Abstract
To investigate the regulation of Drosophila melanogaster behavior by biogenic amines, we have exploited the broad requirement of the vesicular monoamine transporter (VMAT) for the vesicular storage and exocytotic release of all monoamine neurotransmitters. We used the Drosophila VMAT (dVMAT) null mutant to globally ablate exocytotic amine release and then restored DVMAT activity in either individual or multiple aminergic systems, using transgenic rescue techniques. We find that larval survival, larval locomotion, and female fertility rely predominantly on octopaminergic circuits with little apparent input from the vesicular release of serotonin or dopamine. In contrast, male courtship and fertility can be rescued by expressing DVMAT in octopaminergic or dopaminergic neurons, suggesting potentially redundant circuits. Rescue of major aspects of adult locomotion and startle behavior required octopamine, but a complementary role was observed for serotonin. Interestingly, adult circadian behavior could not be rescued by expression of DVMAT in a single subtype of aminergic neurons, but required at least two systems, suggesting the possibility of unexpected cooperative interactions. Further experiments using this model will help determine how multiple aminergic systems may contribute to the regulation of other behaviors. Our data also highlight potential differences between behaviors regulated by standard exocytotic release and those regulated by other mechanisms.
Keywords: octopamine (OA), dopamine (DA), serotonin, courtship, circadian
BOTH invertebrate and mammalian behaviors undergo extensive regulation by monoamine neurotransmitters (reviewed in Joshua et al. 2009; Sara 2009; Barron et al. 2010; Bromberg-Martin et al. 2010; Schultz 2010; Shohamy and Adcock 2010; Haenisch and Bönisch 2011). These include serotonin (5-HT), dopamine (DA), noradrenaline (NE), adrenaline, and histamine in mammals (Southwick et al. 2005; Sara 2009; Daubert and Condron 2010; Schultz 2010; Shohamy and Adcock 2010; Barnes et al. 2011; Haenisch and Bönisch 2011) and 5-HT, DA, histamine, octopamine (OA), and tyramine (TA) in Drosophila as well as other insects (Monastirioti et al. 1996; Blenau and Baumann 2001; Cole et al. 2005; Roeder 2005; Farooqui 2007; Hardie et al. 2007; Barron et al. 2010; Daubert and Condron 2010; Waddell 2010). The function of individual aminergic systems has been studied with both genetic and pharmacologic methods, yet the contribution of each system and the manner in which they may interact to regulate complex behaviors remain unclear. Understanding these interactions will be critical to determine not only the molecular mechanisms by which amines regulate behavior, but also the contribution of each system to the therapeutic effects of antidepressants, antipsychotics, and other psychotropic drugs (Sora et al. 2001; Wilens 2006; Meltzer et al. 2008; Mailman and Murthy 2010; Haenisch and Bönisch 2011).
Studies in mammals illustrate the complex relationship between central aminergic circuits (Mabry and Campbell 1973; Guiard et al. 2008; Leggio et al. 2009; Sesack and Grace 2010). These include the regulation of DA release by 5-HT (Di Matteo et al. 2001; Alex and Pehek 2007; Abdallah et al. 2009), as well as the convergence of 5-HT and DA onto some of the same downstream outputs (Gervais et al. 1999; Boureau and Dayan 2010). However, studies of possible interactions between aminergic systems in mammals are limited by technical constraints usually associated with combining multiple knockout alleles. Most behavioral-genetic models therefore study the effects of inhibiting one or at most two aminergic systems. This approach can limit the analysis of potential interactions: since each of these regulatory systems may be either redundant or replaceable by other aminergic systems, knocking out one component may not alter behavioral phenotypes of interest. For example, the combined contributions of 5-HT, noradrenaline, and DA to the behavioral effects of cocaine remained unclear until double and triple knockouts of the 5-HT, DA, and noradrenaline transporter genes were generated (Mössner et al. 2006).
Behavior in model systems such as Caenorhabditis elegans (Duerr et al. 1999; Sawin et al. 2000; Sanyal et al. 2004; Chase and Koelle 2007; Suo et al. 2009) and Drosophila is also dependent on multiple aminergic regulatory systems (Monastirioti et al. 1996; Blenau and Baumann 2001; Cole et al. 2005; Roeder 2005; Farooqui 2007; Hardie et al. 2007; Barron et al. 2010; Daubert and Condron 2010; Waddell 2010). In Drosophila, molecular-genetic and pharmacologic analyses have demonstrated the importance of DA in sleep, arousal, light perception, circadian entrainment, feeding, and aversive conditioning (Schwaerzel et al. 2003; Andretic et al. 2005; Kume et al. 2005; Neckameyer and Weinstein 2005; Chang et al. 2006; Draper et al. 2007; Bayersdorfer et al. 2010; Hirsh et al. 2010; Riemensperger et al. 2011); of 5-HT in aggression, place memory, circadian rhythms, and sleep (Yuan et al. 2005, 2006; Sitaraman et al. 2008; Alekseyenko et al. 2010); and of OA in sleep, locomotion, female fertility, aggression, place memory, and the fly’s response to environmental stressors (Hirashima et al. 2000; Lee et al. 2003; Fox et al. 2006; Hardie et al. 2007; Crocker and Sehgal 2008; Zhou et al. 2008; Crocker et al. 2010; Sitaraman et al. 2010). However, as in mammals, the relative importance of each aminergic system for particular behaviors and their potential interplay remain unclear.
To explore the function and interaction of aminergic systems in Drosophila, we have taken advantage of the requirement for the vesicular monoamine transporter (VMAT) in the vesicular storage and exocytotic release of all biogenic amines within independent subsets of aminergic neurons (Liu and Edwards 1997; Duerr et al. 1999; Greer et al. 2005). We have previously shown that flies express a single VMAT gene (dVMAT) in 5-HT, DA, and OA neurons and that mutation of dVMAT causes multiple behavioral deficits (Simon et al. 2009). The requirement of DVMAT for normal exocytotic transmitter release in all central aminergic neurons—rather than a single subset—presents a unique opportunity for ablating normal synaptic transmission in all aminergic systems simultaneously and then adding back each one in isolation or in combination. Thus, starting with a loss-of-function dVMAT mutant background, we have systematically added back, or genetically rescued, DVMAT function and vesicular release in specific subsets of aminergic neurons.
Our approach differs from previous studies using single mutations, as well as from those in which toxins or dominant transgenes have been used to silence individual transmitter systems or circuits, and circumvents some important limitations. First, ablating the function of one modulatory system may not reveal deficits if redundant or complementary regulatory circuits compensate for the loss. Second, although genetic ablation studies can determine how loss of particular systems or circuits will affect behavior, they do not describe the minimal circuitry that is both necessary and sufficient to drive any given behavior. Conversely, our approach allows us to determine whether a single system is sufficient to drive a particular behavior, since we start from a baseline of no exocytotic release in any aminergic system and then add back individual amines. Third, unlike some other probes of aminergic processes (Neckameyer and Quinn 1989), dVMAT is not expressed in nonneuronal tissues such as those required for cuticle hardening and pigmentation (Simon et al. 2009). This restricted pattern of expression renders DVMAT particularly useful for studying neuronal processes in the absence of potentially confounding nonneuronal effects (Colas et al. 1999; True 2003; Hsouna et al. 2007). Fourth, mutation of VMAT specifically blocks release of amines while preserving release of peptide neurotransmitters, a potential confound for other techniques that inhibit the exocytotic machinery. Finally, the mutation of dVMAT rather than biosynthetic enzymes also confines the signaling deficit to normal exocytotic release as opposed to other types of release such as efflux.
Our data indicate that restoration of DVMAT in cells that release OA and/or TA is sufficient to rescue larval locomotion, female fertility, and the decreased viability of dVMAT mutants. The findings are consistent with previous studies demonstrating the widespread importance of OA in multiple aspects of invertebrate behavior (Bicker 1999; Roeder 1999; Scheiner et al. 2006; Chase and Koelle 2007; Verlinden et al. 2010). Both DA and OA can individually rescue male courtship and male fertility, suggesting potentially redundant roles for these systems in stimulating male sexual behavior. Individual aminergic systems are able to rescue distinct components of the dVMAT mutant startle response, consistent with the notion that they serve complementary roles. In contrast, only combinations of aminergic systems are able to detectably rescue the defects we observe in circadian behavior, suggesting unexpected cooperative interactions. These data may be generally relevant to the mechanisms by which aminergic systems interact to modulate other complex behaviors and the response to psychotropic drugs.
Materials and Methods
Drosophila husbandry
For larval locomotion assays, the w1118 mutant outcrossed into Canton-S (CS) for 10 generations (w1118CS10) was used as the wild-type (WT) control, and for rhythmicity assays, the w mutant was used as the WT control. For all other experiments WT controls were Canton-S (CS). The dVMAT null, homozygous for the loss-of-function allele (dVMATP1), and the UAS-DVMAT transgene have been previously described (Oh et al. 2003; Chang et al. 2006; Romero-Calderon et al. 2008; Simon et al. 2009). Note that the UAS-DVMAT transgene used here encodes the neuronal isoform of DVMAT (DVMAT-A); a distinct RNA splice variant of dVMAT (DVMAT-B) is expressed only in a small subset of glia in the visual system and is unlikely to be relevant to the behaviors discussed here (Greer et al. 2005; Romero-Calderon et al. 2008). Gal4 driver lines include those previously shown to drive expression in serotonergic (TrH-Gal4) (Park et al. 2006), dopaminergic (TH-Gal4) (Friggi-Grelin et al. 2003), tyraminergic and octopaminergic (Tdc2-Gal4) (Cole et al. 2005), and both serotonergic and dopaminergic (Ddc-Gal4) (Li et al. 2000) neurons. To reduce the effects of genetic background on behavior, all drivers, as well as the dVMATP1 allele and the UAS-DVMAT transgene, were outcrossed for 5 generations into wild-type background (w1118CS10). The outcrossed transgenes were introduced into the dVMATP1 mutant background to generate the following genotypes, used for the transgenic rescue experiments described in the text: (1) “ubiq-5HT,” w; dVMATP1; daughterless-Gal4, UAS-DVMAT / +; (2) “Ddc,” w; dVMATP1, DDC-Gal4; UAS-DVMAT; (3) “Tdc,” w; dVMATP1, Tdc2-Gal4; UAS-DVMAT; (4) “TH,” w; dVMATP1; TH-Gal4, UAS-DVMAT; (5) “TrH,” w; dVMATP1, TrH-Gal4; UAS-DVMAT; (6) “Tdc + TH,” w; dVMATP1 / Tdc2-GAL4, dVMATP1; UAS-DVMAT / TH-Gal4, UAS-DVMAT; (7) “Tdc + TrH,” w; dVMATP1, TrH-Gal4 / dVMATP1, Tdc2-Gal4; UAS-DVMAT; (8) “TH + TrH,” w; dVMATP1 / TrH-GAL4, dVMAP1; UAS-DVMAT / TH-Gal4, UAS-DVMAT; and (9) “UAS,” w; dVMATP1; UAS-DVMAT. All lines were maintained on standard agar-molasses–based fly food made by the University of California, Los Angeles (UCLA) fly facility: 10.7 g/liter agar, 28.6 g/liter yeast, 71 ml/liter molasses, 71 g/liter cornmeal, 5.7 ml/liter propionic acid, and 16 ml/liter of a 10% g/ml solution of methyl-paraben used as an antifungal agent.
Western blots
Western blots were performed as previously described (Chang et al. 2006). Briefly, adult flies (4 days posteclosion) were anesthetized using CO2, and four heads per genotype were homogenized in SDS–PAGE sample buffer. One head equivalent of homogenate from each fly line was loaded onto a polyacrylamide gel, followed by transfer to nitrocellulose. The membrane was incubated with 1:1000 mouse anti-HA (Covance Research Products, Denver, CO) overnight at 4° and then incubated with 1:1000 mouse anti–β-tubulin (Accurate Chemical and Scientific, Westbury, NY) for 1 hr at room temperature. Membranes were incubated in secondary antibody for 1 hr at room temperature, using 1:1000 anti-mouse HRP-conjugated antibodies (Amersham Biosciences, Piscataway, NJ). The protein bands were detected using SuperSignal West Pico Luminol/Peroxide (Thermo Scientific, Rockford, IL) and exposed for 15–60 sec on Kodak (Rochester, NY) Biomax Light Film.
HPLC
HPLC analysis of dopamine and serotonin was performed as previously described (Chang et al. 2006). For all experiments to measure dopamine and serotonin we used four 2- to 3-day-old female heads per sample. Fly heads were manually collected and homogenized in 0.1 M perchloric acid containing 0.1% EDTA, using a glass-on-glass microtissue grinder (Kontes). Insoluble debris was sedimented by centrifugation and the supernatant filtered through a 0.22-μm Millipore (Bedford, MA) MC cartridge. To measure octopamine, brains were dissected from four 2- to 3-day-old females for each sample and homogenized as above. Octopamine was analyzed by HPLC coupled to electrochemical detection (Antec Leyden, Palm Bay, FL; oxidation potential 0.95 V, glassy carbon electrode against Ag/AgCl reference), using a reverse-phase column (SC-5ODS, 150 × 3.0 mm with a 4 × 5-mm AC-ODS precolumn maintained at 25°; Eicom, San Diego, CA) pumped at 0.5 ml/min with mobile phase consisting of 0.1 M citrate-acetate buffer (pH 3.5), 13% methanol (v/v), and 130 mg/liter sodium-1-octane sulfonate.
Viability
To obtain flies for viability assays, 10 males and 10 females heterozygous for the dVMAT mutation were mated for 3 days at ∼23° in bottles containing standard fly food. For all genotypes, the percentage of progeny homozygous for dVMATP1 was compared to the number of homozygous progeny predicted by standard Mendelian ratios. The numbers of F1 dVMAT homozygote and total progeny were summed over 19 days postmating. Bottles containing 500–1000 progeny were scored as standard culture conditions for calculating percentage of survival in Supporting Information, Figure S3F.
Anatomical analysis
To image dopaminergic neurons, WT and dVMAT mutant wandering third-instar larvae were dissected and fixed in 4% paraformaldeheyde and then probed with rabbit anti-DTH (Neckameyer et al. 2000), a gift of W. Neckameyer (St. Louis University School of Medicine), followed by anti-rabbit Alexaflour 488 (Invitrogen Molecular Probes, Eugene, OR). For serotonergic and octopaminergic neuron counts, one copy of UAS-mCD8-GFP and one copy of either TrH-Gal4 or Tdc2-Gal4, respectively, were expressed either in the WT or in the dVMAT mutant background, and the endogenous fluorescent signal of GFP was used to count cell bodies in larval brains from wandering third-instar larvae fixed in 4% paraformaldehyde. All images were obtained using a Zeiss (Thornwood, NY) confocal microscope, using either a 20× Plan-Apochromat (0.75 NA) or a 40×/0.75 EC Plan-Neofluar objective. For some preparations, additional quantification was performed using an AxioSkop Zeiss upright microscope and manually focusing through the entire depth of the cluster.
Larval locomotion
To obtain larvae homozygous for dVMATP1 in the presence or absence of additional rescue transgenes, 20 males and 20 females were mated for 1 day at ∼23° in a 6-cm plastic dish filled with 20 ml of standard molasses fly food. Approximately 1 week later, third-instar larvae were collected. To assay larval locomotion, a single third-instar larva was placed on a 14.5-cm diameter plastic dish filled with standard fly food and allowed to acclimate for 1 min. The lid of the dish was covered with a 5 × 5-mm grid and the number of grid lines crossed by the larva was manually recorded over a period of 5 min. Experiments were performed blindly with respect to genotype. All larvae were then allowed to pupate and eclose as adults: individuals homozygous for the dVMAT mutation were confirmed post hoc by the absence of the CyO chromosome. Only data collected from dVMATP1 homozygotes were used for further analysis.
Neurotransmitter feeding
To make plates for drug administration, molten fly food was mixed with red food dye (Kroger, Cincinnati; 9.6% final concentration) and an aqueous stock solution of neurotransmitter for a final neurotransmitter concentration of 10 mg/ml. Third-instar larvae with colored abdomens were tested using the locomotion assay described above. Larvae were allowed to feed for 4 hr and were tested at the start of feeding, after 2 hr of feeding, and at the end of feeding.
Fertility
To obtain adult flies for fertility assays, 10 males and 10 females heterozygous for the dVMAT mutation were mated for 3 days at ∼25°. Homozygous progeny were sorted under cold anesthesia over ice. To test male fertility, one male candidate (0–5 days old) was mated with 3 WT virgin females in a standard culture vial. To test female fertility, one virgin female candidate (0–4 days old) was mated with 3 Canton-S males in a vial. One to 2 weeks after initial mating, candidates were scored as either fertile or infertile based on whether the vial contained at least one progeny (larva, pupa, or adult). Only vials containing at least one male and at least one female the day after initial mating were scored.
Negative geotaxis
Negative geotaxis assays were performed as described in Simon et al. (2009). Briefly, 2- to 5-day-old males and females were cold anesthetized and allowed to recover for 1 day prior to testing. For each trial, 20 flies were loaded into a choice-test apparatus and the percentage of flies that climbed to the upper tube in 15 sec after tapping the apparatus on the bench top three times was recorded. At least 160 naive flies were tested for each genotype.
Response to startle (puff-o-mat)
Males (2–4 days old) were CO2 anesthetized and allowed to recover for 2 days prior to testing. Flies were reared on a 12-hr day–night cycle at 25°. Temperature for behavioral experiments was maintained at 23°–25°. For each assay, 10 flies were manually loaded into tubes and allowed to acclimate for 10 min prior to filming. Activity was recorded beginning at 1 min before delivery of the puff stimuli, until 3.5 min after stimulus termination. Each air puff (35 psi) lasted 200 msec with a 5-sec interpuff interval. Movies were analyzed using custom locomotor tracking software (described in Lebestky et al. 2009).
Courtship
To obtain courtship candidates, 20 males and 20 females heterozygous for the dVMAT mutation, or WT controls, were mated in bottles for 3 days at ∼25°. Cold anesthesia was used to collect homozygous male progeny, control males, and virgin females. Flies were aged 3–7 days in vials with fly food and were passed into fresh vials both the night before and the morning of testing. Using very brief (∼10 sec) cold anesthesia, a single male was paired with a single WT virgin female in a polypropylene chamber (8 mm inner diameter × 4 mm height) and digitally recorded for a maximum of 30 min or until copulation occurred. All courtship assays were performed in a dedicated test area maintained at ∼23° and ∼80% humidity. Male following of the female and wing song were scored as male courtship behaviors. Courtship index (CI) was calculated as the total time in which the male spent performing courtship behaviors as a percentage of total observation time prior to copulation or as a percentage of 30 min if copulation never occurred within the designated 30-min time period.
Circadian behavior
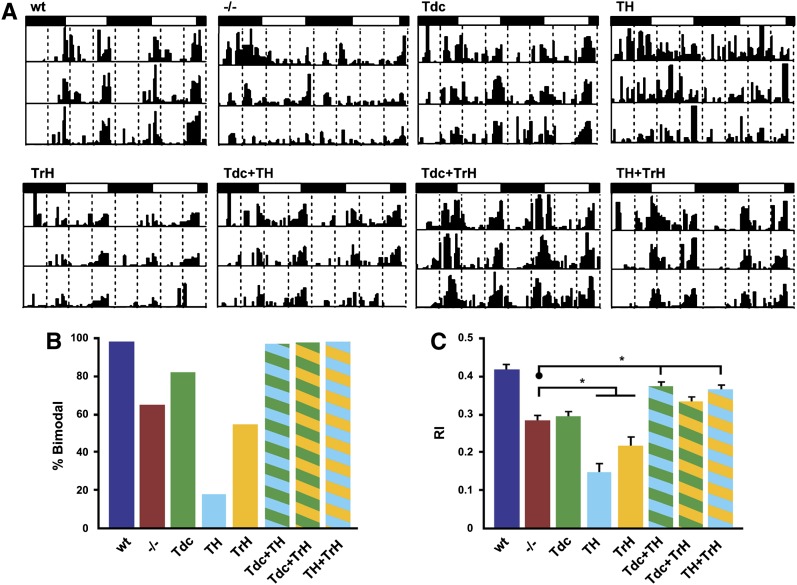
Behavioral data were collected with the Trikinetics Drosophila Activity Monitor (DAM) system. One- to 3-day-old flies were loaded in DAM monitors and activity data were collected for 10–12 days at a constant temperature of 23° inside an enclosed incubator. During each experiment, flies were first maintained under a light:dark (LD) 12:12 schedule for 3–4 days to collect entrainment data, followed by a constant dark period (DD) for 7–8 days. The signal processing toolbox algorithms (Levine et al. 2002) within MATLAB (MathWorks) were used to estimate circadian periods and to visualize actograms of individual flies. Differences in rhythmicity among genotypes were determined using the rhythmicity index (RI) (a measure of robustness), the correlogram (a statistical measure of rhythmicity), and the pattern of activity (as assessed by examining actograms). Activity under LD conditions was scored as bimodal if both the morning and evening activity peaks were at least twofold higher than the baseline (mean) activity in the actograms. Flies were scored as entrained if they satisfied the following three criteria under LD conditions: (1) bimodality in each 24-hr period, (2) statistical significance for the correlogram, and (3) an RI value >0.1.
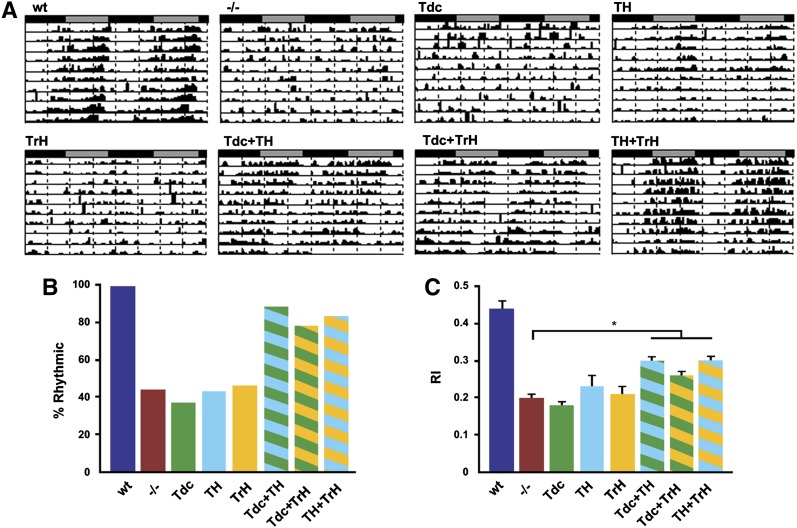
Behavior under DD conditions (see Table 2, page 168). was scored for those flies that were entrained under LD conditions and still alive after 5 days of constant darkness conditions. To increase the sample size under DD conditions for the TH, WT, and the dVMAT mutant, additional flies not tallied in Table 1 (page 168) were tested and included in the tally for Table 2 and the analysis of rhythmicity under DD conditions. The percentage of flies that were rhythmic (% rhythmic) under DD conditions was calculated as NR/NB, where NR is the total number of rhythmic flies and NB is the total number of flies with bimodal behavior (during LD) that survived for at least 5 days of DD. For both LD and DD conditions, RI values were assessed using the Kruskal–Wallis test (nonparametric ANOVA) with Dunn’s multiple-comparisons test (InStat 3, GraphPad).
Table 2 . Locomotor activity data for constant dark (DD).
| DD (days 5–12) | |||
|---|---|---|---|
| Genotype | N | RI ± SEM | % rhythmic |
| WT | 94 | 0.44 ± 0.01** | 99 |
| −/− | 81 | 0.20 ± 0.01* | 44 |
| Tdc | 98 | 0.18 ± 0.01* | 37 |
| TH | 21 | 0.23 ± 0.03* | 43 |
| TrH | 34 | 0.21 ± 0.02* | 46 |
| Tdc+TH | 140 | 0.30 ± 0.01*,** | 88 |
| Tdc+TrH | 204 | 0.26 ± 0.01*,** | 78 |
| TH+TrH | 119 | 0.30 ± 0.01*,** | 83 |
Activity data under DD conditions. A summary of mean activity, RI value and percentage of entrainment or rhythmicity for each genotype is shown for DD conditions. * genotypes that were significantly different from the WT control (Kruskal-Wallace with Dunn's post-hoc for multiple comparisons P < 0.05). ** genotypes that were significantly different from the dVMAT mutant (Kruskal-Wallace with Dunn's post-hoc for multiple comparisons P < 0.05).
Table 1 . Locomotor activity data for LD.
| LD (days 1–4) | ||||
|---|---|---|---|---|
| Genotype | N | Mean activity ± SEM | RI ± SEM | % bimodal |
| WT | 70 | 16.39 ± 0.84** | 0.42 ± 0.01** | 99 |
| −/− | 100 | 11.53 ± 0.62* | 0.29 ± 0.01* | 65 |
| Tdc | 120 | 12.78 ± 0.60* | 0.30 ± 0.01* | 82 |
| TH | 33 | 11.24 ± 0.93* | 0.15 ± 0.02*,** | 18 |
| TrH | 65 | 13.99 ± 0.76 | 0.22 ± 0.01*,** | 55 |
| Tdc+TH | 147 | 18.58 ± 0.81** | 0.37 ± 0.01** | 97 |
| Tdc+TrH | 209 | 19.23 ± 0.49*,** | 0.33 ± 0.01* | 98 |
| TH+TrH | 123 | 14.93 ± 0.43** | 0.36 ± 0.01*,** | 98 |
Activity data under LD conditions. A summary of mean activity, RI and percentage of entrainment or rhythmicity for each genotype is shown for LD conditions. * genotypes that were significantly different from the WT control (Kruskal-Wallace with Dunn's post-hoc for multiple comparisons P < 0.05). ** genotypes that were significantly different from the dVMAT mutant (Kruskal-Wallace with Dunn's post-hoc for multiple comparisons P < 0.05).
Results
Rescue strategy
To determine how exocytotic release of specific amines contributes to the dVMAT mutant phenotype, we compared the effects of expressing UAS-DVMAT with previously developed aminergic drivers. Drivers used here included Tyrosine decarboxylase-Gal4 (Tdc2-Gal4) to express UAS-DVMAT in octopaminergic and tyraminergic neurons, Tyrosine Hydroxylase-Gal4 (TH-Gal4) to express UAS-DVMAT in dopaminergic cells, and Tryptophan Hydroxylase-Gal4 (TrH-Gal4) to express UAS-DVMAT in serotonergic cells. These drivers confer specificity by encoding regulatory regions of genes involved in the biosynthesis of specific neurotransmitters (Friggi-Grelin et al. 2003; Zhang et al. 2004; Cole et al. 2005). Expression of DVMAT using each driver was confirmed on Western blots (Figure S1). As previously reported, we have also used the daughterless-Gal4 (da-Gal4) driver to broadly express UAS-DVMAT throughout the fly and to rescue amine levels and the dVMAT mutant phenotype (Simon et al. 2009). Since UAS-DVMAT driven by da-Gal4 is not expressed in 5-HT neurons, we designate rescue with da-Gal4 in the experiments below as nominally ubiquitous with the notable absence of rescue in serotonergic neurons (“ubiq−5HT”).
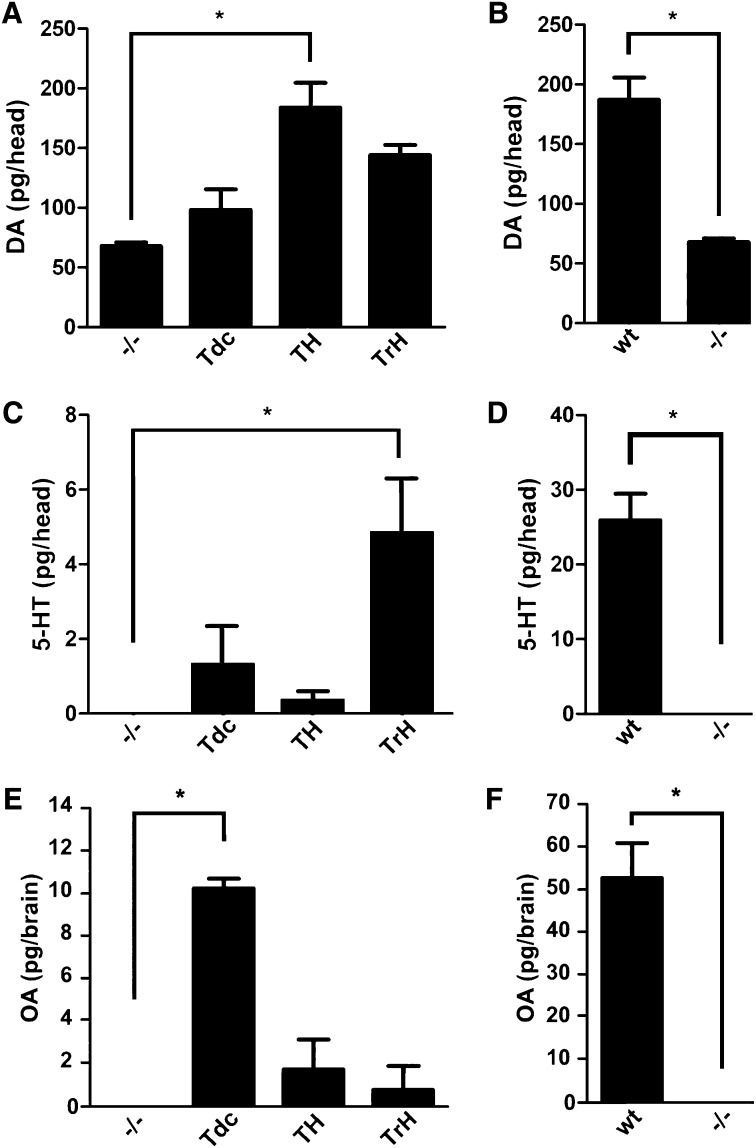
To further demonstrate that expression using TH-, TrH-, and Tdc2-Gal4 conferred functional rescue, we performed HPLC with electrochemical detection (Figure 1). For measurement of DA and 5-HT, homogenates were obtained from whole adult heads as described (Chang et al. 2006). However, since the cuticle contains an unidentified compound with a column retention time similar to that of OA, we measured OA levels in homogenates of dissected adult brains (Hardie and Hirsh 2006). Expression of UAS-DVMAT using Tyrosine Hydroxylase-Gal4 driver (Figure 1A, TH-Gal4) (Friggi-Grelin et al. 2003) restored DA to levels similar to those in WT flies (compare Figure 1A and 1B). We also see a trend toward higher levels of DA, using Tryptophan Hydroxylase-Gal4 to express DVMAT (Figure 1A). Although this difference did not reach statistical significance, we cannot rule out the possibility that the TrH-Gal4 driver used in these studies is expressed at low levels in some dopaminergic cells (Park et al. 2006). Alternatively, it is possible that these systems functionally interact in a manner that we do not yet understand. Expression of UAS-DVMAT using Tryptophan Hydroxylase-Gal4 (Figure 1C, TrH-Gal4) (Park et al. 2006) and Tyrosine decarboxylase2 (Figure 1D, Tdc2-Gal4) (Cole et al. 2005) partially rescued the tissue content of 5-HT and OA, respectively. Rescued levels of 5-HT and OA were lower than those of WT (CS) flies (compare Figure 1, C and D, 1E, and 1F) but may be more specific for the appropriate driver than rescue of DA using TH-Gal4.
Figure 1 .
Neurotransmitter content in genetically rescued dVMAT mutants. Shown is content of DA (A and B), 5-HT (C and D), and OA (E and F) in the dVMAT mutant (−/−) expressing UAS-DVMAT with the indicated drivers (A, C, and E); WT controls are shown for comparison (B, D, and F). Note that the y-axes differ across panels. HPLC with electrochemical detection was used for homogenates of either adult heads (5-HT and DA) or adult brains (OA). Nonparameteric ANOVA was used for analysis (Dunn’s post hoc, *P < 0.05 as indicated) since some mutant values were undetectable.
Octopamine and tyramine are sufficient to rescue the density-dependent survival deficit of the dVMAT mutant
We have previously shown that the dVMAT mutation is lethal under standard culture conditions but can survive under conditions of low density; only 5–10% of homozygous dVMAT mutants reared under standard culture conditions survive to adulthood (Simon et al. 2009). To determine whether this could be due to gross changes in neural development, we quantified the number of larval aminergic neurons in the mutant vs. WT nervous system (Figure S2). We used an antibody to tyrosine hydroxylase to detect DA neurons (Neckameyer et al. 2000). Of the seven DA clusters we analyzed, one (DL2) showed an ∼17% decrease in total cell number (Figure S2, A and B). The other DA clusters did not differ significantly from WT (Figure S2, A and B). Using a membrane-bound form of GFP as an additional marker, we failed to detect any differences between WT and the mutant for either 5-HT (Figure S2, C and D) or OA cell clusters (Figure S2, E and F) in the larvae. Since the DA, 5-HT, and OA systems appeared to be grossly intact, we proceeded with genetic experiments to rescue the storage and release of individual amines, using UAS-DVMAT; however, we cannot rule out the possibility that mutation of dVMAT causes additional defects in the neuropil that could affect our analysis (see Discussion).
To determine which aminergic system(s) is required for survival under normal density culture conditions, we compared the survival of dVMAT mutants rescued with each aminergic driver (Figure S2G and Figure S3). Expression of UAS-DVMAT using Dopa Decarboxylase-Gal4 (Ddc-Gal4 for expression in serotonergic and dopaminergic neurons), TH-Gal4, or TrH-Gal4 did not increase survival under standard (high-density) culture conditions (Figure S3, A–C and F). In contrast, mutants rescued with either Tdc2- or daughterless-Gal4 (Figure S3, D–F) survived at rates comparable to those of WT controls. Thus, although we cannot rule out additional effects of DA and 5-HT on development under low-density conditions (see Figure S3), our data suggest that the exocytotic release of DA and 5-HT may not be required for viability under standard, high-density culture conditions. By contrast, OA and/or TA are sufficient to rescue the density-dependent lethality of the dVMAT mutant under standard culture conditions.
Octopamine is sufficient for the initiation of larval locomotion
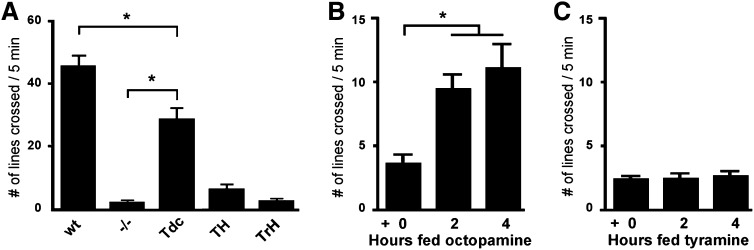
dVMAT mutant larvae have grossly retarded locomotion (Simon et al. 2009), consistent with other studies demonstrating aminergic regulation of crawling and fictive locomotion in intact larvae and ex vivo preparations, respectively (Neckameyer 1996; Fox et al. 2006). 5-HT is important for regulation of locomotion in some invertebrates (Willard 1981; Mackey and Carew 1983; Nusbaum 1986), and it has been previously suggested that both 5-HT and DA might contribute to the regulation of larval motor function in Drosophila (Mackey and Carew 1983; Neckameyer 1996; Cooper and Neckameyer 1999). We do not detect a significant increase in locomotion in larvae that express DVMAT in 5-HT neurons using TrH-Gal4 (Figure 2A, TrH), in DA neurons using TH-Gal4 (Figure 2A, TH), or, in a separate set of experiments, in both DA and 5-HT neurons using Ddc-Gal4 (Figure S4). In contrast, DVMAT expression in OA+TA neurons using Tdc2-Gal4 cells rescued locomotor activity to levels approaching those of the wild-type controls (Figure 2A, Tdc).
Figure 2 .
Octopamine rescues larval locomotion. (A) Expression of DVMAT using Tdc2-Gal4 but neither TH-Gal4 (TH) nor TrH-Gal4 (TrH) rescues larval locomotion to levels significantly higher than those of the dVMAT mutant (−/−), albeit less than those of WT (one-way ANOVA, *P < 0.05, Bonferroni posttest to compare indicated columns). Feeding octopamine (B) but not tyramine (C) or other amines (Figure S5) increased larval locomotion in the dVMAT mutants.
Previous studies have shown that ingested amines can be taken up by cells in the central nervous system (Budnik et al. 1989) and thereby rescue the behavior of mutants deficient in OA synthesis (Monastirioti et al. 1996; Cole et al. 2005). Similarly, 5-HT and DA feeding have been used to study their potential effects on ovarian follicle development (Willard et al. 2006) and DA feeding has been shown to partially rescue the phenotype of the Dopa Decarboxylase (Ddc) mutant (Budnik et al. 1989). To explore the potential roles of particular amines in stimulating larval locomotion, homozygous dVMAT null mutants were fed 10 mg/mL OA or other amines (Figure 2, B and C, and Figure S5, B and C). dVMAT mutant larvae fed OA for either 2 or 4 hr showed enhanced locomotion relative to mutant controls (Figure 2B). In contrast, feeding TA (Figure 2C) had no detectable effect on larval locomotion in the dVMAT mutant. Likewise, vehicle (Figure S5A), DA (Figure S5B), and 5-HT (Figure S5C) had no apparent effects on larval locomotion in the dVMAT mutant. Although we cannot rule out the possibility that amines other than OA failed to rescue because they were inefficiently absorbed, these findings suggest that OA, in the absence of other amines can rescue the larval locomotion defect seen in the dVMAT mutant.
In contrast to our results using the dVMAT mutant, we did not detect a consistent increase in locomotion in wild-type larvae fed OA or any of the other biogenic amines (Figure S5D). The lack of any apparent effect of biogenic amines fed to wild-type larvae demonstrates that any metabolites potentially produced by feeding the neurotransmitters did not have any dramatic untoward effects on behavior.
Storage of octopamine by DVMAT is required for egg release
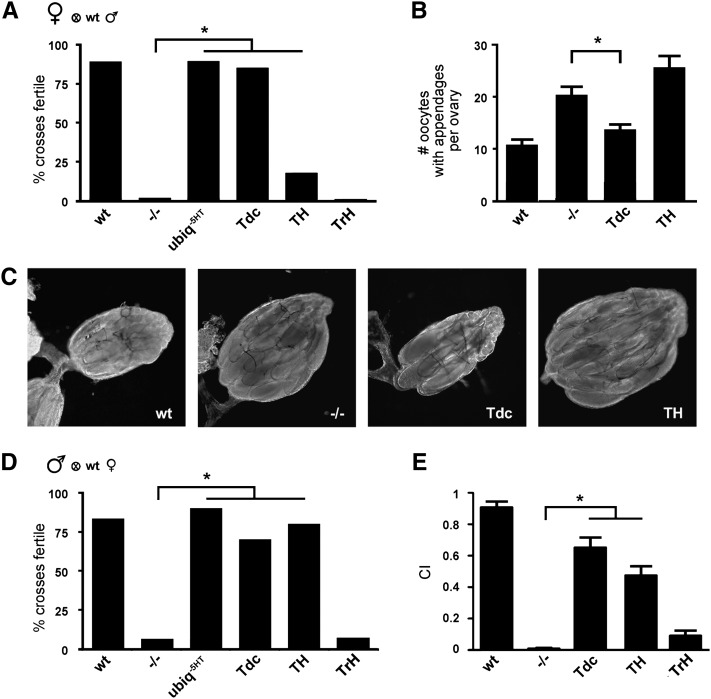
Female flies that lack DVMAT are infertile and retain more eggs in their ovaries than WT females (Simon et al. 2009). Consistent with these earlier findings, we show here that few dVMAT mutants produce progeny (Figure 3A, −/−). DVMAT expression in OA+TA+DA cells using da-Gal4 rescued the fertility defect (Figure 3A, ubiq−5HT), and since da-Gal4 does not appear to drive expression in 5-HT neurons (Simon et al. 2009), these data suggest that 5-HT is dispensable for female fertility. Indeed, rescue of DVMAT expression in 5-HT cells using TrH-Gal4 did not improve fertility of the dVMAT mutant (Figure 3A, TrH). Rescue using TH-Gal4 alone was associated with a small, but statistically significant increase in fertility (Figure 3A, TH). In contrast, expression of DVMAT in TA+OA cells with Tdc2-Gal4 restored female fertility to nearly wild-type levels (Figure 3A, Tdc).
Figure 3 .
Dopamine and octopamine contribute to fertility and sexual behavior. (A) Expression of DVMAT using da-Gal4 (ubiq-5HT) or Tdc2-Gal4 (Tdc) rescues the dVMAT female fertility defect (Fisher’s exact test, *P < 0.05). Rescue using TH-Gal4 (TH) was less robust but significant (*P < 0.05) with respect to the mutant (but unlike either Tdc2-Gal4 or da-Gal4 differed significantly from WT, not shown). Flies expressing DVMAT in serotonergic (TrH) neurons remained infertile. (B and C) dVMAT mutants retain more oocytes than WT controls. Expression of DVMAT using Tdc-Gal4 rescues this deficit (one-way ANOVA, *P < 0.05, Bonferroni posttest). Expression using TH-Gal4 (TH) shows a trend toward retention of more oocytes than the mutant. (D) Expression of DVMAT using da-Gal4 (ubiq−5HT), TH-Gal4 (TH), or Tdc2-Gal4 (Tdc) rescues the dVMAT male fertility defect (Fisher’s exact test, *P < 0.05). (E) Male courtship is reduced in the dVMAT mutant (−/−) and partially rescued using either Tdc2-Gal4 or TH-Gal4 (one-way ANOVA, *P < 0.05, Bonferroni posttest) but not TrH-Gal4.
To further explore the mechanism by which loss of OA storage and release reduces fertility of the dVMAT mutant, we examined oocyte retention in the ovaries of mated female flies (Figure 3, B and C). We find that expression of DVMAT in OA+TA cells (Tdc) rescues the oocyte retention deficit observed in the dVMAT mutant (Figure 3, B and C, Tdc). We also find that feeding OA but not TA or DA rescues the egg retention phenotype in the dVMAT mutant (Figure S6). These results are consistent with previous reports showing that mutation of the biosynthetic enzyme for OA causes egg retention (Monastirioti 2003) and that OA receptors are likely to mediate passage of eggs through the oviduct as well (Lee et al. 2003, 2009; Middleton et al. 2006; Rodríguez-Valentín et al. 2006). Our data further indicate that the vesicular storage and release of OA rather than other signaling mechanisms are important for female fertility and egg laying.
Although OA appeared to exert the most robust effects on female fertility and egg laying, we observe additional, possibly complementary effects of DA. Expression of DVMAT in DA neurons alone modestly but significantly increased the fertility of the dVMAT mutant (Figure 3A) and also showed a trend toward higher numbers of retained oocytes, although this did not reach statistical significance (Figure 3, B and C, TH). These observations may reflect a role for DA in oocyte development, independent of the role of OA in egg laying (Willard et al. 2006).
Dopamine and octopamine play complementary or redundant roles in male sexual behavior
We next used the dVMAT rescue lines to examine male sexual behavior (Figure 3, D and E). We have previously shown that male fertility is dramatically reduced in the dVMAT mutant (Simon et al. 2009); however, the contribution of aminergic circuits to male courtship and mating remains poorly understood. We find that fertility of dVMAT mutant males is restored with the da-Gal4 driver (Figure 3D, ubiq−5HT). In addition, both TH-Gal4 and Tdc2-Gal4 but not TrH-Gal4 partially rescued male fertility (Figure 3D).
We have previously shown that overexpression of DVMAT results in increased courtship activity (Chang et al. 2006). We show here that male courtship behavior is reduced in the dVMAT mutant (Figure 3E, −/−). Courtship is partially rescued using either Tdc2-Gal4 or TH-Gal4 (Figure 3E, Tdc and TH). Conversely, TrH-Gal4 does not significantly rescue the male courtship defect observed in the dVMAT mutant (Figure 3E, TrH). Although neither TH-Gal4 nor Tdc2-Gal4 fully restores courtship to wild-type levels, these data suggest that both DA and OA contribute to important aspects of male sexual behavior. DA has been previously suggested to instigate same-sex courtship (Liu et al. 2009), female receptivity (Neckameyer 1998b; Wicker-Thomas and Hamann 2008), and male to female courtship behavior (Alekseyenko et al. 2010) and OA may modulate the male’s choice between courtship and aggression (Certel et al. 2007). Similarly, since DA and OA can both rescue male fertility and courtship in the dVMAT mutants, we suggest that they serve complementary or perhaps redundant roles in male sexual behavior (see Discussion.)
The dVMAT mutant has altered startle-induced activity
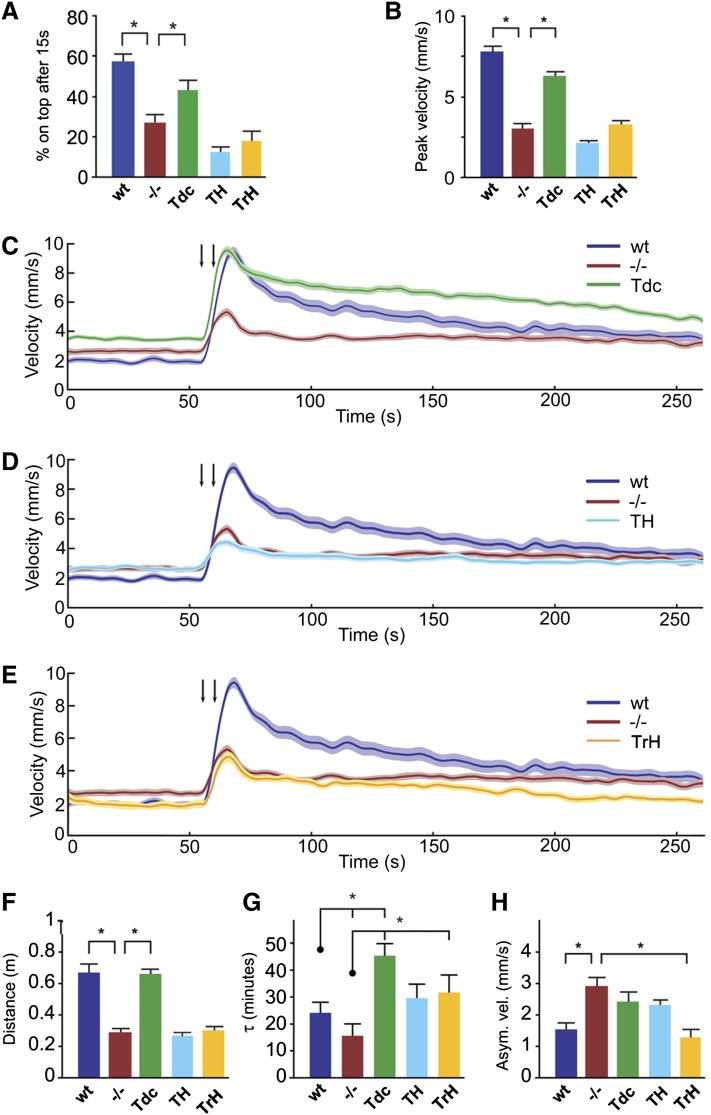
Dopamine has been previously implicated in arousal and startle responsiveness in Drosophila (Kume et al. 2005; Lebestky et al. 2009). Although there are no reports on the role of other amines in the Drosophila startle response per se, OA has been suggested to increase arousal and mediate the fight-or-flight response in other insects (Adamo et al. 1995; Bacon et al. 1995; Stern 1999; Rind et al. 2008). In negative geotaxis assays, the lower percentage of dVMAT mutants that reach the top of a vial in response to mechanical stimulus may be due in part to a diminished startle response (Simon et al. 2009). We find that expression of DVMAT in OA+TA neurons via Tdc2-Gal4 significantly improved performance in negative geotaxis assays whereas rescue with TH-Gal4 or TrH-Gal4 did not (Figure 4A).
Figure 4 .
Restoration of DVMAT in individual aminergic systems rescues selected aspects of adult startle-induced locomotion. (A) Negative geotaxis assay. Given 15 sec to climb after a mechanical startle, flies with DVMAT restored in OA+TA neurons via Tdc2-Gal4 have improved performance compared to dVMAT mutants. Rescue with TH-Gal4 or TrH-Gal4 did not display improved performance (one-way ANOVA, *P < 0.05, Bonferroni posttest; n = 8–23 trials per genotype. (B–H) Phenotypic characterization of startle response to successive air puffs in the puff-o-matt, n = 24 tubes per genotype. (B) Peak velocity minus baseline velocity after air puffs is rescued in flies with DVMAT restored using Tdc2-Gal4. (C–E) Traces show selective restoration of DVMAT using Tdc2-Gal4 (C), TH-Gal4 (D), or TrH-Gal4 (E). (F) DVMAT expression in OA+TA neurons using Tdc2-Gal4 rescues distance traveled after startle, computed by integrating the area under the postpuff curve, after subtracting the prepuff baseline. (G) Poststartle rate to acclimation is reported as the poststartle decay constant τ, and is rescued using TrH-Gal4. Rescue using Tdc2-Gal4 differs from the mutant but is also higher than WT, suggesting a prolongation of poststartle acclimation. (H) Asymptotic velocity represents the estimated final settle velocity after the puff startle. dVMAT flies remain hyperactive after startle. The elevated asymptotic velocity is rescued with DVMAT expression in 5-HT neurons (Kruskal–Wallis ANOVA followed by Mann–Whitney U-test: *P < 0.05).
To further assess the role of OA and other amines in startle-induced locomotion, we utilized a previously described repetitive startle assay (the puff-o-mat), which allows quantification of parameters and phases within the startle response (Lebestky et al. 2009). In response to air puffs, WT controls increase locomotor speed within seconds and then gradually return to a less active state in a response profile that can be characterized by a single exponential (Lebestky et al. 2009). We find that dVMAT mutants show a dramatically reduced peak velocity relative to WT controls (Figure 4, B–E, −/−, shown in red). Also consistent with earlier observations (Simon et al. 2009), dVMAT mutants display a somewhat higher basal locomotion in the prestartle phase, when acclimating to a novel environment, in comparison with WT animals (Figure 4C, time 0–60 sec, compare to WT shown in blue). This result suggests that although the mutants display an abrogated startle response, they are not generally sluggish in all locomotor measures.
Consistent with the results of negative geotaxis assays, we observe significant rescue of the startle response in the puff-o-mat assays by restoring DVMAT expression in OA+TA neurons (Figure 4C, Tdc, shown in green). Further dissection of the startle response shows that selective restoration of DVMAT in OA+TA neurons restores peak response to startle (Figure 4B) and distance traveled (Figure 4F). However, dVMAT mutants rescued with Tdc2-Gal4 continued to show heightened basal locomotor activity relative to that of WT controls (Figure 4C, Tdc, time 0–60 sec; note that the peak velocity value reported in Figure 3B is the difference between peak velocity and basal activity and corrects for differences in basal locomotor activity). Restoration of DVMAT expression using Tdc2-Gal4 also resulted in a prolongation of the startle response, where the slow decay of the startle state following peak velocity is measured as a heightened time constant, τ (Figure 4, C and G).
In contrast to the effects seen using the Tdc2-Gal4 driver, restoration of DVMAT in DA (Figure 4D) and 5-HT neurons (Figure 4E) failed to rescue peak startle responsiveness (Figure 4B) or distance traveled in the immediate, post-startle period (Figure 4F). However, speed to reacclimation after startle, quantified as the time constant τ, appeared to be rescued using TrH-Gal4 (Figure 4G). In addition, asymptotic velocity of dVMAT flies recued with TrH-Gal4 (Figure 4H, yellow bar) was significantly lower than of the dVMAT mutant and indistinguishable from WT. In sum, some aspects of adult locomotion and startle were rescued by expression in OA+TA cells, irrespective of the activity of other systems. In addition, expression using TH-Gal4 showed effects on other aspects of the startle response, suggesting a potentially complementary role for the serotonergic system in regulating arousal or startle behaviors.
Circadian locomotor activity rhythms are abnormal in the dVMAT mutant
For both mammals and flies, the control of circadian rhythmicity is complex and may involve multiple aminergic systems including 5-HT and DA (Meijer and Groos 1988; Morin and Blanchard 1991; Yuan et al. 2005; Hamasaka and Nässel 2006; Nässel and Homberg 2006; Suh and Jackson 2007; Crocker et al. 2010; Hirsh et al. 2010; Kula-Eversole et al. 2010; Gravotta et al. 2011). However, it is unknown whether the different aminergic systems function independently, cooperatively, or in a complementary manner in the regulation of rhythmicity. We first examined locomotor activity rhythms in dVMAT mutants and genetically matched wild-type controls in both LD and free-running (DD) conditions, using the DAM system. Fly activity was monitored for 3 days in a light:dark cycle consisting of 12 hr of light and 12 hr of dark (LD 12:12, Figure 5, Table 1) and then subsequently in DD conditions for an additional 7–8 days for flies able to entrain in LD (Figure 6, Table 2). As expected, WT control flies exhibited normal levels of locomotor activity and robust bimodal activity rhythms in LD with peaks occurring at the beginning (morning) and end (evening) of day; this was observed in actograms for individual flies (Figure 5A, WT) and in plots illustrating average daily profiles of activity for an entire population (Figure S7B, WT). Consequently, the average RI, a measure of the robustness of the rhythm (Levine et al. 2002), was high for wild-type control populations (Figure 5C, blue bar). In contrast, dVMAT mutants had weaker rhythmicity in LD (Figure 5C, red bar) and reduced activity (Figure S7A). In addition, the daily profile of activity was altered in the mutant such that morning activity was proportionally decreased relative to the evening bout of activity (Figure 5A, Figure S7B, −/−). This effect is reflected in the percentage of bimodality for mutant populations (Figure 5B, −/−) and in population averages of daily activity (Figure S7A, −/−, red bar). When transferred to DD, wild-type controls continued to show robust activity rhythms (Figure 6A) with a high average percentage of rhythmicity (Figure 6B) and RI value (Figure 6C). However, even for the dVMAT mutant population that exhibited bimodal rhythms in LD (and were therefore followed under DD conditions, see Materials and Methods), only ∼40% had statistically significant rhythmicity in DD (Figure 6, A and B, −/−). Thus, the average RI value for the mutant population was significantly decreased relative to that for controls in these conditions (Figure 6C). We also observed small (<1 hr) differences in circadian period among certain rescued strains. We have not pursued the origin of these small changes in period length, but it is possible that further experiments will elucidate a relationship to specific aminergic pathways.
Figure 5 .
The dVMAT mutant shows a defect in rhythmicity under LD entrainment conditions that can be rescued using two aminergic drivers. (A) Actograms showing LD locomotor activity profiles for representative flies of each genotype. The white and black rectangles above each graph indicate the light:dark schedule. (B and C) Histograms indicate the percentage of flies showing a bimodal rhythm (B) and the average rhythmicity index (RI). (C) Flies with DVMAT expression in DA (TH) or 5-HT (TrH) neurons showed RI values lower than those of the mutant, suggesting an exacerbation of the phenotype. In contrast, flies with DVMAT expression in OA+TA+DA (Tdc + TH) or 5-HT+DA neurons (TH + TrH) showed RI values significantly higher than those of the mutant, suggesting phenotypic rescue (Kruskal–Wallis, nonparametric ANOVA, with Dunn’s multiple-comparisons test, *P < 0.05).
Figure 6 .
Arhythmicity of the dVMAT mutant in constant darkness (DD) can be rescued using pairs of aminergic drivers. Rhythmicity of the mutant is rescued by simultaneous expression of wild-type DVMAT in two of the three different aminergic neuronal populations, but not using a single driver. (A) Representative DD actograms for each genotype. (B and C) Histograms indicating the percentage of flies that showed rhythmic behavior (rhythmicity, B) and the corresponding average rhythmicity index (RI, C). Flies with DVMAT expression in multiple aminergic neuronal subsets were significantly different from the mutant, suggesting phenotypic rescue (Kruskal–Wallis nonparametric ANOVA, Dunn’s multiple-comparisons test, *P < 0.05).
Interestingly, the circadian molecular oscillator of neurons is normal in the dVMAT mutant in both LD and DD conditions, as assessed by immunohistochemical measurements of PERIOD (PER) abundance (data not shown). Similarly, circadian rhythms in pigment dispersing factor (PDF), a neurotransmitter released from ventral lateral clock neurons, appeared normal in the mutant (data not shown). Both observations are consistent with the idea that the defects we observe here are likely to occur downstream of the pacemaker.
We next tested whether expression of a UAS-DVMAT transgene in DA, OA+TA, or 5-HT cells could rescue rhythmicity of the dVMAT mutant. In contrast to other behaviors described above (Figures 2–5), expression of DVMAT using any of the three individual aminergic drivers was not able to rescue the activity rhythm defects of the dVMAT mutant in LD or DD conditions (Figures 5 and 6, Figure S7, Tables 1 and 2). Indeed, expression of DVMAT using TH-Gal4 or TrH-Gal4 resulted in an average RI less than that of the dVMAT mutant in LD conditions (Figure 5C, TH and TrH), suggesting a worsening of the rhythmicity phenotype. Interestingly, in LD conditions, RI is most decreased in TH-Gal4 rescued flies (Figure 5C), which have the most severely reduced morning peak of activity in LD (Figure S7B). These results are consistent with the idea that DVMAT expression in a single aminergic regulatory system may not be sufficient to rescue the rhythmicity defect of the dVMAT mutant.
Normal circadian rhythmicity requires the expression of DVMAT in multiple aminergic cell types
Given the failure of any single aminergic Gal4 driver to rescue rhythmicity of the dVMAT mutant, it seemed likely that multiple aminergic systems might be required for normal circadian behavior. We therefore examined activity rhythms in dVMAT mutants expressing DVMAT under the control of combinations of Gal4 drivers (Tdc + TH, Tdc + TrH, or TH + TrH) to restore aminergic function in multiple different cell types. Interestingly, simultaneous expression of DVMAT using any two of the three aminergic drivers rescued the abnormal LD phenotypes of the dVMAT mutant (Figure 5, Figure S7D, Tables 1 and 2) with the exception of the RI value for the Tdc + TrH combination (Figure 5C, green and yellow striped bar). Similar to our findings under LD conditions, the arrhythmic phenotype of the dVMAT mutant in DD was rescued only with simultaneous expression of DVMAT in at least two classes of aminergic neurons (Figure 6). Expression of DVMAT in a mutant background under control of any of the three possible combinations of drivers (Tdc + TH, Tdc + TrH, or TH+TrH) resulted in percentage of rhythmicity values (Figure 6B) similar to those of wild-type controls and average RIs (Figure 6C) that were significantly higher than those of dVMAT nulls or mutants carrying DVMAT and a single Gal4 driver (see Discussion).
Discussion
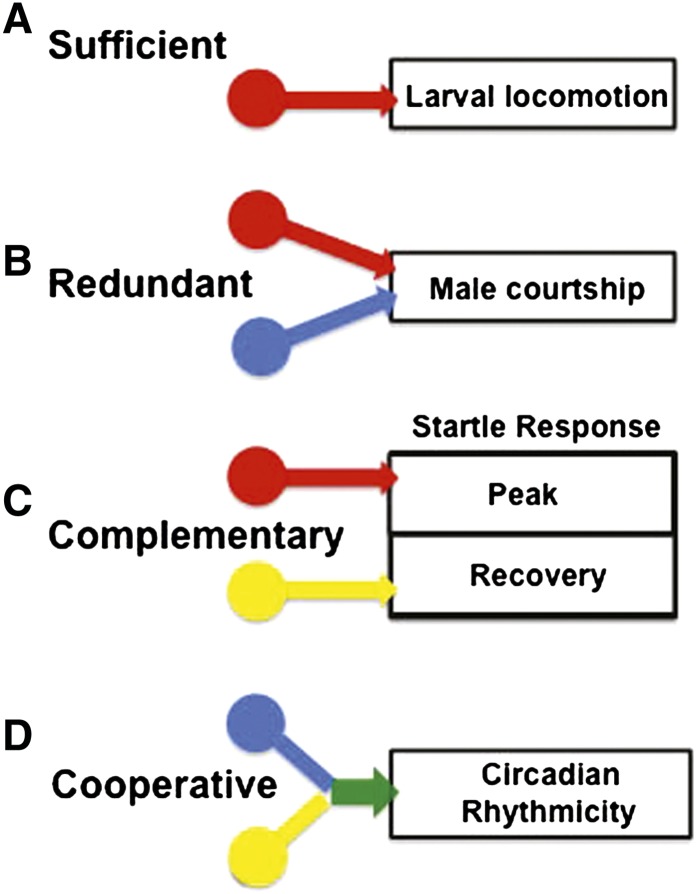
In previous studies, genetic or pharmacological manipulation of individual aminergic systems or peptide transmitters has been the method of choice for determining their functions. Here we have employed a different approach, exploiting the broad requirement of the VMAT for the vesicular release of neurotransmitter from all aminergic neurons. We used the dVMAT null mutant to globally ablate exocytotic amine release and then restored vesicular storage of individual amines or multiple systems, using transgenic rescue techniques. Our results provide a dissection of the functions of individual vs. combinations of aminergic neurotransmitters in the regulation of Drosophila behavior and their potential modes of interaction. We find that certain Drosophila behaviors rely predominantly on a single aminergic system, while others employ multiple aminergic systems that function in concert (see cartoons in Figure 7). Similar regulatory interactions may occur in mammals, for which the interplay between aminergic systems remains poorly understood (Gainetdinov and Caron 2003; Leggio et al. 2009). In the sections below, we discuss in greater detail Drosophila behaviors that (Figure 7A) depend primarily on a single regulatory system, (Figure 7, B and C) employ two or more aminergic systems that may function redundantly (Figure 7B) or in a complementary manner (Figure 7C), or (Figure 7D) appear to require two or more systems working cooperatively. We emphasize that loss of DVMAT and the genetic experiments we have performed pertain only to standard exocytotic release of amines via secretory vesicles. It remains possible that other, novel types of release are active in the dVMAT mutant and important for some developmental processes as we discuss below.
Figure 7 .
Summary of potential interactions between aminergic regulatory systems. The cartoon summarizes potential modes of interaction between aminergic regulatory systems highlighted by the rescue experiments we describe in the text. (A) The stimulation of baseline larval locomotion exemplifies a behavior for which a single aminergic regulatory system (octopamine) appears to be necessary and sufficient. (B) By contrast, rescue of male courtship occurred using two distinct aminergic drivers, suggesting the possibility of redundant regulatory pathways for this behavior. (C) The complex adult startle response is composed of at least two distinct phases, each rescued separately via DVMAT expression in a different aminergic system, thus suggesting that each system serves complementary and distinct functions. (D) The simultaneous expression of DVMAT using two aminergic drivers rescued circadian rhythmicity, whereas single drivers failed, suggesting possible cooperative interactions between regulatory systems. Colors indicate inputs from neurons that release octopamine (red), dopamine (blue), and serotonin (yellow) and cooperative effects between dopaminergic and serotonergic circuits (green).
OA alone may be sufficient to rescue some behaviors
We find that expression of DVMAT via Tdc2-Gal4 alone is sufficient to rescue the larval lethality of the dVMAT mutant that occurs under standard, high-density culture conditions, indicative of a developmental role for OA and possibly TA. This result also suggests that the vesicular release of DA and 5-HT may be dispensable for larval development and adult survival under standard culture conditions, an observation that is surprising given the fundamental role of DA in many physiological processes and the lethal effects of genetic (pale) (Kobayashi et al. 1995) or pharmacologic inhibition of tyrosine hydroxylase activity (Neckameyer 1996; Pendleton et al. 1996). Similarly, it has been proposed that 5-HT has a critical role in fly development (Colas et al. 1999; Sykes and Condron 2005; Willard et al. 2006; Schaerlinger et al. 2007). In contrast to these results, it has been shown that mutants of aromatic l-amino acid decarboxylase/Dopa decarboxylase (AADC/DDC)—which converts 5-hydroxy-l-tryptophan (5-HTP) to 5-HT (Hodgetts and O’Keefe 2006)—and flies with increased or decreased 5-HT survive beyond third-instar larval stages (Budnik et al. 1986; Sykes and Condron 2005; Daubert et al. 2010; Neckameyer 2010). Moreover, a recent study showed that pale mutants lacking DA synthesis in the nervous system (but not cuticle-forming tissue) survived to adulthood, despite observed deficits in phototaxis, arousal, and avoidance of shock-associated odor (Riemensperger et al. 2011). Similarly, we find that flies unable to store or exocytotically release DA and 5-HT can survive through larval and pupal development and eclose as viable adults. We suggest that the severe developmental phenotype of the 5HT2Dro mutant may reflect a requirement for nonvesicular release of 5-HT (Schaerlinger et al. 2007). In addition, consistent with Riemensperger et al. (2011), we find that the neuronal storage and exocytotic release of DA are not required for development. The phenotype of the dVMAT mutant vs. that caused by deletion of tyrosine hydroxylase activity in the nervous system (Riemensperger et al. 2011) may differ in other important respects; future comparative studies of these mutants may allow a dissection of the respective roles for standard vesicular release of DA vs. other nonexocytotic mechanisms in the adult brain.
Similar to larval survival, the larval locomotion deficits of the dVMAT mutant can be rescued using either da-Gal4 or, more specifically, Tdc2-Gal4 to express DVMAT in OA+TA neurons. Conversely, although DA and 5-HT application may modulate firing patterns at the neuromuscular junction (Cooper and Neckameyer 1999; Dasari and Cooper 2004), we find that expression of DVMAT using Ddc-, TH-, or TrH-Gal4 does not significantly rescue the dVMAT larval locomotion phenotype. In addition, we find that feeding larvae the neurotransmitter OA, but not DA or other amines to dVMAT mutants can partially rescue larval locomotion. At present we cannot rule out the possibility that OA is absorbed more readily than other amines. This caveat aside, our data are consistent with the critical role for OA in regulating motor behaviors in many invertebrates, in part by initiating the central pattern generators (CPGs) for crawling, walking, and flying (Sombati and Hoyle 1984; Hashemzadeh-Gargari and Friesen 1989; Ramirez and Pearson 1991; Orchard et al. 1993; Baudoux et al. 1998; Roeder 1999). Once initiated by OA or other modulatory neurotransmitters, CPGs propagate a stereotypic behavior independent of sensory input (Marder and Bucher 2001; Grillner 2003).
In the Drosophila larva, both TA and OA have been proposed to have opposing effects on locomotion, with OA stimulating and TA possibly inhibiting the CPG (Saraswati et al. 2004; Fox et al. 2006). We do not detect a decrease (or increase) in locomotion when either dVMAT mutant or WT larvae are fed TA. It is possible that larvae absorb TA less efficiently than OA or perhaps that a distinct, sensitized background will be needed to detect the behavioral effects of feeding TA.
The apparent dominance of OA vs. either DA or 5-HT for the behaviors we have studied here may be surprising, but is consistent with a range of other studies in invertebrates demonstrating the fundamental importance of OA in a variety of basic behaviors (Bicker 1999; Roeder 1999; Scheiner et al. 2006; Chase and Koelle 2007; Verlinden et al. 2010). It is conceivable that we failed to detect the importance of 5-HT in some cases because of the limited rescue of 5-HT levels using the relatively weak TrH-Gal4 driver. However, for both survival and larval locomotion, the contribution of serotonin was also tested using the Ddc-Gal4 driver, which drives expression in both DA and 5-HT neurons and also failed to rescue the dVMAT phenotype. DVMAT expression using TrH-Gal4 significantly altered both startle and circadian behaviors, thus demonstrating functional effects of UAS-DVMAT expression in 5-HT cells using this driver despite the limited biochemical rescue of 5-HT levels. We also note that the degree to which TrH-Gal4 rescued 5-HT levels was similar to the degree to which Tdc2-Gal4 rescued OA levels.
We cannot rule out the possibility that the loss of DA neurons mitigated the ability of DA feeding to rescue larval locomotion. We observe a small but significant loss of DA neurons in the dVMAT mutant larvae; similarly, we see a small decrease in the number of neurons in some DA clusters in adults (Lawal et al. 2010). It is also possible that additional changes in the aminergic neuropil could mitigate some aspects of our chemical rescue data. Changes in the branching pattern of serotonergic processes have been observed in the Ddc mutant (Budnik et al. 1989) and in larval brains exposed to excess exogenous 5-HT (Sykes and Condron 2005), and serotonin may generally function as a trophic agent (Daubert and Condron 2010). In addition, under some conditions, exogenous octopamine can increase the number of filopodia or synaptopods seen on octopaminergic processes (Koon et al. 2011). Similar developmental changes caused by abnormal amine release in the dVMAT mutants could potentially block its ability to respond to exogenous neurotransmitter. This concern would not apply to the genetic rescue experiments that make up the bulk of our data.
Complementary aminergic circuits
dVMAT mutant adults show deficits in motor behavior, but the adult dVMAT phenotype is more complex than that of the larvae. Adult flies have periodic bouts of flying, walking, and relatively stationary grooming and all of these behaviors may undergo aminergic regulation. Startle-induced locomotion is likely to be particularly important for the escape of adult flies from predators and survival, and DA has been previously shown to play an important role for this behavior in Drosophila (Lebestky et al. 2009) as well as for arousal in general (Riemensperger et al. 2011; Andretic et al. 2005). We note that dVMAT mutants show mildly elevated locomotion in the open field (Simon et al. 2009) and at baseline in the startle assay we have used here. For both of these assays, it is possible that handling of the flies increased motor behavior, since true baseline activity recorded over the course of many hours revealed a small decrease in the dVMAT mutant relative to WT (data not shown).
The dVMAT mutant showed a robust decrease in startle-induced locomotion, using a puff of air to stimulate locomotion as previously described (Lebestky et al. 2009). Expression of UAS-DVMAT using Tdc2-Gal4 rescued some of these deficits, including peak velocity and distance traveled in response to startle, clearly demonstrating an important role for OA and perhaps TA in some aspects of adult startle response and locomotion. These data are consistent with the proposed role for OA in both arousal and the fight-or-flight response in other insects (Adamo et al. 1995; Bacon et al. 1995; Stern 1999; Rind et al. 2008).
Although the results were less dramatic than those seen using Tdc2-Gal4, expression of DVMAT using TrH-Gal4 revealed significant rescue effects in the absence of Tdc2-Gal4. Effects associated with TrH-Gal4 were detectable during return to “baseline” activity after startle. The behavior of mutants expressing DVMAT with TrH-Gal4 suggests that 5-HT neurons may be important for regulating at least some parameters of the poststartle behavior, including the time constant (τ) and asymptotic velocity. We suggest that the complexity of adult locomotor behavior may require several complementary aminergic systems to regulate specific aspects of this behavior; for example, OA may initiate a fight-or-flight response whereas other amines may be responsible for shaping the appropriate response after initiation. This complexity and the nature of the interactions between regulatory inputs will be an important consideration for future efforts to more precisely map the underlying circuitries.
Consideration of potential interactions between regulatory systems is also critical for comparing behavioral phenotypes across mutants that affect aminergic signaling in different ways. Indeed, some differences between our current data and previously published results may depend on complex interactions between aminergic circuits that we do not fully understand. For example, although mutation of a single DA receptor decreased the adult startle response (Lebestky et al. 2009) and DA has been implicated in arousal (Andretic et al. 2005; Riemensperger et al. 2011), genetic rescue of DVMAT in DA cells did not detectably affect startle behavior. Similarly, although we find that the absence of DA input does not compromise negative geotaxis, the loss of DA cells inhibits this behavior (e.g., Feany and Bender 2000). The internal states of these lines are likely to differ, and the contribution of particular regulatory circuits may also differ dramatically. We suggest that these results are not contradictory, but rather may indicate that removal vs. restoration of specific regulatory pathways may have very different effects on behavior. More generally, these differences underscore the importance of understanding the manner in which each regulatory system interacts with each other and with the nervous system as a whole.
Similar to larval locomotion, expression of UAS-DVMAT in OA cells (using Tdc2-Gal4) and feeding OA rescued the infertility of adult dVMAT females. Previous genetic studies have demonstrated a critical role for OA in female fertility (Monastirioti et al. 1996; Neckameyer 1996; Lee et al. 2003; Monastirioti 2003; Willard et al. 2006; Gruntenko et al. 2007; Hardie et al. 2007). OA is likely to act at several sites in the female reproductive system to enable egg laying, and activation of different types of OA receptors may mediate contraction of the musculo-epithelial net surrounding the ovary, relaxation of the oviduct, and lubrication of the oviduct via epithelial cells that line the lumen (Lee et al. 2003, 2009; Middleton et al. 2006; Rodríguez-Valentín et al. 2006).
A possible role for 5-HT and/or DA in female fertility and egg development has been suggested previously (Neckameyer 1996; Willard et al. 2006). We find that 5-HT is neither required nor sufficient to rescue the fertility defect of the dVMAT mutant (see also Simon et al. 2009). However, consistent with a possible role for DA in early development, fertility and ovary size in the dVMAT mutant are modestly increased when DVMAT expression is restored solely in dopaminergic neurons. Ovary size may increase because dopamine enhances or accelerates oocyte development (Willard et al. 2006). In the absence of octopaminergic signaling to allow their release, a larger number of late-stage oocytes may be retained in the ovary.
Information on the potential contribution of biogenic amines to normal male sexual function is relatively limited (Hall 1994; Yamamoto et al. 1997; Greenspan 2000; Lasbleiz et al. 2006). One recent report suggests that the 5-HT7 receptor is involved in male courtship (Becnel et al. 2011). Overexpression or elimination of one aminergic system at a time has also demonstrated possible dopaminergic effects on courtship conditioning, a learned behavior (Neckameyer 1998a), and on aberrant male–male courtship (Liu et al. 2008). One recent report has demonstrated that standard male–female courtship is decreased when DA cells are silenced (Alekseyenko et al. 2010) and OA has been shown to modulate the male’s choice between courtship and aggressive behaviors (Certel et al. 2007). Using genetic rescue of individual aminergic systems in the dVMAT mutant, we demonstrate a role for both DA and OA in normal male sexual activity. Interestingly, the expression of UAS-DVMAT using either TH-Gal4 or Tdc2-Gal4 rescues the dVMAT mutant male courtship phenotype to a similar degree. We speculate that DA and OA may serve some redundant or overlapping roles in activating male courtship. Mechanistically, it is possible that DA and OA inputs converge upon a similar common pathway or stimulate courtship via distinct pathways. Although both DA and OA have been implicated in male courtship (Neckameyer 1998b; Certel et al. 2007; Alekseyenko et al. 2010), the notion that they might serve redundant roles would have been less obvious in previous studies in which only one of these system was disrupted at any one time.
Possible cooperative effects between aminergic systems
Our results suggest that interactions between aminergic systems may be particularly important for the regulation of circadian rhythms. An earlier study indicated that 5-HT is required for light input to the clock circuitry via the 5-HT1B receptor and glycogen synthase kinase 3β signaling (Yuan et al. 2005). More recently, it has been shown that flies with reduced neural DA exhibit defects in circadian light sensitivity, circadian entrainment, and free-running rhythmicity [i.e., in DD after entrainment (Hirsh et al. 2010)]. We find that dVMAT mutants lacking vesicular release of 5-HT, DA, and OA show decreased activity in the DAMS monitor (Figure S7A) and exhibit an altered profile of daily activity in LD. Specifically, there is an unusually high percentage of night-time activity (Figure S7, B and C) and the morning bout of activity is proportionally decreased relative to the wild-type profile or missing entirely in many mutants. Although both defects are likely to contribute to a decrease in rhythmicity, the defect in morning activity appears to be more severe. A relatively selective effect on morning vs. evening activity is of interest because different groups of clock neurons are postulated to control the two bouts of activity (Nitabach and Taghert 2008), although there is still ongoing debate about the precise composition of the evening and morning circadian oscillators (e.g., Shafer and Taghert 2009; Sheeba et al. 2010). In addition to the LD phenotype, most dVMAT individuals—even those that exhibit bimodal activity in LD—are weakly rhythmic or arrhythmic in free-running DD conditions (Figure 6, Table 2), consistent with the idea that biogenic amines are also essential for free-running rhythmicity.
As both DA and 5-HT are required for normal circadian light sensitivity, a deficit in this process might contribute to the phenotype of dVMAT. However, neuronal PER cycling can be synchronized to LD and persists in DD in the dVMAT mutant (data not shown), suggesting that loss of dVMAT primarily modulates clock output rather than directly affecting the circadian pacemaker itself. It remains possible that DVMAT-dependent amine release contributes to the so-called “positive masking” response to lights on—a clock-independent, direct stimulation of locomotor activity (Wheeler et al. 1993; Rieger et al. 2003)—since most dVMAT mutant individuals do not exhibit the short, light-induced bout of activity that is observed at the beginning of day in wild-type flies (see Figure 5A). Of note, increased activity is observed in mutant individuals in response to the lights-off signal, so the effect on masking may be selective for the lights-on signal. To our knowledge, it is not known whether distinct circuits control responses to lights on vs. lights off in Drosophila, although DA has been shown to be required for the circadian entrainment in low light (Hirsh et al. 2010).
Surprisingly, we were unable to detect partial rescue effects for rhythmicity with any single driver. Rather, restoration of DVMAT expression in two classes of aminergic neurons was required to detectably rescue circadian behavior. One trivial explanation for this observation would be that the methods we used to quantify circadian behavior were less sensitive than those used for other behaviors. We think this is unlikely, since in some cases single drivers did indeed show a detectable effect, but worsened rather than rescued the mutant phenotype. Rather, we speculate that the apparent requirement for at least two systems may reflect an unexpected cooperativity between the aminergic circuits that regulate circadian rhythms. We suggest that the potential for nonadditive, synergistic interactions may be important to consider in future efforts to map the circuitry underlying rhythmic behavior in the fly.
The failure of a transgene expressing TH to rescue circadian rhythmicity in the TH-deficient pale mutant has been attributed to a hypothesized lack in the temporal cycling of the TH transcript (Hirsh et al. 2010). Similarly, we cannot rule out the possibility that some of the circadian deficits we observed were caused by incomplete temporal regulation of DVMAT expression. It is unclear, however, how this effect would explain the requirement for two rather than a single aminergic driver in the rescue experiments we report here.
Another interesting aspect of these data is that any combination of two Gal4 drivers (TrH + TH, Tdc + TH, or Tdc + TrH) was sufficient to rescue the circadian phenotypes of the dVMAT mutant (with the exception of the RI value for LD entrainment of the Tdc + TrH combination, which failed to reach statistical significance). This demonstrates that two of the three aminergic systems suffice for rhythmicity and that no one specific combination is absolutely required for circadian behavior. This result contrasts with that of some other behaviors we examined in which particular systems were necessary and sufficient (larval locomotion, survival, female fertility), but is similar to male sexual behavior in which both OA and DA were capable of stimulating courtship and rescuing fertility. Our data suggest the possibility that there are redundant aminergic inputs to the circuitry regulating clock-based as well as courtship circuitries. In the case of the clock circuits, previous results indicate plasticity in the clock neurons’ regulation of locomotor activity (Sheeba et al. 2010), and network properties of the circadian circuitry may permit flexibility such that the absence of one set of synaptic interactions does not disrupt behavior.
The possibility that two aminergic systems can control the same behavior has important implications in the fly as well as in mammalian systems. In mammals, the prediction that DA circuits were responsible for psychostimulant-based reward behavior has matured into a view that DA as well as NE and 5-HT are involved (Torres et al. 2003). Similarly, some behaviors in the fly may undergo control by multiple regulatory circuits, whose input would vary depending on shifting environmental conditions or internal states.
Supplementary Material
Acknowledgments
Work performed at University of California (UCLA) was supported by National Institute of Mental Health grant R01 MH076900 (to D.E.K.) and National Institute of Environmental Health (NIEHS) grant R01 ES015747 (to D.E.K.), an Independent Investigator Award from The Brain and Behavior Research Foundation (to D.E.K.), a Center Grant from the NIEHS (P01 ES016732; PI, M. F. Chesselet), a Ruth L. Kirschstein National Research Service Award GM07185 (to A.C.) and a Postdoctoral Training Grant from the UCLA Molecular Toxicology Program, and United States Department of Health and Human Services Ruth L. Kirschstein Institutional National Research Service Award T32 ES015457 (to H.O.L.). Work performed at Tufts was supported by National Institutes of Health (NIH) grant R01 HL59873 (to F.R.J.), NIH grant R01 NS065900 (to F.R.J.), and a Center Grant to the Tufts Center for Neuroscience Research (NIH grant P30 NS047243; PI, F.R.J.). F.N. was supported by NIH training grant T32 HD049341.
Footnotes
Communicating editor: R. Anholt
Literature Cited
- Abdallah L., Bonasera S. J., Hopf F. W., O’Dell L., Giorgetti M., et al. , 2009. Impact of serotonin 2C receptor null mutation on physiology and behavior associate with nigrostriatal dopamine pathway function. J. Neurosci. 29: 8156–8165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo S. A., Linn C. E., Hoy R. R., 1995. The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J. Exp. Biol. 198: 1691–1700 [DOI] [PubMed] [Google Scholar]
- Alekseyenko O. V., Lee C., Kravitz E. A., 2010. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE 5: e10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex K. D., Pehek E. A., 2007. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 113: 296–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R., van Swinderen B., Greenspan R. J., 2005. Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15: 1165–1175 [DOI] [PubMed] [Google Scholar]
- Bacon J. P., Thompson K. S., Stern M., 1995. Identified octopaminergic neurons provide an arousal mechanism in the locust brain. J. Neurophysiol. 74: 2739–2743 [DOI] [PubMed] [Google Scholar]
- Barnes J. M., Dean A. J., Nandam L. S., O’Connell R. G., Bellgrove M. A., 2011. The molecular genetics of executive function: role of monoamine system genes. Biol. Psychiatry 69: e127–e143 [DOI] [PubMed] [Google Scholar]
- Barron A. B., Sovik E., Cornish J. L., 2010. The roles of dopamine and related compounds in reward seeking behaviour across animal phyla. Front. Behav. Neurosci. 4: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoux S., Duch C., Morris O. T., 1998. Coupling of efferent neuromodulatory neurons to rhythmical leg motor activity in the locust. J. Neurophysiol. 79: 361–370 [DOI] [PubMed] [Google Scholar]
- Bayersdorfer F., Voigt A., Schneuwly S., Botella J. A., 2010. Dopamine-dependent neurodegeneration in Drosophila models of familial and sporadic Parkinson’s disease. Neurobiol. Dis. 40: 113–119 [DOI] [PubMed] [Google Scholar]
- Becnel J., Johnson O., Luo J., Nässel D. R., Nichols C. D., 2011. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS ONE 6: e20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker G., 1999. Biogenic Amines in the Brain of the Honeybee: Cellular Distribution, Development, and Behavioral Functions, pp. 166–178. John Wiley & Sons, New York [Google Scholar]
- Blenau W., Baumann A., 2001. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch. Insect Biochem. Physiol. 48: 13–38 [DOI] [PubMed] [Google Scholar]
- Boureau Y. L., Dayan P., 2010. Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology 36: 74–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin E. S., Matsumoto M., Hikosaka O., 2010. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68: 815–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V., Martin-Morris L., White K., 1986. Perturbed pattern of catecholamine-containing neurons in mutant Drosophila deficient in the enzyme dopa decarboxylase. J. Neurosci. 6: 3682–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V., Wu C. F., White K., 1989. Altered branching of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin and dopamine. J. Neurosci. 9: 2866–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certel S. J., Savella M. G., Schlegel D. C. F., Kravitz E. A., 2007. Modulation of Drosophila male behavioral choice. Proc. Natl. Acad. Sci. USA 104: 4706–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.-Y., Grygoruk A., Brooks E. S., Ackerson L. C., Maidment N. T., et al. , 2006. Over-expression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol. Psychiatry 11: 99–113 [DOI] [PubMed] [Google Scholar]
- Chase D., Koelle M., 2007. Biogenic amine neurotransmitters in C. elegans (February 20, 2007), pp. 1–15 in WormBook, edited by The C. elegans Research Community, www.wormbook.org
- Colas J.-F., Launay J.-M., Vonesch J.-L., Hickel P., Maroteaux L., 1999. Serotonin synchronises convergent extension of ectoderm with morphogenetic gastrulation movements in Drosophila. Mech. Dev. 87: 77–91 [DOI] [PubMed] [Google Scholar]
- Cole S. H., Carney G. E., McClung C. A., Willard S. S., Taylor B. J., et al. , 2005. Two Functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 280: 14948–14955 [DOI] [PubMed] [Google Scholar]
- Cooper R. L., Neckameyer W. S., 1999. Dopaminergic modulation of motor neuron activity and neuromuscular function in Drosophila melanogaster. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 122: 199–210 [DOI] [PubMed] [Google Scholar]
- Crocker A., Sehgal A., 2008. Octopamine regulates sleep in Drosophila through protein kinase A-dependent mechanisms. J. Neurosci. 28: 9377–9385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A., Shahidullah M., Levitan I. B., Sehgal A., 2010. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65: 670–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S., Cooper R. L., 2004. Modulation of sensory-CNS-motor circuits by serotonin, octopamine, and dopamine in semi-intact Drosophila larva. Neurosci. Res. 48: 221–227 [DOI] [PubMed] [Google Scholar]
- Daubert E. A., Condron B. G., 2010. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci. 33: 424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert E. A., Heffron D. S., Mandell J. W., Condron B. G., 2010. Serotonergic dystrophy induced by excess serotonin. Mol. Cell. Neurosci. 44: 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo V., Cacchio M., Di Giulio C., Espositio E., 2001. Role of serotonin2C receptors in the control of brain dopaminergic function. Pharmacol. Biochem. Behav. 71: 727–734 [DOI] [PubMed] [Google Scholar]
- Draper I., Kurshan P. T., McBride E., Jackson F. R., Kopin A. S., 2007. Locomotor activity is regulated by D2-like receptors in Drosophila: an anatomic and functional analysis. Dev. Neurobiol. 67: 378–393 [DOI] [PubMed] [Google Scholar]
- Duerr J. S., Frisby D. L., Gaskin J., Duke A., Asermely K., et al. , 1999. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J. Neurosci. 19: 72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui T., 2007. Octopamine-mediated neuromodulation of insect senses. Neurochem. Res. 32: 1511–1529 [DOI] [PubMed] [Google Scholar]
- Feany M. B., Bender W. W., 2000. A Drosophila model of Parkinson’s disease. Nature 404: 394–398 [DOI] [PubMed] [Google Scholar]
- Fox L. E., Soll D. R., Wu C.-F., 2006. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine beta hydroxlyase mutation. J. Neurosci. 26: 1486–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggi-Grelin F., Coulom H., Meller M., Gomez D., Hirsh J., et al. , 2003. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54: 618–627 [DOI] [PubMed] [Google Scholar]
- Gainetdinov R. R., Caron M. G., 2003. Monoamine transporters: from genes to behavior. Annu. Rev. Pharmacol. Toxicol. 43: 261–284 [DOI] [PubMed] [Google Scholar]
- Gervais J., Soghomonian J.-J., Richard D., Rouillard C., 1999. Dopamine and serotonin interactions in the modulation of the expression of the immediate-early transcription factor, nerve growth factor-inducible B, in the striatum. Neuroscience 91: 1045–1054 [DOI] [PubMed] [Google Scholar]
- Gravotta L., Gavrila A., Hood S., Amir S., 2011. Global depletion of dopamine using intracerebroventricular 6-hydroxydopamine injection disrupts normal circadian wheel-running patterns and PERIOD2 expression in the rat forebrain. J. Mol. Neurosci. 45: 162–171 [DOI] [PubMed] [Google Scholar]
- Greenspan R. J., 2000. Courtship in Drosophila. Annu. Rev. Genet. 34: 205–232 [DOI] [PubMed] [Google Scholar]
- Greer C. L., Grygoruk A., Patton D. E., Ley B., Romero-Calderon R., et al. , 2005. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J. Neurobiol. 64: 239–258 [DOI] [PubMed] [Google Scholar]
- Grillner S., 2003. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 4: 573–586 [DOI] [PubMed] [Google Scholar]
- Gruntenko N., Karpova E., Alekseev A., Chentsova N., Bogomolova E., et al. , 2007. Effects of octopamine on reproduction, juvenile hormone metabolism, dopamine, and 20-hydroxyecdysone contents in Drosophila. Arch. Insect Biochem. Physiol. 65: 85–94 [DOI] [PubMed] [Google Scholar]
- Guiard B. P., El Mansari M., Blier P., 2008. Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus ceruleus, and dorsal hippocampus. Mol. Pharmacol. 74: 1463–1475 [DOI] [PubMed] [Google Scholar]
- Haenisch B., Bönisch H., 2011. Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol. Ther. 129: 352–368 [DOI] [PubMed] [Google Scholar]
- Hall J. C., 1994. The mating of a fly. Science 264: 1702–1714 [DOI] [PubMed] [Google Scholar]
- Hamasaka Y., Nässel D. R., 2006. Mapping of serotonin, dopamine, and histamine in relation to different clock neurons in the brain of Drosophila. J. Comp. Neurol. 494: 314–330 [DOI] [PubMed] [Google Scholar]
- Hardie S. L., Hirsh J., 2006. An improved method for the separation and detection of biogenic amines in adult Drosophila brain extracts by high performance liquid chromatography. J. Neurosci. Methods 153: 243–249 [DOI] [PubMed] [Google Scholar]
- Hardie S. L., Zhang J. X., Hirsh J., 2007. Trace amines differentially regulate adult locomotor activity, cocaine sensitivity, and female fertility in Drosophila melanogaster. Dev. Neurobiol. 67: 1396–1405 [DOI] [PubMed] [Google Scholar]
- Hashemzadeh-Gargari H., Friesen W. O., 1989. Modulation of swimming activity in the medicinal leech by serotonin and octopamine. Comp. Biochem. Physiol. C Comp. Pharmacol. 94: 295–302 [DOI] [PubMed] [Google Scholar]
- Hirashima A., Sukhanova M. J., Rauschenbach I. Y., 2000. Genetic control of biogenic-amine systems in Drosophila under normal and stress conditions. Biochem. Genet. 38: 163–176 [PubMed] [Google Scholar]
- Hirsh J., Riemensperger T., Coulom H., Iché M., Coupar J., et al. , 2010. Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr. Biol. 20: 209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts R. B., O’Keefe S. L., 2006. DOPA DECARBOXYLASE: a model gene-enzyme system for studying development, behavior, and systematics. Annu. Rev. Entomol. 51: 259–284 [DOI] [PubMed] [Google Scholar]
- Hsouna A., Lawal H. O., Izevbaye I., Hsu T., O’Donnell J. M., 2007. Drosophila dopamine synthesis pathway genes regulate tracheal morphogenesis. Dev. Biol. 308: 30–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M., Adler A., Bergman H., 2009. The dynamics of dopamine in control of motor behavior. Curr. Opin. Neurobiol. 19: 615–620 [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Morita S., Sawada H., Mizuguchi T., Yamada K., et al. , 1995. Targeted disruption of the tyrosine-hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J. Biol. Chem. 270: 27235–27243 [DOI] [PubMed] [Google Scholar]
- Koon A. C., Ashley J., Barria R., DasGupta S., Brain R., et al. , 2011. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat. Neurosci. 14: 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kula-Eversole E., Nagoshi E., Shang Y., Rodriguez J., Allada R., et al. , 2010. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc. Natl. Acad. Sci. USA 107: 13497–13502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K., Kume S., Park S. K., Hirsh J., Jackson F. R., 2005. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25: 7377–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasbleiz C., Ferveur J.-F., Everaerts C., 2006. Courtship behaviour of Drosophila melanogaster revisited. Anim. Behav. 72: 1001–1012 [Google Scholar]
- Lawal H. O., Chang H. Y., Terrell A. N., Brooks E. S., Pulido D., et al. , 2010. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol. Dis. 40: 102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebestky T., Chang J.-S. C., Dankert H., Zelnik L., Kim Y.-C., et al. , 2009. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron 64: 522–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-G., Seong C.-S., Kim Y.-C., Davis R. L., Han K.-A., 2003. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev. Biol. 264: 179–190 [DOI] [PubMed] [Google Scholar]
- Lee H.-G., Rohila S., Han K.-A., 2009. The octopamine receptor OAMB mediates ovulation via Ca2+ / calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS ONE 4: e4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio G. M., Cathala A., Neny M., Rouge-Pont F., Drago F., et al. , 2009. In vivo evidence that constitutive activity of serotonin2C receptors in the medial prefrontal cortex participates in the control of dopamine release in the rat nucleus acumbens: differential effects of inverse agonist vs. antagonist. J. Neurochem. 111: 614–623 [DOI] [PubMed] [Google Scholar]
- Levine J. D., Funes P., Dowse H. B., Hall J. C., 2002. Resetting the circadian clock by social experience in Drosophila melanogaster. Science 298: 2010–2012 [DOI] [PubMed] [Google Scholar]
- Li H., Chaney S., Forte M., Hirsh J., 2000. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr. Biol. 10: 211–214 [DOI] [PubMed] [Google Scholar]
- Liu Y., Edwards R. H., 1997. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu. Rev. Neurosci. 20: 125–156 [DOI] [PubMed] [Google Scholar]
- Liu T., Dartevelle L., Yuan C., Wei H., Wang Y., et al. , 2008. Increased dopamine level enhances male-male courtship in Drosophila. J. Neurosci. 28: 5539–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Dartevelle L., Yuan C., Wei H., Wang Y., et al. , 2009. Reduction of dopamine level enhances the attractiveness of male Drosophila to other males. PLoS ONE 4: e4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabry P. D., Campbell B. A., 1973. Serotonergic inhibition of catecholamine-induced behavioral arousal. Brain Res. 49: 381–391 [DOI] [PubMed] [Google Scholar]
- Mackey S., Carew T., 1983. Locomotion in Aplysia: triggering by serotonin and modulation by bag cell extract. J. Neurosci. 3: 1469–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman R. B., Murthy V., 2010. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity. Curr. Pharm. Des. 16: 488–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E., Bucher D., 2001. Central pattern generators and the control of rhythmic movements. Curr. Biol. 11: R986–R996 [DOI] [PubMed] [Google Scholar]
- Meijer J. H., Groos G. A., 1988. Responsiveness of suprachiasmatic and ventral lateral geniculate neurons to serotonin and imipramine: a microiontophoretic study in normal and imipramine-treated rats. Brain Res. Bull. 20: 89–96 [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y., Huang M., Giuseppe Di Giovann V. D. M., Ennio E., 2008. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems, pp. 177–197 in Progress in Brain Research edited by G. Di Giovann, V. Di Matteo and E. Ennio. Elsevier, Amsterdam/New York
- Middleton C. A., Nongthomba U., Parry K., Sweeney S. T., Sparrow J. C., et al. , 2006. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M., 2003. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 264: 38–49 [DOI] [PubMed] [Google Scholar]
- Monastirioti M., Linn J. C. E., White K., 1996. Characterization of Drosophila tyramine beta -hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 16: 3900–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin L. P., Blanchard J., 1991. Serotonergic modulation of the hamster wheelrunning rhythm: response to lighting conditions and food deprivation. Brain Res. 566: 186–192 [DOI] [PubMed] [Google Scholar]
- Mössner R., Simantov R., Marx A., Lesch K. P., Seif I., 2006. Aberrant accumulation of serotonin in dopaminergic neurons. Neurosci. Lett. 401: 49–54 [DOI] [PubMed] [Google Scholar]
- Nässel D., Homberg U., 2006. Neuropeptides in interneurons of the insect brain. Cell Tissue Res. 326: 1–24 [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., 1996. Multiple roles for dopamine in Drosophila development. Dev. Biol. 176: 209–219 [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., 1998a. Dopamine and mushroom bodies in Drosophila: experience-dependent and -independent aspects of sexual behavior. Learn. Mem. 5: 157–165 [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., 1998b. Dopamine modulates female sexual receptivity in Drosophila melanogaster. J. Neurogenet. 12: 101–114 [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., 2010. A trophic role for serotonin in the development of a simple feeding circuit. Dev. Neurosci. 32: 217–237 [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., Quinn W. G., 1989. Isolation and characterization of the gene for Drosophila tyrosine hydroxylase. Neuron 2: 1167–1175 [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., Weinstein J. S., 2005. Stress affects dopaminergic signaling pathways in Drosophila melanogaster. Stress 8: 117–131 [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., Woodrome S., Holt B., Mayer A., 2000. Dopamine and senescence in Drosophila melanogaster. Neurobiol. Aging 21: 145–152 [DOI] [PubMed] [Google Scholar]
- Nitabach M. N., Taghert P. H., 2008. Organization of the Drosophila circadian control circuit. Curr. Biol. 18: R84–R93 [DOI] [PubMed] [Google Scholar]
- Nusbaum M. P., 1986. Synaptic basis of swim initiation in the leech. III. Synaptic effects of serotonin-containing interneurones (cells 21 and 61) on swim CPG neurones (cells 18 and 208). J. Exp. Biol. 122: 303–321 [DOI] [PubMed] [Google Scholar]
- Oh S.-W., Kingsley T., Shin H.-h., Zheng Z., Chen H.-W., et al. , 2003. A P-element insertion screen identified mutations in 455 novel essential genes in Drosophila. Genetics 163: 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard I., Ramirez J.-M., Lange A. B., 1993. A multifunctional role for octopamine in locust flight. Annu. Rev. Entomol. 38: 227–249 [Google Scholar]
- Park J., Lee S. B., Lee S., Kim Y., Song S., et al. , 2006. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441: 1157–1161 [DOI] [PubMed] [Google Scholar]
- Pendleton R. G., Robinson N., Roychowdhury R., Rasheed A., Hillman R., 1996. Reproduction and development in are dependent upon catecholamines. Life Sci. 59: 2083–2091 [DOI] [PubMed] [Google Scholar]
- Ramirez J. M., Pearson K. G., 1991. Octopaminergic modulation of interneurons in the flight system of the locust. J. Neurophysiol. 66: 1522–1537 [DOI] [PubMed] [Google Scholar]
- Rieger D., Stanewsky R., Helfrich-Förster C., 2003. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J. Biol. Rhythms 18: 377–391 [DOI] [PubMed] [Google Scholar]
- Riemensperger T., Isabel G., Coulom H., Neuser K., Seugnet L., et al. , 2011. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA 108: 834–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rind F. C., Santer R. D., Wright G. A., 2008. Arousal facilitates collision avoidance mediated by a looming sensitive visual neuron in a flying locust. J. Neurophysiol. 100: 670–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Valentín R., López-González I., Jorquera R., Labarca P., Zurita M., et al. , 2006. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J. Cell. Physiol. 209: 183–198 [DOI] [PubMed] [Google Scholar]
- Roeder T., 1999. Octopamine in invertebrates. Prog. Neurobiol. 59: 533–561 [DOI] [PubMed] [Google Scholar]
- Roeder T., 2005. Tyramine and octopamine: ruling behavior and metabolism. Annu. Rev. Entomol. 50: 447–477 [DOI] [PubMed] [Google Scholar]
- Romero-Calderon R., Uhlenbrock G., Borycz J., Simon A. F., Grygoruk A., et al. , 2008. A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system. PLoS Genet. 4: e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S., Wintle R. F., Kindt K. S., Nuttley W. M., Arvan R., et al. , 2004. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J. 23: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara S. J., 2009. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10: 211–223 [DOI] [PubMed] [Google Scholar]
- Saraswati S., Fox L. E., Soll D. R., Wu C.-F., 2004. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J. Neurobiol. 58: 425–441 [DOI] [PubMed] [Google Scholar]
- Sawin E. R., Ranganathan R., Horvitz H. R., 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631 [DOI] [PubMed] [Google Scholar]
- Schaerlinger B., Launay J. M., Vonesch J. l., Maroteaux L., 2007. Gain of affinity point mutation in the serotonin receptor gene 5–HT2Dro accelerates germband extension movements during Drosophila gastrulation. Dev. Dyn. 236: 991–999 [DOI] [PubMed] [Google Scholar]
- Scheiner R., Baumann A., Blenau W., 2006. Aminergic control and modulation of honeybee behavior. Curr. Neuropharmacol. 4: 259–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W., 2010. Dopamine signals for reward value and risk: basic and recent data. Behav. Brain Funct. 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., et al. , 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23: 10495–10502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack S. R., Grace A. A., 2010. Cortico-basal ganglia reward network: Microcircuitry. Neuropsychopharmacology 35: 27–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer O. T., Taghert P. H., 2009. RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: the anatomical basis of a neuropeptide’s circadian functions. PLoS ONE 4: e8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V., Fogle K. J., Holmes T. C., 2010. Persistence of morning anticipation behavior and high amplitude morning startle response following functional loss of small ventral lateral neurons in Drosophila. PLoS ONE 5: e11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D., Adcock R. A., 2010. Dopamine and adaptive memory. Trends Cogn. Sci. 14: 464–472 [DOI] [PubMed] [Google Scholar]
- Simon A. F., Daniels R., Romero-Calderon R., Grygoruk A., Chang H.-Y., et al. , 2009. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics 181: 525–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D., Zars M., LaFerriere H., Chen Y.-C., Sable-Smith A., et al. , 2008. Serotonin is necessary for place memory in Drosophila. Proc. Natl. Acad. Sci. USA 105: 5579–5584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D., Zars M., Zars T., 2010. Place memory formation in Drosophila is independent of proper octopamine signaling. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 196: 299–305 [DOI] [PubMed] [Google Scholar]
- Sombati S., Hoyle G., 1984. Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J. Neurobiol. 15: 481–506 [DOI] [PubMed] [Google Scholar]
- Sora I., Hall F. S., Andrews A. M., Itokawa M., Li X.-F., et al. , 2001. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc. Natl. Acad. Sci. USA 98: 5300–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick S. M., Vythilingam M., Charney D. S., 2005. The psychobiology of depression and resilience to stress: implications for prevention and treatment*. Annu. Rev. Clin. Psychol. 1: 255–291 [DOI] [PubMed] [Google Scholar]
- Stern M., 1999. Octopamine in the Locust Brain: Cellular Distribution and Functional Significance in an Arousal Mechanism, pp. 135–141 John Wiley & Sons, New York; [DOI] [PubMed] [Google Scholar]
- Suh J., Jackson F. R., 2007. Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron 55: 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo S., Culotti J. G., Van Tol H. H. M., 2009. Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J. 28: 2437–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes P. A., Condron B. G., 2005. Development and sensitivity to serotonin of Drosophila serotonergic varicosities in the central nervous system. Dev. Biol. 286: 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres G. E., Gainetdinov R. R., Caron M. G., 2003. Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci. 4: 13–25 [DOI] [PubMed] [Google Scholar]
- True J. R., 2003. Insect melanism: the molecules matter. Trends Ecol. Evol. 18: 640–647 [Google Scholar]
- Verlinden H., Vleugels R., Marchal E., Badisco L., Pflüger H.-J., et al. , 2010. The role of octopamine in locusts and other arthropods. J. Insect Physiol. 56: 854–867 [DOI] [PubMed] [Google Scholar]
- Waddell S., 2010. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 33: 457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. A., Hamblen-Coyle M. J., Dushay M. S., Hall J. C., 1993. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J. Biol. Rhythms 8: 67–94 [DOI] [PubMed] [Google Scholar]
- Wicker-Thomas C., Hamann M., 2008. Interaction of dopamine, female pheromones, locomotion and sex behavior in Drosophila melanogaster. J. Insect Physiol. 54: 1423–1431 [DOI] [PubMed] [Google Scholar]
- Wilens T., 2006. Mechanism of action of agents used in attention-deficit/hyperactivity disorder. J. Clin. Psychiatry 67(Suppl. 8): 32–38 [PubMed] [Google Scholar]
- Willard A., 1981. Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J. Neurosci. 1: 936–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard S. S., Koss C. M., Cronmiller C., 2006. Chronic cocaine exposure in Drosophila: Life, cell death and oogenesis. Dev. Biol. 296: 150–163 [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Jallon J.-M., Komatsu A., 1997. Genetic dissection of sexual behavior in Drosophila Melanogaster. Annu. Rev. Entomol. 42: 551–585 [DOI] [PubMed] [Google Scholar]
- Yuan Q., Lin F., Zheng X., Sehgal A., 2005. Serotonin modulates circadian entrainment in Drosophila. Neuron 47: 115–127 [DOI] [PubMed] [Google Scholar]
- Yuan Q., Joiner W. J., Sehgal A., 2006. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr. Biol. 16: 1051–1062 [DOI] [PubMed] [Google Scholar]
- Zhang X., Beaulieu J.-M., Sotnikova T. D., Gainetdinov R. R., Caron M. G., 2004. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 305: 217. [DOI] [PubMed] [Google Scholar]
- Zhou C., Rao Y., Rao Y., 2008. A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci. 11: 1059–1067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.