Abstract
Introduction
This review aims to give an overview of available published evidence concerning the association between physical activity and asthma in children, adolescents and adults.
Methods
We included all original articles in which both physical activity and asthma were assessed in case-control, cross-sectional or longitudinal (cohort) studies. Excluded were studies concerning physical fitness, studies in athletes, therapeutic or rehabilitation intervention studies such as physical training or exercise in asthma patients. Methodological quality of the included articles was assessed according to the Newcastle-Ottawa Scale (NOS).
Results
A literature search was performed until June 2011 and resulted in 6,951 publications derived from PubMed and 1,978 publications from EMBASE. In total, 39 studies met the inclusion criteria: 5 longitudinal studies (total number of subjects n = 85,117) with physical activity at baseline as exposure, and asthma incidence as outcome. Thirty-four cross-sectional studies (n = 661,222) were included. Pooling of the longitudinal studies showed that subjects with higher physical activity levels had lower incidence of asthma (odds ratio 0.88 (95% CI: 0.77–1.01)). When restricting pooling to the 4 prospective studies with moderate to good study quality (defined as NOS≥5) the pooled odds ratio only changed slightly (0.87 (95% CI: 0.77–0.99)). In the cross-sectional studies, due to large clinical variability and heterogeneity, further statistical analysis was not possible.
Conclusions
The available evidence indicates that physical activity is a possible protective factor against asthma development. The heterogeneity suggests that possible relevant effects remain hidden in critical age periods, sex differences, or extremes of levels of physical activity (e.g. sedentary). Future longitudinal studies should address these issues.
Introduction
The prevalence of asthma has increased significantly during the past decades [1]. Concurrently, the prevalence of overweight has increased, while physical activity levels have decreased substantially [2], [3]. In 2005, less than half (49.1%) of US adults met the CDC/ACSM (Centers for Disease Control and Prevention/American College of Sports Medicine) physical activity recommendation (at least 30 minutes of moderately intense activity on five days per week or vigorously intense activity for a minimum of 20 minutes on three days each week) [4]. Physical inactivity is an important risk factor, because it is potentially modifiable and therefore an opportunity for prevention. Several studies have shown that training improves cardiopulmonary fitness, asthma symptoms and quality of life in asthmatic subjects [5]. This evidence suggests that training and high levels of physical activity play a role in the course and severity of asthma. Besides this, an etiological relation between physical activity levels and development of incident asthma might also be possible. Different hypotheses have been suggested to explain the possible protective character of physical activity against asthma development such as reducing airway inflammation, a central feature of asthma [6]. Another explanation is that physical activity could positively influence the patency of bronchioles: poor mucociliary clearance from decreased epithelial stimulation secondary to decreased activity can cause excess mucus and airway edema. Decreased deep inspiration and sigh rate during physical inactivity could lead to smooth muscle latching and subsequent increased risk of asthmatic symptoms [7].
We performed a systematic literature review to evaluate the potential causal relation between physical (in)activity and asthma development, and a pooled analysis to estimate the effect size.
Methods
Search strategy
We conducted an electronic search in PubMed (US National Library of Medicine) and EMBASE to obtain all publications on studies that reported on physical activity and asthma published until June 2011. The PubMed search used the Medical Subject Headings (MeSH) terms “motor activity” or text word terms “activity”, “physical activity”, “physical exercise” or “sedentary”, as well as the MeSH term “asthma” or text word terms “asthma”, “asthmatic”, “wheeze” or “wheezing”. The EMBASE search used the MeSH terms “motor activity” or “physical activity” or text word terms “physical activity”, “physical exercise”, “sedentary”, as well as the MeSH terms “asthma” or “wheezing” or the text word terms “asthma”, “asthmatic”, “wheeze” or “wheezing”. These terms were searched using limits that included all articles published in the English language. There were no age restrictions.
We conformed to the MOOSE (Meta-analysis Of Observational Studies in Epidemiology) guidelines for reporting [8] and PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement [9].
Inclusion
Our primary research question concerned the role of habitual physical activity in the development of incident asthma. Therefore, we searched for longitudinal studies in which the exposure (physical activity) precedes the outcome (onset of asthma). In addition, we included studies that looked into asthma prevalence in different physical activity levels. For this goal, we searched cross-sectional studies that investigated physical activity levels in subjects with asthma compared to controls. For maximal sensitivity, a broad inclusion strategy was used. Inclusion criteria were: original articles in which physical activity as well as asthma was studied, and a control group consisting of healthy subjects or general population. Excluded were studies that did not concern habitual physical activity such as studies in athletes, physical fitness, therapeutic or rehabilitation intervention studies such as physical training in asthma patients. Two investigators (ME, MM) independently assessed whether articles met the inclusion criteria. In case of disagreement, consensus was reached through discussion.
Quality assessment and data extraction
Methodological quality of included articles was assessed according to the Newcastle-Ottawa Scale (NOS). This instrument was developed to assess the quality of nonrandomized studies. Its content validity and inter-rater reliability has been established [10]. The NOS gives predefined criteria, some of which have to be further specified for the specific topic. We specified these criteria in a consensus meeting with all authors (criteria are presented in figure S1 and S2) before assessing the studies. In short, longitudinal studies were assessed for quality of selection (representativeness, selection of controls, ascertainment of exposure, no asthma at start of study); comparability (confounding); and outcome (assessment of outcome, length and adequacy of follow-up). Gender, weight, and smoking were identified as important confounders. Studies could be awarded a maximum score of 9 points. Studies with scores of 5 points or more were considered to be of moderate to good study quality. However, all studies were used for analysis, irrespective of NOS score. Quality assessment was done by all five authors using the NOS. Each single article was assessed by at least three authors independently. In case of disagreement the other two authors were consulted. Quality assessment was completed before data extraction was started. Data were extracted from the full text article. Quantitative results were extracted from text and tables, choosing preferably those adjusted for important confounders (gender, weight, and smoking). Data-extraction in the longitudinal studies was performed independently by two authors (ME, CT). If essential data were lacking in the original studies, their authors were contacted.
Statistical analysis
Analyses were performed using the statistical software Review Manager version 5 [11]. Heterogeneity among studies was assessed using the chi-square test (significant at p<0.05) and the Higgins I2 test [12]. A random effects model with the Mantel–Haenszel method was used for pooling the results of different studies. Pooled odds ratios (OR) with 95% confidence intervals (CI) were calculated for the longitudinal studies and a subgroup of the cross-sectional studies, namely those studies that used a motion sensor for measuring physical activity levels. We decided to refrain from statistical pooling of the other cross-sectional studies because of substantial clinical and methodological heterogeneity.
Results
Literature search
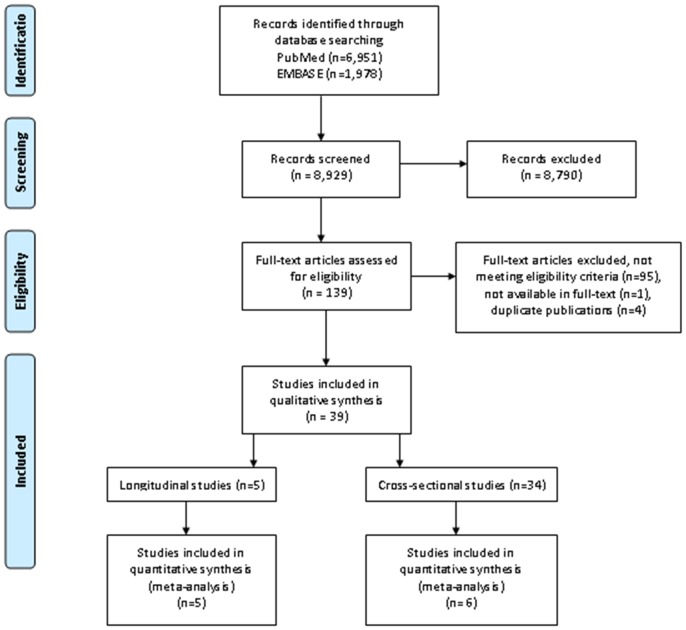
The search resulted in 6,951 publications derived from PubMed, and 1,978 studies from EMBASE. Based on titles and abstracts, 8,790 articles were excluded at first screening because they did not meet the eligibility criteria, such as experimental studies with intermediate outcomes (such as inflammatory markers) but no asthma as clinical outcome, case reports and studies with case series without control group, and studies of exercise induced asthma in athletes. Full-text copies of the remaining 139 potentially relevant studies were obtained. Ninety-five studies were excluded because they did not meet the inclusion criteria. Four were excluded because they were duplicate publications of the same studies. One study was not available in full-text. The remaining 39 studies were included for this systematic review. Five studies were longitudinal studies and 34 were cross-sectional in design (figure 1).
Figure 1. flow diagram of study inclusion.
Study quality
All 39 studies were assessed using the adjusted NOS scale (see figure S1 and S2 for the adjusted NOS scales), of which the majority (79%) scored 5 points or more, indicating a moderate to good study quality (table S1 and S2). When grouped by study quality, we could not detect a clear pattern in study results or study characteristics (tables 1 and 2). The authors of 4 articles [13], [14], [15], [16] were contacted to obtain essential data that were lacking in the original studies, of which 3 replied [14], [15], [16].
Table 1. Overview of longitudinal studies on physical activity at baseline and incident asthma.
| Study basics | Physical activity | Asthma | Confounders | Follow up (yrs) | NOS | Odds ratio (95% CI) | Reference | ||||
| Reference | Country | n | Age | Population | Measurement | Diagnosis | |||||
| Beckett 2001 | US | 4,547 | 18–30 | African American and white adults | Questionnaire #c | Asthma medication or doctor's diagnosis (self reported) | Gender, other #h | 10 | 7 | 2nd quintile: aHR 0.81 (0.57–1.15)3rd quintile: aHR 0.84 (0.59–1.20)4th quintile: aHR 0.94 (0.66–1.35)5th quintile: aHR 1.08 (0.75–1.55) | Highest quintile PA |
| Benet 2011 | France | 51,080 | 40–65 | Women | Questionnaire #d | Asthma attacks and doctor's diagnosis (self reported) | Weight, smoking, other #i | 9 | 7 | 2nd tertile: aHR 1.03 (0.83–1.27)3rd tertile: aHR 1.00 (0.81–1.24) | Lowest tertile PA |
| Huovinen 2003 | Finland | 9,671 | 25–52 | Twins#b | Questionnaire #e | Doctor's diagnosis (insurance register) | Gender, weight, smoking, other #j | 9 | 7 | Men:Occasional: aOR 0.86 (0.41–1.81)Conditioning: aOR 0.54 (0.22–1.33)Women:Occasional: aOR 1.83 (0.74–4.51)Conditioning: aOR 1.42 (0.51–3.93) | Sedentary |
| Lucke 2007 #a | Australia | 19,021 | 18–75 | Women | Questionnaire #f | Doctor's diagnosis (self reported) | None stated | 5–7 | 6 | Older (age 70–75):Nil/low PA: OR 1.15 (0.92–1.47)Mid-aged (age 45–50):Nil/low PA: OR 1.28 (1.09–1.56) *Younger (age 18–23):Nil/low PA: OR 1.12 (0.82–1.54) | Moderate/high PA |
| Thomsen 2006 | Denmark | 798 | 12–41 | Discordant twin pairs | Questionnaire #g | Asthma (self reported) | None stated | 8 | 3 | Dizygotic twin pairs:High PA: OR 1.48 (0.84–2.61)Monozygotic twin pairs:High PA: OR 0.35 (0.13–0.91) * | Low PA |
Overview of study characteristics, study quality based on the Newcastle-Ottawa Scale (NOS) and odds ratios of longitudinal studies on physical activity and asthma incidence. Note that Beckett and Lucke use high physical activity levels as reference category, while Benet, Huovinen and Thomsen use low physical activity levels as reference category.
CI; confidence interval, PA; physical activity, aHR; adjusted hazard ratio, aOR; adjusted odds ratio, OR; odds ratio.
P<0.05.
Data provided by the authors.
Analyses did not account for the correlation between twin pairs but twins were considered unrelated subjects.
PA assessed through questionnaires using the Physical Activity History Score (validated); categorized in five equal levels (quintiles).
PA defined as time spent in household and leisure time PA, converted to metabolic equivalents (METs); categorized in three equal levels (tertiles).
Three categories of PA: Sedentary: Respondents estimating their own leisure time physical activity as practically non-existent. Conditioning: Respondents who exercised at least 6 times per month for at least 30 min with a mean intensity corresponding to walking. Occasional: Respondents who did not meet the criteria of sedentary or conditioning.
Two categories of PA: Nil/low PA: <600 METs (metabolic equivalents) per week (this reflects 30 minutes of moderate activity on five days each week). Moderate/high PA: >600 METs per week.
Two categories of PA: Low PA: <2 hours per week of light leisure time exercise activities. High PA: >2 hours per week of light leisure time exercise activities.
other confounders: age, race, centre, and maximal education.
other confounders: menopausal status, education level, working status, and co-morbidities.
other confounders: age, atopy, and respiratory symptoms.
Table 2. Overview of cross-sectional studies on physical activity and asthma prevalence.
| Study basics | Physical activity | Asthma | Confounders | NOS | Odds ratio (95% CI) | Reference | Author's conclusions | |||
| Reference | Country | n | Age | Measurement | Diagnosis | |||||
| Adults Questionnaire | ||||||||||
| Chen 2001 | Canada | 16,813 | >12 | Questionnaire #c | Doctor's diagnosis (self reported) | Weight, smoking, other | 6 | It was concluded that asthmatics were not consistently inactive compared with non-asthmatics. | ||
| Dogra 2008 | Canada | 21,636 | 65–79 | Questionnaire #f | Doctor's diagnosis (self reported) | Gender, weight, other | 5 | Older asthmatics were less active than their non-asthmatic peers. | ||
| Ford 2003 | U.S. | 165,123 | >18 | Questionnaire #b d f | Doctor's diagnosis (self reported) | Gender, weight, smoking, other | 8 | Participants with current asthma were significantly more often considered to be inactive and had significantly lower estimated energy expenditure compared with respondents who never had asthma. | ||
| Kilpeläinen 2006 | Finland | 10,667 | 18–25 | Questionnaire #a | Doctor's diagnosis (self reported) | Gender, weight, smoking, other | 7 | Men:Moderate PA: aOR 0.62 (0.42–0.92)*Vigorous PA: aOR 0.77 (0.56–1.07)Women:Moderate PA: aOR 0.77 (0.56–1.07)Vigorous PA: aOR 1.19 (0.88–1.60) | low PA | Moderate leisure time physical activity was associated with lower risk of asthma in men, but not among women |
| Mälkiä 1998 | Finland | 7,193 | >30 | Questionnaire #d | Doctor's diagnosis (self reported) and spirometry | Gender | 7 | The intensity of physical activity was lower in theasthmatic subjects than in those who were not asthmatic. | ||
| Ritz 2010 | U.S. | 40 | 21–38 | Questionnaire #a | Doctor's diagnosis | Other | 2 | No differences were found between asthma and controls in physical activity. | ||
| Strine 2007 | U.S. | 354,025 | >18 | Questionnaire #f | Doctor's diagnosis (self reported) | Gender, weight, smoking, other | 7 | No leisure time PA in past 30 days: aOR 1.2 (1.1–1.2)* | Leisure time PA in past 30 days | Moreover, persons who (…) were physically inactive were slightly more likely to have asthma than those without (…) these behaviors. |
| Teramoto 2011 | U.S. | 3,840 | >18 | Questionnaire #b f | Doctor's diagnosis (self reported) | Gender, weight | 6 | No regular PA: aOR 3.01 (1.63–5.55)***No leisure time PA in past 30 days: aOR 2.17 (1.40–3.37)*** | Regular PA in past 30 days | It was found that asthmatic people spent significantly less time on moderate and vigorous physical activity than their nonasthmatic counterparts. |
| Vogt 2008 | U.S. | 4,925 | > 18 | Questionnaire #b | Doctor's diagnosis (self reported) | Gender, weight, smoking, other | 7 | [No significant relation between physical activity and asthma diagnosis.] | ||
| Children Questionnaire | ||||||||||
| Bener 1996 | United Arab Emirates | 729 | 6–14 | Questionnaire #f | Asthma symptoms (self reported) | None stated | 3 | Environmental risk factors associated with asthma were (…) physical exercise. (…) | ||
| Cheng 2010 | China | 232 | 7–14 | Questionnaire #a | Doctor's diagnosis and spirometry | Gender | 4 | Asthmatic children took part in less exercise than their healthy peers. | ||
| Chiang 2006 | China | 429 | 9–11 | Questionnaire #a b | Doctor's diagnosis | Gender, weight, other | 5 | MVPA>90 min/week:Healthy controls: OR 1.24 (0.57–2.71)VPA>60 min/week:Healthy controls: OR 2.03 (1.31–3.15)** | Diagnosed asthma | Asthma interferes with children's ability to participate in vigorous physical activity but not in moderate-to-vigorous physical activity. |
| Corbo 2008 | Italy | 20,016 | 6–7 | Questionnaire #a e | Asthma symptoms (self reported) | Gender, weight, smoking, other | 7 | Rarely PA: aOR 1.05 (0.85–1.29)1–2 times/week: aOR 1.13 (0.93–1.38)>3 times/week: aOR 1.33 (0.99–1.77) | No PA | Our data support the hypothesis that (…) spending a lot of time watching television (…) increases the risk of asthma symptoms in children. Wheeze or asthma was not associated with regular sports activity. |
| Gannotti 2007 | U.S. | 15,300 | 9 | Questionnaire #a e | Doctor's diagnosis (self reported) | None stated | 5 | For children with asthma (…), the most frequent perception of parents was that their children were as active as their peers. Days per week of aerobic activity, number of structured activities per week, and playing sports with parents three times a week or more did not vary significantly between children with and without disabilities [including asthma]. | ||
| Glazebrook 2006 | U.K. | 117 | 7–14 | Questionnaire #a | Doctor's diagnosis and Peak Flow variability | Gender, weight, other | 2 | We found that children attending a hospital clinic for asthma (…) were significantly less active than a comparison group with other medical conditions. | ||
| Jones 2006 | U.S. | 13,222 | high school (grades 9–12) | Questionnaire #b e f | Doctor's diagnosis (self reported) | Gender, other | 6 | Sufficient VPA:Current asthma: OR 1.1 (1.0–1.3)Sufficient MPA:Current asthma: OR 1.1 (0.9–1.3) | No asthma | No significant differences were found for participation in sufficient vigorous or moderate physical activity or strengthening exercises among students with and without current asthma. |
| Kitsantas 2000 | U.S. | 135 | 14–18 | Questionnaire #a | Doctor's diagnosis | Gender (only girls included) | 6 | It was found that asthmatic girls (…) participated less often in vigorous activities than nonasthmatic girls. | ||
| Lang 2004 | U.S. | 243 | 6–12 | Questionnaire #a f | Doctor's diagnosis (self reported) | None stated | 4 | Children with asthma were less active than their peers. | ||
| Nystad 1997 | Norway | 4,585 | 7–16 | Questionnaire #a | Doctor's diagnosis (self reported) | Gender, other | 5 | PA 1–3 times/week: aOR 0.9 (0.5–1.4)PA>3 times/week: aOR 1.1 (0.6–1.9) | PA<3 times/month | The data suggest that asthmatic children are as physically active as their peers. |
| Ownby 2007 | U.S. | 636 | 8–10 | Questionnaire #d | Doctor's diagnosis (self reported) | Gender, other | 7 | Higher levels of physical activity were related to more diagnosed asthma. | ||
| Priftis 2007 | Greece | 700 | 10–12 | Questionnaire #a d e | Asthma symptoms (self reported) | Gender, weight, other | 7 | Not participating in any PA: Asthmatic boys: aOR 2.17 (1.34–3.54)* Asthmatic girls: aOR 1.63 (0.86–3.11) | No asthma | Multiple logistic regression analysis revealed that (…) sedentary lifestyle is associated with asthma symptoms only in boys. |
| Romieu 2004 | U.S. | 7,851 | 2–16 | Questionnaire #e | Doctor's diagnosis (self reported) | Gender, weight, smoking, other | 7 | Television watching >4 hours/day: aOR 2.67 (0.97–7.31) | Television watching <3 hours/day | [No significant relation between physical activity and and asthma diagnosis.] |
| Tsai 2007 | China | 2,218 | 11–12 | Questionnaire #a e | Doctor's diagnosis (self reported) | Gender, weight, other | 6 | Boys:PA 1–2 times/week: aOR 0.74 (0.42–1.32)PA>3 times/week: aOR 0.55 (0.30–1.03)PA every day: aOR 0.76 (0.43–1.35)Girls:PA 1–2 times/week: aOR 1.63 (0.69–3.84)PA>3 times/week: aOR 2.27 (0.92–5.59)PA every day: aOR 1.74 (0.67–4.47) | PA low (<1 time/week) | Results of the present study suggest that sedentary life is associated with increased risk of respiratory symptoms. [No significant relation between physical activity and asthma diagnosis.] |
| Tsai 2009 | China | 1,287 | 11–12 | Questionnaire #a e | Doctor's diagnosis (self reported) | Gender, weight, smoking, other | 7 | PA>30 min, times/week: aOR 1.02 (0.96–1.09) | PA <30 min times/week | The number of respiratory symptoms was positively correlated with (…) self-reported sedentary time per weekend-day in girls. [No significant relation between physical activity and asthma diagnosis.] |
| Vlaski 2008 | Macedonia | 3,026 | 13–14 | Questionnaire #a e | Doctor's diagnosis (self reported) | Gender, weight, smoking, other | 7 | VPA 1–2 times/week: aOR 1.84 (0.94–3.60)VPA>3 times/week: aOR 1.13 (0.40–3.23) | VPA occasionally/never | The findings support the aggravating role of sedentary regimen and poor physical fitness on asthma symptoms. [No significant relation between physical activity and asthma diagnosis.] |
| Vogelberg 2007 | Germany | 2,910 | 16–18 | Questionnaire #a e | Asthma symptoms (self reported) | Gender, weight, smoking, other | 6 | Sport>3 times/week: aOR 0.8 (0.5–1.3) | Sport <1 time/month | In the bivariate analyses, exercising more than once per week (…) was inversely related to new onset of wheeze. The association between physical activity and new onset of wheeze disappeared when active smoking was taken into account. |
| Weston 1989 | New Zealand | 408 | 11–13 | Questionnaire #a | Doctor's diagnosis (self reported) | None stated | 4 | Asthmatic children were significantly more active than nonasthmatic children for all activities and for school activities. | ||
| Children Motion sensor | ||||||||||
| Berntsen 2009 | Norway | 174 | 13–14 | Accelero-meter SenseWear #a c | Doctor's diagnosis (self reported) | Gender, other | 7 | Neither aerobic fitness, total energy expenditure nor hours in moderate to very vigorous intensity physical activity during week and weekend differed between adolescents with and without asthma. | ||
| Eijkemans 2008 | The Netherlands | 305 | 4–5 | Accelero-meter Actigraph and Questionnaire #a | Asthma symptoms (self reported) | Smoking, other | 6 | Total activity (counts/minute)Boys:Recent wheeze: aGMR 1.06 (0.94–1.20) Girls:Recent wheeze: aGMR 0.99 (0.85–1.14) | Never wheeze | Our data provide no evidence that asthmatic symptoms induce a lower physical activity level. |
| Firrincieli 2005 | U.S. | 54 | 3–5 | Accelero-meter Actiwatch #a | Asthma symptoms (self reported) | None stated | 5 | Physical activity measured with the motion sensor was decreased among children with a history of wheezing. | ||
| Rundle 2009 | U.S. | 437 | 4 | Accelero-meter Actiwatch #a e | Doctor's diagnosis or wheeze (both self reported) | Weight, other | 5 | Quartile of mean activity counts/minuteQuartile 2: aOR 0.85 (0.45–1.63)Quartile 3: aOR 1.03 (0.54–1.96)Quartile 4: aOR 0.91 (0.46–1.80) | Quartile 1 (lowest PA) | In cross-sectional analyses (…) asthma symptoms were not associated with physical activity in this age group. |
| Vahlkvist 2009 | Denmark | 214 | 6–14 | Accelero-meter RT3 #a d | Asthma symptoms (self reported) and FEV variability | None stated | 4 | No statistically significant differences were found between the two groups [asthma vs no asthma] in overall daily activity, time spent in high or vigorous activity (…) | ||
| Van Gent 2007 | The Netherlands | 1,614 | 7–10 | Accelero-meter PAM and Questionnaire #a | Doctor's diagnosis (self reported) and FEV variability | None stated | 6 | Childhood asthma does not appear to be associated with a decreased level of daily physical activity in our study population. | ||
| Walders-Abramson 2009 | U.S. | 118 | 10–16 | Pedometer Omron #a | Doctor's diagnosis (self reported) and asthma medication | None stated | 7 | We found similar rates of objectively measured physical activity among youth with well controlled asthma and controls. |
Overview of study characteristics, study quality based on the Newcastle-Ottawa Scale (NOS), odds ratios and author's conclusions of cross-sectional studies on physical activity and asthma prevalence. Odds ratios are noted here only if odds ratios or equivalents with 95% confidence intervals are specified in the article. Author's conclusions are noted only if the author mentions a conclusion on the relation between physical activity and asthma prevalence. If not, a conclusion was drawn based on the data in the article. In this case the conclusion is noted between [ ].
CI; confidence interval, PA; physical activity, aOR; adjusted odds ratio, aHR; adjusted hazard ratio, OR; odds ratio, aGMR; adjusted geometric mean ratio, MVPA; moderate to vigorous physical activity, VPA; vigorous physical activity.
P<0.05.
P<0.01.
P<0.001.
frequency of physical activity (PA).
participation of enough PA to meet the recommendations for PA.
Energy Expenditure (EE).
Metabolic Equivalent of Task (MET).
physical inactivity (e.g. TV watching, computer play).
physically active vs. physically not active group.
Longitudinal studies
Study characteristics and odds ratios of the longitudinal studies [14], [17], [18], [19], [20] are summarized in table 1. All 5 studies looked into physical activity levels of subjects at baseline and incident asthma during follow up. Follow up duration ranged between 5 and 10 years. Physical activity was assessed by questionnaires. Different reference categories, subgroups and confounders were used (table 1). Asthma diagnosis was defined as doctor's diagnosis, either through self report or linkage to an insurance registry.
Statistical analysis and pooling
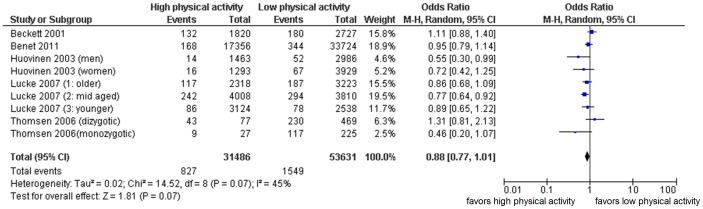
Data of the 5 longitudinal studies were pooled using a random effects model (figure 2). Data on studies with more than two groups of different physical activity levels were converted into two groups, namely low physical activity and high physical activity, of which low physical activity was used as reference category. In case of an uneven number of groups, the reference category consisted of the lowest physical activity levels including the middle group.
Figure 2. pooling of longitudinal data: physical activity at baseline and risk of asthma incidence.
M-H; Mantel-Haenszel method, Random effects, CI; confidence interval. Not adjusted for potential confounders. Low physical activity used as reference category. Note that odds ratios are different of those in table 1 because reference categories were reversed and/or the number of categories was converted into two categories per study. For example Beckett et al. and Lucke et al. use high physical activity as reference category; in our meta-analysis we standardized low physical activity as reference category. In studies were more than two categories of physical activity were used (such as Beckett et al. who used 5 levels of physical activity), these were converted into two categories (in case of Becket et al. we converted the highest two levels into high physical activity, and the lowest three levels into low physical activity).
Pooled odds ratio was 0.88 (95% CI: 0.77–1.01). Chi-square test for heterogeneity was borderline significant (p = 0.07). Higgins I2 index was 45%, indicating moderate inconsistency. These results are not adjusted for potential confounders as the majority of studies did not provide adjusted results. When we restricted analysis to the studies with moderate to good study quality, identified by NOS scores of 5 or higher, 4 studies remained [14], [17], [18], [19]. Sensitivity analysis showed a consistent result: the pooled odds ratio did not change much (0.87 (95% CI: 0.77–0.99)) but did reach statistical significance.
Cross-sectional studies
Study characteristics and results of the 34 cross-sectional studies [13], [15], [16], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [ 39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52] are summarized in table 2. The vast majority (25 studies) examined children of different age spans, 8 studies included only adults, and one study included both children (12 years and older) and adults (table 2).
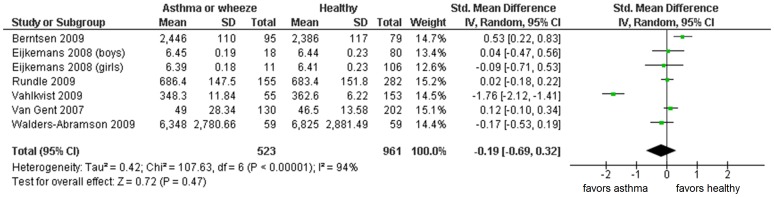
Physical activity was assessed by motion sensors in 7 studies [13], [16], [22], [28], [42], [48], [49], all examining children (n = 2916). In these studies, asthma was defined as self reported doctor's diagnosis or asthma symptoms. Two studies [48], [49] combined self reported asthma diagnosis with spirometry. Not enough data were available for pooling in one study (i.e. standard deviations were missing despite contacting the authors). [13] Data of the other 6 studies using motion sensors were pooled: standard mean difference −0.19 (95% CI −0.69; +0.32) (figure 3). Testing for heterogeneity showed a highly significant result (p<0.00001) and high inconsistency (I2 = 94%).
Figure 3. pooling of cross-sectional data using motion sensors: physical activity measured by motion sensors and asthma prevalence.
Random effects, CI; confidence interval. Not adjusted for potential confounders. Low physical activity used as reference category.
The other 27 studies used only questionnaires to assess physical activity levels. Methods were diverse (table 2): the majority focused on activity by counting frequency and duration of activity per time unit (month, week, day). [15], [24], [25], [26], [30], [31], [33], [34], [35], [37], [39], [40], [46], [47], [50], [52] Others looked into the proportion of subjects that was physically active [21], [27], [29], [32], [35], [43], [44] or met the physical activity recommendation. [25], [29], [32], [44], [51] A relatively small number of studies focused on energy expenditure per day [23] or metabolic equivalent of task (MET). [29], [36], [38], [39] Besides physical activity, inactivity (television watching, sedentary time) was also investigated by 9 studies. [15], [26], [30], [32], [39], [41], [46], [47], [50] Asthma was defined as self reported doctor's diagnosis or asthma symptoms. Only three studies combined questionnaire based asthma diagnosis with spirometry. [24], [31], [36]
We decided to refrain from statistical pooling due to heterogeneity of study designs, populations, and measurement methods for both physical activity and asthma outcome.
In total, 13 studies (564,394 subjects in total) reported a statistically significant association between high physical activity levels and lower asthma prevalence. [13], [24], [25], [27], [29], [31], [33], [34], [35], [36], [39], [43], [44] In contrast, 3 studies (total of 1,773 subjects) found a statistically significant association between high physical activity levels and higher asthma prevalence. [21], [38], [52] Eighteen studies (95,055 subjects) obtained no significant results. [15], [16], [22], [23], [26], [28], [30], [32], [37], [40], [41], [42], [46], [47], [48], [49], [50], [51]
Discussion
This systematic review gives an overview of the published evidence concerning the association between physical activity and asthma. Our primary research question was aimed at the etiological association between different physical activity levels and subsequent asthma incidence. In an extensive search, we only found 5 longitudinal studies that met the inclusion criteria and could be of use in answering this question. Although the number of longitudinal studies was small, the total accrued number of subjects was considerable (n = 85,117). Pooling showed that subjects with higher physical activity levels might have lower risk of developing asthma.
Thirty-four studies were cross-sectional in design. Due to large clinical variability and heterogeneity we had to refrain from further statistical analysis, except for a small group of studies using a motion sensor to measure physical activity. Despite this limitation, however, we can draw some conclusions: a substantial number of included cross-sectional studies, with the largest total study population, did find an association between high physical activity levels and low asthma prevalence. This seems consistent with physical activity being protective against asthma. However, we can not rule out publication bias. Moreover, cross-sectional studies are not suited to give insight into the causal relation between physical activity and subsequent asthma incidence. Besides the hypothesis that subjects with higher physical activity levels have a lower risk of developing asthma (protective), reverse causality is also possible. There are several hypotheses why asthma patients (with asthma as exposure) could have lower physical activity levels (outcome), such as fear for symptoms of shortness of breath, wrongful education, or by asthma that is not well regulated.
In contrast to the studies that were cross-sectional in design, this reverse causality does not play a role in interpreting the results of the 5 longitudinal studies. In all 5 studies physical activity levels were measured before asthma was diagnosed. However, the results could be influenced by protopathic bias (e.g. physical activity restricted by respiratory complaints that precede an asthma diagnosis) or earlier diagnosis of asthma through exercise-induced symptoms. The first would lead to overestimation of the true association between low physical activity levels and subsequent asthma development; whereas the second would lead to an underestimation. Unfortunately, none of the longitudinal studies addressed these biases.
Limitations
It is important to realize that there are several limitations to this review. First of all, due to the fact that this research is based on published material, publication bias is an important factor. Furthermore, studies showed substantial heterogeneity in different areas such as population (number, age, gender, race, duration of follow-up), exposure variables (physical activity measured by questionnaires, whether or not validated, or measured by motion sensors) and outcome variables (asthma diagnosis as self reported doctor's diagnosis, asthma symptoms or spirometry). Analysis showed borderline statistical heterogeneity. The small number of longitudinal studies prevented us from performing meta-regression or subgroup analysis. Confounding is an important issue, because other risk factors (such as smoking and obesity) could be associated with both low habitual physical activity as well as asthma development. First and second-hand cigarette smoke exposure is already established as an independent risk factor for developing asthma. [53] It is suggested that obesity is a risk factor for asthma development. [54] In our meta-analysis of longitudinal studies, pooling of results adjusted for confounders was not sensible because only three studies presented such results. However, adjusted odds ratios were never lower than unadjusted odds ratios (see table S3), so that a pooled effect for the adjusted results would be higher than the odds ratio of 0.88 (95% CI: 0.77–1.01) found for the unadjusted result.
Another limitation might be the fact that the validity of the NOS score recently has been questioned by Stang who believes that the NOS provides a quality score that has unknown validity at best. [55] We noted that some methodological pitfalls were not well represented in the NOS scale: reverse causation and protopathic bias or confounding by indication (e.g. advice to remain physically active for children with respiratory complaints).
Conclusion
In conclusion, the results of available published evidence indicate that high physical activity levels are a possible protective factor against asthma development. The heterogeneity suggests that possible relevant effects remain hidden in critical age periods, sex differences, or extremes of levels of physical activity (e.g. sedentary). Future longitudinal studies should address these issues.
Supporting Information
NOS scale physical activity and asthma longitudinal studies. NOS: Newcastle-Ottawa Scale. Adjusted NOS scale for physical activity and asthma in longitudinal studies.
(DOC)
NOS scale physical activity and asthma cross-sectional studies. NOS: Newcastle-Ottawa Scale. Adjusted NOS scale for physical activity and asthma in cross-sectional studies.
(DOC)
PRISMA 2009 Checklist. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) checklist adjusted for this study.
(DOC)
NOS scores of longitudinal studies. NOS: Newcastle-Ottawa Scale. Result of quality assessment of longitudinal studies on physical activity and asthma using NOS scores. We refer to figure S1 for the adjusted NOS for longitudinal studies, which was used as a scoring list.
(DOC)
NOS scores of cross-sectional studies. NOS: Newcastle-Ottawa Scale. Result of quality assessment of cross-sectional studies on physical activity and asthma using NOS scores. We refer to figure S2 for the adjusted NOS for cross-sectional studies, which was used as a scoring list.
(DOC)
Data extraction of longitudinal studies. CI; confidence interval, PA; physical activity, aHR; adjusted hazard ratio, OR; odds ratio; aOR; adjusted odds ratio, BMI; body mass index. Data extraction of longitudinal studies concerning baseline physical activity and asthma incidence. * p<0.05, #a used as reference category for pooling in this review, #1 adjusted for age, race, sex, center, and maximal education, #2 adjusted for BMI, smoking status, menopausal status, education level, working status, co-morbidities, #3 adjusted for age, atopy, and respiratory symptoms.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Burr ML, Wat D, Evans C, Dunstan FD, Doull IJ (2006) Asthma prevalence in 1973, 1988 and 2003. Thorax 61: 296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241. [DOI] [PubMed] [Google Scholar]
- 3. Livingstone MB, Robson PJ, Wallace JM, McKinley MC (2003) How active are we? Levels of routine physical activity in children and adults. Proc Nutr Soc 62: 681–701. [DOI] [PubMed] [Google Scholar]
- 4. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, et al. (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39: 1423–1434. [DOI] [PubMed] [Google Scholar]
- 5. Ram FS, Robinson SM, Black PN, Picot J (2005) Physical training for asthma. Cochrane Database Syst Rev CD001116. [DOI] [PubMed] [Google Scholar]
- 6. Ford ES (2002) Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology 13: 561–568. [DOI] [PubMed] [Google Scholar]
- 7. Lucas SR, Platts-Mills TA (2005) Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol 115: 928–934. [DOI] [PubMed] [Google Scholar]
- 8. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 2 November 2012.
- 11.Higgins JPT GSe Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available: http://www.cochrane-handbook.org. Accessed 2 November 2012.
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Firrincieli V, Keller A, Ehrensberger R, Platts-Mills J, Shufflebarger C, et al. (2005) Decreased physical activity among Head Start children with a history of wheezing: use of an accelerometer to measure activity. Pediatr Pulmonol 40: 57–63. [DOI] [PubMed] [Google Scholar]
- 14. Lucke J, Waters B, Hockey R, Spallek M, Gibson R, et al. (2007) Trends in women's risk factors and chronic conditions: findings from the Australian Longitudinal Study on Women's Health. Womens Health (Lond Engl) 3: 423–432. [DOI] [PubMed] [Google Scholar]
- 15. Vogelberg C, Hirsch T, Radon K, Dressel H, Windstetter D, et al. (2007) Leisure time activity and new onset of wheezing during adolescence. Eur Respir J 30: 672–676. [DOI] [PubMed] [Google Scholar]
- 16. Walders-Abramson N, Wamboldt FS, Curran-Everett D, Zhang L (2009) Encouraging physical activity in pediatric asthma: a case-control study of the wonders of walking (WOW) program. Pediatr Pulmonol 44: 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beckett WS, Jacobs DR Jr, Yu X, Iribarren C, Williams OD (2001) Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med 164: 2045–2050. [DOI] [PubMed] [Google Scholar]
- 18. Benet M, Varraso R, Kauffmann F, Romieu I, Anto JM, et al. (2011) The effects of regular physical activity on adult-onset asthma incidence in women. Respir Med 105: 1104–1107. [DOI] [PubMed] [Google Scholar]
- 19. Huovinen E, Kaprio J, Koskenvuo M (2003) Factors associated to lifestyle and risk of adult onset asthma. Respir Med 97: 273–280. [DOI] [PubMed] [Google Scholar]
- 20. Thomsen SF, Ulrik CS, Kyvik KO, Larsen K, Skadhauge LR, et al. (2006) Risk factors for asthma in young adults: a co-twin control study. Allergy 61: 229–233. [DOI] [PubMed] [Google Scholar]
- 21. Bener A, Abdulrazzaq YM, Al-Mutawwa J, Debuse P (1996) Genetic and environmental factors associated with asthma. Hum Biol 68: 405–414. [PubMed] [Google Scholar]
- 22. Berntsen S, Carlsen KC, Anderssen SA, Mowinckel P, Hageberg R, et al. (2009) Norwegian adolescents with asthma are physical active and fit. Allergy 64: 421–426. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Dales R, Krewski D (2001) Leisure-time energy expenditure in asthmatics and non-asthmatics. Respir Med 95: 13–18. [DOI] [PubMed] [Google Scholar]
- 24. Cheng BL, Huang Y, Shu C, Lou XL, Fu Z, et al. (2010) A cross-sectional survey of participation of asthmatic children in physical activity. World J Pediatr 6: 238–243. [DOI] [PubMed] [Google Scholar]
- 25. Chiang LC, Huang JL, Fu LS (2006) Physical activity and physical self-concept: comparison between children with and without asthma. J Adv Nurs 54: 653–662. [DOI] [PubMed] [Google Scholar]
- 26. Corbo GM, Forastiere F, De Sario M, Brunetti L, Bonci E, et al. (2008) Wheeze and asthma in children: associations with body mass index, sports, television viewing, and diet. Epidemiology 19: 747–755. [DOI] [PubMed] [Google Scholar]
- 27. Dogra S, Meisner BA, Baker J (2008) Psychosocial predictors of physical activity in older aged asthmatics. Age Ageing 37: 449–454. [DOI] [PubMed] [Google Scholar]
- 28. Eijkemans M, Mommers M, de Vries SI, van Buuren S, Stafleu A, et al. (2008) Asthmatic symptoms, physical activity, and overweight in young children: a cohort study. Pediatrics 121: e666–672. [DOI] [PubMed] [Google Scholar]
- 29. Ford ES, Heath GW, Mannino DM, Redd SC (2003) Leisure-time physical activity patterns among US adults with asthma. Chest 124: 432–437. [DOI] [PubMed] [Google Scholar]
- 30. Gannotti M, Veneri D, Roberts D (2007) Weight status and physical activity in third graders with chronic health conditions. Pediatr Phys Ther 19: 301–308. [DOI] [PubMed] [Google Scholar]
- 31. Glazebrook C, McPherson AC, Macdonald IA, Swift JA, Ramsay C, et al. (2006) Asthma as a barrier to children's physical activity: implications for body mass index and mental health. Pediatrics 118: 2443–2449. [DOI] [PubMed] [Google Scholar]
- 32. Jones SE, Merkle SL, Fulton JE, Wheeler LS, Mannino DM (2006) Relationship between asthma, overweight, and physical activity among U.S. high school students. J Community Health 31: 469–478. [DOI] [PubMed] [Google Scholar]
- 33. Kilpelainen M, Terho EO, Helenius H, Koskenvuo M (2006) Body mass index and physical activity in relation to asthma and atopic diseases in young adults. Respir Med 100: 1518–1525. [DOI] [PubMed] [Google Scholar]
- 34. Kitsantas A, Zimmerman BJ (2000) Self-efficacy, activity participation, and physical fitness of asthmatic and nonasthmatic adolescent girls. J Asthma 37: 163–174. [DOI] [PubMed] [Google Scholar]
- 35. Lang DM, Butz AM, Duggan AK, Serwint JR (2004) Physical activity in urban school-aged children with asthma. Pediatrics 113: e341–346. [DOI] [PubMed] [Google Scholar]
- 36. Malkia E, Impivaara O (1998) Intensity of physical activity and respiratory function in subjects with and without bronchial asthma. Scand J Med Sci Sports 8: 27–32. [DOI] [PubMed] [Google Scholar]
- 37. Nystad W (1997) The physical activity level in children with asthma based on a survey among 7–16 year old school children. Scand J Med Sci Sports 7: 331–335. [DOI] [PubMed] [Google Scholar]
- 38. Ownby DR, Peterson EL, Nelson D, Joseph CC, Williams LK, et al. (2007) The relationship of physical activity and percentage of body fat to the risk of asthma in 8- to 10-year-old children. J Asthma 44: 885–889. [DOI] [PubMed] [Google Scholar]
- 39. Priftis KN, Panagiotakos DB, Antonogeorgos G, Papadopoulos M, Charisi M, et al. (2007) Factors associated with asthma symptoms in schoolchildren from Greece: the Physical Activity, Nutrition and Allergies in Children Examined in Athens (PANACEA) study. J Asthma 44: 521–527. [DOI] [PubMed] [Google Scholar]
- 40. Ritz T, Rosenfield D, Steptoe A (2010) Physical activity, lung function, and shortness of breath in the daily life of individuals with asthma. Chest 138: 913–918. [DOI] [PubMed] [Google Scholar]
- 41. Romieu I, Mannino DM, Redd SC, McGeehin MA (2004) Dietary intake, physical activity, body mass index, and childhood asthma in the Third National Health And Nutrition Survey (NHANES III). Pediatr Pulmonol 38: 31–42. [DOI] [PubMed] [Google Scholar]
- 42. Rundle A, Goldstein IF, Mellins RB, Ashby-Thompson M, Hoepner L, et al. (2009) Physical activity and asthma symptoms among New York City Head Start Children. J Asthma 46: 803–809. [PMC free article] [PubMed] [Google Scholar]
- 43. Strine TW, Balluz LS, Ford ES (2007) The associations between smoking, physical inactivity, obesity, and asthma severity in the general US population. J Asthma 44: 651–658. [DOI] [PubMed] [Google Scholar]
- 44. Teramoto M, Moonie S (2011) Physical Activity Participation among Adult Nevadans with Self-Reported Asthma. J Asthma 517–522. [DOI] [PubMed] [Google Scholar]
- 45. Trzcieniecka-Green A, Bargiel-Matusiewicz K, Wilczynska-Kwiatek A (2009) Quality of life and activity of children suffering from bronchial asthma. Eur J Med Res 14 Suppl 4: 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsai HJ, Tsai AC, Nriagu J, Ghosh D, Gong M, et al. (2007) Associations of BMI, TV-watching time, and physical activity on respiratory symptoms and asthma in 5th grade schoolchildren in Taipei, Taiwan. J Asthma 44: 397–401. [DOI] [PubMed] [Google Scholar]
- 47. Tsai HJ, Tsai AC (2009) The association of BMI and sedentary time with respiratory symptoms and asthma in 5th grade schoolchildren in Kaohsiung, Taiwan. J Asthma 46: 9–15. [DOI] [PubMed] [Google Scholar]
- 48. Vahlkvist S, Pedersen S (2009) Fitness, daily activity and body composition in children with newly diagnosed, untreated asthma. Allergy 64: 1649–1655. [DOI] [PubMed] [Google Scholar]
- 49. van Gent R, van der Ent CK, van Essen-Zandvliet LE, Rovers MM, Kimpen JL, et al. (2007) No differences in physical activity in (un)diagnosed asthma and healthy controls. Pediatr Pulmonol 42: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 50. Vlaski E, Stavric K, Seckova L, Kimovska M, Isjanovska R (2008) Influence of physical activity and television-watching time on asthma and allergic rhinitis among young adolescents: preventive or aggravating? Allergol Immunopathol (Madr) 36: 247–253. [DOI] [PubMed] [Google Scholar]
- 51. Vogt R, Bersamin A, Ellemberg C, Winkleby MA (2008) Evaluation of risk factors and a community intervention to increase control and treatment of asthma in a low-income semi-rural California community. J Asthma 45: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weston AR, Macfarlane DJ, Hopkins WG (1989) Physical activity of asthmatic and nonasthmatic children. J Asthma 26: 279–286. [DOI] [PubMed] [Google Scholar]
- 53. DiFranza JR, Aligne CA, Weitzman M (2004) Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics 113: 1007–1015. [PubMed] [Google Scholar]
- 54. Flaherman V, Rutherford GW (2006) A meta-analysis of the effect of high weight on asthma. Arch Dis Child 91: 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25: 603–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NOS scale physical activity and asthma longitudinal studies. NOS: Newcastle-Ottawa Scale. Adjusted NOS scale for physical activity and asthma in longitudinal studies.
(DOC)
NOS scale physical activity and asthma cross-sectional studies. NOS: Newcastle-Ottawa Scale. Adjusted NOS scale for physical activity and asthma in cross-sectional studies.
(DOC)
PRISMA 2009 Checklist. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) checklist adjusted for this study.
(DOC)
NOS scores of longitudinal studies. NOS: Newcastle-Ottawa Scale. Result of quality assessment of longitudinal studies on physical activity and asthma using NOS scores. We refer to figure S1 for the adjusted NOS for longitudinal studies, which was used as a scoring list.
(DOC)
NOS scores of cross-sectional studies. NOS: Newcastle-Ottawa Scale. Result of quality assessment of cross-sectional studies on physical activity and asthma using NOS scores. We refer to figure S2 for the adjusted NOS for cross-sectional studies, which was used as a scoring list.
(DOC)
Data extraction of longitudinal studies. CI; confidence interval, PA; physical activity, aHR; adjusted hazard ratio, OR; odds ratio; aOR; adjusted odds ratio, BMI; body mass index. Data extraction of longitudinal studies concerning baseline physical activity and asthma incidence. * p<0.05, #a used as reference category for pooling in this review, #1 adjusted for age, race, sex, center, and maximal education, #2 adjusted for BMI, smoking status, menopausal status, education level, working status, co-morbidities, #3 adjusted for age, atopy, and respiratory symptoms.
(DOC)