Abstract
Cholesterol efflux from macrophages and the vascular wall is the initial step of the cardiovascular protective reverse cholesterol transport process. This study demonstrates a mass spectrometry based assay to measure the cellular and media content of [d7]-cholesterol and unlabeled cholesterol that can be used to measure cholesterol efflux from cell lines. Using a triple quadrupole ESI-MS instrument in direct infusion mode, product ion scanning for m/z 83, neutral loss (NL) 375.5 scanning and NL 368.5 scanning were used to detect cholesterol (as an acetylated derivative), [d7]-cholesteryl ester (CE) and unlabeled CE, respectively. The same mass of [d7]-cholesterol was substituted for [3H]-cholesterol under standard efflux assay conditions. At the end of [d7]-cholesterol loading, the intracellular mass of [d7]-cholesterol was 2-fold greater than unlabeled cholesterol, and the intracellular [d7]-CE profile is similar to unlabeled CE. Efflux of cholesterol to apolipoprotein A-I and high-density lipoproteins was similar when comparing efflux of either [d7]-cholesterol or [3H]-cholesterol as measured by following efflux of the tracers only. This technique also can be used to assess the efflux of unlabeled cholesterol to acceptors in media that are initially cholesterol-free (e.g., apolipoprotein A-I). Taken together, this mass spectrometry based assay provides new molecular detail to assess cholesterol efflux.
Keywords: cholesterol, cholesteryl esters, mass spectrometry, macrophages, cholesterol efflux, lipoproteins
INTRODUCTION
The efflux of cholesterol via the ATP binding cassette transporter A1 (ABCA1) and ABCG1, from monocyte-derived macrophages to acceptors within plasma, such as apolipoprotein (apo) A-I and high-density lipoproteins (HDL), is a key first step in the anti-atherogenic process of reverse cholesterol transport [1; 2]. Cell culture-based assay systems for cholesterol efflux traditionally involve loading cells with radiolabeled cholesterol through direct addition to culture media or addition to media containing acetylated low-density lipoproteins [3; 4; 5; 6], followed by monitoring radiolabel flux from cells to media containing acceptors of cholesterol such as apoA-I, HDL, and sera. Although the analyses of cell and media radiolabel content from cholesterol efflux assays by scintillation counting provide rapid results, data can only reflect the sum of radiolabeled cholesterol and cholesteryl esters (CE). Potential changes in the mass of both radiolabeled and unlabeled free cholesterol and CE molecular species (i.e, changes in the radio specific activity) are problematic using traditional radiolabeled assays for cholesterol efflux. Determining the radiospecific activity for cholesterol and CE is essential in most cases to accurately assess changes in cholesterol efflux under conditions where cellular unlabeled free cholesterol and CE molecular species are changing.
The use of mass spectrometry (MS) to identify different classes of lipids has evolved into a powerful tool enabling quantification of individual species of different classes of lipids [7; 8]. Previous lipidomic studies using electrospray ionization (ESI)-MS have shown that different CE molecular species as ammoniated adducts can be identified by parent ion scanning of natural cholestane (m/z 369.3) [9; 10]. Free cholesterol does not efficiently ionize under ESI conditions, however the cholesterol derivative, cholesteryl acetate, can be readily detected by ESI-MS [11]. Tandem ESI-MS (ESI-MS/MS) was previously used to quantify ammoniated adducts of cholesterol, derivatized with acetyl chloride, by selected reaction monitoring, and to quantify ammoniated adducts of CE using parent ion scanning (m/z 369.3) from plasma and cell samples [11; 12]. In contrast to ammoniated adducts, sodiated adducts have a different fragmentation pattern favouring the formation of the product ion of the sodiated fatty acid. Thus, sodiated CE molecular species can be quantified by monitoring the neutral loss (NL) of natural cholestane (m/z 368.5) [13]. We have adapted this method to quantify CE molecular species as well as in parallel, quantify cholesterol as its acetylated derivative employing parent ion scanning for sodiated acetate (m/z 83). This MS approach provides unparalleled measures of cholesterol and CE mass and efflux using murine J774 macrophages labeled with deuterated cholesterol.
MATERIALS AND METHODS
Reagents and Materials
HPLC grade chloroform and methanol were purchased from Burdick and Jackson (Morristown, NJ). Long chain fatty acyl chlorides including myristate (14:0), palmitate (16:0), palmitoleate (16:1), heptadecanoate (17:0), stearate (18:0), oleate (18:1), linoleate (18:2), arachidonate (20:4) were purchased from NuChek Prep (Elysian, MN). Acetyl chloride and fatty acid-free bovine serum albumin (BSA) were purchased from Sigma Chemical (St. Louis, MO). CE molecular species were purchased from NuChek Prep. [25,26,26,26,27,27,27-d7]-cholesterol ([d7]-cholesterol) and [25,26,26,26-d4]-cholesterol ([d4]-cholesterol) were purchased from Avanti Polar Lipids (Alabaster, AL) and C/D/N Isotopes (Pointe-Claire, QC, Canada), respectively. Both apoA-I and HDL were purchased from Biomedical Technologies Inc. (Stoughton, MA).
Synthesis of [d7]-CE Molecular Species
[d7]-CE molecular species were synthesized under anhydrous conditions using dichloromethane as solvent. In brief, 52 µmol [d7]-cholesterol, 110 µmol of acyl chloride, and 160 µmol 4-dimethylaminopyridine were mixed in 2 ml of anhydrous dichloromethane under nitrogen. Reaction mixtures were stirred at 37°C for 1h. Reactions were terminated by Bligh-Dyer extraction [14], and synthesized [d7]-CE molecular species were purified on preparative silica gel G plates (1 mm thickness) using a mobile phase comprised of petroleum ether/ethyl ether/acetic acid (80/20/1, v/v/v) (rf for CE=0.9). Individual [d7]-CE molecular species were subsequently quantified following modified acid methanolysis by gas chromatography-flame ionization detection using a known amount of 20:0 CE added during derivatization as an internal standard [15]. Fatty acid methyl esters were separated on an HP-5 column (Agilent, 30m × 0.32mm ID, 0.25 µ) with helium as carrier gas at a flow rate of 1.1 ml/min with thermal gradient elution. Initial oven temperature was 180°C, which was held for 4 min followed by a thermal gradient at 4°C per min to 200°C with this temperature subsequently held for 1 min, followed by another thermal gradient at 4°C per min to 240°C with this temperature subsequently held for 7 min before returning oven temperature to initial conditions. Purified [d7]-CE molecular species were stored in chloroform under nitrogen at −20°C.
Derivatization of free cholesterol to cholesteryl acetate
Lipid mixtures or lipid extracts of cell and media samples were derivatized with acetyl chloride as previously described [11]. Briefly, lipid extracts were dissolved in 0.5 ml chloroform in glass test tubes with screw cap. Cholesterol derivatization reactions were performed by adding 5 µl acetyl chloride to samples and incubating at room temperature for 90 min under nitrogen. Reactions were terminated by adding 1ml of deionized water. Following 10 min at room temperature, 3 ml of water and 4 ml of chloroform were added to reaction products, and samples were mixed by vortexing and then centrifuged at 600 gmax for 10 min. The lower chloroform phase was collected and chloroform extracts were dried under nitrogen. Dried extracts were resuspended in 200 µl chloroform under nitrogen at −20°C until further analysis.
Cell culture line and culture conditions
The J774 murine macrophage cell line was a generous gift from Dr. George Rothblat (Children’s Hospital of Philadelphia). Cells were maintained at 37°C with 5% CO2 in growth medium comprised of RPMI-1640 medium (Sigma, R8758) containing 10% v/v fetal bovine serum (FBS)(Atlanta Biologicals, S11050) and 1% antibiotic/antimycotic reagent (Sigma, A5955).
Uptake of deuterated cholesterol by macrophage J774
J774 cells were seeded on 6-well plates at 95% confluency in growth medium one day before uptake experiments. Cells were treated with RPMI-1640 media containing 15 µg/ml [d7]-cholesterol and 1% FBS for 0, 6, 12 or 24 h. At the end of each experimental interval, media were collected and centrifuged at 1000 × gmax for 8 min to remove any cell debris. Supernatants were stored at −80°C before lipid analyses. Cells were scraped from plates in 1.5 ml ice-cold saline (750 µl twice) following two sequential washes with 0.2% BSA (in RPMI-1640) and then PBS. Cell suspensions were stored at −80°C before further analysis. Lipids from cell culture media (1.5 ml) and 1.2 ml of cell suspension from each experimental condition were extracted in the presence of 17:0 CE, 17:0-[d7]-CE and [d4]-cholesterol as internal standards by a modified Bligh-Dyer lipid extraction method with saline in the aqueous phase. The pooled chloroform extracts (from sequential extractions) were dried under nitrogen, resuspended in 250 µl of chloroform, and stored under nitrogen at −20°C until analysis. For MS analyses of CE molecular species, 50 µl of the chloroform extract was mixed with 200 µl of methanol and NaOH to a concentration of 10 µM. To quantify each CE molecular species, the ion intensity of each CE molecular species was divided by the ion intensity of 17:0-[d7]-CE and this ratio was corrected by the response factors determined for each synthetically-prepared [d7]-CE molecular species in comparison to the 17:0-[d7]-CE internal standard.
Macrophage cholesterol efflux with stable or radio isotope labeled cholesterol
J774 cells were seeded to over 95% confluency in 6-well plates in growth medium. The following day, medium was changed to RPMI medium containing 1% FBS, 1% of antibiotic/antimycotic reagent and either 15 µg/ml of [d7]-cholesterol or [3H]-cholesterol (1 µCi/ml) (with 15 µg/ml of unlabeled cholesterol added). Labeling was also performed in either the presence or the absence of both the liver X receptor (LXR) agonist, T0901317 (1 µM), and retinoic X receptor (RXR) agonist, 9-cis retinoic acid (1 µM) (collectively referred to LXR/RXR agonists). Cells were labeled for 24 h and were subsequently washed three times with RPMI-1640 media containing 0.2% BSA prior to efflux conditions. For efflux studies, cells were incubated with different efflux acceptors (20 µg/ml apoA-I or 50 µg/ml HDL) in RPMI-1640 medium containing 0.2% BSA for 6 h. In control groups, cells were incubated in the absence of the acceptors (only 0.2% BSA was present). At the end of the efflux interval, medium was collected and centrifuged at 1000 × gmax for 5 min at 4°C. Supernatants were collected and stored at −80°C until further analysis. For experiments employing stable isotope labeling, cells on the plate were washed once with RPMI-1640 medium containing 0.2% BSA and then washed one more time with ice-cold PBS before their harvest in 1.5 ml saline. Cell suspensions (1.2 ml) were subjected to Bligh-Dyer lipid extraction in the presence of internal standards (0.4µg of 17:0 CE, 0.4µg of [d7]-17:0 CE and 4µg of [d4]-cholesterol). Similarly, cell culture media (700 µl) was extracted by a modified Bligh-Dyer lipid extraction with 0.125µg of [d7]-17:0 CE, 0.125µg of 17:0 CE (for efflux to HDL, 2µg of 17:0 CE was used) and 0.6µg of [d4]-cholesterol added as internal standards. Typically for cholesterol and CE mass analyses of efflux studies, concentration ranges of 160 – 3200 ng per ml of methanol/chloroform (4/1, v/v) are infused for ESI analyses. For [3H]-cholesterol-labeled cells, radiolabel associated with cells was determined by incubating cells in 1ml isopropanol for 1h with agitation, followed by scraping cells and measuring radioactivity in an aliquot using liquid scintillation spectrometry.
Analysis of deuterated CE and unlabeled CE using collision activated dissociation (CAD) analysis with electrospray ionization mass spectrometry
Direct-infusion ESI-MS of CE was performed in positive ion mode using a Thermo Fisher TSQ Quantum Ultra with Xcalibur data acquisition software. Samples were analyzed at a flow rate of 3.5 µl/min. Tune parameters were optimized for CE analyses, and were set at spray voltage = 3800 V, sheath gas = 8 (arbitrary units), ion sweep gas pressure = 0.5 (arbitrary units), and capillary temperature = 270°C. In MS/MS mode, the collision energy for analyses of CE molecular species was set at 18 eV for cholesterol acetate and 25 eV for long chain CE molecular species. Spectra for MS/MS scan modes (NL 368.5 and 375.5 for CE and [d7]-CE, respectively; and product ion (PI) m/z 83 for acetylated cholesterol derivatives) were acquired over 3–5 min with a scan rate of 0.5 scans/s.
LDH analysis
Following a 24 h incubation of J774 cells with media supplemented with either no additions, 15 µg/ml [d7]-cholesterol or 500 µM palmitic acid, cell medium was removed, centrifuged, and assayed for general cell death using a lactate dehydrogenase (LDH) release assay (Sigma, St. Louis, MO) following the manufacturer’s instructions.
RESULTS
Direct infusion ESI-MS analyses of unlabeled and stable isotope labeled cholesterols derivatized to acetylated cholesterol
Cholesterol was derivatized to acetylated cholesterol since cholesterol does not readily form molecular ions that can be detected by ESI-MS, and acetylated cholesterol is detectable due to the dipole of the acetyl ester. Figure 1 A-C show the collisionally-activated dissociation (CAD) spectra of the acetylated derivatives of natural, [d4]- and [d7]-cholesterols, respectively (e.g., CAD of m/z 451.73, 455.43 and 458.83, respectively). Fragment ions (from CAD) including natural cholestane (m/z 369.69), [d4]-cholestane (m/z 373.44), [d7]-cholestane (m/z 376.73) and sodiated acetate (m/z 83) were observed in these CAD spectra with similar abundance, which are all anticipated ions from fragmentation of each acetylated cholesterol derivative. The uniform product ion (m/z 83) of the stable isotope labeled and natural cholesterol acetylated derivatives indicated that each of these cholesteryl ester derivatives is detectable in one MS/MS scan (i.e., PI 83 scanning). This common product ion was scanned in samples with fixed levels of [d4]-cholesterol and varying levels of either [d7]-cholesterol or free cholesterol in the presence and absence of plasma lipid in the matrix that were subsequently derivatized to their acetylated analogs. Calibration lines for [d7]-cholesterol compared to [d4]-cholesterol are shown in Figures 1D and 1E, which provide evidence that under this derivatization condition and PI scan detection, [d7]-cholesterol can be detected with a linear response signal compared to the internal standard ([d4]-cholesterol). The slopes in both analyses were near unity, and the presence of plasma lipid in the matrix did not impact outcomes. Similarly, in the absence of plasma the response calibration line for natural cholesterol in comparison to [d4]-cholesterol was linear (y = 1.111x + 0.002, R2 = 0.999).
Figure 1. Quantification of [d7]- and unlabeled cholesterol by ESI-MS/MS.
Either unlabeled cholesterol (A), [d4]-cholesterol (B), or [d7]-cholesterol (C) (mass of each 2.5 nmol) were converted to their acetylated derivatives and subjected to CAD analysis as their sodiated adducts in 250 µl of 4/1 methanol/chloroform at 18 eV and 1 atmosphere of argon in the collision cell as described in “Materials and Methods”. Based on the common product ion shown in Panels A-C, precursor ion m/z 83.03 was used to quantify acetylated cholesterol derivatives (D & E). Selected concentrations of [d7]-cholesterol to a fixed concentration of [d4]-cholesterol in the presence (E) and absence (D) of plasma lipid extract (from 10 µl of plasma) were sequentially converted to their acetylated derivatives, and analyzed as their sodiated adduct in 4/1 methanol/chloroform by PI m/z 83.03 scanning.
The quantification of 2.5 nmol of both [d4]- and [d7]-cholesterol was assessed in the presence of plasma lipid, J774 cell lipid, or [d7]-CE matrices – added prior to cholesterol derivatization and analyses by ESI-MS/MS. Table I shows data demonstrating that the addition of plasma does not alter the quantification of [d7]-cholesterol. Similarly, J774 cell total lipid extracts from 300 and 600 µg cell protein did not impact the quantification of [d7]-cholesterol. Data shown in Table I also reveal that increasing amounts of cholesterol from both J774 cell and plasma lipid extracts do not interfere with [d7]-cholesterol quantification. Also, synthetic [d7]-CE species (16:0 and 17:0) were added to derivatization reactions to ensure that the acetylation reaction does not result in the conversion of long chain CE to acetylated cholesterol derivatives. Adding a 20-fold molar excess of [d7]-CEs did not alter the measured [d7]-cholesterol. Furthermore, [d7]-cholesterol was not detectable in reactions with 50 nmol of [d7]-CE and no added [d7]-cholesterol.
TABLE 1.
Plasma and J774 Lipids, and Excess CE Do Not Alter Quantification of[d7]-Cholesterol
| Derivatized Lipids | Measured Cholesterol | |
|---|---|---|
| Natural cholesterol | [d7]-cholesterol | |
| 2.5 nmol [d7]-cholesterol | N.D. | 2.56 ± 0.04 nmol |
| 2.5 nmol [d7]-cholesterol + 10 µl human plasma extract |
11.38 ± 0.36 nmol | 2.66 ± 0.11 nmol |
| 2.5 nmol [d7]-cholesterol + J774 lipid extract from 30µg cell protein |
38.60 ± 0.70 nmol | 2.64 ± 0.09 |
| 2.5 nmol [d7]-cholesterol + J774 lipid extract from 600µg cell protein |
72.87 ± 0.52 nmol | 2.46 ± 0.02 nmol |
| 2.5 nmol [d7]-cholesterol + 2.5 nmol d7 16:0 CE and 2.5 nmol d7 17:0 CE |
N.D. | 2.50 ± 0.05 nmol |
| 2.5 nmol [d7]-cholesterol + 25 nmol d7 16:0 CE and 25 nmol d7 17:0 CE |
N.D. | 2.56 ± 0.02 nmol |
| 2.5 nmol d7 16:0 CE and 2.5 nmol d7 17:0 CE |
N.D. | N.D. |
| 25 nmol d7 16:0 CE and 25 nmol d7 17:0 CE |
N.D. | N.D. |
Values are means ± S.E.M. N.D. = not detectable
Direct infusion ESI-MS of sodiated adducts of [d7]-CE molecular species
We have previously shown that NL scanning of 368.5 can be used to specifically detect naturally-occurring CE molecular species using either sodiated or lithiated adducts [13; 16]. Accordingly, we examined the CAD fragmentation of synthetically-prepared, [d7]-CE molecular species to confirm that they can be assessed using NL of 375.5 corresponding to the loss of the [d7]-cholestane from the [d7]-CE. Figure 2 A-C show the CAD spectra of synthetic 17:0-[d7]-CE, 18:1-[d7]-CE, 20:4-[d7]-CE, respectively. For these three molecular species, [d7]-cholestane (m/z 376.4) and sodiated fatty acid ions (m/z 293.17, 305.06 and 327.16 respectively) were observed. These results demonstrate that NL 375.5 scanning can be exploited to detect [d7]-CE molecular species. Figure 2D shows the NL 375.5 scan of an equimolar mixture of [d7]-CE molecular species (each at 2 µM) as sodiated adducts. These [d7]-CE species with varying degrees of unsaturation have disparate ionization efficiencies due to both the interaction of sodium cation with double bonds [17] and the effect of the degree of unsaturation on the carboxyl ester dipole ion, similar to the effects previously observed with their corresponding natural CE species [13; 16]. Accordingly, the response of varying amounts of each [d7]-CE molecular species was compared to that of 17:0-[d7]-CE (internal standard) to determine response factors (Table 2).
Figure 2. CAD and NL scans of sodiated adducts of [d7]-CE molecular species using direct-infusion ESI-MS analysis.
CAD analysis was performed at a collisional energy of 25 eV on [d7]-17:0 CE (A), [d7]-18:1 CE (B), and [d7]-20:4 CE (C). The concentrations of the [d7]-CE were 10 µM. The NaOH added prior to ESI analysis was 10 µM. Neutral loss of 375.5 MS/MS analyses of an equimolar mixture of the cholesteryl esters (each at 2 µM) as sodiated adducts is shown in D. Spectra were obtained using the neutral loss 375.5 at a collisional energy of 25 eV as described in “Materials and Methods”. Differential ionization efficiencies (in D) are associated with CE molecular species containing different levels of unsaturation.
Table 2.
Response factors for sodiated adducts of [d7]-CE molecular species
| CEs | NL 375.5 [M+Na]+ (amu) | Response Factor |
|---|---|---|
| 14:0 | 626.73 | 0.97 |
| 16:0 | 654.77 | 1.05 |
| 16:1 | 652.75 | 1.65 |
| 18:0 | 682.80 | 1.04 |
| 18:1 | 680.78 | 1.64 |
| 18:2 | 678.76 | 2.47 |
| 20:4 | 702.76 | 3.73 |
Linear regression of ion inte nsity responses using NL 375.5 scans f or each deuterated CE over a concentration range of 0.1 to 10 µM was performed with comparisons to the [d7]-CE internal standard (17:0-[d7]-CE), which was constant at 2 µM. In all cases, the coefficient of determination (R2) was greater than 0.998. Response factors were based on the slope of the lines.
Uptake of deuterated cholesterol by macrophage J774
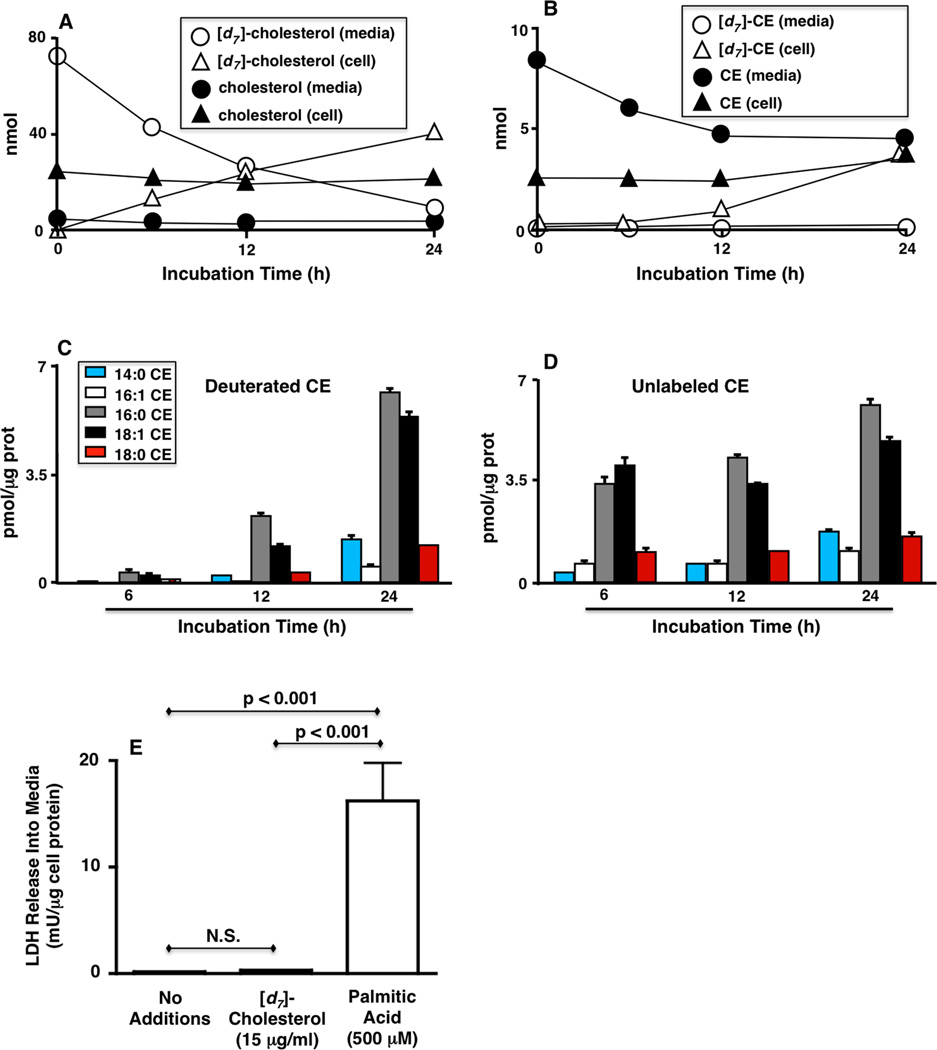
We used PI scanning for m/z 83 and NL scanning of 375.5 to assess the uptake of a similar mass of media [d7]-cholesterol by J774 cells to that used in procedures to load J774 cells with [14C]-cholesterol prior to efflux studies [18]. Using PI scanning for m/z 83, [d7]-cholesterol mass was shown to decrease in the media with its concomitant accumulation in the cells (Figure 3A). These same analyses revealed no changes in the levels of unlabeled (natural) cholesterol levels in the media and cells under these conditions (Figure 3A). Using NL 375.5 and 368.5 scanning for quantification, a modest decrease in media natural CE was observed accompanied by an increase in both natural CE and [d7]-CE in the cells over the experimental interval (Figure 3B). Very minimal [d7]-CE was detected in the media under these [d7]-cholesterol uptake conditions. Taken together, these data show [d7]-cholesterol is taken up by J774 cells and is subsequently, in part, incorporated into the CE pool. Furthermore, detailed inspection of the natural and deuterated intracellular CE molecular species revealed that following 24 h of [d7]-cholesterol labeling, the distribution of [d7]-cholesterol in the various CE molecular species was similar to that of the natural CE molecular species (Figures 3C and D). After 24 h of [d7]-cholesterol labeling, the predominant [d7]-CE molecular species were 16:0 and 18:1 CE, which is similar to the enrichment of these molecular species in the natural CE pool. Further studies showed that the 3-fold higher levels of intracellular cholesterol following uptake of [d7]-cholesterol was not cytotoxic since LDH levels in the media were insignificantly increased compared to cells that were not loaded with cholesterol as well as the comparison to the positive control for this experiment (24 h treatment with 500 µM palmitic acid) (Figure 3E). These uptake conditions with [d7]-cholesterol are similar to those employed for loading J774 for efflux studies with radiolabel, and thus, the present studies that concomitantly assess the masses of labeled cholesterol, labeled CE, unlabeled cholesterol, and unlabeled CE show: 1) the majority of [d7]-cholesterol is incorporated into the cell and remains free cholesterol; 2) over time the cellular specific activity of [d7]-cholesterol is changing with nearly 2-fold more [d7]-cholesterol present compared to natural cholesterol following 24 h of labeling; 3) following 24 h, [d7]-CE and naturally occurring CE masses are near equal in the cell; and 4) further inspection of the CE molecular species reveals a similar profile between natural and [d7]-CE molecular species only following 24 h of labeling with [d7]-cholesterol.
Figure 3. Uptake of [d7]-cholesterol by J774 cells.
Cells were incubated with 15 µg [d7]-cholesterol for indicated times. At the end of incubation intervals, [d7]-cholesterol, [d7]-CE, unlabeled cholesterol and CE were extracted from cells and media and quantified by ESI-MS as described in “Materials and Methods”. The cell-associated molecular species distribution of [d7]-CE (C) and CE (D) were determined using NL 375.5 and 368.5 scanning, respectively. LDH release into the media was determined as described in “Materials and Methods” (E). Values are the means + S.E.M. (n=3 or 4). For Panels A, B, and E, the S.E.M. bars are within the size of the symbol indicating the mean.
Cholesterol efflux from J774 cells
The utility of labeling J774 cells with [d7]-cholesterol to subsequently measure cholesterol efflux was determined, and compared to the conventional assay employing [3H]-cholesterol. J774 cells were labeled for 24 h with either [d7]-cholesterol or [3H]-cholesterol with the same mass of cholesterol added under both conditions (15 µg/ml cell culture media). Following this labeling interval, cholesterol efflux to BSA, HDL or apoA-I was determined (efflux for 6 h). Figure 4A and B show comparable efflux rates when comparing these two assay systems with these three acceptors. Both assays systems show increased efflux in cells treated in the presence of LXR/RXR agonists during cholesterol labelling, which is indicative of increased efflux activities via ABCA1 (toward apoA-I and HDL) and ABCG1 (toward HDL). Additionally, data in Figure 4A (solid bars) show the efflux of unlabeled cholesterol mass to BSA and apoA-I, which was determined by dividing the mass of cholesterol in the media by the total unlabeled cholesterol mass present in media and cells. It should be noted that unlabeled cholesterol present in the media following efflux to apoA-I in both the presence and absence of LXR/RXR agonists is derived from efflux of cholesterol from cells. As shown in Figure 5A, the cell culture media levels of unlabeled cholesterol to apoA-I following efflux is 1–2 orders of magnitude greater than the cholesterol associated with apoA-I in the media prior to efflux (48 ± 2 pmol/ml).
Figure 4. Cholesterol efflux from J774 cells.
Cells were incubated with 15 µg [d7]-cholesterol or 15 µg [3H]-cholesterol (1µCi) for 24h. At the end of labeling interval, cells were sequentially washed with unlabeled media and then efflux was begun by adding media containing BSA, apoA-I or HDL. As indicated, some conditions included LXR/RXR agonists treatment during both labeling and efflux intervals. Following a 6h efflux interval media was removed, and [d7]-cholesterol, [d7]-CE, unlabeled cholesterol and CE were extracted from cells and media and quantified by ESI-MS as described in “Materials and Methods”. Alternatively, radiolabeled lipids were extracted from the cells in isopropanol and [3H] content was subsequently determined by liquid scintillation spectrometry. The efflux of [d7]-cholesterol and unlabeled cholesterol (A) were determined by dividing the mass of either [d7]-cholesterol and [d7]-CE, or unlabeled cholesterol and CE, in the media by that in both media and cells. Similarly, [3H]-cholesterol (B) efflux was determined by dividing dpm in the media by the total dpm in media and cells. Values are the means + S.E.M. (n=3).
Figure 5. Distribution of unlabeled and [d7]-cholesterol in J774 cells and media following efflux determinations.
The distribution of [d7]-cholesterol and unlabeled cholesterol and their respective CE molecular species in media (A) and cells (B) were determined by ESI-MS as described in “Materials and Methods”. Using the total of [d7]-cholesterol and unlabeled cholesterol and their respective CE molecular species as the denominator, the [d7]-cholesterol (in the media, numerator) and unlabeled cholesterol (in the media, numerator) efflux rates over 6h were determined (Panel C). Values are the means + S.E.M. (n=3).
Interestingly, using this calculation for the unlabeled efflux to apoA-I with LXR/RXR agonists present (i.e., based solely on the unlabeled cholesterol and CE in the media and cells), significantly greater (p < 0.001) efflux of unlabeled cholesterol is observed compared to the concomitantly measured [d7]-cholesterol efflux (Figure 4A). Further inspection of the [d7]-cholesterol and unlabeled cholesterol in media and cells following efflux to BSA or apoA-I reveals that the total unesterified cholesterol (unlabeled and [d7]-cholesterol) pool in the cells contains approximately twice as much [d7]-cholesterol compared to unlabeled cholesterol (Figure 5B), consistent with data shown in Figure 3A. Additionally, the cholesterol associated with media apoA-I contains more [d7]-cholesterol compared to unlabeled cholesterol (Figure 5A). Also, data shown in Figure 5B reveals that the addition of LXR/RXR agonists reduces the cellular mass levels of CE (both [d7]-labeled and unlabeled molecular species). Finally, if the flux of [d7]-cholesterol or natural cholesterol is calculated based on the total cholesterol in media and cells including both [d7]- and unlabeled cholesterol and CE, then the relative flux of [d7]-cholesterol from the J774 cells to apoA-I in the presence and absence of LXR/RXR agonists is greater than that of the unlabeled cholesterol (compare Figures 4A and Figure 5C). Thus, this calculation, which may better reflect efflux of labeled cholesterol, provides an alternate conclusion of relative flux in comparisons to the efflux calculated and shown in Figure 4A.
DISCUSSION
We present a new approach for examining cholesterol efflux from J774 macrophages using direct infusion ESI-MS/MS methods to quantify cholesterol and CE from cells and media. These studies required first developing accurate methodology to accurately measure both d7-cholesterol and d7-CE by ESI-MS. In the presence of varying amounts of natural, unlabeled cholesterol, and CE, both d7-cholesterol and d7-CE can be accurately measured by direct infusion ESI-MS/MS by exploiting PI 83 scanning for the acetylated d7-cholesterol derivative, and NL 375.5 scanning for d7-CE. It should be noted that using these MS/MS scan modes requires optimal tuning of both mass analyzers (in this case, quadrupoles 1 and 3 in a triple quadrupole instrument). Using cells labeled with d7-cholesterol, sodiated adducts of d7-CE from cells and media were quantified by comparisons to the response of the internal standard, d7-17:0 CE by monitoring the neutral loss of d7-cholestane (m/z 375.5). In addition, sodiated adducts of the unlabeled CE relative to the unlabeled internal standard, 17:0 CE, were quantified by monitoring the NL of cholestane (m/z 368.5). Unlabeled and d7-cholesterol from cells and media were quantified in the presence of d4-cholesterol (internal standard), by derivatizing the cholesterol species into cholesteryl acetates, followed by scanning for parent ions that yield the sodiated acetate product ion (m/z 83). This technique can be used for simultaneous quantification of sodiated cholesteryl acetate (m/z 451.7) and sodiated d7-cholesteryl acetate (m/z 458.8). Data shown in Table 1 demonstrate the accurate and reproducible measurement of these lipids despite the presence of several exogenous lipid matrices. Furthermore, these scan modes used to measure both natural and d7-cholesterol uptake, esterification and efflux are summarized in Table 3.
Table 3.
Summary of Scan Modes Used for Natural and Deuterated Cholesterol and CE Detection and Quantification in Uptake and Efflux Studies
| Target Compound | Infused Compound Observed |
Parent Ion Observed | CAD Ions Observed | Scan Type Used To Measure Target Compound |
|---|---|---|---|---|
| Cholesterol | Cholesterol acetate | Sodiated cholesterol acetate m/z 451.73 |
Sodiated acetate (m/z 83.01) Cholestane (m/z 369.69) |
Positive Ion Mode- PI 83 |
| [d7]-Cholesterol | [d7]-Cholesterol acetate |
Sodiated [d7]- cholesterol acetate m/z 458.83 |
Sodiated acetate (m/z 83.02) [d7]-Cholestane (m/z 376.73) |
Positive Ion Mode- PI 83 |
| 18:1 CE | 18:1 CE | Sodiated 18:1 CE m/z 673.6 |
Sodiated oleate (m/z 305.1) Cholestane (m/z 369.4) |
Positive Ion Mode- NL 368.5 |
| [d7]-18:1 CE | [d7]-18:1 CE | Sodiated [d7]-18:1 CE m/z 680.7 |
Sodiated oleate (m/z 305.1) [d7]-Cholestane (m/z 376.4) |
Positive Ion Mode- NL 375.5 |
Each indicated target compound was subjected to direct infusion ESI-MS/MS with indicated scan modes including collisionally-activated dissociation (CAD), precursor ion (PI) and neutral loss (NL). Cholesterol and [d7]-cholesterol were first converted to their acetylated derivatives prior to ESI-MS/MS. All infused compounds were assessed as their sodiated adducts. Similar strategies (NL 368.5 and 375.5, respectively) were used for other natural and [d7]-CE as is depicted for 18:1 CE and [d7]-18:1 CE targets.
During the development of this technique, it was recognized that correction factors are needed for CE quantification since CE molecular species of varying fatty acid length and degrees of unsaturation have disparate ionization efficiencies compared to the internal standard d7-17:0 CE. Increased unsaturation leads to increased ionization due to both the interaction of sodium cation with double bonds [17] and an enhanced dipole property of the CE [11, 13; 16; 19; 20]. Accordingly, for these studies individual molecular species of d7-CE molecular species were synthesized and compared to d7-17:0 CE to determine response factors. Our data show that sodiated adducts of d7-CE also share this property (Table 2). The response factors determined for the sodiated adducts of d7-CE molecular species versus d7-17:0 CE are similar to previously reported response factors for natural species of sodiated CE versus 17:0 CE [13]. For cholesteryl acetate, both natural- and d7-cholesterol acetylated derivatives ionize similarly to that of the d4-cholesterol internal standard derivative as demonstrated by their linear responsiveness shown in Figures 1D and 1E.
Either [3H]- or [14C]-cholesterol are conventionally used for quantifying cholesterol efflux from macrophages and other cells. We examined several aspects of assays for [14C]-cholesterol efflux by substituting an equal molar mass of d7-cholesterol [18]. Over 85% of the d7-cholesterol in the cell culture media was taken up by J774 cells over a 24 h labeling period. The increase in cellular d7-cholesterol has little impact on natural cholesterol levels in the media and cells. However, over this 24 h labeling period, cell culture media-associated unlabeled CE (associated with the FBS) decreased and cell-associated d7-CE increased. Furthermore, comparisons of the CE molecular species profile indicated that only after 24 h is the cellular d7-CE pool both similar in mass and molecular species profile with the cellular unlabeled CE pool. It should, however, be noted that the cell-associated unesterified d7-cholesterol and unlabeled cholesterol pools were over 10- and 5-fold greater than the cell-associated d7-CE and unlabeled CE pools, respectively, following a 24 h labeling interval. The increase in unesterified cholesterol and the relatively low flux of cholesterol into CE pools was not accompanied by cell death, and it might be speculated that the imported cholesterol is stored in cholesterol rich crystals as has been previously observed [21]. With these observations in mind, these data provide insights into the pools that d7-cholesterol is incorporated into during standard labeling intervals used for the [3H]-cholesterol-based assay, and it is likely that [3H]-cholesterol has a similar distribution in J774 cells during labeling intervals.
We examined the efflux of total d7-cholesterol (free and esterified) from J774 cells to HDL and apoA-I, in the absence or presence of LXR/RXR stimulation to increase ABCA1- and ABCG1-mediated transport. Data show that our method of quantifying cholesterol efflux using d7-cholesterol is comparable to the values obtained for cholesterol efflux using [3H]-cholesterol. As expected, LXR/RXR stimulation increased the percent of d7-cholesterol effluxed to HDL and apoA-I. Although rapid information can be gained through the traditional use of [3H]-cholesterol in efflux studies, a key advantage of this new method is that the molecular detail of labeled and unlabeled cholesterol can be followed by mass measurements. In our study, the treatment of J774 cells with LXR and RXR agonists, prior to and during efflux reduces the d7-CE pool and this was a uniform decrease amongst the major CE molecular species, including 16:0, 18:0 and 18:1 CE (data not shown). These results suggest that d7-CE pools are mobilized following ABCA1 expression and in the presence of extracellular apoAI. In addition to d7-cholesterol efflux, efflux of unlabeled cholesterol to apoA-I can be simultaneously determined by these mass measurements. It is clear from our study that the efflux of d7-cholesterol from cells is greater than that of unlabeled cholesterol when calculating efflux based on the total cholesterol and CE (both labeled and unlabeled). In contrast, the d7-cholesterol efflux calculated based only on the total d7-cholesterol and d7-CE was considerably slower, and this measurement of isotope only would be similar to that expected to be determined with the conventional [3H]-cholesterol-based efflux assay system. These data for the flux of unlabeled and d7-cholesterol mass highlight considerations that should be made before drawing conclusions when using the [3H]-cholesterol-based efflux assay. For example, the use of tracers (tritiated or deuterated) for the measure of cholesterol efflux may not always represent the efflux of unlabeled cholesterol in the cells. Additionally, investigations employing tracers must be aware of assumptions regarding the distribution of the tracer and the unlabeled cholesterol including changes in the relative mass of labeled and unlabeled cholesterol, and both the metabolic and subcellular distribution of labeled and unlabeled cholesterol in the cell. Previous studies by others have measured cholesterol mass measurements for efflux (including the use of GC/MS) and these studies have also concluded that isotope distribution is not identical to the unlabeled cholesterol pools in cells [22; 23]. Other studies have employed GC-MS to quantify cholesterol mass efflux to HDL with media cholesterol efflux determined by the difference in media cholesterol present in media with and without exposure to cells, and these studies have indicated caution in the interpretation of cholesterol efflux based on isotope measurements [24; 25].
In summary, we have developed a novel and informative mass spectrometric method to assess cholesterol efflux, while monitoring the levels of media and cell molecular species of CE. Using product ion scanning for the acetylated cholesterol derivatives enables the accurate measurement of both natural and d7-cholesterol in one MS/MS scan mode. This capability is important for determining the specific activity of the stable isotope in the cell. Furthermore, this method can be applied to measure unlabeled cholesterol efflux to acceptors that do not contain cholesterol such as apoA-I.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health Grants HL107797 to Á.B, as well as HL-074214, HL-098907, HL-111906 and RR-019232 to D.A.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Bailey JM. Lipid metabolism in cultured cells. IV. Serum alpha globulins and cellular cholesterol exchange. Exp Cell Res. 1965;37:175–182. doi: 10.1016/0014-4827(65)90168-0. [DOI] [PubMed] [Google Scholar]
- 4.Stein Y, Glangeaud MC, Fainaru M, Stein O. The removal of cholesterol from aortic smooth muscle cells in culture and Landschutz ascites cells by fractions of human high-density apolipoprotein. Biochim Biophys Acta. 1975;380:106–118. doi: 10.1016/0005-2760(75)90049-1. [DOI] [PubMed] [Google Scholar]
- 5.Henriksen T, Evensen SA, Blomhoff JP, Torsvik H, Carlander B. The effect of serum lipoproteins on cholesterol content and sterol exchange in cultured human endothelial cells. Biochim Biophys Acta. 1979;574:312–320. doi: 10.1016/0005-2760(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 6.Brown MS, Goldstein JL, Krieger M, Ho YK, Anderson RG. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 1979;82:597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross RW, Han X. Lipidomics at the interface of structure and function in systems biology. Chem Biol. 2011;18:284–291. doi: 10.1016/j.chembiol.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annema W, Dikkers A, Freark de Boer J, Gautier T, Rensen PC, Rader DJ, Tietge UJ. ApoE promotes hepatic selective uptake but not RCT due to increased ABCA1-mediated cholesterol efflux to plasma. J Lipid Res. 2012;53:929–940. doi: 10.1194/jlr.M020743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffin K, Obukowicz M, Raz A, Shieh JJ. Electrospray/Tandem mass spectrometry for quantitative analysis of lipid remodeling in essential fatty acid deficient mice. Anal Biochem. 2000;279:179–188. doi: 10.1006/abio.1999.4452. [DOI] [PubMed] [Google Scholar]
- 10.Kalo P, Kuuranne T. Analysis of free and esterified sterols in fats and oils by flash chromatography, gas chromatography and electrospray tandem mass spectrometry. J Chromatogr A. 2001;935:237–248. doi: 10.1016/s0021-9673(01)01315-2. [DOI] [PubMed] [Google Scholar]
- 11.Liebisch G, Binder M, Schifferer R, Langmann T, Schulz B, Schmitz G. High throughput quantification of cholesterol and cholesteryl ester by electrospray ionization tandem mass spectrometry (ESI-MS/MS) Biochim Biophys Acta. 2006;1761:121–128. doi: 10.1016/j.bbalip.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Schifferer R, Liebisch G, Bandulik S, Langmann T, Dada A, Schmitz G. ApoA-I induces a preferential efflux of monounsaturated phosphatidylcholine and medium chain sphingomyelin species from a cellular pool distinct from HDL(3) mediated phospholipid efflux. Biochim Biophys Acta. 2007;1771:853–863. doi: 10.1016/j.bbalip.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Bowden JA, Shao F, Albert CJ, Lally JW, Brown RJ, Procknow JD, Stephenson AH, Ford DA. Electrospray ionization tandem mass spectrometry of sodiated adducts of cholesteryl esters. Lipids. 2011;46:1169–1179. doi: 10.1007/s11745-011-3609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Ichihara K, Fukubayashi Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid Res. 2010;51:635–640. doi: 10.1194/jlr.D001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowden JA, Albert CJ, Barnaby OS, Ford DA. Analysis of cholesteryl esters and diacylglycerols using lithiated adducts and electrospray ionization-tandem mass spectrometry. Anal Biochem. 2011;417:202–210. doi: 10.1016/j.ab.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Applegate KR, Glomset JA. Effect of acyl chain unsaturation on the conformation of model diacylglycerols: a computer modeling study. J Lipid Res. 1991;32:1635–1644. [PubMed] [Google Scholar]
- 18.Mahlberg FH, Glick JM, Lund-Katz S, Rothblat GH. Influence of apolipoproteins AI, AII, and Cs on the metabolism of membrane and lysosomal cholesterol in macrophages. J Biol Chem. 1991;266:19930–19937. [PubMed] [Google Scholar]
- 19.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 20.Hutchins PM, Barkley RM, Murphy RC. Separation of cellular nonpolar neutral lipids by normal-phase chromatography and analysis by electrospray ionization mass spectrometry. J. Lipid Res. 2008;49:804–813. doi: 10.1194/jlr.M700521-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellner-Weibel G, Yancey PG, Jerome WG, Walser T, Mason RP, Phillips MC, Rothblat GH. Crystallization of free cholesterol in model macrophage foam cells. Arterioscler Thromb Vasc Biol. 1999;19:1891–1898. doi: 10.1161/01.atv.19.8.1891. [DOI] [PubMed] [Google Scholar]
- 22.Sparrow CP, Baffic J, Lam MH, Lund EG, Adams AD, Fu X, Hayes N, Jones AB, Macnaul KL, Ondeyka J, Singh S, Wang J, Zhou G, Moller DE, Wright SD, Menke JG. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. J Biol Chem. 2002;277:10021–10027. doi: 10.1074/jbc.M108225200. [DOI] [PubMed] [Google Scholar]
- 23.Mendez AJ, Uint L. Apolipoprotein-mediated cellular cholesterol and phospholipid efflux depend on a functional Golgi apparatus. J Lipid Res. 1996;37:2510–25124. [PubMed] [Google Scholar]
- 24.Matsuura F, Wang N, Chen W, Jiang XC, Tall AR. HDL from CETPdeficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J Clin Invest. 2006;116:1435–1442. doi: 10.1172/JCI27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yvan-Charvet L, Pagler TA, Wang N, Senokuchi T, Brundert M, Li H, Rinninger F, Tall AR. SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL. J Lipid Res. 2008;49:107–114. doi: 10.1194/jlr.M700200-JLR200. [DOI] [PubMed] [Google Scholar]