Abstract
Sperm competition arises as a result of complex interactions among male and female factors. While the roles of some male factors are known, little is known of the molecules or mechanisms that underlie the female contribution to sperm competition. The genetic tools available for Drosophila allow us to identify, in an unbiased manner, candidate female genes that are critical for mediating sperm competition outcomes. We first screened for differences in female sperm storage and use patterns by characterizing the natural variation in sperm competition in a set of 39 lines from the sequenced Drosophila Genetic Reference Panel (DGRP) of wild-derived inbred lines. We found extensive female variation in sperm competition outcomes. To generate a list of candidate female genes for functional studies, we performed a genome-wide association mapping, utilizing the common single-nucleotide polymorphisms (SNPs) segregating in the DGRP lines. Surprisingly, SNPs within ion channel genes and other genes with roles in the nervous system were among the top associated SNPs. Knockdown studies of three candidate genes (para, Rab2, and Rim) in sensory neurons innervating the female reproductive tract indicate that some of these candidate female genes may affect sperm competition by modulating the neural input of these sensory neurons to the female reproductive tract. More extensive functional studies are needed to elucidate the exact role of all these candidate female genes in sperm competition. Nevertheless, the female nervous system appears to have a previously unappreciated role in sperm competition. Our results indicate that the study of female control of sperm competition should not be limited to female reproductive tract-specific genes, but should focus also on diverse biological pathways.
Keywords: sperm competition, nervous system, biased sperm use
IN many organisms, sperm competition is an important source of reproductive variation and is critical to the reproductive success of both males and females. Sperm competition occurs when a female mates with and stores sperm from multiple males. Multiple mating creates a situation in which the males’ sperm “compete” for successful fertilizations. While the ultimate outcome of a sperm competition depends on a complex interaction between male and female factors, the sexes often have different interests. Males are essentially in competition with other males, and while natural selection drives males to become better competitors with each other, it may result in males damaging the female through toxicity of seminal proteins (Wigby and Chapman 2004; Mueller et al. 2007). On the other hand, females benefit from simply being able to produce the most and highest-fitness offspring, and this may entail a specific response to male-produced molecules that influence sperm competition. The sexual conflict that arises from these different evolutionary goals of males and females implies that the genetic response to selection on sperm competition may be totally distinct in males and females. It remains largely unknown what genes drive this sexual antagonism underlying sperm competition.
Identifying the genes that are important to the success of sperm competition for each sex will lead to a full understanding of the genetic architecture and evolutionary dynamics of sperm competition. Due to the numerous genetic and experimental tools available, Drosophila has been a particularly good model for studying the basic biology of sperm competition. Previous work in Drosophila has demonstrated that male genotype (Clark et al. 1995, 1999; Fiumera et al. 2005, 2007; Civetta et al. 2008; Chow et al. 2010), female genotype (Clark et al. 1995, 1999; Clark and Begun 1998; Civetta et al. 2008; Chow et al. 2010), and male × female genotype interactions (Clark and Begun 1998; Clark et al. 1999; Chow et al. 2010) are all important to the outcome of sperm competition and that each aspect involves dozens of genes.
Functional and association studies demonstrated that several male-derived accessory gland proteins (Acps) play important roles in sperm competition (Harshman and Prout 1994; Fiumera et al. 2005, 2007; Wong et al. 2008; Avila and Wolfner 2009; Chow et al. 2010). However, while different female genotypes associate with differences in sperm competition outcomes, little is known about the specific genes or gene variants that underlie the differences in the female side of sperm competition. Gene expression analyses in singly mated (Lawniczak and Begun 2004; McGraw et al. 2004, 2008, 2009; Kapelnikov et al. 2008) and doubly mated (Innocenti and Morrow 2010) females found numerous genes regulated by mating. These mating-regulated genes may include some that are important for sperm competition. Similarly, genes expressed specifically in the female sperm storage organs (Allen and Spradling 2008; Prokupek et al. 2008, 2009, 2010; Schnakenberg et al. 2011) might produce products that influence sperm competition.
Many studies of the female role in sperm competition have focused on the female reproductive tract. A priori, a focus on the female reproductive tract makes sense because this is where sperm competition occurs. In Drosophila females, sperm are stored in two types of organs, the spermatheca and the seminal receptacle, and there must be numerous interactions between the female molecules, the male molecules, and sperm within these organs (Wolfner 2009, 2011). Indeed, a recent study found that polymorphisms in genes expressed in the female reproductive tract or female genes with signatures of selection are associated with female sperm use (Giardina et al. 2011). However, to date, only one female gene has been directly implicated in sperm competition, the Sex Peptide Receptor (SPR) (Chow et al. 2010). Overall, it is unknown whether these interactions are necessary and/or sufficient for sperm competition.
The outcome of sperm competition depends on multiple interactions at the behavioral, pheromonal, and molecular levels (Sirot et al. 2009). Given the complexities of each step, we hypothesized that female-derived molecules and interactions important for sperm competition might not be limited to the female reproductive tract. Thus we performed an unbiased screen for candidate genes that affect sperm competition outcomes in females. We found and quantified high levels of natural genetic variation in outcomes of sperm competition trials. We then carried out association tests to identify single-nucleotide polymorphisms (SNPs) that contributed to variation in the female effect on sperm competition. We found a striking overrepresentation of neuronal genes, and we validated several of these in additional experiments. These results provide clues about the mechanisms used by females to actively control sperm competition.
Materials and Methods
Drosophila melanogaster fly cultures
Thirty-nine lines from the fully sequenced Drosophila Genetics Reference Panel (DGRP) were used in this study (Supporting Information, Table S1) (Ayroles et al. 2009; Mackay et al. 2012). The DGRP is a collection of wild-derived, inbred Drosophila lines. Sperm competition assays were carried out with females from the DGRP lines. To eliminate male genetic variation, all DGRP female genotypes were mated to the same first and second males from the standard laboratory lines, cn bw and bwD, respectively. All flies were collected as virgins under CO2 anesthesia and aged 4–7 days in single-sex vials of 20–30 flies. All flies were maintained on standard agar–dextrose–yeast media and housed at 24° on a 12-h light/dark cycle.
Sperm competition assays
Thirty females from each DGRP line were given the opportunity to mate doubly to males from the cn bw and bwD homozygous, inbred laboratory stocks. Sperm competition assays were performed by methods similar to those in previous reports (Figure S5) (Fiumera et al. 2005, 2007; Chow et al. 2010; Giardina et al. 2011). DGRP females all have wild-type red eyes, cn bw males have white eyes, and the doubly heterozygous F1 progeny sired by cn bw males and DGRP females have red eyes. bwD males are homozygous for a dominant mutation that causes brown eyes. The bwD allele was crossed into a homozygous laboratory background and the bwD males in this background are good sperm displacers as second males (Chow et al. 2010). Progeny sired by bwD males and DGRP females all inherit one copy of the dominant bwD allele, resulting in brown eyes.
For the first mating, cn bw males were mass mated with DGRP females for 12 h (overnight on day 0). For each DGRP line, three parallel mass matings of 10 males and 10 females was performed. On day 1, cn bw males were discarded and each female was aspirated into an individual vial (vial 1). At the end of day 1, two bwD males were placed with each female for 12 hr overnight (second mating). On day 2, each female was aspirated into a new vial (vial 2) and allowed to lay eggs for 48 hr. On days 4, 6, and 8, each female was aspirated into a new vial (vials 3, 4, and 5). On day 10, females were discarded. The adult progeny were scored for eye color to ascertain the sperm competition and progeny phenotypes of the parental lines.
We scored the following parameters: Total progeny is the number of progeny in vials 1–5 from each doubly mated female irrespective of eye color (red + brown) (30 measurements per DGRP line). Remating rate is the proportion of the 30 females that mate with a second male (single proportion per DGRP line), as determined by the presence of one or more brown-eyed progeny in vials 1–5. P1 is the proportion of progeny from the first male after the second mating, calculated as the proportion of red-eyed progeny after the second mating (red/(red + brown)). P1 scores were calculated from vials 2–5 of the crosses that remated. The second mating occurs in vial 1, and vial 1 is excluded from the P1 score because it is impossible to determine which eggs were laid before the second mating. In the cases of P1 = 0, it was observed that there was at least 1 red-eyed progeny in vial 1 (confirming that the first mating had occurred) and subsequently no red-eyed progeny in vials 2–5. Similarly, in cases of P1 = 1, it was observed that there was at least 1 brown-eyed progeny in vial 1 (confirming the second mating) and subsequently no brown-eyed progeny in vials 2–5.
Statistical analysis
All statistical analysis was performed in R (version 2.8.1, R Development Core Team). To identify a line effect, analysis of variance (ANOVA) was used to apply a simple linear model to P1 score and progeny number, similar to the analysis previously described (Chow et al. 2010; Clark et al. 1999; Grueber et al. 2007). The mean of the phenotype (yi) for females from the ith DGRP line was
where the indexes for the female genotypes are each of the 38 DGRP lines (one of the original 39 lines displayed a remating rate of 0; thus all sperm competition results henceforth include only 38 lines), where μ is the overall mean, and where εij is the error term. For progeny counts, j = 1–30. For P1 the ANOVA was fitted for individual flies that remated and thus j = number of remated females. Permutation tests based on chi-square statistics were applied to test for significant heterogeneity among lines in remating rate (Fiumera et al. 2005, 2007; Giardina et al. 2011).
To test the significance of SNP associations with sperm competition phenotypes, the lines were partitioned into two groups based on their genotype at each SNP (two groups representing either allele for each SNP) (Mackay et al. 2012). This is equivalent to pooling the lines into two bins for each SNP, with each bin representing one or the other allele at the SNP. From these collapsed data, the linear model to test for SNP interactions in P1 score was
where yi represents the P1 score, where μ is the overall mean, and where εij is the error term. All SNPs for which the minor allele frequency was <0.10 were excluded from the analysis. A total of 2,600,361 SNPs were tested. The number of SNPs tested in 38 lines results in a significant multiple-testing problem. However, rather than draw meaning from the P-value scores, we merely used this statistical association to generate a list of candidate genes for subsequent functional studies. Nevertheless, we did use the nominal P-values from these tests and removed all SNPs with P > 10−6 from further consideration. We also calculated a false discovery rate (FDR), but because of the very large number of SNPs being tested with just 38 lines, none of the FDR-corrected Q-statistics was <0.2.
To test for gene expression enrichment in neurological tissues, expression levels for each gene were taken from FlyAtlas (Chintapalli et al. 2007). Tissues were categorized as either “neuronal” (brain, eye, thoracicoabdominal ganglion, and larval CNS) or “nonneuronal” (all other tissues). A paired t-test was performed to test for neuronal tissue enrichment.
RNAi functional testing
To achieve tissue-specific knockdown of select candidate genes, we generated RNAi females by crossing ppk-GAL4/TM3, Sb (Grueber et al. 2007) females to UAS-RNAi–generating males (Vienna Drosophila RNAi Center; para, line 104775; SK, line 103985; Rab2, line 105358; Rim, line 39384). Control females were generated by crossing females from the same ppk-GAL4/TM3, Sb driver line to the AttP RNAi background line (Vienna Drosophila RNAi Center). Control and knockdown flies were identical with the exception of the insertion of an RNAi transgene at the predetermined AttP site. Only Sb+ progeny were used from each cross. Sperm competition experiments using RNAi and control females were performed in an identical way to the tests of DGRP females. RNAi females and control females were mated first to cn bw males and then to a second bwD male. Forty females of each genotype (RNAi and control) were assayed. To test the efficacy of RNAi knockdown for each gene, Tubulin-GAL4/TM3, Sb females were crossed to UAS-RNAi–generating males. Lethality was scored based on deviation from 1:1 for Tubulin-Gal4:TM3,Sb genotypes. Since all candidate genes in this study are essential genes, only lines that demonstrated complete or near complete lethality with Tubulin-GAL4–driven RNAi were used.
Results
Females from 39 lines of the DGRP (lines are listed in Table S1) were tested in double-mating trials as described in Materials and Methods. Thirty females were used for each of the 39 lines, resulting in a total of 1170 double-mating opportunities. One of the lines never engaged in a second mating, and we discuss only results for the 38 lines that successfully doubly mated.
Progeny number
Progeny number was determined for 11 days following the first mating. A total of 132,217 progeny were counted and scored (123 ± 73 per cross) (Figure S1A). Total progeny number showed a significant DGRP line effect (F37,485 = 4.0, P < 3.92 × 10−13) (Figure S1B and Table S1).
Remating rate
Female remating rate for each DGRP line is the proportion of the 30 females that remated with the second bwD male. The average remating rate across the 39 lines was 0.48 (including the single line that showed no remating). There was a significant DGRP line effect on remating rate (χ2 = 27, P = 0.003) (Figure S2 and Table S1)
P1 score
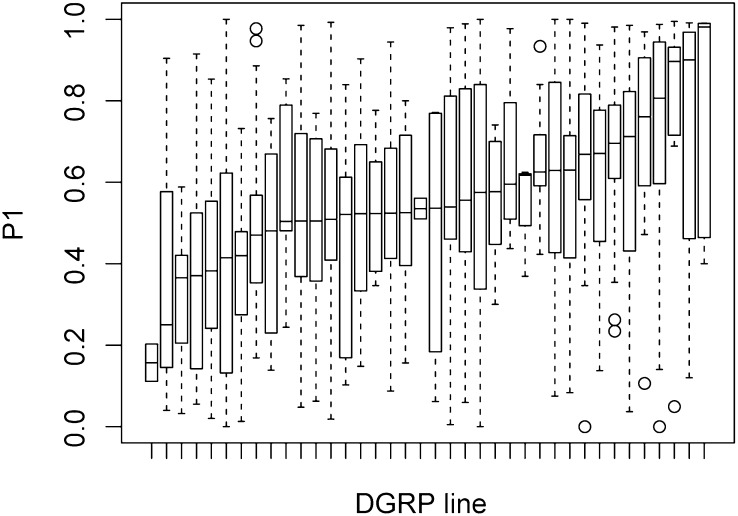
The P1 score is the proportion of progeny sired by the first male after the second mating. P1 was calculated from all females that remated with the second bwD male (presence of ≥1 brown-eyed progeny). A single DGRP line (RAL313) displayed zero remating among the 30 replicate females; thus, this line is excluded from the P1 analysis (38 lines remain). A total of 524 females remated with the second male. The average P1 score across all lines was 0.53 ± 0.27 (Figure S3). There was a significant effect of DGRP line on the P1 score (F37,485 = 1.98, P = 0.0003) (Figure 1 and Table S1). Mean P1 for each DGRP line varied from <0.2 to >0.9.
Figure 1 .
DGRP lines vary widely in female effect on P1 scores. Each box plot represents P1 scores from 30 females from a single DGRP line. P1 score is the proportion of first-male progeny after the second mating. Mean P1 scores vary up to sixfold among genotypes. Female DGRP genotype has a significant effect on P1 score (P = 0.0003). In the box plots, the boxes represent the interquartile range, the whiskers represent 1.5 × interquartile range, and open circles are outliers. See Table S1 for line identity.
Association of female SNPs with sperm competition
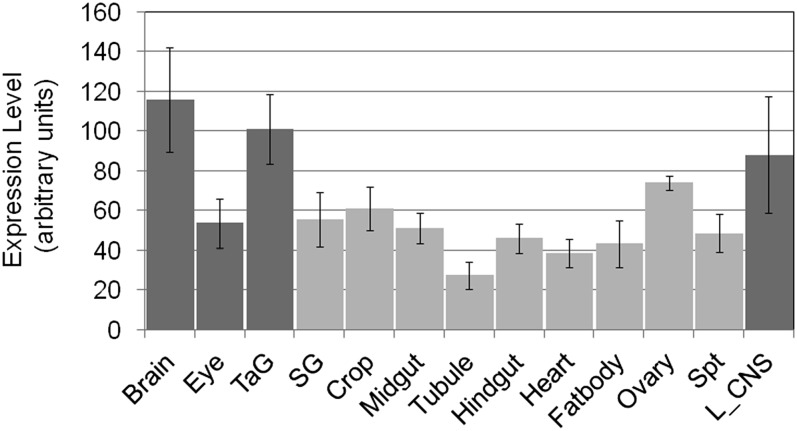
To generate an unbiased, prioritized list of candidate female genes involved in sperm competition, we performed an association study. The mean P1 score from each of the 38 doubly mating DGRP lines was used as the phenotype to associate with SNPs genome-wide. Each polymorphism in the genome allowed the partitioning of the DGRP lines by allele identity. ANOVA testing was performed to test for evidence that a SNP was associated with P1 score (see Table S4 for all SNPs with nominal P < 0.05). This test suffers from a severe multiple-testing problem, so rather than be concerned about the precise meaning of the P-values from the tests, we simply use the rank order of the test statistics to flag genes that might be worth pursuing for subsequent study. Thirty-three genes were represented among the most significant candidate SNPs associated with P1 score (Table 1; arbitrary cutoff of P < 10−6). All these candidate SNPs lie in noncoding regions (introns, UTRs, or intergenic) or are synonymous substitutions, suggesting that the SNPs most likely affect gene expression. Strikingly, 15 of these 33 genes have a specific neurological function or are enriched in expression in the nervous system (Table 1), as determined by literature review and gene function. An analysis of Gene Ontology (GO) terms did not yield enrichment of specific functional classes. This is not particularly surprising, as neurological genes in general do not fall into specific classes; instead, genes that are important for neurological functions fall into numerous functional GO categories. To further explore the neurological enrichment observed, we examined the expression of candidate genes in neurological tissues. We observed that the 33 genes associated with variation in sperm competition have a significantly higher mean expression level in neurological tissues compared to nonneurological tissues (P = 0.0019) (Figure 2). To identify whether any genes associated with sperm competition also influenced other parameters of reproductive success, we compared our sperm competition genome-wide association (GWA) with results from GWA of progeny number (Table S2). and remating rate measured in these lines (Table S3). There was no overlap between the genes associated with the three phenotypes, suggesting that, in this set of DGRP lines, the genetic architecture of these phenotypes might be independent.
Table 1 . Genes with SNPs associated with sperm competition.
| Rank ordera | P-value* | Gene | FlyBase ID | Chr position | Gene position | Neurob |
|---|---|---|---|---|---|---|
| 1 | 5.95E-07 | para | FBgn0260993 | X: 16,381,784 | Intron | Y |
| 2 | 1.33E-06 | caup | FBgn0015919 | 3L: 12,601,187 | Upstream | Y |
| 3 | 1.69E-06 | SK | FBgn0029761 | X: 5,257,979 | Intron | Y |
| 4 | 2.38E-06 | Cyp313a2 | FBgn0038006 | 3R: 8,062,040 | Synonymous | N |
| 5 | 2.43E-06 | Ddr | FBgn0053531 | 2L: 6,310,282 | Intron | Y |
| 6 | 4.06E-06 | Msp-300 | FBgn0261836 | 2L: 5,198,158 | Intron | Y |
| 7 | 4.63E-06 | CG42796 | FBgn0261929 | 3R: 4,454,906 | Downstream | Y |
| 8 | 4.66E-06 | CG31872 | FBgn0051872 | 2L: 10,643,524 | 3′-UTR | N |
| 9c | 4.88E-06 | CG9850 | FBgn0034903 | 2R: 19,676,975 | Intron | N |
| 10c | CG15800 | FBgn0034904 | 2R:19,676,975 | Downstream | N | |
| 11 | 6.35E-06 | CG15765 | FBgn0029814 | X: 5,713,699 | Intron | Y |
| 12 | 6.42E-06 | CG33298 | FBgn0032120 | 2L: 9,508,912 | Intron | N |
| 13 | 6.49E-06 | W | FBgn0003997 | 3L: 18,169,110 | Intron | N |
| 14 | 6.83E-06 | Rab2 | FBgn0014009 | 2R: 2,585,188 | Intron | Y |
| 15 | 6.90E-06 | spz5 | FBgn0035379 | 3L: 2,889,912 | Intron | Y |
| 16 | 6.90E-06 | Shab | FBgn0262593 | 3L: 2,927,299 | Intron | Y |
| 17 | 6.90E-06 | btsz | FBgn0053555 | 3R: 10,635,250 | Synonymous | N |
| 18 | 6.90E-06 | sima | FBgn0015542 | 3R: 25,901,368 | Intron | N |
| 19 | 6.90E-06 | CG32532 | FBgn0052532 | X: 19,433,004 | Intron | Y |
| 20 | 6.92E-06 | CG32264 | FBgn0052264 | 2R: 20,505,670 | Intron | Y |
| 21d | 6.92E-06 | CG10858 | FBgn0035458 | 3L: 3,753,223 | Intron | N |
| 22d | CG13594 | FBgn0035041 | 3L: 3,753,223 | Intron | Y | |
| 23 | 7.00E-06 | CG6163 | FBgn0036155 | 3L: 11,352,338 | Downstream | N |
| 24 | 7.41E-06 | sti | FBgn0002466 | 3L: 12,506,163 | Synonymous | N |
| 25 | 8.15E-06 | CG32834 | FBgn0052834 | 2R: 18,871,486 | Synonymous | N |
| 26 | 8.15E-06 | RFeSP | FBgn0021906 | 2L: 1,613,800 | Intron | N |
| 27e | 8.15E-06 | CG33095 | FBgn0053095 | 3R: 20,928,676 | Downstream | N |
| 28e | CG34027 | FBgn0054027 | 3R: 20,928,676 | Downstream | N | |
| 29 | 8.15E-06 | uif | FBgn0031879 | 2L: 6,987,724 | Intron | N |
| 30 | 9.01E-06 | Rbp6 | FBgn0260943 | 3L: 17,073,613 | Intron | N |
| 31 | 9.01E-06 | CG10962 | FBgn0030073 | X: 8,931,792 | Intron | N |
| 32 | 9.34E-06 | Zasp66 | FBgn0035917 | 3L: 8,631,316 | Intron | Y |
| 33 | 9.54E-06 | Rim | FBgn0053547 | 3R: 10,635,250 | Intron | Y |
Chr, chromosome; Neuro, neurological function; Y, yes; N, no. * P-value cutoff of P < 10−6.
Rank order of the most significant associated SNP in a particular gene.
See text for description of neurological functions.
These three SNPs each can be each associated with two different genes.
Figure 2 .
Average expression of candidate genes associated with sperm competition in different tissues. The mean expression levels of the 33 candidate genes are higher in neuronal tissues (dark shading) than in nonneuronal tissues (light shading) (P = 0.0019). Tissue-specific expression data were taken from FlyAtlas. TaG, thoracicoabdominal ganglion; SG, salivary gland; Spt, spermatheca; L_CNS, larval central nervous system. Mean ± SD is shown.
SNPs in genes with potential roles in sperm storage associated with sperm competition:
We predicted that at least some of the top candidate genes should have known functions in sperm competition. Two of the 18 nonneurological genes with SNPs associated with sperm competition may have specific functions in sperm competition. These two genes, CG32834 and CG10962, are both spermathecae-specific genes in the adult fly [FlyAtlas (Chintapalli et al. 2007)]. CG32834 encodes a predicted protease and CG10962 encodes a protein predicted to be involved in oxidation reduction. Both classes of protein may play roles in maintaining sperm in storage by activating sperm or maintaining sperm while in storage. The observation that at least some of the top 50 associated candidate SNPs are in genes with potential roles in sperm storage lends support to the validity of our methods and results.
Candidate neurological genes associated with sperm competition:
The 15 neurological genes whose SNPs associate with sperm competition outcome fall into diverse functional categories. Five of these genes are either ion channels (para, SK, and Shab) or neuronal trafficking genes (Rab2 and Rim). para is the only gene in the D. melanogaster genome to encode a voltage-gated sodium channel (Loughney et al. 1989). para is homologous to the mammalian α-subunits of voltage-gated sodium channels and plays an important role in neuronal excitability (Goldin et al. 2000). SK (small conductance calcium-activated potassium channel) encodes a non-voltage–dependent potassium channel critical to excitable cells by linking Ca2+ concentrations within a cell to membrane hyperpolarization (Stocker 2004). Shab encodes the Shaker cognate b-subunit and is solely responsible for the delayed rectifier current in neurons and muscles of Drosophila (Hegde et al. 1999).
Rab2 and Rim both encode nervous system-specific trafficking proteins. Rab2 is a Rab GTPase that is mainly expressed in the Drosophila nervous system (Zhang et al. 2007). It is required for proper vesicle transport from the endoplasmic reticulum to the Golgi. Rab2 is also involved in the biogenesis of dense core vesicles, a form of neuropeptide release (Edwards et al. 2009; Sumakovic et al. 2009). Rim is an adaptor protein localized to the presynaptic zone of a neuron. Rim interacts with various Rab proteins to regulate the release of neurotransmitters (Wang and Sudhof 2003).
The 10 remaining neurological candidate genes have a variety of neuronal functions. Two genes are broadly involved in muscle development (Msp-300 and Zasp66) (Rosenberg-Hasson et al. 1996). Three other genes are involved in the development of the nervous system (caup, Ddr, and spz5) (Gomez-Skarmeta and Modolell 1996; Gomez-Skarmeta et al. 1996; Zhu et al. 2008; Mummery-Widmer et al. 2009). Finally, 5 of the genes with SNPs associated with sperm competition levels are highly expressed in the nervous system [FlyAtlas (Chintapalli et al. 2007)], but have not been functionally tested for neuronal function (CG42796, CG15765, CG32532, CG32264, and CG13594).
Some additional nonneurological genes associated with sperm competition:
Sixteen of 18 nonneurological genes fall into various functional classes and do not give a clear indicator of pathways or systems that could affect sperm competition. For example, genes like Wrinkled, which plays a role in cell death (Bilak and Su 2009), and sticky, which is involved in cytokinesis (Naim et al. 2004), contain SNPs associated with sperm competition in females, but it is not obvious how or whether these genes may affect differential sperm use. Several of the nonneurological genes, such as CG31872 and CG15800, are male-specific genes expressed mainly in the accessory glands or testis, respectively. Our experimental design did not allow for estimation of a false positive rate and instead uses this first tier of association testing merely as a means to nominate candidate genes. Future experimentation is needed to establish a functional role of these genetic variants in sperm competition.
Functional testing
To validate potential roles of neurological genes in sperm competition in females, we used RNAi to ask whether reduction in the expression of these genes changes sperm competition outcomes. These candidates could be potentially involved in the development of neural networks specific for sperm competition and/or they could function at the time of sperm competition. Given that knockdown of at least some of these neural genes is lethal or affects behaviors unrelated to sperm competition (e.g., locomotion behaviors that could affect courtship or mating) (Loughney et al. 1989; Lloyd et al. 2000; Schulte et al. 2010), we chose to test a specific hypothesis: that neural genes might influence sperm competition through neurons that innervate the female reproductive tract. This approach likely underestimates the extent of contribution of these genes that we found, but is a simple, initial, and direct test of function.
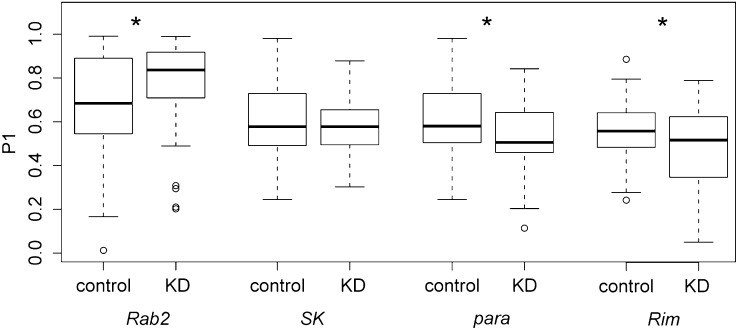
Candidate gene function was tested by knocking down expression in the ppk+ sensory neurons that innervate the female reproductive tract. We tested candidates in these neurons because SPR, the only female gene known to be important for sperm competition, signals through them (Hasemeyer et al. 2009; Yang et al. 2009). Knockdown was achieved with the ppk-GAL4 driver that is specifically expressed in this subset of neurons (Grueber et al. 2007). Knockdown females were compared to controls in a sperm competition experiment identical to the DGRP experiment described above. Because we sought to test candidates in neurons (rather than muscle), we chose candidate genes with clear functions in neurons. Four of the neurological candidates, with various levels of significance in the association study (Figure S4), were chosen for functional testing: Rab2, SK, para, and Rim.
Knockdown of three of these genes significantly affected P1 score. When Rab2 was knocked down in ppk+ neurons, knockdown females had a significant increase in P1 score compared to control females [control median P1 = 0.685, N = 38; knockdown (KD) median P1 = 0.836, N = 40; P = 0.04] (Figure 3). Thus loss of Rab2 function in ppk+ neurons results in an increase in first-male progeny compared to controls. Knockdown of para in sensory neurons resulted in reduction in P1 score compared to that in control females (control median P1 = 0.580, N = 40; KD median P1 = 0.506, N = 42; P = 0.008) (Figure 3). Similarly, knockdown of Rim in sensory neurons also resulted in a significant reduction in P1 score (control median P1 = 0.560, N = 44; KD median P1 = 0.519, N = 41; P = 0.017) (Figure 3). Knockdown of para or Rim in ppk+ neurons results in production of fewer first-male progeny after a second mating.
Figure 3 .
Level of first-male sperm precedence (P1 score) in RNAi knockdown of select neurological candidates. Three of the four candidates chosen for functional analysis affect P1 score when knocked down in sensory neurons innervating the female reproductive tract. KD, knockdown; *P < 0.05.
We observed no difference in P1 score when SK was knocked down in ppk+ neurons (control median P1 = 0.578, N = 40; KD median P1 = 0.578, N = 40; P = 0.344) (Figure 3). The previous success of this ppk-GAL4 driver and the lethality of a ubiquitous knockdown of SK (data not shown) suggest that knockdown is occurring, although we cannot gauge its extent. It is unclear whether SK knockdown level was insufficient to give a sperm competition phenotype, whether SK’s role in sperm competition is not through ppk+ neurons, whether compensatory mechanisms are at play, or whether the SK association was a false positive.
Discussion
Despite overwhelming evidence that the female genotype affects sperm competition (Clark and Begun 1998; Clark et al. 1999; Fiumera et al. 2005, 2007; Civetta et al. 2008; Chow et al. 2010), little is known about the molecular mechanisms that underlie these female contributions. We sought to identify potential candidate pathways and molecules in the female that play a role in sperm competition. To do this, we took advantage of the variation captured in the DGRP resource (Mackay et al. 2012). The DGRP is a collection of 192 wild-derived inbred lines from a single population. Full-genome sequence, transcript abundance, and many other attributes have been measured in all these strains. Many studies, including ours, have used this resource to successfully identify novel genetic variation important for adaptive traits (Ayroles et al. 2009, 2011; Mackay et al. 2012; Swarup et al. 2012). This collection is an important resource for understanding how variation at the genome level mediates phenotypic variation. We found extensive variation in the female control of sperm competition, and this phenotypic variation associated with genotypic variation. By taking this unbiased approach, we found, for the first time, that genes involved in the function and/or development of the nervous system appear to play a critical role in sperm competition.
Although numerous male × female interactions must take place before sperm competition occurs, sperm from different males directly compete once they enter the female reproductive tract and mix in the sperm storage organs (Manier et al. 2010). Studies of these organs have given clues about how females might be biasing sperm use. For example, after the second mating, females eject some first-male sperm (Manier et al. 2010). Visualization of sperm in storage showed that sperm move between storage organs and mixing within the seminal receptacle (Manier et al. 2010). Any of these processes could act to bias sperm use. In some insects, the female preferentially places a certain male’s sperm in an advantageous position within the sperm storage organs (Bloch Qazi 2003; Bussiere et al. 2010). These dynamic sperm movements might be influenced, in some part, by male seminal proteins or the morphology of sperm themselves, but it is also likely that muscle contractions and active movements within the female tract are important factors that facilitate such movement.
Previous studies have demonstrated that an intact female nervous system is required for proper sperm storage in Drosophila: as females with masculinized nervous systems showed abnormal sperm storage (Arthur et al. 1998). In Tribolium beetles, isolated female abdomens showed abnormal sperm storage, also suggesting the need for nervous system input (Bloch Qazi et al. 1998). While these experiments do not shed light on the specific mechanism(s) in which the female nervous system affects sperm competition, they suggest that females are not passive players in sperm storage. This point is underscored by recent work showing that proper sperm storage requires the neuromodulators octopamine and tyramine within the female (Avila et al. 2012). Also the male-derived accessory gland protein, Acp36DE interacts with unknown female factors to bias sperm competition. Acp36DE is required for uterine conformation in mated females (Avila and Wolfner 2009). These conformational changes play a role in moving sperm into storage. Neurological control of sperm storage organs and other parts of the female reproductive tract suggests that females actively store and maintain sperm. These various female actions suggest several routes by which the female could affect the outcome of sperm competition.
To validate our set of candidate genes, we chose to test a specific mechanism (one of many possibilities) by which the female nervous system may be involved in sperm competition. Selected candidates were knocked down in ppk+ sensory neurons innervating the female reproductive tract. This class of neurons is of particular relevance to sperm competition for several reasons: (1) in locusts, sensory neurons innervating the female reproductive tract play a role in sperm and egg release, both of which are important for differential sperm usage (Clark and Lange 2001); (2) silencing these sensory neurons in Drosophila increases postmating receptivity, so they affect the amount of remating and thus the timing and amount of competing sperm the female receives (Hasemeyer et al. 2009; Yang et al. 2009); and (3) the seminal protein Sex Peptide (SP) causes at least some postmating changes through the Sex Peptide Receptor (SPR) in these neurons (Hasemeyer et al. 2009; Yang et al. 2009). We have previously shown that the interaction between SP and SPR is critical for sperm competition outcomes (Chow et al. 2010). Taken together, these lines of evidence suggest that ppk+ neurons compose a promising site at which to begin dissecting female neuronal control of sperm competition.
P1, the proportion of offspring sired by the first male in doubly mated females, is the endpoint of complex interactions between males and females that extends beyond the reproductive tract and reproductive proteins. While we present evidence that the nervous system may influence P1 through variation in ppk+ neuron function, it is impossible to know for sure the specific mechanism driving the P1 score association with variation in neurological genes. For example, because we did not observe the matings, we cannot be certain that the variation in P1 score does not arise from precopulatory interactions, such as a female’s ability to detect male social signals, which would require the female sensory systems. Differences in P1 score could also arise from variation in copulation duration, where duration may determine the amount of sperm a male transfers (Gilchrist and Partridge 2000). This can also be altered based on the female’s detection of pheromones transferred during the copulation or her ability to remove a male’s sperm, both requiring the nervous system. Similarly, as discussed above, female sperm storage could be influenced by the nervous system. Nevertheless, our results demonstrate, for the first time, that the final P1 score of a sperm competition may rely heavily on the female nervous system.
The results from our functional tests should be viewed as a “proof of principle.” The SNP associations we identified were either noncoding or synonymous SNPs, indicating that the effect of each SNP is likely on temporal or spatial expression levels of the genes with which they associated (unless they affect splicing in some as yet unknown way). Since the majority of the top 50 candidates are essential genes, the presence of potential regulatory variation and the lack of nonsynonymous polymorphism are unsurprising, as severe mutations in these genes would likely be eliminated from the population. Given these observations, the knockdown of candidate genes does not test the function of each SNP, but instead tests whether perturbing the particular gene’s expression could affect sperm competition. Three of four tested candidates had a significant effect on sperm competition when knocked down in a specific set of sensory neurons innervating the female reproductive tract. This is not proof that the SNPs identified in this study function by altering the expression of these genes in sensory neurons innervating the reproductive tract, but a demonstration of one possible mechanism by which these genes could act.
Our results have important implications for future studies of sperm competition. Although it is accepted that both males and females contribute to sperm competition, efforts to understand the female contribution have focused on female-specific molecules and the female reproductive tract. Our results demonstrate the importance of expanding research beyond female-specific reproductive systems to more global and basic aspects of the female’s physiology. The results of this study are the first indication that one role of the female nervous system is to bias sperm use, suggesting an active role by the female during sperm competition. A comprehensive view of the complex interactions between males and females and the evolution of sperm competition cannot be achieved until we fully understand the mechanisms behind the phenotypes observed.
Supplementary Material
Acknowledgments
We thank J. Ayroles, F. Avila, G. Findlay, C. D. Rubinstein, L. Sirot, and anonymous reviewers for helpful comments. This work was funded by National Institutes of Health/National Institute of Child Health and Human Development (NIH/NICHD) grant R01-HD059060 (to M.F.W. and A.G.C.). For part of this research, C.Y.C. was supported by a traineeship under an NIH/NICHD training grant in reproductive genomics (T32-HD052471). Subsequently he was supported by NIH/National Research Service Award fellowship 1F32GM093663-01.
Footnotes
Communicating editor: L. Cooley
Literature Cited
- Allen A. K., Spradling A. C., 2008. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development 135: 311–321 [DOI] [PubMed] [Google Scholar]
- Arthur B. I., Jr, Hauschteck-Jungen E., Nothiger R., Ward P. I., 1998. A female nervous system is necessary for normal sperm storage in Drosophila melanogaster: a masculinized nervous system is as good as none. Proc. R. Soc. Lond. 265: 1749–1753 [Google Scholar]
- Avila F. W., Wolfner M. F., 2009. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc. Natl. Acad. Sci. USA 106: 15796–15800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila F. W., Bloch Qazi M. C., Rubinstein C. D., Wolfner M. F., 2012. A requirement for the neuromodulators octopamine and tyramine in Drosophila melanogaster female sperm storage. Proc. Natl. Acad. Sci. USA 109: 4562–4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroles J. F., Carbone M. A., Stone E. A., Jordan K. W., Lyman R. F., et al. , 2009. Systems genetics of complex traits in Drosophila melanogaster. Nat. Genet. 41: 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroles J. F., Laflamme B. A., Stone E. A., Wolfner M. F., Mackay T. F., 2011. Functional genome annotation of Drosophila seminal fluid proteins using transcriptional genetic networks. Genet. Res. 93: 387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilak A., Su T. T., 2009. Regulation of Drosophila melanogaster pro-apoptotic gene hid. Apoptosis 14: 943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Qazi M., Aprille J. R., Lewis S. M., 1998. Female role in sperm storage in the red flour beetle, Tribolium castaneum. Comp. Biochem. Physiol. 120: 641–647 [Google Scholar]
- Bloch Qazi M. C., 2003. A potential mechanism for cryptic female choice in a flour beetle. J. Evol. Biol. 16: 170–176 [DOI] [PubMed] [Google Scholar]
- Bussiere L. F., Demont M., Pemberton A. J., Hall M. D., Ward P. I., 2010. The assessment of insemination success in yellow dung flies using competitive PCR. Mol. Ecol. Resour. 10: 292–303 [DOI] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720 [DOI] [PubMed] [Google Scholar]
- Chow C. Y., Wolfner M. F., Clark A. G., 2010. The genetic basis for male × female interactions underlying variation in reproductive phenotypes of Drosophila. Genetics 186: 1355–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta A., Rosing K., Fisher J., 2008. Differences in sperm competition and sperm competition avoidance in Drosophila melanogaster. Anim. Behav. 75: 1739–1746 [Google Scholar]
- Clark A. G., Begun D. J., 1998. Female genotypes affect sperm displacement in Drosophila. Genetics 149: 1487–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. G., Aguadé M., Prout T., Harshman L. G., Langley C. H., 1995. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics 139: 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. G., Begun D. J., Prout T., 1999. Female x male interactions in Drosophila sperm competition. Science 283: 217–220 [DOI] [PubMed] [Google Scholar]
- Clark J., Lange A. B., 2001. Evidence of a neural loop involved in controlling spermathecal contractions in Locusta migratoria. J. Insect Physiol. 47: 607–616 [DOI] [PubMed] [Google Scholar]
- Edwards S. L., Charlie N. K., Richmond J. E., Hegermann J., Eimer S., et al. , 2009. Impaired dense core vesicle maturation in Caenorhabditis elegans mutants lacking Rab2. J. Cell Biol. 186: 881–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera A. C., Dumont B. L., Clark A. G., 2005. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics 169: 243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera A. C., Dumont B. L., Clark A. G., 2007. Associations between sperm competition and natural variation in male reproductive genes on the third chromosome of Drosophila melanogaster. Genetics 176: 1245–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina T. J., Beavis A., Clark A. G., Fiumera A. C., 2011. Female influence on pre- and post-copulatory sexual selection and its genetic basis in Drosophila melanogaster. Mol. Ecol. 20: 4098–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A. S., Partridge L., 2000. Why it is difficult to model sperm displacement in Drosophila melanogaster: the relation between sperm transfer and copulation duration. Evolution 54: 534–542 [DOI] [PubMed] [Google Scholar]
- Goldin A. L., Barchi R. L., Caldwell J. H., Hofmann F., Howe J. R., et al. , 2000. Nomenclature of voltage-gated sodium channels. Neuron 28: 365–368 [DOI] [PubMed] [Google Scholar]
- Gomez-Skarmeta J. L., Modolell J., 1996. Araucan and caupolican provide a link between compartment subdivisions and patterning of sensory organs and veins in the Drosophila wing. Genes Dev. 10: 2935–2945 [DOI] [PubMed] [Google Scholar]
- Gomez-Skarmeta J. L., Diez del Corral R., de la Calle-Mustienes E., Ferre-Marco D., Modolell J., 1996. Araucan and caupolican, two members of the novel Iroquois complex, encode homeoproteins that control proneural and vein-forming genes. Cell 85: 95–105 [DOI] [PubMed] [Google Scholar]
- Grueber W. B., Ye B., Yang C. H., Younger S., Borden K., et al. , 2007. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development 134: 55–64 [DOI] [PubMed] [Google Scholar]
- Harshman L. G., Prout T., 1994. Sperm displacement without sperm transfer in Drosophila melanogaster. Evolution 48: 758–766 [DOI] [PubMed] [Google Scholar]
- Hasemeyer M., Yapici N., Heberlein U., Dickson B. J., 2009. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61: 511–518 [DOI] [PubMed] [Google Scholar]
- Hegde P., Gu G. G., Chen D., Free S. J., Singh S., 1999. Mutational analysis of the Shab-encoded delayed rectifier K(+) channels in Drosophila. J. Biol. Chem. 274: 22109–22113 [DOI] [PubMed] [Google Scholar]
- Innocenti P., Morrow E. H., 2010. The sexually antagonistic genes of Drosophila melanogaster. PLoS Biol. 8: e1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapelnikov A., Zelinger E., Gottlieb Y., Rhrissorrakrai K., Gunsalus K. C., et al. , 2008. Mating induces an immune response and developmental switch in the Drosophila oviduct. Proc. Natl. Acad. Sci. USA 105: 13912–13917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawniczak M. K., Begun D. J., 2004. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome 47: 900–910 [DOI] [PubMed] [Google Scholar]
- Lloyd T. E., Verstreken P., Ostrin E. J., Phillippi A., Lichtarge O., et al. , 2000. A genome-wide search for synaptic vesicle cycle proteins in Drosophila. Neuron 26: 45–50 [DOI] [PubMed] [Google Scholar]
- Loughney K., Kreber R., Ganetzky B., 1989. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58: 1143–1154 [DOI] [PubMed] [Google Scholar]
- Mackay T. F., Richards S., Stone E. A., Barbadilla A., Ayroles J. F., et al. , 2012. The Drosophila melanogaster Genetic Reference Panel. Nature 482: 173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manier M. K., Belote J. M., Berben K. S., Novikov D., Stuart W. T., et al. , 2010. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science 328: 354–357 [DOI] [PubMed] [Google Scholar]
- McGraw L. A., Gibson G., Clark A. G., Wolfner M. F., 2004. Genes regulated by mating, sperm or seminal proteins in mated female Drosophila melanogaster. Curr. Biol. 14: 1509–1514 [DOI] [PubMed] [Google Scholar]
- McGraw L. A., Clark A. G., Wolfner M. F., 2008. Post-mating gene expression profiles of female Drosophila melanogaster in response to time and to four male accessory gland proteins. Genetics 179: 1395–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw L. A., Gibson G., Clark A. G., Wolfner M. F., 2009. Strain-dependent differences in several reproductive traits are not accompanied by early postmating transcriptome changes in female Drosophila melanogaster. Genetics 181: 1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. L., Page J. L., Wolfner M. F., 2007. An ectopic expression screen reveals the protective and toxic effects of Drosophila seminal fluid proteins. Genetics 175: 777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery-Widmer J. L., Yamazaki M., Stoeger T., Novatchkova M., Bhalerao S., et al. , 2009. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature 458: 987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim V., Imarisio S., Di Cunto F., Gatti M., Bonaccorsi S., 2004. Drosophila citron kinase is required for the final steps of cytokinesis. Mol. Biol. Cell 15: 5053–5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokupek A., Hoffmann F., Eyun S. I., Moriyama E., Zhou M., et al. , 2008. An evolutionary expressed sequence tag analysis of Drosophila spermatheca genes. Evolution 62: 2936–2947 [DOI] [PubMed] [Google Scholar]
- Prokupek A. M., Kachman S. D., Ladunga I., Harshman L. G., 2009. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster. Insect Mol. Biol. 18: 465–475 [DOI] [PubMed] [Google Scholar]
- Prokupek A. M., Eyun S. I., Ko L., Moriyama E. N., Harshman L. G., 2010. Molecular evolutionary analysis of seminal receptacle sperm storage organ genes of Drosophila melanogaster. J. Evol. Biol. 23: 1386–1398 [DOI] [PubMed] [Google Scholar]
- Rosenberg-Hasson Y., Renert-Pasca M., Volk T., 1996. A Drosophila dystrophin-related protein, MSP-300, is required for embryonic muscle morphogenesis. Mech. Dev. 60: 83–94 [DOI] [PubMed] [Google Scholar]
- Schnakenberg S. L., Matias W. R., Siegal M. L., 2011. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 9: e1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte J., Sepp K. J., Jorquera R. A., Wu C., Song Y., et al. , 2010. DMob4/Phocein regulates synapse formation, axonal transport, and microtubule organization. J. Neurosci. 30: 5189–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot L. K., LaFlamme B. A., Sitnik J. L., Rubinstein C. D., Avila F. W., et al. , 2009. Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv. Genet. 68: 23–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M., 2004. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat. Rev. Neurosci. 5: 758–770 [DOI] [PubMed] [Google Scholar]
- Sumakovic M., Hegermann J., Luo L., Husson S. J., Schwarze K., et al. , 2009. UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans. J. Cell Biol. 186: 897–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup S., Harbison S. T., Hahn L. E., Morozova T. V., Yamamoto A., et al. , 2012. Extensive epistasis for olfactory behaviour, sleep and waking activity in Drosophila melanogaster. Genet. Res. 94: 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sudhof T. C., 2003. Genomic definition of RIM proteins: evolutionary amplification of a family of synaptic regulatory proteins(small star, filled). Genomics 81: 126–137 [DOI] [PubMed] [Google Scholar]
- Wigby S., Chapman T., 2004. Female resistance to male harm evolves in response to manipulation of sexual conflict. Evolution 58: 1028–1037 [DOI] [PubMed] [Google Scholar]
- Wolfner M. F., 2009. Battle and ballet: molecular interactions between the sexes in Drosophila. J. Hered. 100: 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner M. F., 2011. Precious essences: female secretions promote sperm storage in Drosophila. PLoS Biol. 9: e1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A., Albright S. N., Giebel J. D., Ram K. R., Ji S., et al. , 2008. A role for Acp29AB, a predicted seminal fluid lectin, in female sperm storage in Drosophila melanogaster. Genetics 180: 921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Rumpf S., Xiang Y., Gordon M. D., Song W., et al. , 2009. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61: 519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Schulze K. L., Hiesinger P. R., Suyama K., Wang S., et al. , 2007. Thirty-one flavors of Drosophila rab proteins. Genetics 176: 1307–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Pennack J. A., McQuilton P., Forero M. G., Mizuguchi K., et al. , 2008. Drosophila neurotrophins reveal a common mechanism for nervous system formation. PLoS Biol. 6: e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.