Abstract
Smoking causes endothelial dysfunction and systemic microvascular disease with resultant end-organ damage in the kidneys, eyes and heart. Little is known about microvascular changes in smoking-related lung disease. We tested if microvascular changes in the retina, kidneys and heart were associated with obstructive spirometry and low lung density on computed tomography. The Multi-Ethnic Study of Atherosclerosis recruited participants age 45–84 years without clinical cardiovascular disease. Measures of microvascular function included retinal arteriolar and venular caliber, urine albumin-to-creatinine ratio and, in a subset, myocardial blood flow on magnetic resonance imaging. Spirometry was measured following ATS/ERS guidelines. Low attenuation areas (LAA) were measured on lung fields of cardiac computed tomograms. Regression models adjusted for pulmonary and cardiac risk factors, medications and body size. Among 3,397 participants, retinal venular caliber was inversely associated with forced expiratory volume in one second (FEV1) (P<0.001) and FEV1/forced vital capacity (FVC) ratio (P = 0.04). Albumin-to-creatinine ratio was inversely associated with FEV1 (P = 0.002) but not FEV1/FVC. Myocardial blood flow (n = 126) was associated with lower FEV1 (P = 0.02), lower FEV1/FVC (P = 0.001) and greater percentage LAA (P = 0.04). Associations were of greater magnitude among smokers. Low lung function was associated with microvascular changes in the retina, kidneys and heart, and low lung density was associated with impaired myocardial microvascular perfusion. These cross-sectional results suggest that microvascular damage with end-organ dysfunction in all circulations may pertain to the lung, that lung dysfunction may contribute to systemic microvascular disease, or that there may be a shared predisposition.
Introduction
Chronic obstructive pulmonary disease (COPD) recently surpassed stroke as the third leading cause of death in the United States [1]. Smoking is the major cause of COPD [2]; however, a minority of smokers develop COPD [3]. Cigarette smoke injures the airways and lung parenchyma by direct chemical exposure and inflammation induced predominantly via neutrophils, macrophages and T cells [4], [5].
Smoking is a major cause of endothelial dysfunction and microvascular disease throughout the body, caused in part by impairment of vascular endothelial growth factor (VEGF), with subsequent generation of reactive oxygen species and diminished nitric oxide release [6]. While it is known that smoking-related microvascular disease contributes to end-organ damage in the brain, kidney, heart and eyes [7], [8], it is unclear whether similar microvascular changes in the lung may contribute to COPD pathogenesis.
Animal studies suggest that endothelial dysfunction might contribute to COPD and emphysema [9], [10]. Several mechanisms have been proposed both in vitro and in vivo [11], [12], [13], [14]. Impaired flow mediated dilation (FMD) in large systemic arteries was associated with early COPD and emphysema [15],[16], as was impaired left ventricular filling [17] and increased pulmonary perfusion heterogeneity [18]. Endothelial microparticles reflective of endothelial apoptosis were increased in smokers with isolated reductions in diffusing capacity [19] and in COPD [20]. Together, these findings may suggest early pulmonary endothelial and microvascular damage in patients with COPD.
Whereas methods for assessing pulmonary microvascular compromise in the general population are limited, validated measures of systemic microvascular function are available for the retina, kidneys and heart. Retinal microvascular abnormalities include narrowing of retinal arterioles, predominantly from hypertension, and widening of retinal venules, predominantly from diabetes, inflammation and smoking. Both measures predict cardiovascular events in middle-aged individuals [21], notably in women (a 20 µm increase or decrease in retinal venular or arteriolar caliber, respectively, was associated increased risk of coronary heart disease) [22]. Microalbuminuria is a measure of renal microvascular damage that predicts cardiovascular events [23], as well as all-cause mortality in general population studies [24]. A two-fold increase in urine albumin excretion was associated with a relative risk of 1.29 for cardiovascular mortality, and 1.12 for overall mortality [25]. Myocardial blood flow (MBF) on magnetic resonance imaging (MRI) is a measure of cardiac microvascular perfusion, impairments in which can cause myocardial ischemia [26].
We examined the association of retinopathy, microalbuminuria and myocardial blood flow, respectively, with lung function and lung density on computed tomography (CT) in a large, multi-ethnic cohort free of clinical cardiovascular disease. We hypothesized that these measures of systemic microvascular changes were associated with reduced lung function and lower lung density, and that relationships would be of greater magnitude among smokers.
Materials and Methods
Study sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study of white, African-American, Hispanic and Asian (predominantly of Chinese decent) adults [27]. In 2000–2002, MESA recruited 6,814 men and women ages 45–84 years old from six U.S. communities: Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles, CA. Exclusion criteria included clinical cardiovascular disease, weight greater than 300 lbs, pregnancy and impediment to long-term participation. All measures were ascertained at baseline except as noted below.
The MESA Lung Study enrolled 3,965 MESA participants of 4,484 selected who were sampled randomly among those who consented to genetic analyses, underwent baseline measures of endothelial function, and attended an examination during the MESA-Lung recruitment period in 2004–2006 (99%, 89%, and 91% of the MESA cohort, respectively). Asians were over-sampled.
Similar to prior studies [17],[28], we excluded a priori 322 participants with a restrictive pattern of spirometry, defined as a forced vital capacity (FVC) less than the lower limit of normal (LLN) [29], with a forced expiratory volume in one second (FEV1)/FVC ratio above the LLN, since the primary hypothesis related to obstructive lung disease.
Ethics Statement
The protocols of MESA and all studies described herein were approved by the Institutional Review Boards of all collaborating institutions (Columbia University, New York, NY; Johns Hopkins University, Baltimore, MD; Northwestern University, Chicago, IL; University of California, Los Angeles, CA; University of Minnesota, Twin Cities, MN; Wake Forest University, Winston-Salem, NC) and the National Heart, Lung and Blood Institute (NHLBI). Written informed consent was obtained from all study participants.
Microvascular Measures in the Retina, Kidney and Heart
Retinal Vascular Caliber
Retinal vascular caliber was measured from digital retinal photographs of both eyes of each participant in 2002–03 [30]. All arterioles and venules coursing through an area one half to one full disc diameter from the optic disc margin (Figure 1) were measured using a computer-based program by trained graders masked to participant characteristics at a central reading center. Vascular caliber was summarized as the central retinal artery equivalent (CRAE) and the central retinal vein equivalent (CRVE), two well-established, reproducible indicators of the average caliber of retinal vessels [30].
Figure 1. Digital photograph a MESA participant's retina showing central retinal vascular caliber.
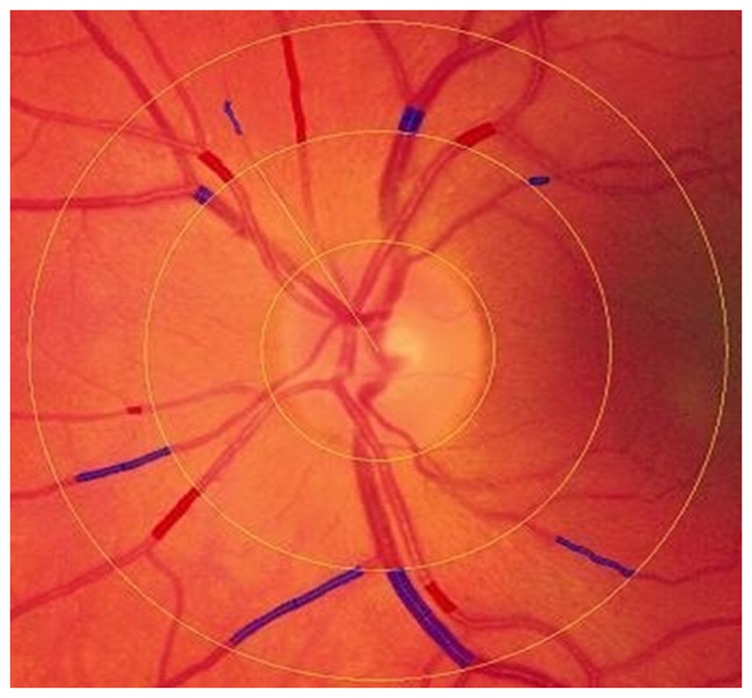
The caliber of retinal vessels is a calculated average of measurements of all arterioles and venules coursing through an area one half to one full disc diameter from the optic disc margin. Red shaded areas are used to compute the central retinal artery equivalent (CRAE) and blue shaded areas are used to compute the central retinal vein equivalent (CRVE).
Urine Albumin-to-Creatinine Ratio and Albuminuria
Urine albumin and creatinine were measured at the baseline examination by nephelometry and the rate Jaffe reaction. Spot urine albumin (µg/mL)-to-creatinine (µg/mL) ratios (ACRs) were calculated. Previously published, gender-specific categories of ACR were used to define albuminuria as high-normal urine albumin excretion, microalbuminuria and macroalbuminuria [31].
Myocardial Blood Flow
All participants at one field center were asked to participate in the myocardial perfusion study; 222 agreed and underwent the study, of whom 126 met inclusion criteria for the present report. MBF was measured using gadolinium-enhanced cardiac MRI (Figure S1) at rest and again during maximum adenosine-induced vasodilation (hyperemia) [32]. All imaging was performed on a 1.5 T magnet with a 2-coil anterior surface coil array positioned on the chest as a 2-coil phased array integrated in the patient bed for posterior signal reception. Image acquisition was gated electrocardiographically. MBF was determined by model-independent deconvolution and expressed as mL/g/min [33].
Spirometry
Spirometry was conducted in 2004–2006 and in accordance with the American Thoracic Society/European Respiratory Society guidelines [34] using a dry-rolling-sealed spirometer with software that performed automated quality checks in real time (Occupational Marketing, Inc., Houston, TX). All spirometry exams were performed pre-bronchodilator and were reviewed by one investigator [35].
Percent Low Attenuation Area
CT lung density was measured as percent lung low attenuation area (%LAA) on the lung fields of cardiac CT scans, which imaged approximately 70% of the lung volume from the carina to each lung base. Cardiac CT scans were performed at full inspiration on multi-detector (MD) and electron-beam CT scanners [36]. Two scans were performed on each participant; the scan with the higher air volume was used for analyses except in cases of discordant scan quality, in which case the higher quality scan was used.
Image attenuation was assessed using modified Pulmonary Analysis Software Suite [37] at a single reading center by trained readers without knowledge of other participant information. The attenuation of each pixel in the lung regions was linearly corrected, such that the mean attenuation outside the body was −1000 and aortic was 50 Hounsfield Units (HU). %LAA was defined as the percentage of the total voxels in the lung below −910 HU. This threshold was chosen based upon pathology comparisons [38] and the generally mild degree of emphysema in the sample. Sensitivity analyses were performed using %LAA defined as the percentage of the total voxels in the lung below −950 HU.
%LAA measures from the carina to lung base are highly correlated (r = 0.99) with full-lung measures on the same full-lung scans in smokers [39]. %LAA measures from cardiac scans correlated with those from full-lung scans from the same MESA participants [40] and have been used in this cohort to confirm multiple prior hypotheses [17], [28].
Smoking
Cigarette, cigar and pipe smoking was self-reported using standardized questionnaire items [41]. Pack-years of cigarettes were calculated as age of starting to age of quitting or current age×(cigarettes-per-day/20). Cigarettes-per-day was assessed twice over a 4-year interval by questionnaire; the greater of the two measures were used in calculations. Current smoking was confirmed by urinary cotinine levels (Immulite 2000 Nicotine Metabolite Assay; Diagnostic Products Corp., Los Angeles, CA) [42].
Covariates
Information on age, gender, race/ethnicity, educational attainment, occupational exposure to dust, fumes, or smoke, environmental tobacco smoke exposure, family history of emphysema, medical history and medication use were self-reported using standardized questionnaire items [41], [43], as recommended. Height, weight and resting blood pressure were measured using standard techniques, the latter using the Dinamap Monitor PRO 100 (Critikon, Tampa, FL). Serum glucose and lipids, including high- and low-density lipoproteins, were measured after 12-hour fast. The presence of diabetes mellitus was defined as fasting serum glucose level >126 mg/dL or current use of any diabetes medication.
Statistical Analysis
The cohort was stratified by quartile of CRVE for descriptive purposes. Multivariate mean differences in lung function and %LAA were estimated using generalized linear models that regressed CRVE, CRAE, log-transformed ACR and MBF on spirometric and %LAA measurements after adjustment for age, gender, race/ethnicity, height, body mass index (BMI), waist and hip circumference. For CT analyses, models were also adjusted for scanner type and mAs. Multivariate models were then additionally adjusted for cigarette smoking status, cigarette pack years and urine cotinine, and subsequently the potential confounders listed in the footnotes to the tables. The presence of effect modification by smoking was tested with the – 2 log likelihood test of nested models with and without interaction terms for smoking. Analyses stratified by smoking status and restricted to individuals with airflow limitation as defined by FEV1/FVC<0.7 were also performed.
95% confidence intervals and P-values were estimated from generalized linear models. A P-value<0.05 was considered to indicate statistical significance. This threshold was not adjusted for multiple comparisons; rather, all analyses are reported, as recommended [44].
Statistical analyses were performed in SAS 9.2 (Cary, NC) and R Statistical Software, version 2.10.0 (R Foundation, Vienna, Austria) was used to generate graphs.
Results
Figure S2 shows the recruitment of 3,965 participants in the MESA Lung Study and exclusions. There were no significant differences between participants with and without retinal and renal measures (data not shown) whereas those with MBF measures were younger, more often White or Hispanic, and more likely to report symptoms of chronic bronchitis (Table S1).
The overall sample of 3,643 was 61.1 years old at the baseline examination and 51% women. Fourteen percent were current smokers and 53% had ever smoked cigarettes. The mean FEV1 was 95.7% of predicted values and mean FEV1/FVC ratio was 74.5%.The median % LAA (<−910 HU) was 18.6 (interquartile range, IQR, 10.0, 29.3).
Table 1 shows the characteristics of the study sample by quartile of CRVE. Participants with a larger CRVE (4th quartile) were more likely to be African-American or Hispanic, of younger age, higher BMI, and have lower education attainment. Tobacco exposure and diabetes were associated with larger CRVE, as were occupational exposure to dust and measures of inflammation.
Table 1. Characteristics of participants in the MESA Lung Study according to retinal vascular caliber as measured by central retinal vein equivalent (CRVE).
| Characteristic | Quartile of Central Retinal Vein Equivalent | |||
| 1st Quartile 123.6–199.7 | 2nd Quartile 199.7–214.2 | 3rd Quartile 214.2–228.1 | 4th Quartile 228.1–315.2 | |
| N = 849 | N = 849 | N = 850 | N = 849 | |
| Age, mean (SD), years | 63.7 (10.0) | 60.9 (9.6) | 60.2 ( 9.5) | 59.7 ( 9.6) |
| Male gender, % | 47.4 | 50.1 | 49.4 | 49.7 |
| Race/ethnicity, % | ||||
| - White (Non-Hispanic) | 52.1 | 41.8 | 28.9 | 19.4 |
| - African American | 15.1 | 20.6 | 29.5 | 39.8 |
| - Asian | 15.2 | 16.5 | 17.1 | 16.6 |
| - Hispanic | 17.7 | 21.1 | 24.5 | 24.2 |
| Education, years, % | ||||
| - <High school | 12.5 | 14.4 | 18.6 | 19.7 |
| - High school & beyond | 87.5 | 67.6 | 81.4 | 80.3 |
| Body mass index, mean (SD), kg/m2 | 27.2 (4.9) | 27.7 (4.9) | 28.2 (5.4) | 28.7 (5.6) |
| Waist circumference, mean (SD), cm | 95.6 (13.6) | 96.3 (13.6) | 97.5 (13.9) | 98.5 (14.7) |
| Height, mean (SD), cm | 166.3 (10.0) | 167.0 (10.3) | 166.3 (10.1) | 166.7 (9.8) |
| Cigarette smoking,% | ||||
| - Never | 51.5 | 52.5 | 46.1 | 39.2 |
| - Past | 41.1 | 38.2 | 38.9 | 36.4 |
| - Current | 7.4 | 9.3 | 15.1 | 24.4 |
| Pack-years,* median (IQR) | 15.0 (5.0, 33.0) | 17.0 (6.0,32.3) | 16.5 (6.3,34.2) | 20.0 (7.6, 37.0) |
| Cotinine,† median (IQR), ng/mL | 2351 (226, 6479) | 3058 (769,6012) | 3891 (1127,7039) | 4864 (1594, 9203) |
| Environmental tobacco exposure, % | 41.6 | 39.4 | 44.0 | 46.6 |
| Family history of emphysema, % | 5.2 | 3.7 | 6.4 | 3.9 |
| Asthma before age 45 years, % | 7.5 | 7.4 | 8.1 | 8.5 |
| Hypertension, % | 44.6 | 40.4 | 40.3 | 41.3 |
| Blood pressure, mean (SD), mmHg | ||||
| - Systolic | 125.2 (19.6) | 124.1 (20.1) | 124.1 (19.0) | 124.2 (19.4) |
| - Diastolic | 70.7 (10.2) | 72.3 (10.1) | 72.1 (9.7) | 71.9 (9.8) |
| Diabetes mellitus, % | 7.3 | 9.1 | 10.7 | 14.4 |
| Fasting plasma glucose, median (IQR),mg/100 mL | 95 (89,103) | 95 (89,103) | 97 (90,106) | 98 (92,108) |
| High-density lipoprotein, mean (SD), mg/dL | 53.6 (15.5) | 51.4 (15.3) | 50.4 (14.8) | 48.3 (13.2) |
| Low-density lipoprotein, mean (SD), mg/dL | 116.0 (29.8) | 117.1 (30.7) | 118.6 (30.9) | 118.8 (31.8) |
Abbreviations: SD = standard deviation; IQR = interquartile range.
Definitions: Hypertension = physician diagnosis of hypertension or systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg; Diabetes mellitus = physician diagnosis of diabetes or fasting plasma glucose >126 mg/dl.
Among ever smokers.
Among current smokers.
Retinal Vascular Caliber
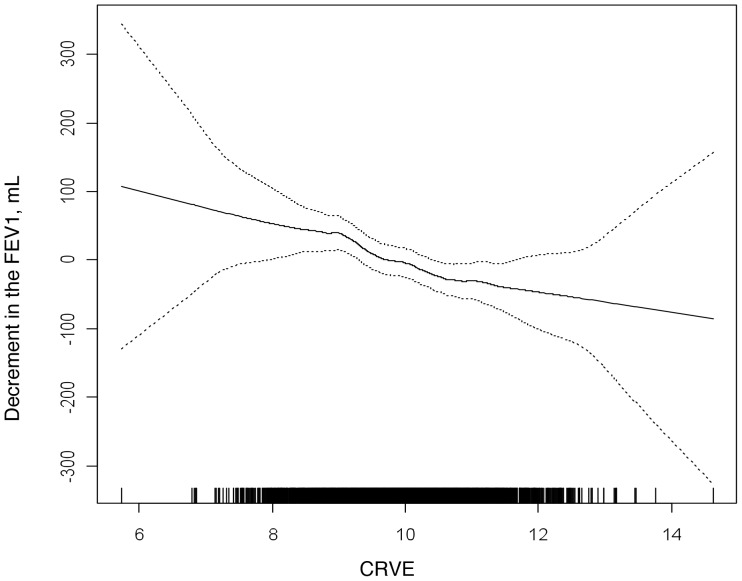
Table 2 shows measures of lung function stratified by quartiles of CRVE and mean difference in lung function per standard deviation (SD) unit of CRVE. There were highly significant associations between CRVE and all spirometric measures. These associations were somewhat attenuated after adjustment for smoking variables but remained statistically significant after adjustment for multiple measures of smoking in addition to other potential confounders. The association of CRVE to the FEV1 was linear without evidence for a threshold effect (Figure 2).
Table 2. Mean differences in lung function and percent low attenuation area (%LAA) by retinal vascular caliber as measured by central retinal vein equivalent (CRVE).
| Quartile of Central Retinal Vein Equivalent | Mean difference per 1 SD unit of CRVE (95% CI) | P-value | ||||
| 1st Quartile 123.6–199.7 | 2nd Quartile 199.7–214.2 | 3rd Quartile 214.2–228.1 | 4th Quartile 228.1–315.2 | |||
| N = 849 | N = 849 | N = 850 | N = 849 | |||
| FEV1,mL | ||||||
| Model 1† | 0 | −37 | −78 | −129 | −53 (−69,−38) | <0.001 |
| Model 2‡ | 0 | −31 | −55 | −81 | −33 (−48,−18) | <0.001 |
| Model 3§ | 0 | −35 | −46 | −71 | −28 (−43,−14) | <0.001 |
| FEV1/FVC,% * | ||||||
| Model 1† | 0 | −0.9 | −1.5 | −1.9 | −0.8 (−1.0,−0.5) | <0.001 |
| Model 2‡ | 0 | −0.8 | −1.0 | −0.9 | −0.3 (−0.6,−0.1) | 0.02 |
| Model 3§ | 0 | −0.9 | −0.9 | −0.8 | −0.3 (−0.6,−0.0) | 0.04 |
| LAA, % | ||||||
| Model 1† | 0 | −1.1 | −1.4 | −1.6 | −0.6 (−1.0,−0.2) | 0.004 |
| Model 2‡ | 0 | −1.1 | −1.2 | −1.0 | −0.3 (−0.8, 0.1) | 0.11 |
| Model 3§ | 0 | −1.1 | −1.1 | −1.0 | −0.3 (−0.8, 0.1) | 0.13 |
Abbreviations: SD = standard deviation; CRVE = Central Retinal Vein Equivalent; CI = confidence interval.
Includes 16 fewer participants than FEV1 analysis.
Model 1: Adjusted for age, gender, race/ethnicity, body mass index, height, waist and hip circumference and, for CT analyses, CT scanner type.
Model 2: Adjusted for all the variables in model 1 plus cigarette smoking status, cigarette pack years and urine cotinine.
Model 3: Adjusted for all the variables in model 2 plus cigar-years, pipe-years, environmental tobacco exposure, occupational exposure to dust, asthma before age 45, family history of emphysema, chronic bronchitis, educational attainment, diabetes mellitus, fasting blood glucose, hypertension, systolic blood pressure, diastolic blood pressure, high-density lipoprotein, low-density lipoprotein, C reactive protein, fibrinogen, aspirin use, beta blocker use, angiotensin II receptor blocker and/or angiotensin converting enzyme inhibitor use, statin use, diuretic use, hormone replacement therapy use, bronchodilator use, oral or inhaled steroid use.
Figure 2. Multivariate association of the central retinal vein equivalent (CRVE) and the forced expiratory volume in one second (FEV1).
The association of CRVE to the FEV1 was linear in the fully adjusted model, and without evidence for a threshold effect. Covariates include age, gender, race/ethnicity, height, BMI, waist and hip circumference, cigarette smoking status, pack-years, urine cotinine, cigar-years, pipe-years, environmental tobacco exposure, occupational exposure to dust, asthma before age 45, family history of emphysema, chronic bronchitis, educational attainment, diabetes mellitus, fasting blood glucose, hypertension, systolic blood pressure, diastolic blood pressure, high-density lipoprotein, low-density lipoprotein, C reactive protein, fibrinogen, aspirin use, beta blocker use, angiotensin II receptor blocker and/or angiotensin converting enzyme inhibitor use, statin use, diuretic use, hormone replacement therapy use, bronchodilator use, and oral or inhaled steroid use. Dotted lines are 95% confidence intervals.
The relationship of CRVE with the FEV1 was modified by smoking status (P-interaction = 0.008): a 93 mL decrement in current smokers (95% CI {−148, −38}; P<0.001), 48 mL decrement in former smokers (95% CI {−73, −23}; p<0.001), and 4 mL decrement in never smokers per SD unit of CRVE (95% CI {−23, 15}; P = 0.67).
There was no evidence that CRVE was associated with %LAA after adjustment for smoking variables and other potential confounders. CRAE was associated with neither lung function nor %LAA (Table S2).
Albuminuria
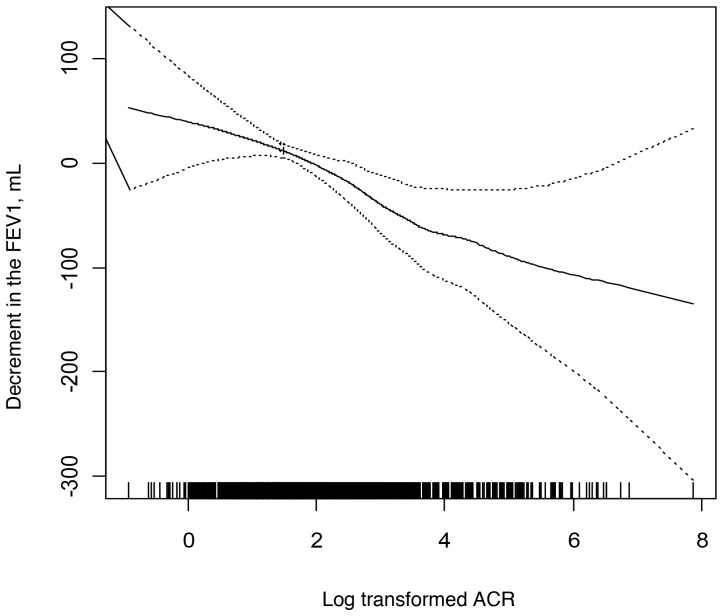
Table 3 shows measures of lung function and %LAA by urine albumin excretion (UAE) as defined by degree of albuminuria. Mean difference in FEV1 was −25 mL per SD unit of log-transformed ACR (95% CI {−40, −9}, P = 0.002).There were highly significant associations of UAE and ACR, respectively, to the FEV1 and FVC before and after adjustment for smoking variables and other covariates, but not to the FEV1/FVC ratio. The association of ACR to the FEV1 was linear on a log scale (Figure 3).
Table 3. Mean differences in lung function and percent low attenuation area (%LAA) by renal function, as measured by urine albumin excretion (UAE) categories defined by albumin-to-creatinine ratio (ACR).
| Urine Albumin Excretion | P-value | ||||
| None | High normal | Micro-albuminuria | Macro-albuminuria | ||
| N = 2802 | N = 396 | N = 394 | N = 35 | ||
| FEV1,mL | |||||
| Model 1† | 0 | −79 | −106 | −109 | <0.0001 |
| Model 2‡ | 0 | −80 | −97 | −108 | 0.0002 |
| Model 3§ | 0 | −58 | −70 | −49 | 0.0006 |
| FEV1/FVC,% * | |||||
| Model 1† | 0 | −0.6 | −0.7 | 1.1 | 0.18 |
| Model 2‡ | 0 | −0.6 | −0.5 | 1.1 | 0.65 |
| Model 3§ | 0 | −0.5 | −0.7 | 0.5 | 0.12 |
| LAA, % | |||||
| Model 1† | 0 | −0.3 | −2.1 | −1.2 | 0.0022 |
| Model 2‡ | 0 | −0.4 | −2.1 | −1.5 | 0.24 |
| Model 3§ | 0 | −0.1 | −1.3 | −0.3 | 0.11 |
Abbreviations: SD = standard deviation; ACR = albumin-to-creatinine ratio; CI = confidence interval.
Definitions: 1) High normal urine albumin excretion: ACR 9–16.9 mg/g in men and 13–24.9 mg/g in women; 2) Microalbuminuria: ACR 17–250 mg/g in men and 25–354.9 mg/g in women; and 3) Macroalbuminuria: ACR ≥250 mg/g in men and ≥355 mg/g in women.
Includes 17 fewer participants than FEV1 analysis.
Model 1: Adjusted for age, gender, race/ethnicity, body mass index, height, waist and hip circumference and, for CT analyses, CT scanner type.
Model 2: Adjusted for all the variables in model 1 plus cigarette smoking status, cigarette pack years and urine cotinine.
Model 3: Adjusted for all the variables in model 2 plus cigar-years, pipe-years, environmental tobacco exposure, occupational exposure to dust, asthma before age 45, family history of emphysema, chronic bronchitis, educational attainment, diabetes mellitus, fasting blood glucose, hypertension, systolic blood pressure, diastolic blood pressure, high-density lipoprotein, low-density lipoprotein, C reactive protein, fibrinogen, aspirin use, beta blocker use, angiotensin II receptor blocker and/or angiotensin converting enzyme inhibitor use, statin use, diuretic use, hormone replacement therapy use, bronchodilator use, oral or inhaled steroid use.
Figure 3. Multivariate association of the log-transformed albumin-to-creatinine ratio (ACR) and the forced expiratory volume in one second (FEV1).
The association of ACR to FEV1 was linear on a log scale in the fully adjusted multivariate model. Covariates include age, gender, race/ethnicity, height, BMI, waist and hip circumference, cigarette smoking status, pack-years, urine cotinine, cigar-years, pipe-years, environmental tobacco exposure, occupational exposure to dust, asthma before age 45, family history of emphysema, chronic bronchitis, educational attainment, diabetes mellitus, fasting blood glucose, hypertension, systolic blood pressure, diastolic blood pressure, high-density lipoprotein, low-density lipoprotein, C reactive protein, fibrinogen, aspirin use, beta blocker use, angiotensin II receptor blocker and/or angiotensin converting enzyme inhibitor use, statin use, diuretic use, hormone replacement therapy use, bronchodilator use, and oral or inhaled steroid use. Dotted lines are 95% confidence intervals.
Smoking status did not modify the associations of ACR with lung function (P-interaction>0.10) although the magnitude of the association was greater in smokers: a decrement in the FEV1 of 67 mL (95% CI {−121, −13}; P = 0.02) in current smokers, 48 mL (95% CI {−71, −26}; P<0.001) in former smokers and 18 ml (95% CI { −36, 0.1}; P = 0.06) in never smokers.
Associations with %LAA were not statistically significant in fully adjusted models for UAE (Table 3) or for ACR (−0.3% per 1 SD unit of ACR, 95% CI {−0.8, 0.1}; P = 0.13).
Myocardial Perfusion
Mean differences in lung function and %LAA per SD unit of resting and hyperemic MBF are shown in Table 4. In fully adjusted models, hyperemic MBF was positively associated with measures of airflow limitation (both FEV1 and FEV1/FVC) and inversely associated with %LAA. Findings were essentially unchanged after additional adjustment of hyperemic MBF for resting MBF (FEV1 112 mL, 95% CI {14, 210}; P-value 0.03; FEV1/FVC 2.9%, 95% CI {1.1, 4.6}; P = 0.002; and %LAA −2.6%, 95% CI {−5.5, 0.2}; P = 0.07). In addition, %LAA was associated with resting MBF. Associations were qualitatively of greater magnitude among smokers (data not shown).
Table 4. Mean differences in lung function and percent low attenuation area (%LAA) by myocardial blood flow (MBF) at rest and during hyperemia.
| Resting MBF, ml/g/min (95% CI) | P-value | Hyperemic MBF, ml/g/min (95% CI) | P-value | |
| FEV1,mL | ||||
| Model 1† | 150 (−226, 526) | 0.43 | 62 (−47, 171) | 0.26 |
| Model 2‡ | 112 (−245, 469) | 0.54 | 94 (−8, 197) | 0.07 |
| Model 3§ | 118 (−244, 481) | 0.52 | 113 (16, 211) | 0.02 |
| FEV1/FVC,% | ||||
| Model 1† | 5.7 (−0.6, 11.9) | 0.08 | 2.0 (−0.2, 3.8) | 0.03 |
| Model 2‡ | 4.3 (−1.5, 10.0) | 0.14 | 2.5 (0.8, 4.1) | 0.003 |
| Model 3§ | 5.1 (−1.7, 11.8) | 0.14 | 3.0 (1.2, 4.7) | 0.001 |
| LAA, % | ||||
| Model 1† | −9.4 (−19.6, 0.9) | 0.07 | −1.7 (−4.6, 1.3) | 0.27 |
| Model 2‡ | −8.1 (−17.7, 1.5) | 0.10 | −1.4 (−4.2, 1.4) | 0.34 |
| Model 3§ | −14.7 (−25.4, −3.9) | 0.008 | −3.1 (−6.0, 0.1) | 0.04 |
Abbreviations: SD = standard deviation; MBF = myocardial blood flow; CI = confidence interval;
Model 1: Adjusted for age, gender, race/ethnicity, body mass index, height, waist and hip circumference and, for CT analyses, CT scanner type.
Model 2: Adjusted for all the variables in model 1 plus cigarette smoking status, cigarette pack years and urine cotinine.
Model 3: Adjusted for all the variables in model 2 plus cigar-years, pipe-years, environmental tobacco exposure, occupational exposure to dust, asthma before age 45, family history of emphysema, chronic bronchitis, educational attainment, diabetes mellitus, fasting blood glucose, hypertension, systolic blood pressure, diastolic blood pressure, heart rate, high-density lipoprotein, low-density lipoprotein, C reactive protein, fibrinogen, aspirin use, beta blocker use, angiotensin II receptor blocker and/or angiotensin converting enzyme inhibitor use, statin use, diuretic use, hormone replacement therapy use, bronchodilator use, oral or inhaled steroid use.
Supplemental Analyses
After the exclusion of participants with diabetes, the magnitude of associations for the FEV1 and FEV1/FVC ratio generally increased. Exclusion of participants with hypertension had little impact on the results and there was no evidence for effect modification by race/ethnicity or gender (data not shown).
The relationship between CRVE and FEV1/FVC was similar among 783 participants with airflow limitation compared to all participants but did not attain statistical significance (−0.02% per 1 SD unit CRVE, P = 0.06). The association between FEV1 and ACR among those with airflow limitation was attenuated (a decrement in FEV1 of 13 mL per 1 SD unit ACR; P = 0.58).
Using a threshold of −950 HU to define %LAA, the association with resting MBF remained significant (P = 0.004), the relationship with hyperemic MBF did not (P = 0.12), and the statistical significance of all other results were unchanged (data not shown). Sensitivity analysis using an inflated measure of pack-years also did not alter the results.
Discussion
Microvascular changes in the retina, kidneys and heart were associated with decrements in lung function in this large, population-based cohort free of clinical cardiovascular disease. In addition, impaired myocardial microvascular function on MRI scanning was associated with lower lung function and lower lung density on CT scan.
We assessed three complementary measures of systemic microvascular disease in the retinal, renal and myocardial circulations. The first, retinal vascular caliber, has been widely used as a marker for changes in the microvasculature [45], [46]. No prior studies have examined retinal vascular caliber in COPD, to our knowledge, although one study found impaired hemodynamic changes of extraocular orbital arteries in COPD patients compared to healthy controls [47].
Retinal venular caliber, which is typically considered reflective of early diabetic microvascular changes [46], was linearly related to an obstructive pattern of spirometry. In studies of the effect of hyperglycemia on retinal blood flow, venular vasodilation is thought to occur due to oxidative stress and local “pseudohypoxia” [48]. In addition, endothelial dysfunction, abnormal nitric oxide levels and inflammatory mediators interfere with vascular auto-regulation, leading to vasodilation of the retinal veins [49]. Retinal venular caliber in MESA was associated with brachial artery FMD, a measure of endothelial dysfunction [50], and we have previously shown that brachial artery FMD is associated with lung function and %LAA [15]. The observed association of CVRE with lung function may therefore reflect endothelial dysfunction or hypoxemia, although it is unlikely that many participants in this population-based study were significantly hypoxemic.
In contrast, there was no evidence for an association of lung function with retinal arteriolar caliber, which is typically narrowed in hypertension and hypertensive arteriopathies [30].
The second microvascular measure, albuminuria, reflects endothelial dysfunction and microvascular damage in the renal circulation [51]. A prior small study found a significant relationship between microalbuminuria and COPD exacerbations [52], and in another, microalbuminuria was greater among 129 COPD patients compared to 51 controls [53]. The lack of association in the present study between albuminuria and FEV1/FVC may reflect residual confounding by obesity. Although we did not detect an association with low lung density in this healthy cohort, severe emphysema, as measured by %LAA on quantitative CT, was recently shown to be associated with reduced glomerular filtration rate in smokers [54].
The third measure, myocardial perfusion, is thought to be affected by impairment of smooth muscle relaxation and endothelial dysfunction at a microcirculatory level in the absence of coronary epicardial stenosis [32]. In the present study, coronary vasodilatation with adenosine demonstrated an association of hyperemic MBF with lung function, which suggests impaired cardiac microvascular perfusion. The relationship of resting MBF to %LAA also suggests impairment of microvascular blood flow in the heart. This association is unlikely to reflect any epicardial abnormalities since there is no evidence for an association of %LAA with coronary artery calcium in this cohort [28]. A previous thallium-201 imaging study reported reversible perfusion defects in COPD patients attributable to reduced thallium uptake due to cellular dysfunction [55]. The present study adds to this finding by linking the reduced contrast uptake to microvascular dysfunction, as gadolinium is an extracellular, blood-borne tracer that allows direct estimation of MBF and microvascular function without regard to cellular function.
Taken in totality, findings from this large study with a unique combination of microvascular measures suggest that there are diffuse systemic microvascular changes associated with decrements in lung function in healthy individuals, especially in smokers. While the cross-sectional design of the study prevents definitive conclusions about causality and the direction of the association (i.e. whether microvascular changes contribute to airflow limitation or the reverse), we speculate smoking could cause microvascular disease with resultant end-organ damage to the lungs via related mechanisms by which it causes microvascular disease in the systemic circulation. Although this concept is relatively unexplored in COPD as classically defined by airflow obstruction, this mechanism is biologically plausible given the proximal dose of tobacco smoke to the pulmonary circulation and the small probability that the pulmonary microcirculation is immune to the effects of tobacco smoke.
The few existing biopsy studies on pulmonary microvascular structure in COPD demonstrate microvascular remodeling and redistribution [56], [57], [58], [59] and functional studies show that airway blood flow and vasodilator response is reduced in COPD [60]. In a mouse model, tobacco smoke-induced alterations in lung vascular structure and function preceded emphysema, and were independent of hypoxia [61].
Alternatively, decrements in lung function and structure might cause systemic microvascular disease, potentially via hypoxemia [53], which was not measured in the present study. Another possibility is a shared susceptibility to cigarette smoke; that is, persons with a susceptibility to smoking-related systemic microvascular disease are also susceptible to smoking-related lung disease. For example, genome-wide association studies of lung function have found significant associations for variants in the receptor for advanced glycation end products (RAGE) gene, which is also implicated in microvascular disease [62].
There are several limitations to our study. There is the potential for residual confounding in observational studies, particularly by smoking. However, dose of current smoking was precisely measured by the assessment of cotinine levels and sensitivity analyses using inflated measures of pack-years yielded similar results (data not shown). An additional limitation is that %LAA was assessed on cardiac scans, an approach which has been previously validated in this cohort and which has confirmed prior hypotheses [17], [28], [40]. Smoking-related emphysema has an apical predominance and the apices were less accurately measured than the bases in the current study because the coronary calcium scans assessed the lower 70% of the lung. This may explain why associations of the retina and kidney were more evident for measures of lung function than lung density, which is the opposite of what we found in a study of endothelial dysfunction among smokers using full-lung scans [15]. Finally, spirometry was measured approximately four years after the other measures, which could have resulted in temporally dissociated relationships; however, this is unlikely to have had a significant impact on the results given the small change in spirometric values over this time period. Despite these limitations, the large number of participants, including 783 with airflow limitation, contributes to both the power and precision of findings.
In conclusion, low lung function was associated with microvascular changes in the retina, kidney and heart, and low lung density was associated with impaired myocardial microvascular perfusion. These findings suggest that obstructive decrements in lung function may be characterized by defects in the microvascular circulation, which may provide an important link between cardiovascular and pulmonary disease.
Supporting Information
Measurement of myocardial blood flow (MBF) using magnetic resonance imaging (MRI).Figure S1a. The three MRI images from a MESA participant show selected phases for measurement of MBF depicting 1) the transit of a contrast bolus in the right ventricle, 2) peak enhancement of the left ventricular blood pool, and 3) maximum myocardial enhancement. Figure S1b–d. Signal intensity versus time curves were generated in a region of the left ventricle (Fig. S1b) and the anterior myocardial sector (Fig. S1d). MBF was determined by optimizing the shape of the maximum tissue impulse response (Fig. S1c) in conjunction with arterial input (convolution operator symbolized by ∶ in Fig. S1b). The line of best fit is shown in Fig. S1d.
(TIF)
Recruitment of the MESA Lung Study and exclusions for the current study sample. 3,965 participants were recruited from the overall MESA cohort to the MESA Lung Study. Subsequent exclusions based on available/acceptable measurements and restriction on spirometry yielded 3,397 participants in the retinal analysis and 3,627 participants in the renal analysis. Given the availability myocardial blood flow (MBF) measurements in a subset of MESA-Lung participants at one study site, there were 126 participants available for cardiac analysis.
(TIF)
Comparison of characteristics of participants in overall sample with and without myocardial perfusion measurements.
(DOCX)
Lung function and percent low attenuation area (%LAA) by retinal vascular caliber as measured by central retinal artery equivalent (CRAE).
(DOCX)
Funding Statement
This study was funded by National Institutes of Health R01-HL077612, R01-HL093081, and R01-HL075476. MESA is supported by N01-HC95159-HC95169 and UL1-TR000040. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Miniño AM, Murphy SL, Xu J, Kochanek KD (2011) Deaths: final data for 2008. Natl Vital Stat Rep 59 10: 1–126. [PubMed] [Google Scholar]
- 2. Forey BA, Thornton AJ, Lee PN (2011) Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med 11 36: 1–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rennard SI, Vestbo J (2008) Natural histories of chronic obstructive pulmonary disease. Proc Am Thorac Soc 5: 878–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, et al. (1998) Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med 158: 1277–1285. [DOI] [PubMed] [Google Scholar]
- 5. Pesci A, Balbi B, Majori M (1998) Inflammatory cells and mediators in bronchial lavage of patients with chronic obstructive pulmonary disease. Eur Respir J 12: 380–386. [DOI] [PubMed] [Google Scholar]
- 6. Michaud SE, Dussault S, Groleau J, Haddad P, Rivard A (2006) Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: Role of NO and reactive oxygen species. J Mol Cell Cardiol 41: 275–284. [DOI] [PubMed] [Google Scholar]
- 7. O'Rourke MF, Safar ME (2005) Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 46: 200–204. [DOI] [PubMed] [Google Scholar]
- 8. Gooding KM, Tooke JE, von Lany H, Mitra M, Ling R, et al. (2010) Capillary pressure may predict preclinical changes in the eye. Diabetologia 53: 2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshida T, Tuder RM (2007) Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 87 3: 1047–1082. [DOI] [PubMed] [Google Scholar]
- 10. Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, et al. (2008) Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem 283 43: 29447–29460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schweitzer KS, Hatoum H, Brown MB, Gupta M, Justice MJ, et al. (2011) Mechanisms of lung endothelial barrier disruption induced by cigarette smoke: role of oxidative stress and ceramides. Am J Physiol Lung Cell Mol Physiol 301 6: L836–L846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Damico R, Simms T, Kim BS, Tekeste T, Amankwan H, et al. (2011) P53 mediates cigarette smoke-induced apoptosis of pulmonary endothelial cells inhibitory effects of macrophage migration inhibitor factor. Am J Respir Cell Mol Biol 44 3: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nana-Sinkam SK, Lee JD, Sotto-Santiago S, Stearman RS, Keith RL, et al. (2007) Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med 175 7: 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma J, Young DM, Marentette JO, Rastogi P, Turk J, et al. (2012) Lung endothelial cell platelet-activating factor production and inflammatory cell adherence are increased in response to cigarette smoke component exposure. Am J Physiol Lung Cell Mol Physiol 302: L47–L55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, et al. (2007) Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: The Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med 176: 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, et al. (2008) Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178 12: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 17. Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, et al. (2010) Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med 362: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alford SK, van Beek EJ, McLennan G, Hoffman EA (2010) Heterogeneity of pulmonary perfusion as a mechanistic image-based phenotype in emphysema susceptible smokers. Proc Natl Acad Sci U S A 107 16: 7485–7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, et al. (2011) Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med 184: 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takahashi T, Kobayashi S, Fujino N, Suzuki T, Ota C, et al. (2012) Increased circulating endothelial microparticles in COPD patients: a potential biomarker for COPD exacerbation susceptibility. Thorax doi:10.1136/thoraxjnl-2011-201395. [DOI] [PubMed] [Google Scholar]
- 21. Wang JJ, Liew G, Klein R, Rochtchina E, Knudtson MD, et al. (2007) Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J 28: 1984–1992. [DOI] [PubMed] [Google Scholar]
- 22. McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, et al. (2009) Meta-analysis: Retinal vessel caliber and risk for coronary heart disease. Ann Intern Med 151: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, et al. (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426. [DOI] [PubMed] [Google Scholar]
- 24. Hillege HL, Fidler V, Diercks GF, van Gilst W, de Zeeuw D, et al. (2002) Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782. [DOI] [PubMed] [Google Scholar]
- 25. Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, et al. (2007) Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med 167 22: 2490–2496. [DOI] [PubMed] [Google Scholar]
- 26. Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, et al. (2002) Abnormal subendocardial perfusion in Cardiac Syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 346: 1948–1953. [DOI] [PubMed] [Google Scholar]
- 27. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, et al. (2002) Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol 156: 871–881. [DOI] [PubMed] [Google Scholar]
- 28. Barr RG, Ahmed FS, Carr JJ, Hoffman EA, Jiang R, et al. (2012) Subclinical atherosclerosis: airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J 39 4: 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hankinson J, Odencrantz J, Fedan K (1999) Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 159: 179–187. [DOI] [PubMed] [Google Scholar]
- 30. Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, et al. (2006) Retinal vascular caliber, cardiovascular risk factors, and inflammation: The Multi-Ethnic Study of Atherosclerosis (MESA). Invest Ophthalmol Vis Sci 47: 2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kramer H, Jacobs DR Jr, Bild D, Post W, Saad MF, et al. (2005) Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension 46: 38–43. [DOI] [PubMed] [Google Scholar]
- 32. Wang L, Jerosch-Herold M, Jacobs DR Jr, Shahar E, Folsom AR (2006) Coronary risk factors and myocardial perfusion in asymptomatic adults: The Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 47: 565–572. [DOI] [PubMed] [Google Scholar]
- 33. Jerosch-Herold M, Swingen C, Seethamraju RT (2002) Myocardial blood flow quantification with MRI by model-independent deconvolution. Med Phys 29: 886–897. [DOI] [PubMed] [Google Scholar]
- 34. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. (2005) Standardisation of spirometry. Eur Respir J 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 35. Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, et al. (2010) Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Chest 137: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, et al. (2005) Calcified coronary artery plaque measurement with cardiac CT in population-based studies: Standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology 234: 35–43. [DOI] [PubMed] [Google Scholar]
- 37. Hu S, Hoffman EA, Reinhardt JM (2001) Automatic lung segmentation for accurate quantitation of volumetric x-ray CT images. IEEE Trans Med Imaging 20: 490–498. [DOI] [PubMed] [Google Scholar]
- 38. Coxson HO, Mayo JR, Behzad H, Moore BJ, Verburgt LM, et al. (1995) Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Applied Physiol 79: 1525–1530. [DOI] [PubMed] [Google Scholar]
- 39. Reddy S, Barr RG, Berbaum K, McLennan G, van Bee E, et al. (2005) Quantitative evaluation of emphysema presence and distribution via coronary calcium CT compared with full lung study. Abstract presented at the International Conference of the Radiological Society of North America [Google Scholar]
- 40. Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, et al. (2009) Reproducibility and validity of lung density measures from cardiac CT scans–the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol 16: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferris BG (1978) Epidemiology standardization project. Am Rev Respir Dis 118 Supp 2: 1–120. [PubMed] [Google Scholar]
- 42. Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, et al. (2010) The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: A cross-sectional study. Ann Intern Med 152: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bakke PS, Rönmark E, Eagan T, Pistelli F, Annesi-Maesano I, et al. (2011) Recommendations for epidemiological studies on COPD ERS Task Force Report. Eur Respir J 38 6: 1261–1277. [DOI] [PubMed] [Google Scholar]
- 44. Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1: 43–46. [PubMed] [Google Scholar]
- 45. Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, et al. (2001) Retinal microvascular abnormalities and incident stroke: The Atherosclerosis Risk in Communities Study. Lancet 358: 1134–1140. [DOI] [PubMed] [Google Scholar]
- 46. Nguyen TT, Wang JJ, Sharrett AR, Islam FM, Klein R, et al. (2008) Relationship of retinal vascular caliber with diabetes and retinopathy: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 31: 544–549. [DOI] [PubMed] [Google Scholar]
- 47. Ozer T, Altin R, Ugurbas SH, Ozer Y, Mahmutyazicioglu K, et al. (2006) Color Doppler evaluation of the ocular arterial flow changes in chronic obstructive pulmonary disease. Eur J Radiol 57: 63–68. [DOI] [PubMed] [Google Scholar]
- 48. Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, et al. (1993) Hyperglycemic pseudohypoxia and diabetic complications. Diabetes 42: 801–813. [DOI] [PubMed] [Google Scholar]
- 49. Chester AH, Borland JA, Buttery LD, Mitchell JA, Cunningham DA, et al. (1998) Induction of nitric oxide synthase in human vascular smooth muscle: Interactions between proinflammatory cytokines. Cardiovasc Res 38: 814–821. [DOI] [PubMed] [Google Scholar]
- 50. Nguyen TT, Islam FM, Farouque HM, Klein R, Klein BE, et al. (2010) Retinal vascular caliber and brachial flow-mediated dilation: The Multi-Ethnic Study of Atherosclerosis. Stroke 41: 1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stehouwer CD, Smulders YM (2006) Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol 17: 2106–2111. [DOI] [PubMed] [Google Scholar]
- 52. Polatli M, Cakir A, Cildag O, Bolaman AZ, Yenisey C, et al. (2008) Microalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbation. J Thromb Thrombolysis 26: 97–102. [DOI] [PubMed] [Google Scholar]
- 53. Casanova C, de Torres JP, Navarro J, Aguirre-Jaime A, Toledo P, et al. (2010) Microalbuminuria and hypoxemia in patients with COPD. Am J Respir Crit Care Med 182 8: 1004–1010. [DOI] [PubMed] [Google Scholar]
- 54. Chandra D, Stamm JA, Palevsky PM, Leader JK, Fuhrman CR, et al. (2012) The relationship between pulmonary emphysema and kidney function in smokers. Chest 142 3: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mehrotra PP, Weaver YJ, Higginbotham EA (1983) Myocardial perfusion defect on thallium-201 imaging in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol 2: 233–239. [DOI] [PubMed] [Google Scholar]
- 56. Calabrese C, Bocchino V, Vatrella A, Marzo C, Guarino C, et al. (2006) Evidence of angiogenesis in bronchial biopsies of smokers with and without airway obstruction. Respir Med 100: 1415–1422. [DOI] [PubMed] [Google Scholar]
- 57. Zanini A, Chetta A, Saetta M, Baraldo S, Castagnetti C, et al. (2009) Bronchial vascular remodelling in patients with COPD and its relationship with inhaled steroid treatment. Thorax 64: 1019–1024. [DOI] [PubMed] [Google Scholar]
- 58. Hashimoto M, Tanaka H, Abe S (2005) Quantitative analysis of bronchial wall vascularity in the medium and small airways of patients with asthma and COPD. Chest 127: 965–972. [DOI] [PubMed] [Google Scholar]
- 59. Soltani A, Reid DW, Sohal SS, Wood-Baker R, Weston S, et al. (2010) Basement membrane and vascular remodelling in smokers and chronic obstructive pulmonary disease: A cross-sectional study. Respir Res 11: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mendes ES, Campos MA, Wanner A (2006) Airway blood flow reactivity in healthy smokers and in ex-smokers with or without COPD. Chest 129: 893–898. [DOI] [PubMed] [Google Scholar]
- 61. Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, et al. (2011) Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 147: 293–305. [DOI] [PubMed] [Google Scholar]
- 62. Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, et al. (2010) Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Measurement of myocardial blood flow (MBF) using magnetic resonance imaging (MRI).Figure S1a. The three MRI images from a MESA participant show selected phases for measurement of MBF depicting 1) the transit of a contrast bolus in the right ventricle, 2) peak enhancement of the left ventricular blood pool, and 3) maximum myocardial enhancement. Figure S1b–d. Signal intensity versus time curves were generated in a region of the left ventricle (Fig. S1b) and the anterior myocardial sector (Fig. S1d). MBF was determined by optimizing the shape of the maximum tissue impulse response (Fig. S1c) in conjunction with arterial input (convolution operator symbolized by ∶ in Fig. S1b). The line of best fit is shown in Fig. S1d.
(TIF)
Recruitment of the MESA Lung Study and exclusions for the current study sample. 3,965 participants were recruited from the overall MESA cohort to the MESA Lung Study. Subsequent exclusions based on available/acceptable measurements and restriction on spirometry yielded 3,397 participants in the retinal analysis and 3,627 participants in the renal analysis. Given the availability myocardial blood flow (MBF) measurements in a subset of MESA-Lung participants at one study site, there were 126 participants available for cardiac analysis.
(TIF)
Comparison of characteristics of participants in overall sample with and without myocardial perfusion measurements.
(DOCX)
Lung function and percent low attenuation area (%LAA) by retinal vascular caliber as measured by central retinal artery equivalent (CRAE).
(DOCX)