisiae in understanding peroxisome-related processes in humans.
Outlook and perspectives
For many years the peroxisome field has struggled, and in fact still struggles, with major controversies regarding the mode of peroxisome biogenesis and development. One topic currently under heavy debate are the mechanisms by which peroxisomes are formed. Data in several yeast species indicate that, following their induction, peroxisomes predominantly proliferate by fission, whereas other data suggest that all organelles are derived from the ER, as suggested for mammalian peroxisomes. Potentially, ER-derived peroxisomes are capable of one or only a few fission events. Clearly, this point needs urgent elucidation and requires the identification and analysis of novel components that are essential for this process. Similarly, novel approaches will help to resolve the question of how the various PMPs reach the peroxisome membrane. It is attractive to suggest that sorting of at least some PMPs may be associated with lipid transfer from the ER, since the organelles increase in size during maturation; on the other hand, PMP and lipid import may also be separate processes. Notably, a major constituent of the peroxisomal membrane is cardiolipin, which is synthesized in the inner mitochondrial membrane, adding another level of complexity to the peroxisome assembly process.
The question of how matrix proteins enter peroxisomes has long been enigmatic but seems now to be have been cracked by the observations of the Erdmann group that these proteins enter via a transient pore, formed by Pex5 and Pex14 molecules (Meinecke et al. 2010). However, many questions remain, for example, regarding the composition and regulation of this pore. Particularly interesting is the excellent suggestion by Gould and Collins (2002) who proposed that receptor and matrix proteins may form large complexes (pre-import complex) prior to the membrane translocation step. An estimated pore diameter of up to 90 Å would indeed allow the import of such complexes. The formation of such complexes at the organelle membrane may also explain why the pool of matrix precursor proteins in the cytosol is invariably extremely low. In the end, detailed protein structure information will be required to end the debate over the function of this fascinating and unique protein translocation machinery.
For the identification of novel peroxisome components, transcriptome studies have been proven to be of low value. Also, in silico prediction methods need improved programs. A major limitation in proteomics studies to identify novel components of the peroxisomal import and assembly machinery is the insufficient purity of the organelle fractions that are obtained by currently available fractionation protocols. Moreover, because of the occurrence of dual localizations of proteins, e.g., of Fis1 and Rho1, which are dually localized to mitochondria and peroxisomes, or Pex30, which is present in peroxisomes and the ER, certain proteins can erroneously be regarded as contaminants. Also, current procedures to isolate peroxisomal fractions are time-consuming and involve many purification steps, which may result in the dissociation of proteins that only weakly or transiently interact with the peroxisomal surface. Moreover, it is difficult to isolate intact organelles, and leakage of matrix proteins during the isolation procedure is invariably observed. An additional potential drawback may be the use of density centrifugation in current protocols since only organelles of high density are isolated; several data, however, indicate the presence of organelle subpopulations (Veenhuis et al. 1989), and immature developing organelles that do not cofractionate with the dense mature organelle fractions exist. In fact, the lighter, nascent organelles may carry the bulk of proteins important for peroxisome biogenesis and dynamics (Erdmann and Blobel 1995; Cepinska et al. 2011) and therefore are missed in the current isolation procedures. Procedures that are independent of peroxisome size or density would help in isolating the whole peroxisome population of cells. Immunopurification and FACS-based methods have been attempted, but need further improvement.
In general, the importance of peroxisomes has long been greatly underestimated. We anticipate that due to the technical drawbacks in identifying all relevant peroxisomal components the atlas of peroxisome functions is still far from being complete. This assumption is supported by the recent identification of several novel crucial functions, i.e., non-metabolic activities that are required to cope with stress conditions other than oxidative stress, especially in plants. Among these are roles in reactive nitrogen species signaling, aging, antiviral innate immunity, and plant defense against pathogens (Dixit et al. 2010; Lazarow 2011). Of particular interest also are data indicating that peroxisomes play a role in aging in various species, including humans (Aksam et al. 2009; Bonekamp et al. 2009; Titorenko and Terlecky 2011). It is therefore important to analyze cells that have been grown under various conditions, including stress scenarios, to identify novel potential peroxisome functions in stress and aging.
Despite technical limitations, peroxisomes are among the best-characterized organelles in terms of composition, biogenesis, inheritance, and turnover in yeast. The field of lipid droplet research, on the other hand, is still in its infancy, despite the important role of this organelle in lipid homeostasis and its implication in prevalent metabolic diseases in mammals. Only models of how the neutral lipid of the LD core is formed exist, and although increasing evidence suggests a close functional interaction of LDs and the ER, the specific molecular mechanisms of LD formation are obscure (Farese and Walther 2009; Walther and Farese 2009, 2012). As discussed above for peroxisomes, the biochemical characterization of LD composition is limited since current LD isolation methods based on cell fractionation are time-consuming and may lead to cross-contamination with other cellular compartments or to loss of transiently associated components. Since LDs are typically isolated by flotation due to the low density of the TAG and SE core components, nascent LDs that contain less TAG and SE cannot be isolated by that method. As a consequence, the “time window” for LD isolation during cell cultivation is rather narrow and limited to the late-log/early stationary phase of growth. Thus, in addition to the mechanisms that drive LD formation, such fundamental concerns as determining the mechanism of protein targeting to LDs are largely unresolved. Anabolic LD enzymes are frequently also associated with the ER, whereas catabolic LD enzymes are exclusively LD resident: Which signals regulate the distribution of proteins between the ER and LDs? How are ER-resident proteins excluded from LDs? Evidence suggests that hydrophobic stretches of amino acids are required to drive LD association, but the topology of these potential transmembrane domains in the monolayer leaflet of the LD surface remains obscure. Since LDs may also serve as an overflow compartment for (misfolded) hydrophobic proteins, experimental use of episomally expressed fusion constructs to investigate targeting sequences may be misleading. Wild-type cells are characterized by a remarkably homogeneous size distribution of LDs (Czabany et al. 2008). What limits the size of LDs? Do LDs fuse in vivo? Are there specific subpopulations of LDs, harboring either SEs or TAGs, and are there also differences in the protein content between LDs? Yeast provides the unique opportunity to deplete LDs and induce their formation by regulated expression of acyltransferases that drive TAG synthesis (Jacquier et al. 2011), thus enabling studies on the early events of LD biogenesis and their interaction with the ER. Further refinement of isolation procedures and proteomic and lipidomic analysis will provide better insight into composition under various nutritional conditions (Connerth et al. 2009; Grillitsch et al. 2011). The exploitation of components involved in the physical interaction between LDs and mitochondria or peroxisomes (Pu et al. 2011) may also contribute to a better understanding of the interconnection between lipid storage and cellular physiology. In conclusion: many challenges remain and are open to further exploration of the biology of these fascinating organelles, the peroxisome and lipid droplets.
Acknowledgments
We thank the members of our laboratories for critical reading of the manuscript and helpful suggestions. We gratefully acknowledge Khaw Aik Kia for the artwork shown in Figures 3 and 6 and Heimo Wolinski and Dagmar Kolb for providing fluorescence and electron micrographs shown in Figure 1. Work in the authors’ laboratories is supported by funds from the Netherlands Organization for Scientific Research (NWO) and the Kluyver Centre for Genomics of Industrial Fermentation (to I.J.v.d.K.), which is part of the Netherlands Genomics Initiative/NWO, and by the Fonds zur Förderung der wissenschaftlichen Förschung in Österreich, FWF (to S.D.K.) (projects F3005-B12 SFB LIPOTOX and Ph.D. program “Molecular Enzymology”).
Footnotes
The numbers indicate the total number of carbon atoms in the acyl chains and the number of double bonds. Yeast produces only mono-unsaturated FAs; thus TAG 52:3 indicates a species containing C18:1 + C18:1 + C16:1 acyl chains.
In the original publication, TAG levels were erroneously printed as “mg TAG/mg cell dry weight” (K. Athenstaedt, personal communication)
Communicating editor: T. N. Davis
Literature Cited
- AbdelRaheim S. R., Cartwright J. L., Gasmi L., McLennan A. G., 2001. The NADH diphosphatase encoded by the Saccharomyces cerevisiae NPY1 nudix hydrolase gene is located in peroxisomes. Arch. Biochem. Biophys. 388: 18–24 [DOI] [PubMed] [Google Scholar]
- Adeyo O., Horn P. J., Lee S., Binns D. D., Chandrahas A., et al. , 2011. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J. Cell Biol. 192: 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A. K., Garg A., 2003. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol. Metab. 14: 214–221 [DOI] [PubMed] [Google Scholar]
- Agarwal A. K., Barnes R. I., Garg A., 2004. Genetic basis of congenital generalized lipodystrophy. Int. J. Obes. Relat. Metab. Disord. 28: 336–339 [DOI] [PubMed] [Google Scholar]
- Agne B., Meindl N. M., Niederhoff K., Einwachter H., Rehling P., et al. , 2003. Pex8p: an intraperoxisomal organizer of the peroxisomal import machinery. Mol. Cell 11: 635–646 [DOI] [PubMed] [Google Scholar]
- Agrawal G., Joshi S., Subramani S., 2011. Cell-free sorting of peroxisomal membrane proteins from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 108: 9113–9118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksam E. B., Koek A., Kiel J. A., Jourdan S., Veenhuis M., et al. , 2007. A peroxisomal lon protease and peroxisome degradation by autophagy play key roles in vitality of Hansenula polymorpha cells. Autophagy 3: 96–105 [DOI] [PubMed] [Google Scholar]
- Aksam E. B., de Vries B., van der Klei I. J., Kiel J. A., 2009. Preserving organelle vitality: peroxisomal quality control mechanisms in yeast. FEMS Yeast Res. 9: 808–820 [DOI] [PubMed] [Google Scholar]
- Albertini M., Rehling P., Erdmann R., Girzalsky W., Kiel J. A., et al. , 1997. Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell 89: 83–92 [DOI] [PubMed] [Google Scholar]
- Albertyn J., Hohmann S., Thevelein J. M., Prior B. A., 1994. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 14: 4135–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Feel W., Chirala S. S., Wakil S. J., 1992. Cloning of the yeast FAS3 gene and primary structure of yeast acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. USA 89: 4534–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. M., Bitterman K. J., Wood J. G., Medvedik O., Sinclair D. A., 2003. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423: 181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonio E. A., Brees C., Baumgart-Vogt E., Hongu T., Huybrechts S. J., et al. , 2009. Small G proteins in peroxisome biogenesis: the potential involvement of ADP-ribosylation factor 6. BMC Cell Biol. 10: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenkov V. D., Mindthoff S., Grunau S., Erdmann R., Hiltunen J. K., 2009. An involvement of yeast peroxisomal channels in transmembrane transfer of glyoxylate cycle intermediates. Int. J. Biochem. Cell Biol. 41: 2546–2554 [DOI] [PubMed] [Google Scholar]
- Athenstaedt K., Daum G., 1997. Biosynthesis of phosphatidic acid in lipid particles and endoplasmic reticulum of Saccharomyces cerevisiae. J. Bacteriol. 179: 7611–7616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athenstaedt K., Daum G., 1999. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem. 266: 1–16 [DOI] [PubMed] [Google Scholar]
- Athenstaedt K., Daum G., 2000. 1-Acyldihydroxyacetone-phosphate reductase (Ayr1p) of the yeast Saccharomyces cerevisiae encoded by the open reading frame YIL124w is a major component of lipid particles. J. Biol. Chem. 275: 235–240 [DOI] [PubMed] [Google Scholar]
- Athenstaedt K., Daum G., 2003. YMR313c/TGL3 encodes a novel triacylglycerol lipase located in lipid particles of Saccharomyces cerevisiae. J. Biol. Chem. 278: 23317–23323 [DOI] [PubMed] [Google Scholar]
- Athenstaedt K., Daum G., 2005. Tgl4p and Tgl5p, two triacylglycerol lipases of the yeast Saccharomyces cerevisiae are localized to lipid particles. J. Biol. Chem. 280: 37301–37309 [DOI] [PubMed] [Google Scholar]
- Athenstaedt K., Daum G., 2006. The life cycle of neutral lipids: synthesis, storage and degradation. Cell. Mol. Life Sci. 63: 1355–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athenstaedt K., Daum G., 2011. Lipid storage: Yeast we can! Eur. J. Lipid Sci. Technol. 113: 1188–1197 [Google Scholar]
- Athenstaedt K., Zweytick D., Jandrositz A., Kohlwein S. D., Daum G., 1999. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 181: 6441–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avers C. J., Federman M., 1968. The occurrence in yeast of cytoplasmic granules which resemble microbodies. J. Cell Biol. 37: 555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo J. E., Schliebs W., 2006. Pex14p, more than just a docking protein. Biochim. Biophys. Acta 1763: 1574–1584 [DOI] [PubMed] [Google Scholar]
- Barreto L., Garcera A., Jansson K., Sunnerhagen P., Herrero E., 2006. A peroxisomal glutathione transferase of Saccharomyces cerevisiae is functionally related to sulfur amino acid metabolism. Eukaryot. Cell 5: 1748–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewska M., Opalinski L., Veenhuis M., van der Klei I. J., 2011. The significance of peroxisomes in secondary metabolite biosynthesis in filamentous fungi. Biotechnol. Lett. 33(10): 1921–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewska M., Williams C., Kikhney A., Opalinski L., van Roermund C. W., et al. , 2012. Peroxisomal proteostasis involves a Lon family protein that functions as protease and chaperone. J. Biol. Chem. 287: 27380–27395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellu A. R., Komori M., van der Klei I. J., Kiel J. A., Veenhuis M., 2001. Peroxisome biogenesis and selective degradation converge at Pex14p. J. Biol. Chem. 276: 44570–44574 [DOI] [PubMed] [Google Scholar]
- Benghezal M., Roubaty C., Veepuri V., Knudsen J., Conzelmann A., 2007. SLC1 and SLC4 encode partially redundant acyl-coenzyme A 1-acylglycerol-3-phosphate O-acyltransferases of budding yeast. J. Biol. Chem. 282: 30845–30855 [DOI] [PubMed] [Google Scholar]
- Binns D., Januszewski T., Chen Y., Hill J., Markin V. S., et al. , 2006. An intimate collaboration between peroxisomes and lipid bodies. J. Cell Biol. 173: 719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns D., Lee S., Hilton C. L., Jiang Q. X., Goodman J. M., 2010. Seipin is a discrete homooligomer. Biochemistry 49: 10747–10755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. N., DiRusso C. C., 2007. Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim. Biophys. Acta 1771: 286–298 [DOI] [PubMed] [Google Scholar]
- Blobel F., Erdmann R., 1996. Identification of a yeast peroxisomal member of the family of AMP-binding proteins. Eur. J. Biochem. 240: 468–476 [DOI] [PubMed] [Google Scholar]
- Bonekamp N. A., Volkl A., Fahimi H. D., Schrader M., 2009. Reactive oxygen species and peroxisomes: struggling for balance. Biofactors 35: 346–355 [DOI] [PubMed] [Google Scholar]
- Bottger G., Barnett P., Klein A. T., Kragt A., Tabak H. F., et al. , 2000. Saccharomyces cerevisiae PTS1 receptor Pex5p interacts with the SH3 domain of the peroxisomal membrane protein Pex13p in an unconventional, non-PXXP-related manner. Mol. Biol. Cell 11: 3963–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumann H. A., Damen M. J., Versluis C., Heck A. J., de Kruijff B., et al. , 2003. The two biosynthetic routes leading to phosphatidylcholine in yeast produce different sets of molecular species. Evidence for lipid remodeling. Biochemistry 42: 3054–3059 [DOI] [PubMed] [Google Scholar]
- Bozaquel-Morais B. L., Madeira J. B., Maya-Monteiro C. M., Masuda C. A., Montero-Lomeli M., 2010. A new fluorescence-based method identifies protein phosphatases regulating lipid droplet metabolism. PLoS ONE 5(10): e13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackmann C., Norbeck J., Åkeson M., Bosch D., Larsson C., et al. , 2009. CARS microscopy of lipid stores in yeast: the impact of nutritional state and genetic background. J. Raman Spectrosc. 40: 748–756 [Google Scholar]
- Brasaemle D. L., 2007. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559 [DOI] [PubMed] [Google Scholar]
- Braschi E., Goyon V., Zunino R., Mohanty A., Xu L., et al. , 2010. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr. Biol. 20: 1310–1315 [DOI] [PubMed] [Google Scholar]
- Bratschi M. W., Burrowes D. P., Kulaga A., Cheung J. F., Alvarez A. L., et al. , 2009. Glycerol-3-phosphate acyltransferases gat1p and gat2p are microsomal phosphoproteins with differential contributions to polarized cell growth. Eukaryot. Cell 8: 1184–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard C., Hartig A., 2006. Peroxisome targeting signal 1: Is it really a simple tripeptide? Biochim. Biophys. Acta 1763: 1565–1573 [DOI] [PubMed] [Google Scholar]
- Brocard C., Kragler F., Simon M. M., Schuster T., Hartig A., 1994. The tetratricopeptide repeat-domain of the PAS10 protein of Saccharomyces cerevisiae is essential for binding the peroxisomal targeting signal-SKL. Biochem. Biophys. Res. Commun. 204: 1016–1022 [DOI] [PubMed] [Google Scholar]
- Brocard C., Lametschwandtner G., Koudelka R., Hartig A., 1997. Pex14p is a member of the protein linkage map of Pex5p. EMBO J. 16: 5491–5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. W., Titorenko V. I., Rachubinski R. A., 2000. Mutants of the Yarrowia lipolytica PEX23 gene encoding an integral peroxisomal membrane peroxin mislocalize matrix proteins and accumulate vesicles containing peroxisomal matrix and membrane proteins. Mol. Biol. Cell 11: 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg J. S., Espenshade P. J., 2011. Regulation of HMG-CoA reductase in mammals and yeast. Prog. Lipid Res. 50: 403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman G. M., Han G. S., 2006. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem. Sci. 31: 694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman G. M., Han G. S., 2009. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 284: 2593–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman G. M., Han G. S., 2011. Regulation of phospholipid synthesis in the yeast Saccharomyces cerevisiae. Annu. Rev. Biochem. 80: 859–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman G. M., Wu W., 2007. Lipid phosphate phosphatases from Saccharomyces cerevisiae, 305–315 Methods Enzymol. edited by Brown H. A. Academic Press, New York: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright J. L., Gasmi L., Spiller D. G., McLennan A. G., 2000. The Saccharomyces cerevisiae PCD1 gene encodes a peroxisomal nudix hydrolase active toward coenzyme A and its derivatives. J. Biol. Chem. 275: 32925–32930 [DOI] [PubMed] [Google Scholar]
- Cepinska M. N., Veenhuis M., van der Klei I. J., Nagotu S., 2011. Peroxisome fission is associated with reorganization of specific membrane proteins. Traffic 12: 925–937 [DOI] [PubMed] [Google Scholar]
- Chang J., Mast F. D., Fagarasanu A., Rachubinski D. A., Eitzen G. A., et al. , 2009. Pex3 peroxisome biogenesis proteins function in peroxisome inheritance as class V myosin receptors. J. Cell Biol. 187: 233–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K. D., Dyer J. M., Mullen R. T., 2012. Biogenesis and functions of lipid droplets in plants: Thematic Review Series: Lipid Droplet Synthesis and Metabolism: from Yeast to Man. J. Lipid Res. 53: 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa R., Kandasamy P., Oh C. S., Jiang Y., Vemula M., et al. , 2001. The membrane proteins, Spt23p and Mga2p, play distinct roles in the activation of Saccharomyces cerevisiae OLE1 gene expression. Fatty acid-mediated regulation of Mga2p activity is independent of its proteolytic processing into a soluble transcription activator. J. Biol. Chem. 276: 43548–43556 [DOI] [PubMed] [Google Scholar]
- Chen Q., Kazachkov M., Zheng Z., Zou J., 2007. The yeast acylglycerol acyltransferase LCA1 is a key component of Lands cycle for phosphatidylcholine turnover. FEBS Lett. 581: 5511–5516 [DOI] [PubMed] [Google Scholar]
- Choi H. S., Su W. M., Morgan J. M., Han G. S., Xu Z., et al. , 2011. Phosphorylation of phosphatidate phosphatase regulates its membrane association and physiological functions in Saccharomyces cerevisiae: Identification of SER602, THR723, and SER744 as the sites phosphorylated by CDC28 (CDK1)-encoded cyclin-dependent kinase. J. Biol. Chem. 286: 1486–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary V., Jacquier N., Schneiter R., 2011. The topology of the triacylglycerol synthesizing enzyme Lro1 indicates that neutral lipids can be produced within the luminal compartment of the endoplasmatic reticulum: Implications for the biogenesis of lipid droplets. Commun. Integr. Biol. 4: 781–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connerth M., Grillitsch K., Kofeler H., Daum G., 2009. Analysis of lipid particles from yeast. Methods Mol. Biol. 579: 359–374 [DOI] [PubMed] [Google Scholar]
- Connerth M., Czabany T., Wagner A., Zellnig G., Leitner E., et al. , 2010. Oleate inhibits steryl ester synthesis and causes liposensitivity in yeast. J. Biol. Chem. 285: 26832–26841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaki L. S., Reue K., 2010. Lipins: multifunctional lipid metabolism proteins. Annu. Rev. Nutr. 30: 257–272
- Czabany T., Athenstaedt K., Daum G., 2007. Synthesis, storage and degradation of neutral lipids in yeast. Biochim. Biophys. Acta 1771: 299–309 [DOI] [PubMed] [Google Scholar]
- Czabany T., Wagner A., Zweytick D., Lohner K., Leitner E., et al. , 2008. Structural and biochemical properties of lipid particles from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 283: 17065–17074 [DOI] [PubMed] [Google Scholar]
- Daum G., Wagner A., Czabany T., Athenstaedt K., 2007a. Dynamics of neutral lipid storage and mobilization in yeast. Biochimie 89: 243–248 [DOI] [PubMed] [Google Scholar]
- Daum G., Wagner A., Czabany T., Grillitsch K., Athenstaedt K., 2007b. Lipid storage and mobilization pathways in yeast. Novartis Found. Symp. 286: 142–151; discussion 151–144, 162–143, 196–203 [DOI] [PubMed] [Google Scholar]
- Debelyy M. O., Platta H. W., Saffian D., Hensel A., Thoms S., et al. , 2011. Ubp15p, a ubiquitin hydrolase associated with the peroxisomal export machinery. J. Biol. Chem. 286: 28223–28234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., 1965. The separation and characterization of subcellular particles. Harvey Lect. 59: 49–87 [PubMed] [Google Scholar]
- Delille H. K., Schrader M., 2008. Targeting of hFis1 to peroxisomes is mediated by Pex19p. J. Biol. Chem. 283: 31107–31115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet C. H., Vittone E., Scherer M., Houweling M., Liebisch G., et al. , 2012. The yeast acyltransferase Sct1p regulates fatty acid desaturation by competing with the desaturase Ole1p. Mol. Biol. Cell 23: 1146–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Virgilio C., 2012. The essence of yeast quiescence. FEMS Microbiol. Rev. 36: 306–339 [DOI] [PubMed] [Google Scholar]
- de Vries B., Kiel J. A., Scheek R., Veenhuis M., van der Klei I. J., 2007. A conserved alpha helical domain at the N-terminus of Pex14p is required for PTS1 and PTS2 protein import in Hansenula polymorpha. FEBS Lett. 581: 5627–5634 [DOI] [PubMed] [Google Scholar]
- Dhavale T., Jedd G., 2007. The fungal Woronin body, pp. 87–96 Biology of the Fungal Cell, edited by Howard R. J., Gow N. A. R. Springer, Berlin [Google Scholar]
- Dickson R. C., 2010. Roles for sphingolipids in Saccharomyces cerevisiae. Adv. Exp. Med. Biol. 688: 217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel B., Kragt A., 2006. Purification of yeast peroxisomes. Methods Mol. Biol. 313: 21–26 [DOI] [PubMed] [Google Scholar]
- Dixit E., Boulant S., Zhang Y., Lee A. S., Odendall C., et al. , 2010. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141: 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einwachter H., Sowinski S., Kunau W. H., Schliebs W., 2001. Yarrowia lipolytica Pex20p, Saccharomyces cerevisiae Pex18p/Pex21p and mammalian Pex5pL fulfill a common function in the early steps of the peroxisomal PTS2 import pathway. EMBO Rep. 2: 1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejsing C. S., Sampaio J. L., Surendranath V., Duchoslav E., Ekroos K., et al. , 2009. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA 106: 2136–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O., Elofsson A., von Heijne G., Cristobal S., 2003. In silico prediction of the peroxisomal proteome in fungi, plants and animals. J. Mol. Biol. 330: 443–456 [DOI] [PubMed] [Google Scholar]
- Erdmann R., Blobel G., 1995. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J. Cell Biol. 128: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Veenhuis M., Mertens D., Kunau W. H., 1989. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 86: 5419–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Wiebel F. F., Flessau A., Rytka J., Beyer A., et al. , 1991. PAS1, a yeast gene required for peroxisome biogenesis, encodes a member of a novel family of putative ATPases. Cell 64: 499–510 [DOI] [PubMed] [Google Scholar]
- Espenshade P. J., Hughes A. L., 2007. Regulation of sterol synthesis in eukaryotes. Annu. Rev. Genet. 41: 401–427 [DOI] [PubMed] [Google Scholar]
- Fagarasanu A., Fagarasanu M., Eitzen G. A., Aitchison J. D., Rachubinski R. A., 2006. The peroxisomal membrane protein Inp2p is the peroxisome-specific receptor for the myosin V motor Myo2p of Saccharomyces cerevisiae. Dev. Cell 10: 587–600 [DOI] [PubMed] [Google Scholar]
- Fagarasanu A., Mast F. D., Knoblach B., Jin Y., Brunner M. J., et al. , 2009. Myosin-driven peroxisome partitioning in S. cerevisiae. J. Cell Biol. 186: 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakas S., Konstantinou C., Carman G. M., 2011a. DGK1-encoded diacylglycerol kinase activity is required for phospholipid synthesis during growth resumption from stationary phase in Saccharomyces cerevisiae. J. Biol. Chem. 286: 1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakas S., Qiu Y., Dixon J. L., Han G. S., Ruggles K. V., et al. , 2011b. Phosphatidate phosphatase activity plays key role in protection against fatty acid-induced toxicity in yeast. J. Biol. Chem. 286: 29074–29085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Jr, Walther T. C., 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139: 855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei W., Yang H., 2012. Genome-wide screens for gene products regulating lipid droplet dynamics. Methods Cell Biol. 108: 303–316 [DOI] [PubMed] [Google Scholar]
- Fei W., Shui G., Gaeta B., Du X., Kuerschner L., et al. , 2008. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 180: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei W., Du X., Yang H., 2011a. Seipin, adipogenesis and lipid droplets. Trends Endocrinol. Metab. 22: 204–210 [DOI] [PubMed] [Google Scholar]
- Fei W., Li H., Shui G., Kapterian T. S., Bielby C., et al. , 2011b. Molecular characterization of seipin and its mutants: implications for seipin in triacylglycerol synthesis. J. Lipid Res. 52: 2136–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei W., Shui G., Zhang Y., Krahmer N., Ferguson C., et al. , 2011c. A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet. 7: e1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen M., Vastiau I., Brees C., Brys V., Mannaerts G. P., et al. , 2004. Potential role for Pex19p in assembly of PTS-receptor docking complexes. J. Biol. Chem. 279: 12615–12624 [DOI] [PubMed] [Google Scholar]
- Freitag J., Alst J., Bölker M., 2012. Cryptic peroxisomal targeting via alternative splicing and stop codon read-through in fungi. Nature 485: 522–525 [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Ohsaki Y., Cheng J., Suzuki M., Shinohara Y., 2008. Lipid droplets: a classic organelle with new outfits. Histochem. Cell Biol. 130: 263–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo C. M., Smith D. L., Jr, Smith J. S., 2004. Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity. Mol. Cell. Biol. 24: 1301–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino J., Padamsee M., Wilcox L., Oelkers P. M., D’Ambrosio D., et al. , 2009. Sterol and diacylglycerol acyltransferase deficiency triggers fatty acid-mediated cell death. J. Biol. Chem. 284: 30994–31005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A., 2004. Acquired and inherited lipodystrophies. N. Engl. J. Med. 350: 1220–1234 [DOI] [PubMed] [Google Scholar]
- Garg A., Agarwal A. K., 2009. Lipodystrophies: disorders of adipose tissue biology. Biochim. Biophys. Acta 1791: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar M. L., Jesch S. A., Viswanatha R., Antosh A. L., Brown W. J., et al. , 2008. A block in endoplasmic reticulum-to-Golgi trafficking inhibits phospholipid synthesis and induces neutral lipid accumulation. J. Biol. Chem. 283: 25735–25751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar M. L., Hofbauer H. F., Kohlwein S. D., Henry S. A., 2011. Coordination of storage lipid synthesis and membrane biogenesis: evidence for cross-talk between triacylglycerol metabolism and phosphatidylinositol synthesis. J. Biol. Chem. 286: 1696–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto G. J., Jr, Geisbrecht B. V., Gould S. J., Berg J. M., 2000. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nat. Struct. Biol. 7: 1091–1095 [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Murk J. L., Stroobants A. K., Griffith J. M., Kleijmeer M. J., et al. , 2003. Involvement of the endoplasmic reticulum in peroxisome formation. Mol. Biol. Cell 14: 2900–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., et al. , 2003. Global analysis of protein expression in yeast. Nature 425: 737–741 [DOI] [PubMed] [Google Scholar]
- Girzalsky W., Rehling P., Stein K., Kipper J., Blank L., et al. , 1999. Involvement of Pex13p in Pex14p localization and peroxisomal targeting signal 2-dependent protein import into peroxisomes. J. Cell Biol. 144: 1151–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover J. R., Andrews D. W., Rachubinski R. A., 1994. Saccharomyces cerevisiae peroxisomal thiolase is imported as a dimer. Proc. Natl. Acad. Sci. USA 91: 10541–10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A., Barrell B. G., Bussey H., Davis R. W., Dujon B., et al. , 1996. Life with 6000 genes. Science 274(5287): 546; 563–567 [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., et al. , 1973. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science 182: 62–64 [DOI] [PubMed] [Google Scholar]
- Goodman J. M., 2008. The gregarious lipid droplet. J. Biol. Chem. 283: 28005–28009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. M., 2009. Demonstrated and inferred metabolism associated with cytosolic lipid droplets. J. Lipid Res. 50: 2148–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Collins C. S., 2002. Opinion: peroxisomal-protein import: Is it really that complex? Nat. Rev. Mol. Cell Biol. 3: 382–389 [DOI] [PubMed] [Google Scholar]
- Gould S. G., Keller G. A., Subramani S., 1987. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J. Cell Biol. 105: 2923–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Schneider M., Howell S. H., Garrard L. J., et al. , 1990. Peroxisomal protein import is conserved between yeast, plants, insects and mammals. EMBO J. 9: 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillitsch K., Connerth M., Kofeler H., Arrey T. N., Rietschel B., et al. , 2011. Lipid particles/droplets of the yeast Saccharomyces cerevisiae revisited: lipidome meets proteome. Biochim. Biophys. Acta 1811: 1165–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. A., Snapp E. L., Silver D. L., 2010. Structural insights into triglyceride storage mediated by fat storage-inducing transmembrane (FIT) protein 2. PLoS ONE 5: e10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. A., Zhan C., Silver D. L., 2011. Direct binding of triglyceride to fat storage-inducing transmembrane proteins 1 and 2 is important for lipid droplet formation. Proc. Natl. Acad. Sci. USA 108: 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau S., Schliebs W., Linnepe R., Neufeld C., Cizmowski C., et al. , 2009. Peroxisomal targeting of PTS2 pre-import complexes in the yeast Saccharomyces cerevisiae. Traffic 10: 451–460 [DOI] [PubMed] [Google Scholar]
- Gunkel K., van Dijk R., Veenhuis M., van der Klei I. J., 2004. Routing of Hansenula polymorpha alcohol oxidase: an alternative peroxisomal protein-sorting machinery. Mol. Biol. Cell 15: 1347–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Cordes K. R., Farese R. V., Jr, Walther T. C., 2009. Lipid droplets at a glance. J. Cell Sci. 122: 749–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvitz A., Rottensteiner H., 2006. The biochemistry of oleate induction: transcriptional upregulation and peroxisome proliferation. Biochim. Biophys. Acta 1763: 1392–1402 [DOI] [PubMed] [Google Scholar]
- Gurvitz A., Mursula A. M., Yagi A. I., Hartig A., Ruis H., et al. , 1999. Alternatives to the isomerase-dependent pathway for the beta-oxidation of oleic acid are dispensable in Saccharomyces cerevisiae. Identification of YOR180c/DCI1 encoding peroxisomal delta(3,5)-delta(2,4)-dienoyl-CoA isomerase. J. Biol. Chem. 274: 24514–24521 [DOI] [PubMed] [Google Scholar]
- Haan G. J., Baerends R. J., Krikken A. M., Otzen M., Veenhuis M., et al. , 2006. Reassembly of peroxisomes in Hansenula polymorpha pex3 cells on reintroduction of Pex3p involves the nuclear envelope. FEMS Yeast Res. 6: 186–194 [DOI] [PubMed] [Google Scholar]
- Habeler G., Natter K., Thallinger G. G., Crawford M. E., Kohlwein S. D., et al. , 2002. YPL.db: the Yeast Protein Localization database. Nucleic Acids Res. 30: 80–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., et al. , 2011. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 17: 1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G. S., Carman G. M., 2010. Characterization of the human LPIN1-encoded phosphatidate phosphatase isoforms. J. Biol. Chem. 285: 14628–14638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G. S., Wu W. I., Carman G. M., 2006. The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281: 9210–9218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G. S., Siniossoglou S., Carman G. M., 2007. The cellular functions of the yeast lipin homolog Pah1p are dependent on its phosphatidate phosphatase activity. J. Biol. Chem. 282: 37026–37035 [DOI] [PubMed] [Google Scholar]
- Han G. S., O’Hara L., Carman G. M., Siniossoglou S., 2008a. An unconventional diacylglycerol kinase that regulates phospholipid synthesis and nuclear membrane growth. J. Biol. Chem. 283: 20433–20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G. S., O’Hara L., Siniossoglou S., Carman G. M., 2008b. Characterization of the yeast DGK1-encoded CTP-dependent diacylglycerol kinase. J. Biol. Chem. 283: 20443–20453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapala I., Marza E., Ferreira T., 2011. Is fat so bad? Modulation of endoplasmic reticulum stress by lipid droplet formation. Biol. Cell 103: 271–285 [DOI] [PubMed] [Google Scholar]
- Hasslacher M., Ivessa A. S., Paltauf F., Kohlwein S. D., 1993. Acetyl-CoA carboxylase from yeast is an essential enzyme and is regulated by factors that control phospholipid metabolism. J. Biol. Chem. 268: 10946–10952 [PubMed] [Google Scholar]
- Hazra P. P., Suriapranata I., Snyder W. B., Subramani S., 2002. Peroxisome remnants in pex3delta cells and the requirement of Pex3p for interactions between the peroxisomal docking and translocation subcomplexes. Traffic 3: 560–574 [DOI] [PubMed] [Google Scholar]
- Heier C., Taschler U., Rengachari S., Oberer M., Wolinski H., et al. , 2010. Identification of Yju3p as functional orthologue of mammalian monoglyceride lipase in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1801: 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Kohlwein S. D., Carman G. M., 2012. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics 190: 317–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema E. H., van Roermund C. W., Distel B., van den Berg M., Vilela C., et al. , 1996. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 15: 3813–3822 [PMC free article] [PubMed] [Google Scholar]
- Hettema E. H., Girzalsky W., van Den Berg M., Erdmann R., Distel B., 2000. Saccharomyces cerevisiae pex3p and pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 19: 223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges B. D. M., Wu C. C., 2010. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J. Lipid Res. 51: 262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner D., van den Berg M., Philippsen P., Tabak H. F., Hettema E. H., 2001. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J. Cell Biol. 155: 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner D., Schildknegt D., Braakman I., Philippsen P., Tabak H. F., 2005. Contribution of the endoplasmic reticulum to peroxisome formation. Cell 122: 85–95 [DOI] [PubMed] [Google Scholar]
- Hofmann K., 2000. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem. Sci. 25: 111–112 [DOI] [PubMed] [Google Scholar]
- Hoppe T., Matuschewski K., Rape M., Schlenker S., Ulrich H. D., et al. , 2000. Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell 102: 577–586 [DOI] [PubMed] [Google Scholar]
- Horvath S. E., Wagner A., Steyrer E., Daum G., 2011. Metabolic link between phosphatidylethanolamine and triacylglycerol metabolism in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1811: 1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hronska L., Mrozova Z., Valachovic M., Hapala I., 2004. Low concentrations of the non-ionic detergent Nonidet P-40 interfere with sterol biogenesis and viability of the yeast Saccharomyces cerevisiae. FEMS Microbiol. Lett. 238: 241–248 [DOI] [PubMed] [Google Scholar]
- Huber A., Koch J., Kragler F., Brocard C., Hartig A., 2012. A subtle interplay between three Pex11 proteins shapes de novo formation and fission of peroxisomes. Traffic 13: 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., et al. , 2003. Global analysis of protein localization in budding yeast. Nature 425: 686–691 [DOI] [PubMed] [Google Scholar]
- Islinger M., Li K. W., Seitz J., Volkl A., Luers G. H., 2009. Hitchhiking of Cu/Zn superoxide dismutase to peroxisomes: evidence for a natural piggyback import mechanism in mammals. Traffic 10: 1711–1721 [DOI] [PubMed] [Google Scholar]
- Ivnitski-Steele I., Holmes A. R., Lamping E., Monk B. C., Cannon R. D., et al. , 2009. Identification of Nile red as a fluorescent substrate of the Candida albicans ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator superfamily transporter Mdr1p. Anal. Biochem. 394: 87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier N., Choudhary V., Mari M., Toulmay A., Reggiori F., et al. , 2011. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Sci. 124: 2424–2437 [DOI] [PubMed] [Google Scholar]
- Jain S., Stanford N., Bhagwat N., Seiler B., Costanzo M., et al. , 2007. Identification of a novel lysophospholipid acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 282: 30562–30569 [DOI] [PubMed] [Google Scholar]
- Jandrositz A., Petschnigg J., Zimmermann R., Natter K., Scholze H., et al. , 2005. The lipid droplet enzyme Tgl1p hydrolyzes both steryl esters and triglycerides in the yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta 1735: 50–58 [DOI] [PubMed] [Google Scholar]
- Jones J. M., Morrell J. C., Gould S. J., 2004. PEX19 is a predominantly cytosolic chaperone and import receptor for class 1 peroxisomal membrane proteins. J. Cell Biol. 164: 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S., Marelli M., Rachubinski R. A., Goodlett D. R., Aitchison J. D., 2010. Dynamic changes in the subcellular distribution of Gpd1p in response to cell stress. J. Biol. Chem. 285: 6739–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadereit B., Kumar P., Wang W. J., Miranda D., Snapp E. L., et al. , 2008. Evolutionarily conserved gene family important for fat storage. Proc. Natl. Acad. Sci. USA 105: 94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M., McVey M., Guarente L., 1999. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13: 2570–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kal A. J., van Zonneveld A. J., Benes V., van den Berg M., Koerkamp M. G., et al. , 1999. Dynamics of gene expression revealed by comparison of serial analysis of gene expression transcript profiles from yeast grown on two different carbon sources. Mol. Biol. Cell 10: 1859–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kal A. J., Hettema E. H., van den Berg M., Koerkamp M. G., van Ijlst L., et al. , 2000. In silicio search for genes encoding peroxisomal proteins in Saccharomyces cerevisiae. Cell Biochem. Biophys. 32(Spring): 1–8 [DOI] [PubMed] [Google Scholar]
- Kaliszewski P., Żołądek T., 2008. The role of Rsp5 ubiquitin ligase in regulation of diverse processes in yeast cells. Acta Biochim. Pol. 55: 649–662 [PubMed] [Google Scholar]
- Kals M., Natter K., Thallinger G. G., Trajanoski Z., Kohlwein S. D., 2005. YPL.db2: the Yeast Protein Localization database, version 2.0. Yeast 22: 213–218 [DOI] [PubMed] [Google Scholar]
- Karanasios E., Han G. S., Xu Z., Carman G. M., Siniossoglou S., 2010. A phosphorylation-regulated amphipathic helix controls the membrane translocation and function of the yeast phosphatidate phosphatase. Proc. Natl. Acad. Sci. USA 107: 17539–17544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik S. K., Trelease R. N., 2005. Arabidopsis peroxin 16 coexists at steady state in peroxisomes and endoplasmic reticulum. Plant Physiol. 138: 1967–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel J. A., Emmrich K., Meyer H. E., Kunau W. H., 2005a. Ubiquitination of the peroxisomal targeting signal type 1 receptor, Pex5p, suggests the presence of a quality control mechanism during peroxisomal matrix protein import. J. Biol. Chem. 280: 1921–1930 [DOI] [PubMed] [Google Scholar]
- Kiel J. A., Otzen M., Veenhuis M., van der Klei I. J., 2005b. Obstruction of polyubiquitination affects PTS1 peroxisomal matrix protein import. Biochim. Biophys. Acta 1745: 176–186 [DOI] [PubMed] [Google Scholar]
- Kiel J. A., Veenhuis M., van der Klei I. J., 2006. PEX genes in fungal genomes: common, rare or redundant. Traffic 7: 1291–1303 [DOI] [PubMed] [Google Scholar]
- Kienesberger P. C., Oberer M., Lass A., Zechner R., 2009. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J. Lipid Res. 50(Suppl.): S63–S68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. T., van den Berg M., Bottger G., Tabak H. F., Distel B., 2002. Saccharomyces cerevisiae acyl-CoA oxidase follows a novel, non-PTS1, import pathway into peroxisomes that is dependent on Pex5p. J. Biol. Chem. 277: 25011–25019 [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Cuervo A. M., Dunn W. A., Jr, Levine B., van der Klei I., et al. , 2007. How shall I eat thee? Autophagy 3: 413–416 [DOI] [PubMed] [Google Scholar]
- Klose C., Surma M. A., Gerl M. J., Meyenhofer F., Shevchenko A., et al. , 2012. Flexibility of a eukaryotic lipidome: insights from yeast lipidomics. PLoS ONE 7: e35063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach B., Rachubinski R. A., 2010. Phosphorylation-dependent activation of peroxisome proliferator protein PEX11 controls peroxisome abundance. J. Biol. Chem. 285: 6670–6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Tanaka A., Fujiki Y., 2007. Fis1, DLP1, and Pex11p coordinately regulate peroxisome morphogenesis. Exp. Cell Res. 313: 1675–1686 [DOI] [PubMed] [Google Scholar]
- Koffel R., Schneiter R., 2006. Yeh1 constitutes the major steryl ester hydrolase under heme-deficient conditions in Saccharomyces cerevisiae. Eukaryot. Cell 5: 1018–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel R., Tiwari R., Falquet L., Schneiter R., 2005. The Saccharomyces cerevisiae YLL012/YEH1, YLR020/YEH2, and TGL1 genes encode a novel family of membrane-anchored lipases that are required for steryl ester hydrolysis. Mol. Cell. Biol. 25: 1655–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlwein S. D., 2010a. Obese and anorexic yeasts: experimental models to understand the metabolic syndrome and lipotoxicity. Biochim. Biophys. Acta 1801: 222–229 [DOI] [PubMed] [Google Scholar]
- Kohlwein S. D., 2010b. Triacylglycerol homeostasis: insights from yeast. J. Biol. Chem. 285: 15663–15667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlwein S. D., Petschnigg J., 2007. Lipid-induced cell dysfunction and cell death: lessons from yeast. Curr. Hypertens. Rep. 9: 455–461 [DOI] [PubMed] [Google Scholar]
- Komori M., Rasmussen S. W., Kiel J. A., Baerends R. J., Cregg J. M., et al. , 1997. The Hansenula polymorpha PEX14 gene encodes a novel peroxisomal membrane protein essential for peroxisome biogenesis. EMBO J. 16: 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragler F., Langeder A., Raupachova J., Binder M., Hartig A., 1993. Two independent peroxisomal targeting signals in catalase A of Saccharomyces cerevisiae. J. Cell Biol. 120: 665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragt A., Voorn-Brouwer T., van den Berg M., Distel B., 2005. Endoplasmic reticulum-directed Pex3p routes to peroxisomes and restores peroxisome formation in a Saccharomyces cerevisiae pex3Delta strain. J. Biol. Chem. 280: 34350–34357 [DOI] [PubMed] [Google Scholar]
- Krahmer N., Guo Y., Farese R. V., Jr, Walther T. C., 2009. SnapShot: Lipid Droplets. Cell 139: 1024–1024e102 [DOI] [PubMed] [Google Scholar]
- Krikken A. M., Veenhuis M., van der Klei I. J., 2009. Hansenula polymorpha pex11 cells are affected in peroxisome retention. FEBS J. 276: 1429–1439 [DOI] [PubMed] [Google Scholar]
- Kumar A., Agarwal S., Heyman J. A., Matson S., Heidtman M., et al. , 2002. Subcellular localization of the yeast proteome. Genes Dev. 16: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunau W. H., Dommes V., Schulz H., 1995. β-Oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog. Lipid Res. 34: 267–342 [DOI] [PubMed] [Google Scholar]
- Kunze M., Pracharoenwattana I., Smith S. M., Hartig A., 2006. A central role for the peroxisomal membrane in glyoxylate cycle function. Biochim. Biophys. Acta 1763: 1441–1452 [DOI] [PubMed] [Google Scholar]
- Kurat C. F., Natter K., Petschnigg J., Wolinski H., Scheuringer K., et al. , 2006. Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J. Biol. Chem. 281: 491–500 [DOI] [PubMed] [Google Scholar]
- Kurat C. F., Wolinski H., Petschnigg J., Kaluarachchi S., Andrews B., et al. , 2009. Cdk1/Cdc28-dependent activation of the major triacylglycerol lipase Tgl4 in yeast links lipolysis to cell-cycle progression. Mol. Cell 33: 53–63 [DOI] [PubMed] [Google Scholar]
- Kuravi K., Nagotu S., Krikken A. M., Sjollema K., Deckers M., et al. , 2006. Dynamin-related proteins Vps1p and Dnm1p control peroxisome abundance in Saccharomyces cerevisiae. J. Cell Sci. 119: 3994–4001 [DOI] [PubMed] [Google Scholar]
- Kurbatova E., Otzen M., van der Klei I. J., 2009. p24 proteins play a role in peroxisome proliferation in yeast. FEBS Lett. 583: 3175–3180 [DOI] [PubMed] [Google Scholar]
- Lam S. K., Yoda N., Schekman R., 2010. A vesicle carrier that mediates peroxisome protein traffic from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 107: 21523–21528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambkin G. R., Rachubinski R. A., 2001. Yarrowia lipolytica cells mutant for the peroxisomal peroxin Pex19p contain structures resembling wild-type peroxisomes. Mol. Biol. Cell 12: 3353–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lametschwandtner G., Brocard C., Fransen M., Van Veldhoven P., Berger J., et al. , 1998. The difference in recognition of terminal tripeptides as peroxisomal targeting signal 1 between yeast and human is due to different affinities of their receptor Pex5p to the cognate signal and to residues adjacent to it. J. Biol. Chem. 273: 33635–33643 [DOI] [PubMed] [Google Scholar]
- Lass A., Zimmermann R., Oberer M., Zechner R., 2011. Lipolysis: a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 50: 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., 2006. The import receptor Pex7p and the PTS2 targeting sequence. Biochim. Biophys. Acta 1763: 1599–1604 [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., 2011. Viruses exploiting peroxisomes. Curr. Opin. Microbiol. 14: 458–469 [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y., 1985. Biogenesis of peroxisomes. Annu. Rev. Cell Biol. 1: 489–530 [DOI] [PubMed] [Google Scholar]
- Leber R., Zinser E., Zellnig G., Paltauf F., Daum G., 1994. Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast 10: 1421–1428 [DOI] [PubMed] [Google Scholar]
- Leber R., Zinser E., Hrastnik C., Paltauf F., Daum G., 1995. Export of steryl esters from lipid particles and release of free sterols in the yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta 1234: 119–126 [DOI] [PubMed] [Google Scholar]
- Leber R., Landl K., Zinser E., Ahorn H., Spok A., et al. , 1998. Dual localization of squalene epoxidase, Erg1p, in yeast reflects a relationship between the endoplasmic reticulum and lipid particles. Mol. Biol. Cell 9: 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S., Subramani S., 2007. A conserved cysteine residue of Pichia pastoris Pex20p is essential for its recycling from the peroxisome to the cytosol. J. Biol. Chem. 282: 7424–7430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Roberts R., 2001. WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases. Cell. Mol. Life Sci. 58: 2085–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingard M. J., Gidda S. K., Bingham S., Rothstein S. J., Mullen R. T., et al. , 2008. Arabidopsis PEROXIN11c-e, FISSION1b, and DYNAMIN-RELATED PROTEIN3A cooperate in cell cycle-associated replication of peroxisomes. Plant Cell 20: 1567–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr, et al. , 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 100: 3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Tan X., Veenhuis M., McCollum D., Cregg J. M., 1992. An efficient screen for peroxisome-deficient mutants of Pichia pastoris. J. Bacteriol. 174: 4943–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanovic N., Streith I., Wolinski H., Rechberger G., Kohlwein S. D., et al. , 2008. S-adenosyl-L-homocysteine hydrolase, key enzyme of methylation metabolism, regulates phosphatidylcholine synthesis and triacylglycerol homeostasis in yeast: implications for homocysteine as a risk factor of atherosclerosis. J. Biol. Chem. 283: 23989–23999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Managadze D., Wurtz C., Wiese S., Schneider M., Girzalsky W., et al. , 2010. Identification of PEX33, a novel component of the peroxisomal docking complex in the filamentous fungus Neurospora crassa. Eur. J. Cell Biol. 89: 955–964 [DOI] [PubMed] [Google Scholar]
- Manjithaya R., Nazarko T. Y., Farre J. C., Subramani S., 2010. Molecular mechanism and physiological role of pexophagy. FEBS Lett. 584: 1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli M., Smith J. J., Jung S., Yi E., Nesvizhskii A. I., et al. , 2004. Quantitative mass spectrometry reveals a role for the GTPase Rho1p in actin organization on the peroxisome membrane. J. Cell Biol. 167: 1099–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr N., Foglia J., Terebiznik M., Athenstaedt K., Zaremberg V., 2012. Controlling lipid fluxes at glycerol-3-phosphate acyltransferase step in yeast: unique contribution of Gat1p to oleic acid-induced lipid particle formation. J. Biol. Chem. 287: 10251–10264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P. A., Krimkevich Y. I., Lark R. H., Dyer J. M., Veenhuis M., et al. , 1995. Pmp27 promotes peroxisomal proliferation. J. Cell Biol. 129: 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. E., Oh C. S., Kandasamy P., Chellapa R., Vemula M., 2002. Yeast desaturases. Biochem. Soc. Trans. 30: 1080–1082 [DOI] [PubMed] [Google Scholar]
- Martin C. E., Oh C. S., Jiang Y., 2007. Regulation of long chain unsaturated fatty acid synthesis in yeast. Biochim. Biophys. Acta 1771: 271–285 [DOI] [PubMed] [Google Scholar]
- Martin W., Muller M., 1998. The hydrogen hypothesis for the first eukaryote. Nature 392: 37–41 [DOI] [PubMed] [Google Scholar]
- Matsushita M., Nikawa J., 1995. Isolation and characterization of a SCT1 gene which can suppress a choline-transport mutant of Saccharomyces cerevisiae. J. Biochem. 117: 447–451 [DOI] [PubMed] [Google Scholar]
- Matsuzaki T., Fujiki Y., 2008. The peroxisomal membrane protein import receptor Pex3p is directly transported to peroxisomes by a novel Pex19p- and Pex16p-dependent pathway. J. Cell Biol. 183: 1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon M. T., Veenhuis M., Trapp S. B., Goodman J. M., 1990. Association of glyoxylate and beta-oxidation enzymes with peroxisomes of Saccharomyces cerevisiae. J. Bacteriol. 172: 5816–5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew J. A., Goodman J. M., 1994. An oligomeric protein is imported into peroxisomes in vivo. J. Cell Biol. 127: 1245–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer W. H., van der Klei I. J., Veenhuis M., Kiel J. A., 2007. ATG genes involved in non-selective autophagy are conserved from yeast to man, but the selective Cvt and pexophagy pathways also require organism-specific genes. Autophagy 3: 106–116 [DOI] [PubMed] [Google Scholar]
- Meinecke M., Cizmowski C., Schliebs W., Kruger V., Beck S., et al. , 2010. The peroxisomal importomer constitutes a large and highly dynamic pore. Nat. Cell Biol. 12: 273–277 [DOI] [PubMed] [Google Scholar]
- Moir R. D., Gross D. A., Silver D. L., Willis I. M., 2012. SCS3 and YFT2 Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR. PLoS Genet. 8(8): e1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscicka K. B., Klompmaker S. H., Wang D., van der Klei I. J., Boekema E. J., 2007. The Hansenula polymorpha peroxisomal targeting signal 1 receptor, Pex5p, functions as a tetramer. FEBS Lett. 581: 1758–1762 [DOI] [PubMed] [Google Scholar]
- Motley A. M., Hettema E. H., 2007. Yeast peroxisomes multiply by growth and division. J. Cell Biol. 178: 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A. M., Ward G. P., Hettema E. H., 2008. Dnm1p-dependent peroxisome fission requires Caf4p, Mdv1p and Fis1p. J. Cell Sci. 121: 1633–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullner H., Daum G., 2004. Dynamics of neutral lipid storage in yeast. Acta Biochim. Pol. 51: 323–347 [PubMed] [Google Scholar]
- Mullner H., Zweytick D., Leber R., Turnowsky F., Daum G., 2004. Targeting of proteins involved in sterol biosynthesis to lipid particles of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1663: 9–13 [DOI] [PubMed] [Google Scholar]
- Mullner H., Deutsch G., Leitner E., Ingolic E., Daum G., 2005. YEH2/YLR020c encodes a novel steryl ester hydrolase of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 280: 13321–13328 [DOI] [PubMed] [Google Scholar]
- Munck J. M., Motley A. M., Nuttall J. M., Hettema E. H., 2009. A dual function for Pex3p in peroxisome formation and inheritance. J. Cell Biol. 187: 463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. J., 2001. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 40: 325–438 [DOI] [PubMed] [Google Scholar]
- Murphy S., Martin S., Parton R. G., 2009. Lipid droplet-organelle interactions: sharing the fats. Biochim. Biophys. Acta 1791: 441–447 [DOI] [PubMed] [Google Scholar]
- Nagotu S., Krikken A. M., Otzen M., Kiel J. A., Veenhuis M., et al. , 2008a. Peroxisome fission in Hansenula polymorpha requires Mdv1 and Fis1, two proteins also involved in mitochondrial fission. Traffic 9: 1471–1484 [DOI] [PubMed] [Google Scholar]
- Nagotu S., Saraya R., Otzen M., Veenhuis M., van der Klei I. J., 2008b. Peroxisome proliferation in Hansenula polymorpha requires Dnm1p which mediates fission but not de novo formation. Biochim. Biophys. Acta 1783: 760–769 [DOI] [PubMed] [Google Scholar]
- Natter K., Leitner P., Faschinger A., Wolinski H., McCraith S., et al. , 2005. The spatial organization of lipid synthesis in the yeast Saccharomyces cerevisiae derived from large scale green fluorescent protein tagging and high resolution microscopy. Mol. Cell. Proteomics 4: 662–672 [DOI] [PubMed] [Google Scholar]
- Neuberger G., Maurer-Stroh S., Eisenhaber B., Hartig A., Eisenhaber F., 2003a. Motif refinement of the peroxisomal targeting signal 1 and evaluation of taxon-specific differences. J. Mol. Biol. 328: 567–579 [DOI] [PubMed] [Google Scholar]
- Neuberger G., Maurer-Stroh S., Eisenhaber B., Hartig A., Eisenhaber F., 2003b. Prediction of peroxisomal targeting signal 1 containing proteins from amino acid sequence. J. Mol. Biol. 328: 581–592 [DOI] [PubMed] [Google Scholar]
- Neufeld C., Filipp F. V., Simon B., Neuhaus A., Schuller N., et al. , 2009. Structural basis for competitive interactions of Pex14 with the import receptors Pex5 and Pex19. EMBO J. 28: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoff K., Meindl-Beinker N. M., Kerssen D., Perband U., Schafer A., et al. , 2005. Yeast Pex14p possesses two functionally distinct Pex5p and one Pex7p binding sites. J. Biol. Chem. 280: 35571–35578 [DOI] [PubMed] [Google Scholar]
- Ntamack A. G., Karpichev I. V., Gould S. J., Small G. M., Schulz H., 2009. Oleate beta-oxidation in yeast involves thioesterase but not Yor180c protein that is not a dienoyl-CoA isomerase. Biochim. Biophys. Acta 1791: 371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttley W. M., Brade A. M., Eitzen G. A., Veenhuis M., Aitchison J. D., et al. , 1994. PAY4, a gene required for peroxisome assembly in the yeast Yarrowia lipolytica, encodes a novel member of a family of putative ATPases. J. Biol. Chem. 269: 556–566 [PubMed] [Google Scholar]
- Nyathi Y., Baker A., 2006. Plant peroxisomes as a source of signalling molecules. Biochim. Biophys. Acta 1763: 1478–1495 [DOI] [PubMed] [Google Scholar]
- Oeljeklaus S., Reinartz B. S., Wolf J., Wiese S., Tonillo J., et al. , 2012. Identification of core components and transient interactors of the peroxisomal importomer by dual-track stable isotope labeling with amino acids in cell culture analysis. J. Proteome Res. 11: 2567–2580 [DOI] [PubMed] [Google Scholar]
- Oelkers P., Tinkelenberg A., Erdeniz N., Cromley D., Billheimer J. T., et al. , 2000. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 275: 15609–15612 [DOI] [PubMed] [Google Scholar]
- Oelkers P., Cromley D., Padamsee M., Billheimer J. T., Sturley S. L., 2002. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 277: 8877–8881 [DOI] [PubMed] [Google Scholar]
- O’Hara L., Han G. S., Sew P. C., Grimsey N., Carman G. M., et al. , 2006. Control of phospholipid synthesis by phosphorylation of the yeast lipin Pah1p/Smp2p Mg2+-dependent phosphatidate phosphatase. J. Biol. Chem. 281: 34537–34548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S. O., Bostrøm P., Andersson L., Rutberg M., Perman J., et al. , 2009. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim. Biophys. Acta 1791: 448–458 [DOI] [PubMed] [Google Scholar]
- Opalinski L., Kiel J. A., Homan T. G., Veenhuis M., van der Klei I. J., 2010. Penicillium chrysogenum Pex14/17p: a novel component of the peroxisomal membrane that is important for penicillin production. FEBS J. 277: 3203–3218 [DOI] [PubMed] [Google Scholar]
- Opalinski L., Kiel J. A., Williams C., Veenhuis M., van der Klei I. J., 2011. Membrane curvature during peroxisome fission requires Pex11. EMBO J. 30: 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P., 1977. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett. 80: 360–364 [DOI] [PubMed] [Google Scholar]
- Otzen M., Perband U., Wang D., Baerends R. J., Kunau W. H., et al. , 2004. Hansenula polymorpha Pex19p is essential for the formation of functional peroxisomal membranes. J. Biol. Chem. 279: 19181–19190 [DOI] [PubMed] [Google Scholar]
- Otzen M., Wang D., Lunenborg M. G., van der Klei I. J., 2005. Hansenula polymorpha Pex20p is an oligomer that binds the peroxisomal targeting signal 2 (PTS2). J. Cell Sci. 118: 3409–3418 [DOI] [PubMed] [Google Scholar]
- Otzen M., Krikken A. M., Ozimek P. Z., Kurbatova E., Nagotu S., et al. , 2006. In the yeast Hansenula polymorpha, peroxisome formation from the ER is independent of Pex19p, but involves the function of p24 proteins. FEMS Yeast Res. 6: 1157–1166 [DOI] [PubMed] [Google Scholar]
- Otzen M., Rucktäschel R., Thoms S., Emmrich K., Krikken A. M., et al. , 2012. Pex19p contributes to peroxisome inheritance in the association of peroxisomes to Myo2p. Traffic 13(7): 947–959 [DOI] [PubMed] [Google Scholar]
- Outeiro T. F., Lindquist S., 2003. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 302: 1772–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozimek P., Kotter P., Veenhuis M., van der Klei I. J., 2006. Hansenula polymorpha and Saccharomyces cerevisiae Pex5p’s recognize different, independent peroxisomal targeting signals in alcohol oxidase. FEBS Lett. 580: 46–50 [DOI] [PubMed] [Google Scholar]
- Pagac M., de la Mora H. V., Duperrex C., Roubaty C., Vionnet C., et al. , 2011. Topology of 1-acyl-sn-glycerol-3-phosphate acyltransferases SLC1 and ALE1 and related membrane-bound O-acyltransferases (MBOATs) of Saccharomyces cerevisiae. J. Biol. Chem. 286: 36438–36447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri L., Rottensteiner H., Girzalsky W., Scarcia P., Palmieri F., et al. , 2001. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 20: 5049–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish R. W., 1975. The isolation and characterization of peroxisomes (microbodies) from baker’s yeast, Saccharomyces cerevisiae. Arch. Microbiol. 105: 187–192 [DOI] [PubMed] [Google Scholar]
- Pascual F., Carman G. M., 2012. Phosphatidate phosphatase, a key regulator of lipid homeostasis. Biochim. Biophys. Acta (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perktold A., Zechmann B., Daum G., Zellnig G., 2007. Organelle association visualized by three-dimensional ultrastructural imaging of the yeast cell. FEMS Yeast Res. 7: 629–638 [DOI] [PubMed] [Google Scholar]
- Perry R. J., Mast F. D., Rachubinski R. A., 2009. Endoplasmic reticulum-associated secretory proteins Sec20p, Sec39p, and Dsl1p are involved in peroxisome biogenesis. Eukaryot. Cell 8: 830–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterfy M., Phan J., Xu P., Reue K., 2001. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat. Genet. 27: 121–124 [DOI] [PubMed] [Google Scholar]
- Petschnigg J., Wolinski H., Kolb D., Zelling G., Kurat C. F., et al. , 2009. Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast. J. Biol. Chem. 284: 30981–30993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires J. R., Hong X., Brockmann C., Volkmer-Engert R., Schneider-Mergener J., et al. , 2003. The ScPex13p SH3 domain exposes two distinct binding sites for Pex5p and Pex14p. J. Mol. Biol. 326: 1427–1435 [DOI] [PubMed] [Google Scholar]
- Platta H. W., El Magraoui F., Baumer B. E., Schlee D., Girzalsky W., et al. , 2009. Pex2 and pex12 function as protein-ubiquitin ligases in peroxisomal protein import. Mol. Cell. Biol. 29: 5505–5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y., Antonenkov V. D., Glumoff T., Hiltunen J. K., 2006. Peroxisomal beta-oxidation: a metabolic pathway with multiple functions. Biochim. Biophys. Acta 1763: 1413–1426 [DOI] [PubMed] [Google Scholar]
- Prein B., Natter K., Kohlwein S. D., 2000. A novel strategy for constructing N-terminal chromosomal fusions to green fluorescent protein in the yeast Saccharomyces cerevisiae. FEBS Lett. 485: 29–34 [DOI] [PubMed] [Google Scholar]
- Ptacek J., Devgan G., Michaud G., Zhu H., Zhu X., et al. , 2005. Global analysis of protein phosphorylation in yeast. Nature 438: 679–684 [DOI] [PubMed] [Google Scholar]
- Pu J., Ha C. W., Zhang S., Jung J. P., Huh W. K., et al. , 2011. Interactomic study on interaction between lipid droplets and mitochondria. Protein Cell 2: 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S., Daum G., 2010a. Janus-faced enzymes yeast Tgl3p and Tgl5p catalyze lipase and acyltransferase reactions. Mol. Biol. Cell 21: 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S., Daum G., 2010b. Multiple functions as lipase, steryl ester hydrolase, phospholipase, and acyltransferase of Tgl4p from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 285: 15769–15776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S., Grillitsch K., Daum G., 2008. Synthesis and turnover of non-polar lipids in yeast. Prog. Lipid Res. 47: 157–171 [DOI] [PubMed] [Google Scholar]
- Rajakumari S., Rajasekharan R., Daum G., 2010. Triacylglycerol lipolysis is linked to sphingolipid and phospholipid metabolism of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1801: 1314–1322 [DOI] [PubMed] [Google Scholar]
- Rape M., Hoppe T., Gorr I., Kalocay M., Richly H., et al. , 2001. Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin-selective chaperone. Cell 107: 667–677 [DOI] [PubMed] [Google Scholar]
- Raychaudhuri S., Young B. P., Espenshade P. J., Loewen C. J., 2012. Regulation of lipid metabolism: a tale of two yeasts. Curr. Opin. Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reue K., Zhang P., 2008. The lipin protein family: dual roles in lipid biosynthesis and gene expression. FEBS Lett. 582: 90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodin J. A. G., 1954. Correlation of Ultrastructural Organization and Function in Normal and Experimentally Changed Proximal Convoluted Tubule Cells of the Mouse Kidney. Doctoral Thesis, Karolinska Institutet, Stockholm [Google Scholar]
- Riekhof W. R., Wu J., Jones J. L., Voelker D. R., 2007. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 282: 28344–28352 [DOI] [PubMed] [Google Scholar]
- Rockenfeller P., Ring J., Muschett V., Beranek A., Buettner S., et al. , 2010. Fatty acids trigger mitochondrion-dependent necrosis. Cell Cycle 9: 2836–2842 [DOI] [PubMed] [Google Scholar]
- Roggenkamp R., Numa S., Schweizer E., 1980. Fatty acid-requiring mutant of Saccharomyces cerevisiae defective in acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. USA 77: 1814–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottensteiner H., Stein K., Sonnenhol E., Erdmann R., 2003. Conserved function of pex11p and the novel pex25p and pex27p in peroxisome biogenesis. Mol. Biol. Cell 14: 4316–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Oku M., van der Klei I. J., Kiel J. A., 2006. Pexophagy: autophagic degradation of peroxisomes. Biochim. Biophys. Acta 1763: 1767–1775 [DOI] [PubMed] [Google Scholar]
- Saleem R. A., Knoblach B., Mast F. D., Smith J. J., Boyle J., et al. , 2008. Genome-wide analysis of signaling networks regulating fatty acid-induced gene expression and organelle biogenesis. J. Cell Biol. 181: 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem R. A., Long-O’Donnell R., Dilworth D. J., Armstrong A. M., Jamakhandi A. P., et al. , 2010. Genome-wide analysis of effectors of peroxisome biogenesis. PLoS ONE 5: e11953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons F. A., Kiel J. A., Faber K. N., Veenhuis M., van der Klei I. J., 2000. Overproduction of Pex5p stimulates import of alcohol oxidase and dihydroxyacetone synthase in a Hansenula polymorpha Pex14 null mutant. J. Biol. Chem. 275: 12603–12611 [DOI] [PubMed] [Google Scholar]
- Sandager L., Gustavsson M. H., Ståhl U., Dahlqvist A., Wiberg E., et al. , 2002. Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 277: 6478–6482 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H., Leung J., Grimsey N., Peak-Chew S., Siniossoglou S., 2005. The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24: 1931–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraya R., Cepinska M. N., Kiel J. A., Veenhuis M., van der Klei I. J., 2010. A conserved function for Inp2 in peroxisome inheritance. Biochim. Biophys. Acta 1803: 617–622 [DOI] [PubMed] [Google Scholar]
- Saraya R., Krikken A. M., Veenhuis M., van der Klei I. J., 2011. Peroxisome reintroduction in Hansenula polymorpha requires Pex25 and Rho1. J. Cell Biol. 193: 885–900 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schafer A., Kerssen D., Veenhuis M., Kunau W. H., Schliebs W., 2004. Functional similarity between the peroxisomal PTS2 receptor binding protein Pex18p and the N-terminal half of the PTS1 receptor Pex5p. Mol. Cell. Biol. 24: 8895–8906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer H., Nau K., Sickmann A., Erdmann R., Meyer H. E., 2001. Identification of peroxisomal membrane proteins of Saccharomyces cerevisiae by mass spectrometry. Electrophoresis 22: 2955–2968 [DOI] [PubMed] [Google Scholar]
- Schaffer J. E., 2003. Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 14: 281–287 [DOI] [PubMed] [Google Scholar]
- Scharnewski M., Pongdontri P., Mora G., Hoppert M., Fulda M., 2008. Mutants of Saccharomyces cerevisiae deficient in acyl-CoA synthetases secrete fatty acids due to interrupted fatty acid recycling. FEBS J. 275: 2765–2778 [DOI] [PubMed] [Google Scholar]
- Schell-Steven A., Stein K., Amoros M., Landgraf C., Volkmer-Engert R., et al. , 2005. Identification of a novel, intraperoxisomal pex14-binding site in pex13: association of pex13 with the docking complex is essential for peroxisomal matrix protein import. Mol. Cell. Biol. 25: 3007–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliebs W., Kunau W. H., 2004. Peroxisome membrane biogenesis: the stage is set. Curr. Biol. 14: R397–R399 [DOI] [PubMed] [Google Scholar]
- Schliebs W., Kunau W. H., 2006. PTS2 co-receptors: diverse proteins with common features. Biochim. Biophys. Acta 1763: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Schliebs W., Girzalsky W., Erdmann R., 2010. Peroxisomal protein import and ERAD: variations on a common theme. Nat. Rev. Mol. Cell Biol. 11: 885–890 [DOI] [PubMed] [Google Scholar]
- Schneiter R., Brugger B., Sandhoff R., Zellnig G., Leber A., et al. , 1999. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 146: 741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueller N., Holton S. J., Fodor K., Milewski M., Konarev P., et al. , 2010. The peroxisomal receptor Pex19p forms a helical mPTS recognition domain. EMBO J. 29: 2491–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M., Lass A., Zimmermann R., Eichmann T. O., Zechner R., 2009. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am. J. Physiol. Endocrinol. Metab. 297: E289–E296 [DOI] [PubMed] [Google Scholar]
- Sere Y. Y., Regnacq M., Colas J., Berges T., 2010. A Saccharomyces cerevisiae strain unable to store neutral lipids is tolerant to oxidative stress induced by alpha-synuclein. Free Radic. Biol. Med. 49: 1755–1764 [DOI] [PubMed] [Google Scholar]
- Shimozawa N., Tsukamoto T., Suzuki Y., Orii T., Shirayoshi Y., et al. , 1992. A human gene responsible for Zellweger syndrome that affects peroxisome assembly. Science 255: 1132–1134 [DOI] [PubMed] [Google Scholar]
- Shiozawa K., Konarev P. V., Neufeld C., Wilmanns M., Svergun D. I., 2009. Solution structure of human Pex5.Pex14.PTS1 protein complexes obtained by small angle X-ray scattering. J. Biol. Chem. 284: 25334–25342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou S., Santos-Rosa H., Rappsilber J., Mann M., Hurt E., 1998. A novel complex of membrane proteins required for formation of a spherical nucleus. EMBO J. 17: 6449–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoneczny M., Chelstowska A., Rytka J., 1988. Study of the coinduction by fatty acids of catalase A and acyl-CoA oxidase in standard and mutant Saccharomyces cerevisiae strains. Eur. J. Biochem. 174: 297–302 [DOI] [PubMed] [Google Scholar]
- Smith J. J., Marelli M., Christmas R. H., Vizeacoumar F. J., Dilworth D. J., et al. , 2002. Transcriptome profiling to identify genes involved in peroxisome assembly and function. J. Cell Biol. 158: 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger D., Daum G., 2002. Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J. Bacteriol. 184: 519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger D., Daum G., 2003. Triacylglycerol biosynthesis in yeast. Appl. Microbiol. Biotechnol. 61: 289–299 [DOI] [PubMed] [Google Scholar]
- Sorger D., Athenstaedt K., Hrastnik C., Daum G., 2004. A yeast strain lacking lipid particles bears a defect in ergosterol formation. J. Biol. Chem. 279: 31190–31196 [DOI] [PubMed] [Google Scholar]
- South S. T., Baumgart E., Gould S. J., 2001. Inactivation of the endoplasmic reticulum protein translocation factor, Sec61p, or its homolog, Ssh1p, does not affect peroxisome biogenesis. Proc. Natl. Acad. Sci. USA 98: 12027–12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandl J., White D. J., Peychl J., Thiele C., 2009. Live cell multicolor imaging of lipid droplets with a new dye, LD540. Traffic 10: 1579–1584 [DOI] [PubMed] [Google Scholar]
- Spanova M., Czabany T., Zellnig G. N., Leitner E., Hapala I., et al. , 2010. Effect of lipid particle biogenesis on the subcellular distribution of squalene in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 285: 6127–6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanova M., Zweytick D., Lohner K., Klug L., Leitner E., et al. , 2012. Influence of squalene on lipid particle/droplet and membrane organization in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1821: 647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley W. A., Filipp F. V., Kursula P., Schuller N., Erdmann R., et al. , 2006. Recognition of a functional peroxisome type 1 target by the dynamic import receptor pex5p. Mol. Cell 24: 653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley W. A., Fodor K., Marti-Renom M. A., Schliebs W., Wilmanns M., 2007. Protein translocation into peroxisomes by ring-shaped import receptors. FEBS Lett. 581: 4795–4802 [DOI] [PubMed] [Google Scholar]
- Stein K., Schell-Steven A., Erdmann R., Rottensteiner H., 2002. Interactions of Pex7p and Pex18p/Pex21p with the peroxisomal docking machinery: implications for the first steps in PTS2 protein import. Mol. Cell. Biol. 22: 6056–6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S. J., Dodt G., Raymond G. V., Braverman N. E., Moser A. B., et al. , 2006. Peroxisome biogenesis disorders. Biochim. Biophys. Acta 1763: 1733–1748 [DOI] [PubMed] [Google Scholar]
- Stevens P., Monastyrska I., Leao-Helder A. N., van der Klei I. J., Veenhuis M., et al. , 2005. Hansenula polymorpha Vam7p is required for macropexophagy. FEMS Yeast Res. 5: 985–997 [DOI] [PubMed] [Google Scholar]
- Strating J. R., Hafmans T. G., Martens G. J., 2009. Functional diversity among p24 subfamily members. Biol. Cell 101: 207–219 [DOI] [PubMed] [Google Scholar]
- Stukey J. E., McDonough V. M., Martin C. E., 1989. Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J. Biol. Chem. 264: 16537–16544 [PubMed] [Google Scholar]
- Stukey J. E., McDonough V. M., Martin C. E., 1990. The OLE1 gene of Saccharomyces cerevisiae encodes the delta 9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J. Biol. Chem. 265: 20144–20149 [PubMed] [Google Scholar]
- Szymanski K. M., Binns D., Bartz R., Grishin N. V., Li W. P., et al. , 2007. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl. Acad. Sci. USA 104: 20890–20895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., van der Zand A., Braakman I., 2008. Peroxisomes: minted by the ER. Curr. Opin. Cell Biol. 20: 393–400 [DOI] [PubMed] [Google Scholar]
- Tam Y. Y., Rachubinski R. A., 2002. Yarrowia lipolytica cells mutant for the PEX24 gene encoding a peroxisomal membrane peroxin mislocalize peroxisomal proteins and accumulate membrane structures containing both peroxisomal matrix and membrane proteins. Mol. Biol. Cell 13: 2681–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam Y. Y., Torres-Guzman J. C., Vizeacoumar F. J., Smith J. J., Marelli M., et al. , 2003. Pex11-related proteins in peroxisome dynamics: a role for the novel peroxin Pex27p in controlling peroxisome size and number in Saccharomyces cerevisiae. Mol. Biol. Cell 14: 4089–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam Y. Y., Fagarasanu A., Fagarasanu M., Rachubinski R. A., 2005. Pex3p initiates the formation of a preperoxisomal compartment from a subdomain of the endoplasmic reticulum in Saccharomyces cerevisiae. J. Biol. Chem. 280: 34933–34939 [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Kaplan C. P., Gao X., Baker A., 1996. Localization and targeting of isocitrate lyases in Saccharomyces cerevisiae. Biochem. J. 319(Pt. 1): 255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehlivets O., 2011. Homocysteine as a risk factor for atherosclerosis: Is its conversion to s-adenosyl-L-homocysteine the key to deregulated lipid metabolism? J. Lipids 2011: 702853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehlivets O., Hasslacher M., Kohlwein S. D., 2004. S-adenosyl-L-homocysteine hydrolase in yeast: key enzyme of methylation metabolism and coordinated regulation with phospholipid synthesis. FEBS Lett. 577: 501–506 [DOI] [PubMed] [Google Scholar]
- Tehlivets O., Scheuringer K., Kohlwein S. D., 2007. Fatty acid synthesis and elongation in yeast. Biochim. Biophys. Acta 1771: 255–270 [DOI] [PubMed] [Google Scholar]
- Thoms S., Debelyy M. O., Nau K., Meyer H. E., Erdmann R., 2008. Lpx1p is a peroxisomal lipase required for normal peroxisome morphology. FEBS J. 275: 504–514 [DOI] [PubMed] [Google Scholar]
- Titorenko V. I., Terlecky S. R., 2011. Peroxisome metabolism and cellular aging. Traffic 12: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titorenko V. I., Smith J. J., Szilard R. K., Rachubinski R. A., 1998. Pex20p of the yeast Yarrowia lipolytica is required for the oligomerization of thiolase in the cytosol and for its targeting to the peroxisome. J. Cell Biol. 142: 403–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titorenko V. I., Chan H., Rachubinski R. A., 2000. Fusion of small peroxisomal vesicles in vitro reconstructs an early step in the in vivo multistep peroxisome assembly pathway of Yarrowia lipolytica. J. Cell Biol. 148(1): 29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Essner E., 1981. Microbodies: peroxisomes and glyoxysomes. J. Cell Biol. 91: 271s–283s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower R. J., Fagarasanu A., Aitchison J. D., Rachubinski R. A., 2011. The peroxin Pex34p functions with the Pex11 family of peroxisomal divisional proteins to regulate the peroxisome population in yeast. Mol. Biol. Cell 22: 1727–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova N. G., Klass D. M., Salzman J., Brown P. O., 2010. Proteome-wide search reveals unexpected RNA-binding proteins in Saccharomyces cerevisiae. PLoS ONE 5: pii: e12671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., et al. , 2003. Targets of the cyclin-dependent kinase Cdk1. Nature 425: 859–864 [DOI] [PubMed] [Google Scholar]
- Urquhart A. J., Kennedy D., Gould S. J., Crane D. I., 2000. Interaction of Pex5p, the type 1 peroxisome targeting signal receptor, with the peroxisomal membrane proteins Pex14p and Pex13p. J. Biol. Chem. 275: 4127–4136 [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Veenhuis M., 2006a. PTS1-independent sorting of peroxisomal matrix proteins by Pex5p. Biochim. Biophys. Acta 1763: 1794–1800 [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Veenhuis M., 2006b. Yeast and filamentous fungi as model organisms in microbody research. Biochim. Biophys. Acta 1763: 1364–1373 [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Harder W., Veenhuis M., 1991. Methanol metabolism in a peroxisome-deficient mutant of Hansenula polymorpha: a physiological study. Arch. Microbiol. 156: 15–23 [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Hilbrands R. E., Swaving G. J., Waterham H. R., Vrieling E. G., et al. , 1995. The Hansenula polymorpha PER3 gene is essential for the import of PTS1 proteins into the peroxisomal matrix. J. Biol. Chem. 270: 17229–17236 [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Hilbrands R. E., Kiel J. A., Rasmussen S. W., Cregg J. M., et al. , 1998. The ubiquitin-conjugating enzyme Pex4p of Hansenula polymorpha is required for efficient functioning of the PTS1 import machinery. EMBO J. 17: 3608–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zand A., Braakman I., Tabak H. F., 2010. Peroxisomal membrane proteins insert into the endoplasmic reticulum. Mol. Biol. Cell 21: 2057–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zand A., Gent J., Braakman I., Tabak H. F., 2012. Biochemically distinct vesicles from the endoplasmic reticulum fuse to form peroxisomes. Cell 13(149): 397–409 [DOI] [PubMed] [Google Scholar]
- van Roermund C. W., Waterham H. R., Ijlst L., Wanders R. J., 2003. Fatty acid metabolism in Saccharomyces cerevisiae. Cell. Mol. Life Sci. 60: 1838–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zutphen T., Baerends R. J., Susanna K. A., de Jong A., Kuipers O. P., et al. , 2010. Adaptation of Hansenula polymorpha to methanol: a transcriptome analysis. BMC Genomics 11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhuis M., Mateblowski M., Kunau W. H., Harder W., 1987. Proliferation of microbodies in Saccharomyces cerevisiae. Yeast 3: 77–84 [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Sulter G., van der Klei I., Harder W., 1989. Evidence for functional heterogeneity among microbodies in yeasts. Arch. Microbiol. 151: 105–110 [DOI] [PubMed] [Google Scholar]
- Vizeacoumar F. J., Torres-Guzman J. C., Tam Y. Y., Aitchison J. D., Rachubinski R. A., 2003. YHR150w and YDR479c encode peroxisomal integral membrane proteins involved in the regulation of peroxisome number, size, and distribution in Saccharomyces cerevisiae. J. Cell Biol. 161: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizeacoumar F. J., Torres-Guzman J. C., Bouard D., Aitchison J. D., Rachubinski R. A., 2004. Pex30p, Pex31p, and Pex32p form a family of peroxisomal integral membrane proteins regulating peroxisome size and number in Saccharomyces cerevisiae. Mol. Biol. Cell 15: 665–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A., Grillitsch K., Leitner E., Daum G., 2009. Mobilization of steryl esters from lipid particles of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1791: 118–124 [DOI] [PubMed] [Google Scholar]
- Walther T. C., Farese R. V., Jr, 2009. The life of lipid droplets. Biochim. Biophys. Acta 1791: 459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther T. C., Farese R. V., Jr, 2012. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 81: 687–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton P. A., Hill P. E., Subramani S., 1995. Import of stably folded proteins into peroxisomes. Mol. Biol. Cell 6: 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., Waterham H. R., 2006. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 75: 295–332 [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Ferdinandusse S., Brites P., Kemp S., 2010. Peroxisomes, lipid metabolism and lipotoxicity. Biochim. Biophys. Acta 1801: 272–280 [DOI] [PubMed] [Google Scholar]
- Wang D., Visser N. V., Veenhuis M., van der Klei I. J., 2003. Physical interactions of the peroxisomal targeting signal 1 receptor pex5p, studied by fluorescence correlation spectroscopy. J. Biol. Chem. 278: 43340–43345 [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Lu J. F., Steinberg S. J., Gould S. J., Smith K. D., et al. , 1998. Disruption of the Saccharomyces cerevisiae FAT1 gene decreases very long-chain fatty acyl-CoA synthetase activity and elevates intracellular very long-chain fatty acid concentrations. J. Biol. Chem. 273: 18210–18219 [DOI] [PubMed] [Google Scholar]
- Wiebel F. F., Kunau W. H., 1992. The Pas2 protein essential for peroxisome biogenesis is related to ubiquitin-conjugating enzymes. Nature 359: 73–76 [DOI] [PubMed] [Google Scholar]
- Williams C., Distel B., 2006. Pex13p: Docking or cargo handling protein? Biochim. Biophys. Acta 1763: 1585–1591 [DOI] [PubMed] [Google Scholar]
- Williams C., van den Berg M., Distel B., 2005. Saccharomyces cerevisiae Pex14p contains two independent Pex5p binding sites, which are both essential for PTS1 protein import. FEBS Lett. 579: 3416–3420 [DOI] [PubMed] [Google Scholar]
- Williams C., van den Berg M., Geers E., Distel B., 2008. Pex10p functions as an E3 ligase for the Ubc4p-dependent ubiquitination of Pex5p. Biochem. Biophys. Res. Commun. 374: 620–624 [DOI] [PubMed] [Google Scholar]
- Wolinski H., Kohlwein S. D., 2008. Microscopic analysis of lipid droplet metabolism and dynamics in yeast. Methods Mol. Biol. 457: 151–163 [DOI] [PubMed] [Google Scholar]
- Wolinski H., Natter K., Kohlwein S. D., 2009a. The fidgety yeast: focus on high-resolution live yeast cell microscopy. Methods Mol. Biol. 548: 75–99 [DOI] [PubMed] [Google Scholar]
- Wolinski H., Petrovic U., Mattiazzi M., Petschnigg J., Heise B., et al. , 2009b. Imaging-based live cell yeast screen identifies novel factors involved in peroxisome assembly. J. Proteome Res. 8: 20–27 [DOI] [PubMed] [Google Scholar]
- Wolinski H., Kolb D., Hermann S., Koning R. I., Kohlwein S. D., 2011. A role for seipin in lipid droplet dynamics and inheritance in yeast. J. Cell Sci. 124: 3894–3904 [DOI] [PubMed] [Google Scholar]
- Wolinski H., Bredies K., Kohlwein S. D., 2012. Quantitative imaging of lipid metabolism in yeast: from 4D analysis to high content screens of mutant libraries. Methods Cell Biol. 108: 345–365 [DOI] [PubMed] [Google Scholar]
- Woods A., Munday M. R., Scott J., Yang X., Carlson M., et al. , 1994. Yeast SNF1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J. Biol. Chem. 269: 19509–19515 [PubMed] [Google Scholar]
- Yan M., Rachubinski D. A., Joshi S., Rachubinski R. A., Subramani S., 2008. Dysferlin domain-containing proteins, Pex30p and Pex31p, localized to two compartments, control the number and size of oleate-induced peroxisomes in Pichia pastoris. Mol. Biol. Cell 19: 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Bard M., Bruner D. A., Gleeson A., Deckelbaum R. J., et al. , 1996. Sterol esterification in yeast: a two-gene process. Science 272: 1353–1356 [DOI] [PubMed] [Google Scholar]
- Yang L., Ding Y., Chen Y., Zhang S., Huo C., et al. , 2012. The proteomics of lipid droplets: structure, dynamics, and functions of the organelle conserved from bacteria to humans. J. Lipid Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi E. C., Marelli M., Lee H., Purvine S. O., Aebersold R., et al. , 2002. Approaching complete peroxisome characterization by gas-phase fractionation. Electrophoresis 23: 3205–3216 [DOI] [PubMed] [Google Scholar]
- Yu C., Kennedy N. J., Chang C. C., Rothblatt J. A., 1996. Molecular cloning and characterization of two isoforms of Saccharomyces cerevisiae acyl-CoA:sterol acyltransferase. J. Biol. Chem. 271: 24157–24163 [DOI] [PubMed] [Google Scholar]
- Yu H., Braun P., Yildirim M. A., Lemmens I., Venkatesan K., et al. , 2008. High-quality binary protein interaction map of the yeast interactome network. Science 322: 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman S., Lippman S. I., Zhao X., Broach J. R., 2008. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 42: 27–81 [DOI] [PubMed] [Google Scholar]
- Zanghellini J., Natter K., Jungreuthmayer C., Thalhammer A., Kurat C. F., et al. , 2008. Quantitative modeling of triacylglycerol homeostasis in yeast: metabolic requirement for lipolysis to promote membrane lipid synthesis and cellular growth. FEBS J. 275: 5552–5563 [DOI] [PubMed] [Google Scholar]
- Zanghellini J., Ruckerbauer D., Wodlei F., von Grünberg H. H., Jungreuthmayer C., 2010a. Phospholipid demixing: molecular interpretation of lipid droplet biogenesis, pp. 1–28 in Advances in Planar Lipid Bilayers and Liposomes Elsevier: Amsterdam; New York
- Zanghellini J., Wodlei F., von Grünberg H. H., 2010b. Phospholipid demixing and the birth of a lipid droplet. J. Theor. Biol. 264: 952–961 [DOI] [PubMed] [Google Scholar]
- Zaremberg V., McMaster C. R., 2002. Differential partitioning of lipids metabolized by separate yeast glycerol-3-phosphate acyltransferases reveals that phospholipase D generation of phosphatidic acid mediates sensitivity to choline-containing lysolipids and drugs. J. Biol. Chem. 277: 39035–39044 [DOI] [PubMed] [Google Scholar]
- Zechner R., Zimmermann R., Eichmann T. O., Kohlwein S. D., Haemmerle G., et al. , 2012. FAT SIGNALS: lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15: 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehmer J. K., Bartz R., Liu P., Anderson R. G., 2008. Identification of a novel N-terminal hydrophobic sequence that targets proteins to lipid droplets. J. Cell Sci. 121: 1852–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Zou J., 2001. The initial step of the glycerolipid pathway: identification of glycerol 3-phosphate/dihydroxyacetone phosphate dual substrate acyltransferases in Saccharomyces cerevisiae. J. Biol. Chem. 276: 41710–41716 [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., et al. , 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306: 1383–1386 [DOI] [PubMed] [Google Scholar]
- Zinser E., Sperka-Gottlieb C. D., Fasch E. V., Kohlwein S. D., Paltauf F., et al. , 1991. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol. 173: 2026–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser E., Paltauf F., Daum G., 1993. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 175: 2853–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutphen T., Veenhuis M., van der Klei I. J., 2008. Pex14 is the sole component of the peroxisomal translocon that is required for pexophagy. Autophagy 4: 63–66 [DOI] [PubMed] [Google Scholar]
- Zwart K., Veenhuis M., Harder W., 1979. Biogenesis and breakdown of peroxisomes in the yeast Hansenula polymorpha in relation to environmental changes. Antonie van Leeuwenhoek 45: 331–332 [DOI] [PubMed] [Google Scholar]
- Zweytick D., Athenstaedt K., Daum G., 2000a. Intracellular lipid particles of eukaryotic cells. Biochim. Biophys. Acta 1469: 101–120 [DOI] [PubMed] [Google Scholar]
- Zweytick D., Leitner E., Kohlwein S. D., Yu C., Rothblatt J., et al. , 2000b. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 267: 1075–1082 [DOI] [PubMed] [Google Scholar]