Abstract
Background and Purpose
Stroke during pregnancy is an emerging concern. Although females undergo many physiological, endocrine, and neurological alterations during pregnancy, the consequences of such changes on outcome after stroke are unclear. It is predicted that increases in steroid hormones observed during pregnancy may confer protective effects against the neurological and pathological sequelae of stroke.
Methods
We therefore investigated behavioral and histological consequences of a global cerebral ischemia (2-vessel occlusion; 2VO), and how these outcomes correlated with pregnancy-related changes in hormones in Sprague-Dawley rats.
Results
After the 2VO, pregnant rats exhibited poorer memory in a contextual fear conditioning test of learning and memory than sham-treated controls, whereas nonpregnant rats did not. They also showed enhanced CA1 hippocampal neuronal injury. This susceptibility to damage is despite significant pregnancy-associated hypothermia and is probably not associated with alterations in 17β-estradiol or corticosterone levels.
Conclusion
These findings are the first to show enhanced neuronal damage in pregnant animals after global cerebral ischemia. They also suggest that the mechanism may be independent of changes in estrogen, corticosterone, and body temperature.
Keywords: contextual fear conditioning, hippocampus, temperature
During pregnancy the mother undergoes myriad changes to her physiology, including physical and neuroendocrine changes, many of which should be neuroprotective against such neurological insults as stroke. Thus, hypothalamic-pituitary-adrenal (HPA) axis activity is altered and basal corticosterone upregulated in late pregnancy,1,2 estrogen and progesterone rise throughout most of pregnancy and are reduced in the last few days,3,4 the cardiovascular system is remodeled,5,6 and body temperature is chronically lowered.7 There is much to suggest that many, or all, of these changes associated with pregnancy should protect the brain from ischemic damage. For instance, estrogen is known to have neuroprotective effects in the brain after cerebral ischemia and ovariectomized rats sustain more damage,8,9 whereas corticosterone reduces infarct sizes in hypoxic-ischemic neonates.10,11 Hypothermia too is neuroprotective and is one of the few treatments successful in ameliorating ischemic damage in humans.12,13 In spite of this, there is a distinct absence of evidence to suggest that such alterations are associated with a better prognosis after stroke during pregnancy. Stroke, in humans, is a significant cause of maternal mortality and morbidity during late pregnancy,14,15 and significantly more pregnant than nonpregnant women of the same age present with a stroke.14–16
Although stroke in young women is rare,15,17 the current trend toward delayed childbearing18 suggests that age-related neurological events, such as stroke, will become more prevalent during pregnancy and are therefore critical to investigate. Thus, in the present study we asked: is pregnancy associated with a better neurological outcome after global cerebral ischemia in a rat model, and what might be some of the factors influencing cell damage in the pregnant state? Although the 2-vessel occlusion (2VO) with hypotension can be regarded as a model of cardiac arrest, we used this model as we have shown it to produce very consistent damage within the same treatment group in males19,20 and we could examine vulnerability to damage in many brain regions. In addition, there is reported evidence that pregnancy can increase the risk of myocardial infarct 3 to 4 times21 and is associated with increased “arterial ischemic strokes” including cerebral hypoperfusion attributable to heart failure and cardioembolism.22 To determine the impact of pregnancy on the outcome after cerebral ischemia, we examined the effects of a 2VO in pregnant and nonpregnant rats. We assessed learning and memory performance in a test of contextual fear conditioning, and hippocampal cell survival and degeneration along with body temperature and steroid hormone levels to identify some factors influencing cell damage.
Materials and Methods
Animals
Experiments were conducted on 58 female randomly cycling virgin/nulliparous (nonpregnant) or gestational day (G)17 or 19 (pregnant) Sprague-Dawley rats (Charles River; Quebec, Canada). All procedures were in accordance with the Canadian Council on Animal Care regulations and were approved by the University of Calgary Animal Care Committee.
Surgery
All rats were deprived of food overnight before the 2VO to normalize blood glucose.23 Under halothane anesthesia, we implanted a sterile, silicone-coated temperature data logger (SubCue Data loggers), preprogrammed to record temperature every 5 minutes, into the abdominal cavity. Rats were then subjected to a 2VO (carotid arteries occluded simultaneously for 15 minutes) with hypotension (blood pressure [MAP] maintained between 55 and 60 mm Hg by adjustments of the halothane anesthesia) as has been previously described for males.19,20 Initial and 15 minutes MAP, minimum and changes in MAP at reperfusion, and final MAP were not different between nonpregnant and pregnant groups. We estimated brain temperature using an ear thermocouple and maintained it between 37 and 38°C using a heating lamp. Control “sham” rats were treated identically except we did not occlude the carotid arteries or reduce MAP.
Behavioral Testing
One day before surgery, a subset of the rats was used in a contextual fear conditioning test. Rats were placed individually in a conditioning chamber (Coulbourn Instruments) for 3 minutes (baseline) and were then given 3 unsignaled footshocks through a metal grid floor (2 s, 0.5 mA, 60 s interstimulus interval). During the baseline period, spontaneous motor activity (midline chamber crossovers) was recorded. Every 8s after each footshock, rats were scored for defensive freezing— cessation of all movement except that required for breathing. Subjects were returned to their cages 60 s after the last footshock and the conditioning apparatus cleaned with 10% ethanol. Approximately 72 hours after the 2VO, rats were again placed into the conditioning chamber for the extinction trial and were scored for defensive freezing every 8s for 8 minutes. Both postshock and extinction-trial scores were converted to a percentage of time spent freezing.24 Scores are presented as a ratio of extinction to postshock freezing, <1.0 representing reduced freezing during the extinction trial compared with postshock, consistent with impaired performance.
Also ≈72 hours after the 2VO, the remaining rats were assessed for locomotor activity and anxiety in the open field as previously described.19,25 Each rat was observed for 10 minutes for locomotion, middle arena exploration, and rearing. All tests were conducted during the light phase, between 8:00 AM and 11:00 AM.
Perfusions
Immediately after behavioral testing, rats were anesthetized (100 mg/kg i.p. sodium pentobarbital) and, within 3 to 4 minutes, blood (≈1 mL) was removed from the left ventricle of the open field-tested rats for hormone level assessment. The rats were then perfused with 4°C phosphate-buffered saline via the left cardiac ventricle, and the right hemisphere of the brain postfixed in 10% paraformaldehyde. The left hemisphere was snap-frozen for use in other experiments. Number and appearance of the fetuses was also assessed at this point in pregnant animals.
Histology
Extent of ischemic injury was quantified by histological analysis of remaining hippocampal neurons in paraffin-embedded 10 μm toluidine blue-stained coronal sections. Neuronal survival was evaluated by an experimenter, blind to the rats’ treatment group, in the CA1 and the CA3 subfields of the hippocampus ≈4.2 mm caudal to bregma. Numbers of degenerating cells were quantified by analysis of Fluoro-Jade-stained CA1 and CA3 hippocampal sections adjacent to those used for toluidine blue.19,20
Preliminary counts of 4 rostrocaudal regions of the hippocampus from each animal revealed no differences across levels in counts of surviving neurons, provided the relative size of the hippocampus was accounted for. We therefore examined cell damage at one rostrocaudal level only. We also qualitatively examined the dentate gyrus, which was unaffected in each case.
Immunohistochemistry
Sections adjacent to those used for Fluoro-Jade were immunolabeled for microglia/macrophages.26 Briefly, sections were incubated in primary Iba1 antibody (overnight; room temperature; 1:400; rabbit; Wako Chemicals USA, Inc., Richmond, VA), followed by secondary antibody (2 hour; 1:400: donkey anti-rabbit IgG [Cy3]; Jackson ImmunoResearch Laboratories, West Grover, PA, USA).
Because of the extremely high numbers of labeled microglia in the hippocampus, we analyzed, blind to treatment, only a subsection of each hippocampal section as described previously.20 Activated microglia were distinguished from inactive by their shorter, less ramified processes, perikaryal hypertrophy, and ameboid appearance.26
Assessment of Plasma Hormones
Plasma concentrations of 17β-estradiol were analyzed by Calgary Laboratory Services and corticosterone by us using a standard enzyme-linked-immunosorbent assay (ELISA) kit (Assay Designs). The interassay variabilities for these assays were, respectively, 2.3 to 6.2 and 7.8 to 13.1% CV; intraassay variabilities 1.6 to 5.7 and 6.6 to 8.4% CV; lower limit of detection 10 pg/mL and 32 pg/mL. All samples from each experiment were assayed together and in duplicate.
Data Analysis
All behavioral, histological, and hormonal scores were compared using 2-way analyses of variance (ANOVA) with pregnancy and surgery as between factors, followed by 1-way ANOVA with Student-Neuman-Keuls post hoc comparisons as appropriate. Temperature data for the days after the 2VO were averaged from 10:00 AM to 4:00 PM and 10:00 PM to 4:00 AM, giving a mean temperature for day- and nighttime, respectively. These were compared as above. We also determined 2-tailed Pearson correlation coefficients correlating surviving cell numbers with numbers of activated microglia, corticosterone, and 17β-estradiol. Statistical significance was assumed when P<0.05. All data are presented as mean±SEM.
Results
Mortality
Initially we chose G19 as representative of late pregnancy to administer the 2VO. Of the 5 G19 rats subjected to 2VO, 4 unexpectedly died 1 day after the surgery. These deaths were not a direct effect of surgery or anesthetic as all the rats awoke within 1 hour and appeared healthy on the evening after surgery. It is possible that these deaths were attributable to complications with parturition as a consequence of the 2VO. However, we were reluctant to persevere with a pregnancy stage resulting in such high and inexplicable mortality. Therefore we used surgery at G17 as our pregnancy group where survival rate was 100% after surgery. All nonpregnant rats also survived. Both groups had similar initial MAP, and during the 2VO both groups sustained similar reductions in MAP and recovered to the same degree. All pregnant rats had healthy-looking fetuses of consistent size and number for stage of pregnancy.
Body Temperature
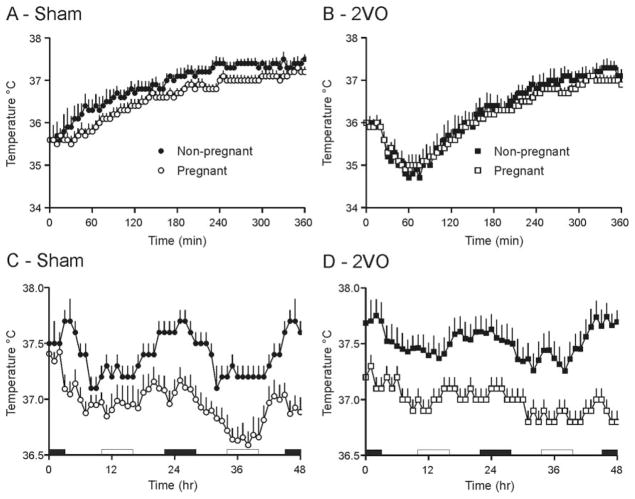
Temperatures at onset and for 6 hours after surgery were similar between both groups of sham and 2VO rats (n=11 to 15; Figure 1A and 1B). Nonpregnant and pregnant 2VO rats displayed indistinguishable hypothermia reaching a nadir at 1 hour after onset of occlusion and returning to initial temperatures by 3 hours, after which temperatures were similar to sham-treated animals.
Figure 1.
Body temperatures in the 6 hours (A and B) and 2 days (C and D) after 2VO or sham surgery in nonpregnant and pregnant rats. C and D, Temperatures are recordings from 5-minute intervals averaged over 1 hour. Time 0 represents midnight on the night of surgery. White bars indicate the daytime period used for temperature calculations, black bars indicate nighttime. Both sham and 2VO pregnant rats showed significant hypothermia in the 2 days after surgery compared with their nonpregnant counterparts, P<0.05.
Irrespective of surgical treatment, nonpregnant rats maintained normal temperatures and circadian temperature rhythmicity. Pregnancy, however, reduced both nighttime (10:00 PM to 4:00 AM; F=48.11, P<0.0001) and daytime (10:00 AM to 4:00 PM; F=13.08, P=0.001) temperatures (Figure 1C and 1D) so that pregnant rats were hypothermic compared with nonpregnant of the same treatment, particularly during the night (F=16.28, P<0.0001).
Learning and Memory
Initial responses to the contextual fear conditioning test for learning and memory were not significantly affected by pregnancy; both the number of midline crossovers and postshock percentage freezing scores being similar between the 2 groups (data not shown).
Performance in the extinction trial was, however, significantly affected by the 2VO (F=9.70, P=0.005; n=5 to 7; Figure 2), but in pregnant rats only (F=4.93, P=0.01). Thus, pregnant-2VO rats froze less in the extinction trial than immediately postshock, reflecting a significantly greater relative memory deficit than their sham counterparts. The other groups all froze slightly more in the extinction trial than immediately postshock, indicated by an extinction to post-shock ratio >1.
Figure 2.

Ratios of extinction to postshock freezing in the contextual fear conditioning test after 2VO or sham surgery in non-pregnant and pregnant rats. The 2VO significantly affected freezing (memory) in pregnant rats only, *P<0.05.
Locomotion and Anxiety
Significant effects of pregnancy were seen on locomotor activity (F=14.36, P=0.001), exploration of the middle of the arena (F=28.89, P<0.0001) and rearing (F=12.88, P=0.001) in the open field test for locomotion and anxiety (n=6 to 9; Figure 3). Thus, nonpregnant sham-treated rats crossed more lines (F=4.87, P=0.008) and displayed more rearing (F=5.79, P=0.004) than pregnant shams. Both sham and 2VO-treated nonpregnant rats explored the middle of the arena more than their pregnant controls (F=9.90, P<0.0001). No significant effects of surgery were seen on any aspect of open field behavior.
Figure 3.
Open field behavior after 2VO or sham surgery in non-pregnant and pregnant rats. A, Locomotor activity (number of lines crossed). B, Middle exploration (number of crosses through the middle). C, Rearing. Pregnancy had a significant effect on locomotor and exploratory activity, *P<0.05.
Cell Survival and Degeneration
As expected from our previous studies in males,19,20 the 2VO significantly affected cell survival in the CA1 region of the hippocampus (F=10.96, P=0.002; n=10 to 16; Figure 4A,C,D). However, subsequent 1-way ANOVA and post-hoc analyses revealed this reduction to be only significant if the rats were pregnant (F=5.17, P=0.004). Of these animals, only 30% of nonpregnant 2VOs had surviving cell numbers less than 2 standard deviations below the mean for the shams, whereas 60% of pregnant rats were thus affected, indicating that there may be a threshold at which significant cell death occurs that is more easily reached in pregnancy.
Figure 4.
Neuronal survival in the CA1 (A, C, and D) and CA3 (B) regions of the hippocampus after 2VO or sham surgery in nonpregnant (C) and pregnant (D) rats. Degenerating (Fluoro-Jade-positive) cell numbers in the CA1 (E, G, and H) and CA3 (F) regions of the hippocampus after 2VO or sham surgery in nonpregnant (G) and pregnant (H) rats. The 2VO significantly affected CA1 cell survival and degeneration in pregnant rats only, *P<0.05. Scale bars=50 μm. Photomicrographs were selected from a nonpregnant rat with many surviving/few degenerating cells and a pregnant rat with few surviving/many degenerating cells reflecting the majority of the respective groups.
Significant numbers of degenerating cells were also seen after the 2VO (F=13.76, P=0.001; Figure 4E, 4G, and 4H) and, again, only pregnant rats were different from their shams (F=6.53, P=0.001). The CA3 hippocampus was unaffected by either pregnancy state or surgical treatment (Figure 4B and 4F).
Activated Microglia
To investigate potential differences in the inflammatory response to 2VO, we examined microglial activation in the CA1 hippocampus. Analyses revealed no significant differences between the pregnant and nonpregnant groups (n=14 to 16) in mean numbers of activated or resting microglia (data not shown). However, we did see a highly significant inverse correlation between numbers of activated microglia and surviving cell numbers in nonpregnant (R=−0.849, P=0.0001) and pregnant (R=−0.985, P=0.0001) 2VO rats. We also saw significant positive correlations between numbers of resting microglia and surviving cells for both groups (R=0.872, P=0.0001 and R=0.950, P=0.0001 respectively; Figure 5).
Figure 5.
Activated (A) and resting microglia (B) vs surviving cell numbers in the CA1 region of the hippocampus after 2VO or sham surgery in nonpregnant or pregnant rats. Photomicrographs are examples of activated (C) and resting (D) microglia in 2 pregnant 2VO rats, the former having high numbers of activated microglia and low cell survival, the latter low numbers of activated and high of resting microglia and high cell survival. Scale bars=25 μm.
Corticosterone and 17β-Estradiol
Pregnancy was associated with a reduction in the potentially neuroprotective ovarian hormone, 17β-estradiol (F=9.088, P=0.008), ie, sham pregnant rats had significantly less 17β-estradiol than sham nonpregnant (F=4.097, P=0.025), a finding not observed in the 2VO-treated rats (n=4 to 8; Figure 6A). However, there were no significant correlations between 17β-estradiol levels and numbers of surviving CA1 cells (nonpregnant-2VO, R=−0.313, P=0.608; pregnant-2VO, R=−0.089, P=0.834).
Figure 6.

Plasma 17β-estradiol (A) and corticosterone (B) after 2VO or sham surgery in nonpregnant and pregnant rats. Pregnant sham-treated rats had significantly less 17β-estradiol than nonpregnant shams, *P<0.05.
Similarly, there was a trend toward reduced levels of the HPA axis activity-dependent hormone, corticosterone in sham pregnant rats that was not seen with the 2VO (n=6 to 9; Figure 6B). However, no significant differences were seen between any of the groups. Again, no correlation between corticosterone and numbers of surviving CA1 cells was revealed (nonpregnant-2VO, R=−0.249, P=0.59; pregnant-2VO, R=−0.127, P=0.744).
Discussion
Our findings demonstrate, for the first time, that pregnancy can negatively impact the outcome after global ischemia in the rat. We show here that pregnant rats are likely to sustain significant damage to the CA1 region of the hippocampus, have significant numbers of CA1 cells still degenerating after 3 days, and display correspondingly large memory deficits compared with sham-treated pregnant rats, whereas their nonpregnant counterparts are not. This damage is probably specifically hippocampal as we saw no corresponding deficits in exploratory behavior in the open field, which would reflect damage to locomotor or anxiety processing brain regions.27,28
One particularly interesting finding from this study is that the hypothermia associated with pregnancy did not confer a neuroprotective effect after the 2VO. Hypothermia has been shown to attenuate ischemic damage in animal models.29 It is also one of the few promising treatments for alleviating such damage in humans,12,13 but we saw no such neuroprotective effect here. We should note that the neuroprotective effects associated with hypothermia are maximized under certain very specific conditions, such as with particular temperatures30 or timing and duration of application.31 Thus, generalized body-temperature attenuation or an alteration of the usual set point may not have the same neuroprotective effects. It is also possible that the hypothermia is neuroprotective in these rats and that the ischemia-induced damage associated with pregnancy we see here would be further exacerbated in its absence.
One of the more obvious candidates to explain altered susceptibility to damage after global ischemia in the pregnant rat is the ovarian hormone, estrogen. Estrogen has been found in numerous cerebral ischemia studies to have neuroprotective effects.8,9 For instance, its administration can rescue ischemia-induced neuronal death,32,33 whereas gonadectomy in females exacerbates the damage.8,32 It has even been demonstrated that damage associated with cerebral ischemia is greater in females when the insult occurs in metestrous, when endogenous estrogen levels are low, than in proestrous, when estrogen levels are high.34
In this investigation we found lower levels of a representative estrogen, 17β-estradiol, in our pregnant than nonpregnant animals. This finding is puzzling as although estrogen rises throughout the course of pregnancy and is dramatically reduced in late pregnancy,3,4 levels should still remain higher than in nonpregnant rats. We have no explanation for why our pregnant estradiol levels are so low. It could be suggested that the higher mean levels of 17β-estradiol in our nonpregnant rats fits with a certain neuroprotection in this group. However, the reduced 17β-estradiol in pregnancy was restored to nonpregnant levels by the 2VO and we saw no correlation between cell death and 17β-estradiol levels in either the pregnant or nonpregnant groups. In addition, our plasma samples were taken 3 days after the 2VO. At the time of the 2VO, the pregnant rats were at G17 where 17β-estradiol levels should have been elevated compared with G203,4 and perhaps likely to provide more neuroprotection rather than less. Complicating the story further, however, estrogen is also potentially neurotoxic under some conditions, such as when the levels are particularly high.32 Therefore, although our study does not completely rule out a role for estrogen in the pregnancy-exacerbated ischemic damage, the lack of correlation between 17β-estradiol levels and damage provides a strong indication that estrogen is not primarily responsible. It will also be important for future studies to examine the role of progesterone in ischemic damage during pregnancy.
Corticosterone is another candidate hormone that is altered during pregnancy and has the potential to provide neuroprotection during cerebral ischemia. Corticosterone or dexamethasone treatment can reduce infarct sizes in neonates after a hypoxic-ischemic challenge.10,35 However, the proposed neuroprotective effects of corticosterone are again far from clear-cut. For instance, recent evidence suggests that neuro-protection by glucocorticoids could be dependent on their relative activation of mineralocorticoid and glucocorticoid receptors, the former being neuroprotective and the latter, neurotoxic.36
In the current study we saw no indication that differential levels of corticosterone could be responsible for enhanced susceptibility to ischemic damage in pregnant rats. There were no significant differences in mean basal plasma concentrations of the hormone and again no correlation with cell damage. We should note that anesthetics have been shown to elevate corticosterone levels.37 However, it is unlikely that this led to such a high corticosterone elevation as to obscure any potential differences between the groups. We have previously demonstrated much higher corticosterone concentrations ≈200 ng/mL than those seen here using the same type of ELISA in awake rats.38 A similar argument exists for corticosterone as for 17β-estradiol—that we measured levels in the plasma 3 days after the 2VO and these levels may not necessarily be indicative of those at the time of surgery. However, this caveat does not suggest that these 3-day measurements have no validity. Certainly the rats are still subject to the protracted effects of the ischemia. We saw substantial numbers of cells still degenerating in the hippocampus and robust microglial activation at this time point that all indicate a poor prognosis beyond 3 days for the pregnant rats. Thus, any neurodegenerative mechanisms we can identify at this time point would be informative. Clearly it is necessary to investigate this system at different time points to comprehensively rule out involvement of the hormones discussed above.
We did see a significant correlation, at our 3-day time point, between cell damage and microglial activation. However, because such a correlation was observed in nonpregnant as well as pregnant rats, we should be wary of overemphasizing the importance of this. It is impossible to tell from these findings whether the activated microglia are a cause or a consequence of cell death and it seems unlikely, in either case, that they play a differential role during pregnancy.
In addition to those described, many other changes do occur during pregnancy that could potentially predispose animals to damage after cerebral ischemia. For instance, one of the other major changes involves alterations to cardiovascular function.5,6 Although we did not see differences in MAP in the pregnant animals during surgery or associated with reperfusion of the brain, this does not necessarily discount the impact of altered vascular properties. During pregnancy changes in cardiovascular function occur to accommodate the large increase in blood volume and cardiac output required.5,6 The effects we see could therefore be attributable to differences in cerebral blood flow and reperfusion after the 2VO. These changes could be particularly relevant to the outcome after stroke when considering that one of the major driving forces for vascular remodeling during pregnancy is upregulation of nitric oxide production,39,40 and this can also have neuroprotective or deleterious effects in the brain after ischemia. Clearly this is another avenue for further research.
Our investigation is exciting in that it is the first to describe an animal model mimicking human susceptibility during pregnancy to the effects of a cerebral ischemia. We provide some very interesting findings suggesting that increased susceptibility to ischemic damage during pregnancy may not be related to some of the major pregnancy-associated and usually neuroprotective changes in physiology; reduced body temperature, 17β-estradiol, corticosterone.
Acknowledgments
We thank Dr Roland Auer for the use of his surgical facilities and Drs Kathryn M. Buller and Jaideep S. Bains for comments on the manuscript.
Sources of Funding
This work was supported by the Canadian Institutes of Health Research (CIHR). S.J.S. is an Alberta Heritage Foundation for Medical Research (AHFMR) postdoctoral fellow and is also supported by a personnel award from the Heart and Stroke Foundation of Canada, the Canadian Stroke Network, CIHR and AstraZeneca Canada Inc. M.A.G. is supported by AHFMR and Natural Sciences and Engineering Council of Canada (NSERC) graduate student awards. Q.J.P. is an AHFMR Medical Scientist.
Footnotes
Disclosures
None.
References
- 1.Wigger A, Lorscher P, Oehler I, Keck ME, Naruo T, Neumann ID. Nonresponsiveness of the rat hypothalamo-pituitary-adrenocortical axis to parturition-related events: inhibitory action of endogenous opioids. Endocrinology. 1999;140:2843–2849. doi: 10.1210/endo.140.6.6784. [DOI] [PubMed] [Google Scholar]
- 2.Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cathey TM, Chung KW. The relationship between ovarian steroids and uterine estrogen receptors during late pregnancy. Life Sci. 1991;49:293–298. doi: 10.1016/0024-3205(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 4.de Lauzon S, Uhrich F, Vandel S, Cittanova N, Jayle MF. Determination of progesterone and of free and conjugated estrogens in pregnant and peudo-pregnant rats. Steroids. 1974;24:31–40. doi: 10.1016/0039-128x(74)90043-9. [DOI] [PubMed] [Google Scholar]
- 5.Carbillon L, Uzan M, Uzan S. Pregnancy, vascular tone, and maternal hemodynamics: a crucial adaptation. Obstet Gynecol Surv. 2000;55:574–581. doi: 10.1097/00006254-200009000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Kelly BA, Bond BC, Poston L. Aortic adaptation to pregnancy: elevated expression of matrix metalloproteinases-2 and -3 in rat gestation. Mol Hum Reprod. 2004;10:331–337. doi: 10.1093/humrep/gah045. [DOI] [PubMed] [Google Scholar]
- 7.Fewell JE. Body temperature regulation in rats near term of pregnancy. Can J Physiol Pharmacol. 1995;73:364–368. doi: 10.1139/y95-046. [DOI] [PubMed] [Google Scholar]
- 8.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 9.Raval AP, Bramlett H, Perez-Pinzon MA. Estrogen preconditioning protects the hippocampal CA1 against ischemia. Neuroscience. 2006;141:1721–1730. doi: 10.1016/j.neuroscience.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Tuor UI. Glucocorticoids and the prevention of hypoxic-ischemic brain damage. Neurosci Biobehav Rev. 1997;21:175–179. doi: 10.1016/s0149-7634(96)00007-3. [DOI] [PubMed] [Google Scholar]
- 11.Bertorelli R, Adami M, Di Santo E, Ghezzi P. MK 801 and dexamethasone reduce both tumor necrosis factor levels and infarct volume after focal cerebral ischemia in the rat brain. Neurosci Lett. 1998;246:41–44. doi: 10.1016/s0304-3940(98)00221-3. [DOI] [PubMed] [Google Scholar]
- 12.Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Mol Neurobiol. 1997;14:171–201. doi: 10.1007/BF02740655. [DOI] [PubMed] [Google Scholar]
- 13.Hemmen TM, Lyden PD. Induced hypothermia for acute stroke. Stroke. 2007;38(Suppl-9) doi: 10.1161/01.STR.0000247920.15708.fa. [DOI] [PubMed] [Google Scholar]
- 14.Jaigobin C, Silver FL. Stroke and pregnancy. Stroke. 2000;31:2948–2951. doi: 10.1161/01.str.31.12.2948. [DOI] [PubMed] [Google Scholar]
- 15.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–516. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 16.Bashiri A, Lazer T, Burstein E, Smolin A, Lazer S, Perry ZH, Mazor M. Maternal and neonatal outcome following cerebrovascular accidents during pregnancy. J Matern Fetal Neonatal Med. 2007;20:241–247. doi: 10.1080/14767050601135030. [DOI] [PubMed] [Google Scholar]
- 17.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 18.Ventura SJ, Abma JC, Mosher WD, Henshaw S. Estimated pregnancy rates for the United States, 1990–2000: an update. Natl Vital Stat Rep. 2004;52:1–9. [PubMed] [Google Scholar]
- 19.Spencer SJ, Auer RN, Pittman QJ. Rat neonatal immune challenge alters adult responses to cerebral ischaemia. J Cereb Blood Flow Metab. 2006;26:456–467. doi: 10.1038/sj.jcbfm.9600206. [DOI] [PubMed] [Google Scholar]
- 20.Spencer SJ, Mouihate A, Pittman QJ. Peripheral inflammation exacerbates damage after global ischemia independently of temperature and acute brain inflammation. Stroke. 2007;38:1570–1577. doi: 10.1161/STROKEAHA.106.476507. [DOI] [PubMed] [Google Scholar]
- 21.James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113:1564–1571. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 22.Mas JL, Lamy C. Stroke in pregnancy and the puerperium. J Neurol. 1998;245:305–313. doi: 10.1007/s004150050224. [DOI] [PubMed] [Google Scholar]
- 23.Voll CL, Auer RN. The effect of postischemic blood glucose levels on ischemic brain damage in the rat. Ann Neurol. 1988;24:638–646. doi: 10.1002/ana.410240508. [DOI] [PubMed] [Google Scholar]
- 24.McKay BE, Lado WE, Martin LJ, Galic MA, Fournier NM. Learning and memory in agmatine-treated rats. Pharmacol Biochem Behav. 2002;72:551–557. doi: 10.1016/s0091-3057(02)00724-4. [DOI] [PubMed] [Google Scholar]
- 25.Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge-effects on behavioural indices of adult rat fear and anxiety. Behav Brain Res. 2005;164:231–238. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, Yong VW. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 2003;23:10107–10115. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi E, Takeshita S. Effects of illumination and handling upon rat open field activity. Physiol Behav. 1995;57:699–703. doi: 10.1016/0031-9384(94)00317-3. [DOI] [PubMed] [Google Scholar]
- 29.Colbourne F, Grooms SY, Zukin RS, Buchan AM, Bennett MV. Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc Natl Acad Sci U S A. 2003;100:2906–2910. doi: 10.1073/pnas.2628027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kollmar R, Blank T, Han JL, Georgiadis D, Schwab S. Different degrees of hypothermia after experimental stroke: short- and long-term outcome. Stroke. 2007;38:1585–1589. doi: 10.1161/STROKEAHA.106.475897. [DOI] [PubMed] [Google Scholar]
- 31.Sterz F, Behringer W, Holzer M. Global hypothermia for neuroprotection after cardiac arrest. Acute Card Care. 2006;8:25–30. doi: 10.1080/14628840600621371. [DOI] [PubMed] [Google Scholar]
- 32.Macrae IM, Carswell HV. Oestrogen and stroke: the potential for harm as well as benefit. Biochem Soc Trans. 2006;34:6–5. doi: 10.1042/BST0341362. [DOI] [PubMed] [Google Scholar]
- 33.Gibson CL, Gray LJ, Murphy SP, Bath PM. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26:1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- 34.Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2000;278:H290–H294. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- 35.Tuor UI, Del Bigio MR. Protection against hypoxic-ischemic damage with corticosterone and dexamethasone: inhibition of effect by a glucocorticoid antagonist RU38486. Brain Res. 1996;743:258–262. doi: 10.1016/s0006-8993(96)01054-2. [DOI] [PubMed] [Google Scholar]
- 36.Almeida OF, Conde GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J. 2000;14:779–790. doi: 10.1096/fasebj.14.5.779. [DOI] [PubMed] [Google Scholar]
- 37.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–E828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 38.Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 2005;19:1519–1521. doi: 10.1096/fj.04-3569fje. [DOI] [PubMed] [Google Scholar]
- 39.Weiner CP, Thompson LP. Nitric oxide and pregnancy. Semin Perinatol. 1997;21:367–380. doi: 10.1016/s0146-0005(97)80003-1. [DOI] [PubMed] [Google Scholar]
- 40.Xu DL, Martin PY, St John J, Tsai P, Summer SN, Ohara M, Kim JK, Schrier RW. Upregulation of endothelial and neuronal constitutive nitric oxide synthase in pregnant rats. Am J Physiol. 1996;271:R1739–R1745. doi: 10.1152/ajpregu.1996.271.6.R1739. [DOI] [PubMed] [Google Scholar]